Abstract
Background:
Testicular cancer represents the most common malignancy in young adult men. In the current study, we sought to analyze and compare the prognostic value of lymph node ratio (LNR) as well as positive lymph node counts (LNC) to understand its clinical significance in testicular germ cell tumors.
Methods:
We employed eligibility criteria to recruit a total of 931 patients, with testicular cancer, from 2010 to 2015 from The Surveillance, Epidemiology, and End Results (SEER) database. We then used the X-Tile program to calculate LNR and LNC cutoff values and discriminate survival. We then calculated the overall and cancer specific survival rates and analyzed the association between LNR/LNC and clinical pathological characteristics using the χ2 test. Finally, we assessed the relationships between clinical pathological factors and patient survival using univariate Cox proportional hazard analysis.
Results:
Univariate analysis revealed a significant association between prognosis with age (HR, 5.169; 95% CI, 1.758-15.200; P = 0.003), AJCC stage (III vs I: HR, 9.298; 95% CI, 2.691-32.131; P < 0.001), M stage (HR, 7.897; 95% CI, 3.417-18.251; P < 0.001) and LNR (HR, 3.009; 95% CI, 1.275-7.098; P = 0.012). On the other hand, LNC (HR, 1.743; 95% CI, 0.687-4.420; P = 0.242) was not significantly associated with prognosis. Analysis of the association between LNR/LNC and clinical pathological characteristics showed that high LNR patients tended to have significantly larger tumor sizes (χ2 = 7.877, P = 0.005), as well as advanced T (χ2 = 13.195, P = 0.004), N ( χ2 = 86.775, P < 0.001), M (χ2 = 19.948, P < 0.001) and 7th AJCC (χ2 = 103.074, P < 0.001) stages. In addition, high LNC patients were significantly associated with T (χ2 = 8.799, P = 0.032), N (χ2 = 74.390, P < 0.001) and 7th AJCC (χ2 = 111.759, P < 0.001) stages.
Conclusion:
LNR was a better predictor for long-term prognosis and was closely associated with clinical pathological characteristics than LNC in patients with testicular germ cell tumors.
Keywords: lymph node ratio, positive lymph node counts, testicular germ cell tumor, prognosis
Introduction
Testicular cancer is the most common solid malignancy in young adult men, with a high incidence over the past several decades.1-5 Of all histological types of this cancer, malignant testicular germ cell tumors are the most common, while non-germ cell tumors are exceedingly rare.6-8 Although survival rate is up to 95% in all patients with testicular cancer, and 80% of those diagnosed with metastatic disease owing to advanced progress in treatment of patients in recent years, approximately 10% of patients remain incurable.9 In future, more effective treatment strategies and better prognostic predictors for these patients need to be further investigated.
The American Joint Committee on Cancer (AJCC) staging system is the most common staging method for malignant tumors worldwide. However, new predictors, such as the positive lymph node counts (LNC) and lymph node ratio (LNR), have recently emerged and showed promising prognostic value for patients with various cancers.10-22 The positive LNC refers to the number of lymph nodes with metastasis of the primary tumor while the lymph node ratio is the ratio of positive lymph node counts among the total regional lymph nodes examined in the surgery. Although numerous studies have demonstrated the potential prognostic value for LNC and LNR in various cancers, their clinical prognostic roles in testicular germ cell tumor remain unknown.
In the present study, we obtained clinical pathological information of testicular germ cell tumor patients from 2010 to 2015 using the Surveillance, Epidemiology, and End Results (SEER) database. We analyzed these data with the aim of comparing the prognostic impacts of LNR and LNC, to understand their potential association as well as other clinical pathological characteristics in testicular germ cell tumor patients.
Materials and Methods
Patients and Eligibility Criteria
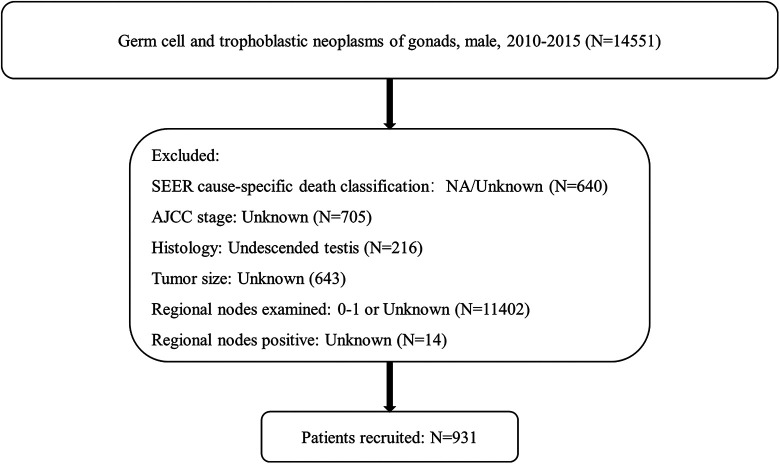
We used the SEER*Stat software program to identify 14,551 men diagnosed with germ cell or trophoblastic gonadal neoplasms, between 2010 and 2015. Patients were excluded if they met the following criteria: (1) SEER cause-specific death classification: NA/Unknown; (2) AJCC stage: Unknown; (3) Histology: Undescended testis; (4) Tumor size: Unknown; (5) Regional nodes examined: 0 -1 or Unknown; (6) Regional nodes positive: Unknown. At the end of exclusion, a total of 931 patients were recruited in the study (Figure 1).
