Keywords: COVID-19, endocrine system, SARS, SARS-CoV-2, virus
Abstract
The current COVID-19 pandemic is probably the worst the world has ever faced since the start of the new millennium. Although the respiratory system is the most prominent target of SARS-CoV-2 (the contagion of COVID-19), extrapulmonary involvement are emerging as important contributors of its morbidity and lethality. This article summarizes the impact of SARS-CoV and SARS-CoV-2 on the endocrine system to facilitate our understanding of the nature of coronavirus-associated endocrinopathy. Although new data are rapidly accumulating on this novel infection, many of the endocrine manifestations of COVID-19 remain incompletely elucidated. We, hereby, summarize various endocrine dysfunctions including coronavirus-induced new onset diabetes mellitus, hypocortisolism, thyroid hormone, and reproductive system aberrations so that clinicians armed with such insights can potentially benefit patients with COVID-19 at the bedside.
INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a highly transmissible and pathogenic novel coronavirus that emerged in late 2019 and has caused a pandemic of acute respiratory disease, named “coronavirus disease 2019” (COVID-19), which threatens human health and public safety. At this time of writing, the total confirmed cases are approaching 60 million round the world, with total number of reported deaths well exceeding a million (1). The contagion of COVID-19 has been named “severe acute respiratory syndrome coronavirus subtype 2” or “SARS-CoV-2”, not only because it assaults predominantly the respiratory system reminiscent of SARS and the Middle East respiratory syndrome (MERS), but also because genomic analysis revealed all three positive-sense, single-stranded RNA viruses to belong under the same genus, Betacoronavirus (2). From a phylogenetic perspective, SARS-CoV-2 and SARS-CoV are both of the same clade (3). Hence, the biology and clinical aspects of SARS can inform and serve as useful yardsticks for predicting and extrapolating the behavior of COVID-19.
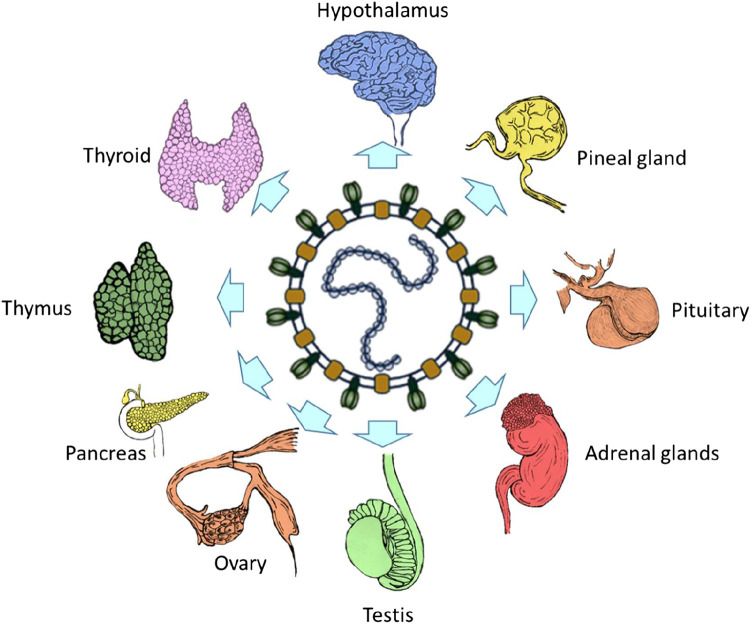
Despite its name, there is cogent evidence that SARS-CoV has extrapulmonary manifestations (4). During the SARS outbreak in 2003, many patients suffered sequelae in the gastrointestinal tract (5), the cardiovascular system (6), the coagulation system (7), the immune system (8), the nervous system (9), and even the endocrine system (10). Indeed, it is very likely that systemic viremia and an over-reactive immune response contributed to the pathogenesis of the lesions in key endocrine glands (Fig. 1). In the same vein, there is wisdom of hindsight distilled from historical parallels in guiding the manner we tackle this ongoing crisis. By contrast, MERS, while exacting a higher fatality rate compared with SARS and COVID-19, had not been associated with overt endocrine sequelae; despite the broad tissue distribution of dipeptidyl peptidase-4 (DPP4) receptors which serves as the portal of cell entry for MERS-CoV, this coronavirus has not been detected in any endocrine tissues in an autopsy study correlating clinicopathologic, immunohistochemical, ultrastructural, and molecular findings (11). Combining our experience combating both SARS and COVID-19 at the forefront with updated literature, we present this timely article devoted to features of SARS-induced endocrinopathy to better understand and predict COVID-19 effects on target organs of the endocrine system.
Figure 1.
The endocrine system as a target of betacoronaviruses (e.g., severe acute respiratory syndrome coronavirus, SARS-CoV).
RELEVANT CORONAVIRUS STRUCTURAL AND MOLECULAR BIOLOGY
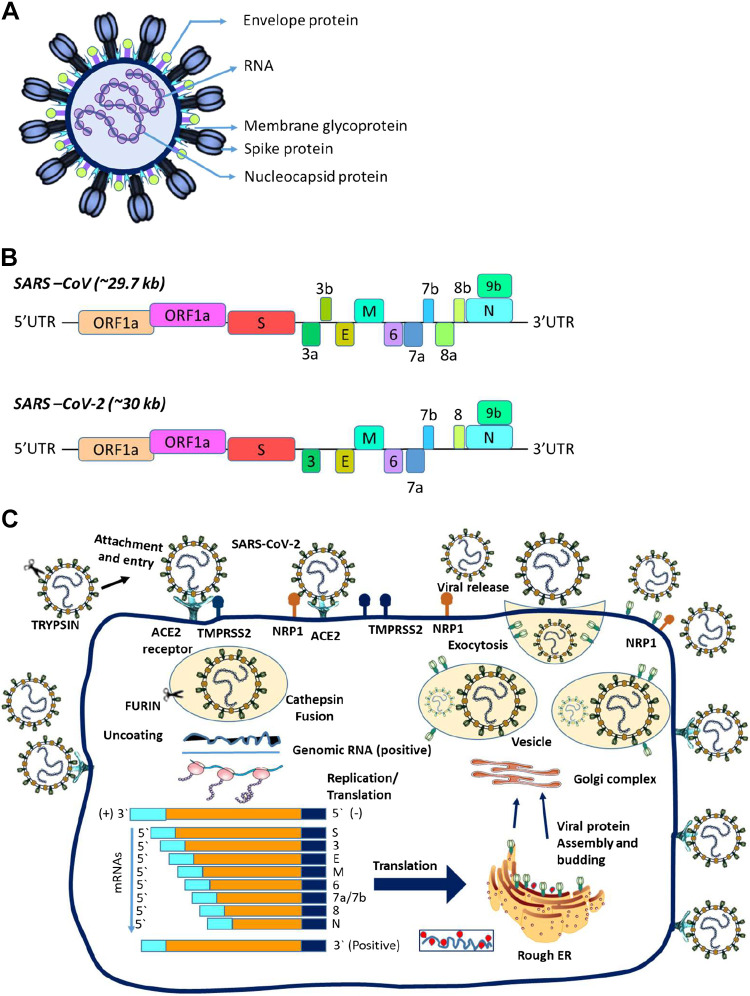
Coronavirus virion particles are spheroidal with dimensions ∼80–120 nm across, and possess morphologically characteristic clubbed-shaped peplomers otherwise termed “S” spike glycoproteins extending 17–20 nm outward from the lipid bilayer surface envelope reminiscent of a “crown” on electron microscopy and atomic force microscopy (12–14) (Fig. 2A). To aid further understanding of the predilection of SARS-CoV and SARS-CoV-2 for the human host, it is necessary to appreciate the structural details of the S spike glycoprotein. Each S protein comprises three monomers fused as a trimer to form the spike (15). The molecularly divergent distal “bulb” amino-terminal half portion is the S1 fragment critical for binding to host cell surface receptors (16) whereas the highly conserved “stalk-like” carboxy-terminal half portion is the S2 fragment of the spike protein that has both an ectodomain and a transmembrane domain responsible for fusion to the host cell membrane (17) (Fig. 2B). Cathepsins are involved in the cleavage of S into S1 and S2 subunits to expose S2 for fusion to cell membrane via host proteases (18). This process is accomplished by the synergistic activity of cathepsins along with other host proteases, cell surface transmembrane protease serine (TMPRSS) proteases, furin, trypsin, and factor Xa, following which the internalized virion undergoes replication within the infected host cell and finally exits the cell via a lysosomal-based exocytosis pathway to complete its life cycle (Fig. 2C). The lipid bilayer multi-spanning M glycoprotein through which the S spike protein structure is inserted and anchored represents the largest constituent of the virion. The coronavirus M protein is interestingly the first polytopic viral membrane ever to be described in the virology field (19), and its glycosylation status plays a role in organ tropism (20) and possesses a capacity of alpha-interferon induction (21). The E protein of SARS-CoV is an integral membrane protein whereas the N protein is a phosphoprotein that binds to viral RNA in a helical nucleocapsid conformation and enhances replication efficiency (22).
