Abstract
Background
Coronavirus disease (COVID-19) infection represents a worldwide critical health burden from the sanitary perspective. This disease's symptoms range from a mild flu-like form to a severe life-threatening respiratory disease and respiratory failure. Several patients, however, remain paucisymptomatic. Among the symptoms that seem relevant are the changes in taste and smell, regardless of the disease's severity.
Methods
Data from patients affected by COVID-19 infection, hospitalized from 15 to 29 April, 2020, were analyzed. Questionnaires about smell, taste, and nasal function were administered to all, and a proportion also received the Quick olfactory Sniffin’ Sticks Test (q-Sticks) to objectivate the presence of anosmia or hyposmia. The results of instruments and Q-Sticks were then compared.
Results
Thirty-seven patients (20 males, 54.1%), with a mean age 0f 69.19 years (SD = 17.96; median 76, IQR: 63–82) were evaluated. Among the patients, 8 (22%) were asymptomatic. Out of the remaining 29 patients, 28 (97%) had fever, 19 (66%) asthenia, 11 (38%) dry cough, 10 (34%) dyspnea, and 6 (21%) gastroenteric symptoms. The q-Sticks test was performed on 27 patients and showed that 6 with anosmia, and 16 patients had hyposmia, where only 5 (14%) patients complained of loss of smell by conducting the questionnaires.
Conclusion
Although olfactory disturbances may be secondary to other factors, a sudden onset of anosmia or hyposmia should be assessed as a possible symptom of COVID-19 infection. The use of questionnaires or anamnestic collection is sometimes not enough, while adding to them a simple test such as the q-Sticks test can provide more accurate and reliable data. A simple, easy-to-perform, and reliable tool (q-Sticks) for olfactory disorders assessment can be administered to identify the real size of anosmia in patients with COVID-19 infection and detect the early stage of infection or paucisymptomatic patients, therefore becoming important to reduce the spreading of the pandemic.
Keywords: COVID-19, Anosmia, Hyposmia, ENT, Q-stick test
Abbreviations: ACE-2, angiotensin-converting enzyme 2; COVID-19, coronavirus disease 2019; UPSIT, University of Pennsylvania Smell Identification Test
Introduction
Since the end of 2019, coronavirus disease 2019 (COVID-19) infection, caused by a new type of coronavirus (SARS-CoV-2), has affected people from different countries, representing a major health problem worldwide and becoming a pandemic.1 The most common symptoms of this disease are fever, cough, and dyspnea. In several cases, the disease's severity leads to hospitalization, different treatment intensity regimes, and critical care unit admissions2 due to severe respiratory illnesses; however, the majority show mild-to-moderate symptoms or remain asymptomatic.3 The minor symptoms reported are fatigue, myalgia, arthralgia, headache, diarrhea, sore throat, and sudden loss of taste, olfactory, and smell function.4 Anosmia and ageusia are reported between 34% and 68% of COVID-19 positive patients in the recently published surveys from Germany, Great Britain, Iran, Italy, and the United States.5, 6, 7, 8, 9 A multicenter European study reported that 85.6% of patients had an olfactory dysfunction related to the infection.10 Moein et al used The University of Pennsylvania Smell Identification Test (UPSIT) to test the olfactory quality and reported smell dysfunctions in 98% of positive COVID-19 patients.11
According to the aforementioned studies, smell dysfunction seems to be strongly associated with COVID-19 infection. Therefore, in this pandemic era, the search for COVID-19 appears meaningful in the differential diagnosis of patients who complain of sudden onset of anosmia or ageusia. Moreover, it is important to point out asymptomatic patients, thereby reducing the risk of infection transmission.12
The aim of the present work was to identify a simple, easy-to-perform, and reliable tool for identifying olfactory disorders in COVID-19 positive patients. Generally, anosmia is reported by patients or by administering specific olfactory sticks with different pure smells to confirm the diagnosis.
We tested 27 patients with Hummel's quick olfactory Quick olfactory Sniffin’Sticks Test (q-Sticks), which is based on the recognition of 3 odors.13 Q-Sticks is simple to apply and a fast test to do, with 96% specificity in differentiation between anosmia, hyposmia, and normosmia.13 We compared the result of q-Sticks to a questionnaire about smell capability to observe if there is a difference between the patient's subjective perception and an objective test for anosmia in patients with COVID-19, and possibly hypothesize the use of olfactory tests as early screening in suspected subjects.
