Abstract
Background
The purpose of this study was to investigate the prevalence of osteosarcopenia in the over 60-year-old community and to evaluate whether osteosarcopenia is associated with disability, frailty and depression.
Methods
This study was performed using the baseline data of Namgaram-2, among the 1010 surveyed subjects, 885 study subjects who were 60 years or older and had all necessary tests performed were selected. The Kaigo-Yobo checklist (frailty), World Health Organization Disability Assessment Schedule (WHODAS) and Geriatric Depression Scale-Short Form-Korean (GDSSF-K) were used. The Asian Working Group for Sarcopenia (AWGS 2019) were applied in this study. Osteopenia was measured using data from dual energy X-ray absorptiometry (DEXA) and osteopenia was diagnosed when the T-score was less than − 1.0.
The study subjects were divided into four groups: the normal group, in which both sarcopenia and osteopenia were undiagnosed, osteopenia only, sarcopenia only and the osteosarcopenia group, which was diagnosed with both sarcopenia and osteopenia.
Results
Of the 885 subjects over 60 years old evaluated, the normal group comprised 34.0%, the only osteopenia group 33.7%, the only sarcopenia group 13.1%, and the osteosarcopenia group 19.2%. WHODAS (17.5, 95% CI: 14.8-20.1), Kaigo-Yobo (3.0, 95% CI: 2.6-3.4), and GDSSF mean score (4.6, 95% CI: 3.9-5.4) were statistically significantly higher in the osteosarcopenia group compared the other groups. Partial eta squared (ηp2) of WHODAS (0.199) and Kaigo-Yobo (0.148) values according to Osteosarcopenia were large, and GDSSF (0.096) was medium
Conclusions
Osteosarcopenia is a relatively common disease group in the older adults community that may cause deterioration of health outcomes. Therefore, when evaluating osteopenia or sarcopenia in the older adults, management of those in both disease groups should occur together.
Keywords: Disability, Frailty, Depression, Osteosarcopenia
Background
As people live longer, the world’s population is projected to age rapidly in most regions [1]. Korea became an aging society in 2018, and by 2026 Korea will become a super-aged society [2]. Older adults are increasing the prevalence of many chronic diseases, including osteoporosis and sarcopenia [3].
Although osteoporosis-based clinical outcomes and treatment recommendation studies have been increasingly popular in recent decades, sarcopenia and related studies have been less frequent [4]. In addition, sarcopenia has recently been classified as M62.84 in the ICD-10-CM since 2016 [5].
Sarcopenia is most often seen in metabolic disorders such as diabetes mellitus, obesity, and other serious diseases, including congestive heart failure, chronic renal failure, and chronic obstructive pulmonary disease (COPD) [6–8]. Sarcopenia is substantially correlated with osteopenia (or osteoporosis) in older populations. Several studies have shown that sarcopenia and osteopenia (osteosarcopenia, OS) share similar risk factors and biological pathways [9]. OS is associated with severe physical impairment, which presents a major threat to the loss of freedom in later life. The combination of these two conditions exacerbates adverse health effects and creates a significant problem for the older adults. This combination has been presented as a “hazardous duet” that adds the predisposition of falling from sarcopenia to bone weakness in those with osteopenia [10].
In recent years, questionnaire research tools (World Health Disability Assessment Schedule (WHODAS), Kaigo-Yobo) have been developed to define disability and frailty as health outcomes and to measure these outcomes easily and effectively [11, 12]. OS patients have a higher risk of fracture from falling than those with osteopenia and sarcopenia alone. Studies have also shown that sarcopenia acts as a major risk factor for frailty [13]. However, a study on the relationships among OS, disability and frailty is lacking.
Therefore, the purpose of this study was to investigate the prevalence of OS in the over 60-year-old community and to evaluate whether OS is associated with disability, frailty and depression.
Methods
Participants
This study was performed using the baseline data of Namgaram-2, which was developed to study the relationship between the prevalence of musculoskeletal disorders and activity limitations in the older adults in 6 rural villages [14, 15].
