Abstract
Aims
Azithromycin is widely used broad spectrum antibiotic recently used in treatment protocol of COVID-19 for its antiviral and immunomodulatory effects combined with Hydroxychloroquine or alone. Rat models showed that Azithromycin produces oxidative stress, inflammation, and apoptosis of myocardial tissue. Rosuvastatin, a synthetic statin, can attenuate myocardial ischemia with antioxidant and antiapoptotic effects. This study aims to evaluate the probable protective effect of Rosuvastatin against Azithromycin induced cardiotoxicity.
Main method
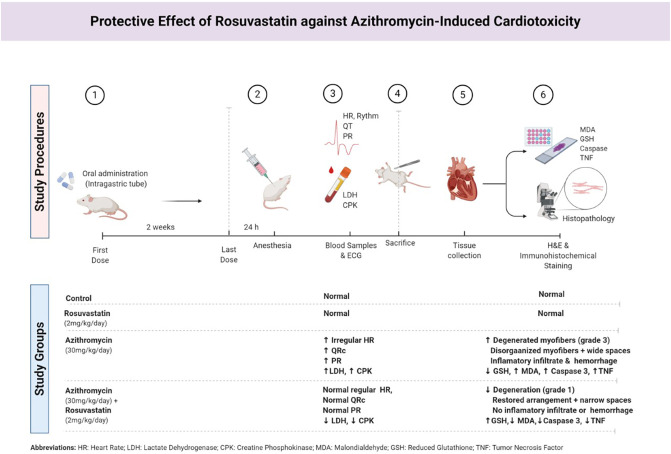
Twenty adult male albino rats were divided randomly into four groups, five rats each control, Azithromycin, Rosuvastatin, and Azithromycin +Rosuvastatin groups. Azithromycin 30 mg/kg/day and Rosuvastatin 2 mg/kg/day were administrated for two weeks by an intragastric tube. Twenty-four hours after the last dose, rats were anesthetized and the following measures were carried out; Electrocardiogram, Blood samples for Biochemical analysis of lactate dehydrogenase (LDH), and creatine phosphokinase (CPK). The animals sacrificed, hearts excised, apical part processed for H&E, immunohistochemical staining, and examined by light microscope. The remaining parts of the heart were collected for assessment of Malondialdehyde (MDA) and Reduced Glutathione (GSH).
Key findings
The results revealed that Rosuvastatin significantly ameliorates ECG changes, biochemical, and Oxidative stress markers alterations of Azithromycin. Histological evaluation from Azithromycin group showed marked areas of degeneration, myofibers disorganization, inflammatory infiltrate, and hemorrhage. Immunohistochemical evaluation showed significant increase in both Caspase 3 and Tumor necrosis factor (TNF) immune stain. Rosuvastatin treated group showed restoration of the cardiac muscle fibers in H&E and Immunohistochemical results.
Significance
We concluded that Rosuvastatin significantly ameliorates the toxic changes of Azithromycin on the heart.
Keywords: Azithromycin, Rosuvastatin, Heart, Rat
1. Introduction
Azithromycin (macrolide group) is a widely used broad spectrum antibiotic. It was approved by the Food and Drug Administration (FDA) for respiratory tract and sexually transmitted infections [1,2]. There are several arguments supporting the potential effectiveness of Azithromycin in COVID-19 infection, including its antiviral activity and immunomodulatory effects when combined with Hydroxychloroquine or alone [3,4]. Azithromycin appears to decrease the virus entry into cells [5,6]. It can also enhance the immune response against viruses by up-regulating the production of type I and III interferons (especially interferon-β and interferon-λ) and genes involved in virus recognition such as MDA5 and RIG-I [6,7]. These mechanisms are universally involved in the innate response against infectious agents and potentially against COVID-19. It is extensively distributed into the tissue, especially in lungs,where average concentrations in both extracellular fluids and within cells are much higher than in plasma [8]. In 2013, the FDA published warning notifications about the QT prolongation risk of Azithromycin and the cautions that should be considered for patients who are also taking QT-prolonging medications [9,10]. Patients treated with Azithromycin had a greater risk for cardiovascular death, which was significantly greater with Azithromycin than with either amoxicillin or ciprofloxacin. There were 47 extra cardiovascular deaths per 1 million courses comparing to amoxicillin. Moreover, for patients with the highest risk for cardiovascular disease, there were 245 additional cardiovascular deaths per 1 million. Further risk factors for cardiac effects include old age, high doses, and rapid administration leading to higher serum concentrations, a history of cardiac disease, and risk factors for cardiovascular disease such as diabetes. This increased mortality risk is due to the cardiotoxicity with Azithromycin due to cardiac arrhythmias and sudden death [11]. Rat models showed that Azithromycin could produce oxidative stress, inflammation, and apoptosis of the myocardial tissue leads to ECG changes, myocardial infarction and death [12,13].