Patients and methods
Patients aged ≥18 years, hospitalized in Allergy and Respiratory Clinic due to COVID-19 infection from 15 to 29 April, 2020, were evaluated for inclusion. All patients had a positive rt-PCR COVID test from the rhino-pharyngeal swab. The characteristics of the onset of symptoms were deduced by the clinical diary. For the classification of patients in the 3 categories (normal, anosmia, and hyposmia), we considered the clinical history and the results of a submitted questionnaire. We confirmed the results with the q-Sticks test and excluded those who already had anosmia before COVID-19 infection. Only patients with anosmia and hyposmia were included in the analysis.
A self-made, non-validated questionnaire (repository table 1) to evaluate smell and taste perception subjectively was administered in the period of COVID-19 infection. In this questionnaire, the possible appearance of alterations and characteristics of duration and intensity of smell was linked to certain odors and tastes. The quick olfactory tests, used for the trial, were not natural, and the sticks commercialized by MediSense (Admiraal de Ruyterlaan 5, GN Groningen, The Netherlands) were used. Four pure odors samples (fish, coffee, clove, and rose) were used to have an objective evaluation of smell perception. The 3 odors (coffee, clove, and rose) to be tested were selected based on data from other studies14,15 and being discernible regardless of age and ethnicity. Fish was added as a well recognizable and particularly strong sample. During smell administration, the scent was set at a distance of 1–2. cm from the two nostrils for about 3 s. The capability to distinguishing different smells was evaluated for the 4 scents using 4 direct questions about the possible choice for each stick. The interval between smell introductions was of at least 30s.
Categorical variables were expressed as counts and percentages. Continuous variables were summarized using mean and standard deviation (SD) and median and interquartile range (IQR). Data were analyzed using Excel (Microsoft) by the t-student test, Chi-Square test, or Fisher's exact test when necessary, assuming p < 0.05 as significant value.
Results
A total of 37 patients were enrolled in the study, 20 men (54.1%) and 17 women (45.9%), with a mean age (SD) of 69.19 (17.96) and median (IQR) of 76 (63–82) years. The q-Sticks was carried out in 27 patients, and the remaining declined to participate.
Regarding COVID-19 presentation in the whole cohort of observed patients, 8 (22%) were asymptomatic at the beginning of the disease and hospitalized for other reasons. Subsequently, they were tested positive for COVID-19 following observation of unexpected desaturation, imaging, or hematological findings suggesting alterations during hospitalization. Of the remaining 29 patients, 28 (97%) had a fever, 19 (66%) complained of asthenia, 11 (38%) dry cough, ten (34%) dyspnea, and 6 (21%) gastroenteric symptoms such as nausea, vomiting or diarrhea. The main comorbidities were hypertension in 21 (57%), diabetes in 15 (41%), COPD in 5 (14%), and cancer in 2 (5%) patients (several of them have more than 1 comorbidity). The main otorhinolaryngological comorbidities were chronic rhino-sinusitis in eight (22%). Anosmia and hyposmia were already present in 4 (11%) patients and ageusia in 4 patients (11%) due to past ischemic stroke (Table 1). Based on questionnaires, an additional 5 (14%) patients complained of loss of smell and 11 (30%) of taste, particularly to savory and sour flavors after COVID-19 infection.
Table 1.
Comparison between patients with anosmia and hyposmia.