At the time of the study, subjects who were diagnosed with chronic diseases and are currently receiving cancer treatment were excluded from the study. Only those with no cognitive problems were included in the study. The cognitive performance evaluation for participants with no cognitive function disorders was performed via an interview study utilizing the Korean version of the Mini-Mental State Examination for Dementia Screening (MMSE-DS) [16]. All surveys were evaluated by a medical professional.
After providing written informed consent, participants completed a questionnaire to assess cognitive function. Subsequently, fasting blood samples were collected from participants, followed by physical function evaluation. Among the 1,010 surveyed subjects, 885 were 60 years or older and had all necessary tests performed. These 885 population were selected as study subjects.
All investigations were conducted after obtaining participant consent and after being reviewed by the Institutional Review Board of our institution (approval number: GIRB-A16-0012).
Materials
Data on social demographic variables such as gender, age, marital status, economic level perceived by the person, and health behavior variable such as smoking was acquired. Also, survey results, such as one on whether or not the subject was affected by hypertension and diabetes, were used. In addition, the blood test measured hemoglobin, cholesterol, albumin, uric acid, γ-GTP, and creatinine.
Frailty (Kaigo-Yobo checklist in Korean older adults)
The Kaigo-Yobo checklist [17] was developed by researchers at the Tokyo Senior Research Institute, and the reliability and validity in Korean older adults have been confirmed [18]. The checklist consists of 15 questions and is a survey instrument that focuses on social activities and evaluation of daily life. The Kaigo-Yobo checklist is a complex domain phenotype aging assessment tool and comprises questions only. The checklist’s 15 items consist of four products for nutritional status; three items for fall; two items for activities; two items for social relationships; and one item each for general health status, communication, mobility, and leisure activities. Furthermore, the appropriate answer to each query was ‘Yes’ or ‘No,‘ and a score of one point was possible for each query. The final score is in the range of 0–15 points, and the higher the score was indicated the higher the degree of frailty.
WHODAS-12
The World Health Organization (WHO) developed the WHO Disability Assessment Schedule (WHODAS) based on the International Classification of Functioning, Disability, and Health (ICF). WHODAS-12 measures the difficulties caused by health conditions by dividing the difficulties into six areas (cognition, mobility, self-care, getting along, life activities, and participation) [19].
All questions were measured with a 5-point Likert item system (1 point, not very difficult to 5 points, very severe). The final score is in the range of 0-100 points, and the higher the score, the higher the degree of disability in everyday life.
Depressive symptoms
To understand the symptoms of depression in the older adults, the Geriatric Depression Scale-Short Form-Korean (GDSSF-K) adapted and developed for aged people in Korea was used [20]. The GDSSF-K has the advantage of comprising items that are easy for the older adults to understand relative to other current depression measurement tools. This scale of 15 items is graded from 0 to 15 points; higher values indicate worse depression.
Osteosarcopenia
Although several criteria have been proposed to define sarcopenia, the recent criteria of the Asian Working Group for Sarcopenia (AWGS 2019) were applied in this study [21].
Dual energy X-ray absorptiometry (DEXA) was used to measure muscle mass. In addition, the measured total appendicular skeletal muscle mass (ASM), excluding bone and fat, divided by the square of the height (m2) was calculated (ASM/Ht2) and used as the skeletal muscle mass index (SMI). Sarcopenia was defined as SMI less than 7.0 kg/m2 in men and less than 5.4 kg/m2 in women. Muscle strength was evaluated by grip strength, and the measurement was conducted using the Smedley-type dynamometer (TKK 5401; Takei Scientific Instruments Co., Tokyo, Japan). Both hands were evaluated, twice each. Grip strength was used for analysis as one of the four measured values. The maximum value was used as a reference level, for men below 28 kg and for women below 18 kg.
Osteopenia was measured using data from dual energy X-ray absorptiometry (DEXA) and osteopenia was diagnosed when the T-score was less than − 1.0 [22].
