Abstract
Background:
Depression is a major nonmotor symptom of Parkinson’s disease (PD). However, few treatments exist for PD depression. Monoamine oxidase-B inhibitors (MAOB-Is) provide symptomatic relief for the motor symptoms of PD and exert antidepressive effects. The present meta-analysis of randomized controlled trials (RCTs) investigated the effects of MAOB-Is on depressive symptoms in patients with PD.
Methods:
Articles on PD-management-related RCTs using one of three MAOB-Is approved by the US Food and Drug Administration, that is, selegiline, rasagiline, and safinamide, were identified. The primary outcomes were the benefits of MAOB-Is for depressive symptoms. Subgroup analysis included the effects of MAOB-Is on patients in the early versus middle-to-late stages of PD and the effect of short-term versus long-term treatment.
Results:
Overall, six studies were included, four of which were conducted on patients with early stage PD. Overall, MAOB-Is significantly reduced the severity of depressive symptoms [standardized mean difference (SMD): −0.14, 95% confidence interval (CI): −0.21 to −0.06, p < 0.001]. Subgroup analysis indicated that the positive effect of MAOB-Is was significant in patients with early stage PD (SMD: −0.20, 95% CI: −0.31 to −0.09, p < 0.001), but not in those with middle-to-late-stage PD (SMD: −0.07, 95% CI: −0.17 to 0.03, p = 0.18). The antidepressive effect was significant for short-term treatment, that is, 90–120 days (SMD: −0.23, 95% CI: −0.35 to −0.10, p < 0.001), but not long-term treatment, that is, 24 weeks to 18 months (SMD: −0.08, 95% CI: −0.18 to 0.01, p = 0.09).
Conclusion:
In addition to the treatment of PD motor symptoms, MAOB-Is may help reduce the severity of depressive symptoms in PD, especially in patients with early stage PD. Considering the tolerability and simultaneous benefits of MAOB-Is, further RCTs are warranted to confirm their therapeutic effects in moderate-to-severe PD depression.
Keywords: depressive symptoms, monoamine oxidase-B inhibitors, Parkinson’s disease
Background
Parkinson’s disease (PD) is the second most common neurodegenerative disease.1 Its cardinal symptoms include rigidity, bradykinesia, rest tremor, and postural instability. In addition to involving motor symptoms, PD is accompanied by numerous nonmotor symptoms (NMSs), such as anosmia, sleep disturbance, constipation, dementia, and depression.2 Most motor symptoms of PD can be favorably managed using various treatments. By contrast, the NMSs of PD are under-recognized and poorly managed due to a lack of effective treatments.3 In patients with PD, NMSs usually increase the disease burden and affect quality of life more strongly than traditional motor symptoms.4
Depression is a crucial NMS of PD and affects approximately 30% of patients with PD on average,5 although the prevalence varies across studies. The onset of depression may occur before, along with, or much after the onset of PD motor symptoms.2 Poor motor performance, fluctuation in motor symptoms, and psychological stress after PD diagnosis may precipitate reactive depression in patients with PD. Moreover, biologically, the degeneration of dopaminergic systems, which is the main pathogenetic mechanism of PD, is also the mechanism of depression development in patients with PD.6
The current treatment for PD depression includes antidepressants and dopamine agonists. Tricyclic acid antidepressants have achieved successful treatment responses in clinical trials.5 Nevertheless, the clinical application of tricyclic antidepressants in patients with PD is unpopular due to their notorious side effects. Serotonergic antidepressants are preferred, but the lack of evidence of their effects and concerns regarding aggravated motor symptoms have limited their use.7 Pramipexole, a nonergot dopamine agonist, is effective for PD depression through its potent D3 receptor stimulation. However, its side effects, including impulse control disorder, are problematic for some patients with PD.8
Monoamine oxidase-B inhibitors (MAOB-Is) provide substantial symptomatic relief for motor symptoms and exert potential neuroprotective effects in PD.9,10 Moreover, they are effective in treating major depression in patients without PD,11,12 even though they are generally not considered first-line therapy due to their side effects and dietary limitations. As MAOB metabolizes the neurotransmitter dopamine,13 its activity is linked to both PD and depression. MAOB is elevated in the brain of patients with PD14 and is associated with negative emotionality.15
Currently, three MAOB-Is have been approved by the US Food and Drug Administration (FDA) for managing PD: two irreversible MAOB-Is, selegiline and rasagiline, and one reversible MAOB-I, safinamide. Although evidence of consistent antidepressive effects of MAOB-Is in patients with PD is lacking, a trend of improvement in depression has been observed.13,14,16 We hypothesized that MAOB-Is are beneficial for depressive symptoms in patients with PD. In the present study, we performed a meta-analysis of randomized controlled trials (RCTs) to investigate the effect of MAOB-Is on depression in patients with PD.
