Abstract
Background:
Extracorporeal membrane oxygenation (ECMO) may be urgently used as a last resort form of life support when all other treatment options for potentially reversible cardiopulmonary injury have failed.
Objective:
To examine the range and frequency of neurological injury in ECMO-treated adults.
Design:
Retrospective clinicopathological cohort study.
Setting:
Mayo Clinic, Rochester, Minnesota.
Patients:
A prospectively collected registry of all patients 15 years or older treated with ECMO for 12 or more hours from January 2002 to April 2010.
Intervention:
Patients were analyzed for potential risk factors for neurological events and death using logistic regression and Cox proportional hazards models.
Main Outcome Measures:
Neurological diagnosis and/or death.
Results:
A total of 87 adults were treated (35 female [40%]; median age, 54 years [interquartile range, 31]; mean duration of ECMO, 91 hours [interquartile range, 100]; overall survival >7 days after ECMO, 52%). Neurological events occurred in 42 patients who received ECMO (50%; 95% confidence interval [CI], 39%−61%). Diagnoses included subarachnoid hemorrhage, ischemic watershed infarctions, hypoxic-ischemic encephalopathy, unexplained coma, and brain death. Death in patients who received ECMO who did not require antecedent cardiopulmonary resuscitation was associated with increased age (odds ratio,1.24 per decade; 95% CI, 1.03–1.50; P=.02) and lower minimum arterial oxygen pressure (odds ratio,0.79; 95% CI, 0.68–0.92; P=.03). Although stroke was rarely diagnosed clinically, 9 of 10 brains studied at autopsy demonstrated hypoxic-ischemic and hemorrhagic lesions of vascular origin.
Conclusion:
Severe neurological sequelae occur frequently in adult ECMO-treated patients with otherwise reversible cardiopulmonary injury (conservative estimate, 50%) and include a range of potentially fatal neurological diagnoses that may be due to the precipitating event and/or ECMO treatment.
Extracorporeal membrane oxygenation (ECMO) is a form of mechanical circulatory support that can be life-saving in people with potentially reversible heart or lung injury. Extracorporeal membrane oxygenation is nearly always used urgently, when all other treatment options for cardiopulmonary injury have failed and mortality is otherwise expected. Standard ECMO treatment involves venous drainage from the femoral vein or left atrium with artificial extracirculatory oxygen exchange. Return to the body is through the same veins (venovenous) or arterial system via the femoral artery or ascending aorta (venoarterial). Compared with cardiopulmonary bypass circuitry, ECMO is transportable, smaller, and closed to the atmosphere and can treat a patient for several days to weeks.1
Extracorporeal membrane oxygenation is a mainstay of intensive treatment in neonatal and some pediatric patients in whom the technique has been studied extensively and is considered a standard of care. In infants, intracranial hemorrhage is the most common cause of death in patients who receive ECMO, surpassing cardiopulmonary failure.2 There has been limited published experience with adult patients treated with ECMO. Potential indications include postcardiopulmonary arrest with cardiopulmonary resuscitation, a bridge for heart or lung transplant, severe respiratory failure (as in the recent novel influenza A(H1N1) outbreak),3 cardiogenic shock, and postsurgical cardiopulmonary failure following cardiotomy.
Neurological consequences of ECMO in adults are likely common but uncharacterized. At one academic medical center, combined ischemic and hemorrhagic stroke affected 7% of adult patients and significantly increased the odds ratio of death (4.94; 95% confidence interval, 1.65–14.80).4 A meta-analysis of 135 adults from 20 separate publications who received ECMO following cardiopulmonary resuscitation (CPR) noted that neurological sequelae were “poorly described in the reviewed literature.”5 At the extreme, brain death occurred in 7% to 21% of cases of ECMO-treated adults in some academic centers.4,6–8
In contrast, longer-term neurological follow-up of ECMO-treated adult patients demonstrates the possibility of unimpaired survival. In a recent study, nearly half (43%) of 28 adults who survived 30 days or longer were without clinical findings at long-term follow-up.9 Because ECMO is a resource-intense, high-cost, and difficult-to-access treatment, a detailed clinicopathological study of neurological sequelae could assist neurologists who are asked to evaluate and prognosticate on ECMO-treated adults. Herein, we identify the range, frequency, potential risk factors, and outcomes of neurological injury and associated survival among ECMO-treated adults at a single institution from an 8-year period.
METHODS
PATIENTS
The institutional review board approved this study. The Mayo Clinic in Rochester, Minnesota, began a prospective registry of all patients treated with ECMO in January 2002. Patients 15 years and older at the time of ECMO were included in the study. Patients were selected for ECMO treatment at the Mayo Clinic by their treating physicians, usually the anesthesiologist and cardiothoracic surgeon involved in their care. There is no protocol for choosing patients for a trial of ECMO beyond the physicians’ belief that the underlying etiology of cardiac or respiratory failure is potentially reversible and the patient has not had catastrophic neurological injury. Patients were required to have received ECMO treatment for at least 12 hours to reduce the influence of precipitating events alone.
DATA COLLECTION
Medical records, including clinical notes, intraoperative monitoring reports, laboratory databases, brain imaging studies, and pathological specimens from cerebral autopsy, were reviewed in June 2010. All patients who were alive on June 10, 2010, were censored on that date. Clinical variables included age, sex, duration of ECMO treatment (hours), reason for ECMO, presurgical or pre-event modified Rankin Scale (mRS)10 and cerebral performance category scores, lowest systolic blood pressure, and lowest arterial oxygen pressure while undergoing ECMO.