Figure 1.
Flow diagram indicating target patients selected from SEER database.
We evaluated the following variables: age, marital status, race, tumor size, the 7th AJCC/TNM stages, histology, lymph-vascular invasion status, lymph node ratio, positive lymph node counts and primary site. LNR = LNC/lymph node examined counts. The endpoints we used was overall survival (OS) and cancer specific survival (DFS), which were determined by vital status and SEER cause-specific death classification, respectively. Detailed information of patients in Table 1.
Table 1.
Patient and Demographics Details.
| Characteriscs | NO. of patients (n = 931) |
|---|---|
| Age (y) | |
| ≤27 | 477 (51.2%) |
| >27 | 454 (48.8%) |
| Martial status | |
| Married | 299 (32.1%) |
| Single | 567 (60.9%) |
| Unknown | 26 (2.8%) |
| Separated/Divorced/Widowed | 39 (4.2%) |
| Race | |
| White | 855 (91.8%) |
| Other | 76 (8.2%) |
| Tumor size (cm) | |
| ≤4 | 507 (54.5%) |
| >4 | 424 (45.5%) |
| T stage | |
| T0/T1 | 517 (55.5%) |
| T1 | 318 (34.2%) |
| T3/T4 | 79 (8.5%) |
| TX | 17 (1.8%) |
| N stage | |
| N0/N1 | 602 (64.7%) |
| N2/N3 | 329 (35.3%) |
| M stage | |
| M0 | 765 (82.2%) |
| M1 | 166 (17.8%) |
| 7th AJCC stage | |
| I | 336 (36.1%) |
| II | 390 (41.9%) |
| III | 205 (22.0%) |
| Histology | |
| Germ cell neoplasms | 901 (96.8%) |
| Trophoblastic neoplasms | 30 (3.2%) |
| Lymph-vascular Invasion | |
| Unknown | 112 (12.0%) |
| Negative | 458 (49.2%) |
| Positive | 361 (38.8%) |
| Primary site | |
| Testis, NOS | 451 (48.4%) |
| Descended testis | 480 (51.6%) |
| Pathological grade | |
| Unknown | 899 (96.6%) |
| I | 4 (0.4%) |
| II | 1 (0.1%) |
| III | 18 (1.9%) |
| IV | 9 (1.0%) |
Cutoff Values for LNR and LNC
We employed the X-tile program to determine the optimal cutoff value. For OS, the optimal cutoff value of LNR was 0.1538, with values ≤0.1538 regarded as low while those >0.1538 taken as high LNR. The optimal cutoff value of LNC was 2, with values ≤2 regarded as low while those >2 taken as high LNC. For DFS, the optimal cutoff value of LNR was the same as overall survival. The optimal cutoff value for LNC was 0, with 0 regarded as low LNC while values >0 considered high LNC.
Statistical Analysis
We performed comparisons on demographic, clinical pathologic between the LNR or LNC groups using the χ2 test. OS and DFS were estimated using the Kaplan–Meier method and compared using the log-rank test. Cox proportional hazards analysis was used to calculate hazard ratios (HRs) at a 95% confidence interval (CI) for the prognostic factors of survival outcomes. All statistical analyses were conducted using SPSS version 25 (IBM Corporation, NY, USA), with two-sided P values <0.05 considered to be statistically significant.
Results
Characteristics of the Study Cohorts
A total of 931 patients were enrolled in this study according to the eligible criteria (Figure 1). Detailed description of the demographics and clinical pathological characteristics are displayed in Table 1.