| Total (37) | Q-Sniff tested patients (27) |
p-valueb | |||
|---|---|---|---|---|---|
| Anosmia(6)a | Hyposmia (16)a | Normal (5)a | |||
| Age (SD) | 69 (18) | 65 (23) | 78 (13) | 64 (23) | n.s. |
| Male | 20 (54) | 1 (17) | 9 (56) | 3 (60) | n.s. |
| Smokersb | 11 (30) | 2 (33) | 4 (25) | 2 (40) | n.s. |
| Symptomatic | 29 (78) | 6 (100) | 10 (63) | 4 (80) | n.s. |
| Fever | 28 (76) | 5 (83) | 10 (63) | 4 (80) | n.s. |
| Cough | 11 (30) | 1 (17) | 3 (19) | 3 (60) | n.s. |
| Dyspnoea | 10 (27) | 1 (17) | 4 (25) | 2 (40) | n.s. |
| Gastro-enteric symptoms | 6 (16) | 1 (17) | 2 (13) | 1 (20) | n.s. |
| Asthenia | 21 (57) | 5 (83) | 8 (50) | 1 (20) | n.s. |
| DM | 15 (41) | 2 (33) | 8 (50) | 2 (40) | n.s. |
| COPD | 5 (14) | 2 (33) | 2 (13) | 1 (20) | n.s. |
| Hypertension | 21 (57) | 4 (67) | 9 (56) | 2 (40) | n.s. |
| Prednisone | 24 (65) | 5 (83) | 12 (75) | 0 | n.s. |
| Heparin | 29 (78) | 4 (67) | 16 (100) | 2 (40) | n.s. |
| Subjective smell alteration | 5 (14) | 1 (17) | 1 (6) | 3 (60) | n.s. |
| Subjective taste alteration | 11 (30) | 3 (50) | 2 (13) | 3 (60) | n.s. |
| RS comorbidities | 15 (41) | 2 (33) | 6 (38) | 1 (40) | n.s. |
| CRS | 7 (19) | 1 (17) | 4 (25) | 2 (40) | n.s. |
| Nasal Obstruction+ | 1 (3) | 0 | 0 | 0 | n.s. |
| Rhinorrhea+ | 1 (3) | 1 (17) | 0 | 0 | n.s. |
All data are expressed in absolute number and percentage (%) where not otherwise reported.
Patients in this category are the only one who has been tested with q-Sticks test, excluded the one with anosmia, before COVID-19 infection. + ENT symptoms occurred after COVID-19 infection. § analysis about group with anosmia and hyposmia.
Current of former smokers. P-value significance if < 0.05, n. s. = not significant
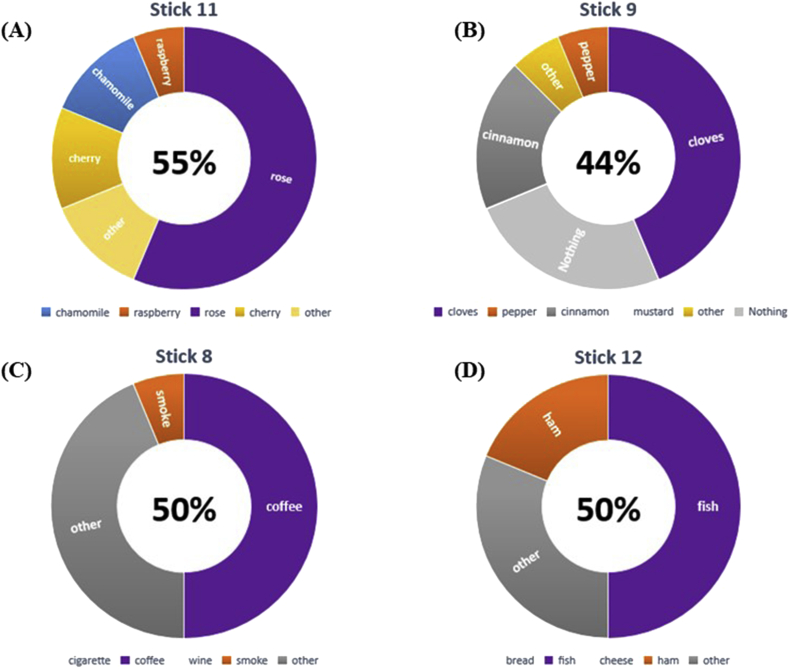
The q-Sticks test results revealed that 3 of the 5 patients, subjectively reported in the questionnaire a lack of smell during COVID-19 infection, recognized the 4 proposed odors allowing us to redefine them as normal smell. One patient was able to recognize three out of four odors and was reclassified to hyposmia. The remaining 1 continued to be in the anosmia group. Moreover, the q-Sticks tests identified 5 new patients with impairment in the perception of smell and could not recognize any of the 4 sticks. Also, 16 patients were unable to recognize several proposed odors and were therefore reallocated as hyposmia; among them, only 1 was conscious of the anosmia based on the clinical history and questionnaire results. (Fig. 1). Compared to only 5 patients who complained of a subjective alteration of the sense of smell, 22 patients were found to have anosmia and hyposmia when using q-Sticks tests. Most of them (16 patients) could not recognize most of the odors, while 6 could not recognize any odor and therefore reclassified into hyposmia and anosmia groups, respectively. Of note, 2 patients, self-defined as having anosmia, had an erroneous perception and recognized all the odors once tested using the q-Sticks test. Finally, analyzing the odors presented to patients revealed that among the hyposmia, 6 (38%) were able to recognize 1, 4 (25%) recognized 2, and 6 (38%) recognized 3 out of 4 odors. The distribution per stick is summarized in Fig. 1.