Rosuvastatin, a synthetic statin, showed with other statins a variable range of activities such as anti-inflammatory, antioxidant, ion channel stabilization, and autonomic nervous system regulation [14].
Statins are established as performing the most critical role in cardiovascular illness in nearly all patient risk groups. The benefit of using statins acutely before developments of myocardial injury may be anticipated and good conditions. The potential benefits of limiting myocardial injury in these settings are evident [15]. Rosuvastatin produced a significant rise in myocardial levels of adenosine in both normal and ischemic regions, significantly improved myocardial 6-Keto-PGF1α, levels which interpret into cardioprotective effect. Rosuvastatin decreased post infarction activation of the NLRP-3 inflammasome [16].
This action can be via immune regulatory or inflammatory pathways [17,18].The co-administration of Rosuvastatin in rats showed a cardio-protective role against myocardial apoptosis, improvement of the hemodynamic parameters as serum lactate dehydrogenase (LDH), and lipid profile reduction [14].
This study was designed to evaluate Rosuvastatin's probable protective effect against Azithromycin induced cardiotoxicity in adult male albino rats.
2. Materials and methods
Chemicals:
Azithromycin: Zithromax 250 mg, Pfizer, Germany.
Rosuvastatin: Rosuvast 20 mg, Chemipharm, Egypt.
Animals: Twenty adult male Sprague Dawley albino rats weighing 200–250 g, aged 10–12 weeks, obtained from the animal house of Faculty of Veterinary Medicine, Suez Canal University. They kept in a ventilated room, housed in wire mesh cages at room temperature, received food and water ad libitum. They left two weeks to accommodate at Human Anatomy and Embryology Department, Faculty of Medicine, Suez Canal University.
Ethical consideration: All experiments were carried out by the guidelines of the Institutional Animals Ethics Committee of Faculty of Medicine Suez Canal University.
Experimental design: twenty adult male albino rats randomly divided into four groups, five rats each. Azithromycin and Rosuvastatin were dissolved in distilled water and the intragastric route administrated all drugs.
Control group: received distilled water 1 ml/100 g/day for two weeks [12].
Azithromycin group: received Azithromycin 30 mg/kg/day for two weeks [12].
Rosuvastatin group: received Rosuvastatin 2 mg/kg/day for two weeks [19].
Azithromycin and Rosuvastatin group: rats received Azithromycin and Rosuvastatin as previously.
Twenty four hours after the last dose, rats were anesthetized by intraperitoneal injection of 60 mg/kg ketamine,and 5 mg/kg xylazine [12],and the following measures were carried out:
Electrocardiogram: ECG machine ECG-903A at the Department of Physiology, Faculty of Veterinary Medicine, Suez Canal University. Needle electrodes were located at the right wrist, right ankle, and left ankle of the anesthetized rats.
Biochemical analysis: Blood samples were collected from retro-orbital plexus and centrifuged to assess cardiac function using serum lactate dehydrogenase (LDH) and creatine phosphokinase (CPK).
Assessment of oxidative stress markers: The remaining parts of each heart were collected to be used for evaluation of Malondialdehyde (MDA) and Reduced Glutathione (GSH) using bio diagnostic kits, Egypt according to Beutler et al. [20] and Uchiyama and Mihara [21].
Histological evaluation: The anesthetized animals were sacrificed by cervical dislocation, and hearts were excised. The apical part of each heart was fixed and stained by hematoxylin and eosin (H&E) and examined by a light microscope.
Immunohistochemical evaluation: The same paraffin embedded samples were processed for immunohistochemical staining for detection of caspase 3 as a marker of apoptosis [22] and tumor necrosis factor (TNF) as a marker of inflammation [23]. The slides investigated using FIJI image processing software [24].
Statistical analysis: Statistical analysis was performed using the SPSS computer software (version 17). ANOVA and Bonferroni Post Hoc tests were used. Statistical significance was considered at p-value <0.05.
3. Results
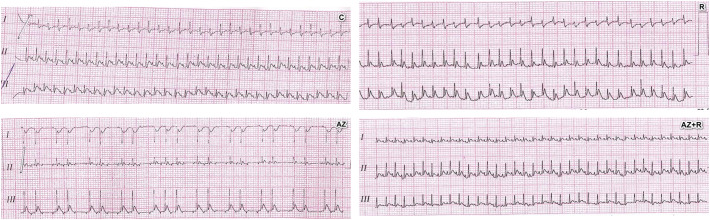
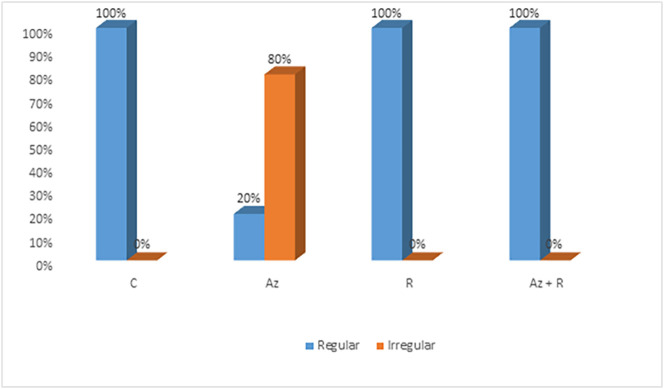
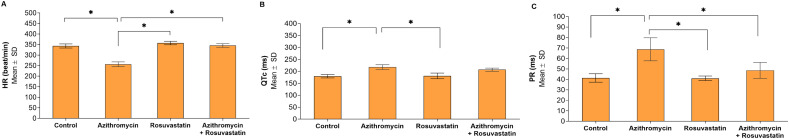
Electrocardiogram (ECG): Azithromycin treated group showed a statistically significant decrease in heart rate with 80% irregular rhythm when compared with the control group (summary of the used experimental methods and results were illustrated in Fig. 1 ). Regarding QTc interval in Azithromycin treated group was significantly prolonged when compared with the control group. Moreover, the PR segment showed a statistically significant increase when compared with all other groups.
