Abstract
Introduction
The coronavirus, which first appeared in 2019, developed into a pandemic during 2020. It remains unclear to what extent the pandemic endangers the safety of kidney transplantation programs. In this study, we evaluated the short-term outcomes of our patients receiving a kidney transplant during the first phase and compared them with patients who received a kidney transplant immediately before the coronavirus pandemic.
Materials and Methods
Our retrospective study includes 34 kidney transplant recipients between October 1, 2019, and April 30, 2020. Nineteen patients from the phase immediately prior to the first coronavirus wave (pre-corona group), and 15 patients from the phase of the first coronavirus wave (corona group) were studied. We retrospectively evaluated demographic data, postoperative short-term outcomes and complications, immunosuppression regime, coronavirus infection status, and behavior during the first phase of the pandemic.
Results
There were no differences between the 2 groups regarding short-term outcomes and postoperative complications or in immunosuppressive medication. After the introduction of intensified hygienic conditions and routine swabs prior to transplantation, no nosocomial SARS-CoV-2 infections occurred. In the outpatient setting, none of the patients developed a SARS-CoV-2 infection. The majority of patients performed voluntary quarantine.
Conclusions
The short-term outcomes after kidney transplantation during the first phase of the coronavirus pandemic were comparable to pre-pandemic patients, and no SARS-CoV-2-associated death or transplant failure occurred in our small cohort. We considered patient compliance with hygiene and self-isolation measures very high. Nevertheless, in further phases of the pandemic, the continuation of the living kidney donation program must be critically evaluated.
Coronavirus disease 2019 (COVID-19) is caused by a very infective virus first identified in December 2019, and was declared a global pandemic by the World Health Organization on March 11, 2020 [1,2]. The global COVID-19 pandemic creates major challenges for national and international health systems. Not only must the management of the acute COVID-19 disease be organized and ensured, but other equally urgent diseases must be treated and therapies performed at the same time. In this field of conflict, it is necessary to consider for each individual patient whether therapy is necessary and whether the increased risk of becoming infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) during treatment and afterwards is justifiable. Elective therapies must also be considered in view of the limited resources of medical staff, intensive care units (ICUs), and medical equipment [3,4].
However, kidney transplantation is the most effective and cost-effective treatment for terminal renal failure [5]. Transplanted patients require lifelong immune suppression and intensive medical care, especially in the first weeks after transplantation. Initially after transplantation, patients are highly immunosuppressed, and thus, have an increased risk to viral diseases such as cytomegalovirus or Epstein-Barr virus [6,7]. Consequently, this group of recently transplanted patients also represents a high-risk group for a severe case of COVID-19 [8].
There were no national or international guidelines for kidney transplantation during the first wave of the COVID-19 pandemic. First protocols for standardized procedures in organizational and hygiene processes around organ transplantation are only in the initial phase of development [9]. The pandemic is expected to continue until the successful introduction of a vaccine. The question arises for us as a medium-volume kidney transplantation center and as a superregional COVID-19 treatment center: what are the outcomes after kidney transplantation during the pandemic? Additionally, is there an increased number of COVID-19 infections in the vulnerable first phase after kidney transplantation?
To answer these questions, we compared the patients who underwent kidney transplantation during the first wave of the COVID-19 pandemic with the patients who were transplanted just before the appearance of COVID-19 in Germany.
Material and Methods
Study Design
In Germany, the first case of SARS-CoV-2 was reported on January 24, 2020. During the pandemic period, we continued our kidney transplantation program, interrupting only the living donor kidney transplant program from mid-March 2020 until mid-May 2020. We retrospectively identified 15 recipients transplanted in this period of the first wave of the pandemic in Germany and compared them with 19 renal transplant recipients in the direct pre-corona time period (October 23, 2019-January 2020) (Fig 1 ). Follow-up was finished by the end of July 2020. The donors in our living kidney transplant program were not from prison, paid, or coerced. In all recipients, we applied immunosuppression after our standard protocol, including a triple maintenance immunotherapy with tacrolimus, mycophenolate acid, and steroids. Induction therapy was applied risk-adjusted: none, low risk as defined in a former report; basiliximab, standard risk and thymoglobulin; and immunized recipients [10]. All recipients were only treated in 2-bed rooms in our transplant ward, and only shared the room with other transplanted patients to reduce contact with other patients and staff. Visitors were restricted to 2 people per recipient, and from mid-March 2020 onward, completely prohibited. After discharge, we suggested voluntary quarantine. After the advice of the German Transplant Society, beginning March 23, 2020, all recipients were tested for SARS-CoV-2 before transplantation.