Figure 2.
A: shows the virion structure of severe acute respiratory syndrome coronavirus (SARS-CoV) and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). B: shows the similarities and differences in the amino acid sequences of the two viruses. C: illustrates the life cycle of these two coronaviruses.
CELL INVASION GATEWAYS FOR COVID-19 AND SIMILARITIES TO SARS
Angiotensin-Converting Enzyme-2
Coronaviruses exploit host cell membrane protein receptors to gain entry into the interior of the cell. The most well-described gateway for both SARS-CoV and SARS-CoV-2 is angiotensin-converting enzyme-2 (ACE2). ACE2 is a type I transmembrane zinc-dependent monocarboxypeptidase with homology to ACE, a key player in the renin-angiotensin-aldosterone system (RAAS) and a target for the treatment of hypertension because it cleaves angiotensin-II into angiotensin-(1–7) which binds Mas-receptors to negatively regulate the RAAS (23). Unlike MERS-CoV, which engages surface dipeptidylpeptidase-subtype 4 (DPP4) and sialoside attachment receptors for host cell entry (24), SARS-CoV and SARS-CoV-2 seek out ACE2 as receptors for cell invasion (25). ACE2 is abundantly expressed in human kidneys, adrenals, adipose tissues, thyroid, endothelium, pancreas, testis, ovary, and pituitary (26, 27). ACE2 possesses highly similar binding motifs for the S protein indispensable for SARS-CoV and SARS-CoV-2 invasion (25, 28, 29).
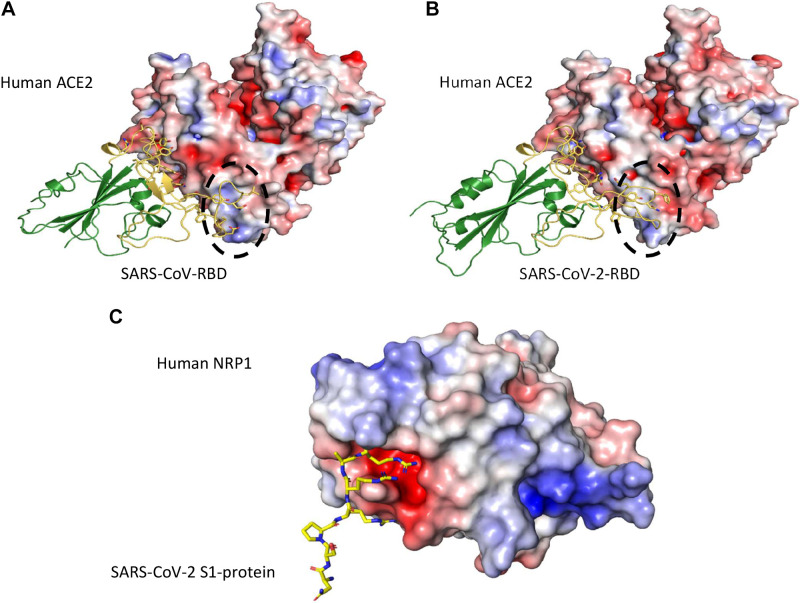
ACE2 interaction with the spike protein was first made available for SARS-CoV shortly after the 2002–2004 SARS outbreak, and had since been steadily built up for more than a decade, before being accumulated explosively in response to the 2019 SARS-CoV-2 pandemic (25, 29–34). Such information details both similarities and differences between SARS-CoV and SARS-CoV-2 in their receptor binding (29, 31). These two viruses both utilize their receptor-binding domains (RBDs), residing at the C-terminal half of the S1 fragment to bind ACE2 with nanomolar affinities; the binding sites on ACE2, located at the N-terminal peptidase domain, are nearly identical. Major differences lie within the two viruses’ respective ∼70 residue-long receptor-binding motifs (RBMs), which are extended insertions grafted onto the core of RBD, sharing ∼50% sequence identity and adopting different conformations upon ACE2 binding (Fig. 3, A and B). Consequently, the viruses’ ACE2 binding affinities are folds apart (20–30 folds), with SARS-CoV-2 being the tighter binder (25, 31, 32, 35–37), which corroborates with the drastically higher transmissibility of SARS-CoV-2 (38). Taking SARS-CoV and MERS-CoV as examples, moderate changes in their RBM sequences have led to zero cross-reactivity against the receptor for the other (39).
Figure 3.
Angiotensin-converting enzyme-2 (ACE2) binding by the receptor-binding domains (RBDs) of severe acute respiratory syndrome coronavirus (SARS-CoV) (A) (PDB ID: 2AJF) and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (B) (PDB ID: 6M0J). The RBD cores are in green, whereas the receptor-binding motifs (RBMs) are highlighted in gold. Major conformational differences between the RBDs are circled with dashed lines. In (C) the interaction between the C-terminus of SARS-CoV-2 S1 (residues 679–685, NSPRRAR, yellow sticks) and human transmembrane receptor Neuropilin-1 (electrostatic surface), based on recently published complex structure (PDB ID: 7JJC) (51) is illustrated. These figures are generated using PyMOL (36), with the APBS plugin for electrostatic surface calculations (35).
Transmembrane Protease Serine-2
TMPRSS2 facilitates cellular entry of SAR-CoV and SARS-CoV-2 into the host. TMPRSS2 is an androgen-regulated gene (40, 41) involved in the priming of the viral spike protein. This process is critical for virulence as it diminishes viral recognition by neutralizing antibodies and also helps in activating SARS-CoV-2 for virus cell fusion. That the activated androgen receptor regulates the transcriptional activity of the TMPRSS2 gene could partly explain the differential susceptibility of males for COVID-19 (42, 43). TMPRSS2 works synergistically along with “a disintegrin and metalloproteinase domain-containing protein-17” (ADAM17) required for the shedding of ACE2 (44). It has been shown that single nucleotide polymorphisms (SNPs) in TMPRSS2 might influence SARS-CoV-2 entry into the cell (45). Some newly reported target sites of COVID-19 mediated by TMPRSS2 are hepatobiliary and pancreatic tissues (46), which deserves further mention below.
Neuropilin-1
Neuropilin-1 (NRP1), a highly conserved type 1 transmembrane protein, plays important roles in the development of the nervous and cardiovascular system as well as in tumorigenesis through interaction with its established binding partners, such as vascular endothelial growth factor (VEGF) and semaphorin 3A (Sema3A). Cell entry of SARS-CoV-2 depends on priming by host cell proteases (47, 48). There is limited knowledge about the virus-host interactions that determine cellular entry of SARS-CoV-2. Viruses display considerable redundancy and flexibility because they can exploit weak multivalent interactions to enhance affinity. Although the focus to date has been almost entirely on the role of ACE2 in SARS-CoV-2 entry, the expression pattern of ACE2 does not match tissue tropism of SARS-CoV-2 (49). This raises the possibility that cofactors are required to facilitate virus-host cell interactions in cells with low ACE2 expression. In the case of SARS, the C-type lectin receptor CD209L (L-SIGN) was found to serve as an alternative gateway for the cellular entry of SARS-CoV by tethering to the mannose glycans of the S-protein (50). Recently, by applying site-directed mutagenesis and monoclonal antibodies, it was shown that NRP1 could represent such an ACE2 potentiating factor (51); the high expression of NRP1 on epithelial cells strategizes it as an entry receptor (52). Reports demonstrated that the SARS-CoV-2 S protein binds to the b1b2 ectodomain of the NRP1 (Fig. 3C). The subsequent entry of SARS-CoV-2 into cells is facilitated by a polybasic amino acid sequence (682RRAR685) termed the “C-end rule” (CendR) motif within NRP1 (53). Corroborating evidence for its putative role as an entry receptor is its upregulation in COVID-19 biological samples versus healthy controls as shown by “omic” analyses (53). That SARS-CoV-2 shows a much greater infectivity relative to SARS-CoV is probably explained in part by NRP1 binding to the CendR peptide in S1. Such a discovery meant that novel therapeutic approaches that target this mechanism could be developed. From the Human Protein Atlas, we find medium expression of NRP1 in the parathyroids, adrenals, and testis, and low expression of NRP1 in the thyroid. As VEGF is a natural endogenous ligand of NRP1, it is also possible that the pituitary could well be a target of SARS-CoV-2 since VEGF receptors are also present in the pituitary gland (54).
