Abstract
Purpose
To report a single-center series of patients with type B aortic dissection treated with the Multilayer Flow Modulator (MFM).
Materials and Methods
Over a 36-month period, 23 patients (median age 53 years; 20 men) with complicated type B aortic dissections (2 acute, 5 subacute, and 16 chronic) were treated with the MFM. Primary endpoints of rupture or dissection-related death, overall mortality, and reintervention were evaluated using the Kaplan-Meier method; estimates for freedom from the endpoints are reported with the 95% confidence interval (CI). Secondary outcomes included technical success, adverse events, and aortic remodeling. Clinical and imaging data were collected preoperatively, directly postoperatively, and annually to 36 months for analysis using computational fluid dynamics (CFD).
Results
Initial technical success was 91.3%. The estimates of the endpoints at 12 months were 100% for freedom from rupture or aortic-related death, 95.7% for freedom from overall mortality, and 91.3% for freedom from reintervention. No device-related neurological or systemic complications occurred, and no additional reinterventions were needed during follow-up. A total of 144 branches overstented by the MFM remained patent. Morphologic analysis of the aortic dissection showed progressive true lumen volume increase (75.9%, p<0.001) with concomitant false lumen volume decrease (42.8%, p<0.001); the CFD analyses showed increased laminar flow.
Conclusion
In the current series, the MFM provided a safe and feasible treatment option for complicated acute, subacute, and chronic type B aortic dissections, with high technical success, low mortality, and active aortic remodeling. Further studies should elucidate the long-term safety of the MFM and its effectiveness in a larger patient cohort.
Keywords: acute complicated dissection, aortic remodeling, false lumen, lumen volume, multilayer stent, true lumen, type B aortic dissection
Introduction
Despite recent developments in surgical and endovascular techniques, there is still no consensus regarding the most optimal protocol to manage different types of aortic dissections. In general current practice, the DISSECT criteria guide physicians in the treatment decision process based on 6 critical characteristics of dissections.1 In type B aortic dissections (TBAD), acute dissections are defined as within 14 days of the onset of symptoms, subacute between 2 weeks and 3 months, and chronic after 3 months.1,2 Complicated TBAD refer to the presence of rupture and/or hypotension or shock, periaortic hematoma, rapid aortic expansion, renovisceral ischemia, lower limb malperfusion, paraplegia, paraparesis, recurrent or refractory pain, and refractory hypertension despite adequate medical therapy.2
The first step in the treatment of uncomplicated acute TBAD is medical therapy to reduce blood pressure, heart rate, and pain.3 Although conservative therapy is preferred in many countries,3,4 this approach often fails to prevent aortic rupture, leading to death in 20% of patients. Up to 40% of the surviving patients suffer from progressive aneurysm formation.5 The conventional open surgical approach is associated with a high rate of complications and mortality, especially in complicated acute TBAD with involvement of the visceral branches.6,7 Therefore, in these complicated acute cases, thoracic endovascular repair (TEVAR) is increasingly performed.2
In subacute and chronic TBAD (CTBAD), which includes residual TBAD after type A dissection repair, up to 50% of patients may suffer aorta-related complications such as false lumen (FL) or aneurysm enlargement or rupture and retrograde dissection.8 Again, the primary goal of treatment is reduction of blood pressure,9 combined with statin use, antiplatelet therapy, and smoking cessation.10 If imaging shows rupture, dissection progression, or malperfusion in CTABD, emergency repair should be considered.8 Also, thoracic aortic diameters >60 mm should be considered an indication for elective surgery.2
As proposed for acute TBAD, endovascular repair and hybrid approaches provide alternatives to open surgery in subacute and CTABD cases. However, the frequency of imaging surveillance, the incidence of primary endoleaks, incomplete coverage of distally dissected segments, reintervention rates, and mortality leave room for improvement.11,12 Furthermore, long-term follow-up is lacking, and fenestrated/branched endovascular repair is limited by the high treatment costs and the need for time-consuming personalized device manufacturing, thereby excluding use in emergency situations.13
Multilayer flow-diverting aortic stents represent a relatively new concept, targeting vessel reconstruction rather than direct aneurysm or dissection exclusion. These stents can be used in acute, subacute, or chronic TBAD. The Multilayer Flow Modulator (MFM; Cardiatis, Isnes, Belgium) is a self-expanding wire mesh made of cobalt alloy and formed into a complex 3-layered web of varying porosity (up to ~65%) that modulates blood flow within the device in the aortic lumen. The first-generation MFM was received with uncertainty, as early reports highlighted stent migration.14 More recently, the MFM has been used outside the instructions for use (IFU) in individual cases of aortic dissections15 on the assumption that the MFM promotes laminar blood flow inside the stent and the dissected aorta, contributing to aortic remodeling.16,17 One key step in remodeling is obliteration of the FL, which the MFM achieves through structural support. This leads to attachment of the dissection flap to the aortic wall and modulation of flow through the true lumen (TL). Simultaneously, the open-cell design of the MFM ensures patent flow into the aortic branches.17–19
So far, there is limited experience using MFM stents in the treatment of acute, subacute, and chronic TBAD.15 The current single-center study comprised consecutive patients with an aortic dissection treated over a 3-year period using the second-generation MFM stent.
Materials and Methods
Study Design and Patient Population
A prospective single-center study was initiated in 2014 enrolling consecutive patients with acute, subacute, or chronic complicated TBAD who underwent endovascular repair using the second-generation MFM stents. Both primary TBADs and residual TBADs after surgery for type A dissections were included. As noted in the literature, residual type B dissections were grouped with the CTABD cases.
Patients were enrolled based on their clinical situation, taking into account onset of dissection, comorbidity, and dissection morphology per the DISSECT criteria.1 If patients were suitable for TEVAR, endovascular treatment using MFM was considered for the patient. Although use of the MFM for aortic dissections is outside the IFU, the same indications as for aortic aneurysms were utilized, namely (1) a minimum 2-cm landing zone in nondiseased aortic wall proximally and distally to the intended placement zone of the MFM and (2) an aneurysm diameter <65 mm (if present). No dilation outside the MFM was performed.