The study subjects were divided into four groups [23]: the normal group, in which both sarcopenia and osteopenia were undiagnosed, and the osteosarcopenia group, which was subdivided into those diagnosed with both sarcopenia and osteopenia, osteopenia only, and sarcopenia only.
Statistical analysis
The general characteristics of the participants generated descriptive statistics; the chi-square test was conducted on categorical variables, and the ANOVA test was performed on continuous variables. Post-hoc analysis was performed using the Tukey method. The odds ratios (OR) of osteosarcopenia prevalence according to socioeconomic status were analyzed by logistic regression.
The comparison of WHODAS, Kaigo-Yobo, and GDSSF values among the four groups of study subjects occurred after adjustment for gender, age, marital status, economic level, smoking status, hypertension, diabetes, hemoglobin, uric acid, albumin, r-GTP, creatinine, and cholesterol [24–26]. Adjusted means and their 95 % confidence intervals (CIs) were estimated by general linear model (GLM) and the post-hoc test is based on Turkey method. And Partial eta squared (ηp2) was calculated to determine the effect size, using the 0.0099, 0.0588, and 0.1379 considered as small, medium, and large effect sizes [27]. The SAS Version 9.4 program (SAS Institute Inc., Cary, NC) was used as the analysis tool, and the significance level was set to 0.05.
Result
General characteristics
Of the 885 older adults people over 60 years old evaluated, 594 (67.1 %) were women. The average participant age was 70.3 ± 6.2 years, 561 (63.4 %) participants had spouses, and 577 (69.7 %) had a higher than average economic status. The smoking rate was 7.5 %, 447 (50.5 %) were diagnosed with hypertension, and 192 (21.7 %) were diagnosed with diabetes.
The diagnosis of osteopenia and osteoporosis (below T-score – 1.0) occurred in 468 subjects (47.1 %), and sarcopenia was diagnosed in 286 (32.3 %) (Table 1).
Table 1.
General characteristics (Total number = 885)
| Variables | Number | % | |
|---|---|---|---|
| Sex | Male | 291 | 32.9 % |
| Female | 594 | 67.1 % | |
| Age (years) | mean±SD | 70.3±6.2 | |
| Spouse | Yes | 561 | 63.4 % |
| No | 324 | 36.6 % | |
| Economic status | Low | 268 | 30.3 % |
| Middle | 465 | 52.5 % | |
| High | 152 | 17.2 % | |
| Smoking | Non-smoker | 819 | 92.5% |
| Smoker | 66 | 7.5% | |
| Hypertension | No | 438 | 49.5% |
| Yes | 447 | 50.5% | |
| Diabetes mellitus | No | 693 | 78.3% |
| Yes | 192 | 21.7% | |
Osteosarcopenia according to general characteristics
The normal group comprised 34 % (301/885), the only osteopenia group 33.7 % (298/885), the only sarcopenia group 13.1 % (116/885), and the OS group 19.2 % (116/885) (Table 2).
Table 2.