STATISTICAL ANALYSIS
The Cox proportional hazards model was used to assess the effect of the measured covariates on the risk of death. Model checking was performed by inspection of residual plots and tests for the proportional hazards assumption.
A variable that is etiologically associated with earlier deaths and unassociated with neurological events will be observed to be protective against neurological events in a typical analysis; however, individuals who died could no longer be observed to have neurological outcomes. This constitutes a semicompeting risk scenario11 and posed a threat to the validity of standard analyses of neurological events. To address this issue, our criteria for a variable to be considered predictive of neurological events were (1) association with earlier death and (2) association with more neurological events before death. This is conservative with respect to the semicompeting risk biases with our model assumptions. Logistic regression was used to estimate the probability of observing a neurological event before death.
The statistical package R (R for Mac OS X GUI 1.35, version 2.10.1; R Foundation for Statistical Computing, Vienna, Austria)12 was used. All statistical tests of hypothesis were performed using 2-sided alternatives with a predetermined type 1 error rate of α=.05.
PATHOLOGICAL EVALUATION OF BRAIN TISSUE
Brain autopsies were performed using standardized techniques. Following 7 to 10 days of immersion fixation in 10% buffered formalin, the brains were examined grossly. Representative microscopic sections from the dura and selected neocortical and subcortical areas including the frontal neocortex, basal ganglia, hippocampus, cerebellum, and brainstem were processed routinely in paraffin, and sections were stained with hematoxylin-eosin.
RESULTS
DEMOGRAPHIC AND SURVIVAL DATA
The flow diagram of all patients who received ECMO following either CPR (n=14; 16%) or cardiothoracic surgery (n=73; 84%) is shown in Figure 1. Patients were predominantly male (n=52; 60%) and middle-aged (median age, 54 years; interquartile range, 31). The average duration of ECMO treatment was 91 hours (interquartile range, 100). Primary precipitating factors for ECMO treatment were cardiac valve and/or aortic repair (44%), cardiopulmonary arrest with resuscitation (16%), ventricular repair (8%), coronary artery bypass graft surgery (7%), heart or lung transplant (7%), other pulmonary disease/surgery (7%), surgical procedures for congenital heart disease (6%), pericardectomy (2%), and cardiac device placement (2%).
Figure 1.
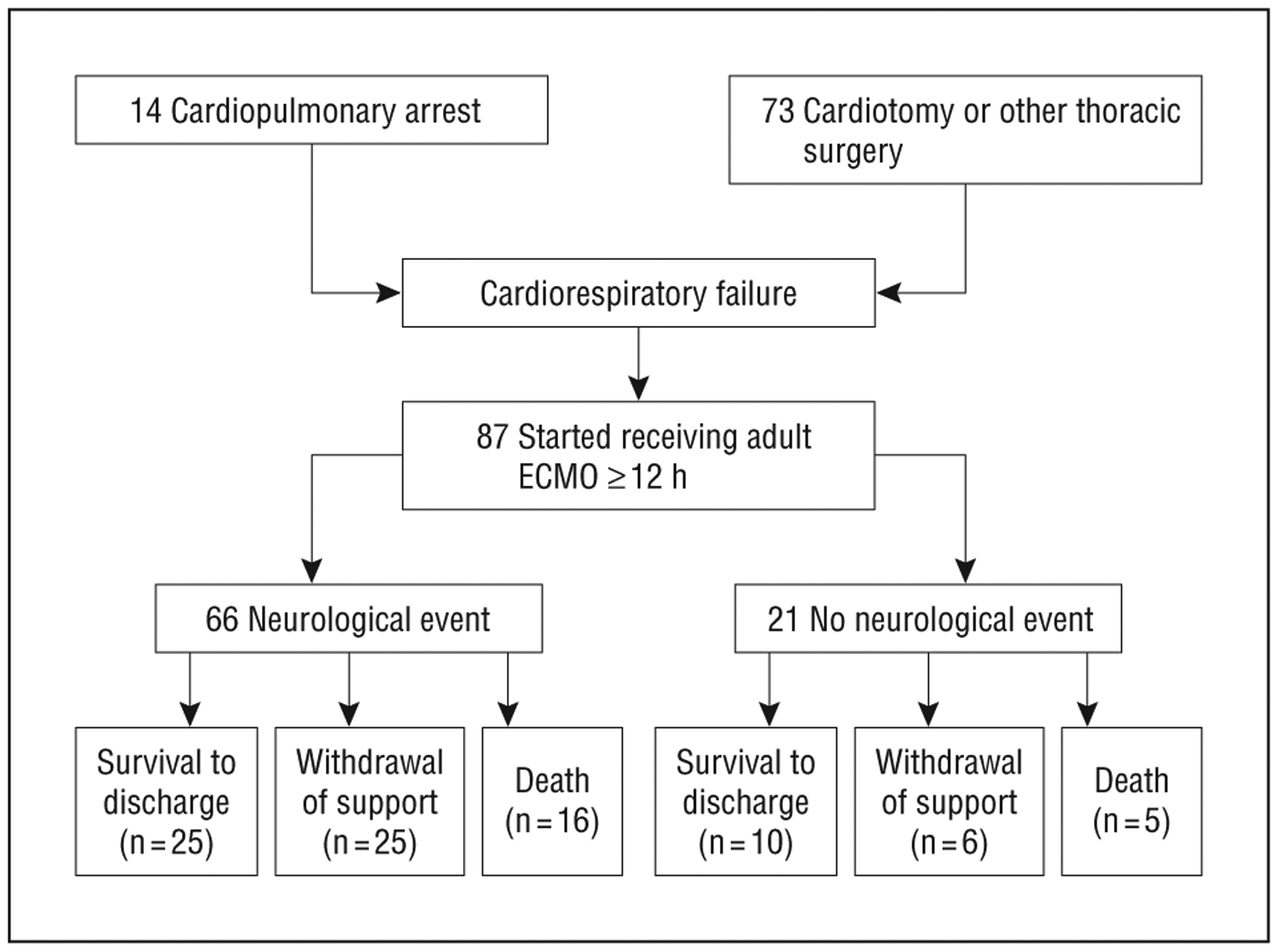
Flow diagram of adult patients treated with extracorporeal membrane oxygenation (ECMO) (n=87).
