Abstract
In anticancer therapy, the effectiveness of therapeutics is limited by mutations causing drug resistance. KRAS mutations are the only determinant for cetuximab resistance in patients with colorectal cancer (CRC). However, cetuximab treatment has not been fully successful in the majority of patients with wild-type (WT) KRAS. Therefore, it is important to determine new predictive mutations in CRC treatment. In the present study, the association between AKT1/β-catenin (CTNNB1) mutations with the drug resistance to cetuximab and other chemotherapeutics used in the CRC treatment was investigated by using site-directed mutagenesis, transfection, western blotting and cell proliferation inhibition assay. Cetuximab resistance was higher in the presence of AKT1 E17K, E49K and L52R mutations, as well as CTNNB1 T41A, S45F and S33P mutations compared with that of respective WT proteins. AKT1/CTNNB1 mutations were also associated with oxaliplatin, irinotecan, SN-38 and 5-fluorouracil resistance. Furthermore, mutant cell viability in oxaliplatin treatment was more effectively inhibited compared with that of the other chemotherapeutic drugs. In conclusion, AKT1/CTNNB1 mutations may be used as an important predictive biomarker in CRC treatment.
Keywords: cetuximab, AKT1, β-catenin, drug resistance, colorectal cancer
Introduction
Colorectal cancer (CRC) is the third most common malignancy and is the second most common cause of cancer-associated death worldwide (1). In CRC treatment, drug resistance is an important problem limiting the efficacy of therapeutics (2). Mutations in specific genes are one of the mechanisms contributing to drug resistance. Therefore, it is important to identify the mutations responsible for drug resistance (3).
The epidermal growth factor receptor (EGFR) is an attractive target for cancer treatment due to its function in several critical signal networks (4). Thus, numerous drugs inhibiting EGFR activity have been developed for cancer treatment. For example, cetuximab is a recombinant, chimeric and monoclonal antibody that specifically targets the extracellular domain of human EGFR (5). KRAS, which is one of the proteins involved in the EGFR pathway, is a predictive biomarker for EGFR targeting monoclonal antibodies in CRC treatment (6). The KRAS mutation status of patients with CRC is the most important determinant of cetuximab resistance (7). Although KRAS mutations are largely responsible for cetuximab resistance, there are still patients with wild-type (WT) KRAS who are drug-resistant (8). Therefore, it is important to investigate the possible effects on drug resistance in CRC of other variations in different candidate genes, such as AKT1 and CTNNB1.
AKT1, encoded by the AKT1 gene, is a protein kinase involved in the PI3K/AKT pathway and serves an important role in cellular processes, such as cell proliferation, viability, proliferation, metabolism and angiogenesis. Several mutations in the Pleckstrin homology (PH) domain of AKT1 increase the binding of AKT1 to membrane phospholipids, and this binding causes abnormal activation of AKT1 (9–11). The abnormal activation of the PI3K/AKT pathway is a common cause of resistance to numerous anticancer agents, including conventional chemotherapy and other biological agents such as doxorubicin, paclitaxel and bevacizumab (12). Epidemiological studies reported that AKT1 was found to be mutated in 0.9–6.0% of patients with CRC (9,8,13). The most studied AKT1 E17K mutation was present in 0.7–6.0% of patients with CRC (9,14–16). To the best of our knowledge, there is no study study reporting the frequency of AKT1 E49K and L52R mutations in patients with CRC. β-catenin, encoded by the CTNNB1, gene is one of the major components of the canonical Wnt signaling pathway (17). The mutations, especially in the phosphorylation sites of β-catenin, are called exon 3 mutations, such as S33, T41, S45 and S37, and lead to the stabilization of the protein (17). As a result, β-catenin translocates to the nucleus and initiates the expression of Wnt target genes, such as cyclin D1, c-Myc and CD44, that play a key role in tumor progression (3). Previous studies reported that the frequencies of CTNNB1 mutations were 1.1–6.0% in CRCs (18–20). Although nearly all CTNNB1 missense mutations are localized in exon 3 (21), there is no large-scale analysis study in the literature reporting in detail on the frequency of T41A, S45F and S33P, to the best of our knowledge.
Taken together, mutations causing drug resistance should be identified to improve the response of anti-EGFR treatment and chemotherapy. Although studies have focused on drug resistance and mutations (22–26), only KRAS exon 2 mutations have been demonstrated as predictive biomarkers in CRC treatment. Therefore, in the present study, the contribution of the AKT1/CTNNB1 mutations to resistance to cetuximab, oxaliplatin, irinotecan, SN-38 and 5-fluorouracil (5-FU) was investigated.
Materials and methods
Materials
Human AKT1 and CTNNB1 pCMV6-entry vectors with C-terminal Myc-DDK tag were purchased from OriGene Technologies Inc. (cat. nos. RC220361 and RC208947, respectively). Erbitux (cetuximab, 100 mg/20 ml solution for infusion; Merck KGaA) was purchased from a local pharmacy in Istanbul, Turkey. Oxaliplatin, irinotecan, SN-38 and 5-FU were purchased from Sigma-Aldrich (Merck KGaA).
Cell culture
The human colorectal cancer cell lines Caco-2 (HTB-37), HT-29 (HTB-38) and HCT 116 (CCL-247) were obtained from the American Type Culture Collection. The cell lines were maintained in RPMI-1640 medium (Wisent, Inc.) supplemented with 10% fetal bovine serum (Capricorn Scientific GmbH), a non-essential amino acid solution (Sigma-Aldrich; Merck KGaA), 100 U/ml penicillin and 100 µg/ml streptomycin (Sigma-Aldrich; Merck KGaA). Cells were cultured at 37°C in a humidified atmosphere containing 5% CO2.
AKT1 and CTNNB1 expression constructs
The AKT1 and CTNNB1 mutations were generated by site-directed mutagenesis (QuikChange Site-Directed Mutagenesis kit; cat. no. 200519; Agilent Technologies, Inc.) using a PCR protocol, following the manufacturer's recommendations. The primers used in the present study were purchased from Integrated DNA Technologies, Inc. (Table I).
Table I.
Mutagenic amplification primer sequences.
| Gene mutation | Primer sequence, 5′→3′ |
|---|---|
| AKT1 | |
| Glu17Lys | F: GCACAAACGAGGGAAGTACATCAAGAC |
| R: GTCTTGATGTACTTCCCTCGTTTGTGC | |
| Glu49Lys | F: GATGTGGACCAACGTAAGGCTCCCCTCAAC |
| R: GTTGAGGGGAGCCTTACGTTGGTCCACATC | |
| Leu52Arg | F: CGTGAGGCTCCCCGCAACAACTTCTCTG |
| R: CAGAGAAGTTGTTGCGGGGAGCCTCACG | |
| CTNNB1 | |
| Thr41Ala | F: CATTCTGGTGCCACTGCCACAGCTCCTTCTC |
| R: GAGAAGGAGCTGTGGCAGTGGCACCAGAATG | |
| Ser45Phe | F: CTACCACAGCTCCTTTTCTGAGTGGTAAAG |
| R: CTTTACCACTCAGAAAAGGAGCTGTGGTAG | |
| Ser33Pro | F: CAGTCTTACCTGGACCCTGGAATCCATTC |
| R: GAATGGATTCCAGGGTCCAGGTAAGACTG | |
F, forward; R, reverse.