Fig. 1.
Percentage of patients able to recognize the right smell for all sticks. Data represent the results of the 16 patients with hyposmia, to the q-Sticks test. (A) Stick n° 11: Rose, Raspberry, Chamomile, Cherry; (B) Stick n° 9: Cloves, Pepper, Cinnamon, Mustard; (C) Stick n° 8: Coffee, Smoke, Cigarette, Wine; (D) Stick n° 12: Fish, Ham, Bread, Cheese. In violet the right smell relative to the stick presented to patients, other possibilities signed in other colors. In the middle of the graph, the percentage of patients able to recognize the right smell.
Discussion
The COVID-19 infection can be transmitted from human-to-human via respiratory droplets when an infected person coughs or sneezes and also normally speaks at a short distance.16 Also, the transmission has been described from infected surfaces, as the virus can survive on surfaces up to 96 h.17 Most of the patients infected with COVID-19 are asymptomatic or have mild symptoms, whereas only 5–10% of them show severe respiratory failure and multi-organ involvement with mortality of 0.7–1.2%.18,19
Exposed individuals to COVID-19 infection may become infectious around day 3 but develop symptoms on day 5. Furthermore, most of them remain mild symptomatic or develop more severe symptoms, not before day 9. The strategies for reducing the pandemic and controlling the infection vary from isolating infectious subjects, active contact tracing, quarantining close contacts, reducing the contact period, implementing travel restrictions, mandating physical distancing, and enforcing total lockdown.20 Despite these restrictive measures adopted to varying degrees in the world's different countries, we are still far from controlling the epidemic. One of the principal problems detected in the fight against the virus is the failure to recognize asymptomatic or paucisymptomatic patients who remain a possible vehicle for spreading viruses. Among these patients, there are those experienced only a subjective alteration of smell and taste. Loss of smell and taste are 2 of the symptoms recently highlighted as an early manifestation of COVID-19 infection.10 Olfactory disorders during other viral infections such as rhinovirus, Epstein-Barr virus, and some coronaviruses have already been described and are related to an inflammatory reaction of the nasal mucosa.21,22 What is peculiar of olfactory disorder during COVID-19 is that there is no link with other nasal symptoms that typically affect patients during flu and nasal viral infections. Anosmia is the only symptom without associated nasal congestion, obstruction, or rhinorrhea.23 Although there is no certain evidence regarding the pathophysiology of COVID-19 infection, the mechanism may be linked directly to the commitment to the olfactory tract's nerve endings by immune and inflammatory responses secondary to the infection.24 The role of ACE-2 receptor, already described due to its possible implications in the lung disease, has been recently also associated in the development of anosmia. The presence of this receptor has been found in the olfactory neuroepithelium relative to nasal respiratory or tracheal epithelial cells.25
A simple, fast, and validated tool that can detect olfactory disorders can determine early-stage infections, leading to early diagnosis and limiting the pandemic's spread.
Several methods have been used for assessing olfactory function or dysfunction and can be listed in 3 different types of testing: self-reporting, olfactory testing, and instrumental exams.26
Specific questionnaires on olfactory diseases, such as the Questionnaire of Olfactory Disorders, allow obtaining statistical and epidemiological data on how the disease affects the quality of life. Clinical data can be collected during an ENT consultation and over a phone call or by online surveys. Nevertheless, the questionnaires'data are often unreliable compared to specific psychophysical testing.27, 28, 29 The first published studies about self-reported surveys have found olfactory disorders in patients with COVID-19 infection ranging from 34% to 85%.5, 6, 7, 8, 9, 10
Psychophysical testing has provided a reliable assessment of overall olfactory function, a more detailed diagnosis, and odor thresholds, discrimination, and identification.30 The UPSIT and the extended versions of Sniffing Test are the most used in specialized centers; however, they are not widely used because they take considerable time, require skilled clinicians, and are expensive. Moein et al used UPSIT to test the olfactory quality and documented a smell dysfunction in 98% of patients with positive COVID-19 confirming the strong relationship between anosmia and hyposmia and COVID-19.11
Instrumental tests include electrophysiology and functional imaging but are used for medico-legal assessment or in research settings. In this study, the short olfactory test based on identifying 3 odors, published by Hummel in 2010, was adopted.13 This test is easy to perform, takes a shorter time, and does not require special skills. The self-reporting tests on smell dysfunction were also applied to check if there were significant differences.