Relationship Between Lymph Node Ratio and Positive Lymph Node Counts With Overall and Cancer Specific Survival
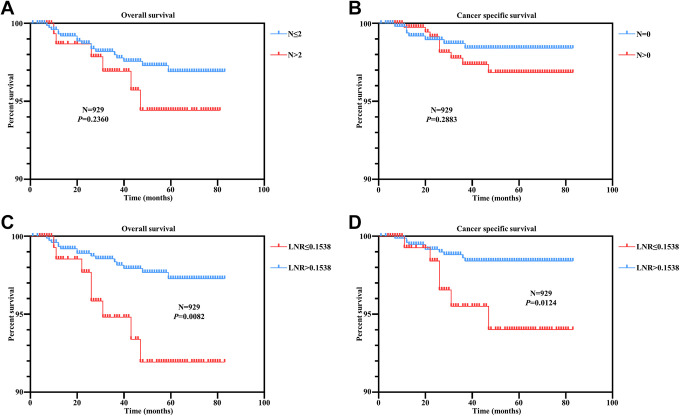
Firstly, we analyzed the relationship between LNR and LNC by constructing a scatter plot, and found that they were significantly correlated (r = 0.5610, P < 0.0001) (Figure S1). This result proved that LNR could include the most information of LNC. To evaluate the role of LNR and LNC in predicting disease prognosis respectively, we conducted Kaplan–Meier analysis for overall survival. We found no significant correlation between LNC status with overall survival time in patients (Figure 2A). On the other hand, high LNR patients had significantly shorter survival time compared to low LNR patients (Figure 2C). Furthermore, analysis of cancer specific survival revealed a consistent pattern with our overall survival results (Figure 2B and 2D). These results indicated that LNR, rather than LNC, is a promising prognostic predictor for male germ cell tumors patients.
Figure 2.
A correlation between Lymph node ratio and positive lymph node counts with overall and cancer specific survival in male germ cell tumors. (A) Overall survival analysis according to the LNC status. (B) Cancer specific survival analysis according to the LNC status. (C) Overall survival analysis according to the LNR status. (D) Cancer specific survival analysis according to the LNR status.
The Relationship Between LNR/LNC and Clinical Pathological Characteristics in Male Germ Cell Tumors
To further understand the roles of LNR and LNC status in different clinical pathological characteristics in male germ cell tumors patients, we first used the χ2 test to compare LNR status and characteristics of patients (Table 2). We found that high LNR patients tended to have significantly larger tumor sizes (χ2 = 7.877, P = 0.005), advanced T (χ2 = 13.195, P = 0.004), N stage ( χ2 = 86.775, P < 0.001), M stage (χ2 = 19.948, P < 0.001) and 7th AJCC (χ2 = 103.074, P < 0.001) stages. In addition, no significant association was observed with regard to the other characteristics. Next, we used the χ2 test to compare LNC and characteristics of patients, and found a significant association between LNC and T (χ2 = 8.799, P = 0.032), N (χ2 = 74.390, P < 0.001) and 7th AJCC (χ2 = 111.759, P < 0.001) stages (Table 2). These results demonstrated that LNR, rather than LRC, was much more closely correlated with worse clinical pathological characteristics of male germ cell tumors. Consequently, LNR represents a promising indicator for worse biological behavior in patients.
Table 2.
Correlation Between LNR/LNC and Clinical Pathology Characteristics in Germ Cell and Trophoblastic Tumors.
| Variable | LNR | χ2 | P Value | LNC | χ2 | P Value | ||
|---|---|---|---|---|---|---|---|---|
| ≤0.1538 | >0.1538 | 0-2 | >2 | |||||
| Age | ||||||||
| ≤27 | 407 (85.3%) | 70 (14.7%) | 0.750 | 0.386 | 391 (82.0%) | 86 (18.0%) | 0.489 | 0.484 |
| >27 | 378 (83.3%) | 76 (16.7%) | 380 (83.7%) | 74 (16.3%) | ||||
| Martial status | ||||||||
| Married | 257 (86.0%) | 42 (14.0%) | 5.583 | 0.134 | 253 (84.6%) | 46 (15.4%) | 3.982 | 0.263 |
| Single | 468 (82.5%) | 99 (17.5%) | 460 (81.1%) | 107 (18.9%) | ||||
| Unknown | 25 (96.2%) | 1 (3.8%) | 24 (92.3%) | 2 (7.7%) | ||||
| Separated/Divorced/Widowed | 35 (89.7%) | 4 (10.3%) | 34 (87.2%) | 5 (12.8%) | ||||
| Race | ||||||||
| White | 723 (84.6%) | 132 (15.4%) | 0.470 | 0.493 | 708 (82.8%) | 147 (17.2%) | <0.001 | 0.985 |
| Other | 62 (81.6%) | 14 (18.4%) | 63 (82.9%) | 13 (17.1%) | ||||
| Tumor size | ||||||||
| ≤4 cm | 443 (87.4%) | 64 (12.6%) | 7.877 | 0.005 | 426 (84.0%) | 81 (16.0%) | 1.144 | 0.285 |
| >4 cm | 342 (80.7%) | 82 (19.3%) | 345 (81.4%) | 79 (18.6%) | ||||
| T stage | ||||||||
| T0/T1 | 447 (86.5%) | 70 (13.5%) | 13.195 | 0.004 | 445 (86.1%) | 72 (13.9%) | 8.799 | 0.032 |
| T1 | 267 (84.0%) | 51 (16.0%) | 250 (78.6%) | 68 (21.4%) | ||||
| T3/T4 | 61 (77.2%) | 18 (22.8%) | 63 (79.7%) | 16 (20.3%) | ||||
| TX | 10 (58.8%) | 7 (41.2%) | 13 (76.5%) | 4 (23.5%) | ||||
| N stage | ||||||||