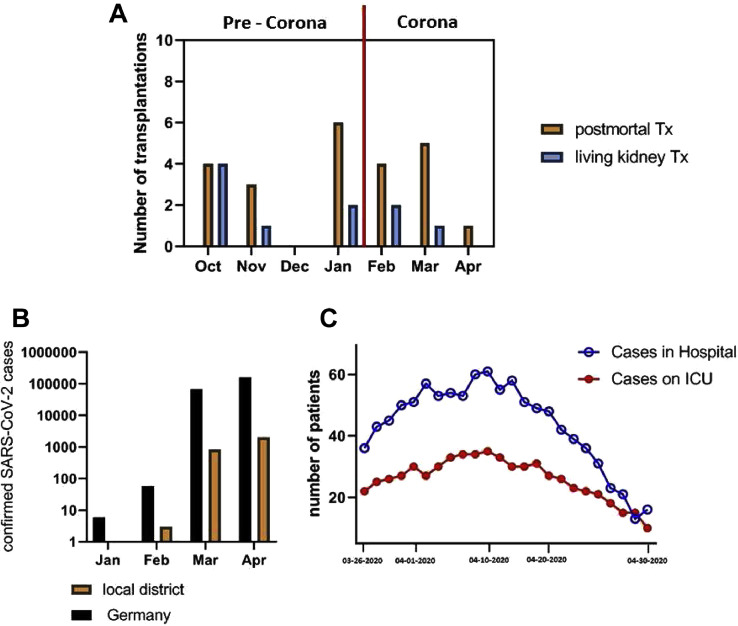
Fig 1.
(A) Kidney transplantation rates (living and postmortem) in the period before and during the first wave of the COVID-19 pandemic. (B) The number of SARS-CoV-2 infections in Germany and in our local district during the first wave. (C) The hospitality rate of SARS-CoV-2 infected patients in our hospital and in the ICU. Tx, transplantation.
The kidney was transplanted using the established technique, typically comprising of implantation into the right iliac fossa. Until postoperative day 5, all recipients received a fixed dosage of 12,500 units of heparin intravenously per day, starting 6 hours after the end of surgery.
Standard Immunosuppressive Protocol
All recipients received prednisone starting with 250 mg intraoperatively, 125 mg on the first postoperative day, and 50 mg on postoperative day 2, with a quick taper to 15 mg on day 12. Tacrolimus trough levels were targeted at 8 ng/mL to 10 ng/mL, and mycophenolate acid started with a fixed dosage of 2 g daily, taken the evening prior to transplantation. If receiving induction therapy, a dose of 20 mg of basiliximab was administered intraoperatively and on day 4. In immunized recipients, 1.5 mg/kg body weight antithymocyte globulin was administered intraoperatively.
Clinical Data Collection, Definitions, and Statistical Analysis
Clinical data were retrospectively collected from clinical records. Follow-up data were collected from the responsible nephrologists throughout Germany and from the patients themselves. Delayed graft function was defined by the need of at least 1 dialysis treatment during the first postoperative week. Postoperative complications were categorized using Clavien-Dindo Classification [11,12]. Grade I describes any deviation from the normal postoperative course without need of pharmalogical treatment. Grade II describes requiring pharmacologic treatment or blood transfusions. Grade III describes requiring surgical, endoscopic, or radiologic intervention without general anesthesia (IIIa) or with general anesthesia (IIIb). Grade IV are life-threatening complications with single-organ dysfunction, including dialysis or multiple organ dysfunction, and Grade V is the death of the patient.
If possible, data were expressed as median and 95% confidence interval. The group comparison for calculated data was performed with Mann-Whitney U test, and the analysis for categorical data with 2-tailed Fisher exact test. The statistical analysis was calculated using MedCalc version 19.0.7 (MedCalc Software LTD, Ostend, Belgium). Statistical significance was considered a P value of < .05.
Number of Treated COVID-19 Patients in our Hospital and ICU
At the peak of the first wave of the COVID-19 pandemic (March 30, 2020-April 17, 2020), our hospital treated an average of 53.6 patients, including 30.8 patients at the ICU every day throughout this time period (Fig 1C).
In our Clinic for Surgery, SARS-CoV-2 positive patients, as well as recently kidney transplanted patients, were treated on the same ward, but in separated areas with separated staff. The kidney transplanted patients were separated from all the other patients at all times. In the high phase in calendar week 19 (May 4, 2020 to May 9, 2020), 12 COVID-19 positive patients and 3 newly kidney transplanted patients were treated simultaneously in our Surgical Clinic.