Materials and methods
Inclusion criteria
This study included RCTs that investigated the therapeutic effects of US FDA-approved MAOB-Is, specifically, selegiline, rasagiline, and safinamide, on patients with PD. RCTs were required to report patient inclusion and exclusion criteria, the process of randomization, the dosage and duration of treatment, and a comprehensive assessment of depression. RCTs with fewer than 40 participants were excluded.
Literature search strategy
We searched for RCTs published before March 2020 in the PubMed, Embase, Web of Science, Scopus, and Cochrane Library databases following the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines. The search keywords are available in the Supplemental material. Only studies published in English were included.
Data extraction
Baseline and outcome data were independently retrieved by two reviewers (CTH and YHH). Furthermore, data regarding study design, study population characteristics, inclusion and exclusion criteria, MAOB-I dosage, treatment duration, and outcome assessment were extracted. Decisions recorded individually by the reviewers were compared, and disagreements were resolved by a third reviewer (EWL).
Appraisal of methodological quality
Two reviewers (CTH and JHC) independently assessed the methodological quality of each study by using the Cochrane risk of bias tool (RoB 2.0). Studies were graded as having high risk of bias, some concerns, or low risk of bias. The grade was estimated by assessing five domains: bias arising from the randomization process, bias due to deviation from the intended intervention, bias due to missing outcome data, bias in the measurement of outcomes, and bias in selecting reported results. The results of the quality assessment are provided in the Supplemental material.
Outcomes
The primary outcome was change in depressive symptoms on treatment with MAOB-Is in patients with PD. Subgroup analysis included the effect on patients with de novo or early stage PD versus middle-to-late-stage PD and the effect of short-term (90–120 days) versus long-term (24 weeks to 18 months) MAOB-I treatment on depressive symptoms. To categorize the status of patients with PD for the first subgroup analysis, we followed the inclusion criteria of all the included RCTs. In general, presence of motor fluctuation was categorized as the middle-to-late stage, whereas disease duration of fewer than 5 years was considered the early stage. The distribution of the modified Hoehn and Yahr stage (see Supplemental material) for the study participants was also considered. If more than one depression inventory was employed in a single study, the most widely used scale among the enrolled studies was selected for the outcome analysis [this was the Hamilton Depression Rating Scale (HDRS) in our analysis].
Statistical analysis
Data were recorded and analyzed using Review Manager 5.3 (The Cochrane Collaboration, Oxford, UK). A meta-analysis was performed according to the PRISMA guidelines, and a random-effects model was applied to assess the outcomes. The standard deviation was calculated using the confidence interval (CI) limits provided, standard errors, or interquartile ranges. The effect sizes of continuous outcomes are reported as standardized mean differences (SMDs). The precision of effect sizes is reported at the 95% CI. A statistically significant result was indicated by p < 0.05 or a 95% CI not including zero in the weighted mean difference.
Statistical heterogeneity and inconsistency in treatment effects across studies were evaluated using the Cochrane Q test and I2 statistics, respectively. Statistical significance was set at p < 0.05 for the Cochrane Q test. Statistical heterogeneity across studies was assessed using the I2 test, which quantifies the proportion of total outcome variability across studies.