| N0/N1 | 557 (92.5%) | 45 (7.5%) | 86.775 | <0.001 | 546 (90.7%) | 56 (9.3%) | 74.390 | <0.001 |
| N2/N3 | 228 (69.3%) | 101 (30.7%) | 225 (68.4%) | 104 (31.6%) | ||||
| M stage | ||||||||
| M0 | 664 (86.8%) | 101 (13.2%) | 19.948 | <0.001 | 641 (83.8%) | 124 (16.2%) | 2.876 | 0.090 |
| M1 | 121 (72.9%) | 45 (27.1%) | 130 (78.3%) | 36 (21.7%) | ||||
| 7th AJCC stage | ||||||||
| I | 336 (100.0%) | 0 (0.0%) | 103.074 | <0.001 | 336 (100.0%) | 0 (0.0%) | 111.759 | <0.001 |
| II | 304 (77.9%) | 86 (22.1%) | 278 (71.3%) | 112 (28.7%) | ||||
| III | 145 (70.7%) | 60 (29.3%) | 157 (76.6%) | 48 (23.4%) | ||||
| Histology | ||||||||
| Germ cell neoplasms | 757 (84.0%) | 144 (16.0%) | 1.905 | 0.167 | 745 (82.7%) | 156 (17.3%) | 0.323 | 0.570 |
| Trophoblastic neoplasms | 28 (93.3%) | 2 (6.7%) | 26 (86.7%) | 4 (13.3%) | ||||
| Lymph-vascular Invasion | ||||||||
| Unknown | 98 (87.5%) | 14 (12.5%) | 3.264 | 0.196 | 94 (83.9%) | 18 (16.1%) | 1.129 | 0.569 |
| Negative | 392 (85.6%) | 66 (14.4%) | 384 (83.8%) | 74 (16.2%) | ||||
| Positive | 295 (81.7%) | 66 (18.3%) | 293 (81.2%) | 68 (18.8%) | ||||
| Primary site | ||||||||
| Testis, NOS | 376 (83.4%) | 75 (16.6%) | 0.594 | 0.441 | 379 (84.0%) | 72 (16.0%) | 0.917 | 0.338 |
| Descended testis | 409 (85.2%) | 71 (14.8%) | 392 (81.7%) | 88 (18.3%) | ||||
Overall and Cancer Specific Survival Rates in Male Germ Cell Tumors
To identify the factors that could impact both overall and cancer specific survival rates, we performed a univariate Cox proportional hazards regression analysis on the dataset. We found that age (HR, 5.169; 95% CI, 1.758-15.200; P = 0.003), AJCC stage (III vs I: HR, 9.298; 95% CI, 2.691-32.131; P < 0.001), M stage (HR, 7.897; 95% CI, 3.417-18.251; P < 0.001) and LNR (HR, 3.009; 95% CI, 1.275-7.098; P = 0.012) had an influence on the overall survival. However, LNC (HR, 1.743; 95% CI, 0.687-4.420; P = 0.242) had no impact on the overall survival (Table 3). Univariate Cox proportional hazards regression analysis for cancer specific survival also showed that age (HR, 4.621; 95% CI, 1.317-16.217; P = 0.017), race (HR, 3.846; 95% CI, 1.240-11.928; P = 0.020), AJCC stage (HR, 24.192; 95% CI, 3.164-184.982; P = 0.002), M stage (HR, 15.268; 95% CI, 4.923-47.348; P < 0.001) and LNR (HR, 3.368; 95% CI, 1.224-9.269; P = 0.019) had an influence on the survival time, while LNC (HR, 1.639; 95% CI, 0.529-5.083; P = 0.392) showed no influence (Table 3, right). Intriguingly, we found that tumor size, as well as T and N stages were not good prognostic predictors in male germ cell tumor. A possible explanation for this is that male germ cell tumor has better prognosis compared to other malignant tumors, and as such these characteristics could not provide enough information of patients. Next, we conducted multivariate Cox proportional hazards regression analysis by including age, LNR and AJCC stage in our model (Table 4). We found that age (For overall survival: HR, 5.920; 95% CI, 2.007-17.456; P = 0.001. For cancer specific survival: HR, 5.505; 95% CI, 1.563-19.385; P = 0.008) and AJCC stage (For overall survival (III vs I): HR, 8.811; 95% CI, 2.400-32.345; P = 0.001. For cancer specific survival (III vs I): HR, 23.448; 95% CI, 2.927-187.855; P = 0.003) were independent prognostic factors for both overall and cancer specific survival. However, we found that LNR (For overall survival: HR, 1.643; 95% CI, 0.667-4.046; P = 0.280. For cancer specific survival: HR, 1.513; 95% CI, 0.535-4.276; P = 0.435) was not an independent prognostic factor in our model for both overall and cancer specific survival. This result shows that our data haven’t provided enough evidence for identifying LNR as an independent prognostic factor in male germ cell tumors. Further large scale data analysis shall be done to provide more information for us to clarify the prognostic significance of LNR in male germ cell tumors. Overall, these results demonstrated that LNR, rather than LNC, was a promising prognostic factor compared to some conventional clinical pathological characteristics, such as T and N stages in male germ cell tumor.
