Abstract
OBJECTIVE –
We compared the clinical outcomes and cost-efficiency of surgical approaches (sternotomy-Open, video assisted thoracoscopic surgery-VATS, and robotic assisted thoracic surgery-RATS) for thymectomy.
METHODS –
A retrospective review of 220 consecutive patients who underwent thymectomy from 1/1/2007 and 1/31/2017. Surgical approach was determined by the surgeon, but we only included cases that could be resected using any of the three approaches.
RESULTS –
Open approach was used in 69 patients, while minimally invasive technique (MIS) was used in 151 (97: VATS, 54: RATS). Open surgery was associated with greater total hospital cost ($22,847±20,061 vs. $14,504±10,845, p<0.001). Open group also revealed longer duration of ICU (1.2±2.8 vs. 0.2±1.3 days, p<0.001) and hospital stay (4.3±4.0 vs. 2.0±2.6 days, p<0.001). There were no differences in major adverse clinical outcomes. Long-term recurrence-free survival following resection of thymoma was similar between the groups.
CONCLUSIONS –
Minimally invasive techniques were equally efficacious compared to the Open approach in the resection of the thymus. Additionally, their use was associated with decreased hospital length of stay and reduced cost. Hence the use of minimally invasive approaches should be encouraged in the resection of thymus.
Introduction
Thymectomy is indicated for a variety of indications, including thymic malignancies, benign thymic lesions, as well as myasthenia gravis [1]. While sternotomy is generally considered to be the traditional gold standard for thymectomy [2,3], minimally invasive approaches such as video-thoracoscopy (VATS) [4–13] as well as robotic-assisted thoracic surgery (RATS) [14–16,21–24] have gained popularity in the last decade, particularly for myasthenia gravis or tumors less than 6 cm. However, the efficacy and safety of these minimally invasive surgical approaches, in comparison to the traditional approaches, remains unclear. Additionally, the long-term efficacy of VATS and RATS in resection of thymoma is unknown. Indeed, much of the surgical literature on the resection of thymus discusses outcomes with the traditional open approach, with limited studies investigating outcomes of minimally invasive surgical techniques (MIS) [4–5,7–8,11–13,17–20]. Hence, we sought to investigate the efficacy and safety of the different resection approaches utilized for thymectomy. We were adequately poised to conduct these studies given sufficient volume of such procedures and the experience of surgeons in the three most commonly utilized approaches, namely sternotomy, VATS, and RATS.
An additional concern associated with the use of minimally invasive techniques, particularly RATS, relates to their cost-efficiency. Indeed, costs associated with the robotic equipment have been viewed as a major impediment in the utilization of this approach for thymectomy. Hence, in addition to comparing the clinical outcomes, we studied the cost-efficiency of the three different surgical approaches and how it compared to the cost of hospitalization. Our data suggests that minimally invasive techniques are comparable to open techniques in safety and efficacy and do not incur significantly increased operating costs.
Methods:
This is a single center, retrospective review, of a prospectively maintained database including 220 consecutive patients who underwent resection for anterior mediastinal masses between 1/1/2007 and 1/31/2017. While the surgical approach was determined by the surgeons depending on their experience and preference, we only included cases for this study that were amenable for all three approaches. Patients who were only candidates for open approaches, for instance due to tumor size or complexity, were not excluded to avoid a confounding bias. Specifically, patients with tumor greater than 6 cm, those with invasion into the cardiac or vascular structures, or who received neo-adjuvant therapy, were excluded since only open approaches were offered to these patients. Our goal was to evaluate the three surgical approaches in cases when all three were feasible to avoid the obvious confounding bias. Open surgery group included thoracotomy or sternotomy, whereas minimally invasive surgical (MIS) techniques included VATS and RATS. We do not offer transcervical thymectomy and did not have any patients who were treated with this approach. We also performed a procedure-specific cost comparison. The capital cost of the purchase of the camera towers with lenses, maintenance contract, and cost of operating room renovation to be suitable for minimally invasive surgery rooms were excluded. Additionally, capital cost for purchase of the robot console, amortization cost, and the maintenance contract for the robot were excluded to perform an unbiased procedure-specific cost comparison.
Study subjects
We included 220 consecutive patients who underwent thymectomy between January 2007 and January 2017. Patient data, including demographics, preoperative characteristics, postoperative complications, and outcomes were collected from our prospectively maintained database. Approval to conduct this review was obtained from our institutional review board, which waived the consent requirement because the study was retrospective. Clinical data collection was performed by direct extraction from the electronic medical record (EMR). Four independent researchers collected missing data points to limit single user bias, while cost data was directly absconded from the hospital billing division. In order to adequately capture all target surgeries, procedure codes were used as primary extraction criteria and final diagnoses were reliant on final surgical pathological evaluation.