Fig. 1.
Summary of the used experimental methods and results.
The addition of Rosuvastatin ameliorate the effect of Azithromycin, restored the normal heart rate, rhythm, the QTc interval, and PR interval to the nearly normal value (became comparable with the control group) (Fig. 2, Fig. 3, Fig. 4 ).
Fig. 2.
Changes in electrocardiogram among groups: C: control, Az: Azithromycin treated group, R: Rosuvastatin and Az + R: Azithromycin + Rosuvastatin.
Fig. 3.
Distribution of heart rhythm among group: C: control, Az: Azithromycin treated group, R: Rosuvastatin and Az + R: Azithromycin + Rosuvastatin.
Fig. 4.
Comparisons of Electrocardiogram parameters between study groups (N = 20). *Statistically significant at p < 0.05. A: HR; B: QTc; C: PR.
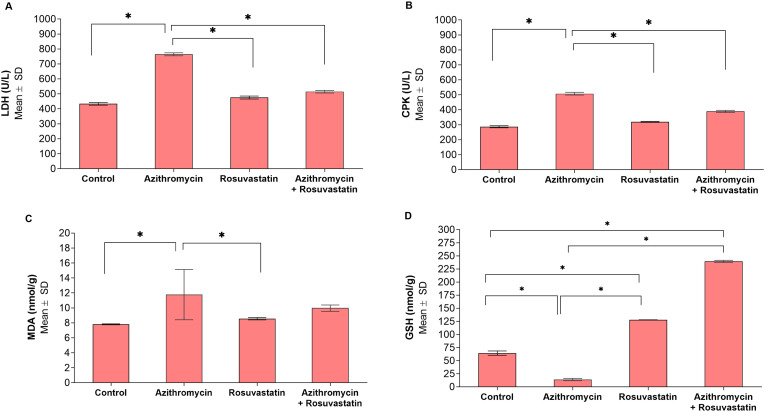
Biochemical analysis: Data analysis showed that the Azithromycin treated group was associated with a significant increase in LDH and CPK levels compared with other groups. Moreover, Azithromycin treated group displayed a significant increase in heart tissue concentration of (MDA), as well as a significantly lower heart tissue concentration of (GSH) (Fig. 5 ).
Fig. 5.
Comparisons of biochemical and oxidative stress markers between study groups (N = 20). *Statistically significant at p < 0.05. A: LDH; B: CPK; C: MDA; D: GSH.
On the other hand, administration of Rosuvastatin significantly ameliorated all the biochemical and Oxidative stress markers alterations induced by Azithromycin. It was noticed that Rosuvastatin showed antioxidant activity as it significantly increased (GSH) in Rosuvastatin treated group compared with the Control group (Fig. 5).