Osteosarcopenia prevalence according to general characteristics
| Variables | Normal | Only Osteopenia | Only Sarcopenia | Osteo-sarcopenia | p- value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | (%) | N | (%) | N | (%) | N | (%) | |||
| Total | 301 | 34.0 | 298 | 33.7 | 116 | 13.1 | 170 | 19.2 | ||
| Sex | Male | 172 | 57.1 | 75 | 25.2 | 26 | 22.4 | 18 | 10.6 | <0.001 |
| Female | 129 | 42.9 | 223 | 74.8 | 90 | 77.6 | 152 | 89.4 | ||
| OR (female/male) | OR=1 | OR=4.0 (p<0.001) | OR=4.6 (p<0.001) | OR=11.3 (p<0.001) | ||||||
| Age (years) | mean±SD | 68.2±5.1 | 68.8±5.9 | 73.1±5.5 | 75.0±5.8 | <0.001 | ||||
| OR (unit: years) | OR=1 | OR=1.0 (p=0.192) | OR=1.2 (p<0.001) | OR=1.2 (p<0.001) | ||||||
| Spouse | Yes | 241 | 80.1 | 193 | 64.8 | 54 | 46.6 | 73 | 42.9 | <0.001 |
| No | 60 | 19.9 | 105 | 35.2 | 62 | 53.4 | 97 | 57.1 | ||
| OR (no/yes) | OR=1 | OR=2.2 (p<0.001) | OR=4.6 (p<0.001) | OR=5.3 (p<0.001) | ||||||
| Economic status | Low | 76 | 25.3 | 87 | 29.2 | 45 | 38.8 | 60 | 35.3 | 0.002 |
| Middle | 153 | 50.8 | 168 | 56.4 | 56 | 48.3 | 88 | 51.8 | ||
| High | 72 | 23.9 | 43 | 14.4 | 15 | 12.9 | 22 | 12.9 | ||
| OR (middle/low) | OR=1 | OR=1.0 (p=0.090) | OR=0.6 (p=0.858) | OR=0.7 (p=0.431) | ||||||
| OR (high/low) | OR=1 | OR=0.5 (p=0.004) | OR=0.4 (p=0.009) | OR=0.4 (p=0.003) | ||||||
| Smoking | No | 272 | 90.4 | 286 | 96.0 | 114 | 98.3 | 166 | 97.6 | <0.001 |
| Yes | 29 | 9.6 | 12 | 4.0 | 2 | 1.7 | 4 | 2.4 | ||
| OR (yes/no) | OR=1 | OR=0.9 (p=0.588) | OR=0.3 (p=0.053) | OR=0.6 (p=0.194) | ||||||
| Hypertension | No | 141 | 46.8 | 178 | 59.7 | 46 | 39.7 | 73 | 42.9 | <0.001 |
| Yes | 160 | 53.2 | 120 | 40.3 | 70 | 60.3 | 97 | 57.1 | ||
| OR (yes/no) | OR=1 | OR=0.6 (p=0.160) | OR=1.3 (p=0.187) | OR=1.2 (p=0.414) | ||||||
The distribution of osteosarcopenia by male and female was significantly different (p < 0.001), and compared to the normal group, the ORs of the other 3 groups and sex were significantly associated with 4.0 (p < 0.001), 4.6 (p < 0.001), 11.3 (p < 0.001), respectively. and age was statistically higher in the OS group (75 ± 5.8 years, P < 0.001) compared to other groups. There was also a statistical difference by spouse’s presence (p < 0.001) and at the economic level, the low group had a significant difference of osetosarcoipenia (22.4 %), while the group with a high economic level (14.5 %) (p = 0.002). The distribution of osteosarcopenia was also significantly different depending on whether hypertension and diabetes were present (p < 0.001).
WHODAS, Kaigo-Yobo, GDSSF scores according to the presence of osteosarcopenia after adjusting covariates
WHODAS disability mean scores were normal (8.6, 95 % CI: 6.3–11.0), only osteopenia (9.4, 95 % CI: 7.1–11.8), only sarcopenia (16.4, 95 % CI: 13.5–19.3), and OS (17.5, 95 % CI: 14.8–20.1), the OS group had statistically significantly higher WHODAS scores compared to the other groups (P < 0.001), and the effect size (partial eta squared (ηp2)) of the WHODAS mean according to the four groups was 0.199, which was large.
Kaigo-Yobo frailty mean scores were normal (1.6, 95 % CI: 1.2-2.0), only osteopenia (1.9, 95 % CI: 1.5–2.2), only sarcopenia (2.7, 95 % CI: 2.3–3.2), and OS (3.0, 95 % CI: 2.6–3.4), the OS group had statistically significantly higher depressive scores compared to the other groups (P < 0.001), and the effect size of the frailty means according to the four groups was 0.148, which was large.
The GDSSF depression mean scores were normal (2.7, 95 % CI: 2.1–3.4), only osteopenia (3.1, 95 % CI: 2.4–3.7), only sarcopenia (4.3, 95 % CI: 3.5–5.1), and OS (4.6, 95 % CI: 3.9–5.4), the OS group had statistically significantly higher depressive scores compared to the other groups (P < 0.001), and the effect size of the depressive means according to the four groups was 0.096, which was medium (Table 3).