Statistical analysis
Patient demographics, postoperative complications, and outcomes were compared between the Open approach and MIS approach, which included VATS and RATS approaches. Continuous variables were compared using analysis of variance (ANOVA) and reported as mean (standard deviation). Categorical variables were compared using chi-square test and reported as number (percentage). P-values <0.05 were accepted as statistically significant. Statistical analyses were performed using Stata/MP14 (StataCorp, College Station, TX).
Results:
Demographic profile of study cohort
We first compared the demographic profile of patients in the Open and MIS groups. As discussed in the methods, the study cohort only included patients that were candidates for both open and MIS approaches, although the procedure was offered to the patients based on the surgeon’s preference and experience. Overall, a total of 220 patients underwent surgical resection for thymic lesions. The mean age was 48.6 ± 16.2 years for the entire cohort, with patients in the Open approach being older (53.2 ± 16.8 vs. 46.5 ± 15.4, p=0.004). Mean duration of follow-up was 6.7 years with interquartile range (3.6,7.5). There were no other statistically significant differences in patient characteristics between Open and MIS groups, nor between VATS and RATS (Table 1,2).
Table 1.
Characteristics of Patients
| Variable | Overall (n=220) | Open (n=69) | MIS (n=151) | P value |
|---|---|---|---|---|
| Age, years | 48.6 ± 16.2 | 53.2 ± 16.8 | 46.5 ± 15.4 | 0.004 |
| Female | 111 (50%) | 31 (45%) | 80 (53%) | 0.26 |
| BMI, kg/m2 | 31.6 ± 20.6 | 31.3 ± 23.7 | 31.7 ± 19.0 | 0.90 |
| Hypertension | 82 (37%) | 31 (45%) | 51 (34%) | 0.12 |
| Hyperlipidemia | 42 (19%) | 18 (26%) | 24 (16%) | 0.07 |
| Diabetes mellitus | 23 (10%) | 10 (14%) | 13 (9%) | 0.18 |
| Chronic heart failure | 4 (2%) | 1 (1%) | 3 (2%) | 0.77 |
| Coronary artery disease | 12 (5%) | 6 (9%) | 6 (4%) | 0.15 |
| PVD | 6 (3%) | 3 (4%) | 3 (2%) | 0.32 |
| Chronic obstructive pulmonary disease | 8 (4%) | 4 (6%) | 4 (3%) | 0.24 |
Continuous data are shown as means ±standard deviation (SD). BMI, body mass index; BSA, PVD, peripheral vascular disease
Table 2.
Characteristics of Patients
| Variable | MIS (n=151) | VATS (n=97) | Robotic (n=54) | P value |
|---|---|---|---|---|
| Age, years | 46.5 ± 15.4 | 47.4 ± 15.2 | 44.9 ± 15.8 | 0.350 |
| Female | 80 (53%) | 55 (57%) | 25 (46%) | 0.22 |
| BMI, kg/m2 | 31.7 ± 19.0 | 32.4 ± 22.9 | 30.5 ± 8.3 | 0.55 |
| Hypertension | 51 (34%) | 35 (36%) | 16 (30%) | 0.40 |
| Hyperlipidemia | 24 (16%) | 16 (16%) | 8 (15%) | 0.78 |
| Diabetes mellitus | 13 (9%) | 9 (9%) | 4 (7%) | 0.69 |
| Chronic heart failure | 3 (2%) | 1 (1%) | 2 (4%) | 0.26 |
| Coronary artery disease | 6 (4%) | 3 (3%) | 3 (6%) | 0.46 |
| PVD | 3 (2%) | 2 (2%) | 1 (2%) | 0.92 |
| Chronic obstructive pulmonary disease | 4 (3%) | 3 (3%) | 1 (2%) | 0.65 |
Continuous data are shown as means ±standard deviation (SD). BMI, body mass index; BSA, PVD, peripheral vascular disease
Minimally invasive thymectomy is comparable to open procedures with regards to mortality and morbidity
The 30-day mortality was no different in the two groups, Open (1.4%) and MIS (0.7%, p=0.71), with 10 patients lost to early follow-up within the first 30 days. Additionally, there was no significant difference in the unexpected return to the operating room (4.0% Open vs. 1.0% MIS, p=0.16). The only statistically significant difference with regards to complications was found in the development of de novo atrial fibrillation (MIS: 3.0% vs. Open: 10.0%, p=0.03). There were no neurological complications or major cardiovascular complications. The 30-day re-admission was also no different between the two groups (3.0% Open vs. 5.0% MIS, p=0.57).