The overall survival rate of adult patients who received ECMO was 52% at 7 days and 44% at 30 days following discontinuation of ECMO. Survival differed between those who had CPR prior to ECMO and those who did not (median, 15 days vs 19 days; Figure 2). Withdrawal of support occurred in 36% of ECMO-treated patients (Figure 3). When comparing baseline characteristics of adult survivors of ECMO, patients who died within 1 week of discontinuation, and patients withdrawn from ECMO, there was no significant difference between groups (Table 1).
Figure 2.
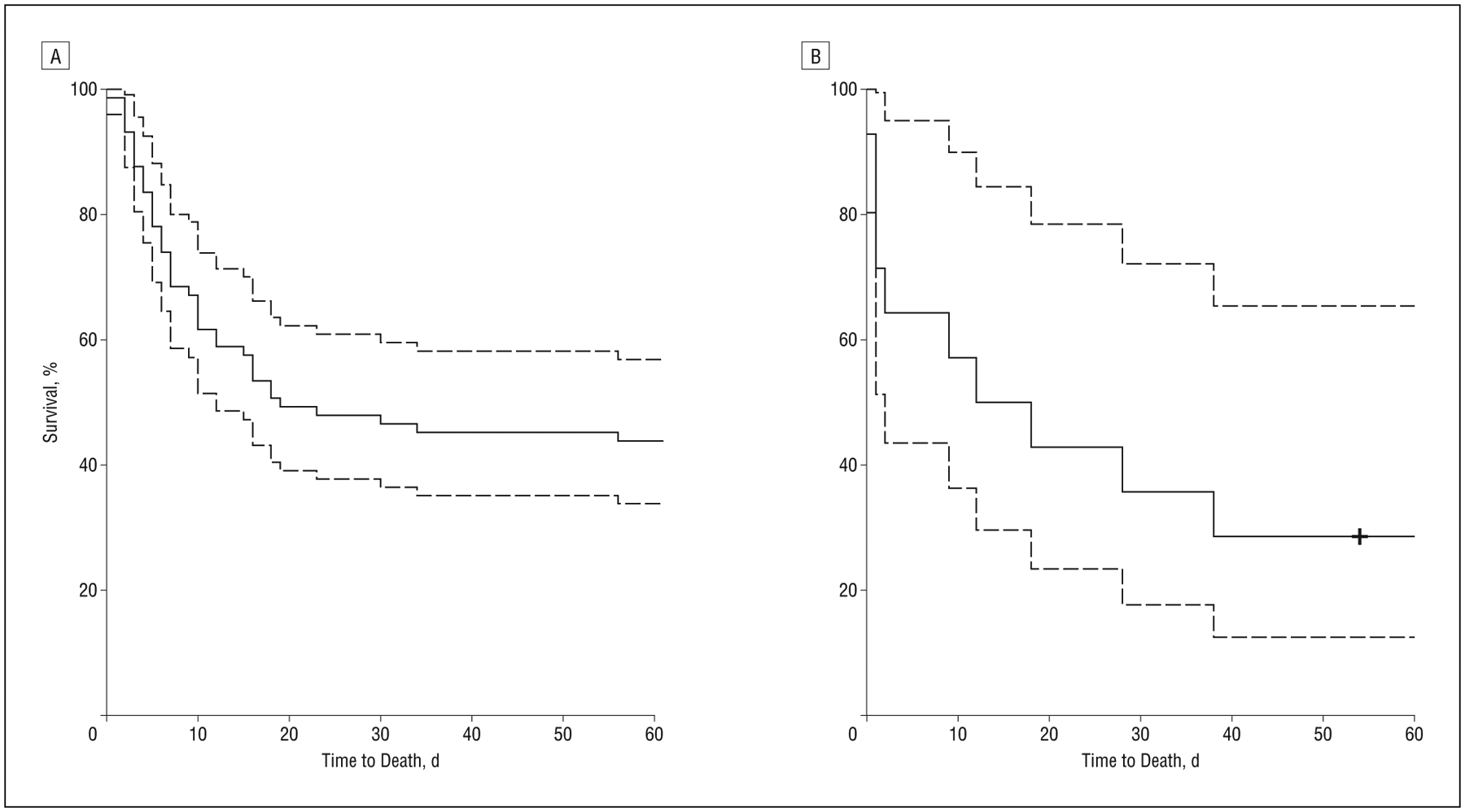
Plot of Kaplan-Meier curve of time from extracorporeal membrane oxygenation (ECMO) initiation to death for all adult patients treated with ECMO by reason for ECMO treatment: after thoracic surgery (A; n=73) and after CPR (B; n=14).