The bacterial transformation was performed using the heat-shock method with One Shot TOP10F' chemically competent Escherichia coli cells (Invitrogen; Thermo Fisher Scientific, Inc.). The transformation was carried out using the protocol recommended by the manufacturer. Briefly, the transformation mixture was cultured on LB agar (Sigma-Aldrich; Merck KGaA) with kanamycin (Sigma-Aldrich; Merck KGaA) in 100-mm petri dishes. Then, the transformant colonies were harvested and replicated in LB Broth liquid medium (Sigma-Aldrich; Merck KGaA). The DNA was isolated from transformed bacteria using the Plasmid Mini kit (Qiagen GmbH), following the manufacturer's instructions. The mutant constructs were sequenced by RefGen Gene Research and Biotechnology Company (http://www.refgen.com/). After sequencing confirmation, plasmids were purified using the Plasmid Maxi kit (Qiagen GmbH), following the manufacturer's instructions.
Cell transfection
Caco-2 cells were transiently transfected with Myc-DDK-tagged AKT1/CTNNB1 constructs (10 µg) by using Transfast transfection reagent (cat. no. E243; Promega Corporation) by optimizing the protocol recommended by the manufacturer. The cells were also transfected with an empty vector (10 µg) (pCMV6; cat. no. PS100001; OriGene Technologies, Inc.) as a control. Briefly, the cells (3×106) were plated in 100-mm petri dishes. After the cells were incubated for 1 day, cells were incubated with transfection reagent for 1 h at 37°C and added more growth medium to the cells. The cells were incubated for a further 48 h. At the end of the incubation period, transfected Caco-2 cells were harvested and used for analysis, such as cell proliferation inhibition and western blotting.
Cell proliferation inhibition assay
The cell proliferation inhibitory effects of cetuximab and chemotherapeutics were evaluated using the 3-[4,5-dimethylthiazol-2-yl]-5-[3-carboxymethoxyphenyl]-2-[4-sulfophenyl]-2H-tetrazolium, inner salt (MTS) assay (Promega Corporation). Caco-2 cells (3×104), HT-29 cells (3×104) and HCT 116 cells (2×104) were plated in 96-well plates. The following day, the cells were exposed to different concentrations of the drugs (cetuximab, 50–500 µg/ml; oxaliplatin, 0.5–50 µg/ml; irinotecan, 0.5–150 µg/ml; SN-38, 0.039–10 µg/ml; 5-FU, 1–150 µg/ml) for 72 h. Then, MTS/Phenazine methosulfate reagent (v:v; 20:1) was added and incubated for 1–4 h at 37°C. The absorbance was read on a microplate reader (Biotek Instruments, Inc.) at 490 nm. The percentage of cell growth inhibition was calculated as:
Western blot analysis
To obtain whole cell lysates, Caco-2 cells (1×107) were lysed with 100 µl of RIPA lysis buffer (50 mM Tris-HCl pH: 8, 150 mM sodium chloride, 1% Nonidet P-40, 0.5% sodium desoxycholate and 0.1% sodium dodecyl sulfate) and a protease inhibitor cocktail (Sigma-Aldrich; Merck KGaA). The lysate was centrifuged at 15,000 × g for 10 min at 4°C. The supernatant containing the dissolved proteins was put in a new microcentrifuge tube. Protein concentrations of cell lysates were determined using the bicinchoninic acid (BCA) assay (Sigma-Aldrich; Merck KGaA). Briefly, a 10-µl sample and 80 µl of BCA working reagent were added to the wells. The plate was incubated in the dark for 15 min at 60°C and then the absorbance was read against the blank at 562 nm (Biotek Instruments, Inc.). The total protein content of the cell lysates was calculated using the standard curve of bovine serum albumin (range, 200–1,000 µg/ml). The cell lysates (20 µg total protein per well) were separated with SDS-PAGE (any kD precast polyacrylamide gel, cat. no. 4569033; Bio-Rad Laboratories, Inc.) and transferred to polyvinylidene difluoride (PVDF) membranes (Bio-Rad Laboratories, Inc.) using the semi-dry method with electrophoretic Trans-Blot Turbo Transfer system (Bio-Rad Laboratories, Inc.) and blocked with 5% skimmed milk in 0.5% TBS-Tween-20 (TBST) at room temperature for 1 h. The membranes were incubated overnight with mouse anti-DDK (cat. no. TA50011-100, 1:1,000; OriGene Technologies, Inc.) monoclonal primary and goat anti-mouse horseradish peroxidase-conjugated polyclonal secondary antibodies (cat. no. sc-2005; 1:10,000; Santa Cruz Biotechnology, Inc.) at 4°C. The mouse monoclonal anti-GAPDH (cat. no. ab9484; 1:2,000; Abcam) was the loading control. Then, immunoblotted proteins were detected using a luminol-based enhanced chemiluminescence substrate (cat. no. 34075; Thermo Fisher Scientific, Inc.). The membrane was visualized using a gel imaging device Fusion FX (Vilber Lourmat). The protein bands were quantified using the Bio1D software version 15.05 (Vilber Lourmat). The data were expressed as a percentage of the WT.
Statistical analysis
All experiments were performed in triplicate from independent assays. The data are expressed as the mean ± standard deviation. The statistical analyses were performed using SPSS version 22 (IBM Corp). The Kruskal-Wallis test was used to determine the statistical difference and significance was assessed at the level of P<0.05. The Mann-Whitney U test with Bonferroni's correction was used as a post hoc test following the Kruskal-Wallis analysis and the new statistical significance level was set at P<0.017 (0.05/3) to determine the statistical difference.
Results
Cell viability against cetuximab treatment
Before the cell transfection, an MTS assay was performed in all CRC cell lines (Caco-2, HT-29 and HCT 116) in order to determine the dose of cetuximab to be applied. The results exhibited that the cetuximab had no cytotoxic effect on HT-29 and HCT 116 cell lines in the dose range of 50–500 µg/ml (Fig. S1). Therefore, HT-29 and HCT 116 cells were excluded from the drug resistance analysis, and the cell transfection was performed with only Caco-2 cell line. The cetuximab did not indicate any cytotoxic effect on HT-29 and HCT 116.