Minimally invasive thymectomy is associated with reduced length of stay and costs
We found that patients who had the MIS approach had a shorter duration of intensive care unit stay compared to the Open approach (0.2 ± 1.3 vs. 1.2 ± 2.8 days, p<0.001) as well as reduced overall hospital length of stay (2.0 ± 2.6 days vs 4.3 ± 4.0, p<0.001). Of note, our discharge criteria were no different between the two groups and included: a) adequate pain control and oral intake, b) return of bowel function, c) removal of all chest tubes, and, d) clearance from our physical therapist for discharge. The operative times were also shorter for the MIS approach compared to open surgery (171 ± 82 vs. 224 ± 100 min, p<0.001). Finally, when comparing total hospital admission costs, Open surgical approach resulted in greater total cost (Open: $22,847 ± 20,061 vs. MIS: $14,504 ± 10.845, p<0.001) (Table 3). In our practice, ICU bed was required for the first day for all patients who underwent sternotomy during the entire study period. This has not changed throughout the study period. Nevertheless, we recognize that this might not be a standard practice in all institutions. Accordingly, we modelled our cost analysis to exclude the costs of ICU stay in the Open cohort. With this modeling, our average costs associated with the Open approach were $19,947 ± 18,061, still significantly higher than the minimally invasive group (p<0.01)
Table 3.
Overall Cohort Outcomes
| Variable | Overall (n=220) | Open (n=69) | Minimum (n=151) | P value |
|---|---|---|---|---|
| Hospital stay (days) | 2.9 ± 3.4 | 4.3 ± 4.0 | 2.0 ± 2.6 | <0.001 |
| Operative time (min) | 187.2 ± 91.5 | 223.5 ± 100.4 | 170.7 ± 82.0 | <0.001 |
| ICU stay (days) | 0.5 ± 2.0 | 1.2 ± 2.8 | 0.2 ± 1.3 | <0.001 |
| Post-op complication | ||||
| Re-operation | 5 (2%) | 3 (4%) | 2 (1%) | 0.16 |
| Arrhythmia | 12 (5%) | 7 (10%) | 5 (3%) | 0.03 |
| Surgical site infection | 3 (1%) | 2 (3%) | 1 (1%) | 0.18 |
| Neurological deficit | 0 | 0 | 0 | 1.00 |
| Acute kidney injury | 1 (0%) | 1 (1%) | 0 | 0.13 |
| 30 Day readmission | 13 (6%) | 5 (8%) | 8 (5%) | 0.57 |
| Total cost | 171,201 ± 14,987 | 22,847 ± 20,061 | 14,504 ± 10,845 | <0.001 |
| Non Thymoma | 179 (81%) | 51 (74%) | 128 (85%) | 0.08 |
| Thymoma | 41 (19%) | 18 (26%) | 23 (15%) | |
| WHO classification | ||||
| A | 4 (10%) | 0 | 4 (17%) | 0.18 |
| AB | 11 (27%) | 6 (33%) | 5 (22%) | 0.63 |
| B1 | 10 (24%) | 2 (11%) | 8 (35%) | 0.16 |
| B2 | 6 (15%) | 2 (11%) | 4 (17%) | 0.90 |
| B3 | 8 (20%) | 6 (33%) | 2 (9%) | 0.11 |
| C | 2 (5%) | 2 (11%) | 0 | 0.40 |
| R0 resection | 41 (100%) | 18 (100%) | 23 (100%) | 1.00 |
Non Thymoma pathologies included: Thymic cyst, normal thymus, thymic hyperplasia
Robotic thymectomy and video-thoracoscopy are comparable in morbidity and costs
We next compared the efficacy of the VATS and RATS approaches to investigate if one was superior. We found that RATS had a shorter hospital length of stay (1.3 ± 0.8 vs. 2.4 ± 3.2 days, p=0.01). However, the total operative time, which included time from skin incision to skin closure, trended towards being higher with the RATS approach (188 ± 78 vs 161 ± 83 min, p=0.06). There were no differences between VATS and RATS in both morbidity and mortality. Additionally, total costs were not significantly different between the VATS and RATS approaches (Table 4).
Table 4.