The herein report showed that the subjective perception of anosmia is susceptible to error. Administering questionnaires alone with a subjective evaluation of patients’ smell identified only 5 patients with alteration odors recognition. When an objective test (the q-Sticks) was used, 22 patients were affected by anosmia and hyposmia; 6 and 16 patients, respectively. Also, 2 patients, self-defined with anosmia, were reallocated to the normal smell group. The recognition of the 4 different odors among the 16 patients hyposmia was also variable ranging from 1 to 3 out of the 4 odors.
The subjective perception of smell and taste disorders has already been widely discussed in the past, where factors such as individual differences in the perceptive and verbal abilities of the subjects and the characteristics of the stimuli themselves seem to play an important role.31 There are also studies that have shown how the effect of anxiety is able to change the perception of odors themselves,32 also in healthy people.33 It is in fact described in the literature how, in conditions of anxiety-stress, odors that we can define as neutral, can become unpleasant and require a longer time to be recognized, accompanied by a delay in the recognition of odors by the olfactory cortex (anterior pyriform and orbital-frontal) and emotion-relevant pregenual anterior cingulate cortex. At the same time, in a state of anxiety there is a greater adaptation of the sensory olfactory relay, strengthening the connections between amygdala and all levels of the olfactory cortical hierarchy.32 Covid-19 infection has been demonstrated to be strongly related with an increase of anxiety and depression, both in affected patients and in those not affected by virus.34 The relationship between anxiety and covid-19 can also play a different role, where subjectively, for fear of having a symptom of the disease is not recognized. The objectification of the anosmia/hyposmia with a test, such as the one carried out on our patients, reduces the possible bias of the subjective perception of the symptom. Other possible confounding factor, able to explain observed results, could be represented by subjective verbal and expression abilities of odor perception. Furthermore several authors have pointed out that could be an objective difficulty in describing an odor, although recognized as a category. An inadequate vocabulary in the description of the presented odors may, again, have caused a difficulty in recognizing the odor presented during the test.32
Finally the role of smoking could also be implicated in the taste and smell perception,35 despite that in our cohort of patient the prevalence of smoking attitude is equally distributed in the groups (Table 1).
This study's limitations are related to the relatively small sample size and the testing of hospitalized patients, who are likely to suffer from a more severe disease than those treated and quarantined at home. However, we find it interesting that a large number of patients in our sample, including asymptomatic patients, were unaware that they had olfactory problems even though they subjectively thought that they recognized odors perfectly.
In this study, although the above mentioned limited number of patients enrolled, the results, objectively through a validated test used to identify the ability to recognize odors, identified how hyposmia and anosmia are frequent symptoms in patients with COVID-19. Also, we confirmed that patients’ subjective perception is not completely reliable and incompetent to determine the diagnosis of anosmia and hyposmia, particularly in patients with COVID-19 infection. The introduction of a simple olfactory test (q-Sticks) to all populations, such as the one described, may help find fearful or asymptomatic patients, who are still a major infection source that is difficult to control.
Funding
None to disclose for all Authors.
Author contributions
DB, GP, RFC designed the project and wrote manuscript; FB, ET, FC, MF, AI, AC, DM, AMR reviewed and approved manuscript.
Ethical aspects
All patients sign an informed consent of hospital where is specified if patients allow the use of the hospitalization data for research, and we use only the one accepting this point. We performed routinary analisis, test and anamnestic data collection. The in text mentioned "questionnaires", are the routinary anamnestic collection data, performed in all covid-19 patients.
Data availability
Partial data are available on request.
Declaration of competing interest
None to disclose for all Authors.
Acknowledgements
CIPRO (Centro Interprofessionale Pneumologico di Ricerca ed Organizzazione) Genova. All medical doctors and all nurses for the clinical management of patients involved in the study. Novartis contributed unconditionally to the publication of the manuscript.