Authorization for this trial was issued by the Department for Clinical Trials and Medical Infrastructure of the Romanian Ministry of Health (number DM4948/37). The research was carried out in accordance with the Declaration of Helsinki (revised 1983). The implementation of the study was supervised by the ethics committee of the Lucian Blaga University of Sibiu and by the ethics committee of the Polisano European Hospital (Sibiu, Romania), where the procedures were performed. Starting from May 2018 the new rules defined in the European Union General Data Protection Regulation (GDPR) were embraced and respected within current study. All patients gave written informed consent for the procedure and use of their anonymized data for research.
Between April 2014 and February 2019, 23 patients (median age 53 years; 20 men) with complicated TBAD were enrolled. All patients presented with multiple comorbidities (Table 1) and were considered high surgical risk for the intervention. The majority of patients (22 of 23) were categorized as American Society of Anesthesiologists class III; 1 patient was class IV. Three patients had Marfan syndrome. MFM stents were deployed in 2 acute cases, 5 subacute cases, and 16 chronic cases (including 9 residual TBAD).
Table 1.
Baseline Characteristics of the 23 Patients in the Study.a
| Age, y | 53 (27–72) |
| Men | 20 |
| Current smoker | 10 |
| Hypertension | 18 |
| Diabetes | 1 |
| Dyslipidemia | 11 |
| Renal insufficiency | 3 |
| COPD | 4 |
| Previous aortic procedure | 4 |
| Bicuspid aortic valve | 2 |
| NYHA class II / III | 20 / 3 |
| LVEF, % | 60 (42–70) |
| Symptom onset (acute / subacute / chronic) | 2 / 5 / 16 |
| Primary diagnosis (TBAD / post TAAD) | 18 / 5 |
| Dissection into iliac arteries | 9 |
| Hemoglobin, g/dL | 13.1 (9.1–16.7) |
| WBC count, ×103/μL | 8.3 (5.7–15.1) |
| Platelet count, ×103/μL | 253.0 (120.0–523.0) |
| Prothrombin time, s | 87.7 (68.7–120.0) |
| CRP, mg/L | 2.3 (0.2–24.0) |
Abbreviations: COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; TAAD, type A aortic dissection; TBAD, type B aortic dissection; WBC, white blood cells.
Continuous data are presented as the median (minimum-maximum); categorical data are given as the count.
MFM Implantation
Preoperatively, computed tomography (CT) scans were assessed using EndoSize virtual deployment software (Thernava SAS, Rennes, France). MFM sizing was based on spanning the dissected aorta with overlap proximally and distally in healthy tissue. The proximal and distal landing zones and the inherent shortening of the MFM were considered in calculating the length and number of devices required for a specific case. If multiple stents were needed, the overlap of the stents was at least 6 cm for straight aortas and 8 cm for angulated aortas.
Femoral artery access was obtained via bilateral groin incisions. A 5-F pigtail catheter (Medtronic, Minneapolis, MN, USA) was gradually advanced in the TL using a torque movement, and 2 to 3 mL of 40% contrast agent were injected to ensure that the catheter was situated in the TL until the ascending aorta was reached. Resistance during guidewire navigation indicated re-entry of the catheter into the FL. Next, the pigtail catheter was replaced with a rigid 0.33-mm guidewire (Lunderquist; Cook Medical, Bloomington, IN, USA), which was positioned in the coronary sinus.
Next, the MFM stent was deployed in the TL through a 20-F introducer sheath (Sentrant; Medtronic). For multiple stents, the device with the smaller diameter was deployed first followed by the larger stent, mindful of adequate overlap. No additional steps were necessary if any major side branches arose from the FL (which is typically the case). There was no need for rapid pacing or pharmacologically controlled hypotension, regardless of the anatomical placement of the aortic stent, since the MFM is an open-cell stent that will not occlude blood flow during deployment. Following deployment of MFM stents, a compliant polyurethane aortic remodeling balloon (Reliant; Medtronic) was used to carefully affix the stent to the aortic wall. Balloon molding was performed only within the MFM.
Follow-up visits in the clinic were scheduled at 1, 3, and 6 months postoperatively and yearly thereafter. During each follow-up visit, clinical data including cardiovascular risk factors, medical history, blood values, adverse events, and high-resolution computed tomography (CT) scans were obtained.
Morphological and CFD Analysis
Morphologic and flow computational fluid dynamics (CFD) simulations were performed by 2 teams of CFD analysts following a standardized aortic dissection protocol as previously published by the current study group.20 The analysts were not involved in the clinical treatment of the patients and were not informed of the postoperative clinical outcomes. CFD simulations were performed of all CT scans preoperatively and during each follow-up visit for all included patients.
In MIMICS software (version 20.0; Materialise, Leuven, Belgium), morphologic dissection remodeling was analyzed by measuring changes in the transverse diameter of the TL and the FL [at the plane of maximum compression (PMC)], changes in volumes of the TL and the FL, and changes in the false lumen index of the volumes [FLI: FLv/(FLv+TLv)]. The PMC is a proxy for the maximum amount of compression on the TL as a consequence of the aortic dissection. The anatomical location of this plane varies from one patient to another, being situated at the inferior thoracic level in the majority of patients.20
In Ansys Workbench software (version 18.2; Ansys, Canonsburg, PA, USA), CFD flow models of all cases were compiled to assess flow and remodeling of the lumens before and after endovascular treatment using MFM stents as previously shown.20 CFD flow models were not quantitatively analyzed; however, they functioned as visualization to support the quantitative measurements.
Study Outcomes
DEFINE group definitions were used to assess clinical endpoints of this study.21 Primary endpoints at 12 months were rupture or dissection-related death, overall mortality, and reintervention. Secondary outcomes were (1) technical success (successful coverage of the entry site with the MFM, branch patency without complications at 30 days, and no procedure-related adverse events or major complications); (2) adverse events (paraplegia, stroke, upper limb ischemia, mesenteric infarction, renal failure, and reintervention); and (3) mechanical stent outcomes and aortic remodeling, including TL and FL diameters at the PMC, TL and FL volumes, and the FLI.