Histological evaluation:
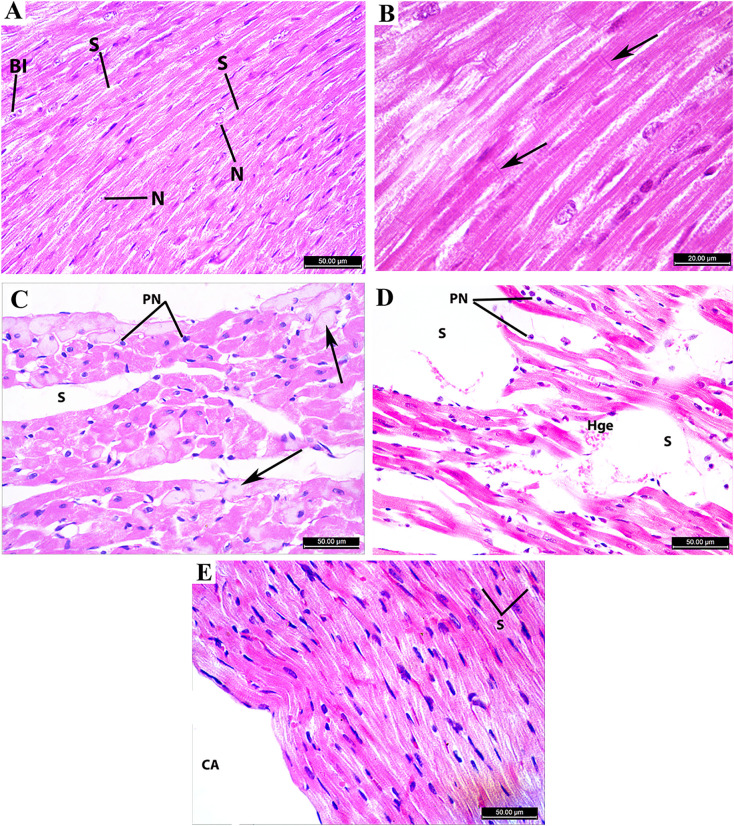
Control and Rosuvastatin groups: Light microscopical examination of heart ventricle in control and Rosuvastatin groups showed normal appearance of cardiac muscle fibers arranged in bundles with narrow spaces in between. Myofibers are connected by intercalated discs. The cytoplasm appeared acidophilic; nuclei were oval, large, and pale. Degree of degeneration = 0 (Fig. 6 ).
Fig. 6.
Photomicrograph of the heart ventricle (A & B): Control group showing normal cardiac muscle fibers with acidophilic cytoplasm and oval, large pale nuclei (N). Some cardiomyocytes are binuclear (BI). Myofibers are arranged in bundles with narrow spaces in between (S) and connected with intercalated discs (arrows). (C & D): Azithromycin group showing areas of severe degeneration in cardiac muscle fibers (arrows), pyknotic nuclei (PN), distortion of the typical arrangement of myofibers with massive wide spaces (S), and hemorrhage (Hge).
(E): Azithromycin + Rosuvastatin group showing the restored arrangement of cardiac muscle fibers with narrow spaces in between (S). Ventricle cavity (CA). A, C, D, E (H&E; ×400) and B (H&E; ×1000).
Azithromycin treated group: Light microscopical examination of heart ventricle in Azithromycin group showed marked areas of degeneration. Myofibers disorganization with wide spaces in between, inflammatory infiltrate, and hemorrhage. Degree of degeneration = 3 (Fig. 6).
Azithromycin + Rosuvastatin treated group: Light microscopical examination of heart ventricle in the (Azithromycin + Rosuvastatin) treated group showed restoration of the cardiac muscle fibers arrangement with narrow spaces in between. Degree of degeneration = 1 (Fig. 6).
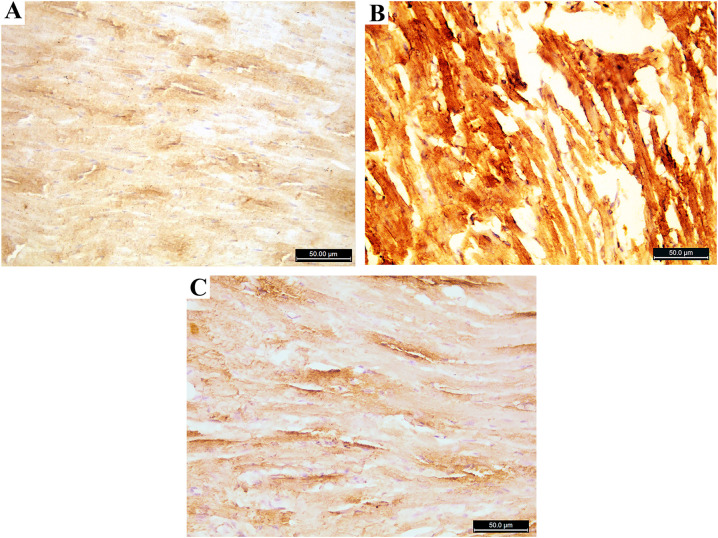
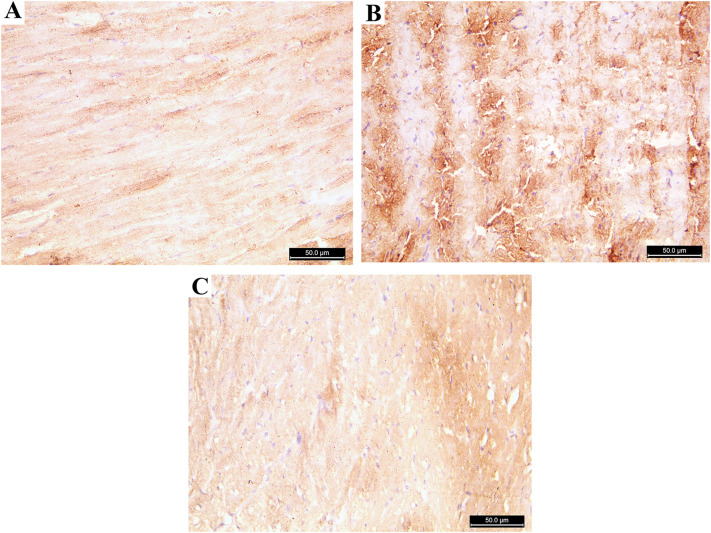
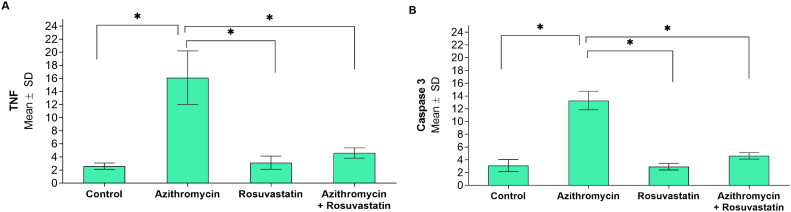
Immunohistochemical evaluation: The Azithromycin treated group showed a significant increase in both Caspase 3 and Tumor necrosis factor (TNF) immune stain compared to the control group. The addition of Rosuvastatin restores nearly the normal immune reaction of both Caspase 3 and Tumor necrosis factor (Fig. 7, Fig. 8, Fig. 9 ).