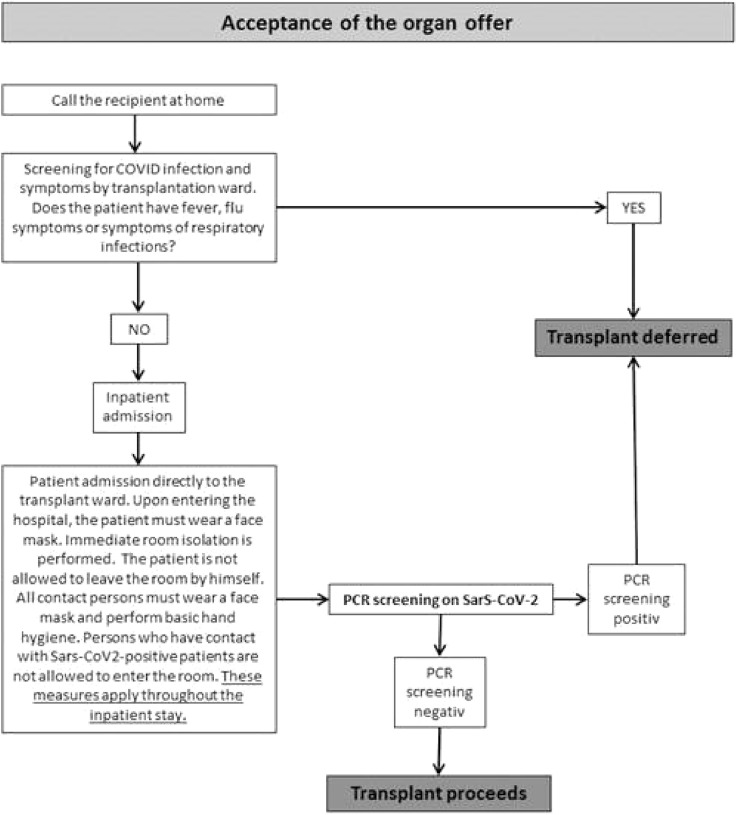
From April 1, 2020 onwards, all patients who were admitted to kidney transplantation were room-isolated and swabbed for COVID-19 before transplantation (Fig 2 ). If the swab was negative, the transplantation was performed. However, the transplanted patients were monitored directly postoperatively in our Surgical ICU, which also took care of patients with SARS-CoV-2 infection. Patients were isolated in their rooms and the nursing staff was not allowed to handle patients with COVID-19 at the same time as transplanted patients.
Fig 2.
Outpatient recipient screening and assessment algorithm during the COVID-19 pandemic in our hospital.
Results
Baseline Characteristics
The 2 patient groups did not differ statistically in the parameters collected (Table 1 ). The donor age, donor and recipient sex distribution, and donor and recipient body mass index were balanced. The ASA score (Classification of the American Society of Anesthesiologists), as a score for preoperative physical status, did not differ between the 2 groups. Both groups had the same amount of pulmonary predisposition (26.3% vs 13.3%). In the pre-corona group (pre-C), 5 patients abused nicotine, while in the corona group (C-group), 2 patients abused nicotine. In the C-group, 1 patient suffered from chronic obstructive pulmonary disease.
Table 1.
Characteristics of the Patients and Creatinine Outcome 30 Days Postoperative in the Pre-C and C-Groups
| Pre-C Group (n=19) | C-Group (n=15) | P value | |
|---|---|---|---|
| Sex donor | 10 female (52.6%) | 7 female (46.7%) | 1.000 |
| 9 male (47.4%) | 8 male (53.3%) | ||
| Age donor, y | 50.9 ± 11.8 | 54.7 ± 10.6 | .3323 |
| BMI donor | 29.2 ± 6.5 kg/m2 | 25.1 ± 10.6 kg/m2 | .0315 |
| Sex recipient | 7 female (36.8%) | 4 female (26.7%) | .7152 |
| 12 male (63.2%) | 11 male (73.3%) | ||
| Age recipient, y | 53.1 ± 12.9 | 55.4 ± 10.4 | .5710 |
| BMI recipient | 25.8 ± 4.3 kg/m2 | 25.8 ± 4.7 kg/m2 | .9667 |
| ASA-Score | 2:0 | 2:1 (6.7%) | .4412 |
| 3:17 (89.5%) | 3:12 (80.0%) | .6343 | |
| 4:2 (10.5%) | 4:2 (13.3%) | 1.000 | |
| Previous pulmonary diseases | 5 (26.3%) | 2 (13.3%) | .4263 |
| Tx-type | .4513 | ||
| Living donation | 7 (36.8%) | 3 (20%) | |
| Postmortem | 12 (63.3%) | 12 (80%) | |
| Immunosuppression with Prograf, CellCept, Decortin | 19 (100%) | 15 (100%) | 1.000 |
| Immunotherapy induction | |||
| No | 3 (15.8%) | 1 (6.7%) | .6128 |
| Simulect | 9 (47.4%) | 11 (73.3%) | .1706 |
| Antithymozytenglobulin | 6 (31.6%) | 3 (20%) | .6974 |
| Eculizumab | 1 (5.3%) | 0 | 1.000 |
| Cold ischemia time, min | 395.5 ± 260.8 | 532.2 ± 349.8 | .2007 |
| Warm ischemia time, min | 25.9±8.8 | 24.7 ± 10.7 | .7299 |
| CMV status donor | .4764 | ||
| Negative | 8 (42.1%) | 4 (26.7%) | |
| Positive | 11 (57.9%) | 11 (73.3%) | |
| CMV recipient | .4764 | ||
| Negative | 8 (42.1%) | 4 (26.7%) | |
| Positive | 11 (57.9%) | 11 (73.3%) | |
| HLA mismatch | 2.47 ± 1.31 | 2.4 ± 1.5 | .8795 |
| Panel reactive antibodies | |||
| 0-5 | 14 (73.7%) | 8 (53.3%) | .2883 |
| 6-84 | 3 (15.8%) | 6 (40.0%) | .1392 |
| ≥85 | 2 (10.5%) | 1 (6.7%) | 1.000 |
| Postoperative blood transfusion | 0.74 ± 1.19 | 0.6 ± 0.83 | .7084 |
| Creatinine in serum 30 d posttransplantation | 2.05 ± 1.99 (0.86-9.97) | 1.49 ± 0.45 (0.912.71) | .2949 |
Data are n (%). P values are estimated 2-tailed Fisher exact test for categorical data.