Results
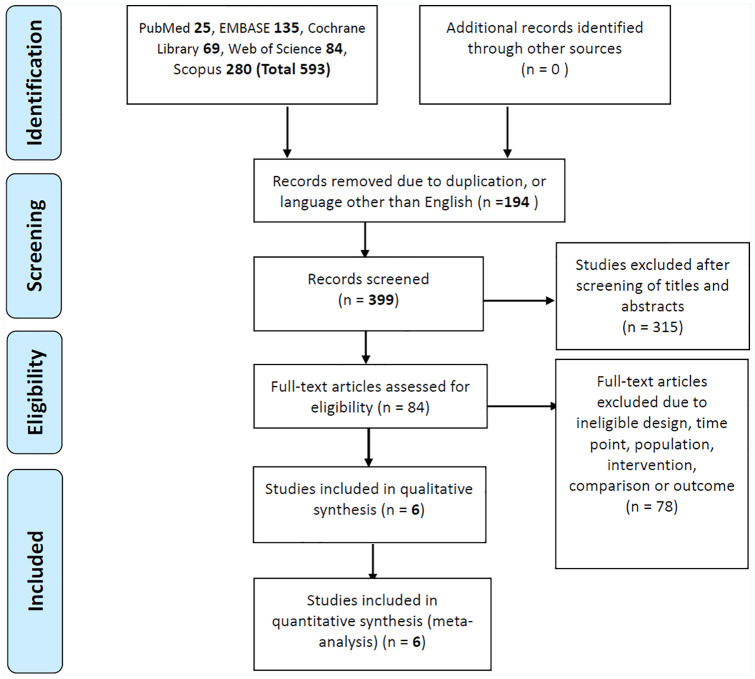
Figure 1 displays the flowchart of study selection. Our initial search yielded 399 studies, of which 315 were deemed ineligible after the titles and abstracts had been screened. Another 78 reports were excluded due to ineligible designs, time points, populations, interventions, comparisons, or outcomes. The remaining six eligible RCTs were included in our analysis. Three of them used safinamide,15,17,18 two used selegiline (deprenyl),19,20 and one used rasagiline.16 These RCTs were published between 1989 and 2016 and had sample sizes ranging from 93 to 800 (Table 1). The participants’ mean age was approximately 60 years, and nearly all study cohorts had male preponderance. None of the RCTs allowed antidepressant use among the participants. Four studies recruited patients in the early stages of PD (de novo PD in one study), and two recruited patients in the middle-to-late stages of PD (as identified by the presence of motor fluctuation). The treatment duration ranged from 90 days to 18 months. For the outcome assessment, five of six studies assessed depressive symptoms using the HDRS, and one used the Beck Depression Inventory (BDI).
Figure 1.
Flowchart of study selection.
Table 1.
Characteristics of the included studies.
| Author (original trial) | Inclusion criteria | No. patients (male, %) | Age, years mean ± SD (if provided) | H & Y stage | Intervention | Outcome |
|---|---|---|---|---|---|---|
| Parkinson Study Group19
(DATATOP) |
Stage I or II for fewer than 5 years | E: 399 (67) C: 401 (66) |
E: 61.1 ± 9.7 C: 61.1 ± 9.4 |
E: 1.6 ± 0.5 C: 1.7 ± 0.5 |
Selegiline 10 mg for 90 days | HAMD |
| Allain et al.20
(French Selegiline Multicenter Trial) |
No treatment, stage less than III | E: 48 (52.1) C: 45 (55.6) |
E: 64.5 ± 8.5 C: 65.4 ± 10.1 |
E: 1.8 ± 0.5 C: 1.7 ± 0.4 |
Selegiline 10 mg for 90 days | HAMD |
| Schapira et al.18
(Study 017) |
Stage I to III for fewer than 5 years | E1: 80 (67.5) E2: 69 (62.3) C: 78 (60.3) |
E1: 56.6 E2: 59.8 C: 57.0 |