Minimally Invasive Cohort Outcomes
| Variable | Minimum (n=151) | VATS (n=97) | Robotic (n=54) | P value |
|---|---|---|---|---|
| Hospital stay (days) | 2.0 ± 2.6 | 2.4 ± 3.2 | 1.3 ± 0.82 | 0.01 |
| Operative time (min) | 170.7 ± 82.0 | 161.1 ± 82.5 | 187.5 ± 78.3 | 0.06 |
| ICU stay (days) | 0.2 ± 1.3 | 0.3 ± 1.6 | 0.1 ± 0.41 | 0.23 |
| Post-op complication | ||||
| Re-operation | 2 (1%) | 2 (2%) | 0 | 0.29 |
| Arrhythmia | 5 (35) | 5 (5%) | 0 | 0.82 |
| Surgical site infection | 1 (1%) | 1 (1%) | 0 | 0.45 |
| Neurological deficit | 0 | 0 | 0 | 1.00 |
| Acute kidney injury | 0 | 0 | 0 | 1 |
| 30 Day Readmission | 8 (5%) | 1 1%) | 7 (13%) | <0.001 |
| Total cost | 14,504± 10,845 | 14,743 ± 13,113 | 14,075 ± 4,439 | 0.71 |
| Non Thymoma | 128 (85%) | 85 (88%) | 43 (80%) | 0.28 |
| Thymoma | 23 (15%) | 12 (12%) | 11 (20%) | |
| WHO classification | ||||
| A | 4 (3%) | 1 (1%) | 3 (6%) | 0.51 |
| AB | 5 (3%) | 5 (5%) | 0 | 0.01 |
| B1 | 8 (5%) | 2 (2%) | 6 (11%) | 0.14 |
| B2 | 4 (3%) | 3 (3%) | 1 (2%) | 0.20 |
| B3 | 2 (1%) | 1 (1%) | 1 (2%) | 0.94 |
| C | 0 | 0 | 0 | 1.00 |
| R0 resection | 23 (1005) | 12 (100%) | 11 (100%) | 1.00 |
Non Thymoma pathologies included: Thymic cyst, normal thymus, thymic hyperplasia
Minimally invasive thymectomy has comparable efficacy to open procedures
We first performed a subgroup analysis by evaluating the outcomes between open and MIS groups for thymoma and non-thymoma disease category separately. As shown in Tables 5 & 6, MIS approaches resulted in shorter length of stay and operative times for both these disease categories. Given that the surgeons and peri-operative protocols did not change throughout the study period, it is unlikely that chronological changes in our practice impacted our results demonstrating shorter length of stay observed with the RATS approach. Nevertheless, to confirm that, we compared the performance of the first half of the surgical cases using each of the techniques with the second half. In doing so, we could study whether time-dependent improvements in peri-operative management influenced our results. As shown in Tables 7&8, we did not find significant differences in each of the techniques with regards to the cases performed in the first or second half.
Table 5.
Open and MIS approaches for non-thymoma
| Non-Thymoma | ||||
|---|---|---|---|---|
| Variable | Overall (n=179) | Open (n=51) | MIS (n=128) | P value |
| Hospital stay | 2.8 ± 3.6 | 4.7 ± 4.5 | 2.1 ± 2.9 | <0.001 |
| Operative time | 180.6 ± 90.7 | 215.2 ± 99.6 | 166.5 ± 82.9 | 0.01 |
| Intensive care unit stay | 0.5 ± 2.1 | 1.1 ± 3.1 | 0.2 ± 1.4 | <0.001 |
Table 6.
Open and MIS approaches for Thymoma
| Thymoma | ||||
|---|---|---|---|---|
| Variable | Overall (n=41) | Open (n=18) | MIS (n=23) | P value |
| Hospital stay | 3.0 ± 2.2 | 5.0 ± 2.0 | 1.5 ± 0.8 | <0.001 |
| Operative time | 216.2 ± 89.3 | 245.0 ± 99.6 | 193.0 ± 72.9 | 0.03 |
| Intensive care unit stay | 0.6 ± 1.5 | 1.2 ± 1.9 | 0.2 ± 1.0 | 0.07 |
Table 7.
Comparison of operative times for surgical cases performed in the first and second halves with each surgical approach.
| Variable | Overall | Early phase | Late phase | P value |
|---|---|---|---|---|
| Open | 223.5 ± 100.4 | 231.9 ± 11.2 | 214.8 ± 86.9 | 0.48 |
| VATS | 161.1 ± 82.5 | 168.6 ± 83.6 | 154.1 ± 80.9 | 0.39 |
| Robotic | 187.5 ± 78.3 | 198.6 ± 78.2 | 177.2 ± 77.0 | 0.32 |
Table 8.