Abbreviations: ASA-score, Classification of the American Society of Anesthesiologists; BMI, body mass index; C-group, corona group; CMV, cytomegalovirus; pre-C group, pre-corona group; Tx, transplantation.
Immunosuppression was the same in both groups. There was also no statistical difference in induction therapy; 47.4% had basiliximab induction in the pre-C group, and 73.3% in the C-group (P = .1706). The cold and warm ischemia times also showed no significant difference, although the cold ischemia time in the pre-C group (395.5 ± 260.8 minutes) was markedly lower than in the C-group (532.2 ± 349.8 minutes). This was due to the higher rate of living donations in the pre-C group. There was also no significant differences in cytomegalovirus status (CMV status). There were 57.9% cytomegalovirus-positive recipients in the pre-C group and 73.3% in the C-group (P = .4764). Both groups had an almost identical HLA mismatch status (2.47 ± 1.31 vs 2.4 ± 1.5, P = .8795) and panel reactive antibodies were not significantly different as well.
Postoperative Outcomes and Complications
In both groups, there were no significant differences in postoperative complications and in the postoperative creatinine level (Tables 1 and 2 ). Three patients in the pre-C group suffered postoperative complications that needed intervention (Clavien-Dindo Grades IIIa and IIIb). Among those, 2 patients received surgical treatment of the lymphocele, and 1 patient showed a urine leakage which was treated with a percutaneous nephrostomy. In the C-group, 2 patients suffered from a complication that needed intervention. Two patients got a lymphocele; one of them had to be relieved laparoscopically, and 1 patient was treated with a percutaneous drainage.
Table 2.
Postoperative Complications Showed in Clavien-Dindo Classification
| Pre-C Group (n=19) | C-Group (n=15) | P value | |
|---|---|---|---|
| I-II | 15 (78.9%) | 13 (86.7%) | 1.000 |
| IIIa | 1 (5.3%) | 1 (6.7%) | 1.000 |
| IIIb | 2 (10.5%) | 1 (6.7%) | 1.000 |
| IVa | 1 (5.3%) | 0 | 1.000 |
| IVb | 0 | 0 | 1.000 |
| V | 0 | 0 | 1.000 |
Data are n (%). P values are estimated 2-tailed Fisher exact test for categorical data.
The rate of any non-surgical complications was also identical in both groups (31.6% vs 46.7%, P = .4836). In the pre-C group, 2 patients developed bacterial pneumonia and 1 patient developed a cellular rejection, which made this patient require dialysis again. Another cellular rejection was successfully treated with cortisone therapy. One patient developed esophagitis, which was also treated conservatively.
In the C-group, 3 patients developed new-onset diabetes after kidney transplantation, and 2 patients a suspected rejection, which regressed after cortisone therapy. One patient developed segmental colitis.
In both patients with COVID-19 positive swabs, prerenal kidney failure occurred (maximum creatinine 2.1 mg/dL), which was completely regressive on volume therapy. Postoperative blood transfusion was balanced in both groups as well.
Serum creatinine 30 days after transplantation was also not statistically significantly different in both groups, and was 2.05 ± 2.23 (0.86-9.97) mg/dL in the pre-C group, and 1.49 ± 0.45 (0.91-2.71) mg/dL in the C-group.
COVID-19 Status in the Cohort
In both groups, there was no significant difference in the incidence of COVID-19 (Table 3 ). Only 2 patients (13%) had COVID-19 after transplantation during their hospital stay in the C-group, but neither of the 2 patients needed ICU treatment. In both patients, the first positive swab was taken during the initial postoperative phase (12 and 25 days postoperatively), and both patients had mild pulmonary symptoms and prerenal kidney failure with a maximum creatinine level of 2.1 mg/dL.