Effects of AKT1 and CTNNB1 mutations on drug resistance
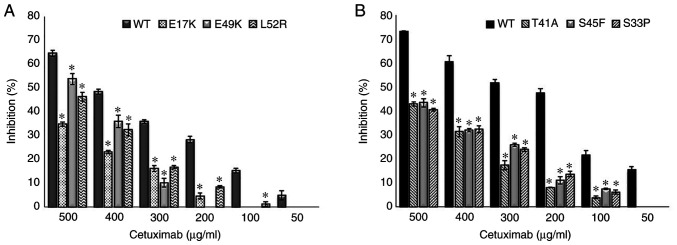
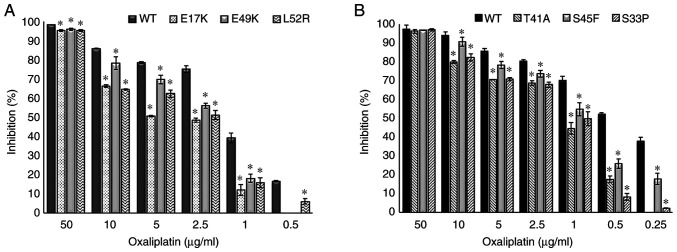
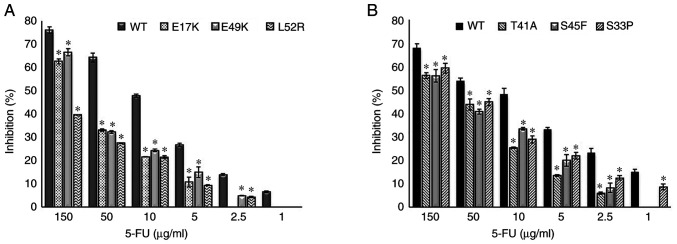
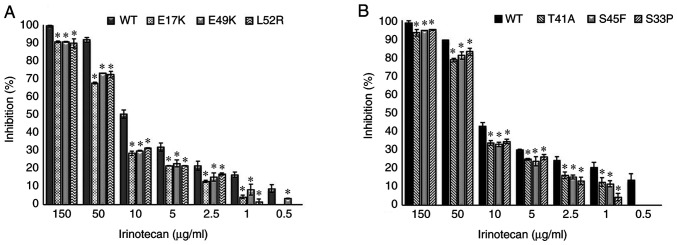
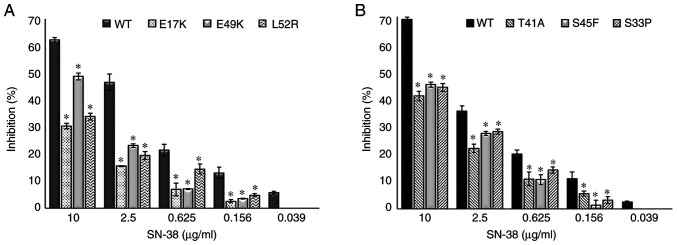
The empty vector, PCMV6, was used as a control and it did not affect drug resistance. The cetuximab-induced cell death (inhibition percentage) in AKT1 WT was found to be significantly higher compared with that of the AKT1 E17K, E49K and L52R at all cetuximab doses (50–500 µg/ml) (all P<0.02) (Fig. 1A). Similarly, the cell death caused by cetuximab in CTNNB1 WT was found to be significantly higher compared with that of the CTNNB1 T41A, S45F and S33P mutations at all doses (all P<0.02) (Fig. 1B). The results showed that all the AKT1/CTNNB1 mutations caused cetuximab resistance. Furthermore, all AKT1/CTNNB1 mutations had a significant effect on oxaliplatin, irinotecan, SN-38 and 5-FU treatment at all drug dose levels, except for 50 µg/ml oxaliplatin in all CTNNB1 mutations (Figs. 2–5). Taken together, AKT1/CTNNB1 mutations caused cetuximab, oxaliplatin, irinotecan, SN-38 and 5-FU resistance.
Figure 1.
Effect of AKT1/CTNNB1 mutations on cetuximab resistance. Effect of (A) AKT1 E17K, E49K and L52R and, (B) CTNNB1 T41A, S45F and S33P on cetuximab resistance. *P<0.02 vs. respective WT. WT, wild-type. CTNNB1, β-catenin.
Figure 2.
Effect of AKT1/CTNNB1 mutations on oxaliplatin resistance. Effect of (A) AKT1 E17K, E49K and L52R and (B) CTNNB1 T41A, S45F and S33P on oxaliplatin resistance. *P<0.02 vs. respective WT. CTNNB1, β-catenin; WT, wild-type.
Figure 5.
Effect of AKT1/CTNNB1 mutations on 5-FU resistance. Effect of (A) AKT1 E17K, E49K and L52R (B) CTNNB1 T41A, S45F and S33P on 5-FU resistance. *P<0.02 vs. respective WT. CTNNB1, β-catenin; WT, wild-type; 5-FU, 5-fluorouracil.
Transfected Caco-2 cells with AKT1 E17K, E49K and L52R, and CTNNB1 T41A, S45F and S33P mutations were found to be most resistant to 5-FU at 10 µg/ml compared with the other chemotherapeutic drugs (Table II). The AKT1/CTNNB1 mutant Caco-2 cells were most effectively inhibited by oxaliplatin (Table II).
Table II.
Effects of chemotherapeutic drugs on mutations at 10 µg/ml.
| Cell proliferation inhibition, % | |||||
|---|---|---|---|---|---|
| Mutation | Oxaliplatin | Irinotecan | SN-38 | 5-FU | P-value |
| E17K | 66.22±0.67 | 28.56±1.31 | 30.38±1.09 | 21.59±0.06 | 0.019 |
| E49K | 78.25±3.30 | 29.95±0.26 | 48.68±1.29 | 24.39±0.55 | 0.016 |
| L52R | 64.48±0.28 | 31.52±0.23 | 33.81±1.22 | 21.52±0.58 | 0.016 |
| T41A | 79.88±0.68 | 33.83±1.30 | 41.07±1.71 | 25.36±0.24 | 0.016 |
| S45F | 90.59±2.42 | 32.92±1.10 | 45.18±0.89 | 33.36±0.54 | 0.024 |
| S33P | 82.25±1.86 | 34.55±1.20 | 44.23±1.39 | 28.89±1.50 | 0.016 |
Data are expressed as mean ± standard deviation (n=3). Data are cell proliferation inhibition (%) values obtained when drugs are administered at a dose of 10 µg/ml for 72 h. 5-FU, 5-fluorouracil.