Comparison of hospital length of stay for surgical cases performed in the first and second halves with each surgical approach.
| Variable | Overall | Early phase | Late phase | P value |
|---|---|---|---|---|
| Open | 4.3 ± 4.0 | 5.5 ± 5.2 | 4.0 ± 1.8 | 0.12 |
| VATS | 2.4 ± 3.2 | 2.2 ± 1.8 | 2.6 ± 4.1 | 0.57 |
| Robotic | 1.3 ± 0.82 | 1.3 ± 0.8 | 1.3 ± 0.8 | 0.83 |
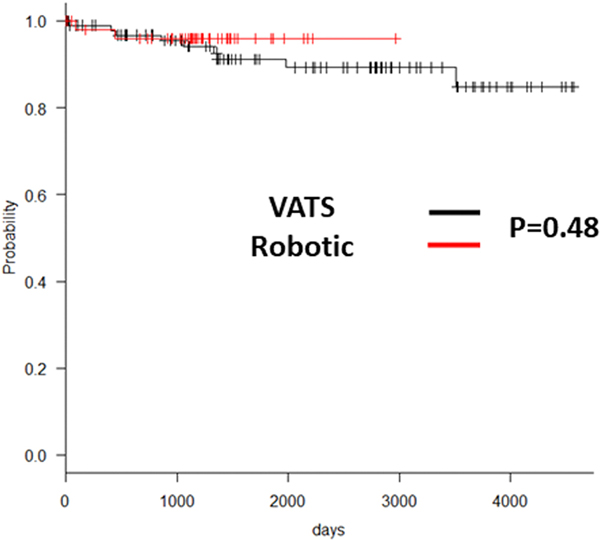
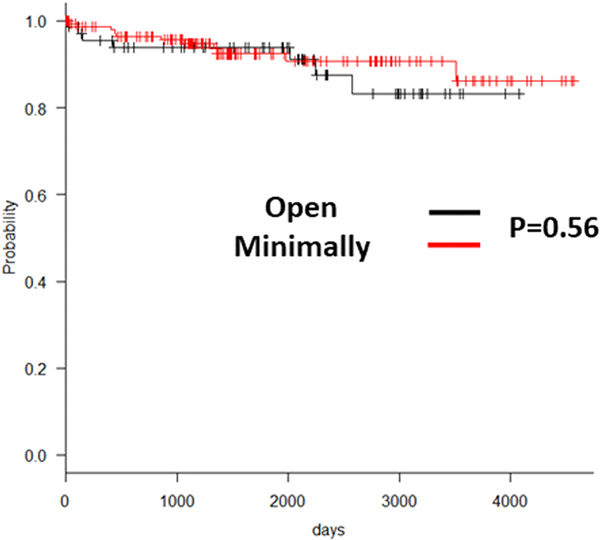
Having investigated the safety and cost-effectiveness of the MIS approach, we next evaluated the efficacy of the MIS approaches in achieving effective thymectomy. It is known that incomplete thymectomy for thymoma results is higher local recurrence rates [1,2]. Hence, we hypothesized that incomplete thymectomy in any of the study groups would result in reduced recurrence-free survival rates. Accordingly, we analyzed long-term survival in patients with thymoma to determine the effectiveness of the different approaches to accomplish complete thymectomy. In our study cohort, 19% of the patients were found to have a pathological diagnosis of thymoma as the etiology of the thymic mass (Table 3,4). There was no difference between the WHO classification of thymoma between the study groups (Table 3,4). When we evaluated the long-term 5-year and 10-year recurrence-free survival of patients in the Open and MIS group, we did not find significant differences between them (Open: 94.2% and 89.8%, MIS: 94.0% and 92.7%, p=0.98 and 0.47). This suggested that MIS and Open approaches may be equally effective in achieving complete thymectomy. Next, to evaluate the efficacy between VATS and RATS, we further studied the difference in the 5-year and 10-year recurrence-free survival between these two groups and found no difference (VATS: 94.8% and 90.7%, RATS: 96.2% and 96.2%, p=0.89 and 0.20). As shown in Figure 2, both VATS and RATS had an excellent and comparable long-term survival. Additionally, in all cases, R0 resection was achieved based on pathologic review, regardless of surgical technique used.
Figure 2.
Kaplan Meyer curve comparing recurrence-free survival between the VATS and RATS surgical cohorts.