Table 3.
Clinical Features and Outcomes After the First Wave of the COVID-19 Pandemic
| Pre-C Group (n=19) | C-Group (n=15) | P value | |
|---|---|---|---|
| COVID-19 status at the end of FU | .1872 | ||
| Negative | 19 (100%) | 13 (87%) | |
| Positive | 0 | 2 (13%) | |
| COVID-19 swab performed until the end of FU | .1392 | ||
| Yes | 3 (15.8%) | 6 (33.3%) | |
| No | 16 (84.2%) | 9 (66.7%) | |
| COVID-19 swab performed preoperatively | .0294 | ||
| Yes | 0 | 4 (26.7%) | |
| No | 19 (100%) | 11 (73.3%) | |
| Confirmed COVID-19 infection | .1872 | ||
| Yes | 0 | 2 (13.3%) | |
| No | 19 (100%) | 13 (86.7%) | |
| Stay on ICU due to COVID-19 | 0 | 0 | 1.000 |
| Voluntarily underwent home quarantine | |||
| Yes | 13 (68.4%) | 12 (80.0%) | .6974 |
| No | 6 (31.6%) | 1 (6.7%) | .1039 |
| Ordered quarantine | 0 | 2 (13.3%) | .1872 |
| Proven contact with a COVID-19 infected person | |||
| Yes | 0 | 0 | 1.000 |
| No | 19 (100%) | 15 (100%) | 1.000 |
Data are n (%). P values are estimated 2-tailed Fisher exact test for categorical data.
In total, 26% of the patients (9/34) in the whole cohort received a COVID-19 swab in the hospital or in ambulant settings. In the pre-C group, 68.4% of patients and 80.0% of patients in the C-group voluntarily underwent home quarantine till the end of follow-up. None of the patients in both groups were known to be in contact with a COVID-19 positive person.
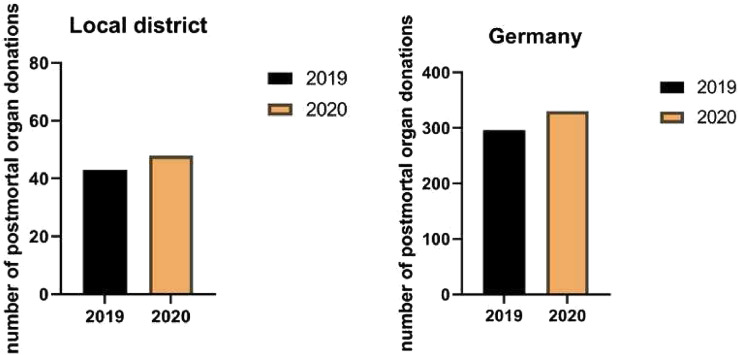
Number of Organ Donations in Baden-Württemberg and in Germany
The numbers of organ donations from January to May in 2019 and 2020 are shown in Fig 3 . Contrary to expectations, there was an increase in organ withdrawals compared with the previous year (296 vs 330 in Germany, 43 vs 48 in the local district, P = n.s.).
Fig 3.
Postmortem organ donation rates in our local district and in Germany during the first wave of the COVID-19 pandemic (P value = not significant).
Discussion
The first COVID-19 infection was detected in Germany on January 24, 2020. The impact of the SARS-CoV-2 pandemic on the health sector and kidney transplantation programs was abrupt, unpredictable, and incalculable. In addition to this challenging new situation, the initial asymptomatic phase of COVID-19 infection and the limitations in testing capacity in the early phase of the pandemic aggravated the situation [[13], [14], [15], [16]]. All these factors of unpredictability have influenced the work in our transplant center.
Although there are impressive data on the better outcomes of preemptive kidney transplantation, it was challenging for us to justify the increased risk of SARS-CoV-2 infection for both donors and recipients in the first wave of the pandemic in Germany. For this reason, in an unclear pandemic situation, we, among others, largely stopped our living donation program in the peak phase of the first pandemic wave [17,18]. An additional reason for this decision was to save as much intensive care capacity and medical staff as possible in case of an upcoming deficit. The aim of this study was to evaluate whether kidney transplantation during the first wave of the COVID-19 pandemic was associated with impaired perioperative and short-term outcomes.
Contrary to reports from France, Spain, and other parts of the world, a rapidly declining number of organ donations and transplants had been found [[17], [18], [19]]. Surprisingly, in Germany and in our district, we did not make these observations (Fig 1). Contrary to expectations, more organ donations were made in Germany and in our district in the same period as the year before. It must be said, however, that the number of organ donations in Germany has been at a historic low level in recent years, and enormous efforts have been made by politicians to improve the situation [20]. The topic of organ donation has been widely discussed publicly across various media platforms. However, it should be noted that there has been no decrease in the number of people willing to donate organs and the number of organ removals in Germany and in our district, as it continues to rise, despite the pandemic situation.