Western blot analysis
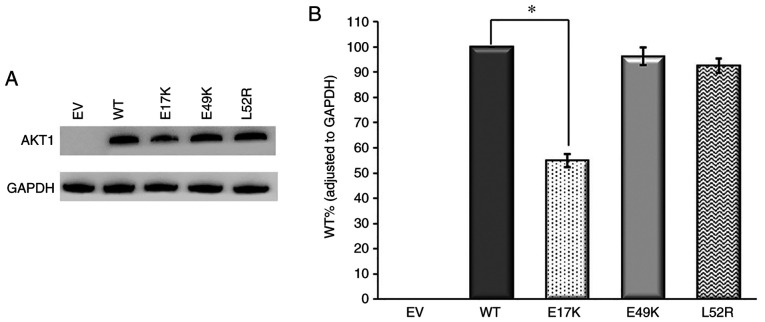
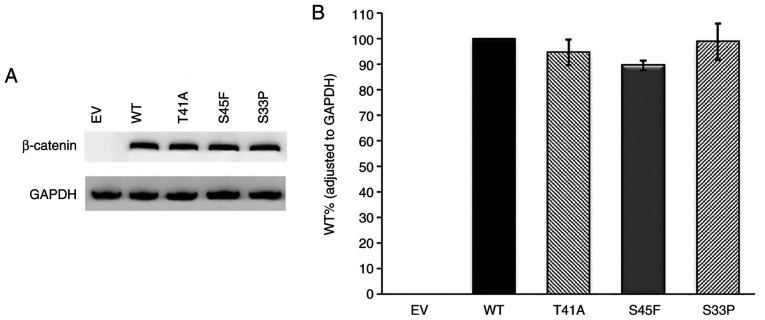
First of all, western blot analysis was performed on the empty vector and AKT1/CTNNB1 WT samples. The pCMV6 empty vector with Myc-DDK tag does not contain related genes. As a result, no band was observed in the empty vector, it was found that the relevant proteins were expressed in the WT gene containing vectors (Figs. 6 and 7). This confirmed that the transfection was performed effectively. Subsequently, western blot analysis of AKT1 and CTNNB1 variants were performed.
Figure 6.
Western blot analysis of AKT1 variants. (A) Immunoreactive AKT1 and GAPDH protein bands and (B) AKT1 WT, E17K, E49K and L52R protein levels. GAPDH was used as the internal control for gene expression. Anti-DDK monoclonal antibody was used for AKT1 detection. n=3. *P<0.02 vs. respective WT. EV, empty vector; WT, wild-type.
Figure 7.
Western blot analysis of β-catenin variants. (A) Immunoreactive β-catenin and GAPDH protein bands and (B) WT, T41A, S45F and S33P β-catenin protein levels. GAPDH was used as the internal control for gene expression. Anti-DDK monoclonal antibody was used for β-catenin detection. n=3. EV, empty vector; WT, wild-type.
According to the western blot results, AKT1 E17K mutant immunoreactive protein levels were lower compared with that of the WT (P<0.02). However, E49K and L52R mutant protein levels were similar to that of the WT (P>0.02) (Fig. 6). Also, there was no statistically significant difference between WT and mutant β-catenin protein levels. Based on these findings, CTNNB1 mutations did not have any effect on protein levels (Fig. 7). Among all the mutant immunoreactive proteins, only AKT1 E17K showed a lower protein level compared with the WT protein.
Discussion
CRC is one of the most common types of cancer worldwide (27). EGFR plays an important role in CRC (28). Therefore, EGFR has begun to be targeted in CRC treatment. However, resistance to chemotherapy/EGFR-targeting therapies is a major problem in current cancer treatment (29). So far, numerous genes have been investigated in terms of their potential to be a predictive marker in drug resistance (22–26). Cetuximab is a monoclonal antibody that specifically inhibits EGFR and has been used as a biological agent in CRC treatment (5). Studies have reported that genes such as EGFR, BRAF, PIK3CA and PTEN may be associated with cetuximab resistance (22–26). However, it still remains unclear whether these genes are valid biomarkers for cetuximab resistance. At present, the only predictive biomarkers used in clinics for the cetuximab treatment are KRAS mutations involved in the EGFR pathway (30). There are a limited number of in vitro studies regarding the association between KRAS mutations and cetuximab resistance. Kumar et al (31) investigated the resistance to cetuximab in KRAS mutant CRC cell lines and reported that cell lines with the KRAS G13D mutation (HCT 116, LoVo and T84) showed intermediate sensitivity between the resistant cell line with the KRAS G12V mutation (SW480) and the sensitive KRAS WT cell line (LIM1215). Nakadate et al (32) examined the proliferation inhibitory effect of cetuximab on six CRC cell lines and reported that cetuximab did not show any cytotoxic effect in KRAS mutant cell lines (SW480 and HCT-15), whereas a cytotoxic effect was observed when KRAS WT cell lines (SW48, COLO 320HSR, COLO 205 and WiDr) were treated with a high dose of cetuximab. Given the findings of previous studies (31,32), KRAS mutations are largely responsible for cetuximab resistance. However, there are still patients with KRAS WT who are cetuximab-resistant (8). Therefore, it is important to investigate the other variations in different possible candidate genes, such as AKT1 and CTNNB1, and how they might affect drug resistance in CRC. Several studies have reported the presence of AKT1 and CTNNB1 mutations in patients with CRC (8–10,14,33). The results of previous studies have indicated the presence of AKT1 E17K and L52R, and CTNNB1 T41A, S45F and S33P mutations in patients with CRC (14,24–37). Furthermore, the AKT1 E49K mutation has been observed in patients with bladder cancer (38), but not in patients with CRC. Based on our survey of the literature, there is no study regarding AKT1/CTNNB1 mutations that may be predictive of cetuximab resistance. Therefore, it was hypothesized that these mutations may affect cancer treatment efficacy due to their important role in CRC.