Discussion:
For decades, sternotomy has been deemed the standard approach that provides the maximum surgical exposure for thymectomy [1]. However, sternotomy can be associated with significant morbidity in patients, particularly in those with disabling myasthenia gravis given that such patients frequently require steroids in the perioperative period. Such concerns have resulted in the development of MIS approaches which has been facilitated by the advances in fiberoptic lighting and digitalization of video-endoscopy [4–6]. There has been a significant increase in the adoption of minimally invasive techniques, including RATS since the first report of robotic-assisted thymectomy in 2001 [7–8,14–17,21–24]. Indeed, by 2013, more than 3500 robotic thymectomy procedures were performed at more than 100 hospitals worldwide [24].
The existing literature consists of single center experiences showing feasibility and safety of minimally invasive approaches. Nevertheless, how they compare to the traditional sternotomy in safety and long-term efficacy as well as cost-efficiency is unknown. Hence, our study presents a novel comparison of the three most commonly used surgical techniques, Open, VATS, and RATS, applied towards thymectomy. Although surgical approach was determined by the surgeon, all three methodologies could be used to treat the patients included in the study. We showed that there were no major adverse outcomes associated with the use of minimally invasive mediastinal surgery. Moreover, a mean clinical follow-up of 6.7 years demonstrated non-inferiority in terms of recurrence-free survival, freedom from 30-day hospital readmission, re-operation, or neurological sequela. MIS techniques were, in fact, associated with improved patient outcomes such as decreased hospital length of stay, decreased ICU stay, and incidence of post-operative arrhythmias. These likely reflect improved patient mobility, pain tolerance, and ability to wean off supplemental oxygenation in part due to smaller surgical incisions, and less musculoskeletal disruption. It is noteworthy, however, that the increased ICU length of stay observed in the Open study group in our manuscript could be related to our practice of admitting all patients undergoing sternotomy to the ICU for the first day. It may be possible to reduce this length of stay if programs could implement direct admission to non-ICU wards. Nevertheless, we believe that the overall length of stay is still likely to be significantly better with the MIS approaches.
In addition to showing clinical equipoise, MIS approaches also demonstrated reduced costs associated with the hospitalization. Our data shows that Open surgery results in higher costs compared to MIS, and that there were no significant differences between VATS and RATS. Although not separately investigated, this likely represents a correlation with total hospital and ICU length of stay, as both MIS strategies resulted in shorter patient recovery times. Our study has the limitation of not being able to include the initial capital costs required for the purchase of the equipment as that information is highly variable between institutions. Additionally, the robot is not exclusively dedicated for the thoracic procedures and we do not have an efficient way of allocating the capital costs directly associated with the thymectomy procedures. Nevertheless, we speculate that such costs could be recovered in due course of time given the reduced total costs associated with the procedure overall.
One of the strengths of our study was that the procedures were performed by surgeons with a unique expertise in the individual procedures that are being studied in this manuscript. If a surgeon was experienced in sternotomy, that surgeon offered it to all patients throughout the study period and did not utilize the VATS or RATS for these mediastinal masses. The same applied to surgeons performing the VATS and RATS approach. The patients were randomly referred to the individual surgeons which mitigated the selection bias. Furthermore, for the purposes of this study we included only those patients that could have been treated by any of the three approaches, as determined by the authors in the study. Hence, the more complex cases such as those with tumor greater than 6 cm, those with invasion into the cardiac or vascular structures, or who received neo-adjuvant therapy, were excluded since only open approaches were offered to these patients. There were no cases where conversion from minimally invasive approach to sternotomy was necessary. We believe this is the main reason why the three groups (namely sternotomy, VATS and RATS) were homogenous in terms of the clinical and demographic profile.
There were two early deaths in our series. The first was for a 79-year old female with history of renal cell carcinoma who presented with a thymic/anterior mediastinal mass. She underwent an uncomplicated thoracotomy and thymectomy, en bloc with the mass, which ultimately was proven to be metastatic clear cell renal cell carcinoma. She was discharged to home on post-operative day 2 without any in-hospital complications. She failed to show up to her one-week follow-up appointment was later reported deceased at home 24 days after surgery without known mechanism of death. The second mortality was from a 44 year old male with C3–5 quadriplegia who presented with a mediastinal mass and underwent a VATS resection of thymectomy with the mass. Final path revealed thymic cyst with granulation tissue without evidence of malignancy. However, the patient shortly thereafter developed bone pain and subsequently was found to have metastatic melanoma. The patient rendered himself DNI/DNR. He ultimately deteriorated and was transitioned to comfort care and passed away on post-operative day 27.