Since April 1, 2020, our clinic has carried out intensified protective interventions, including preoperative SARS-CoV-2-screenings, strict room isolation, and prohibiting visitors (Fig 2). Since the introduction of these preventive interventions, no nosocomial or early postoperative infection with SARS-CoV-2 in the clinical setting has occurred. Contrary to international literature indicating an increased mortality and risk of organ dysfunction after NTX and COVID-19 infection, all patients at our center fully recovered and regained complete graft function after initial mild prerenal renal failure without requiring ICU treatment [[21], [22], [23]]. These observations were also made by Georgiades et al [24] in the United Kingdom, among others [[24], [25], [26]]. However, it should be noted that our cohort, as well as the cohort in Georgiades et al [24], were very small, and certainly no general statements can be made. In literature, there are also numerous reports of larger cohorts that discuss a possible severe course of SARS-CoV-2 infection after transplantation [27].
Nosocomial infections are mainly caused by contact with transplanted patients with asymptomatic carriers. Adapa et al [28] also reported that patients with kidney problems and after kidney transplantation had an increased vulnerability to viral and SARS-CoV-2 infections [28]. The 2 positive patients described in our study tested positive the week before the intensified screening when a routine swab and strict room isolation was first initiated. The nosocomial path of infection in these 2 patients was not retrospectively detectable, but was probably caused by leaving the room and the transplantation area. In a review that collected intercontinental data, Kumar et al [29] discussed and positively evaluated the maintenance of transplantation programs, and recommended a hygiene concept adapted to the local situation like we had done. With the interventions we have introduced since April 1, 2020, we have successfully reduced the transmission rate, and have been able to improve patient safety from a possible COVID-19 infection.
A further aspect, which is currently highly discussed, is the question of whether in a pandemic situation immunosuppression should be modified to minimize the increased risk of a viral infection [28]. An association and consensus paper of all Italian transplant centers and other groups recommended a reduction of immunosuppression in positively tested patients [18,30]. In the 2 infected COVID-19 patients in our cohort, the immunosuppression regime was not reduced due to mild symptoms. Other centers also described a continued triple immunosuppression in infected COVID-19 patients, contrary to the recommendations in the literature [31].
The short-term results after kidney transplantation during the first phase of the COVID-19 pandemic in our hospital are comparable to the results before the pandemic, and to short-term results seen in recent literature and former studies in our center [[32], [33], [34]]. Also, we did not observe increased mortality, organ dysfunction, or infections among the transplanted patients in either group.
In the pre-C and C-group, there were no differences in terms of baseline characteristics, and the surgical and internal complications were comparable as well. Zeuschner et al [34] showed that among older kidney transplant patients, a low body mass index, hypertensive nephropathy, and coronary artery disease represents an increased risk for postoperative ICU stay, and therefore, organ transplantation should be considered in patients with these risk factors if ICU capacity is limited. Chavarot et al [35] described in their study examining whether a worse postoperative outcome was related to immunosuppression or to existing chronic diseases, that severe complications of a COVID-19 infection were more related to preexisting diseases than to chronic immunosuppression. In our cohort, we could not confirm this observation. In both groups, we had an identical cardiac preload of the patients. We did not have a single case of prolonged ICU stay or ICU resumption after discharge from postoperative monitoring. However, the significance of our observations is certainly limited by the small cohort.
In regard to postoperative complications, there were no differences between the two groups before and during the pandemic, and there was no association with a COVID-19 infection. There was also no evidence in the literature for increased surgical complications after NTX and COVID-19 infection [24].
Another question we attempted to answer in this study was whether our patients had an increased rate of COVID-19 infections in the ambulant setting after in-patient discharge. The 2 infections in our cohort were nosocomial infections in the early stages during the first wave. After the first 2 nosocomial infections, no nosocomial or ambulant infections had occurred due to strict adherence to the hygiene and self-quarantine measures of the patients. The majority of patients (68.4% in the pre-C group and 80% in the C-group) performed voluntary quarantine. Michaels et al [3] also recommended a room quarantine of the newly transplanted patients, and a mask obligation for the patients and the persons caring for them, like we did in our hospital. The consensus paper of all Italian transplant centers also recommended strict hygiene measures [18]. No patient in the pre-C group had a clinically apparent infection. A diagnostic swab was taken in 15.8% of the patients in the pre-C group and 33.3% in the C-group after surgery until June 2020. This low number of swabs taken was probably the result of a rather cautious swab policy in Germany. Due to the limited availability of polymerase chain reaction swabs during the first phase of the pandemic, only patients with typical COVID-19 symptoms and proven contact with a COVID-19 infected person were tested in Germany. Due to this constellation, the number of patients tested in our study was surprisingly low. The fact that no transplanted patient in our cohort had to report a high-risk contact shows that the patients were very sensitive to the infection risk, and generally followed the hygiene and behavior recommendations very strictly.