Figure 3.
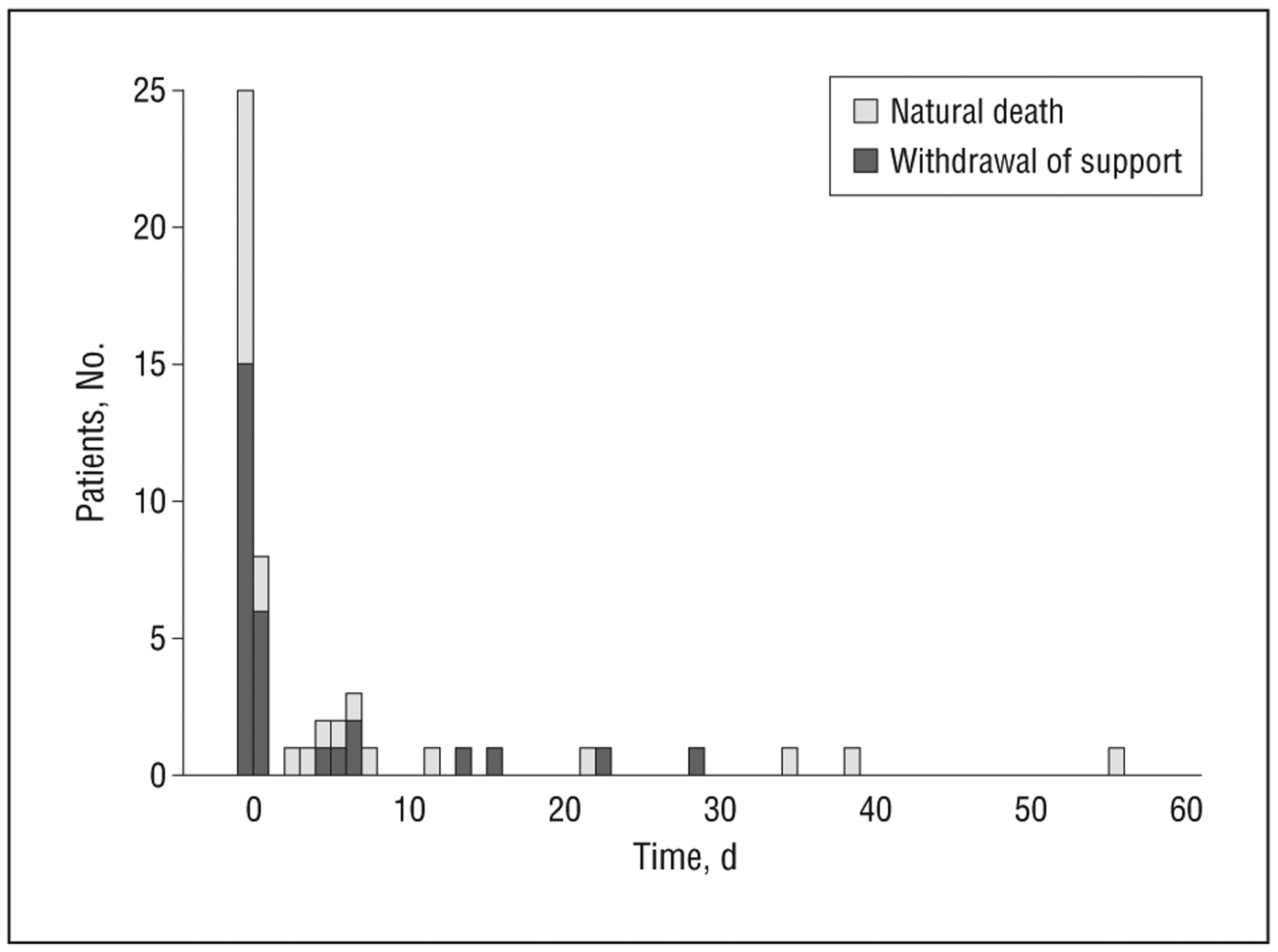
Bar graph of patient survival times among nonsurvivors by reason for extracorporeal membrane oxygenation treatment (n=62).
Table 1.