In the present study, the effects of AKT1 and CTNNB1 mutations on the cetuximab resistance were explored. Although studies have shown the effects of AKT1 E17K, E49K and L52R mutations on the related protein (9,10,38), there are only two studies (8,14) that describe the presence of the AKT1 E17K mutation in patients with cetuximab-resistant CRC. One study was carried out by Hechtman et al (14) and they genotyped 2,631 CRC cases. Two of the three patients carrying the AKT1 E17K mutation (KRAS/BRAF WT) were found to be resistant to cetuximab treatment. The other study only reported one patient with CRC possessing both AKT1 E17K mutation and KRAS WT (exon 2) who did not respond to cetuximab in combination with chemotherapy treatment. In addition to the AKT1 E17K mutation, this patient had also a BRAF G469A mutation (8). Thus, this previous study could not reveal the individual value of the AKT1 E17K mutation for cetuximab resistance since the patient did not respond to cetuximab therapy and also had a BRAF mutation along with the AKT1 E17K mutation. Furthermore, there is no study suggesting that AKT1 E49K and L52R mutations cause cetuximab resistance, to the best of our knowledge. The present findings demonstrated that AKT1 mutations significantly decreased cetuximab-induced cell death compared with the WT. These two studies (8,14) support the present results in terms of AKT1 E17K-cetuximab resistance. Genetic mutations in GSK-3β phosphorylation sites (S33, S37, S45 or T41) of β-catenin are known to inhibit proteasomal degradation of β-catenin coding by CTNNB1 (39). There is only one study demonstrating the association of CTNNB1 T41A mutation with cetuximab resistance (35), in which cetuximab did not inhibit tumor growth in xenograft mice carrying both CTNNB1 T41A and BRAF V600E mutations. Similarly, in the present study, the cytotoxicity of cetuximab was lower in CTNNB1 T41A, S45F and S33P mutant Caco-2 cells compared with the WT cells at all dosage levels. The results of Xu et al (35) strongly support the present results; however, the study could not reveal the individual effect of the CTNNB1 T41A mutation on cetuximab because the mice also had a BRAF mutation along with the CTNNB1 T41A mutation. On the other hand, the effects of S45F and S33P mutations on cetuximab resistance have not been studied before, to the best of our knowledge. Additionally, in the present study, three CRC cell lines (Caco-2, HT-29 and HCT 116) were treated with cetuximab before the cell transfection. However, cetuximab did not show any cytotoxic effects on HT-29 and HCT 116 cells. Since cetuximab did not inhibit proliferation on these cells, HT-29 and HCT 116 cells were excluded from further experiments. In previous studies, the cytotoxic effect of cetuximab has been demonstrated in vitro on CRC cell lines (31,40,41). However, the cytotoxic effect of cetuximab on HT-29 and HCT 116 cells was inconsistent (42–44). Previous studies have shown that the HT-29 cell line has a BRAF V600E mutation (23), and the HCT 116 cell line has a KRAS G13D mutation (43). It is considered that KRAS and BRAF mutations, among the most common mutations in CRC, lead to abnormal activation of the RAS/RAF/MEK/ERK/MAPK cascade, causing cetuximab resistance (45). Although there are studies reporting that KRAS codon 13 mutations do not affect cetuximab resistance as much as KRAS codon 12 mutations, patients with KRAS codon 12 or 13 mutated tumors cannot be treated with cetuximab in Europe or the USA (46). Also, a previous study showed that BRAF mutations weaken the cetuximab response in patients with metastatic CRC. Di Nicolantonio et al (23) reported that 11/79 patients (14%) with KRAS WT who did not respond to cetuximab treatment had BRAF V600E mutation and none of the patients who responded to the treatment had BRAF mutations. Considering that HT-29 has a BRAF mutation (23) and HCT 116 has a KRAS mutation (43), this non-cytotoxic effect against cetuximab may be caused by the gene mutations that cells naturally have.
The present study also evaluated whether AKT1 and CTNNB1 mutations could be responsible for resistance to commonly used chemotherapeutics in the treatment of CRC. The increased activation of AKT1 as a result of E17K, E49K and L52R mutations causes an anti-apoptotic effect. This may cause cell death resistance or a delay in cell death (10). Similarly, as a result of CTNNB1 mutations, the accumulation of β-catenin in the cytoplasm and its translocation to the nucleus activates the expression of target genes, such as cyclin D1, c-myc, CD44 and matrix metalloproteinase 7 (37). In this way, continuous activation of the Wnt/β-catenin pathway causes uncontrolled cell proliferation (47). Moreover, the Wnt signaling promotes the epithelial-mesenchymal transition (EMT) by inducing the expression of EMT transcription factors, such as zinc finger E-box-binding homeobox 1. EMT has been associated with chemotherapy resistance as well as metastasis development in CRC (48). Activation of the Wnt/β-catenin pathway with CTNNB1 mutations may cause resistance to chemotherapeutic drugs by excessive cell proliferation and EMT. Oxaliplatin, irinotecan and 5-FU are frequently used cytotoxic agents in the CRC treatment and they are used in combination with biological agents (49). Oxaliplatin acts as an alkylating agent, producing mainly platinum-DNA adducts that are the major cytotoxic lesions (50). These intra-strand adducts result in the inhibition of DNA replication and transcription (51). Irinotecan is an antitumor pro-drug and activates the metabolite of SN-38 by carboxylesterases. Irinotecan has an anticancer effect by inhibiting DNA topoisomerase I (52). The other cytotoxic agent, 5-FU, exerts its anticancer effects through the inhibition of thymidylate synthase, which is a nucleotide metabolic enzyme (53).
Although studies have shown that there may be numerous candidate genes associated with resistance to oxaliplatin, irinotecan and 5-FU, there are also some conflicting studies (52). There is still a need for further research as the studies are inconsistent, and there are no in vivo studies supporting in vitro results that will allow these findings to be used in treatment. Although Xu et al (35) showed that oxaliplatin and 5-FU do not inhibit tumor growth in xenograft mice carrying both CTNNB1 T41A and BRAF V600E mutations, the effects of CTNNB1 S45F and S33P mutations on oxaliplatin and 5-FU resistance have not been studied before. Furthermore, there is no study showing the contribution of AKT1 E17K, E49K and L52R mutations to resistance to oxaliplatin, irinotecan and 5-FU, to the best of our knowledge. Based on the present study, AKT1 E17K, E49K and L52R, and CTNNB1 T41A, S45F and S33P mutations can be responsible for oxaliplatin, irinotecan and 5-FU resistance. The study demonstrating the association of CTNNB1 T41A mutation with oxaliplatin and 5-FU resistance (35) supports the current findings. There is no study shows the association of AKT1 and CTNNB1 mutations with irinotecan and SN-38 resistance. AKT1 and CTNNB1 mutations may be a reason for resistance to oxaliplatin, irinotecan and 5-FU due to their anti-apoptotic and triggering cell proliferation effects.
Cetuximab is used in combination therapy with the FOLFOX (oxaliplatin/5-FU/leukoverine) and FOLFIRI (irinotecan/5-FU/leukoverine) regimens as well as monotherapy in CRC treatment (54). A number of in vitro and clinical studies demonstrated that the combination therapy with cetuximab and chemotherapeutic agents can increase the beneficial effect in CRC treatment compared with monotherapy (42,55–57). However, some studies have indicated that patients with CRC harboring KRAS mutations do not benefit from combination therapy or monotherapy (cetuximab with FOLFIRI or FOLFOX regimen) (58,59). The present study showed that AKT1/CTNNB1 mutations caused drug resistance to cetuximab, oxaliplatin, irinotecan, SN-38 and 5-FU in the treatment as a monotherapy. One important limitation of the present study is the lack of the results regarding the combination therapy of cetuximab and commonly used chemotherapeutics. Similar to the effect of KRAS mutations, AKT1/CTNNB1 mutations may cause the PI3K/AKT and Wnt signaling pathways to remain active and to be constantly stimulated in cellular proliferation. If the related mutations are present on these genes, this means that the drug resistance will occur no matter what kind of therapy is. As with the patients with CRC harboring KRAS mutations (58,59), monotherapy or combined therapy may not change the outcome of patients with CRC or cells possessing AKT1/CTNNB1 mutations. According to the present results, activation of these two pathways by the mutations may decrease the efficiency of oxaliplatin, irinotecan and 5-FU. The drug resistance caused by AKT1 and CTNNB1 mutations may be overcome by using alternative therapeutics that that exert function on signaling pathways other than the PI3K/AKT and Wnt pathways. Another limitation of the present study is the lack of in vivo models or clinical studies. Further analysis, such as animal models or clinical trials, is necessary to validate the in vitro results and the potential of AKT1/CTNNB1 mutations as biomarkers for CRC treatment.