Statistical Analysis
Nonnormally distributed continuous data are presented as the median with the minimum-maximum range and were compared using the Mann-Whitney U test. Continuous data with a normal distribution are presented as the mean ± standard deviation and were compared using the unpaired Student t test. Categorical data are presented as the count. Interval changes were compared using a Wilcoxon signed-rank test or a paired Student t test for nonnormally and normally distributed data, respectively. There was no correction for multiple testing.
Completeness of follow-up was calculated as the ratio of total observed follow-up person-time to the potential time of follow-up of 36 months maximum in the study.22 The Kaplan-Meier method was used for time-to-event analysis of the primary endpoints. Datasets were truncated at the time point when the at-risk number fell below 10. Statistical significance was defined at p<0.05 (2 sided). Statistical analysis was performed using SPSS software (version 25.0; IBM Corporation, Armonk, NY, USA).
Results
Procedure Details
Technical success was achieved in 21 of 23 patients (91.3%). A 31-year-old hypertensive man with Marfan syndrome and a symptomatic subacute TBAD developed bilateral iliac artery occlusion perioperatively because no introducer sheath was used during successive deployment of 4 stents, which damaged the right external iliac artery. Unfortunately, the arterial lesion was unnoticed, and the patient developed acute bilateral ischemia of the limbs 18 hours postoperatively. Fogarty thrombectomy failed, and a bilateral axillofemoral bypass was performed with good clinical outcome. However, this patient developed retrograde dissection of the ascending aorta 3 months after the initial procedure and underwent a Bentall procedure. The second case without technical success involved a 42-year-old man with a symptomatic subacute TBAD who underwent a Bentall procedure 9 days after the initial MFM procedure because a guidewire had ruptured the aortic valve. This patient was not diagnosed with a known genetic vascular disease or syndrome.
A total of 79 stents were implanted (median 3 per patient, range 1–9) in a median 12.0 minutes (range 5.0–20.0) during procedures that lasted a median 80.0 minutes (50.0–300.0). In 9 cases, the first MFM was deployed immediately distally to the sinotubular junction in the ascending aorta at zone 0 (Figure 1A), completely covering the aortic arch, while in another 9 cases, the first MFM was deployed distally from the innominate artery at zone 1 (Figure 1B). In the 5 cases of residual TBAD after open ascending aorta replacement for type A aortic dissection, an endovascular elephant trunk method was used.19 In these cases, the proximal first stent was deployed within the preexistent Dacron graft and consecutive stents were placed with 6- to 8-cm overlap until reaching healthy landing zone. Stents were deployed distally from the bifurcation into bilateral iliac arteries in 9 cases total. Median overall stent length in the cohort was 461.6 mm (range 191.1–633.8).
Figure 1.
Two cases in which Multilayer Flow Modulator (MFM) devices were implanted. (A) The proximal MFM was deployed just distally to the sinotubular junction in the ascending aorta at zone 0. In this case the complete aortic arch was covered. (B) The proximal MFM was deployed from the innominate artery at zone 1 to the iliac arteries.
The MFMs covered a total of 14 innominate arteries, 18 left common carotid arteries, 22 left subclavian arteries, 44 renal arteries, 23 celiac arteries, and 23 superior mesenteric arteries. Periprocedural and 30-day survival were 100%. Median hospital stay was 4.0 days (range 3.0–6.0). Procedural data are presented in Table 2.
Table 2.
MFM Procedures.a
| Procedure time, min | 80 (50–300) |
| Device implantation time, min | 12 (5–20) |
| Fluoroscopy time, min | 25.9 (7.4–53.4) |
| Radiation, mGy | 1448 (175–5501) |
| Contrast volume, mL | 180.0 (100–500) |
| Bifemoral approach | 23/23 |
| Number of MFM devices | 3 (1–9) |
| Aortic diameter at PLZ, mm | 24.9 (23–30) |
| MFM length, mm | 461.6 (191.1–633.8) |
| Technical success | 21/23 |
Abbreviations: MFM, Multilayer Flow Modulator; PLZ, proximal landing zone.
Continuous data are presented as the median (minimum-maximum); categorical data are given as the count.
Medication and Hypertension Evolution in Follow-up
Postoperatively, all patients were prescribed beta-blockers and antiplatelet drugs. In addition, calcium channel blockers (n=15), ACE inhibitors (n=9), angiotensin II receptor blockers (n=4), central alpha-agonists (n=6), and diuretics (n=6) were prescribed.
On admission, 3 patients had systolic stage 1 (130–139 mmHg) hypertension and 11 had stage 2 (>140 mmHg). Postoperatively, 3 patients had systolic stage 1 and 5 had stage 2. At 12 months, 3 had systolic stage 1 hypertension, and none had stage 2.
Adverse Events
There was no paraplegia, upper limb ischemia, or mesenteric infarction related to the endovascular aortic repair despite covering spinal and intercostal arteries in all patients. There was no renal impairment or failure even though 44 renal arteries were completely covered by MFM stents; however, 3 patients were postoperatively at risk according to the RIFLE criteria. There was no procedure-related major stroke (despite covering 54 supra-aortic branches), but a Marfan patient suffered a minor stroke without sequelae due to spontaneous dissection of a cerebral artery. One case of reactionary pericardial effusion requiring percutaneous drainage occurred (total 3 reinterventions including the 2 technical failures). In follow-up, no additional reinterventions were needed, and no complications occurred related to progression of the disease at the latest follow-up.