Table 3.
Univariate Cox Regression Analysis of Overall Survival and Disease Free Survival in Germ Cell and Trophoblastic Tumors.
| Variables | Overall survival | Cancer specific survival | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P | HR | 95%CI | P | |
| Martial status | ||||||
| Single vs Married | 1.742 | 0.638-4.755 | 0.279 | 1.655 | 0.534-5.133 | 0.383 |
| Unknown vs Married | 2.824 | 0.329-24.214 | 0.344 | 0.000 | 0.000-0.000 | 0.991 |
| Separated/Divorced/Widowed vs Married | 1.379 | 0.161-11.808 | 0.769 | 0.000 | 0.000-0.000 | 0.987 |
| Age | ||||||
| >27 vs ≤27 | 5.169 | 1.758-15.200 | 0.003 | 4.621 | 1.317-16.217 | 0.017 |
| Race | ||||||
| Other vs White | 2.374 | 0.808-6.982 | 0.116 | 3.846 | 1.240-11.928 | 0.020 |
| LNC | ||||||
| >2 vs 0-2 | 1.743 | 0.687-4.420 | 0.242 | 1.639 | 0.529-5.083 | 0.392 |
| LNR | ||||||
| >0.1538 vs ≤0.1538 | 3.009 | 1.275-7.098 | 0.012 | 3.368 | 1.224-9.269 | 0.019 |
| Tumor size | ||||||
| >4 cm vs ≤4 cm | 1.857 | 0.804-4.289 | 0.148 | 1.536 | 0.572-4.123 | 0.395 |
| 7th AJCC stage | ||||||
| II vs I | 1.541 | 0.368-6.448 | 0.554 | 1.858 | 0.168-20.490 | 0.613 |
| III vs I | 9.298 | 2.691-32.131 | <0.001 | 24.192 | 3.164-184.982 | 0.002 |
| T stage | ||||||
| T2 vs T0+T1 | 1.505 | 0.612-3.705 | 0.374 | 1.252 | 0.434-3.610 | 0.677 |
| T3+T4 vs T0+T1 | 2.671 | 0.838-8.519 | 0.097 | 1.666 | 0.354-7.847 | 0.518 |
| TX vs T0+T1 | 0.000 | 0.000-0.000 | 0.980 | 0.000 | 0.000-0.000 | 0.982 |
| N stage | ||||||
| N2+N3 vs N0+N1 | 0.846 | 0.348-2.056 | 0.712 | 0.642 | 0.207 -1.991 | 0.443 |
| M stage | ||||||
| M1 vs M0 | 7.897 | 3.417-18.251 | <0.001 | 15.268 | 4.923-47.348 | <0.001 |
| Primary site | ||||||
| Descended testis vs Testis, NOS | 0.941 | 0.415-2.134 | 0.885 | 0.786 | 0.293-2.111 | 0.633 |
| Histology | ||||||
| Trophoblastic neoplasms vs Germ cell neoplasms | 2.838 | 0.665-12.108 | 0.159 | 4.221 | 0.959-18.578 | 0.057 |
| Lymph-vascular Invasion | ||||||
| Negative vs Unknown | 0.739 | 0.238-2.291 | 0.600 | 0.569 | 0.147-2.200 | 0.414 |
| Positive vs Unknown | 0.527 | 0.154 -1.800 | 0.307 | 0.599 | 0.150-2.396 | 0.469 |
Table 4.