E1: 1.9 ± 0.6 E2: 1.8 ± 0.6 C: 1.9 ± 0.7 |
Safinamide 100 (E1) or 200 (E2) mg for 18 months |
HAMD |
| Borgohain et al.15 | Middle-to-late-stage PD, experiencing motor fluctuations |
E1: 223 (70.4) E2: 224 (72.8) C: 222 (72.1) |
E1: 60.1 ± 9.65 E2: 60.1 ± 9.19 C: 59.4 ± 9.41 |
E1: 2.8 ± 0.6 E2: 2.8 ± 0.6 C: 2.8 ± 0.7 |
Safinamide 50 (E1) or 100 (E2) mg for 24 weeks | HAMD |
| Barone et al.16 | Stage ⩾1 and ⩽3; BDI-I ⩾15 | E: 58 (46.6) C: 65 (58.5) |
E: 66.0 ± 8.74 C: 66.1 ± 8.35 |
E: 1.8 ± 0.6 C: 1.9 ± 0.6 |
Rasagiline 1 mg for 12 weeks | BDI |
| Schapira [2017]17 | Disease duration >3 years, stages I–IV, off time >1.5 h/day | E: 274 (62.4) C: 275 (68.4) |
E: 61.7 ± 9.0 C: 62.1 ± 8.9 |
E: 2.5 ± 0.6 C: 2.5 ± 0.6 |
Safinamide 100 mg for 24 weeks | HAMD |
BDI, Beck Depression Inventory; C, control group; E, experimental group; HAMD, Hamilton Depression Rating Scale; H & Y, Hoehn and Yahr (Supplemental material Table S3); PD, Parkinson’s disease; SD, standard deviation.
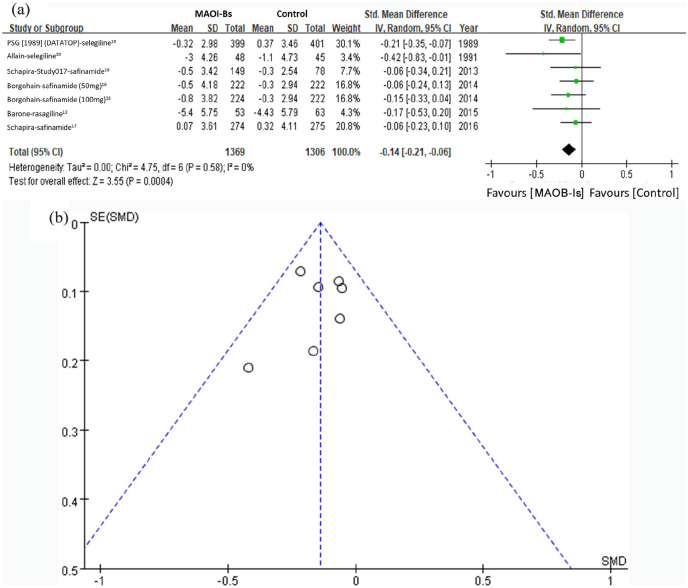
The primary outcome of the meta-analysis was the overall effect of MAOB-Is on depressive symptoms in patients with PD. Seven comparisons were made with the six selected studies. The MAOB-Is significantly improved depressive symptoms when they were assessed using either the HDRS or BDI (SMD: −0.14, 95% CI: −0.21 to −0.06, p < 0.001) (Figure 2(a)). The heterogeneity of this analysis was nonsignificant, with I2 = 0%. The funnel plot indicated no evidence of publication bias (Figure 2(b)).
Figure 2.
Effect of MAOB-Is on the depressive symptoms in patients with PD. (a) Forest plots of the meta-analysis result for the overall effect of MAOB-Is on depressive symptoms in patients with PD; (b) funnel plot with pseudo 95% confidence limit of the overall effect of MAOB-Is on depressive symptoms in patients with PD.
CI, confidence interval; df, degrees of freedom; MAOB-I, monoamine oxidase-B inhibitor; PD, Parkinson’s disease; SD, standard deviation; SE, standard error; SMD, standard mean difference.