In summary, the long-term survival equipoise between Open and MIS techniques, improved short-term recovery, and lower associated costs, demonstrate the safety and cost-efficiency in the use of minimally invasive surgical techniques for thymectomy. In the light of our study and those from other recent institutions, a randomized trial comparing the Open and Minimally invasive approaches may not be considered feasible. However, a systematic review and meta-analysis might be a logical next step and has been proposed [24]. We also propose that the surgeons should consider the use of VATS and RATS based on their own experiences and expertise as in our view the current data shows equivalence between them. While we observed slightly improved benefits with the RATS approach, we recognize that there is a learning curve associated with the use of this technique. However, in our analysis, we did not exclude the early thymectomies performed using the RATS approach and, therefore, it incorporates the initial learning experience outcomes. Additionally, there are intangible costs and resources necessary with the initial setup of RATS. The approach of unilateral VATS can be highly effective if conducted properly.
Limitations:
This is a retrospective study subject to inherent bias of study design, data collection, analysis and quality assurance. Our analysis did not include transcervical thymectomy which is offered at a small number of centers nationally. This approach could be highly effective in thymectomy for myasthenia gravis, as evident by published literature [25]. However, our center does not offer this technique. Additionally, our standard approach is sternotomy and not thoracotomy. Therefore, we do not have patients who underwent thymectomy using the thoracotomy approach.
Figure 1.
Kaplan Meyer curve comparing recurrence-free survival between the open vs. minimally invasive surgical cohorts.
Acknowledgments
Funding/ Support
This work was supported by the National Institutes of Health, NIH HL145478, HL147290, and HL147575 (to AB). Funds were used to support salary for A.B. We are grateful to Ms. Elena Susan for the formatting and submission of the manuscript.
Abbreviations
- Open
sternotomy/thoracotomy
- VATS
unilateral video assisted thoracoscopic surgery
- RATS
robotic assisted VATS
- MIS
minimally invasive technique
Footnotes
COI/ Disclosures
The authors have no related conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wolfe GI, Kaminski HJ, Aban IB, Minisman G, Kuo HC, Marx A, et al. Randomized Trial of Thymectomy in Myasthenia Gravis. N Engl J Med. 2016;375(6):511–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davenport E, Malthaner RA. The role of surgery in the management of thymoma: a systematic review. Ann Thorac Surg. 2008;86(2):673–84. [DOI] [PubMed] [Google Scholar]
- 3.Venuta F, Rendina EA, Anile M, de Giacomo T, Vitolo D, Coloni GF. Thymoma and thymic carcinoma. Gen Thorac Cardiovasc Surg. 2012;60(1):1–12. [DOI] [PubMed] [Google Scholar]
- 4.Ersen E, Kilic B, Kara HV, Iscan M, Sarbay I, Demirkaya A, et al. Comparative study of video-assisted thoracoscopic surgery versus open thymectomy for thymoma and myasthenia gravis. Wideochir Inne Tech Maloinwazyjne. 2018;13(3):376–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ye B, Tantai JC, Ge XX, Li W, Feng J, Cheng M, et al. Surgical techniques for early-stage thymoma: video-assisted thoracoscopic thymectomy versus transsternal thymectomy. J Thorac Cardiovasc Surg. 2014;147(5):1599–603. [DOI] [PubMed] [Google Scholar]
- 6.Melfi FM, Fanucchi O, Mussi A. Minimally invasive mediastinal surgery. Ann Cardiothorac Surg. 2016;5(1):10–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Louie BE, Wilson JL, Kim S, Cerfolio RJ, Park BJ, Farivar AS, et al. Comparison of Video-Assisted Thoracoscopic Surgery and Robotic Approaches for Clinical Stage I and Stage II Non-Small Cell Lung Cancer Using The Society of Thoracic Surgeons Database. Ann Thorac Surg. 2016;102(3):917–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshino I, Hashizume M, Shimada M, Tomikawa M, Tomiyasu M, Suemitsu R, et al. Thoracoscopic thymomectomy with the da Vinci computer-enhanced surgical system. J Thorac Cardiovasc Surg. 2001;122(4):783–5. [DOI] [PubMed] [Google Scholar]