CLINICO-PATHOLOGICAL SPECTRUM OF SARS AND COVID-19 ENDOCRINOPATHY
Hypothalamus-Pituitary-Adrenal Axis
The lack of detailed postmortem studies contributed to the paucity of knowledge surrounding the pathogenesis of endocrinopathy associated with SARS and COVID-19. There is only a dearth of reports of involvement of the hypothalamus and pituitary by SARS (55, 56). Using light microscopy, electron microscopy, and real-time reverse transcription-polymerase chain reaction (RT-PCR), autopsy series previously confirmed SARS-CoV genome sequences associated with cytopathic effects in neuronal cytoplasm of the hypothalamus. Clinical observation of an impaired adrenocorticotropic hormone (ACTH) and TSH response to hypocortisolism and hypothyroidism, respectively, implied that the hypothalamus-pituitary-adrenal (HPA) axis was probably involved either directly by SARS-CoV or indirectly due to hypophysitis caused by autoimmunity triggered by the virus (55). Little has been documented on the hypothalamic-pituitary effects of COVID-19, though a French group has recently confirmed from autopsy findings that the hypothalamus is a highly probable target of SARS-CoV-2 based on its rich expression of ACE2 and TMPRSS2, especially in the paraventricular nucleus (57). CT and MRI imaging have also revealed evidence of COVID-19 infecting the brain with serious consequences (58). A Chinese group successfully detected the presence of SARS-CoV-2 genome in the cerebrospinal fluid in a patient with COVID-19, thereby confirming that SARS-CoV-2 does indeed infiltrate into the brain, and thence can involve any part of the brain, including the hypothalamus and pituitary (59). Although HPA axis compromised with hypocortisolism can contribute to mortality in SARS and COVID-19, an overly excessive endogenous cortisol response itself poses a caveat, lest high cortisol be misinterpreted to portend a better prognosis. A group has shown via Cox proportional hazards regression analysis that cortisol stress responses were predictive of death. Kaplan–Meier survival analysis revealed a sharp dichotomy in death probability, with significantly better median survival for serum cortisol lower than 744 nmol/L during acute COVID-19 infection, a scenario correlative of illness severity. This should not be misconstrued as justification to avoid prescribing exogenous steroids when there are overwhelming life-saving indications as discussed in the next section (60). Several recent publications have also confirmed that the adrenals are a frequent site of COVID-19 related lesions in the body based on radiological and autopsy evidence (61–64).
Hypothalamus-Pituitary-Thyroid Axis
The thyroid is another endocrine gland reportedly disordered in SARS previously (56, 65) and now in COVID-19 (66). In 2004, a group from Guangzhou in China produced postmortem evidence of cytopathic effects of SARS-CoV on endocrine organs including the thyroid, parathyroid, pancreas, and adrenals using a combination of RT-PCR, immunohistochemistry, in situ hybridization, and transmission electron microscopy (67). Among those who deceased from SARS, the thyroids showed destruction of the follicular epithelium with extensive exfoliation of apoptotic cells into the follicular lumen. Thyroid follicular damage was occasionally very severe, associated with complete loss of parafollicular C-cells as shown by total absence of calcitonin immunostaining. This explains why serum T3 and T4 were decreased in 94% and 46%, respectively, in a group of patients with SARS during the acute phase of the disease, followed by persistence of low-serum T3 and T4 in 90% and 38% among convalescent cases (68). Autopsies of patients with SARS showed marked destruction of the follicular and parafollicular cells of the thyroid (65).
The ongoing COVID-19 pandemic yields interesting and important insights on SARS-CoV-2 and thyroid pathology. The available data suggest that the disease spectrum can range from direct viral destructive effects to immune-mediated mechanisms on the thyroid precipitated by COVID-19 (69–72). Leow et al. (56) showed previously that SARS-CoV could inflict pituitary lesions either directly or indirectly and contribute to secondary thyroid and adrenal insufficiency, which could be treated using levothyroxine and hydrocortisone replacement (56). It appears that the SARS-induced thyroid aberrations were largely transient and fully resolved after several months. Current published data indicate a similar pathophysiological phenomenon associated with COVID-19. Mounting evidence points to the fact that COVID-19 might have a greater impact on the hypothalamus-pituitary-thyroid axis than previously suspected. This is particularly so following the discovery of ACE-2 mRNA in thyroid cells (73). ACE-2 mRNA in thyroid follicular cells was confirmed by analyzing primary cultures of thyroid cells, where the expression is similar to those found in tissues. The finding accounts for the recently described COVID-19-related subacute thyroiditis or De Quervain’s thyroiditis that is often thought to have a viral origin (73); it can present with thyrotoxicosis before a hypothyroid phase sets in weeks to months later (74). Hence, subacute thyroiditis is now considered to be a sequela closely associated with COVID-19 (75). Other reports also indicate autoimmune thyroiditis may develop after the “cytokine storm” induced by SARS-CoV-2 infection which could result in the development of primary hypothyroidism. Common thyroid manifestations of COVID-19 thus include overt thyrotoxicosis, Graves’ orbitopathy, and hypothyroidism. Graci et al. (76) in a recent report has even suggested that COVID-19 could be considered as an endocrine disorder, to make sense of the nonspecific response of the immune system to the SARS-CoV-2 virus, which is far different from infections such as influenza (77).
Reproductive Axis
Next, on the list of endocrine gland targets of the coronavirus is the testis which has been established to express ACE2 and NRP1 copiously (78). In addition, the MAS receptor is present on the acrosome and tail of human spermatozoa and plays a role in the acrosome reaction and the maintenance of sperm motility, thereby underscoring the role of ACE2 in sperm biology (79). The release of TMPRSS2 in prostasomes secreted into semen from the prostate during ejaculation together with ACE2 present on the sperm plasma membrane would thus allow SARS-CoV-2 to infect sperm cells (80). With respect to coronavirus invasion of the testis, there were men with symptoms consistent with acute orchitis in some male cases of SARS (81). Similarly, at least one case of orchitis has been reported in a young man with COVID-19 (82). Postmortem examination of men who succumbed to COVID-19 interestingly also revealed seminiferous tubular injury, vacuolation of Sertoli cells, reduced Leydig cells, and lymphocytic infiltrates in 11 of 12 deceased males, of whom one had demonstrable SARS-CoV-2 by RT-PCR within testicular tissues (83). How SARS-CoV-2 might inhibit sperm motility, permanently damage the testis, and negatively influence fertility remains undetermined. A subsequent study confirmed the frequent presence of SARS-CoV-2 in semen of men with acute COVID-19 as well as those convalescing from it (84). This finding, together with the significantly high rates of COVID-19 infection between sex partners, implies the possibility of a sexual route of transmission, though there is no current strong evidence supporting such a route of transmission as one of clinical concern as yet (85). In females, ACE2 is expressed in the ovary, uterus, placenta, vagina, and breast tissues. ACE2 is present in ovarian stroma, granulosa cells, and oocytes (86), and ACE2 mRNA has been shown to be detectable in the ovaries of premenopausal and postmenopausal women (87). Unlike males, TMPRSS2 appears to be absent in human oocytes which means that infection of the female germline by SARS-CoV-2 is rather improbable except in the situation where the ovum is fertilized by an infected spermatozoon. Despite such theoretical concerns, there have not been any published reports of teratogenic effects and embryopathy directly attributable to SARS-CoV-2 as yet in contrast to Zika virus infection (88).