Table 3.
WHODAS, Kaigo-Yobo, GDSSF score according to the presence of osteosarcopenia
| Normal | Only Osteopenia | Only Sarcopenia | Osteosarcopenia | Effect size | p value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mean | SE | 95 % CI | mean | SE | 95 % CI | mean | SE | 95 % CI | mean | SE | 95 % CI | Partial eta squared | ||
| aWHODAS | 8.6 | 1.20 | 6.3–11.0 | 9.4 | 1.20 | 7.1–11.8 | 16.4 | 1.48 | 13.5–19.3 | 17.5 | 1.35 | 14.8–20.1 | 0.199 | < 0.001 |
| aKaigo-Yobo | 1.6 | 0.18 | 1.2–2.0 | 1.9 | 0.18 | 1.5–2.2 | 2.7 | 0.23 | 2.3–3.2 | 3.0 | 0.21 | 2.6–3.4 | 0.148 | < 0.001 |
| aGDSSF | 2.7 | 0.34 | 2.1–3.4 | 3.1 | 0.34 | 2.4–3.7 | 4.3 | 0.42 | 3.5–5.1 | 4.6 | 0.38 | 3.9–5.4 | 0.096 | < 0.001 |
aAdjusting factors: Age, Sex, Spouse, Economic status, Smoking, Hypertension, Diabetes mellitus, Osteopenia, Sarcopenia, Hb, Uric acid, Albumin, γ-GTP, Creatine, Cholesterol
WHODAS World Health Organization Disability Assessment Schedule, GDSSF Geriatric Depression Scale-Short Form
Osteosarcopenia according to the presence of frailty
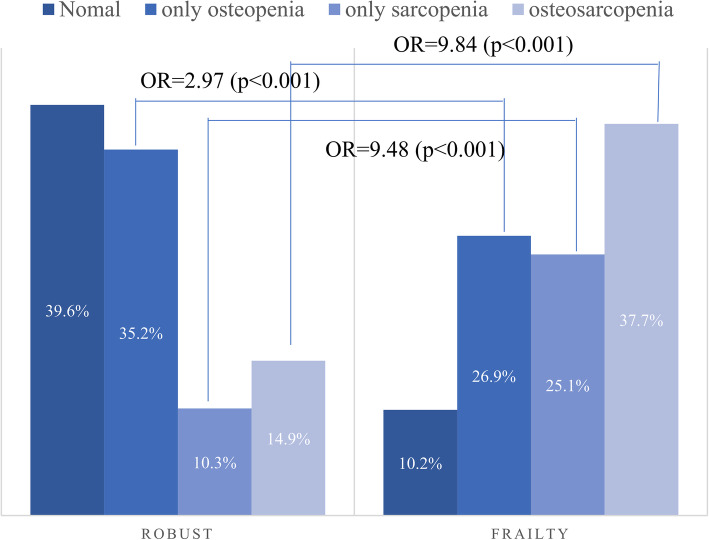
The prevalence of OS (37.7 %) in the frailty group was statistically significantly higher than that of OS (14.9 %) in the robust group. (P < 0.001) (Fig. 1) And, compared to the robust group, the OR between the normal group and only osteopenia was 2.97 (p < 0.001) in the frailty group, the OR with only sarcopenia was 9.48 (p < 0.001) and the OR with the osteosarcopenia group was 9.84 (p < 0.001).
Fig. 1.
Osteosarcopenia according to the presence of frailty-
Discussion
The main findings of this study were: (1) the prevalence of OS in the community using the new AWGS 2019 criteria was 19.2 %; (2) the prevalence of OS was significantly higher in older women; (3) Hb and albumin levels were lower in subjects with OS; and (4) WHODAS, Kaigo-Yobo, and GDSSF scores were all higher in the OS group after adjusting for general characteristics that were different among the four groups. Therefore, we suggest that osteosarcopenia exacerbates frailty, disability and depression, thereby increasing susceptibility to various chronic diseases.