Multivariate Cox Regression Analysis of Overall Survival and Disease Free Survival in Germ Cell and Trophoblastic Tumors.
| Variables | Overall survival | Cancer specific survival | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P | HR | 95%CI | P | |
| Age | ||||||
| >27 vs ≤27 | 5.920 | 2.007-17.456 | 0.001 | 5.505 | 1.563-19.385 | 0.008 |
| LNR | ||||||
| >0.1538 vs ≤0.1538 | 1.643 | 0.667-4.046 | 0.280 | 1.513 | 0.535-4.276 | 0.435 |
| 7th AJCC stage | ||||||
| II vs I | 1.367 | 0.318-5.879 | 0.674 | 1.682 | 0.149-18.916 | 0.674 |
| III vs I | 8.811 | 2.400-32.345 | 0.001 | 23.448 | 2.927-187.855 | 0.003 |
Discussion
Testicular cancer is the most common form of cancer diagnosed in men aged between 15 and 35 years, with germ cell tumor accounting for up to 95% of all cases.9 Testicular germ cell tumors are classified into 3 types; I, II and III on the basis of histological composition, germ cell lineage as well as age of onset.1 The survival rate in testicular germ cell tumors patients is approximately 90%, with the combination of surgery and cisplatin-based chemotherapy. However, better risk classification system, referring to patients with advanced stages and malignant phenotypes remains to be further investigated for better management and treatment of the disease in these patients.6,8 Traditional biological factors, including gene methylation, gene expression and driver gene mutations, have previously been used to predict outcomes of patients with this condition, although scientists and clinicians have recently identified some non-biological factors as independent prognostic predictors.23 Studies have shown that neutrophil-to-lymphocyte ratio,24 hospital case volume,25 patterns of care,26 insurance status27 and vitamin D status28 are significantly associated with survival time of testicular germ cell tumor patients. These non-biological factors provide unique information for precise treatment and management of patients, necessitating their utilization in the management of patients.
LNR, the ratio of positive lymph node counts among the total regional lymph nodes examined in the surgery, has been shown to have prognostic value in various cancers, including lung,29,30 bladder,31 renal,32 breast,33,34 colorectal,16 and gastric15 cancers. However, its prognostic role in testicular germ cell tumors remains unknown. In the current study, we provide the first systematic analysis of the prognostic value of LNR, relative to LNC in testicular germ cell tumors using data from SEER database. Our results showed that LNR, rather than LNC, could predict both overall and cancer specific survival time for patients. Particularly, high LNR patients had significantly shorter overall and cancer specific survival times. However, we found that LNR was not an independent prognostic factor by multivariate Cox proportional hazards regression analysis by including age, LNR and AJCC stage in our model. There are reasons leading to this result: I) there were many patients lacking regional node examination in the database, and our data only represented partial characteristics of male germ cell tumors; II) our recruitment criteria excluded patients with regional nodes examined: 0 -1, which also led to a loss of representation; III) our sample size was relatively small and limitative, which only included the patients of America from 2010 to 2015, and further large population analysis shall be done to better clarify the prognostic significance of LNR for male germ cell tumors. Another finding was that tumor size, as well as T and N stages were not significantly correlated with patients’ outcomes. This could have been due to the fact that our criteria excluded many patients, leading to a loss of representation. Another hypothesis was that tumor size, as well as T and N stages alone were not enough to represent all features of patients, thus not an appropriate prognostic factor in testicular germ cell tumors although this remains to be confirmed using studies involving larger populations. Further, we found a significant association between higher LNR with larger tumor size, advanced T, N, M and 7th AJCC stages, indicating that LNR is a good factor which could reflect the most of the clinical pathological characteristics in testicular germ cell tumors. However, we also noted that our criteria excluded many patients: Regional nodes examined: 0 -1 or Unknown, which may have led to a limitation of our population. In future, additional studies on the clinical data from other sources should be performed to support our conclusion.
In summary, our findings demonstrated that LNR, rather than LNC, is a promising prognostic factor for patients with testicular germ cell tumors. This provides a new non-biological biomarker for patients’ prognosis, which could guide future approaches for better treatment and management for testicular germ cell tumor patients.
Supplemental Material
Supplemental Material, Figure_S1 for Lymph Node Ratio Rather Than Positive Lymph Node Counts Has Better Prognostic Value in Patients With Testicular Germ Cell Tumors by Chuyang Huang, Qian Long, Yangxun Pan, Leilei Wu, Xiaonan Wang, Hailin Xu and Fufu Zheng in Technology in Cancer Research & Treatment
Acknowledgments
We thank all members of the Deng’s laboratory for their advice and technical assistance.
Authors’ Note: Chuyang Huang, Qian Long, and Yangxun Pan contributed equally to this article. CH and FZ conceived and designed the project. QL, YP, LW, XW and HX analyzed and interpreted the data. CH and FZ wrote the paper. All authors read and approved the final manuscript. All data generated or analyzed during this study are included either in this article or in the additional files. The content of this manuscript has not been previously published and is not under consideration for publication elsewhere. This article is in compliance with ethical standards.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the funds from the Natural Science Foundation of Guangdong Province (2016A030311002, 2017A030313615).