Outcomes in Follow-up
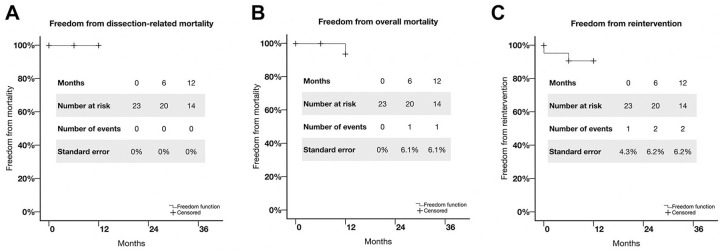
Completeness of follow-up at 12 months was 95.3%. Maximum follow-up was 36 months for 5 patients, 24 months for 4 patients, 12 months for 7 patients, and 6 months for 7 patients. Freedom from rupture or dissection-related death was 100% at 12 months (Figure 2A). Freedom from overall mortality was estimated at 95.7% at 12 months (Figure 2B). One patient died after stopping his medication and ignoring follow-up; a hypertensive crisis led to a massive intracerebral hemorrhage in the first year after the procedure. The freedom from reintervention (details below) estimate was 91.3% at 12 months (Figure 2C). Based on 5 patients at risk at 36 months, freedom from dissection-related mortality was estimated as 100%, freedom from overall mortality as 95.7% (standard error 6.1%), and freedom from reintervention as 91.3% (standard error 6.2%).
Figure 2.
Kaplan-Meier estimates of freedom from (A) dissection-related mortality, (B) overall mortality, and (C) reintervention.
Mechanical Stent Outcomes and Aortic Remodeling
In all cases, no device migration, device kinking, or fracture of the devices were observed at the latest follow-up. Morphologic 3-dimensional reconstructions of a case constructed from preoperative, first postoperative, and latest follow-up CT scans are presented in Figure 3A. The CFD velocity maps (Figure 3B) show a decrease in turbulent flow with a concomitant velocity increase in the TLs and a decrease in the FLs compared with the preoperative CT scan.
Figure 3.
(A) Morphologic reconstructions and (B) velocity maps of the patient presented in Figure 1A. (A) Three-dimensional reconstructions of the case constructed in MIMICS software. A progressive true lumen (TL) increase and concomitant false lumen (FL) decrease were observed directly postoperatively and during the latest follow-up. (B) Velocity maps of the case constructed in ANSYS software. Aside from the identical observations as in A, progressively increased laminar flow was observed in both lumens, with increased velocity directly postoperatively and during the latest follow-up.
At 12 months, there was a 221.4% increase in the TL diameter (Table 3) and a concomitant 38.0% decrease in the FL diameter at the PMC compared with preoperative dimensions (p<0.001; Figure 4). Furthermore, there was a 75.9% increase in the TL volume, a concomitant 42.8% decrease in the FL volume, and a 59.0% decrease of the FLI (p<0.001). Box plots of these outcomes are presented in Figure 5.
Table 3.
Changes in Dissection Morphology Over Time.a
| Variable | Preoperative | Postoperative Changeb | Change at 12 Monthsb |
|---|---|---|---|
| PMC TL diameter, mm | 7.0 | 14.7 [210.3% (−97.5 to 1372.4)] | 15.5 [221.4% (3 to 1395.7)] |
| PMC FL diameter, mm | 22.9 | −10.9 [−47.6% (−74.2 to 28.8)] | −8.7 [−38.0% (−100 to 37.4)] |
| TL volume, mm3 | 8.3×104 | 1.0×105 [110.8% (25.6 to 662.5)] | 6.3×104 [75.9% (36.4 to 659.4)] |
| FL volume, mm3 | 1.8×105 | −7.0×104 [−38.9% (−80.2 to 86.9)] | 7.7×104 [−42.8 (−99.8 to 55.8)] |
| TLIc | 0.22 | 0.50 [227.3% (−6.5 to 1239.0)] | 0.46 [209.1% (−8.4 to 904.6)] |
| FLIc | 0.78 | −0.50 [−64.1% (−76.4 to −17.4)] | −0.46 [−59.0% (−100.0 to −22.3)] |
Abbreviations: FL, false lumen; FLI, false lumen index; PMC, plane of maximum compression; TL, true lumen; TLI, true lumen index.
Continuous data are presented as the median [percent difference from preoperative values (range)]; FLI and TLI are based on volume measurements and are dimensionless.
p<0.001 vs preoperative.
TLI was included as a direct control for FLI: FLI=FLv/(FLv+TLv) and TLI=TLv/(FLv+TLv).
Figure 4.
The plane of maximum compression is shown in the axial slice in which the maximum compression of the true lumen (TL) is observed preoperatively. (A) In the preoperative scan, the TL (green) is collapsed by compression from the false lumen (FL; red). (B) In the first postoperative scan, the TL (green) was increased after deployment of the MFM with a concomitant decrease of the FL (red). Nonetheless, the false lumen was still larger than the TL. (C) At the latest scan at 36 months, the TL (green) nearly covered the full aortic area, while the FL (red) almost completely disappeared.
Figure 5.
Median values and error bars of the true lumen (TL) and false lumen (FL) measurements in 3-dimensional reconstructions. (A) A statistically significant increase of the TL diameter was observed at the plane of maximum compression (PMC). (B) A statistically significant increase of the TL volume was observed on the first postoperative and 12-month follow-up (FU) scans. Concomitantly, a statistically significant decrease was observed for the FL diameter at (C) the PMC. (D) The FL volume and (E) the false lumen index (FLI) at the first postoperative and latest follow-up scans. The asterisk indicates p<0.001 compared with the preoperative values.