Baseline Characteristics of Adult Patients Receiving ECMO by Survival Outcome
| Characteristic | Neurological Events | No Neurological Events | P Valuea | Withdrawal | Survival Until End | Death During or ≤7 d After Discontinuation | P Valueb |
|---|---|---|---|---|---|---|---|
| Patients, % | 50 | 50 | 36 | 40 | 24 | ||
| Patients, No. | 42 | 42 | 31 | 35 | 21 | ||
| Patient characteristics | |||||||
| Age, median (25th, 75th percentiles), y | 59 (43, 70) | 49 (36, 70) | .29 | 57 (44, 70) | 52 (33, 62) | 50 (37, 72) | .30 |
| Female, No. (%) | 18 (43) | 16 (38) | .82 | 9 (29) | 15 (43) | 11 (52) | .22 |
| Reason for ECMO, No. (%) | .77 | .23 | |||||
| Postcardiopulmonary resuscitation | 8 (19) | 6 (14) | 4 (13) | 4 (11) | 6 (29) | ||
| Postthoracic surgery | 34 (81) | 36 (86) | 27 (87) | 31 (89) | 15 (71) | ||
| Duration of ECMO treatment, median (25th, 75th percentiles), h | 90 (46,157) | 91 (59,137) | .91 | 107 (68,162) | 86 (63,126) | 83 (42,182) | .51 |
| Pre-ECMO modified Rankin Scale score, median (25th, 75th percentiles) | 2 (2, 3) | 2 (0, 3) | .67 | 3 (1,3.5) | 2 (0, 2.75) | 2 (1.5,3.5) | .17 |
| Pre-ECMO cerebral performance category score, median (25th, 75th percentiles) | 2 (1,2) | 2 (1,3) | .70 | 2 (1,3) | 1 (1,2) | 2 (1,2.5) | .06 |
| Lowest Pao2, median (25th, 75th percentiles) | 71 (50, 84) | 73 (59, 86) | .45 | 66 (42, 84) | 75 (60, 88) | 72 (54, 85) | .37 |
| Lowest systolic blood pressure, median (25th, 75th percentiles) | 63 (53, 68) | 57 (49,71) | .40 | 59 (49, 69) | 63 (48, 72) | 58 (50, 66) | .73 |
Abbreviations: ECMO, extracorporeal membrane oxygenation; PaO2, arterial oxygen pressure.
Two-sample binomial-based tests for proportions and Wilcoxon rank sum test for medians of non–normally distributed continuous measures.
Likelihood ratio test in logistic regression for proportions and Kruskal-Wallis test for medians of non–normally distributed continuous measures.
NEUROLOGICAL OUTCOMES
Neurological injury occurred in 42 patients (42 of 84; 50%; 95% confidence interval, 39%−61%). Three patients had prior neurological events and were excluded from predictive analyses of neurological events. Patients presented with new onset of coma, new loss of brainstem reflexes, or failure to awaken after completion of ECMO. Diagnostic categories were diffuse anoxic brain injury (n=9), encephalopathy (n=11), comatose without further explanation (n=11), and myoclonus (n=1). There were no seizures noted. Patients with a diagnosis of brain death (n=3)13 or stroke (n=7) are summarized in Table 2. Among patients postthoracic surgery, female sex, older age, nadir systolic blood pressure, nadir arterial oxygen pressure while receiving ECMO, and presurgical or pre-event mRS score did not predict neurological events. Age and nadir arterial oxygen pressure showed a trend toward significance (Table 3). Neurological examination was performed at the discretion of the treating physicians and performed in 69 patients (79%). Of patients who had neurological events, 59% died while receiving ECMO or within 7 days of discontinuation.
Table 2.
Life-threatening Neurological Diagnoses of Adult Patients Treated With ECMO
| Sex/Age, y | Reason for ECMO | Neurological Diagnosis | Onset During ECMO, d | Outcome |
|---|---|---|---|---|
| M/32 | Cardiopulmonary arrest + CPR | Brain death | 1 | Death |
| M/50 | Cardiopulmonary arrest + CPR | Brain death | 1 | Death |
| F/69 | Cardiac valve repair + CABG | Brain death | 3 | Death/WOS |
| M/45 | Fontan conversion, mitral valve repair, patch angioplasty of pulmonary artery | Subarachnoid hemorrhage | 7 | WOS |
| M/70 | Aortic + mitral valve replacement | Intraventricular hemorrhage, watershed infarction | 22 d After ECMO discontinuation | Survived 4 mo |
| M/70 | Aortic valve replacement + CABG | Bihemispheric ischemic infarcts | 5 d After ECMO discontinuation | Death |
| F/60 | Infected prosthetic valve, aortic root and valve replacement, pulmonary artery defect repair | Subarachnoid hemorrhage | 2 d After ECMO discontinuation | Survival |
| M/75 | Mitral valve replacement | Frontal lobe ischemic infarction | 3 | Death due to multiorgan failure |
| F/76 | Pericardiectomy | Subarachnoid hemorrhage | 8 | WOS, diagnosed with neurological disease at autopsy |
| M/41 | Cardiopulmonary arrest + CPR | Putaminal infarction, cerebral edema, uncal herniation | 1 | WOS, diagnosed with neurological disease at autopsy |
Abbreviations: CABG, coronary artery bypass graft; CPR, cardiopulmonary resuscitation; ECMO, extracorporeal membrane oxygenation; WOS, withdrawal of support.
Table 3.
Odds Ratio of Observing Neurological Events Among Adult Patients Treated With ECMO Who Did Not Receive Antecedent CPR (n = 70)
| Neurological Event | OR (95% Cl) | P Value |
|---|---|---|
| Age (by decade increase) | 1.35 (0.99–1.91) | .07 |
| Female | 1.39 (0.48–4.14) | .54 |
| Lowest sBP | 1.02 (0.98–1.06) | .36 |
| Lowest arterial oxygen pressure | 0.77 (0.56–1.00) | .07 |
| Presurgical mRS score | 0.91 (0.65–1.27) | .58 |
Abbreviations: CI, confidence interval; CPR, cardiopulmonary resuscitation; ECMO, extracorporeal membrane oxygenation; mRS, modified Rankin Scale; sBP, systolic blood pressure.