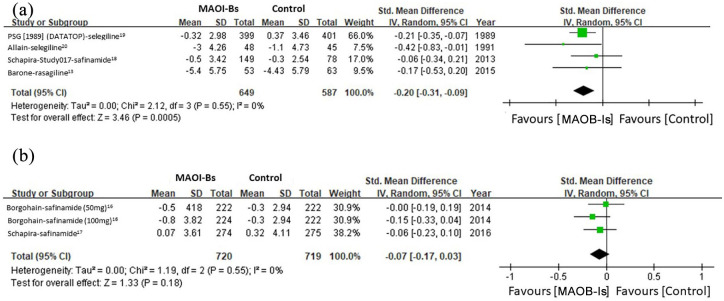
Subgroup analysis was conducted after stratifying patients based on their PD stage and the MAOB-Is’ pharmacological characteristics. For patients with early stage PD, the pooled results revealed significant beneficial effects of MAOB-Is on depressive symptoms (SMD: −0.20, 95% CI: −0.31 to −0.09, p < 0.001) without significant heterogeneity (I2 = 0%) (Figure 3(a)). For patients with middle-to-late-stage PD, the beneficial effect of MAOB-Is on depressive symptoms was nonsignificant (SMD: −0.07, 95% CI: −0.17 to 0.03, p = 0.18) (Figure 3(b)).
Figure 3.
Subgroup analysis of the effect of MAOB-Is on depressive symptoms in patients with (a) early stage and (b) middle-to-late stage PD.
CI, confidence interval; df, degrees of freedom; MAOB-I, monoamine oxidase-B inhibitor; PD, Parkinson’s disease; SD, standard deviation.
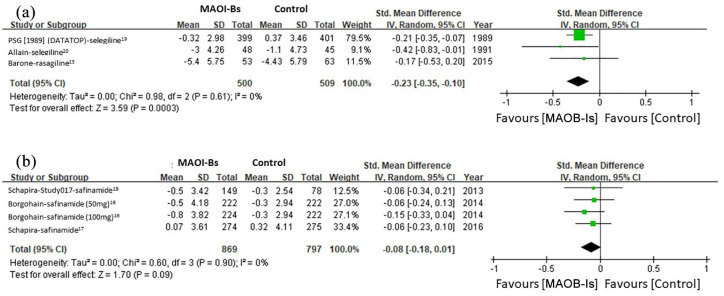
Another subgroup analysis was performed according to the treatment duration to assess the correlation between this duration and the antidepressive effect. For treatment duration of fewer than 24 weeks, the pooled result revealed significant benefits of MAOB-Is in depressive symptoms (SMD: −0.23, 95% CI: −0.35 to −0.10, p < 0.001) without significant heterogeneity (I2 = 0%) (Figure 4(a)). For treatment duration of 24 weeks or longer, the beneficial effect of MAOB-Is on depressive symptoms was marginal (SMD: −0.08, 95% CI: −0.18 to 0.01, p = 0.09) without significant heterogeneity (I2 = 0%) (Figure 4(b)).
Figure 4.
Subgroup analysis of the effect of MAOB-Is on depressive symptoms in patients with treatment duration of (a) <24 weeks and (b) ⩾24 weeks.
CI, confidence interval; df, degrees of freedom; MAOB-I, monoamine oxidase-B inhibitor; SD, standard deviation.
Discussion
The present study demonstrated that MAOB-Is had significantly beneficial effects on depressive symptoms in patients with PD, especially those with early stage PD or under short-term treatment. For patients with middle-to-late-stage PD or longer treatment duration, the effect was nonsignificant. Our results thus indicate that in addition to the well-known symptomatic effect of relieving PD motor symptoms, MAOB-Is may alleviate depressive symptoms in patients with PD over the short term but not the long term.
Dysfunction of the dopaminergic system is an essential contributor to depression. The levels of plasma homovanillic acid, a dopamine metabolite, are low in people with depression.21 Moreover, compensatory upregulation of D2 dopamine receptor density is noted in the basal ganglia and cerebellums of those with depression.22 Thus, modulation of the dopaminergic system has a potent antidepressive effect.23 Depression is a prevalent NMS in PD and may be noted even before the onset of motor symptoms, thereby supporting the notion that depression is a nonmotor presentation of dopaminergic dysfunction in PD. MAOB-Is, particularly selegiline, exhibit remarkable antidepressive efficacy for major depression;24 however, their effect on PD depression has not yet been investigated directly. Unlike traditional tricyclic acid antidepressants and novel selective serotonergic reuptake inhibitors, which worsen the motor performance of patients with PD, MAOB-Is exert notable symptomatic effects on PD motor symptoms. The effects of safinamide17 and rasagiline16 on mood fluctuation and depressive symptoms in patients with PD have been investigated; however, the results have been inconsistent. The present study revealed that MAOB-Is alleviate not only motor symptoms but also depressive symptoms. This finding further supports the dopamine deficiency theory regarding PD depression. Further clinical trials should assess the antidepressive therapeutic effect of MAOB-Is in patients with PD, especially those with moderate and severe depression.