Fig. 7.
Photomicrograph of the heart ventricle stained with caspase 3 A: control group is showing few positive cytoplasmic immune reactions. B: Azithromycin treated group showing strong positive immune reactions C: Azithromycin and Rosuvastatin treated group shows few positive immune reactions. (caspase 3; ×400).
Fig. 8.
Photomicrograph of the heart ventricle stained with TNF α A: control group showing few positive cytoplasmic immune reaction B: Azithromycin treated group showing strong positive immune reactions for TNF α. C: Azithromycin and Rosuvastatin treated group showing few positive immune reactions (TNF α; ×400).
Fig. 9.
Comparisons of immune-histochemical markers between the study groups (N = 20). *Statistically significant at p < 0.05. A: TNF; B: Caspase 3.
4. Discussion
Azithromycin is a second-generation macrolide that is widely used as a broad spectrum antibiotic [25,26]. Rat models showed that Azithromycin produces oxidative stress, inflammation, and apoptosis of the myocardial tissue. This causes ECG changes, myocardial infarction, and death [12,13].
Rosuvastatin is one of the statin group, which has a lipid-lowering activity [27]. It is effectively prevents of cardiovascular diseases and has strong anti-oxidative and anti-inflammatory properties [28].
The current study revealed irregular heart rhythm, a significant decrease in HR, prolonged QT, and PR intervals in Azithromycin treated group compared to the control. These changes run parallel with studies done by [12,29]. Contrary to this result, El-Shitany and El-Desoky [13] reported an increase in HR in the Azithromycin treated group. The prolongation of the PR interval induced by Azithromycin can be explained by its action on β-adrenergic receptor and calcium channels,while the prolongation of QT interval in early stages of acute myocardial ischemia is due to dysfunction of the potassium channel in human, but its role in rats is doubtful [12].
In the present work, HR increased in Rosuvastatin + Azithromycin group, and the heart rhythm became regular. Similar results reported by [14].
Regarding LDH is one of the cardiac biomarkers, it is released from the damaged myocardium into the blood caused by Azithromycin via inducing ischemia [12]. The current study showed a significant increase in, LDH in the Azithromycin group when compared to the Control group. This result is similar to the findings of [13].
In the present work LDH level decrease in Rosuvastatin + Azithromycin group compared to Azithromycin group. A similar result revealed Rosuvastatin ability to increase antioxidant activities and reduce lipid peroxidation, which prevents cardiac tissue damage [30].
Regarding CPK, its leakage into the blood occurs in cases of acute myocardial infarction and muscle injury [31]. The current study showed a significant increase in CPK level in the Azithromycin group when compared to the Control group. This result was similar to the findings of [13]. They explained this damaging of the cardiac muscle fibers caused by the oxidant and apoptotic activities of Azithromycin.
The current work showed a significant decrease in CPK level in the Rosuvastatin+ Azithromycin group compared to the Azithromycin group. This result was agreed with [32,33], who found that Rosuvastatin had a protective effect on myocardial fibrosis, reduce creatine kinase and decrease injury due to its antioxidant and anti-inflammatory activities.
The level of MDA is a good indicator of lipid peroxidation in blood; its elevation is an indicator of oxidative stress [34]. GSH is a soluble antioxidant present in the cytoplasm, nucleus, and mitochondria. It reduces ROS during the enzymatic and non-enzymatic reactions. Its level decreased in the case of oxidative stress [35].
The current study showed a significant increase in MDA and decreased GSH levels in the Azithromycin group compared to the Control group. Similar findings were reported by [12,13,36].
In this work, Rosuvastatin + Azithromycin group showed an insignificant decrease in the MDA and increased GSH levels compared to the Azithromycin group. This was in agreement with [30,37]; they suggested that Rosuvastatin has an antioxidant role which can protect the cell from lipid peroxidation.