Discussion
This study showed that the MFM could treat acute, subacute, and chronic TBAD safely, with promising midterm outcomes. As part of the MFM Global Registry, we previously presented our experience with the MFM for endovascular treatment of aortic dissection as a possible alternative to traditional stent-grafts, with low mortality and low major complications on very short-term follow-up.17–19 Multilayer devices induce modulation of blood flow, generating a positive remodeling effect on the dissected vessel. MFM laminates turbulent flow, reduces damaging peak wall stress, promotes the formation of a physiologic thrombus, and preserves branch patency without compromising flow.23,24 Furthermore, the MFM is characterized by high radial force for full-length stent expansion,24 combined with high compliance in the stented suprarenal region for better aortic elastic recoil adaptation.25
The current study represents our single-center experience after completing over 8 years of treating inoperable thoracoabdominal aneurysms and complicated aortic dissection using MFM technology.24,26 Technical success was high, but 1 patient developed a retrograde dissection of the ascending aorta 3 months after the initial procedure. Another patient required a Bentall procedure 9 days after the initial MFM procedure due to a damaged aortic valve. Both events were procedure-related at the beginning of our learning curve. No stents were accidentally placed in the FL despite not using intravascular ultrasound for TL visualization. Furthermore, no device migration, kinking, or fracture was observed, and permeability of all visceral branches was preserved.
In follow-up to 3 years, no aortic rupture or procedure-related mortality was observed and no complications arose related to progression of the disease. One Marfan patient died of a massive intracerebral hemorrhage approximately 12 months after the procedure, but he was already lost to follow-up. Recent cohort studies27–29 show that these patients have a high incidence of cardiovascular and (to a lesser extent) pulmonary comorbidity, leading to a suboptimal systemic state. Therefore, regular follow-up, strict lifestyle advice, and proper medical treatment should be carried out.
Considerable TL volume increase with concomitant FL decrease was observed. Overstented branches originating from both lumens remained patent. In the initial cases with longer follow-up, there was a higher increase in TL size and a corresponding decrease in the FL at their latest follow-up, which can be explained by progressive remodeling. Also, there was an increase in laminar flow during follow-up.
It was already well-established from a variety of studies27,30–34 that optimal medical treatment alone for acute, subacute, and chronic TBAD is considered suboptimal, and endovascular aortic intervention provides a desirable and suitable alternative. Therefore, TEVAR was proposed and studied as a treatment option. Nonetheless, stent-graft–induced new entry tears following TEVAR could lead to distal degeneration of the aortic wall, resulting in late development of new dissections and, potentially, aneurysms.35,36 One should keep in mind that TEVAR for TBAD is prone to retrograde type A aortic dissection, which is known to have disastrous outcomes. This is especially true when using proximal bare metal stents, proximal balloon dilation, or noncompliant, rigid devices.37 Suboptimal aortic remodeling with the PETTICOAT technique38–41 led to the design of the endovascular STABILISE concept in which proximal aortic endografts are combined with a distal bare metal stent. The body of literature considering the STABILISE concept to treat acute and subacute complicated TBAD is growing.42–44 Likewise, the MFM might be used within the STABILISE concept to induce laminar flow and aortic remodeling while preserving branch patency, even without proximal endografts.
Furthermore, early thoracic endografting may be considered selectively in uncomplicated acute TBAD, and promising initial results were presented in the ADSORB trial.45 In spite of favorable initial results, patients remain at risk of long-term disease progression and late complications due to the inability of these endografts to treat distally dissected aortas.46–50 In symptomatic CTABD with low surgical risk, consideration of open repair is advised. In case of contraindications for open repair, TEVAR can be considered, providing that it is performed in dedicated centers.51 In uncomplicated CTBAD with suitable anatomy, preventive TEVAR can be considered to preclude aortic complications and improve late outcomes, as shown in the INSTEAD-XL trial; however, less aortic remodeling is achieved.52–54 The extent of coverage of the dissected descending aorta seems to be related to FL thrombosis, but greater aortic coverage could increase the risk of spinal cord ischemia.55 Potentially, the coverage should be extended below the diaphragm using bare metal stents.56 This can be achieved using the MFM as well. The latest advances in off-the-shelf branched stent-grafts are promising; however, a large variety of costly off-the-shelf stent-grafts is necessary to treat aortic dissections in up to 30% of distinctive anatomical variations.57,58 In patients with Marfan syndrome, endovascular intervention is increasingly advocated59,60 and can be discussed with TBAD patients who need emergent repair. Future studies will show the exact role of TEVAR in TBAD treatment.
Aortic aneurysms were initially defined as the main indication for MFM stents, leading to approval with promising short-term results. Unfortunately, these did not hold true in the long term.24,61 Aortic aneurysms differ from aortic dissections as surgically treated aneurysms are chronic end-stage diseases in which the MFM cannot induce aortic remodeling, but might only lower the pressure on the aortic wall by redirecting the flow. Therefore, the MFM may be much more suitable for treating aortic dissections, as redirecting flow and reducing pressure play large roles in aortic remodeling. While classic endovascular solutions have shown technical difficulties related to branch vessel revascularization, MFM stents are devices that can safely treat the entire dissected aorta without the risk of losing any of the main side branches.17,19,62,63
Limitations
The current study is limited by the nonrandomized, single-center design. However, randomization would be ethically challenging because alternatives are not proven to be superior, and consensus on the gold standard seems to decline.64,65 The MFM is still a controversial device for TBAD repair. However, the outcomes are favorable and suggest that the MFM device could be considered for complicated TBAD cases.
Conclusion
This series investigating the use of the MFM for complicated TBAD proved the stent to be a safe, feasible, and effective treatment alternative with high technical success. No dissection-related death was observed, and there was a low reintervention rate, with no complication related to progression of the disease or end-organ ischemia during midterm follow-up. Despite the number of devices implanted and the high number of covered arteries, all the branches remained patent throughout follow-up. Further studies and longer follow-up are needed to confirm the role of these new-generation flow-modulating devices in the management of aortic dissection.
Acknowledgments
The authors are grateful to all the patients who participated in the current study and to all the surgical residents, scrub nurses, ward nurses, paramedics, and other individuals that took care of our patients. Furthermore, the authors would like to acknowledge Mihai Sandu and Alexandru Slavu for data analysis in MIMICS and ANSYS software and the validation of measurement methodology.
Footnotes
Authors’ Note: This study was presented at Veith Symposium (November 13–17, 2018; New York City, New York, USA) and East Meets West 2019 (September 26–28, 2019; Bucharest, Romania).