Endocrine Pancreatic Islets
More recently, de novo onset of severe diabetes mellitus and diabetic ketoacidosis among patients with COVID-19 who were formerly healthy and nondiabetic stoked fears of permanent beta cell damage from a single episode of COVID-19 (89). This is not unfounded given the high expression of ACE2 in the pancreatic islets, and previous encounters of new-onset diabetes as a sequela of SARS (90). Importantly, the downregulation of ACE2 by SARS-CoV-2 can lead to unopposed angiotensin-II activity on AT-I receptors that suppresses insulin secretion (91). In addition, because it was reported a decade ago that an abnormal allele of NRP1 in pancreatic beta cells led to type 1 diabetes, this could imply that the binding of the S protein of SARS-CoV-2 to NRP1 in pancreatic islets might potentially cripple the insulin secretory pathway and trigger insulin-dependent diabetes or even overt diabetic ketoacidosis (92). A global CoviDiab registry has just been initiated to examine beta cell injury and insulinopenia in those without pre-existing diabetes in whom SARS-CoV-2 is the only etiologic factor (93). These data suggest the importance of proactive monitoring for such endocrine dysfunction among COVID-19 patients (10).
CLINICAL COURSE OF COVID-19 AMONG PATIENTS WITH PRE-EXISTING ENDOCRINE ISSUES
Available reports indicate that individuals with pre-existing endocrine disorders are at higher risk of suffering greater disease severity during COVID-19. Interestingly though, patients with pre-existing hypothyroidism and receiving thyroid hormone therapy were not found to be associated with an increased risk of hospitalization (94). However, patients with other endocrine conditions such as hypocortisolism and diabetes mellitus appeared to have poorer prognosis. Risk factors for hospital admission among patients with COVID-19 with diabetes include older age, higher HbA1c level, hypertension, cardiovascular disease, cerebrovascular disease, chronic pulmonary disease, malignancy, chronic kidney disease (CKD 3A, 3B, 4), and insulin-treated patients were more likely to require hospital admission (95).
Contributory causes included addisonian crisis and impaired immunity (96), which makes these patients more vulnerable to SARS-CoV-2 infection (97). Serum biomarkers (IL-6, serum ferritin, CRP) and D-dimers are also higher in patients with SARS-CoV-2 with underlying diabetes (98). Although clinicians might be deterred from prescribing corticosteroids in accordance with earlier conservative treatment guidelines for COVID-19 (99), glucocorticoids should be instituted in those with features of hypocortisolism on a case-by-case basis, especially since addisonian crisis can be equally life threatening if missed or untreated (100). Intriguingly, emerging promising data from an ongoing clinical trial, Randomized Evaluation of COVID-19 Therapy, otherwise codenamed RECOVERY (ClinicalTrials.gov ID: NCT04381936) showed that low-dose dexamethasone is a life-saving drug and reduced deaths by 20% for patients with COVID-19 requiring oxygen, and even lowered the risk of death for those on mechanical ventilation by ∼30% which is very impressive. Although speculative, low-dose dexamethasone for patients with severe COVID-19 worldwide can thus be life-saving if prescribed at the right timing during the disease course (101). An expected corollary of dexamethasone might be aversion of collateral damage to endocrine glands via suppression of a cytokine-driven hyperinflammatory response during the course of COVID-19 infection.
Among pre-existing endocrine issues, diabetes mellitus probably tops the list, simply because diabetes itself is a worldwide pandemic and it confers an increased mortality in the face of COVID-19 (102). It has been found that SARS-CoV-2 virus is more prevalent and severe in people with diabetes. A recent case-control study demonstrated the virus infection could lead to significant insulin resistance, dehydration, and acute kidney injury. In rare cases, patients could also develop diabetic ketoacidosis (103). Hyperglycemia predisposes to bacterial and viral pathogens such as tuberculosis and influenza (104), since hyperglycemia could trigger the hyper-virulence of certain pathogens (105). In addition, glycemic control tends to be worse among diabetic patients treated with corticosteroids and lopinavir/ritonavir, which can result in cytokine-induced insulin resistance as well as hypokalemia, which can impair insulin secretion itself. Evidence also suggests a heightened tendency of ketoacidosis among patients with COVID-19 with pre-existing diabetes (93, 106).
Related to diabetes mellitus is obesity, in which a recent study revealed that obesity is correlated to higher odds of mechanical ventilation and in-hospital mortality after multivariable adjustment on analyzed data from patients with COVID-19 at 88 hospitals enrolled in the American Heart Association’s COVID-19 Cardiovascular Disease Registry (107). Notably, among patients ≤ 50 yr of age, BMI ≥ 40 kg/m2 was linked to a profoundly elevated risk of death or mechanical ventilation (OR, 1.64 [95% CI, 1.23–2.21]), moderately increased odds in those aged 51–70 (OR, 1.40 [1.10–1.80]), but no significant increase in risk among patients > 70 yr old (OR, 1.28 [0.83–1.95]). Venous thromboembolism and dialysis were also linked to higher BMI, all of which suggest that the generally lower probability of morbidity and mortality in younger people with COVID-19 may not necessarily apply to those with obesity.
The mechanisms of how increased adiposity complicates the clinical course of COVID-19 remain to be elucidated, but is likely to be multifactorial and related to the impact of excess fat on metabolism, immune response, and vascular function. Whether direct viral infection of adipocytes plays a mechanistic role in COVID-19 severity among obese people is open to question. In this regard, it is remarkable that adipose tissues show the highest level of NRP1 mRNA transcripts among all the organs of the human body examined, as shown by a mean protein-coding transcripts per million (pTPM) of 101 in the Genotype-Tissue (GTEx) RNA-seq database and 388.6 Scaled Tags per Million in another public data set, the Functional Annotation of Mammalian Genomes 5 (FANTOM5) Cap Analysis of Gene Expression (CAGE).
Correspondingly, a previous study on rats also demonstrated the expression of NRP1 on the surface membranes of adipocytes and parenchymal nerves found within adipose tissues (108, 109). Adipose tissues also rank among the top ten tissues with the highest abundance of ACE2 mRNA based on a pTPM of 8.8 in GTEx and an estimated 5.6 scaled tags per million in FANTOM5 CAGE (110). Consistent with this is the finding of ACE2 protein expression in adipocytes (111) as well as TMPRSS2 within adipose tissues (112). There is thus biological plausibility for higher host susceptibility to more severe COVID-19 among those with greater adiposity relative to those who are lean.
As this COVID-19 pandemic evolves, an increasing number of infected patients are observed to be afflicted with a range of different endocrine disorders worldwide. Thus, collaborations between experts from different countries are crucial as the collective wisdom and best practices shared across geographical boundaries can help tackle this pandemic better, such as illustrated by a recent survey of clinical endocrinologists (113). This raises the following important research questions that demand further investigations:
Does SARS-CoV-2 invade the hypothalamus directly and induce endocrine dysfunction, given the fact that other coronaviruses have been proven to exhibit neurotropism through their invasion into the central nervous system, and that human pluripotent stem cell-derived dopaminergic neurons can be infected by SARS-CoV-2 in vitro (114)?
Is SARS-CoV-2 capable of migrating in a retrograde fashion along the olfactory pathway and spread trans-synaptically into the hypothalamus, as this is one established route of entry into the central nervous system exploited by other coronaviruses (115), and especially because anosmia is a very common feature of those infected by the SARS-CoV-2 virus?
Are the parathyroid glands also susceptible to SARS-CoV-2, and if so, would hypocalcemia be a potential complication in certain cases of COVID-19?
Do adipose tissue depots serve as a reservoir for the coronavirus among asymptomatic carriers with obesity, and whether SARS-CoV-2 undergoes a lytic or lysogenic cycle if it infects white adipocytes?
Are there any drugs or biologics approved for the treatment of endocrine and metabolic diseases potentially able to be repurposed as antiviral agents against SARS-CoV-2?
It is imperative to address such questions with scientific rigor and unravel the molecular mechanisms, especially since this pandemic is still very rampant in many countries and reports of an increasing range of endocrinological aberrations associated with COVID-19 continue to be added to the current literature. Greater clarity of COVID-19-induced endocrinopathies will expectedly aid diagnostic pathways, therapeutic decisions, and drive clinical management with ultimate benefit to patients suffering from this viral assault.
CONCLUSIONS
Although COVID-19 is very widespread, much of its endocrine manifestations are still far from being fully elucidated. Knowledge of SARS-associated endocrinopathy forms a basis for better understanding COVID-19 endocrinopathy. Meanwhile, scholarly contributions on endocrine issues in COVID-19 should be facilitated, while peer-reviewed journals might consider publishing more worthy papers that add useful insights to the scarce literature in this emerging field. Physicians, endocrinologists, and even patients with different types of endocrine conditions can get more up-to-date information regarding the endocrine manifestations of COVID-19 or the effects of COVID-19 on any pre-existing endocrine disorders from the various national, regional, and international endocrine societies and thyroid associations.