Huo et al. [23] conducted a cross-sectional study of 680 community-dwelling older individuals and reported that the percentage with OS was almost 40 %. A study of community-dwelling Chinese elders (older than age 65) found the prevalence of OS to be 10.4 % in men and 15.1 % in women. In this study, we observed a prevalence similar to that of community-dwelling Chinese elders. Although the prevalence of OS varies greatly depending on the criteria for diagnosis of osteopenia or sarcopenia, OS is not uncommon in older adults women in the community. In particular, considering the commonality of concomitant osteopenia (including osteoporosis) and sarcopenia in the community, DEXA analyses should measure not only bone density but also muscle mass.
Our results suggest that, in older adults women suspected of being malnourished (low Hb, albumin levels), there may be a risk of impairment due to reduced muscle mass and strength and decreased bone density. There is also a high risk of disability and frailty in these subjects.
WHODAS disability scores were significantly higher in the only osteopenia, only sarcopenia, and OS groups compared to the normal group. Frailty and depression scores were significantly higher in the only sarcopenia and OS groups than in the normal and osteopenia alone groups. Therefore, BMD test performance should be accompanied by the sarcopenia test.
For OS, fall, fracture and mortality rates have been reported to be higher than those with bone loss only or muscle loss only, but other studies have reported different results [28, 29]. However, there are no studies reporting on associations with disability and frailty among these groups. Compared to the osteopenia alone group, the WHODAS score showed a clinically significant difference of 8.8 points when sarcopenia was present suggesting that depression is more likely to impact people with sarcopenia than osteopenia. This is consistent with the results of previous research that identified a correlation between disability, frailty, and depression and sarcopenia. While osteopenia was assessed only by bone density testing, sarcopenia was diagnosed as having a decrease in muscle mass as well as a decrease in muscle strength (function), which may be lower in disability, frailty, and depression. In other words, osteopenia may not have progressed to the point of requiring treatment for assistance in daily life activities. However, sarcopenia may be associated with more activity limitation than osteopenia (not osteoporosis).
This study has several limitations. First, the design was cross-sectional. Therefore, causative relationships could not be ascertained. Second, Because the study subjects are not representative samples, caution should be used when expanding the results of the study.
Nevertheless, our research has strength. The study is meaningful as the first to analyze the relationship between OS and disability, depression, and frailty. Future research should not only focus on OS diagnosis and clinical outcomes such as falls, fractures, and death, but also on the OS patient’s quality of life and the occurrence of disability.
Conclusions
OS is a relatively common disease in the community and may cause deterioration of health outcomes. Therefore, concurrent evaluation and management of osteopenia and sarcopenia in the older adults is required.
Acknowledgements
This work was supported by the centers for farmer’s safety and health, ministry of agriculture, food, and rural affairs.
Abbreviations
- WHODAS
WHO Disability Assessment Schedule
- GDSSF-K
Geriatric Depression Scale-Short Form-Korean
- ICD-10-CM
International Classification of Disease, Tenth Revision, Clinical Modification
- COPD
Chronic obstructive pulmonary disease
- OS
Osteosarcopenia
- MMSE-DS
Mini-Mental State Examination for Dementia Screening
- ICF
International Classification of Functionin
- AWGS
Asian Working Group for Sarcopenia
- DEXA
Dual energy X-ray absorptiometry
- ASM
Appendicular skeletal muscle mass
- SMI
Skeletal muscle mass index
Authors’ contributions
All authors read and approved the manuscript. Conceived and designed the experiments: KSP and JIY. Analyzed the data: GYL, YMS, Contributed reagents/materials/analysis tools: SHS. Wrote the paper: KSP and JIY.
Funding
The authors have no support or funding to report.
Availability of data and materials
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Ethics approval and consent to participate
All investigations were conducted after obtaining consent which obtained from study participants was written and after being reviewed by the Institutional Review Board of Gyeongsang national university hospital (approval number: GIRB-A16-0012).