Footnotes
Full list of author information is available at the end of the article
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2020.100497.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Ramanathan K., Antognini D., Combes A. Planning and provision of ECMO services for severe ARDS during the COVID-19 pandemic and other outbreaks of emerging infectious diseases. Lancet Respir Med. 2020;8(5):518-526. doi: 10.1016/S2213-2600(20)30121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study [published correction appears in Lancet Respir Med. 2020 Apr;8(4):e26] Lancet Respir Med. 2020;8(5):475-481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young B.E., Ong S.W.X., Kalimuddin S. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020;323(15):1488–1494. doi: 10.1001/jama.2020.4372. [published Online Ahead of Print, 2020 Mar 3] [published Correction Appears in JAMA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wan S., Xiang Y., Fang W. Clinical features and treatment of COVID-19 patients in northeast Chongqing [published online ahead of print, 2020 Mar 21] J Med Virol. 2020 doi: 10.1002/jmv.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lüers J.C., Klußmann J.P., Guntinas-Lichius O. Die Covid-19-Pandemie und das HNO-Fachgebiet: worauf kommt es aktuell an? [The Covid-19 pandemic and otolaryngology: what it comes down to?] Laryngo-Rhino-Otol. 2020 Mar 26 doi: 10.1055/a-1095-2344. [published online ahead of print. 2020. [DOI] [PubMed] [Google Scholar]
- 6.Menni C VA, Freydin MB, Ganesh SA, et al. Loss of smell and taste in combination with other symptoms is a strong predictor of COVID- 19 infection. medRxiv DOI: 10.1101/2020.04.05.20048421
- 7.Bagheri A.A., Farhadi M., Shamshiri A.R. Coincidence of COVID-19 epidemic and olfactory dysfunction outbreak. Med J Islam Repub Iran. 2020;34:62. doi: 10.34171/mjiri.34.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giacomelli A., Pezzati L., Conti F. Self-reported olfactory and taste disorders in SARS-CoV-2 patients: a cross-sectional study. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa330. published online ahead of print, 2020 Mar 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan C.H., Faraji F., Prajapati D.P., Boone C.E., DeConde A.S. Association of chemosensory dysfunction and Covid-19 in patients presenting with influenza-like symptoms. Int Forum Allergy Rhinol. 2020 doi: 10.1002/alr.22579. published online ahead of print, 2020 Apr 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lechien J.R., Chiesa-Estomba C.M., De Siati D.R. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Oto-Rhino-Laryngol. 2020:1–11. doi: 10.1007/s00405-020-05965-1. published online ahead of print, 2020 Apr 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moein S.T., Hashemian S.M.R., Mansourafshar B., Khorram-Tousi A., Tabarsi P., Doty R.L. Smell dysfunction: a biomarker for COVID-19. Int Forum Allergy Rhinol. 2020 Apr 17 doi: 10.1002/alr.22587. ([Epub ahead of print]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Russell B., Moss C., Rigg A., Hopkins C., Papa S., Van Hemelrijck M. Anosmia and ageusia are emerging as symptoms in patients with COVID-19: what does the current evidence say? Ecancermedicalscience. 2020;14:ed98. doi: 10.3332/ecancer.2020.ed98. 2020 Apr 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hummel T., Pfetzing U., Lötsch J. A short olfactory test based on the identification of three odors. J Neurol. 2010 Aug;257(8):1316–1321. doi: 10.1007/s00415-010-5516-5. [DOI] [PubMed] [Google Scholar]
- 14.Konstantinidis I., Hummel T., Larsson M. Identification of unpleasant odors is independent of age. Arch Clin Neuropsychol. 2006;21:615–621. doi: 10.1016/j.acn.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Doty R.L., Shaman P., Kimmelman C.P., Dann M.S. University of Pennsylvania smell identification test: a rapid quantitative olfactory function test for the clinic. Laryngoscope. 1984;94:176–178. doi: 10.1288/00005537-198402000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Yu P., Zhu J., Zhang Z., Han Y., Huang L. A familial cluster of infection associated with the 2019 novel coronavirus indicating potential person-to-person transmission during the incubation period. J Infect Dis. 2020 doi: 10.1093/infdis/jiaa077. [published online ahead of print, 2020 Feb 18] jiaa077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Doremalen N., Bushmaker T., Morris D.H. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. published correction appears in Lancet Respir Med. 2020 Apr;8(4):e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Novel coronavirus pneumonia emergency response epidemiology team. Zhonghua Liuxingbingxue Zazhi. 2020;41(2):145–151. [Google Scholar]
