Abstract
We report the case of a 40-year-old female diagnosed with COVID-19 after presenting to our institution with fever, cough and myalgia for three days. Her nasopharyngeal swab tested positive for SARS COV-2 by real time PCR and her plain chest radiograph was reported as normal. She did not require hospitalization and at telephone follow up she confirmed her illness lasted 11 days. Seven weeks later she presented with chest pain, dyspnea and fever for two days. Her repeat chest imaging showed right upper zone consolidation and this culminated in a microbiological diagnosis of pulmonary tuberculosis. The patient's daughter had been treated for tuberculosis two years earlier and unfortunately family screening for latent TB was not undertaken.
This case appears to confirm the concerns that the CD4+ T-cell depletion associated with COVID-19 may promote the development of active tuberculosis from latent infection much like HIV does. If this effect is widespread it may have a significant impact on the worldwide TB burden. We suggest vigilance to ensure patients are diagnosed early and meticulous contact tracing is undertaken to treat those with latent tuberculosis.
Keywords: COVID-19, Mycobacterium tuberculosis, Latent TB, SARS-COV 2
1. Introduction
One third of the world population is latently infected with Mycobacterium Tuberculosis (MTb) [1],but the majority of infected individuals develop protective immunity, which contains Mtb in a T cell-dependent manner. Progression to tuberculosis disease (TB) results from interactions between the environment, host and the pathogen. An effective T cell response determines whether the infection resolves, remains dormant or develops into clinically evident disease.
Several studies have demonstrated that CD4+ T cells play an essential role in defence against Mtb [2,3]as supported by the evidence that CD4+ T cell depletion is responsible for Mtb reactivation in HIV-infected individuals. Restoration of CD4 by anti-retrovirals has been shown to reduce the risk of TB [4,5].CD8+ T cells may also play a role in control of MTb infection [2].
In a study from Wuhan, 76% of 522 COVID-19 patients had significant depletion in their T-cell lymphocyte counts [6].Both CD4 and CD8 counts were severely reduced, and the surviving T cells appeared to demonstrate “functional exhaustion”. This T cell depletion and dysfunction may promote the development of active TB in patients with LTBI.
In acute infection of SARS-cov-1, a reduction in peripheral lymphocyte especially T lymphocyte was observed. CD8+ T lymphocytes took 2–3 months to recover and CD4+ T lymphocytes may take up to a year [7]. Hypothetically SARS COV 2 might share these changes.
2. Case
A 40-year-old female presented to the primary care physician with fever, cough and body aches. She had close contact with a COVID-19 patient three days prior to her presentation. On examination:
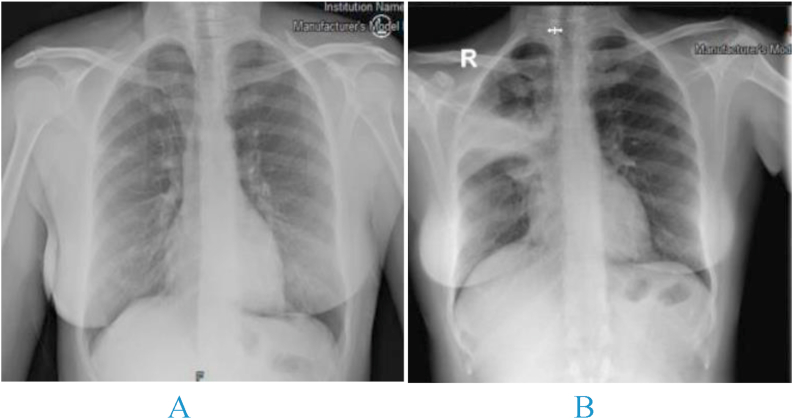
Her oxygen saturations were 98% on ambient air. Other vital signs were also within normal limits. Her nasopharyngeal swab for COVID-19 PCR was positive. Chest radiograph was reported as normal (Fig. 1). The patient was discharged home and treated conservatively. At telephone follow up on she reported that her symptoms lasted 11 days.
Fig. 1.
A) initial chest x-ray time of diagnosis with COVID-19. B) Chest x-ray at second presentation.
Seven weeks later she presented again with a two-day history of right-side pleuritic chest pain, cough, subjective fever and anorexia. Her daughter was treated for fully sensitive pulmonary tuberculosis two years previously. Unfortunately, the family did not undergo screening or receive treatment for latent tuberculosis infection (LTBI). She denied chronic respiratory symptoms. She is a housewife with no environmental exposures of note and no cigarette smoking or alcohol intake. There were no other relevant points in her history.
Her vital signs were within normal limits and her clinical examination was unremarkable with the exception of coarse crackles in the right upper zone on chest auscultation.
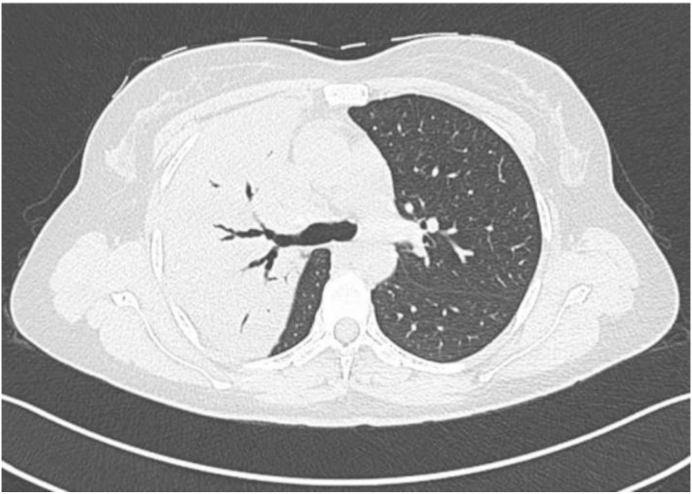
Chest radiograph showed right upper zone opacification and CT confirmed dense consolidation in the anterior and posterior segments of the right upper lobe (Fig. 2). Sputum for MTB PCR was positive with no rifampicin resistance detected. Sputum was negative on AFB staining and culture was positive for TB in the third week. Drug susceptibility testing showed pan-sensitivity. Blood test results were unremarkable with the exception of a C-reactive protein of 60 mg/l. HIV and hepatitis serology were negative.