Participants provided consent for use of data in research projects. The data are centrally housed at GNUH. The data were accessed after an analysis plan for this manuscript was approved by the GIRB Publications Committee.
Consent for publication
Not applicable.
Competing interests
All authors have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.United Nations. Department of Economic and Social Affairs, Population Division. World population ageing, 2019 highlights. 2020.
- 2.Kim KJ, Shin J, Choi J, Won CW. Discrepancies in the Prevalence of Known Frailty Scales: Korean Frailty and Aging Cohort Study. Ann Geriatr Med Res. 2018;22:137–44. doi: 10.4235/agmr.2018.22.3.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curtis E, Litwic A, Cooper C, Dennison E. Determinants of muscle and bone aging. J Cell Physiol. 2015;230:2618–25. doi: 10.1002/jcp.25001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han A, Bokshan SL, Marcaccio SE, DePasse JM, Daniels AH. Diagnostic Criteria and Clinical Outcomes in Sarcopenia Research: A Literature Review. J Clin Med. 2018;7. doi:10.3390/jcm7040070. [DOI] [PMC free article] [PubMed]
- 5.Anker SD, Morley JE, von Haehling S. Welcome to the ICD-10 code for sarcopenia. J Cachexia Sarcopenia Muscle. 2016;7:512–4. doi: 10.1002/jcsm.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Haehling S. Muscle wasting and sarcopenia in heart failure: a brief overview of the current literature. ESC Heart Fail. 2018;5:1074–82. doi: 10.1002/ehf2.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lang T, Streeper T, Cawthon P, Baldwin K, Taaffe DR, Harris TB. Sarcopenia: etiology, clinical consequences, intervention, and assessment. Osteoporos Int. 2010;21:543–59. doi: 10.1007/s00198-009-1059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim SH, Shin MJ, Shin YB, Kim KU. Sarcopenia Associated with Chronic Obstructive Pulmonary Disease. J Bone Metab. 2019;26:65–74. doi: 10.11005/jbm.2019.26.2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirk B, Zanker J, Duque G. Osteosarcopenia: epidemiology, diagnosis, and treatment-facts and numbers. J Cachexia Sarcopenia Muscle. 2020. [DOI] [PMC free article] [PubMed]
- 10.Yoo J-I, Ha Y-C. Review of Epidemiology, Diagnosis, and Treatment of Osteosarcopenia in Korea. J Bone Metab. 2018;25:1. doi: 10.11005/jbm.2018.25.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrer MLP, Perracini MR, Rebustini F, Buchalla CM. WHODAS 2.0-BO: normative data for the assessment of disability in older adults. Rev Saude Publica. 2019;53:19. doi: 10.11606/S1518-8787.2019053000586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kojima G, Taniguchi Y, Kitamura A, Shinkai S. Are the Kihon Checklist and the Kaigo-Yobo Checklist Compatible With the Frailty Index? J Am Med Dir Assoc. 2018;19:797–800.e2. doi: 10.1016/j.jamda.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Greco EA, Pietschmann P, Migliaccio S. Osteoporosis and Sarcopenia Increase Frailty Syndrome in the Elderly. Front Endocrinol (Lausanne). 2019;10. doi:10.3389/fendo.2019.00255. [DOI] [PMC free article] [PubMed]
- 14.Kim M-J, Kang B-H, Park S-H, Kim B, Lee G-Y, Seo Y-M, et al. Association of the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) with Muscle Strength in Community-Dwelling Elderly with Knee Osteoarthritis. Int J Environ Res Public Health. 2020;17. [DOI] [PMC free article] [PubMed]