Patients treated with CPR had different survival outcomes than those who did not receive CPR and were removed from predictive models of adult survival while undergoing ECMO. Death was predicted by increased age and lower arterial oxygen pressure in those who did not have cardiopulmonary arrest and CPR (Table 4). The pre-ECMO cerebral performance category score was removed owing to colinearity between cerebral performance category and mRS. Cerebral performance category ranged from 1 to 4 (median, 2). In these models, only mRS was used to assess functional status.
Table 4.
Cox Proportional Hazards Model for Risk of Death in ECMO Patients (Excluding CPR Patients) (n = 69)
| Potential Risk Factor | HR (95% Cl) | P Value |
|---|---|---|
| Age | 1.24 (0.80–1.03) | .02 |
| Female | 1.16 (0.61–2.05) | .72 |
| Lowest sBP | 0.99 (0.97–1.01) | .27 |
| Lowest arterial oxygen pressure | 0.79 (0.68–0.92) | .003 |
| Presurgical mRS score | 1.14 (0.93–1.40) | .20 |
Abbreviations: CI, confidence interval; CPR, cardiopulmonary resuscitation; ECMO, extracorporeal membrane oxygenation; HR, hazard ratio; mRS, modified Rankin Scale; sBP, systolic blood pressure.
Cerebral imaging was performed in 24 patients overall and was pathologically abnormal in 15 cases (Figure 4A–C). Autopsy of the brain was performed in 10 patients (10 of 40; 25% of nonsurvivors) (Figure 4D–H). The primary pathology, present in 9 of the 10 cases, included a variety of hypoxic-ischemic and hemorrhagic lesions of vascular origin. Acute ischemic cell change (“red-dead” neurons), noted in 7 patients, involved areas of recognized vulnerability including neocortical and subcortical neurons, hippocampal pyramidal cells (sector CA1), and cerebellar Purkinje cells. Diffuse cerebral edema was present in 3 of these patients, with bilateral uncal prominence in 1. Acute and subacute infarcts of variable size and age also were found. In 3 cases, microscopic subacute (organizing) ischemic infarcts involved the thalamus (n=2) (Figure 4E) or basal ganglia (n=1). Hemorrhagic infarcts, likely a result of emboli and/or reperfusion, were present in 3 cases. One patient with suspicion of brain death (an apnea test was not performed) had marked cerebral duskiness, parenchymal disruption, and autolysis of the cerebellar internal granule layer. Seventeen patients (5 of whom underwent autopsy) had multiorgan failure including hepatic dysfunction; 3 of these patients showed associated Alzheimer type 2 gliosis and jaundice of the dura. Hemorrhagic lesions were common and present in 6 brains. All patients receiving ECMO were anticoagulated, and this propensity to bleed was likely accentuated by coexisting hepatic failure. These included diffuse petechial hemorrhages (n=3) (Figure 4F), intradural hemorrhages (n=2), subarachnoid hemorrhages (n=2) (Figure 4G), subdural hemorrhage (n=1), and massive intraventricular hemorrhage (n=1) (Figure 4H). Two patients also had mild, likely age-related, neurodegenerative changes. There was no evidence of venous sinus thrombosis in any of the cases. One case showed no obvious pathologic alterations. In the patients who underwent autopsy, 7 had no neurological examination documented and 3 had normal findings on pre-operative neurological examination.
Figure 4.
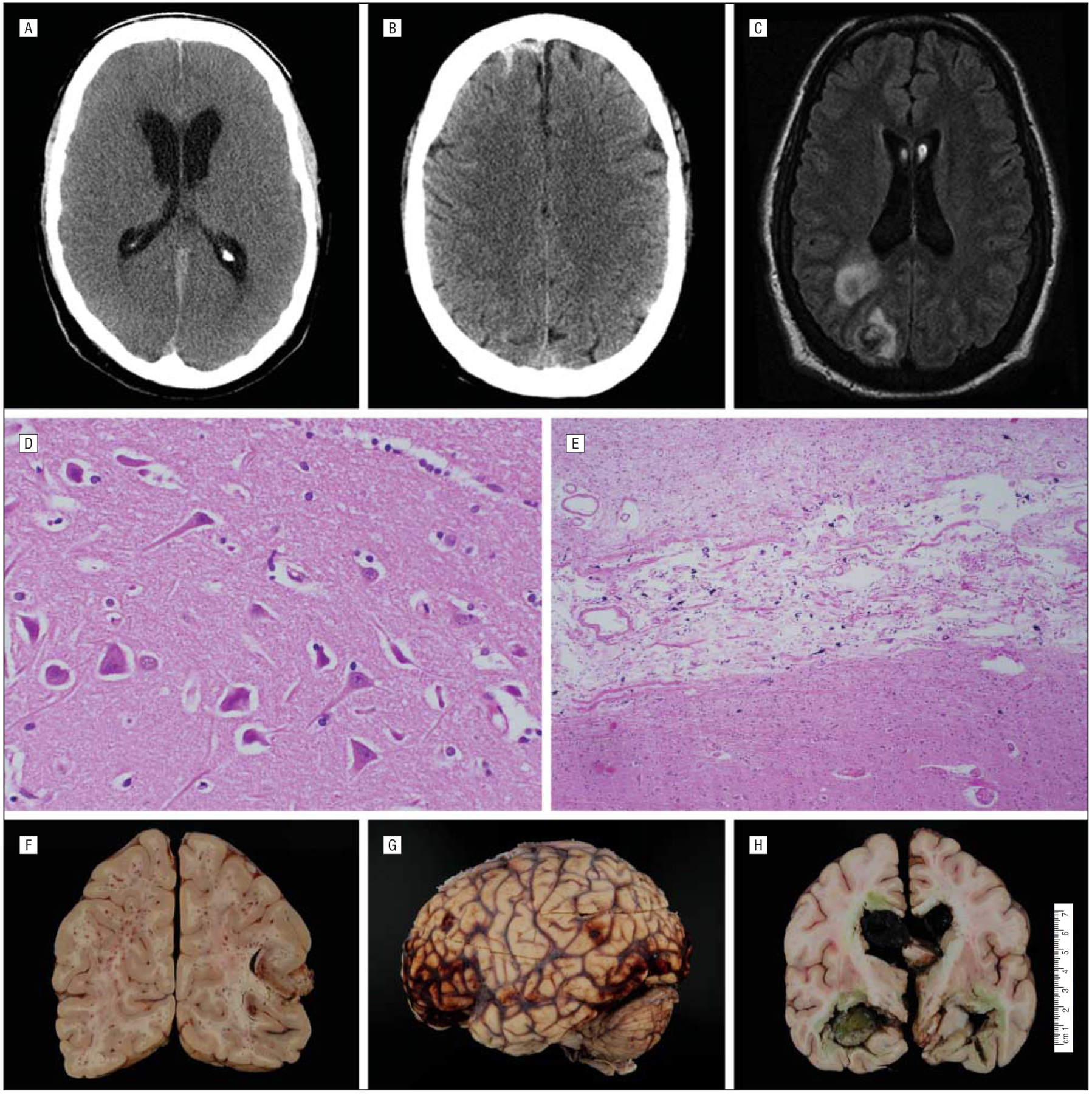