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Competitiveness Operational Programme 2014–2020, financed by the European Regional Development Fund, and by the Romanian Government under the project “Next generation computer aided research in cardiovascular disease management–NextCARDIO,” project code: COP P_37_701.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iDs: Jorn P. Meekel  https://orcid.org/0000-0002-4716-8041
https://orcid.org/0000-0002-4716-8041
Crina Solomon  https://orcid.org/0000-0002-0843-5573
https://orcid.org/0000-0002-0843-5573
Kak K. Yeung  https://orcid.org/0000-0002-8455-286X
https://orcid.org/0000-0002-8455-286X
References
- 1. Dake MD, Thompson M, van Sambeek M, et al. DISSECT: a new mnemonic-based approach to the categorization of aortic dissection. Eur J Vasc Endovasc Surg. 2013;46:175–190. [DOI] [PubMed] [Google Scholar]
- 2. Riambau V, Böckler D, Brunkwall J, et al. Editor’s choice. Management of descending thoracic aorta diseases. Eur J Vasc Endovasc Surg. 2017;53:4–52. [DOI] [PubMed] [Google Scholar]
- 3. Grabenwöger M, Alfonso F, Bachet J, et al. Thoracic endovascular aortic repair (TEVAR) for the treatment of aortic diseases: a position statement from the European Society of Cardiology (ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2012;33:1558–1563. [DOI] [PubMed] [Google Scholar]
- 4. Qin Y-L, Deng G, Li T-X, et al. Treatment of acute type-B aortic dissection: thoracic endovascular aortic repair or medical management alone? JACC Cardiovasc Interv. 2013;6:185–191. [DOI] [PubMed] [Google Scholar]
- 5. Criado FJ, Abul-Khoudoud O. Endograft repair of acute aortic dissection. Promises and challenges. J Cardiovasc Surg (Torino). 2005;46:107–112. [PubMed] [Google Scholar]
- 6. Tsai TT, Fattori R, Trimarchi S, et al. Long-term survival in patients presenting with type B acute aortic dissection: insights from the International Registry of Acute Aortic Dissection. Circulation. 2006;114:2226–2231. [DOI] [PubMed] [Google Scholar]
- 7. Pujara AC, Roselli EE, Hernandez AV, et al. Open repair of chronic distal aortic dissection in the endovascular era: implications for disease management. J Thorac Cardiovasc Surg. 2012;144:866–873. [DOI] [PubMed] [Google Scholar]
- 8. Hata M, Shiono M, Inoue T, et al. Optimal treatment of type B acute aortic dissection: long-term medical follow-up results. Ann Thorac Surg. 2003;75:1781–1784. [DOI] [PubMed] [Google Scholar]
- 9. Genoni M, Paul M, Jenni R, et al. Chronic beta-blocker therapy improves outcome and reduces treatment costs in chronic type B aortic dissection. Eur J Cardiothorac Surg. 2001;19:606–610. [DOI] [PubMed] [Google Scholar]
- 10. Onitsuka S, Akashi H, Tayama K, et al. Long-term outcome and prognostic predictors of medically treated acute type B aortic dissections. Ann Thorac Surg. 2004;78:1268–1273. [DOI] [PubMed] [Google Scholar]
- 11. Parsa CJ, Schroder JN, Daneshmand MA, et al. Midterm results for endovascular repair of complicated acute and chronic type B aortic dissection. Ann Thorac Surg. 2010;89:97–104. [DOI] [PubMed] [Google Scholar]
- 12. Nauta FJ, Trimarchi S, Kamman AV, et al. Update in the management of type B aortic dissection. Vasc Med. 2016;21:251–63. [DOI] [PubMed] [Google Scholar]
- 13. Osman E, Tan KT, Tse L, et al. The in-hospital costs of treating high-risk patients with fenestrated and branched endografts. J Vasc Surg. 2015;62:1457–1464. [DOI] [PubMed] [Google Scholar]
- 14. Lowe C, Worthington A, Serracino-Inglott F, et al. Multilayer flow-modulating stents for thoraco-abdominal and perirenal aneurysms: the UK pilot study. Eur J Vasc Endovasc Surg. 2016;51:225–231. [DOI] [PubMed] [Google Scholar]
- 15. Sultan S, Sultan M, Hynes N. Early mid-term results of the first 103 cases of multilayer flow modulator stent done under indication for use in the management of thoracoabdominal aortic pathology from the independent global MFM registry. J Cardiovasc Surg (Torino). 2014;55:21–32. [PubMed] [Google Scholar]
- 16. Chocron S, Vaislic C, Kaili D, et al. Multilayer stents in the treatment of thoraco-abdominal residual type B dissection. Interact Cardiovasc Thorac Surg. 2011;12:1057–1059. [DOI] [PubMed] [Google Scholar]
- 17. Sultan S, Kavanagh EP, Stefanov F, et al. Endovascular management of chronic symptomatic aortic dissection with the Streamliner Multilayer Flow Modulator: Twelve-month outcomes from the Global Registry. J Vasc Surg. 2017;65:940–950. [DOI] [PubMed] [Google Scholar]
- 18. Stefanov F, Sultan S, Morris L, et al. Computational fluid analysis of symptomatic chronic type B aortic dissections managed with the Streamliner Multilayer Flow Modulator. J Vasc Surg. 2017;65:951–963. [DOI] [PubMed] [Google Scholar]
- 19. Sultan S, Costache V, Ciobanu E, et al. Streamliner Multilayer Flow Modulator for subacute complicated type B dissection using the phantom technique. J Vasc Surg. 2016;63:1635–1636. [DOI] [PubMed] [Google Scholar]
- 20. Costache VS, Yeung KK, Solomon C, et al. Aortic remodeling after total endovascular aortic repair with multilayer stents: computational fluid dynamics analysis of aortic remodeling over 3 years of follow-up. J Endovasc Ther. 2018;25:760–764. [DOI] [PubMed] [Google Scholar]