GRANT
We acknowledge funding assistance from the Singapore Institute for Clinical Sciences, Agency for Science, Technology and Research (A*STAR) to cover the publication charges and open access fee.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N. Kothandaraman, N. Karnani, and M.K.S.L. conceived and designed research; N. Kothandaraman and M.K.S.L. analyzed data; N. Kothandaraman, A.R., and M.K.S.L. interpreted results of experiments; N. Kothandaraman, A.R., and M.K.S.L. prepared figures; N. Kothandaraman, A.R., B.X., W.S.Y., S.S.V., N. Karnani, and M.K.S.L. drafted manuscript; N. Kothandaraman, A.R., B.X., W.S.Y., S.S.V., N. Karnani, and M.K.S.L. edited and revised manuscript; N. Kothandaraman, A.R., B.X., W.S.Y., S.S.V., N. Karnani, and M.K.S.L. approved final version of manuscript.
REFERENCES
- 1.Cases C. https://wwwworldometersinfo/coronavirus/ [Accessed 2020 June 16].
- 2.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al.. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 395: 565–574, 2020. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W.. China Novel Coronavirus Investigating, and Research team . A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382: 727–733, 2020. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Menter T, Haslbauer JD, Nienhold R, Savic S, Hopfer H, Deigendesch N, Frank S, Turek D, Willi N, Pargger H, Bassetti S, Leuppi JD, Cathomas G, Tolnay M, Mertz KD, Tzankov A. Post-mortem examination of COVID19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings of lungs and other organs suggesting vascular dysfunction. Histopathology 77: 198–209, 2020. doi: 10.1111/his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smyk W, Janik MK, Portincasa P, Milkiewicz P, Lammert F, Krawczyk M. COVID-19: focus on the lungs but do not forget the gastrointestinal tract. Eur J Clin Invest, 50: e13276, 2020. doi: 10.1111/eci.13276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu PP, Blet A, Smyth D, Li H. The science underlying COVID-19: implications for the cardiovascular system. Circulation 142: 68–78, 2020. doi: 10.1161/CIRCULATIONAHA.120.047549. [DOI] [PubMed] [Google Scholar]
- 7.Mendoza-Pinto C, Escarcega RO, Garcia-Carrasco M, Bailey DJO, Galvez-Romero JL, Cervera R. Viral infections and their relationship with catastrophic antiphospholipid syndrome: a possible pathogenic Mmechanism of severe COVID-19 thrombotic complications. J Intern Med 288: 737–739, 2020. doi: 10.1111/joim.13123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou Z, Ren L, Zhang L, Zhong J, Xiao Y, Jia Z, Guo L, Yang J, Wang C, Jiang S, Yang D, Zhang G, Li H, Chen F, Xu Y, Chen M, Gao Z, Yang J, Dong J, Liu B, Zhang X, Wang W, He K, Jin Q, Li M, Wang J. Heightened innate immune responses in the respiratory tract of COVID-19 patients. Cell Host Microbe 27: 883–890e2, 2020. doi: 10.1016/j.chom.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paniz-Mondolfi A, Bryce C, Grimes Z, Gordon RE, Reidy J, Lednicky J, Sordillo EM, Fowkes M. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). J Med Virol 92: 699–702, 2020. doi: 10.1002/jmv.25915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mongioi LM, Barbagallo F, Condorelli RA, Cannarella R, Aversa A, La Vignera S, Calogero AE. Possible long-term endocrine-metabolic complications in COVID-19: lesson from the SARS model. Endocrine 68: 467–470, 2020. doi: 10.1007/s12020-020-02349-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ng DL, Al Hosani F, Keating MK, Gerber SI, Jones TL, Metcalfe MG, Tong S, Tao Y, Alami NN, Haynes LM, Mutei MA, Abdel-Wareth L, Uyeki TM, Swerdlow DL, Barakat M, Zaki SR. Clinicopathologic, immunohistochemical, and ultrastructural findings of a fatal case of Middle East respiratory syndrome coronavirus infection in the United Arab Emirates. Am J Pathol 186: 652–658, 2016. doi: 10.1016/j.ajpath.2015.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Almeida JD, Berry DM, Cunningham CH, Hamre D, Hofstad MS, Mallucci L, MCintosh K, Tyrrell DAJ. Coronaviruses. Nature 220: 650, 1968. doi: 10.1038/220650b0. [DOI] [Google Scholar]
- 13.McIntosh K. Coronaviruses: a comparative review. Curr Top Microbiol Immunol 63: 85–129, 1974. doi: 10.1007/978-3-642-65775-7_3. [DOI] [Google Scholar]
- 14.Sugiyama K, Amano Y. Morphological and biological properties of a new coronavirus associated with diarrhea in infant mice. Arch Virol 67: 241–251, 1981. doi: 10.1007/BF01318134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song HC, Seo MY, Stadler K, Yoo BJ, Choo QL, Coates SR, Uematsu Y, Harada T, Greer CE, Polo JM, Pileri P, Eickmann M, Rappuoli R, Abrignani S, Houghton M, Han JH. Synthesis and characterization of a native, oligomeric form of recombinant severe acute respiratory syndrome coronavirus spike glycoprotein. JVI 78: 10328–10335, 2004. doi: 10.1128/JVI.78.19.10328-10335.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang L, Junker D, Hock L, Ebiary E, Collisson EW. Evolutionary implications of genetic variations in the S1 gene of infectious bronchitis virus. Virus Res 34: 327–338, 1994. doi: 10.1016/0168-1702(94)90132-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Groot RJ, Luytjes W, Horzinek MC, van der Zeijst BA, Spaan WJ, Lenstra JA. Evidence for a coiled-coil structure in the spike proteins of coronaviruses. J Mol Biol 196: 963–966, 1987. doi: 10.1016/0022-2836(87)90422-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belen-Apak FB, Sarialioglu F. The old but new: can unfractioned heparin and low molecular weight heparins inhibit proteolytic activation and cellular internalization of SARS-CoV2 by inhibition of host cell proteases? Med Hypotheses 142: 109743, 2020. doi: 10.1016/j.mehy.2020.109743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armstrong J, Niemann H, Smeekens S, Rottier P, Warren G. Sequence and topology of a model intracellular membrane protein, E1 glycoprotein, from a coronavirus. Nature 308: 751–752, 1984. doi: 10.1038/308751a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Haan CA, de Wit M, Kuo L, Montalto-Morrison C, Haagmans BL, Weiss SR, Masters PS, Rottier PJ. The glycosylation status of the murine hepatitis coronavirus M protein affects the interferogenic capacity of the virus in vitro and its ability to replicate in the liver but not the brain. Virology 312: 395–406, 2003. doi: 10.1016/S0042-6822(03)00235-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laude H, Gelfi J, Lavenant L, Charley B. Single amino acid changes in the viral glycoprotein M affect induction of alpha interferon by the coronavirus transmissible gastroenteritis virus. J Virol 66: 743–749, 1992. doi: 10.1128/JVI.66.2.743-749.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Almazán F, Galán C, Enjuanes L. The nucleoprotein is required for efficient coronavirus genome replication. JVI 78: 12683–12688, 2004. doi: 10.1128/JVI.78.22.12683-12688.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riordan JF. Angiotensin-I-converting enzyme and its relatives. Genome Biol 4: 225, 2003. doi: 10.1186/gb-2003-4-8-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park YJ, Walls AC, Wang Z, Sauer MM, Li W, Tortorici MA, Bosch BJ, DiMaio F, Veesler D. Structures of MERS-CoV spike glycoprotein in complex with sialoside attachment receptors. Nat Struct Mol Biol 26: 1151–1157, 2019. doi: 10.1038/s41594-019-0334-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 181: 281–292e286, 2020. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 203: 631–637, 2004. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turner AJ. ACE2 bell Biology, regulation, and physiological functions. Protect Arm Renin Angiotensin Syst (RAS) 185–189, 2015. [Google Scholar]