Histological examination of this study showed marked areas of degeneration, myofibers disorganization, wide spaces in between, inflammatory infiltrate, and hemorrhage in Azithromycin groups, similar results reported by [12,13].
Rosuvastatin + Azithromycin group showed restoration of the arrangement of the cardiac muscle fibers compared to Azithromycin group. This improvement is similar to the finding observed by [14,30].
Regarding immunohistochemical evaluation, the current study showed that caspase 3 staining in the Azithromycin group was significantly increased compared to the Control group. This result was following the findings of [13,38]. They reported that Azithromycin produces reactive oxygen species (ROS), which lead to apoptosis.
In the present study, Rosuvastatin + Azithromycin group showed a significant decrease in caspase 3 staining compared to the Azithromycin group;this agrees with [14].
TNF was assessed in heart tissue, as it is a proinflammatory cytokine. Its detection in cells and tissues is associated with inflammatory diseases [39].
This study showed that TNF staining in the Azithromycin group is significantly increased when compared to Control. This result is similar to the findings [13,40].The contrary result was reported by [41,42].
The immunomodulatory effects of Azithromycin vary according to the phase of an inflammatory response. When administered in a healthy state or early during a bacterial infection, it could promote host defense by activating leukocytes and endothelial cells; at a later stage, it leads to inhibition of inflammation and promotes its resolution [43].
In this study, TNF staining was significantly decreased in Rosuvastatin + Azithromycin compared to the Azithromycin group that agreed with [44,45]. They explained its anti-inflammatory properties by prevention of the macrophage's infiltration.
5. Conclusion
The present study concluded that Rosuvastatin significantly ameliorates the ECG changes, biochemical, and Oxidative stress markers alterations of Azithromycin. Rosuvastatin treated group showed restoration of the cardiac muscle fibers in H&E and Immunohistochemical results.
CRediT authorship contribution statement
All authors have been personally, equally, and actively involved in substantive work leading to the manuscript and will hold themselves jointly and individually responsible for its content.
Declaration of competing interest
There are no conflicts of interest.
References
- 1.Li X., Wang M., Liu G., Ma J., Li C. Association of macrolides with overall mortality and cardiac death among patients with various infections: a meta-analysis. European Journal of Internal Medicine. 2016;28:32–37. doi: 10.1016/j.ejim.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Zimmermann P., Ziesenitz V.C., Curtis N., Ritz N. The immunomodulatory effects of macrolides—a systematic review of the underlying mechanisms. Front. Immunol. 2018;9:1–13. doi: 10.3389/fimmu.2018.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andreani J., Le Bideau M., Duflot I., Jardot P., Rolland C., Boxberger M., et al. In vitro testing of combined hydroxychloroquine and azithromycin on SARS-CoV-2 shows synergistic effect. Microb. Pathog. 2020;145 doi: 10.1016/j.micpath.2020.104228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bleyzac N., Goutelle S., Bourguignon L., Tod M. Azithromycin for COVID-19: more than just an antimicrobial? Clinical Drug Investigation. 2020;40:683–686. doi: 10.1007/s40261-020-00933-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Du X., Zuo X., Meng F., Wu F., Zhao X., Li C., et al. Combinatorial screening of a panel of FDA-approved drugs identifies several candidates with anti-Ebola activities. Biochem. Biophys. Res. Commun. 2020;522:862–868. doi: 10.1016/j.bbrc.2019.11.065. [DOI] [PubMed] [Google Scholar]
- 6.Li C., Zu S., Deng Y.-Q., Li D., Parvatiyar K., Quanquin N., et al. Azithromycin protects against Zika virus infection by upregulating virus-induced type I and III interferon responses. Antimicrob. Agents Chemother. 2019;63 doi: 10.1128/AAC.00394-19. (e00394-19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gautret P., Lagier J.-C., Parola P., Hoang V.T., Meddeb L., Mailhe M., et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020;56 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodvold K.A., Danziger L.H., Gotfried M.H. Steady-state plasma and bronchopulmonary concentrations of intravenous levofloxacin and azithromycin in healthy adults. Antimicrob. Agents Chemother. 2003;47:2450. doi: 10.1128/AAC.47.8.2450-2457.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mosholder A.D., Mathew J., Alexander J.J., Smith H., Nambiar S. Cardiovascular risks with azithromycin and other antibacterial drugs. N. Engl. J. Med. 2013;368:1665–1668. doi: 10.1056/NEJMp1302726. [DOI] [PubMed] [Google Scholar]