In addition to dopamine deficiency, PD depression is caused by multiple environmental factors. Impairment of motor performance or marked motor fluctuation results in distress and loss of self-confidence. Moreover, sleep disturbance, anxiety, or stress following the diagnosis of PD may contribute to the development of depression. According to the treatment recommendations of Timmer et al. for PD depression, before medication is prescribed, PD depression must be managed by taking an overview of the general condition and through multidisciplinary care. After these two essential steps, management of motor symptoms and optimization of dopaminergic medication is the priority, followed by the administration of antidepressants.6 MAOB-Is are well known to exert symptomatic treatment effects on PD motor fluctuation, such as reducing ‘off’ periods and the frequency of the ‘on-off’ phenomenon.25,26 Thus, a single MAOI-B prescription has dual effects and is a superior solution for PD depression compared with other treatment options or combinations.
The heterogeneity of the present meta-analysis was nonsignificant, both in the overall and subgroup analyses, which indicated that our results are reliable. The included studies did have a large time gap, two were conducted in the late 20th century and the remaining four in the 2010s, which may have resulted from the lack of approval of novel MAOB-Is for the management of PD during the late 1990s and first decade of the 21st century. A study found that 2-year safinamide treatment reduced depressive symptoms,14 thus indicating that longer-term treatment with MAOB-Is alleviates depressive symptoms. However, this effect was not seen in our study.
The present study has some limitations. First, we did not control for confounding caused by motor improvement. The pooled studies in the present meta-analysis revealed significant motor improvements in PD, which may be an etiology of depression relief. However, the successful management of the motor symptoms of PD with dopaminergic medication is not always accompanied by improvement in the severity of PD depression. Currently, pramipexole is the only dopaminergic medication proven to be effective in treating PD depression. Levodopa has symptomatic effects but fails to improve PD depression.27 Other dopamine agonists (ropinirole, apomorphine, and rotigotine) do not exert antidepressive effects on PD depression. In addition to improving motor function, MAOB-Is may ameliorate PD depression through other mechanisms such as serotonin and norepinephrine reuptake inhibition. Moreover, our subgroup analysis identified significant benefit for patients with early stage PD, who experience much less motor dysfunction than those with middle-to-late-stage PD. Second, different studies used different assessments for depressive symptoms. As no PD-specific depression questionnaires are available, conventional depression inventories demonstrate inconsistent sensitivity and specificity in diagnosing PD depression.6 Moreover, the timing of administration of the scale also has a considerable impact on scores. As motor symptoms and NMSs fluctuate during the day, the depression scale scores were likely to be high during off periods due to poor motor performance or other discomfort, leading to heterogeneous results. Although the present study standardized the scores of each depression scale for statistical use, the meta-analysis has an inherent shortcoming that could not be completely overcome. Finally, safinamide, in addition to inhibiting MAOB, modulates glutamatergic signaling, which also has an antidepressive effect.
Conclusion
The present meta-analysis demonstrated that MAOB-Is relieve PD depression, especially in patients with early stage PD: this beneficial effect potentially results from the combined effects of dopaminergic system stimulation, serotonin and norepinephrine reuptake inhibition, and motor performance improvement. Double-blind RCTs are required to directly assess the antidepressive effects of MAOB-Is in patients with different stages of PD.
Supplemental Material
Supplemental material, sj-pdf-1-tpp-10.1177_2045125320985993 for The effect of monoamine oxidase-B inhibitors on the alleviation of depressive symptoms in Parkinson’s disease: meta-analysis of randomized controlled trials by Yao Hsien Huang, Jia Hung Chen, El Wui Loh, Lung Chan and Chien Tai Hong in Therapeutic Advances in Psychopharmacology
Acknowledgments
This manuscript was edited by Wallace Academic Editing.