Overall, in the present study, human AKT1 and CTNNB1 somatic missense mutations were generated in vitro, and the possible effect of these mutations on cetuximab, oxaliplatin, irinotecan, SN-38 and 5-FU resistance was explored. The expression of AKT1 and CTNNB1 mutations in Caco-2 cells resulted in a significant decrease in drug-induced cell death. These findings provide evidence that patients with CRC harboring AKT1 and CTNNB1 mutations may have resistance to cetuximab and other frequently used chemotherapeutics. Therefore, these mutations may serve as novel predictive biomarkers responsible for drug resistance.
Supplementary Material
Figure 3.
Effect of AKT1/CTNNB1 mutations on irinotecan resistance. Effect of (A) AKT1 E17K, E49K and L52R (B) CTNNB1 T41A, S45F and S33P on irinotecan resistance. *P<0.02 vs. respective WT. CTNNB1, β-catenin; WT, wild-type.
Figure 4.
Effect of AKT1/CTNNB1 mutations on SN-38 resistance. Effect of (A) AKT1 E17K, E49K and L52R and (B) CTNNB1 T41A, S45F and S33P on SN-38 resistance. *P<0.02 vs. respective WT. CTNNB1, β-catenin; WT, wild-type.
Acknowledgments
The authors would like to thank the biostatistics specialist Mrs. Ayca Pamukcu Beyhan from Milli Savunma University for her guidance in the statistical analysis.
Funding
The present study was supported by The Scientific Research Projects Coordination Unit of Istanbul University (grant no. TDK-2016-20002). Gozde Hasbal-Celikok was funded by The Scientific and Technological Research Council of Turkey PhD Scholarship Program (grant no. BIDEB 2211-C).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author upon reasonable request.
Authors' contributions
PAS, GHC and AC designed the experiments, GHC, PAS and GAU performed the experiments. GHC and PAS analyzed and interpreted the data. GHC, PAS and AC contributed to writing the manuscript and revising it critically for important intellectual content. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Longley DB, Johnston PG. Molecular mechanisms of drug resistance. J Pathol. 2005;205:275–292. doi: 10.1002/path.1706. [DOI] [PubMed] [Google Scholar]
- 3.Arqués O, Chicote I, Puig I, Tenbaum SP, Argilés G, Dienstmann R, Fernández N, Caratù G, Matito J, Silberschmidt D, et al. Tankyrase inhibition blocks Wnt/β-catenin pathway and reverts resistance to PI3K and AKT inhibitors in the treatment of colorectal cancer. Clin Cancer Res. 2016;22:644–656. doi: 10.1158/1078-0432.CCR-14-3081. [DOI] [PubMed] [Google Scholar]
- 4.Banck MS, Grothey A. Biomarkers of resistance to epidermal growth factor receptor monoclonal antibodies in patients with metastatic colorectal cancer. Clin Cancer Res. 2009;15:7492–7501. doi: 10.1158/1078-0432.CCR-09-0188. [DOI] [PubMed] [Google Scholar]
- 5.Graham J, Muhsin M, Kirkpatrick P. Cetuximab. Nat Rev Drug Discov. 2004;3:549–550. doi: 10.1038/nrd1445. [DOI] [PubMed] [Google Scholar]
- 6.Chong CR, Jänne PA. The quest to overcome resistance to EGFR-targeted therapies in cancer. Nat Med. 2013;19:1389–1400. doi: 10.1038/nm.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Mattia E, Toffoli G, Polesel J, D'Andrea M, Corona G, Zagonel V, Buonadonna A, Dreussi E, Cecchin E. Pharmacogenetics of ABC and SLC transporters in metastatic colorectal cancer patients receiving first-line FOLFIRI treatment. Pharmacogenet Genomics. 2013;23:549–557. doi: 10.1097/FPC.0b013e328364b6cf. [DOI] [PubMed] [Google Scholar]
- 8.Hsu HC, Thiam TK, Lu YJ, Yeh CY, Tsai WS, You JF, Hung HY, Tsai CN, Hsu A, Chen HC, et al. Mutations of KRAS/NRAS/BRAF predict cetuximab resistance in metastatic colorectal cancer patients. Oncotarget. 2016;7:22257–22270. doi: 10.18632/oncotarget.8076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carpten JD, Faber AL, Horn C, Donoho GP, Briggs SL, Robbins CM, Hostetter G, Boguslawski S, Moses TY, Savage S, et al. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature. 2007;448:439–444. doi: 10.1038/nature05933. [DOI] [PubMed] [Google Scholar]
- 10.Parikh C, Janakiraman V, Wu WI, Foo CK, Kljavin NM, Chaudhuri S, Stawiski E, Lee B, Lin J, Li H, et al. Disruption of PH-kinase domain interactions leads to oncogenic activation of AKT in human cancers. Proc Natl Acad Sci. 2012;109:19368–19373. doi: 10.1073/pnas.1204384109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yi KH, Axtmayer J, Gustin JP, Rajpurohit A, Lauring J. Functional analysis of non-hotspot AKT1 mutants found in human breast cancers identifies novel driver mutations: Implications for personalized medicine. Oncotarget. 2013;4:29–34. doi: 10.18632/oncotarget.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fruman DA, Chiu H, Hopkins BD, Bagrodia S, Cantley LC, Abraham RT. The PI3K pathway in human disease. Cell. 2017;170:605–635. doi: 10.1016/j.cell.2017.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malapelle U, Pisapia P, Sgariglia R, Vigliar E, Biglietto M, Carlomagno C, Giuffrè G, Bellevicine C, Troncone G. Less frequently mutated genes in colorectal cancer: Evidences from next-generation sequencing of 653 routine cases. J Clin Pathol. 2016;69:767–771. doi: 10.1136/jclinpath-2015-203403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hechtman JF, Sadowska J, Huse JT, Borsu L, Yaeger R, Shia J, Vakiani E, Ladanyi M, Arcila ME. AKT1 E17K in colorectal carcinoma is associated with BRAF V600E but not MSI-H status: A clinicopathologic comparison to PIK3CA helical and kinase domain mutants. Mol Cancer Res. 2015;13:1003–1008. doi: 10.1158/1541-7786.MCR-15-0062-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu Y, Savage RE, Eathiraj S, Meade J, Wick MJ, Hall T, Abbadessa G, Schwartz B. Targeting AKT1-E17K and the PI3K/AKT pathway with an allosteric AKT inhibitor, ARQ 092. PLoS One. 2015;10:e0140479. doi: 10.1371/journal.pone.0140479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gong J, Cho M, Sy M, Salgia R, Fakih M. Molecular profiling of metastatic colorectal tumors using next-generation sequencing: A single-institution experience. Oncotarget. 2017;8:42198–42213. doi: 10.18632/oncotarget.15030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hagen T, Vidal-Puig A. Characterisation of the phosphorylation of beta-catenin at the GSK-3 priming site Ser45. Biochem Biophys Res Commun. 2002;294:324–328. doi: 10.1016/S0006-291X(02)00485-0. [DOI] [PubMed] [Google Scholar]