- 21. Diehm N, Vermassen F, van Sambeek MRHM. Standardized definitions and clinical endpoints in trials investigating endovascular repair of aortic dissections. Eur J Vasc Endovasc Surg. 2013;46:645–650. [DOI] [PubMed] [Google Scholar]
- 22. Clark TG, Altman DG, De Stavola BL. Quantification of the completeness of follow-up. Lancet. 2002;359:1309–1310. [DOI] [PubMed] [Google Scholar]
- 23. Henry M, Polydorou A, Frid N, et al. Treatment of renal artery aneurysm with the multilayer stent. J Endovasc Ther 2008;15:231–6. [DOI] [PubMed] [Google Scholar]
- 24. Vaislic CD, Fabiani JN, Chocron S, et al. Three-year outcomes with the Multilayer Flow Modulator for repair of thoracoabdominal aneurysms: a follow-up report from the STRATO trial. J Endovasc Ther. 2016;23:762–772. [DOI] [PubMed] [Google Scholar]
- 25. Morris L, Stefanov F, Hynes N, et al. An experimental evaluation of device/arterial wall compliance mismatch for four stent-graft devices and a Multilayer Flow Modulator device for the treatment of abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2016;51:44–55. [DOI] [PubMed] [Google Scholar]
- 26. Vaislic CD, Fabiani JN, Chocron S, et al. One-year outcomes following repair of thoracoabdominal aneurysms with the Multilayer Flow Modulator: report from the STRATO trial. J Endovasc Ther. 2014;21:85–95. [DOI] [PubMed] [Google Scholar]
- 27. Durham CA, Cambria RP, Wang LJ, et al. The natural history of medically managed acute type B aortic dissection. J Vasc Surg. 2015;61:1192–1199. [DOI] [PubMed] [Google Scholar]
- 28. Wang GJ, Jackson BM, Foley PJ, et al. National trends in admissions, repair, and mortality for thoracic aortic aneurysm and type B dissection in the National Inpatient Sample. J Vasc Surg. 2018;67:1649–1658. [DOI] [PubMed] [Google Scholar]
- 29. Hosn MA, Goffredo P, Zavala J, et al. Analysis of aortic growth rates in uncomplicated type B dissection. Ann Vasc Surg. 2018;48:133–140. [DOI] [PubMed] [Google Scholar]
- 30. Fattori R, Cao P, De Rango P, et al. Interdisciplinary expert consensus document on management of type B aortic dissection. J Am Coll Cardiol. 2013;61:1661–1678. [DOI] [PubMed] [Google Scholar]
- 31. Jia X, Guo W, Li T-X, et al. The results of stent graft versus medication therapy for chronic type B dissection. J Vasc Surg. 2013;57:406–414. [DOI] [PubMed] [Google Scholar]
- 32. Durham CA, Aranson NJ, Ergul EA, et al. Aneurysmal degeneration of the thoracoabdominal aorta after medical management of type B aortic dissections. J Vasc Surg. 2015;62:900–906. [DOI] [PubMed] [Google Scholar]
- 33. Schwartz SI, Durham C, Clouse WD, et al. Predictors of late aortic intervention in patients with medically treated type B aortic dissection. J Vasc Surg. 2018;67:78–84. [DOI] [PubMed] [Google Scholar]
- 34. Cooper M, Hicks C, Ratchford EV, et al. Diagnosis and treatment of uncomplicated type B aortic dissection. Vasc Med. 2016;21:547–552. [DOI] [PubMed] [Google Scholar]
- 35. Hughes GC. Stent graft-induced new entry tear (SINE): Intentional and NOT. J Thorac Cardiovasc Surg. 2019;157:101–106.e3. [DOI] [PubMed] [Google Scholar]
- 36. Burdess A, Mani K, Tegler G, et al. Stent-graft induced new entry tears after type B aortic dissection: how to treat and how to prevent? J Cardiovasc Surg (Torino). 2018;59:789–796. [DOI] [PubMed] [Google Scholar]
- 37. Eggebrecht H, Thompson M, Rousseau H, et al. Retrograde ascending aortic dissection during or after thoracic aortic stent graft placement: insight from the European registry on endovascular aortic repair complications. Circulation. 2009;120:S276–S281. [DOI] [PubMed] [Google Scholar]
- 38. Nienaber CA, Kische S, Zeller T, et al. Provisional extension to induce complete attachment after stent-graft placement in type B aortic dissection: the PETTICOAT concept. J Endovasc Ther. 2006;13:738–746. [DOI] [PubMed] [Google Scholar]
- 39. Melissano G, Bertoglio L, Rinaldi E, et al. Volume changes in aortic true and false lumen after the ‘PETTICOAT’ procedure for type B aortic dissection. J Vasc Surg. 2012;55:641–651. [DOI] [PubMed] [Google Scholar]
- 40. Sobocinski J, Lombardi JV, Dias NV, et al. Volume analysis of true and false lumens in acute complicated type B aortic dissections after thoracic endovascular aortic repair with stent grafts alone or with a composite device design. J Vasc Surg. 2016;63:1216–1224. [DOI] [PubMed] [Google Scholar]
- 41. Antonello M, Squizzato F, Colacchio C, et al. The PETTICOAT technique for complicated acute stanford type B aortic dissection using a tapered self-expanding nitinol device as distal uncovered stent. Ann Vasc Surg. 2017;42:308–316. [DOI] [PubMed] [Google Scholar]
- 42. Mossop P, Nixon I, Oakes J, et al. Immediate ‘total’ aortic true lumen expansion in type A and B acute aortic dissection after endovascular aortic endografting and GZSD bare stenting. J Thorac Cardiovasc Surg. 2007;134:1360–1362. [DOI] [PubMed] [Google Scholar]
- 43. Hofferberth SC, Nixon IK, Boston RC, et al. Stent-assisted balloon-induced intimal disruption and relamination in aortic dissection repair: the STABILISE concept. J Thorac Cardiovasc Surg. 2014;147:1240–1245. [DOI] [PubMed] [Google Scholar]