- 28.Glowacka I, Bertram S, Herzog P, Pfefferle S, Steffen I, Muench MO, Simmons G, Hofmann H, Kuri T, Weber F, Eichler J, Drosten C, Pohlmann S. Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63. JVI 84: 1198–1205, 2010. doi: 10.1128/JVI.01248-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li F, Li W, Farzan M, Harrison SC. Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science 309: 1864–1868, 2005. doi: 10.1126/science.1116480. [DOI] [PubMed] [Google Scholar]
- 30.Gui M, Song W, Zhou H, Xu J, Chen S, Xiang Y, Wang X. Cryo-electron microscopy structures of the SARS-CoV spike glycoprotein reveal a prerequisite conformational state for receptor binding. Cell Res 27: 119–129, 2017. doi: 10.1038/cr.2016.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, Zhang Q, Shi X, Wang Q, Zhang L, Wang X. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 581: 215–220, 2020. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 32.Shang J, Ye G, Shi K, Wan Y, Luo C, Aihara H, Geng Q, Auerbach A, Li F. Structural basis of receptor recognition by SARS-CoV-2. Nature 581: 221–224, 2020. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song W, Gui M, Wang X, Xiang Y. Cryo-EM structure of the SARS coronavirus spike glycoprotein in complex with its host cell receptor ACE2. PLoS Pathog 14: e1007236, 2018. doi: 10.1371/journal.ppat.1007236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 367: 1444–1448, 2020. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jurrus E, Engel D, Star K, Monson K, Brandi J, Felberg LE, Brookes DH, Wilson L, Chen J, Liles K, Chun M, Li P, Gohara DW, Dolinsky T, Konecny R, Koes DR, Nielsen JE, Head‐Gordon T, Geng W, Krasny R, Wei G-W, Holst MJ, McCammon JA, Baker NA. Improvements to the APBS biomolecular solvation software suite. Protein Science 27: 112–128, 2018. doi: 10.1002/pro.3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schrödinger LLC. The PyMOL Molecular Graphics System, Version 1.8. Schrödinger LLC, 2015. [Google Scholar]
- 37.Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, Graham BS, McLellan JS. Cryo-EM Structure of the 2019-nCoV Spike in the Prefusion Conformation. Science 367: 1260–1263, 2020. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y, Gayle AA, Wilder-Smith A, Rocklov J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Travel Med 27: taaa021, 2020. doi: 10.1093/jtm/taaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu G, Hu Y, Wang Q, Qi J, Gao F, Li Y, Zhang Y, Zhang W, Yuan Y, Bao J, Zhang B, Shi Y, Yan J, Gao GF. Molecular basis of binding between novel human coronavirus MERS-CoV and its receptor CD26. Nature 500: 227–231, 2013. doi: 10.1038/nature12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Montopoli M, Zumerle S, Vettor R, Rugge M, Zorzi M, Catapano CV, Carbone GM, Cavalli A, Pagano F, Ragazzi E, Prayer-Galetti T, Alimonti A. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532). Ann Oncol 31: 1040–1045, 2020. doi: 10.1016/j.annonc.2020.04.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wambier CG, Goren A, Vano-Galvan S, Ramos PM, Ossimetha A, Nau G, Herrera S, McCoy J. Androgen sensitivity gateway to COVID-19 disease severity. Drug Dev Res 81: 771–776, 2020. doi: 10.1002/ddr.21688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clinckemalie L, Spans L, Dubois V, Laurent M, Helsen C, Joniau S, Claessens F. Androgen regulation of the TMPRSS2 gene and the effect of a SNP in an androgen response element. Mol Endocrinol 27: 2028–2040, 2013. doi: 10.1210/me.2013-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mollica V, Rizzo A, Massari F. The pivotal role of TMPRSS2 in coronavirus disease 2019 and prostate cancer. Future Oncol 16: 2029–2033, 2020. doi: 10.2217/fon-2020-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiao L, Sakagami H, Miw N. ACE2: the key molecule for understanding the pathophysiology of severe and critical sonditions of COVID-19: demon or angel? Viruses 12: 491, 2020. doi: 10.3390/v12050491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alireza P, Mohammad M, Akhavan-Niaki H. . First comprehensive computational analysis of functional consequences of TMPRSS2 SNPs in susceptibility to SARS-CoV-2 among different populations. J Biomol Struct Dyn 1–18, In press, 2020. doi: 10.1080/07391102.2020.1767690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patel KP, Patel PA, Vunnam RR, Hewlett AT, Jain R, Jing R, Vunnam SR. Gastrointestinal, hepatobiliary, and pancreatic manifestations of COVID-19. J Clin Virol 128: 104386, 2020. doi: 10.1016/j.jcv.2020.104386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoffmann M, Kleine-Weber H, Pohlmann S. A multibasic cleavage site in the spike protein of SARS-CoV-2 is essential for infection of human lung cells. Mol Cell 78: 779–784e5, 2020. doi: 10.1016/j.molcel.2020.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Millet JK, Whittaker GR. Physiological and molecular triggers for SARS-CoV membrane fusion and entry into host cells. Virology 517: 3–8, 2018. doi: 10.1016/j.virol.2017.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hikmet F, Mear L, Edvinsson A, Micke P, Uhlen M, Lindskog C. The protein expression profile of ACE2 in human tissues. Mol Syst Biol 16: e9610, 2020. doi: 10.15252/msb.20209610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jeffers SA, Tusell SM, Gillim-Ross L, Hemmila EM, Achenbach JE, Babcock GJ, Thomas WD, Jr.,Thackray LB, Young MD, Mason RJ, Ambrosino DM, Wentworth DE, Demartini JC, Holmes KV. CD209L (L-SIGN) is a receptor for severe acute respiratory syndrome coronavirus. Proc Natl Acad Sci U S A 101: 15748–15753, 2004. doi: 10.1073/pnas.0403812101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Daly JL, Simonetti B, Klein K, Chen K-E, Williamson MK, Antón-Plágaro C, Shoemark DK, Simón-Gracia L, Bauer M, Hollandi R, Greber UF, Horvath P, Sessions RB, Helenius A, Hiscox JA, Teesalu T, Matthews DA, Davidson AD, Collins BM, Cullen PJ, Yamauchi Y. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science 370: 861–865, 2020. doi: 10.1126/science.abd3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Plein A, Fantin A, Ruhrberg C. Neuropilin regulation of angiogenesis, arteriogenesis, and vascular permeability. Microcirculation 21: 315–323, 2014. doi: 10.1111/micc.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Teesalu T, Sugahara KN, Kotamraju VR, Ruoslahti E. C-end rule peptides mediate neuropilin-1-dependent cell, vascular, and tissue penetration. Proc Natl Acad Sci U S A 106: 16157–16162, 2009. doi: 10.1073/pnas.0908201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Onofri C, Theodoropoulou M, Losa M, Uhl E, Lange M, Arzt E, Stalla GK, Renner U. Localization of vascular endothelial growth factor (VEGF) receptors in normal and adenomatous pituitaries: detection of a non-endothelial function of VEGF in pituitary tumours. J Endocrinol 191: 249–261, 2006. doi: 10.1677/joe.1.06992. [DOI] [PubMed] [Google Scholar]
- 55.Gu J, Gong E, Zhang B, Zheng J, Gao Z, Zhong Y, Zou W, Zhan J, Wang S, Xie Z, Zhuang H, Wu B, Zhong H, Shao H, Fang W, Gao D, Pei F, Li X, He Z, Xu D, Shi X, Anderson VM, Leong AS. Multiple organ infection and the pathogenesis of SARS. J Exp Med 202: 415–424, 2005. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leow MK, Kwek DS, Ng AW, Ong KC, Kaw GJ, Lee LS. Hypocortisolism in survivors of severe acute respiratory syndrome (SARS). Clin Endocrinol 63: 197–202, 2005. doi: 10.1111/j.1365-2265.2005.02325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chigr F, Merzouki M, Najimi M. Autonomic brain centers and pathophysiology of COVID-19. ACS Chem Neurosci 11: 1520–1522, 2020. doi: 10.1021/acschemneuro.0c00265. [DOI] [PubMed] [Google Scholar]