- 18.Cai ZX, Tang XD, Gao HL, Tang C, Nandakumar V, Jones L, Ye H, Lou F, Zhang D, Sun H, et al. APC, FBXW7, KRAS, PIK3CA, and TP53 gene mutations in human colorectal cancer tumors frequently detected by next-generation DNA sequencing. J Mol Genet Med. 2014;8:145. [Google Scholar]
- 19.Jauhri M, Bhatnagar A, Gupta S, Shokeen Y, Minhas S, Aggarwal S. Targeted molecular profiling of rare genetic alterations in colorectal cancer using next-generation sequencing. Med Oncol. 2016;33:106. doi: 10.1007/s12032-016-0820-2. [DOI] [PubMed] [Google Scholar]
- 20.Kim S, Jeong S. Mutation hotspots in the β-catenin gene: Lessons from the human cancer genome databases. Mol Cells. 2019;42:8–16. doi: 10.14348/molcells.2018.0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anwar M, Kochhar R, Singh R, Bhatia A, Vaiphei K, Mahmood A, Mahmood S. Frequent activation of the β-catenin gene in sporadic colorectal carcinomas: A mutational & expression analysis. Mol Carcinog. 2016;55:1627–1638. doi: 10.1002/mc.22414. [DOI] [PubMed] [Google Scholar]
- 22.Frattini M, Saletti P, Romagnani E, Martin V, Molinari F, Ghisletta M, Camponovo A, Etienne LL, Cavalli F, Mazzucchelli L. PTEN loss of expression predicts cetuximab efficacy in metastatic colorectal cancer patients. Br J Cancer. 2007;97:1139–1145. doi: 10.1038/sj.bjc.6604009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Nicolantonio F, Martini M, Molinari F, Sartore-Bianchi A, Arena S, Saletti P, De Dosso S, Mazzucchelli L, Frattini M, Siena S, Bardelli A. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol. 2008;26:5705–5712. doi: 10.1200/JCO.2008.18.0786. [DOI] [PubMed] [Google Scholar]
- 24.Sartore-Bianchi A, Martini M, Molinari F, Veronese S, Nichelatti M, Artale S, Di Nicolantonio F, Saletti P, De Dosso S, Mazzucchelli L, et al. PIK3CA mutations in colorectal cancer are associated with clinical resistance to EGFR-targeted monoclonal antibodies. Cancer Res. 2009;69:1851–1857. doi: 10.1158/0008-5472.CAN-08-2466. [DOI] [PubMed] [Google Scholar]
- 25.Montagut C, Dalmases A, Bellosillo B, Crespo M, Pairet S, Iglesias M, Salido M, Gallen M, Marsters S, Tsai SP, et al. Identification of a mutation in the extracellular domain of the epidermal growth factor receptor conferring cetuximab resistance in colorectal cancer. Nat Med. 2012;18:221–223. doi: 10.1038/nm0912-1445b. [DOI] [PubMed] [Google Scholar]
- 26.Xu JM, Wang Y, Wang YL, Wang Y, Liu T, Ni M, Li MS, Lin L, Ge FJ, Gong C, et al. PIK3CA mutations contribute to acquired cetuximab resistance in patients with metastatic colorectal cancer. Clin Cancer Res. 2017;23:4602–4616. doi: 10.1158/1078-0432.CCR-16-2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dekker E, Rex DK. Advances in CRC prevention: Screening and surveillance. Gastroenterology. 2018;154:1970–1984. doi: 10.1053/j.gastro.2018.01.069. [DOI] [PubMed] [Google Scholar]
- 28.van Brummelen EMJ, de Boer A, Beijnen JH, Schellens JHM. BRAF mutations as predictive biomarker for response to anti-EGFR monoclonal antibodies. Oncologist. 2017;22:864–872. doi: 10.1634/theoncologist.2017-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: An evolving paradigm. Nat Rev Cancer. 2013;13:714–726. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 30.Nemecek R, Berkovcova J, Radova L, Kazda T, Mlcochova J, Vychytilova-Faltejskova P, Slaby O, Svoboda M. Mutational analysis of primary and metastatic colorectal cancer samples underlying the resistance to cetuximab-based therapy. Onco Targets Ther. 2016;9:4695–4703. doi: 10.2147/OTT.S102891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar SS, Price TJ, Mohyieldin O, Borg M, Townsend A, Hardingham JE. KRAS G13D mutation and sensitivity to cetuximab or panitumumab in a colorectal cancer cell line model. Gastrointest Cancer Res. 2014;7:23–26. [PMC free article] [PubMed] [Google Scholar]
- 32.Nakadate Y, Kodera Y, Kitamura Y, Shirasawa S, Tachibana T, Tamura T, Koizumi F. KRAS mutation confers resistance to antibody-dependent cellular cytotoxicity of cetuximab against human colorectal cancer cells. Int J Cancer. 2014;134:2146–2155. doi: 10.1002/ijc.28550. [DOI] [PubMed] [Google Scholar]
- 33.Stachler MD, Rinehart E, Lindeman N, Odze R, Srivastava A. Novel molecular insights from routine genotyping of colorectal carcinomas. Hum Pathol. 2015;46:507–513. doi: 10.1016/j.humpath.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 34.Chen D, Huang X, Cai J, Guo S, Qian W, Wery JP, Li QX. A set of defined oncogenic mutation alleles seems to better predict the response to cetuximab in CRC patient-derived xenograft than KRAS 12/13 mutations. Oncotarget. 2015;6:40815–40821. doi: 10.18632/oncotarget.5886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu G, Li K, Zhang N, Zhu B, Feng G, Fan Q. Colon cancers carrying BRAF V600E and β-catenin T41A activating mutations are resistant to numerous common anticancer drugs. Oncol Lett. 2018;15:4471–4476. doi: 10.3892/ol.2018.7856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sparks AB, Morin PJ, Vogelstein B, Kinzler KW. Mutational analysis of the APC/beta-catenin/Tcf pathway in colorectal cancer. Cancer Res. 1998;58:1130–1134. [PubMed] [Google Scholar]