We conclude, like Angeletti et al [31], Zeuschner et al [34], and the consensus paper of all Italian transplant centers [18], that kidney transplantations should be maintained in any case with an increased effort in hygiene and behavioral rules for the patients and medical staff. Our data showed, despite the small number of patients, that there were no increases in postoperative complications during the pandemic. Furthermore, our data showed that the compliance of the patients with self-isolation and adherence to the hygiene rules were very high. Despite the rather positive data in this study in regard to patient compliance, it must not be forgotten that a viral infection, in combination with immunosuppression, especially SARS-CoV-2, can cause very severe and life-threatening complications. However, especially in times of a pandemic, whether or not transplantation should be carried out should be carefully discussed individually for each patient.
References
- 1.Cheng Z.J., Shan J. 2019 novel coronavirus: where we are and what we know. Infection. 2020;48:155–163. doi: 10.1007/s15010-020-01401-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization WHO director-general's opening remarks at the media briefing on COVID-19 - 11 March 2020. 2020. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 [accessed 03.12.20]
- 3.Michaels M.G., La Hoz R.M., Danziger-Isakov L., Blumberg E.A., Kumar D., Green M., et al. Coronavirus disease 2019: implications of emerging infections for transplantation. Am J Transplant. 2020;20:1768–1772. doi: 10.1111/ajt.15832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moris D., Shaw B.I., Dimitrokallis N., Barbas A.S. Organ donation during the coronavirus pandemic: an evolving saga in uncharted waters. Transpl Int. 2020;33:826–827. doi: 10.1111/tri.13614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolfe R.A., Ashby V.B., Milford E.L., Ojo A.O., Ettenger R.E., Agodoa L.Y.C., et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725–1730. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 6.Halloran P.F. Immunosuppressive drugs for kidney transplantation. N Engl J Med. 2004;351:2715–2729. doi: 10.1056/NEJMra033540. [DOI] [PubMed] [Google Scholar]
- 7.Humar A., Limaye A.P., Blumberg E.A., Hauser I.A., Vincenti F., Jardine A.G., et al. Extended valganciclovir prophylaxis in D+/R- kidney transplant recipients is associated with long-term reduction in cytomegalovirus disease: two-year results of the IMPACT study. Transplantation. 2010;90:1427–1431. doi: 10.1097/tp.0b013e3181ff1493. [DOI] [PubMed] [Google Scholar]
- 8.Marinaki S., Tsiakas S., Korogiannou M., Grigorakos K., Papalois V., Boletis I. A systematic review of COVID-19 infection in kidney transplant recipients: a universal effort to preserve patients’ lives and allografts. J Clin Med. 2020;9:2986. doi: 10.3390/jcm9092986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galvan N.T.N., Morenom N.F., Garza J.E., Bourgeois S., Hemmersbach-Miller M., Murthy B., et al. Donor and transplant candidate selection for solid organ transplantation during the COVID-19 pandemic. Am J Transplant. 2020;20:3113–3122. doi: 10.1111/ajt.16138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jänigen B.M., Hempel J., Holzner P., Schneider J., Fichtner-Feigl S., Thomusch O., et al. Simultaneous ipsilateral nephrectomy during kidney transplantation in autosomal dominant polycystic kidney disease: a matched pair analysis of 193 consecutive cases. Langenbecks Arch Surg. 2020;405:833–842. doi: 10.1007/s00423-020-01939-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clavien P.A., Sanabria J.R., Strasberg S.M. Proposed classification of complications of surgery with examples of utility in cholecystectomy. Surgery. 1992;111:518–526. [PubMed] [Google Scholar]
- 12.Dindo D., Demartines N., Clavien P.A. Classification of surgical complications. A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bai Y., Yao L., Wei T., Tian F., Jin D.-Y., Chen L., Wang M. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323:1406–1407. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan X., Chen D., Xia Y., Wu X., Li T., Ou X., et al. Asymptomatic cases in a family cluster with SARS-CoV-2 infection. Lancet Infect Dis. 2020;20:410–411. doi: 10.1016/S1473-3099(20)30114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K.W., et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25:2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martino F., Plebani M., Ronco C. Kidney transplant programmes during the COVID-19 pandemic. Lancet Respir Med. 2020;8:e39. doi: 10.1016/S2213-2600(20)30182-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellini M.I., Tortorici F., Capogni M. Kidney transplantation and the lockdown effect. Transpl Int. 2020;33:1142–1143. doi: 10.1111/tri.13639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vistoli F., Furian L., Maggiore U., Caldara R., Cantaluppi V., Ferraresso M., et al. COVID-19 and kidney transplantation: an Italian survey and consensus. J Nephrol. 2020;33:667–680. doi: 10.1007/s40620-020-00755-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loupy A., Aubert O., Reese P.P., Bastien O., Bayer F., Jacquelinet C. Organ procurement and transplantation during the COVID-19 pandemic. Lancet. 2020;396:1395–1396. doi: 10.1016/S0140-6736(20)31040-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eurotransplant Statistics report library. https://statistics.eurotransplant.org/index.php?search_type=donors&search_organ=&search_region=Germany&search_period=2019&search_characteristic=&search_text=&search_collection= accessed 14.01.2021.