- 15.Kim M, Yoo JI, Kim MJ, Na JB, Lee SI, Park KS. Prevalence of Upper Extremity Musculoskeletal Diseases and Disability among Fruit Tree Farmers in Korea: Cross-Sectional Study. Yonsei Med J. 2019;60:870–5. doi: 10.3349/ymj.2019.60.9.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim TH, Jhoo JH, Park JH, Kim JL, Ryu SH, Moon SW, et al. Korean version of mini mental status examination for dementia screening and its’ short form. Psychiatry Investig. 2010;7:102–8. doi: 10.4306/pi.2010.7.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Shinkai S, Watanabe N, Yoshida H, Fujiwara Y, Amano H, Lee S, et al. [Research on screening for frailty: development of “the Kaigo-Yobo Checklist”] Nihon Koshu Eisei Zasshi. 2010;57:345–54. [PubMed] [Google Scholar]
- 18.Hwang HS, Yoon JL, Park BJ, Choi HR, Kwon IS, Shinkai S, et al. The Validity and Reliability of the Kaigo-Yobo Checklist in Korean Elderly. J Korean Geriatr Soc. 2012;16:121. doi: 10.4235/jkgs.2012.16.3.121. [DOI] [Google Scholar]
- 19.Song JM, Lee HJ. Korean Cultural Adaptation of WHODAS 2.0 (36-Item Version): Reliability and Linking to ICF. J Kor Phys Ther. 2018;30:246–55. doi: 10.18857/jkpt.2018.30.6.246. [DOI] [Google Scholar]
- 20.Kim JY, Park JH, Lee JJ, Huh Y, Lee SB, Han SK, et al. Standardization of the Korean Version of the Geriatric Depression Scale: Reliability, Validity, and Factor Structure. Psychiatry Investig. 2008;5:232–8. doi: 10.4306/pi.2008.5.4.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen L-K, Woo J, Assantachai P, Auyeung T-W, Chou M-Y, Iijima K, et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Dir Assoc. 2020;21:300–7.e2. doi: 10.1016/j.jamda.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 22.Kanis JA, Burlet N, Cooper C, Delmas PD, Reginster J-Y, Borgstrom F, et al. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2008;19:399–428. doi: 10.1007/s00198-008-0560-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huo YR, Suriyaarachchi P, Gomez F, Curcio CL, Boersma D, Muir SW, et al. Phenotype of osteosarcopenia in older individuals with a history of falling. J Am Med Dir Assoc. 2015;16:290–5. doi: 10.1016/j.jamda.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y-J, Wang Y, Zhan J-K, Tang Z-Y, He J-Y, Tan P, et al. Sarco-Osteoporosis: Prevalence and Association with Frailty in Chinese Community-Dwelling Older Adults. International Journal of Endocrinology. 2015;2015:1–8. doi: 10.1155/2015/482940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Almeida OP, Flicker L, Rees M. Depression, dementia and cognition in older people. Maturitas. 2014;79:133–5. doi: 10.1016/j.maturitas.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 26.Kirk B, Zanker J, Duque G. Osteosarcopenia: epidemiology, diagnosis, and treatment—facts and numbers. Journal of Cachexia Sarcopenia Muscle. 2020;11:609–18. doi: 10.1002/jcsm.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richardson JT. Eta squared and partial eta squared as measures of effect size in educational research. Educ Res Rev. 2011;6:135–47. doi: 10.1016/j.edurev.2010.12.001. [DOI] [Google Scholar]
- 28.Scott D, Seibel M, Cumming R, Naganathan V, Blyth F, Le Couteur DG, et al. Does Combined Osteopenia/Osteoporosis and Sarcopenia Confer Greater Risk of Falls and Fracture Than Either Condition Alone in Older Men? The Concord Health and Ageing in Men Project. J Gerontol A Biol Sci Med Sci. 2019;74:827–34. doi: 10.1093/gerona/gly162. [DOI] [PubMed] [Google Scholar]
- 29.Balogun S, Winzenberg T, Wills K, Scott D, Callisaya M, Cicuttini F, et al. Prospective associations of osteosarcopenia and osteodynapenia with incident fracture and mortality over 10 years in community-dwelling older adults. Arch Gerontol Geriatr. 2019;82:67–73. doi: 10.1016/j.archger.2019.01.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.