- 58.Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology, 296: E119–E120, 2020. doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou L, Zhang M, Wang J, Gao J. Sars-Cov-2: Underestimated damage to nervous system. Travel Med Infect Dis 36: 101642, 2020. doi: 10.1016/j.tmaid.2020.101642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tan T, Khoo B, Mills EG, Phylactou M, Patel B, Eng PC, Thurston L, Muzi B, Meeran K, Prevost AT, Comninos AN, Abbara ADhillo WS. Association between high serum total cortisol concentrations and mortality from COVID-19. Lancet Diabetes Endocrinol 8: 659–660, 2020. doi: 10.1016/S2213-8587(20)30216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alvarez-Troncoso J, Zapatero Larrauri M, Montero Vega MD, Gil Vallano R, Palmier Pelaez E, Martin Rojas-Marcos P, Martin-Luengo F, Lazaro Del Campo P, Herrero Gil CR, Trigo Esteban E. Case report: COVID-19 with bilateral adrenal hemorrhage. Am J Trop Med Hyg 103: 1156–1157, 2020. doi: 10.4269/ajtmh.20-0722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Freire SM, Borba MGS, Baia-da-Silva DC, Val F, Alexandre MAA, Brito-Sousa JD, Melo GC, Queiroga MVO, Leao Farias ME, Camilo CC, Naveca FG, Xavier MS, Monteiro WM, Augusto Pivoto Joao G, Hajjar LA, Ordi J, Lacerda MVG, Ferreira LCL. Case report: adrenal pathology findings in severe COVID-19: an autopsy study. Am J Trop Med Hyg 103: 1604–1607, 2020.doi: 10.4269/ajtmh.20-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hanley B, Naresh KN, Roufosse C, Nicholson AG, Weir J, Cooke GS, Thursz M, Manousou P, Corbett R, Goldin R, Al-Sarraj S, Abdolrasouli A, Swann OC, Baillon L, Penn R, Barclay WS, Viola P, Osborn M. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. Lancet Microbe 1: e245–e253, 2020. doi: 10.1016/S2666-5247(20)30115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leyendecker P, Ritter S, Riou M, Wackenthaler A, Meziani F, Roy C, Ohana M. Acute adrenal infarction as an incidental CT finding and a potential prognosis factor in severe SARS-CoV-2 infection: a retrospective cohort analysis on 219 patients. Eur Radiol, 1–6, In press, 2020. doi: 10.1007/s00330-020-07226-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wei L, Sun S, Xu CH, Zhang J, Xu Y, Zhu H, Peh SC, Korteweg C, McNutt MA, Gu J. Pathology of the thyroid in severe acute respiratory syndrome. Hum Pathol 38: 95–102, 2007. doi: 10.1016/j.humpath.2006.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brancatella A, Ricci D, Viola N, Sgro D, Santini F, Latrofa F. Subacute thyroiditis after Sars-COV-2 infection. J Clin Endocrinol Metab 105: dgaa276, 2020. doi: 10.1210/clinem/dgaa276 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ding Y, He L, Zhang Q, Huang Z, Che X, Hou J, Wang H, Shen H, Qiu L, Li Z, Geng J, Cai J, Han H, Li X, Kang W, Weng D, Liang P, Jiang S. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol 203: 622–630, 2004. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang W, Ye YX, Yao H. Evaluation and observation of serum thyroid hormone and parathyroid hormone in patients with severe acute respiratory syndrome. J Chin Antituberculos Assoc 25: 232–234, 2003. [Google Scholar]
- 69.Lania A, Sandri MT, Cellini M, Mirani M, Lavezzi E, Mazziotti G. Thyrotoxicosis in patients with COVID-19: the THYRCOV study. Eur J Endocrinol 183: 381–387, 2020. doi: 10.1530/EJE-20-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mattar SAM, Koh SJQ, Rama Chandran S, Cherng BPZ. Subacute thyroiditis associated with COVID-19. BMJ Case Rep 13: e237336, 2020. doi: 10.1136/bcr-2020-237336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Muller I, Cannavaro D, Dazzi D, Covelli D, Mantovani G, Muscatello A, Ferrante E, Orsi E, Resi V, Longari V, Cuzzocrea M, Bandera A, Lazzaroni E, Dolci A, Ceriotti F, Re TE, Gori A, Arosio M, Salvi M. SARS-CoV-2-related atypical thyroiditis. Lancet Diabetes Endocrinol 8: 739–741, 2020. doi: 10.1016/S2213-8587(20)30266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ruggeri RM, Campenni A, Siracusa M, Frazzetto G, Gullo D. Subacute thyroiditis in a patient infected with SARS-COV-2: an endocrine complication linked to the COVID-19 pandemic. Hormones (Athens), 1–3, In press2020. doi: 10.1007/s42000-020-00230-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rotondi M, Coperchini F, Ricci G, Denegri M, Croce L, Ngnitejeu ST, Villani L, Magri F, Latrofa F, Chiovato L. Detection of SARS-COV-2 receptor ACE-2 mRNA in thyroid cells: a cluefor COVID-19-related subacute thyroiditis. J Endocrinol Invest, 1–6, In press2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bellastella G, Maiorino MI, Esposito K. Endocrine complications of COVID-19: what happens to the thyroid and adrenal glands? J Endocrinol Invest 43: 1169–1170, 2020. doi: 10.1007/s40618-020-01311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Campos-Barrera E, Alvarez-Cisneros T, Davalos-Fuentes M, Usui T. Subacute thyroiditis associated with COVID-19. Case Rep Endocrinol 2020: 1–4, 2020. doi: 10.1155/2020/8891539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Grassi T, Varotto E, Galassi FM. COVID-19, a viral endocrinological disease? Eur J Intern Med 77: 156–157, 2020. doi: 10.1016/j.ejim.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Peteranderl C, Herold S, Schmoldt C. Human influenza virus infections. Semin Respir Crit Care Med 37: 487–500, 2016. doi: 10.1055/s-0036-1584801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, , et al. Proteomics. Tissue-based map of the human proteome. Science 347: 1260419–1260419, 2015. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 79.Valdivia A, Cortes L, Beitia M, Totorikaguena L, Agirregoitia N, Corcostegui B, Casis L, Matorras R, Irazusta J, Agirregoitia E. Role of angiotensin-(1-7) via MAS receptor in human sperm motility and acrosome reaction. Reproduction 159: 241–249, 2020. doi: 10.1530/REP-19-0274. [DOI] [PubMed] [Google Scholar]
- 80.Chen YW, Lee MS, Lucht A, Chou FP, Huang W, Havighurst TC, Kim K, Wang JK, Antalis TM, Johnson MD, Lin CY. TMPRSS2, a serine protease expressed in the prostate on the apical surface of luminal epithelial cells and released into semen in prostasomes, is misregulated in prostate cancer cells. Am J Pathol 176: 2986–2996, 2010. doi: 10.2353/ajpath.2010.090665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xu J, Qi L, Chi X, Yang J, Wei X, Gong E, Peh S, Gu J. Orchitis: a complication of severe acute respiratory syndrome (SARS). Biol Reprod 74: 410–416, 2006. doi: 10.1095/biolreprod.105.044776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.La Marca A, Busani S, Donno V, Guaraldi G, Ligabue G, Girardis M. Testicular pain as an unusual presentation of COVID-19: a brief review of SARS-CoV-2 and the testis. Reprod Biomed Online 41: 903–906, 2020. doi: 10.1016/j.rbmo.2020.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ming YSC, Bo H, Jing-Min Z, Hua S, Ya-Jun C, Cao Q, Lin M, Jun H, Li X-, Li FX, Jun-Jie Z, Jun F, Dan-Ju L, Xiao-Na C, Ming Z, Xiu N. Pathological findings in the testes of COVID-19 patients: clinical implications. Eur Urol Focus 6: 1124–1129, 2020. doi: 10.1016/j.euf.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li D, Jin M, Bao P, Zhao W, Zhang S. Clinical characteristics and results of semen tests among men with coronavirus disease 2019. JAMA Netw Open 3: e208292, 2020. doi: 10.1001/jamanetworkopen.2020.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Aitken RJ. COVID-19 and human spermatozoa - potential risks for infertility and sexual transmission. Andrology doi: 10.1111/andr.12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pereira VM, Reis FM, Santos RA, Cassali GD, Santos SH, Honorato-Sampaio K, dos Reis AM. Gonadotropin stimulation increases the expression of angiotensin-(1–7) and MAS receptor in the rat ovary. Reprod Sci 16: 1165–1174, 2009. doi: 10.1177/1933719109343309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Reis FM, Bouissou DR, Pereira VM, Camargos AF, dos Reis AM, Santos RA. Angiotensin-(1-7), its receptor Mas, and the angiotensin-converting enzyme type 2 are expressed in the human ovary. Fertil Steril 95: 176–181, 2011. doi: 10.1016/j.fertnstert.2010.06.060. [DOI] [PubMed] [Google Scholar]