Footnotes
Authors’ contributions: Study conception and design: YHH, JHC, LC, and CTH. Data acquisition and analysis: YHH, EWL, and CTH. Data interpretation: JHC, LC, and CTH. Manuscript writing and revision: YHH, JHC, LC, and CTH. All authors have read and approved the final manuscript.
Availability of data and materials: Please contact the authors of the original studies included in the meta-analysis.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Chien Tai Hong  https://orcid.org/0000-0002-7448-1041
https://orcid.org/0000-0002-7448-1041
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Yao Hsien Huang, Department of Neurology, Shuang Ho Hospital, Taipei Medical University, New Taipei City, Taiwan; Department of Neurology, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan.
Jia Hung Chen, Department of Neurology, Shuang Ho Hospital, Taipei Medical University, New Taipei City.
El Wui Loh, Graduate Institute of Clinical Medicine, College of Medicine, Taipei Medical University, Taipei; Center for Evidence-Based Health Care, Department of Medical Research, Taipei Medical University, New Taipei City.
Lung Chan, Department of Neurology, Shuang Ho Hospital, Taipei Medical University, New Taipei City; Department of Neurology, College of Medicine, Taipei Medical University, Taipei; Department of Neurology, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan.
Chien Tai Hong, Department of Neurology, Shuang Ho Hospital, Taipei Medical University, No.291, Zhongzheng Rd, Zhonghe District, New Taipei City, 23561; Department of Neurology, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan.
References
- 1. de Lau LM, Breteler MM. Epidemiology of Parkinson’s disease. Lancet Neurol 2006; 5: 525–535. [DOI] [PubMed] [Google Scholar]
- 2. Chaudhuri KR, Healy DG, Schapira AH. Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol 2006; 5: 235–245. [DOI] [PubMed] [Google Scholar]
- 3. Schapira AHV, Chaudhuri KR, Jenner P. Non-motor features of Parkinson disease. Nat Rev Neurosci 2017; 18: 435–450. [DOI] [PubMed] [Google Scholar]
- 4. Prakash KM, Nadkarni NV, Lye WK, et al. The impact of non-motor symptoms on the quality of life of Parkinson’s disease patients: a longitudinal study. Eur J Neurol 2016; 23: 854–860. [DOI] [PubMed] [Google Scholar]
- 5. Goodarzi Z, Mrklas KJ, Roberts DJ, et al. Detecting depression in Parkinson disease: a systematic review and meta-analysis. Neurology 2016; 87: 426–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Timmer MHM, van Beek M, Bloem BR, et al. What a neurologist should know about depression in Parkinson’s disease. Pract Neurol 2017; 17: 359–368. [DOI] [PubMed] [Google Scholar]
- 7. Alonso A, Rodríguez LAG, Logroscino G, et al. Use of antidepressants and the risk of Parkinson’s disease: a prospective study. J Neurol Neurosurg Psychiatry 2009; 80: 671–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barone P, Poewe W, Albrecht S, et al. Pramipexole for the treatment of depressive symptoms in patients with Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 2010; 9: 573–580. [DOI] [PubMed] [Google Scholar]
- 9. Olanow CW, Rascol O, Hauser R, et al. A double-blind, delayed-start trial of rasagiline in Parkinson’s disease. N Engl J Med 2009; 361: 1268–1278. [DOI] [PubMed] [Google Scholar]
- 10. Palhagen S, Heinonen E, Hagglund J, et al. Selegiline slows the progression of the symptoms of Parkinson disease. Neurology 2006; 66: 1200–1206. [DOI] [PubMed] [Google Scholar]
- 11. Amsterdam JD. A double-blind, placebo-controlled trial of the safety and efficacy of selegiline transdermal system without dietary restrictions in patients with major depressive disorder. J Clin Psychiatry 2003; 64: 208–214. [DOI] [PubMed] [Google Scholar]