- 20.Peirlinck M., Linka K., Costabal F.S., Kuhl E. Outbreak dynamics of COVID-19 in China and the United States. Biomech Model Mechanobiol. 2020 Apr 27:1–15. doi: 10.1007/s10237-020-01332-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suzuki M., Saito K., Min W.P. Identification of viruses in patients with post- viral olfactory dysfunction. Laryngoscope. 2007;117(2):272–277. doi: 10.1097/01.mlg.0000249922.37381.1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Riel D., Verdijk R., Kuiken T. The olfactory nerve: a shortcut for influenza and other viral diseases into the central nervous system. J Pathol. 2015;235(2):277–287. doi: 10.1002/path.4461. [DOI] [PubMed] [Google Scholar]
- 23.Xydakis M.S., Dehgani-Mobaraki P., Holbrook E.H. Smell and taste dysfunction in patients with COVID-19. Lancet Infect Dis. 2020;S1473–3099(20):30293. doi: 10.1016/S1473-3099(20)30293-0. published online ahead of print, 2020 Apr 15. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han A.Y., Mukdad L., Long J.L., Lopez I.A. Anosmia in COVID-19: mechanisms and significance [published online ahead of print, 2020 Jun 17] Chem Senses. 2020 doi: 10.1093/chemse/bjaa040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen M., Shen W., Rowan N.R. Elevated ACE2 expression in the olfactory neuroepithelium: implications for anosmia and upper respiratory SARS-CoV-2 entry and replication. Eur Respir J. 2020;56(3) doi: 10.1183/13993003.01948-2020. 2001948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hummel T., Whitcroft K.L., Andrews P. Position paper on olfactory dysfunction. Rhinol Suppl. 2017 Mar;54(26):1–30. doi: 10.4193/Rhino16.248. [DOI] [PubMed] [Google Scholar]
- 27.Landis B.N., Hummel T., Hugentobler M., Giger R., Lacroix J.S. Ratings of overall olfactory function. Chem Senses. 2003;28(8):691–694. doi: 10.1093/chemse/bjg061. [DOI] [PubMed] [Google Scholar]
- 28.Philpott C.M., Wolstenholme C.R., Goodenough P.C. Comparsion of subjective perception with objective measurement of olfaction. Otolaryngol Head Neck Surg. 2006 Mar;134(3):488–490. doi: 10.1016/j.otohns.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 29.Wehling E., Lundervold A.J., Espeset T., Reinvang I., Bramerson A., Nordin S. Even cognitively well-function adults are unaware of their olfactory dysfunction: implications for ENT clinicians and researchers. Rhinology. 2015;53(1):89–94. doi: 10.4193/Rhino14.081. [DOI] [PubMed] [Google Scholar]
- 30.Hummel T., Welge-Luessen A. Assessment of olfactory function. Adv Oto-Rhino-Laryngol. 2006;63:84–98. doi: 10.1159/000093752. [DOI] [PubMed] [Google Scholar]
- 31.Kaeppler K., Mueller F. Odor classification: a review of factors influencing perception-based odor arrangements. Chem Senses. 2013 Mar;38(3):189–209. doi: 10.1093/chemse/bjs141. [DOI] [PubMed] [Google Scholar]
- 32.Krusemark E.A., Novak L.R., Gitelman D.R., Li W. When the sense of smell meets emotion: anxiety-state-dependent olfactory processing and neural circuitry adaptation. J Neurosci. 2013 Sep 25;33(39):15324–15332. doi: 10.1523/JNEUROSCI.1835-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takahashi T., Itoh H., Nishikawa Y. Possible relation between olfaction and anxiety in healthy subjects. Psychiatr Clin Neurosci. 2015 Jul;69(7):431–438. doi: 10.1111/pcn.12277. [DOI] [PubMed] [Google Scholar]
- 34.Islam M.S., Ferdous M.Z., Potenza M.N. Panic and generalized anxiety during the COVID-19 pandemic among Bangladeshi people: an online pilot survey early in the outbreak. J Affect Disord. 2020 Nov 1;276:30–37. doi: 10.1016/j.jad.2020.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fraga Da Ré A., Gonçalves Gurgel L., Buffon G. Tobacco influence on taste and smell: systematic review of the literature. Int Arch Otorhinolaryngol. 2018 Jan;22(1):81–87. doi: 10.1055/s-0036-1597921. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Partial data are available on request.