- 88.Woodworth KR, Olsen EO, Neelam V, Lewis EL, Galang RR, Oduyebo T, , et al.; CDC COVID-19 Response Pregnancy and Infant Linked Outcomes Team,COVID-19 Pregnancy and Infant Linked Outcomes Team (PILOT) . Birth and infant outcomes following laboratory-confirmed SARS-CoV-2 infection in pregnancy - SET-NET, 16 Jurisdictions, March 29-October 14, 2020. MMWR Morb Mortal Wkly Rep 69: 1635–1640, 2020. doi: 10.15585/mmwr.mm6944e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chee YJ, Ng SJH, Yeoh E. Diabetic ketoacidosis precipitated by Covid-19 in a patient with newly diagnosed diabetes mellitus. Diabetes Res Clin Pract 164: 108166, 2020. doi: 10.1016/j.diabres.2020.108166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang JK, Lin SS, Ji XJ, Guo LM. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol 47: 193–199, 2010. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Verdecchia P, Cavallini C, Spanevello A, Angeli F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur J Intern Med 76: 14–20, 2020. doi: 10.1016/j.ejim.2020.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hasan NM, Kendrick MA, Druckenbrod NR, Huelsmeyer MK, Warner TF, MacDonald MJ. Genetic association of the neuropilin-1 gene with type 1 diabetes in children: neuropilin-1 expression in pancreatic islets. Diabetes Res Clin Pract 87: e29–e32, 2010. doi: 10.1016/j.diabres.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 93.Rubino F, Amiel SA, Zimmet P, Alberti G, Bornstein S, Eckel RH, Mingrone G, Boehm B, Cooper ME, Chai Z, Del Prato S, Ji L, Hopkins D, Herman WH, Khunti K, Mbanya JC, Renard E. New-onset diabetes in Covid-19. N Engl J Med 383: 789–790, 2020. doi: 10.1056/NEJMc2018688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.van Gerwen M, Alsen M, Little C, Barlow J, Naymagon L, Tremblay D, Sinclair CF, Genden E. Outcomes of patients with hypothyroidism and COVID-19: a retrospective cohort study. Front Endocrinol (Lausanne) 11: 565, 2020. doi: 10.3389/fendo.2020.00565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Al Hayek AA, Robert AA, Matar AB, Algarni A, Alkubedan H, Alharbi T, Al Amro A, Alrashidi SA, Al Dawish M. Risk factors for hospital admission among COVID-19 patients with diabetes. A study from Saudi Arabia. Saudi Med J 41: 1090–1097, 2020. doi: 10.15537/smj.2020.10.25419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pal R, Bhansali A. COVID-19, diabetes mellitus and ACE2: The conundrum. Diabetes Res Clin Pract 162: 108132, 2020. doi: 10.1016/j.diabres.2020.108132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395: 507–513, 2020. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Guo W, Li M, Dong Y, Zhou H, Zhang Z, Tian C, Qin R, Wang H, Shen Y, Du K, Zhao L, Fan H, Luo S, Hu D. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev, 36: e3319, 2020. doi: 10.1002/dmrr.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Veronese N, Demurtas J, Yang L, Tonelli R, Barbagallo M, Lopalco P, Lagolio E, Celotto S, Pizzol D, Zou L, Tully MA, Ilie PC, Trott M, Lopez-Sanchez GF, Smith L. Use of corticosteroids in coronavirus disease 2019 pneumonia: a systematic review of the literature. Front Med (Lausanne) 7: 170, 2020. doi: 10.3389/fmed.2020.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Arlt W, Baldeweg SE, Pearce SHS, Simpson HL. Endocrinology in the time of COVID-19: management of adrenal insufficiency. Eur J Endocrinol 183: G25–G32, 2020. doi: 10.1530/EJE-20-0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.ClinicalTrials.gov. Randomized Evaluation of COVID-19 Therapy. ClinicalTrials.gov, 2020. [Google Scholar]
- 102.Tadic M, Cuspidi C, Sala C. COVID-19 and diabetes: is there enough evidence? J Clin Hypertens 22: 943–948, 2020. doi: 10.1111/jch.13912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Huda MSB, Shaho S, Trivedi B, Fraterrigo G, Chandrarajan L, Zolfaghari P, Dovey TM, Garrett CG, Chowdhury TA. Diabetic emergencies during the COVID-19 pandemic: a case-control study. Diabet Med 38: e14416, 2020. doi: 10.1111/dme.14416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Casqueiro J, Alves C. Infections in patients with diabetes mellitus: A review of pathogenesis. Indian J Endocrinol Metab 16: S27–S36, 2012. doi: 10.4103/2230-8210.94253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cristelo C, Azevedo C, Marques JM, Nunes R, Sarmento B. SARS-CoV-2 and diabetes: new challenges for the disease. Diabetes Res Clin Pract 164: 108228, 2020. doi: 10.1016/j.diabres.2020.108228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li J, Wang X, Chen J, Zuo X, Zhang H, Deng A. COVID-19 infection may cause ketosis and ketoacidosis. Diabetes Obes Metab 22: 1935–1941, 2020. doi: 10.1111/dom.14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hendren NS, de Lemos JA, Ayers C, Das SR, Rao A, Carter S, Rosenblatt A, Walchok JG, Omar W, Khera R, Hegde AA, Drazner MH, Neeland IJ, Grodin JL. Association of body mass index and age with morbidity and mortality in patients hospitalized with COVID-19: results from the American Heart Association COVID-19 cardiovascular disease registry. Circulation; doi: 10.1161/CIRCULATIONAHA.120.051936. [DOI] [PubMed] [Google Scholar]
- 108.Belaid Z, Hubint F, Humblet C, Boniver J, Nusgens B, Defresne MP. Differential expression of vascular endothelial growth factor and its receptors in hematopoietic and fatty bone marrow: evidence that neuropilin-1 is produced by fat cells. Haematologica 90: 400–401, 2005. [PubMed] [Google Scholar]
- 109.Giordano A, Cesari P, Capparuccia L, Castellucci M, Cinti S. Sema3A and neuropilin-1 expression and distribution in rat white adipose tissue. J Neurocytol 32: 345–352, 2003. doi: 10.1023/B:NEUR.0000011328.61376.bb. [DOI] [PubMed] [Google Scholar]
- 110.Uhlen M, Oksvold P, Fagerberg L, Lundberg E, Jonasson K, Forsberg M, Zwahlen M, Kampf C, Wester K, Hober S, Wernerus H, Bjorling L, Ponten F. Towards a knowledge-based Human Protein Atlas. Nat Biotechnol 28: 1248–1250, 2010. doi: 10.1038/nbt1210-1248. [DOI] [PubMed] [Google Scholar]
- 111.Gupte M, Boustany-Kari CM, Bharadwaj K, Police S, Thatcher S, Gong MC, English VL, Cassis LA. ACE2 is expressed in mouse adipocytes and reulated by a high-fat diet. Am J Physiol Regul Integr Comp Physiol 295: R781–788, 2008. doi: 10.1152/ajpregu.00183.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Baughn LB, Sharma N, Elhaik E, Sekulic A, Bryce AH, Fonseca R. Targeting TMPRSS2 in SARS-CoV-2 Infection. Mayo Clin Proc 95: 1989–1999, 2020. doi: 10.1016/j.mayocp.2020.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jasul G Jr, Paz-Pacheco E, Jimeno C, Suastika K, Hussein Z, Mustafa N, Aung A, Robles J, Leow MKHS, Deerochanawong C, Khue NT, Dang TH. ASEAN survey of needs in endocrinology in the time of the COVID-19 pandemic . J ASEAN Federation Endocr Soc 35: 5–15, 2020. doi: 10.15605/jafes.035.01.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yang L, Han Y, Nilsson-Payant BE, Gupta V, Wang P, Duan X, , et al. A human pluripotent stem cell-based platform to study SARS-CoV-2 tropism and model virus infection in human cells and organoids. Cell Stem Cell 27: 125–136e7, 2020. doi: 10.1016/j.stem.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dube M, Le Coupanec A, Wong AHM, Rini JM, Desforges M, Pj T. Axonal transport enables neuron-to-neuron propagation of human coronavirus OC43. J Virol 92: e00404-18, 2018. doi: 10.1128/JVI.00404-18. [DOI] [PMC free article] [PubMed] [Google Scholar]