- 12. Bodkin JA, Amsterdam JD. Transdermal selegiline in major depression: a double-blind, placebo-controlled, parallel-group study in outpatients. Am J Psychiatry 2002; 159: 1869–1875. [DOI] [PubMed] [Google Scholar]
- 13. Elmer L, Schwid S, Eberly S, et al. Rasagiline-associated motor improvement in PD occurs without worsening of cognitive and behavioral symptoms. J Neurol Sci 2006; 248: 78–83. [DOI] [PubMed] [Google Scholar]
- 14. Cattaneo C, Müller T, Bonizzoni E, et al. Long-term effects of safinamide on mood fluctuations in Parkinson’s disease. J Parkinsons Dis 2017; 7: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Borgohain R, Szasz J, Stanzione P, et al. Randomized trial of safinamide add-on to levodopa in Parkinson’s disease with motor fluctuations. Mov Disord 2014; 29: 229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Barone P, Santangelo G, Morgante L, et al. A randomized clinical trial to evaluate the effects of rasagiline on depressive symptoms in non-demented Parkinson’s disease patients. Eur J Neurol 2015; 22: 1184–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schapira AH, Fox SH, Hauser RA, et al. Assessment of safety and efficacy of safinamide as a levodopa adjunct in patients with Parkinson disease and motor fluctuations: a randomized clinical trial. JAMA Neurol 2017; 74: 216–224. [DOI] [PubMed] [Google Scholar]
- 18. Schapira AH, Stocchi F, Borgohain R, et al. Long-term efficacy and safety of safinamide as add-on therapy in early Parkinson’s disease. Eur J Neurol 2013; 20: 271–280. [DOI] [PubMed] [Google Scholar]
- 19. Parkinson Study Group. Effect of deprenyl on the progression of disability in early Parkinson’s disease. N Engl J Med 1989; 321: 1364–1371. [DOI] [PubMed] [Google Scholar]
- 20. Allain H, Cougnard J, Neukirch HC. Selegiline in de novo parkinsonian patients: the French Selegiline Multicenter Trial (FSMT). Acta Neurol Scand Suppl 1991; 136: 73–78. [DOI] [PubMed] [Google Scholar]
- 21. Brown AS, Gershon S. Dopamine and depression. J Neural Transm Gen Sect 1993; 91: 75–109. [DOI] [PubMed] [Google Scholar]
- 22. D’Haenen HA, Bossuyt A. Dopamine D2 receptors in depression measured with single photon emission computed tomography. Biol Psychiatry 1994; 35: 128–132. [DOI] [PubMed] [Google Scholar]
- 23. Pytka K, Podkowa K, Rapacz A, et al. The role of serotonergic, adrenergic and dopaminergic receptors in antidepressant-like effect. Pharmacol Rep 2016; 68: 263–274. [DOI] [PubMed] [Google Scholar]
- 24. Robinson DS, Gilmor ML, Yang Y, et al. Treatment effects of selegiline transdermal system on symptoms of major depressive disorder: a meta-analysis of short-term, placebo-controlled, efficacy trials. Psychopharmacol Bull 2007; 40: 15–28. [PubMed] [Google Scholar]
- 25. Parkinson Study Group. A randomized placebo-controlled trial of rasagiline in levodopa-treated patients with Parkinson disease and motor fluctuations: the PRESTO study. Arch Neurol 2005; 62: 241–248. [DOI] [PubMed] [Google Scholar]
- 26. Hubble JP, Koller WC, Waters C. Effects of selegiline dosing on motor fluctuations in Parkinson’s disease. Clin Neuropharmacol 1993; 16: 83–87. [DOI] [PubMed] [Google Scholar]
- 27. Choi C, Sohn YH, Lee JH, et al. The effect of long-term levodopa therapy on depression level in de novo patients with Parkinson’s disease. J Neurol Sci 2000; 172: 12–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tpp-10.1177_2045125320985993 for The effect of monoamine oxidase-B inhibitors on the alleviation of depressive symptoms in Parkinson’s disease: meta-analysis of randomized controlled trials by Yao Hsien Huang, Jia Hung Chen, El Wui Loh, Lung Chan and Chien Tai Hong in Therapeutic Advances in Psychopharmacology