Fig. 2.
Axial lung window showing dense consolidation of the anterior and posterior segments of the RUL.
3. Discussion
To the best of our knowledge this is the first convincing report of a patient with LTBI who seems to have progressed to active TB following COVID-19. There are several reports and case series of co-infection with TB and COVID-19 including a cohort of cases reported by Tadolini et al. [8] In their study there were 14 patients who had COVID-19 prior to TB, however the diagnosis of COVID-19 preceded that of TB by a median (range) time of 4 (2–10) days. They were therefore unable to comment on the contribution of COVID-19 to the TB diagnosis. They felt that it was the overlap of symptoms between the two conditions that led to the diagnosis of TB. In our case however, the relatively normal chest radiograph obtained during the patient's COVID-19 presentation and the acute onset of symptoms at her second presentation suggest that the onset of active TB was curiously acute. It is noteworthy that two years had lapsed between the contact with TB and this is usually associated with a lower risk of re-activation. It is also interesting that the pattern of TB disease in our patient is unusual with dense consolidation affecting both anterior and posterior segments of the right upper lobe with no cavitation. HIV-seropositive patients with TB are known to present without cavitation which requires vigorous lymphocyte reactivity to the Mtb antigen [9]. This unusual presentation lends weight to the argument that the T lymphocyte depletion and dysfunction associated with COVID-19 may predispose to re-activation of TB.
The COVID-19 pandemic is likely to impact TB burden in a number of ways. It has affected patients on TB treatment due to reduced outpatient consultations and follow up. Although lockdown, social distancing and the widespread use of face masks are also likely to reduce transmission of TB amongst non-household contacts, spread to family may potentially increase. As both COVID-19 and TB spread easily among household contacts and given the potential for prolonged T cell depletion seen post-COVID-19, there may be rapid progression to active TB in household contacts newly infected with MTb who have recently had the SARS-Cov-2 infection.
4. Conclusion
This case appears to suggest that the CD4+ T-cell depletion associated with COVID-19 may be implicated in the progression of LTBI to active TB in a similar manner to HIV. The presentation of TB in these patients may also be atypical however further studies are needed to explore this observation. Given that a significant proportion of the world population has LTBI, the COVID-19 pandemic may lead to a spike in the incidence of active TB. We suggest a preemptive approach to the diagnosis of TB given the possibility of an atypical presentation. Early identification of patients with TB and subsequent contact tracing is essential to help control the spread of TB.
Author contributions
Mohammed Khayat wrote drafting and manuscript. Hanan Fan reviewed the manuscript. Yusuf Vali was responsible for final approval of the version to be submitted.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
Authors declare that they have no competing of interests.
References
- 1.Houben R.M.G.J., Dodd P.J. The global burden of latent tuberculosis infection: a Re-estimation using mathematical modelling. PLoS Med. Oct. 2016;13(10) doi: 10.1371/journal.pmed.1002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behar S.M. 2013. Antigen-Specific CD8+ T Cells and Protective Immunity to Tuberculosis; pp. 141–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewinsohn D.M. Human purified protein derivative-specific CD4+ T cells use both CD95-dependent and CD95-independent cytolytic mechanisms. J. Immunol. Mar. 1998;160(5):2374. http://www.jimmunol.org/content/160/5/2374.abstract [Online]. Available. [PubMed] [Google Scholar]
- 4.Kwan C.K., Ernst J.D. HIV and tuberculosis: a deadly human syndemic. Clin. Microbiol. Rev. Apr. 2011;24(2):351–376. doi: 10.1128/CMR.00042-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suthar A.B. Antiretroviral therapy for prevention of tuberculosis in adults with HIV: a systematic review and meta-analysis. PLoS Med. Jul. 2012;9(7) doi: 10.1371/journal.pmed.1001270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diao B. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) Front. Immunol. May 2020;11 doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin L., Lu L., Cao W., Li T. “Hypothesis for potential pathogenesis of SARS-CoV-2 infection–a review of immune changes in patients with viral pneumonia. Emerg. Microb. Infect. Jan. 2020;9(1):727–732. doi: 10.1080/22221751.2020.1746199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tadolini M. Active tuberculosis, sequelae and COVID-19 co-infection: first cohort of 49 cases. Eur. Respir. J. Jul. 2020;56(1):2001398. doi: 10.1183/13993003.01398-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaisson R.E., Schecter G.F., Theuer C.P., Rutherford G.W., Echenberg D.F., Hopewell P.C. Tuberculosis in patients with the acquired immunodeficiency syndrome: clinical features, response to therapy, and survival. Am. Rev. Respir. Dis. Aug. 1987;136(3):570–574. doi: 10.1164/ajrccm/136.3.570. [DOI] [PubMed] [Google Scholar]