- 37.Abdelmaksoud-Damak R, Miladi-Abdennadher I, Triki M, Khabir A, Charfi S, Ayadi L, Frikha M, Sellami-Boudawara T, Mokdad-Gargouri R. Expression and mutation pattern of β-catenin and adenomatous polyposis coli in colorectal cancer patients. Arch Med Res. 2015;46:54–62. doi: 10.1016/j.arcmed.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 38.Askham JM, Platt F, Chambers PA, Snowden H, Taylor CF, Knowles MA. AKT1 mutations in bladder cancer: Identification of a novel oncogenic mutation that can co-operate with E17K. Oncogene. 2010;29:150–155. doi: 10.1038/onc.2009.315. [DOI] [PubMed] [Google Scholar]
- 39.Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–847. doi: 10.1016/S0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- 40.Jhawer M, Goel S, Wilson AJ, Montagna C, Ling YH, Byun DS, Nasser S, Arango D, Shin J, Klampfer L, et al. PIK3CA mutation/PTEN expression status predicts response of colon cancer cells to the epidermal growth factor receptor inhibitor cetuximab. Cancer Res. 2008;68:1953–1961. doi: 10.1158/0008-5472.CAN-07-5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levy EM, Sycz G, Arriaga JM, Barrio MM, Von Euw EM, Morales SB, González M, Mordoh J, Bianchini M. Cetuximab-mediated cellular cytotoxicity is inhibited by HLA-E membrane expression in colon cancer cells. Innate Immun. 2009;15:91–100. doi: 10.1177/1753425908101404. [DOI] [PubMed] [Google Scholar]
- 42.Balin-Gauthier D, Delord JP, Rochaix P, Mallard V, Thomas F, Hennebelle I, Bugat R, Canal P, Allal C. In vivo and in vitro antitumor activity of oxaliplatin in combination with cetuximab in human colorectal tumor cell lines expressing different level of EGFR. Cancer Chemother Pharmacol. 2006;57:709–718. doi: 10.1007/s00280-005-0123-3. [DOI] [PubMed] [Google Scholar]
- 43.Shigeta K, Hayashida T, Hoshino Y, Okabayashi K, Endo T, Ishii Y, Hasegawa H, Kitagawa Y. Expression of epidermal growth factor receptor detected by cetuximab indicates its efficacy to inhibit in vitro and in vivo proliferation of colorectal cancer cells. PLoS One. 2013;8:e66302. doi: 10.1371/journal.pone.0066302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luca T, Barresi V, Privitera G, Musso N, Caruso M, Condorelli DF, Castorina S. In vitro combined treatment with cetuximab and trastuzumab inhibits growth of colon cancer cells. Cell Prolif. 2014;47:435–447. doi: 10.1111/cpr.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bardelli A, Jänne PA. The road to resistance: EGFR mutation and cetuximab. Nat Med. 2012;18:199–200. doi: 10.1038/nm.2646. [DOI] [PubMed] [Google Scholar]
- 46.Nakamura M, Aoyama T, Ishibashi K, Tsuji A, Takinishi Y, Shindo Y, Sakamoto J, Oba K, Mishima H. Randomized phase II study of cetuximab versus irinotecan and cetuximab in patients with chemo-refractory KRAS codon G13D metastatic colorectal cancer (G13D-study) Cancer Chemother Pharmacol. 2017;79:29–36. doi: 10.1007/s00280-016-3203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yao H, Ashihara E, Maekawa T. Targeting the Wnt/β-catenin signaling pathway in human cancers. Expert Opin Ther Targets. 2011;15:873–887. doi: 10.1517/14728222.2011.577418. [DOI] [PubMed] [Google Scholar]
- 48.Sebio A, Kahn M, Lenz HJ. The potential of targeting Wnt/β-catenin in colon cancer. Expert Opin Ther Targets. 2014;18:611–615. doi: 10.1517/14728222.2014.906580. [DOI] [PubMed] [Google Scholar]
- 49.Ciombor KK, Wu C, Goldberg RM. Recent therapeutic advances in the treatment of colorectal cancer. Annu Rev Med. 2015;66:83–95. doi: 10.1146/annurev-med-051513-102539. [DOI] [PubMed] [Google Scholar]
- 50.Misset JL, Bleiberg H, Sutherland W, Bekradda M, Cvitkovic E. Oxaliplatin clinical activity: A review. Crit Rev Oncol Hematol. 2000;35:75–93. doi: 10.1016/S1040-8428(00)00070-6. [DOI] [PubMed] [Google Scholar]
- 51.Toscano F, Parmentier B, Fajoui ZE, Estornes Y, Chayvialle JA, Saurin JC, Abello J. p53 dependent and independent sensitivity to oxaliplatin of colon cancer cells. Biochem Pharmacol. 2007;74:392–406. doi: 10.1016/j.bcp.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 52.Panczyk M. Pharmacogenetics research on chemotherapy resistance in colorectal cancer over the last 20 years. World J Gastroenterol. 2014;20:9775–9827. doi: 10.3748/wjg.v20.i29.9775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Longley DB, Harkin DP, Johnston PG. 5-Fluorouracil: Mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330–338. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 54.Feldman M, Friedman LS, Brandt LJ. 11th edition. Elsevier Saunders; Philadelphia: 2016. Sleisenger and Fordtran's gastrointestinal and liver disease, pathophysiology, diagnosis, management; pp. 2292–2294. [Google Scholar]
- 55.Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 56.Prewett M, Deevi DS, Bassi R, Fan F, Ellis LM, Hicklin DJ, Tonra JR. Tumors established with cell lines selected for oxaliplatin resistance respond to oxaliplatin if combined with cetuximab. Clin Cancer Res. 2007;13:7432–7440. doi: 10.1158/1078-0432.CCR-07-1768. [DOI] [PubMed] [Google Scholar]
- 57.Ge XJ, Jiang JY, Wang M, Li MY, Zheng LM, Feng ZX, Liu L. Cetuximab enhances the efficiency of irinotecan through simultaneously inhibiting the MAPK signaling and ABCG2 in colorectal cancer cells. Pathol Res Pract. 2020;216:152798. doi: 10.1016/j.prp.2019.152798. [DOI] [PubMed] [Google Scholar]
- 58.Van Cutsem E, Kohne CH, Láng I, Folprecht G, Nowacki MP, Cascinu S, Shchepotin I, Maurel J, Cunningham D, Tejpar S, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: Updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29:2011–2019. doi: 10.1200/JCO.2010.33.5091. [DOI] [PubMed] [Google Scholar]
- 59.Van der Jeught K, Xu HC, Li YJ, Lu XB, Ji G. Drug resistance and new therapies in colorectal cancer. World J Gastroenterol. 2018;24:3834–3848. doi: 10.3748/wjg.v24.i34.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author upon reasonable request.