ORCID iD: Qian Long  https://orcid.org/0000-0002-0089-0862
https://orcid.org/0000-0002-0089-0862
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Cheng L, Albers P, Berney DM, et al. Testicular cancer. Nat Rev Dis Primers. 2018;4(1):29 doi:10.1038/s41572-018-0029-0 [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi:10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 3. Ghazarian AA, Kelly SP, Altekruse SF, Rosenberg PS, McGlynn KA. Future of testicular germ cell tumor incidence in the United States: forecast through 2026. Cancer. 2017;123(12):2320–2328. doi:10.1002/cncr.30597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Trabert B, Chen J, Devesa SS, Bray F, McGlynn KA. International patterns and trends in testicular cancer incidence, overall and by histologic subtype, 1973-2007. Andrology. 2015;3(1):4–12. doi:10.1111/andr.293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Allen C, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3(4):524–548. doi:10.1001/jamaoncol.2016.5688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rajpert-De Meyts E, McGlynn KA, Okamoto K, Jewett MA, Bokemeyer C. Testicular germ cell tumours. Lancet. 2016;387(10029):1762–1774. doi:10.1016/S0140-6736(15)00991-5 [DOI] [PubMed] [Google Scholar]
- 7. Hanna NH, Einhorn LH. Testicular cancer—discoveries and updates. N Engl J Med. 2014;371(21):2005–2016. doi:10.1056/NEJMra1407550 [DOI] [PubMed] [Google Scholar]
- 8. Murray MJ, Turnbull C. Testicular cancer in 2017: sequencing advances understanding. Nat Rev Urol. 2018;15(2):79–80. doi:10.1038/nrurol.2017.209 [DOI] [PubMed] [Google Scholar]
- 9. Adra N, Einhorn LH. Testicular cancer update. Clin Adv Hematol Oncol. 2017;15(5):386–396. [PubMed] [Google Scholar]
- 10. Luo S, Lobo AZ, Tanabe KK, et al. Clinical significance of microscopic melanoma metastases in the nonhottest sentinel lymph nodes. JAMA Surg. 2015;150(5):465–472. doi:10.1001/jamasurg.2014.3843 [DOI] [PubMed] [Google Scholar]
- 11. Thompson RH, Carver BS, Bosl GJ, et al. Evaluation of lymph node counts in primary retroperitoneal lymph node dissection. Cancer. 2010;116(22):5243–5250. doi:10.1002/cncr.25266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Euscher ED, Bassett R, Malpica A. Lymph node counts in endometrial cancer: expectations versus reality. Am J Surg Pathol. 2011;35(6):913–918. doi:10.1097/PAS.0b013e31821899be [DOI] [PubMed] [Google Scholar]
- 13. Zhang W, Zhangyuan G, Wang J, et al. Effect of lymph nodes count in node-positive gastric cancer. J Cancer. 2019;10(23):5646–5653. doi:10.7150/jca.30979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wong JH, Lum SS, Morgan JW. Lymph node counts as an indicator of quality at the hospital level in colorectal surgery. J Am Coll Surg. 2011;213(2):226–230. doi:10.1016/j.jamcollsurg.2011.05.003 [DOI] [PubMed] [Google Scholar]
- 15. Yamashita K, Hosoda K, Ema A, Watanabe M. Lymph node ratio as a novel and simple prognostic factor in advanced gastric cancer. Eur J Surg Oncol. 2016;42(9):1253–1260. doi:10.1016/j.ejso.2016.03.001 [DOI] [PubMed] [Google Scholar]
- 16. Ceelen W, Van Nieuwenhove Y, Pattyn P. Prognostic value of the lymph node ratio in stage III colorectal cancer: a systematic review. Ann Surg Oncol. 2010;17(11):2847–2855. doi:10.1245/s10434-010-1158 -1 [DOI] [PubMed] [Google Scholar]
- 17. Ding X, Hui Z, Dai H, et al. A proposal for combination of lymph node ratio and anatomic location of involved lymph nodes for nodal classification in non-small cell lung cancer. J Thorac Oncol. 2016;11(9):1565–1573. doi:10.1016/j.jtho.2016.05.004 [DOI] [PubMed] [Google Scholar]
- 18. Elbaiomy M, Waheed A, Elkhodary T. Prognostic value of lymph node ratio in lymph node-invaded colon cancer. Ann Oncol. 2019;30(suppl 4):iv34 doi:10.1093/annonc/mdz155.126 [Google Scholar]