- 10.Patel H., Calip G.S., DiDomenico R.J., Schumock G.T., Suda K.J., Lee T.A. Prevalence of cardiac risk factors in patients prescribed azithromycin before and after the 2012 FDA warning on the risk of potentially fatal heart rhythms. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy. 2019;40:107–115. doi: 10.1002/phar.2355. [DOI] [PubMed] [Google Scholar]
- 11.Ray W.A., Murray K.T., Hall K., Arbogast P.G., Stein C.M. Azithromycin and the risk of cardiovascular death. N. Engl. J. Med. 2012;366:1881–1890. doi: 10.1056/NEJMoa1003833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atli O., Ilgin S., Altuntas H., Burukoglu D. Evaluation of azithromycin induced cardiotoxicity in rats. Int. J. Clin. Exp. Med. 2015;8:3681–3690. [PMC free article] [PubMed] [Google Scholar]
- 13.El-Shitany N.A., El-Desoky K. Protective effects of carvedilol and vitamin C against azithromycin-induced cardiotoxicity in rats via decreasing ROS, IL1-β, and TNF-α production and inhibiting NF-κB and caspase-3 expression. Oxidative Med. Cell. Longev. 2016;2016:13. doi: 10.1155/2016/1874762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma H., Pathan R.A., Kumar V., Javed S., Bhandari U. Anti-apoptotic potential of rosuvastatin pretreatment in murine model of cardiomyopathy. Int. J. Cardiol. 2011;150:193–200. doi: 10.1016/j.ijcard.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Ludman A., Venugopal V., Yellon D.M., Hausenloy D.J. Statins and cardioprotection — more than just lipid lowering? Pharmacol. Ther. 2009;122:30–43. doi: 10.1016/j.pharmthera.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Birnbaum Y., Birnbaum G.D., Birnbaum I., Nylander S., Ye Y. Ticagrelor and rosuvastatin have additive cardioprotective effects via adenosine. Cardiovasc. Drugs Ther. 2016;30:539–550. doi: 10.1007/s10557-016-6701-2. [DOI] [PubMed] [Google Scholar]
- 17.Ke D., Fang J., Fan L., Chen Z., Chen L. Regulatory T cells contribute to rosuvastatin-induced cardioprotection against ischemia-reperfusion injury. Coron. Artery Dis. 2013;24:334–341. doi: 10.1097/MCA.0b013e3283608c12. [DOI] [PubMed] [Google Scholar]
- 18.Xie Y., Li Y., Zhao J. Rosuvastatin reduces myocardial ischemia-reperfusion injury by inhibiting miR-155. Int. J. Clin. Exp. Med. 2019;12:7975–7984. [Google Scholar]
- 19.Kim Y.-H., Park S.-M., Kim M., Kim S.H., Lim S.-Y., Ahn J.-C., et al. Cardioprotective effects of rosuvastatin and carvedilol on delayed cardiotoxicity of doxorubicin in rats. Toxicol. Mech. Methods. 2012;22:488–498. doi: 10.3109/15376516.2012.678406. [DOI] [PubMed] [Google Scholar]
- 20.Beutler E., Duron O., Kelly M. Glutathione reagent and method-patent. J. Lab. Clin. Med. 1963;61:882. [PubMed] [Google Scholar]
- 21.Uchiyama M., Mihara M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978;86:271–278. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- 22.Kaushal V., Herzog C., Haun R.S., Kaushal G.P. In: Caspases, Paracaspases, and Metacaspases: Methods and Protocols. Bozhkov P V., Salvesen G., editors. Springer New York; New York, NY: 2014. Caspase protocols in mice; pp. 141–154. [Google Scholar]
- 23.da Hora K., Santos Valença S., Cristóvão Porto L. Immunohistochemical study of tumor necrosis factor-α, matrix metalloproteinase-12, and tissue inhibitor of metalloproteinase-2 on alveolar macrophages of BALB/c mice exposed to short-term cigarette smoke. Exp. Lung Res. 2005;31:759–770. doi: 10.1080/01902140500324828. [DOI] [PubMed] [Google Scholar]
- 24.Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsubouchi K., Araya J., Minagawa S., Hara H., Ichikawa A., Saito N., et al. Azithromycin attenuates myofibroblast differentiation and lung fibrosis development through proteasomal degradation of NOX4. Autophagy. 2017;13:1420–1434. doi: 10.1080/15548627.2017.1328348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Q., Mi G., Hickey D., Li Y., Tu J., Webster T.J., et al. Azithromycin-loaded respirable microparticles for targeted pulmonary delivery for the treatment of pneumonia. Biomaterials. 2018;160:107–123. doi: 10.1016/j.biomaterials.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 27.Wang L., Zhou B., Zhou X., Wang Y., Wang H., Jia S., et al. Combined lowering effects of rosuvastatin and L. acidophilus on cholesterol levels in rat. J. Microbiol. Biotechnol. 2019;29:473–481. doi: 10.4014/jmb.1806.06004. [DOI] [PubMed] [Google Scholar]