- 44. Kahlberg A, Mascia D, Bertoglio L, et al. New technical approach for type B dissection: from the PETTICOAT to the STABILISE concept. J Cardiovasc Surg (Torino). 2019;60:281–288. [DOI] [PubMed] [Google Scholar]
- 45. Brunkwall J, Kasprzak P, Verhoeven E, et al. Endovascular repair of acute uncomplicated aortic type B dissection promotes aortic remodelling: 1 year results of the ADSORB trial. Eur J Vasc Endovasc Surg. 2014;48:285–291. [DOI] [PubMed] [Google Scholar]
- 46. Sailer AM, Nelemans PJ, Hastie TJ, et al. Prognostic significance of early aortic remodeling in acute uncomplicated type B aortic dissection and intramural hematoma. J Thorac Cardiovasc Surg. 2017;154:1192–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. van Bakel TMJ, Figueroa CA, van Herwaarden JA, et al. Commentary: Challenges of thoracic endovascular aortic repair for type B Aortic dissection. J Endovasc Ther. 2018;25:578–580. [DOI] [PubMed] [Google Scholar]
- 48. Zeeshan A, Woo EY, Bavaria JE, et al. Thoracic endovascular aortic repair for acute complicated type B aortic dissection: superiority relative to conventional open surgical and medical therapy. J Thorac Cardiovasc Surg. 2010;140:S109–S115. [DOI] [PubMed] [Google Scholar]
- 49. Verhoye JP, Miller DC, Sze D, et al. Complicated acute type B aortic dissection: midterm results of emergency endovascular stent-grafting. J Thorac Cardiovasc Surg. 2008;136:424–430. [DOI] [PubMed] [Google Scholar]
- 50. Khoynezhad A, Donayre CE, Omari BO, et al. Midterm results of endovascular treatment of complicated acute type B aortic dissection. J Thorac Cardiovasc Surg. 2009;138:625–631. [DOI] [PubMed] [Google Scholar]
- 51. Thrumurthy SG, Karthikesalingam A, Patterson BO, et al. A systematic review of mid-term outcomes of thoracic endovascular repair (TEVAR) of chronic type B aortic dissection. Eur J Vasc Endovasc Surg. 2011;42:632–647. [DOI] [PubMed] [Google Scholar]
- 52. Nienaber CA, Kische S, Rousseau H, et al. Endovascular repair of type B aortic dissection: long-term results of the randomized investigation of stent grafts in aortic dissection trial. Circ Cardiovasc Interv. 2013;6:407–416. [DOI] [PubMed] [Google Scholar]
- 53. Sueyoshi E, Sakamoto I, Hayashi K, et al. Growth rate of aortic diameter in patients with type B aortic dissection during the chronic phase. Circulation. 2004;110:II256–II261. [DOI] [PubMed] [Google Scholar]
- 54. Song J-M, Kim S-D, Kim J-H, et al. Long-term predictors of descending aorta aneurysmal change in patients with aortic dissection. J Am Coll Cardiol. 2007;50:799–804. [DOI] [PubMed] [Google Scholar]
- 55. Kang WC, Greenberg RK, Mastracci TM, et al. Endovascular repair of complicated chronic distal aortic dissections: intermediate outcomes and complications. J Thorac Cardiovasc Surg. 2011;142:1074–1083. [DOI] [PubMed] [Google Scholar]
- 56. Lombardi JV, Cambria RP, Nienaber CA, et al. Prospective multicenter clinical trial (STABLE) on the endovascular treatment of complicated type B aortic dissection using a composite device design. J Vasc Surg. 2012;55:629–640.e2. [DOI] [PubMed] [Google Scholar]
- 57. van der Weijde E, Bakker OJ, Kamman AV, et al. A feasibility study of off-the-shelf scalloped stent-grafts in acute type B aortic dissection. J Endovasc Ther. 2017;24:819–824. [DOI] [PubMed] [Google Scholar]
- 58. Magee GA, Veranyan N, Ham SW, et al. Anatomic suitability for standard, “off-the-shelf” thoracic single side-branched endograft in patients with type B aortic dissection. J Vasc Surg. 2018;68:e36–e37. [DOI] [PubMed] [Google Scholar]
- 59. Tjaden B, Azizzadeh A. Endovascular therapy in Marfan syndrome: PRO. Ann Cardiothorac Surg. 2017;6:672–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pellenc Q, Girault A, Roussel A, et al. Optimising aortic endovascular repair in patients with Marfan syndrome. Eur J Vasc Endovasc Surg. 2020;59:577–585. [DOI] [PubMed] [Google Scholar]
- 61. Ruffino MA, Rabbia C, Italian Cardiatis Registry Investigators Group. Endovascular repair of peripheral and visceral aneurysms with the Cardiatis multilayer flow modulator: one-year results from the Italian Multicenter Registry. J Endovasc Ther. 2012;19:599–610. [DOI] [PubMed] [Google Scholar]
- 62. Calik E, Erkut B. Endovascular repair of a Stanford type A dissection with the Cardiatis multilayer flow modulator. Interact Cardiovasc Thorac Surg. 2019;28:321–323. [DOI] [PubMed] [Google Scholar]
- 63. Schafigh MJ, Kohistani Z, Schiller W, et al. Retrograde Stanford type A dissection caused by a multilayer stent graft in a patient with chronic type B dissection. Interact Cardiovasc Thorac Surg. 2019;28:655–656. [DOI] [PubMed] [Google Scholar]
- 64. Nauta FJ, Trimarchi S, Kamman AV, et al. Update in the management of type B aortic dissection. Vasc Med. 2016;21:251–263. [DOI] [PubMed] [Google Scholar]
- 65. Iannuzzi JC, Stapleton SM, Bababekov YJ, et al. Favorable impact of thoracic endovascular aortic repair on survival of patients with acute uncomplicated type B aortic dissection. J Vasc Surg. 2018;68:1649–1655. [DOI] [PubMed] [Google Scholar]