- 19. Kim J, Park W, Kim JH, et al. Clinical significance of lymph-node ratio in determining supraclavicular lymph-node radiation therapy in pN1 breast cancer patients who received breast-conserving treatment (KROG 14-18): a multicenter study. Cancers (Basel). 2019;11(5). doi:10.3390/cancers11050680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cheraghlou S, Agogo GO, Girardi M. Evaluation of lymph node ratio association with long-term patient survival after surgery for node-positive Merkel cell carcinoma. JAMA Dermatol. 2019;155(7):803–811. doi:10.1001/jamadermatol.2019.0267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu L, Chen F, Chen S, Wang L. The lymph node ratio optimizes staging in patients with small intestinal neuroendocrine tumors. Neuroendocrinology. 2018;107(3):209–217. doi:10.1159/000491017 [DOI] [PubMed] [Google Scholar]
- 22. Pyo JS, Shin YM, Kang DW. Prognostic implication of metastatic lymph node ratio in colorectal cancers: comparison depending on tumor location. J Clin Med. 2019;8(11). doi:10.3390/jcm8111812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huang Y, Sheng H, Zhang J, Liu Q, Ye D, Shi G. Incorporating non-biological factors into the TNM staging system for better prognostication and decision-making in testicular cancer. World J Urol. 2019;37(10):2165–2173. doi:10.1007/s00345-018-2603-1. [DOI] [PubMed] [Google Scholar]
- 24. Tan YG, Sia J, Huang HH, Lau WKO. Neutrophil-to-lymphocyte ratio independently predicts advanced pathological staging and poorer survival outcomes in testicular cancer. Investig Clin Urol. 2019;60(3):176–183. doi:10.4111/icu.2019.60.3.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Woldu SL, Matulay JT, Clinton TN, et al. Impact of hospital case volume on testicular cancer outcomes and practice patterns. Urol Oncol. 2018;36(1):14 e7- e5 doi:10.1016/j.urolonc.2017.08.024 [DOI] [PubMed] [Google Scholar]
- 26. Stokes W, Amini A, Maroni PD, et al. Patterns of care and survival outcomes for adolescent and young adult patients with testicular seminoma in the United States: a national cancer database analysis. J Pediatr Urol. 2017;13(4):386 e1- e7 doi:10.1016/j.jpurol.2016.12.009 [DOI] [PubMed] [Google Scholar]
- 27. Kamel MH, Elfaramawi M, Jadhav S, Saafan A, Raheem OA, Davis R. Insurance status and differences in treatment and survival of testicular cancer patients. Urology. 2016;87:140–145. doi:10.1016/j.urology.2015.06.059 [DOI] [PubMed] [Google Scholar]
- 28. Schepisi G, De Padova S, Scarpi E, et al. Vitamin D status among long-term survivors of testicular cancer. Oncotarget. 2017;8(22):36780–36786. doi:10.18632/oncotarget.14167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Qiu C, Dong W, Su B, Liu Q, Du J. The prognostic value of ratio-based lymph node staging in resected non-small-cell lung cancer. J Thorac Oncol. 2013;8(4):429–435. doi:10.1097/JTO.0b013e3182829c16 [DOI] [PubMed] [Google Scholar]
- 30. Wisnivesky JP, Arciniega J, Mhango G, Mandeli J, Halm EA. Lymph node ratio as a prognostic factor in elderly patients with pathological N1 non-small cell lung cancer. Thorax. 2011;66(4):287–293. doi:10.1136/thx.2010.148601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jin S, Wang B, Zhu Y, et al. Log odds could better predict survival in muscle-invasive bladder cancer patients compared with pN and lymph node ratio. J Cancer. 2019;10(1):249–256. doi:10.7150/jca.27399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Marchioni M, Bandini M, Pompe RS, et al. The impact of lymph node dissection and positive lymph nodes on cancer-specific mortality in contemporary pT2-3 non-metastatic renal cell carcinoma treated with radical nephrectomy. BJU Int. 2018;121(3):383–392. doi:10.1111/bju.14024 [DOI] [PubMed] [Google Scholar]
- 33. Vinh-Hung V, Verkooijen HM, Fioretta G, et al. Lymph node ratio as an alternative to pN staging in node-positive breast cancer. J Clin Oncol. 2009;27(7):1062–1068. doi:10.1200/JCO.2008.18.6965 [DOI] [PubMed] [Google Scholar]
- 34. Wang L, Guyatt GH, Kennedy SA, et al. Predictors of persistent pain after breast cancer surgery: a systematic review and meta-analysis of observational studies. CMAJ. 2016;188(14): E352–E361. doi:10.1503/cmaj.151276 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, Figure_S1 for Lymph Node Ratio Rather Than Positive Lymph Node Counts Has Better Prognostic Value in Patients With Testicular Germ Cell Tumors by Chuyang Huang, Qian Long, Yangxun Pan, Leilei Wu, Xiaonan Wang, Hailin Xu and Fufu Zheng in Technology in Cancer Research & Treatment