- 28.Kanno M., Nakayama M., Zhu W.-J., Hayashi Y., Kazama J.J. Rosuvastatin pretreatment suppresses distant organ injury following unilateral renal ischemia-reperfusion in hypertensive Dahl salt-sensitive rats. Nephrology. 2018;23:1046–1054. doi: 10.1111/nep.13169. [DOI] [PubMed] [Google Scholar]
- 29.Zhang M., Xie M., Li S., Gao Y., Xue S., Huang H., et al. Electrophysiologic studies on the risks and potential mechanism underlying the proarrhythmic nature of azithromycin. Cardiovasc. Toxicol. 2017;17:434–440. doi: 10.1007/s12012-017-9401-7. [DOI] [PubMed] [Google Scholar]
- 30.Yu Y., Jin L., Zhuang Y., Hu Y., Cang J., Guo K. Cardioprotective effect of rosuvastatin against isoproterenol-induced myocardial infarction injury in rats. Int. J. Mol. Med. 2018;41:3509–3516. doi: 10.3892/ijmm.2018.3572. [DOI] [PubMed] [Google Scholar]
- 31.Aujla R.S., Patel R. StatPearls Publishing; StatPearls [Internet]: 2019. Creatine Phosphokinase. [PubMed] [Google Scholar]
- 32.Du X., Hu X., Wei J. Postconditioning with rosuvastatin reduces myocardial ischemia-reperfusion injury by inhibiting high mobility group box 1 protein expression. Experimental and Therapeutic Medicine. 2014;7:117–120. doi: 10.3892/etm.2013.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma Y.-X., Li W.-H., Xie Q. Rosuvastatin inhibits TGF-1 expression and alleviates myocardial fibrosis in diabetic rats. Die Pharmazie-An International Journal of Pharmaceutical Sciences. 2013;68:355–358. [PubMed] [Google Scholar]
- 34.Gaschler M.M., Stockwell B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017;482:419–425. doi: 10.1016/j.bbrc.2016.10.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mirończuk-Chodakowska I., Witkowska A.M., Zujko M.E. Endogenous non-enzymatic antioxidants in the human body. Adv. Med. Sci. 2018;63:68–78. doi: 10.1016/j.advms.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 36.Olayinka E., Ore A. Influence of azithromycin treatment on hepatic lipid peroxidation and antioxidant defence systems of rats. British Journal of Pharmaceutical Research. 2014;4:240–256. [Google Scholar]
- 37.Habibi J., Whaley-Connell A., Qazi M.A., Hayden M.R., Cooper S.A., Tramontano A., et al. Rosuvastatin, a HMG-CoA reductase inhibitor, decreases cardiac oxidative stress and remodeling in Ren2 transgenic rats. Endocrinology. 2007;148:2181–2188. doi: 10.1210/en.2006-1355. [DOI] [PubMed] [Google Scholar]
- 38.Salimi A., Eybagi S., Seydi E., Naserzadeh P., Kazerouni N.P., Pourahmad J. Toxicity of macrolide antibiotics on isolated heart mitochondria: a justification for their cardiotoxic adverse effect. Xenobiotica. 2016;46:82–93. doi: 10.3109/00498254.2015.1046975. [DOI] [PubMed] [Google Scholar]
- 39.Blaser H., Dostert C., Mak T.W., Brenner D. TNF and ROS crosstalk in inflammation. Trends Cell Biol. 2016;26:249–261. doi: 10.1016/j.tcb.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 40.Čulić O., Eraković V., Čepelak I., Barišić K., Brajša K., Ferenčić Ž., et al. Azithromycin modulates neutrophil function and circulating inflammatory mediators in healthy human subjects. Eur. J. Pharmacol. 2002;450:277–289. doi: 10.1016/s0014-2999(02)02042-3. [DOI] [PubMed] [Google Scholar]
- 41.Menzel M., Akbarshahi H., Bjermer L., Uller L. Azithromycin induces anti-viral effects in cultured bronchial epithelial cells from COPD patients. Sci. Rep. 2016;6 doi: 10.1038/srep28698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parnham M.J., Čulić O., Eraković V., Munić V., Popović-Grle S., Barišić K., et al. Modulation of neutrophil and inflammation markers in chronic obstructive pulmonary disease by short-term azithromycin treatment. Eur. J. Pharmacol. 2005;517:132–143. doi: 10.1016/j.ejphar.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 43.Parnham M.J., Haber V.E., Giamarellos-Bourboulis E.J., Perletti G., Verleden G.M., Vos R. Azithromycin: mechanisms of action and their relevance for clinical applications. Pharmacol. Ther. 2014;143:225–245. doi: 10.1016/j.pharmthera.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 44.Cho O., Y-j Jang, Park K.-Y., Heo T.-H. Beneficial anti-inflammatory effects of combined rosuvastatin and cilostazol in a TNF-driven inflammatory model. Pharmacol. Rep. 2019;71:266–271. doi: 10.1016/j.pharep.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 45.Feng J., Zhang Y., Cao H.-M. Effect of rosuvastatin on inflammatory factor, oxidative stress and cardiac function in patients with chronic heart failure. Journal of Hainan Medical University. 2017;23:18–21. [Google Scholar]