- 9.Roviaro G, Varoli F, Nucca O, Vergani C, Maciocco M. Videothoracoscopic approach to primary mediastinal pathology. Chest. 2000;117(4):1179–83. [DOI] [PubMed] [Google Scholar]
- 10.Savcenko M, Wendt GK, Prince SL, Mack MJ. Video-assisted thymectomy for myasthenia gravis: an update of a single institution experience. Eur J Cardiothorac Surg. 2002;22(6):978–83. [DOI] [PubMed] [Google Scholar]
- 11.Manoly I, Whistance RN, Sreekumar R, Khawaja S, Horton JM, Khan AZ, et al. Early and mid-term outcomes of trans-sternal and video-assisted thoracoscopic surgery for thymoma. Eur J Cardiothorac Surg. 2014;45(6):e187–93. [DOI] [PubMed] [Google Scholar]
- 12.Cheng YJ, Kao EL, Chou SH. Videothoracoscopic resection of stage II thymoma: prospective comparison of the results between thoracoscopy and open methods. Chest. 2005;128(4):3010–2. [DOI] [PubMed] [Google Scholar]
- 13.Zahid I, Sharif S, Routledge T, Scarci M. Video-assisted thoracoscopic surgery or transsternal thymectomy in the treatment of myasthenia gravis? Interact Cardiovasc Thorac Surg. 2011;12(1):40–6. [DOI] [PubMed] [Google Scholar]
- 14.Rea F, Marulli G, Bortolotti L, Feltracco P, Zuin A, Sartori F. Experience with the “da Vinci” robotic system for thymectomy in patients with myasthenia gravis: report of 33 cases. Ann Thorac Surg. 2006;81(2):455–9. [DOI] [PubMed] [Google Scholar]
- 15.Fleck T, Fleck M, Muller M, Hager H, Klepetko W, Wolner E, et al. Extended videoscopic robotic thymectomy with the da Vinci telemanipulator for the treatment of myasthenia gravis: the Vienna experience. Interact Cardiovasc Thorac Surg. 2009;9(5):784–7. [DOI] [PubMed] [Google Scholar]
- 16.Marulli G, Schiavon M, Perissinotto E, Bugana A, Di Chiara F, Rebusso A, et al. Surgical and neurologic outcomes after robotic thymectomy in 100 consecutive patients with myasthenia gravis. J Thorac Cardiovasc Surg. 2013;145(3):730–5; discussion 5–6. [DOI] [PubMed] [Google Scholar]
- 17.Cakar F, Werner P, Augustin F, Schmid T, Wolf-Magele A, Sieb M, et al. A comparison of outcomes after robotic open extended thymectomy for myasthenia gravis. Eur J Cardiothorac Surg. 2007;31(3):501–4; discussion 4–5. [DOI] [PubMed] [Google Scholar]
- 18.Ruckert JC, Swierzy M, Ismail M. Comparison of robotic and nonrobotic thoracoscopic thymectomy: a cohort study. J Thorac Cardiovasc Surg. 2011;141(3):673–7. [DOI] [PubMed] [Google Scholar]
- 19.Pennathur A, Qureshi I, Schuchert MJ, Dhupar R, Ferson PF, Gooding WE, et al. Comparison of surgical techniques for early-stage thymoma: feasibility of minimally invasive thymectomy and comparison with open resection. J Thorac Cardiovasc Surg. 2011;141(3):694–701. [DOI] [PubMed] [Google Scholar]
- 20.Liu TJ, Lin MW, Hsieh MS, Kao MW, Chen KC, Chang CC, et al. Video-assisted thoracoscopic surgical thymectomy to treat early thymoma: a comparison with the conventional transsternal approach. Ann Surg Oncol. 2014;21(1):322–8. [DOI] [PubMed] [Google Scholar]
- 21.Mussi A, Fanucchi O, Davini F, Lucchi M, Picchi A, Ambrogi MC, et al. Robotic extended thymectomy for early-stage thymomas. Eur J Cardiothorac Surg. 2012;41(4):e43–6; discussion e7. [DOI] [PubMed] [Google Scholar]
- 22.Bodner J, Wykypiel H, Greiner A, Kirchmayr W, Freund MC, Margreiter R, et al. Early experience with robot-assisted surgery for mediastinal masses. Ann Thorac Surg. 2004;78(1):259–65; discussion 65–6. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura H, Suda T, Ikeda N, Okada M, Date H, Oda M, et al. Initial results of robot-assisted thoracoscopic surgery in Japan. Gen Thorac Cardiovasc Surg. 2014;62(12):720–5. [DOI] [PubMed] [Google Scholar]
- 24.Ismail M, Swierzy M, Ruckert JC. State of the art of robotic thymectomy. World J Surg. 2013;37(12):2740–6. [DOI] [PubMed] [Google Scholar]
- 25.de Perrot M, Bril V, McRae K, Keshavjee S. Impact of minimally invasive trans-cervical thymectomy on outcome in patients with myasthenia gravis. Eur J Cardiothorac Surg. 2003;24(5):677–83. [DOI] [PubMed] [Google Scholar]