- 21.Akalin E., Azzi Y., Bartash R. Covid-19 and kidney transplantation. N Engl J Med. 2020;382:2475–2477. doi: 10.1056/NEJMc2011117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banerjee D., Popoola J., Shah S. COVID-19 infection in kidney transplant recipients. Kidney Int. 2020;97:1076–1082. doi: 10.1016/j.kint.2020.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Columbia University Kidney Transplant Program Early description of coronavirus 2019 disease in kidney transplant recipients in New York. J Am Soc Nephrol. 2020;31:1150–1156. doi: 10.1681/ASN.2020030375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Georgiades F., Summers D.M., Butler A.J., Russell N.K.I., Clatworthy M.R., Torpey N. Renal transplantation during the SARS-CoV-2 pandemic in the UK: experience from a large-volume center. Clin Transplant. 2021;35 doi: 10.1111/ctr.14150. [DOI] [PubMed] [Google Scholar]
- 25.Kolonko A, Dudzicz S, Wiecek A, Król R. COVID-19 infection in solid organ transplant recipients: a single-center experience with patients immediately after transplantation [e-pub ahead of print]. Transpl Infect Dis https://doi.org/10.1111/tid.13381, accessed February 2, 2021. [DOI] [PMC free article] [PubMed]
- 26.Arpali E., Akyollu B., Yelken B., Tekin S., Turkmen A., Kocak B. Case report: a kidney transplant patient with mild COVID-19. Transpl Infect Dis. 2020;22 doi: 10.1111/tid.13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caillard S, Chavarot N, Francois H, Matignon M, Greze C, Kamar N, et al. Is covid-19 infection more severe in kidney transplant recipients? [e-pub ahead of print]. Am J Transplant https://doi.org/10.1111/ajt.16424, accessed February 2, 2021. [DOI] [PMC free article] [PubMed]
- 28.Adapa S., Chenna A., Balla M., Merugu G.P., Koduri N.M., Daggubati S.R., et al. COVID-19 pandemic causing acute kidney injury and impact on patients with chronic kidney disease and renal transplantation. J Clin Med Res. 2020;12:352–361. doi: 10.14740/jocmr4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar D., Manuel O., Natori Y., Egawa H., Grossi P., Han S.H., et al. COVID-19: a global transplant perspective on successfully navigating a pandemic. Am J Transplant. 2020;20:1773–1779. doi: 10.1111/ajt.15876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoek R.A., Manintveld O.C., Betjes M.G., Hellemons M.E., Seghers L., Van Kampen J.A., et al. COVID-19 in solid organ transplant recipients: a single-center experience. Transpl Int. 2020;33:1099–1105. doi: 10.1111/tri.13662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Angeletti A., Trivelli A., Magnasco A., Drovandi S., Sanguineri F., Santaniello M., et al. Risk of COVID-19 in young kidney transplant recipients. Results from a single-center observational study. Clin Transplant. 2020;34 doi: 10.1111/ctr.13889. [DOI] [PubMed] [Google Scholar]
- 32.Jänigen B.M., Salabè C., Glatz T., Thomusch O., Lässle C., Fichtner-Feigl S., et al. Single cohort study: ABO-incompatible kidney transplant recipients have a higher risk of lymphocele formation. Langenbecks Arch Surg. 2019;404:999–1007. doi: 10.1007/s00423-019-01812-y. [DOI] [PubMed] [Google Scholar]
- 33.Veroux M., Zerbo D., Basile G., Gozzo C., Sinagra N., Giaquinta A., et al. Simultaneous native nephrectomy and kidney transplantation in patients with autosomal dominant polycystic kidney disease. PLoS One. 2016;11 doi: 10.1371/journal.pone.0155481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeuschner P., Sester U., Stöckle M., Saar M., Zompolas I., El-Bandar N., et al. Should we perform old-for-old kidney transplantation during the COVID-19 pandemic? The risk for post-operative intensive stay. J Clin Med. 2020;9:1835. doi: 10.3390/jcm9061835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chavarot N., Gueguen J., Bonnet G., Jdidou M., Trimaille A., Burger C., et al. COVID-19 severity in kidney transplant recipients is similar to nontransplant patients with similar comorbidities. Am J Transplant. 2020;00:1–10. doi: 10.1111/ajt.16416. [DOI] [PMC free article] [PubMed] [Google Scholar]