Diagnostic results of adult patients who received extracorporeal membrane oxygenation. The figure shows parafalcine subarachnoid hemorrhage and hydrocephalus on axial-view head computed tomography (A), diffuse subarachnoid hemorrhage on T1-weighted magnetic resonance imaging (B), and septic cerebral emboli on axial-view magnetic resonance imaging (C), which enhances with gadolinium-contrast; acute ischemic cell changes (“red dead neurons”) (D) and microscopic subacute ischemic thalamic infarction on histopathological sectioning (E); and diffuse petechial hemorrhages (F), subarachnoid hemorrhage (G), and massive intraventricular hemorrhage on gross pathological examination (H).
COMMENT
The survival of patients who receive adult ECMO has been previously reported as approximately 50%.5–8,14,15 In this cohort, the proportion of patients surviving to 7 days after ECMO (52%) is consistent with the current literature, though the criteria to be included in this analysis were more stringent. Here, an absolute ECMO duration of 12 hours or more was needed to be included in the assessment of ECMO-related survival predictors and neurological outcomes. By excluding those with less than 12 hours of ECMO treatment, patients dying of the precipitating event, immediately withdrawn from life support, or unable to survive for more than a few hours while receiving ECMO were less likely to be included.
Adult survival following ECMO can be long-lasting. In this cohort, there was a mean duration of ECMO treatment of nearly 4 days, with 44% of patients surviving at least 1 month after ECMO initiation. Patients who were not offered ECMO have expected mortality rates of approximately 100%, making these survival rates comparatively high and of growing importance.
Neurological events affect at least half of ECMO-treated patients and may be predicted by higher age and lower nadir arterial oxygen pressure while receiving ECMO. It is possible that other risk factors exist, but our sample size was not sufficiently large to detect them. We have captured (1) severe neurological events (eg, hemorrhagic stroke), (2) severe neurological presentations without definitive underlying diagnoses in all cases (eg, coma), and (3) important manifestations of potentially severe neurological events that could not be assessed further owing to the patient’s critical status (eg, myoclonus due to presumed anoxic encephalopathy).
It is, however, likely that neurological sequelae are underestimated in this cohort. First, fewer than one-third of patients had brain imaging of any kind, usually owing to their clinical status preventing transport. Second, 21% of patients did not have a formal neurological examination. Neurologists are not routinely involved with ECMO-treated adult patients unless consulted. Third, neurological outcomes that lead to death may not have been observed. This may be especially true in those who died after withdrawal of life support. Our withdrawal-of-support proportion (approximately one-third of patients who received ECMO) is similar to other series, although limited reporting exists. Finally, neuropathological examination revealed a higher degree of neurological sequelae than would have been predicted by clinical examination alone. Nine of the 10 brains studied at autopsy in this series demonstrated ischemic stroke or hypoxic ischemic encephalopathy.
It is not known whether the defined neurological sequelae are a result of the underlying injury that prompted ECMO treatment or the ECMO process itself. Given the emergent setting and, at times, effects of intraoperative anesthesia, there was limited neurological evaluation performed prior to a trial of ECMO. The initial injury sustained may be insurmountable, including hypoxia, hyperpyrexia, hyperglycemia, metabolic acidosis, and other electrolyte disturbances. Other injuries may include high ventilatory pressure, air emboli, subarachnoid hemorrhage, or dissemination of septic emboli. Each patient may experience a different combination of these potentially lethal complications, making inference on the causative role of ECMO difficult. The observation that lower minimum arterial oxygen pressure appears protective against neurological events (odds ratio, <1) and death (hazard ratio, <1) is likely owing to a length bias in observation rather than a physiological phenomenon. Patients who live longer are more likely to have repeated measurements of arterial oxygen pressure over time and more likely to have a lower minimum measurement observed than patients who have less time to be observed. An approach to measure arterial oxygen pressure at predetermined intervals in future studies would be helpful in confirming this assumption.
The use of ECMO and its potential neurological sequelae for survivors has health policy implications. Although it is expensive and currently offered in limited settings, ECMO therapy is likely to be increasingly attempted in adults in the coming years. The prevalence of cardiac disease and cardiac surgery is high, transplant efforts are growing worldwide, and severe flu pandemics have recently affected younger individuals. In 2009, a report of 69 adults from Australia and New Zealand treated with ECMO for acute respiratory distress syndrome in the novel influenza A(H1N1) pandemic found that 48 adults survived to intensive care unit discharge and 32 survived to hospital discharge.3
To our knowledge, this is the first study to provide a detailed analysis of neurological consequences observed in ECMO-treated adults. Necropsy studies of the brains of ECMO-treated adults have not been described, and brain tissue evaluation in general is increasingly limited in neurocritically ill patients. Patients who were deemed to have irreversible failure or catastrophic neurological damage would not be offered ECMO at this institution. This implies that the selected patients were deemed to benefit most from ECMO and had a high degree of neurological sequelae for a chance of survival.
Financial Disclosure:
None reported.
REFERENCES
- 1.Marasco SF, Lukas G, McDonald M, McMillan J, Ihle B. Review of ECMO (extra corporeal membrane oxygenation) support in critically ill adult patients. Heart Lung Circ. 2008;17(suppl 4):S41–S47. [DOI] [PubMed] [Google Scholar]
- 2.Bulas DI, Taylor GA, O’Donnell RM, Short BL, Fitz CR, Vezina G. Intracranial abnormalities in infants treated with extracorporeal membrane oxygenation:update on sonographic and CT findings. AJNR Am J Neuroradiol. 1996;17(2):287–294. [PMC free article] [PubMed] [Google Scholar]
- 3.Davies A, Jones D, Bailey M, et al. ; Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators. Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA. 2009;302(17):1888–1895. [DOI] [PubMed] [Google Scholar]
- 4.Lan C, Tsai PR, Chen YS, Ko WJ. Prognostic factors for adult patients receiving extracorporeal membrane oxygenation as mechanical circulatory support: a 14-year experience at a medical center. Artif Organs. 2010;34(2):E59–E64. [DOI] [PubMed] [Google Scholar]
- 5.Cardarelli MG, Young AJ, Griffith B. Use of extracorporeal membrane oxygenation for adults in cardiac arrest (E-CPR): a meta-analysis of observational studies. ASAIO J. 2009;55(6):581–586. [DOI] [PubMed] [Google Scholar]
- 6.Ko WJ, Lin CY, Chen RJ, Wang SS, Lin FY, Chen YS. Extracorporeal membrane oxygenation support for adult postcardiotomy cardiogenic shock. Ann Thorac Surg. 2002;73(2):538–545. [DOI] [PubMed] [Google Scholar]
- 7.Thiagarajan RR, Brogan TV, Scheurer MA, Laussen PC, Rycus PT, Bratton SL. Extracorporeal membrane oxygenation to support cardiopulmonary resuscitation in adults. Ann Thorac Surg. 2009;87(3):778–785. [DOI] [PubMed] [Google Scholar]
- 8.Massetti M, Tasle M, Le Page O, et al. Back from irreversibility: extracorporeal life support for prolonged cardiac arrest. Ann Thorac Surg. 2005;79(1):178–184. [DOI] [PubMed] [Google Scholar]
- 9.Risnes I, Wagner K, Nome T, et al. Cerebral outcome in adult patients treated with extracorporeal membrane oxygenation. Ann Thorac Surg. 2006;81(4):1401–1406. [DOI] [PubMed] [Google Scholar]
- 10.Rankin J Cerebral vascular accidents in patients over the age of 60, I: general considerations. Scott Med J. 1957;2(4):127–136. [DOI] [PubMed] [Google Scholar]
- 11.Fine JP, Jiang H, Chappell R. On semi-competing risk data. Biometrika. 2001;88(4):907–919. [Google Scholar]
- 12.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2010. [Google Scholar]
- 13.Muralidharan R, Mateen FJ, Shinohara RT, Schears GJ, Wijdicks EF. The challenges with brain death determination in adult patients on extracorporeal membrane oxygenation. Neurocrit Care. 2011;14(3):423–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer S, Bohn D, Rycus P, et al. Extracorporeal membrane oxygenation for primary graft dysfunction after lung transplantation: analysis of the Extracorporeal Life Support Organization (ELSO) registry. J Heart Lung Transplant. 2007; 26(5):472–477. [DOI] [PubMed] [Google Scholar]
- 15.Li J, Long C, Lou S, et al. Venoarterial extracorporeal membrane oxygenation in adult patients: predictors of mortality. Perfusion. 2009;24(4):225–230. [DOI] [PubMed] [Google Scholar]


