Abstract
Background:
Chloroquine (CQ) and primaquine (PQ) remain the frontline drugs for radical cure of uncomplicated P. vivax malaria in the Greater Mekong Sub-region (GMS). Recent reports of decreased susceptibility of P. vivax to CQ in many parts of the GMS raise concerns.
Methods:
From April 2014 to September 2016, 281 patients with uncomplicated P. vivax infection attending clinics in border settlements for internally displaced people in northeast Myanmar were recruited into this study. Patients were treated with standard regimen of 3-day CQ and concurrent 14-day PQ (3.5 mg/kg total dose) as directly observed therapy, and followed for recurrent parasitemia within 28 days post-patency.
Results:
Within the 28-day follow-up period, seven patients developed recurrent parasitemia, resulting in a cumulative rate of parasite recurrence of 2.6%. Five of the seven parasitemias recurred within two weeks, and two of those failed to clear within seven days, indicating high-grade resistance.
Conclusion:
Although failure of CQ/PQ treatment of P. vivax was relatively infrequent in northeast Myanmar, this study nonetheless confirms that CQ/PQ-resistant strains do circulate in this area, some of them of a highly resistant phenotype. It is thus recommended that patients who acquire vivax malaria in Myanmar be treated an artemisinin-combination therapy along with hypnozoitocidal primaquine therapy to achieve radical cure.
Keywords: Plasmodium vivax, chloroquine, efficacy, resistance, directly observed therapy
1. Introduction
Six Southeast Asian countries (Cambodia, China, Laos, Myanmar, Thailand and Vietnam) constituting the Greater Mekong Subregion (GMS) aim to eliminate all malaria from their territories by 2030 [1, 2]. However, this objective is endangered by the increased incidence or proportion of Plasmodium vivax malaria despite strengthened control efforts in this region. Worldwide, approximately 20 million cases of P. vivax occur every year with the majority occurring in the Asian Pacific Region [3, 4]. Dormant hypnozoites cause a latency in P. vivax malaria that endows the species with great resilience of persistence in the face of conventional methods of malaria control [5].
Registered in 1946, the 4-aminoquinoline drug called chloroquine (CQ) had been used for decades as the first-line treatment of acute Plasmodium falciparum or P. vivax malaria. Widespread resistance by P. falciparum led to almost universal abandonment of the drug by around 2005 [6–8]. Resistance to CQ by P. vivax, on the other hand, emerged later and more slowly. Excepting parts of Southeast Asia [6, 7, 9–12], CQ/PQ remains the first-line therapy for uncomplicated vivax malaria in most endemic zones [13–15]. In Indonesia, Papua New Guinea, the Solomon Islands, and Vanuatu, CQ has been replaced by artemisinin-combination therapies (ACTs) [6]. The evolving malaria epidemiology with persistent burden of vivax malaria and the emergence of CQ resistance in P. vivax parasites from many endemic regions warrant continuous monitoring of clinical efficacy [7].
In the GMS, most malaria transmission occurs along international borders [16, 17]. Myanmar had the heaviest malaria burden in this region in 2016 [4]. Its geographical location as the bridge between South Asia and Southeast Asia makes it a strategically important potential jump point for resistant parasites. Although the clinical efficacy of CQ remains relatively high for uncomplicated vivax malaria in the border regions of Myanmar [15, 18], CQ-resistant (CQR) P. vivax parasites have been reported sporadically as early as in 1993 [10, 19, 20]. In recent years, clinical failures after CQ treatment have been reported in multiple regions of Myanmar [21, 22]. In the Kachin State of Myanmar, civil unrest has led to the migration of internally displaced people (IDP) to the China-Myanmar border, which was linked to the recent surge of vivax malaria cases [23]. To determine the potential contributors to this surge, we monitored the therapeutic responses of the parasites to radical cure employing CQ/PQ. We previously measured a 28-day cumulative incidence of 5.2% recurrent parasitemia with CQ/PQ [21]. To continue monitoring the situation, we conducted a more extensive study of the efficacy of CQ/PQ with directly observed therapy (DOT) at the same site.
2. Methods
2.1. Patient enrollment
The study was conducted between April 2014 and September 2016 in two settlements for IDP near the Laiza Township (97.56°E, 24.75°N), Kachin State, Myanmar, along the China-Myanmar border. The settlements, with about 12,000 individuals mostly of Kachin ethnicity, were established in 2010 as a consequence of internal military conflict. This region is subtropical with the hot and rainy season in May – September and dry cooler season in October – April. Malaria transmission is perennial typically with two peaks – a large peak in June and small peak in November. Although all human malaria parasites except the zoonotic species Plasmodium knowlesi were detected, P. vivax has become the predominant parasite species accounting for more than 90% of the clinical cases [24].
Patients from 3 to 59 years of age who presented with fever or history of fever within the last 48 h and symptoms suggestive of malaria at the two clinics serving the IDP settlements were recruited into the study. Screening of patients for malaria infection was done by light microscopy of Giemsa-stained thin and thick blood smears. The smears were first read by field microscopists at the clinics and were further examined at a nearby field laboratory by two more experienced microscopists. Any discrepancies were re-evaluated to obtain a final consensus diagnosis. For parasite enumeration, the smears were examined by two microscopists, who counted the number of parasites per 200 white blood cells (WBCs) or 500 WBCs when the initial number of parasites was less than 99. The average number of parasites per 200/500 WBCs was used to estimate the parasite density (number of parasites/μl of blood), assumed a WBC count of 8000/μl for patients under five years and a WBC count of 5500/μl for patients aged five years and older [25].
This study was approved by the Ethics Review Committees of Kunming Medical University in Kunming and the Bureau of Health in Kachin. Written informed consent was obtained from the patients or the guardians of minors. This study was registered on the Chinese Clinical Trial Registry with Registration Number ChiCTR1900021668.
2.2. Treatment and follow-up
P. vivax treatment followed the locally prescribed regimen for acute vivax malaria, which included three days of CQ and 14 daily doses of PQ. Drugs were manufactured by Remedica Ltd. (Cyprus) as separate packages for CQ and PQ. Both drugs were administered as DOT either at the clinics (during patients’ returning visits on days 1, 2, 3, 7, and 14,) or at patients’ homes (on the rest of the days for the 14-day PQ regimen) by the study staff. Patients were observed for one hour after drug administration and the same dose of drug was re-administered in case the drug was vomited. As the standard practice in this area, glucose-6-phosphate dehydrogenase deficiency was not screened, but subjects were warned about the risk of PQ toxicity and advised to stop PQ if the urine color became dark. CQ treatment regimens were slightly different for three age groups: patients aged 3–7 years (450 mg CQ base on day 1, and 150 mg each on day 2 and 3), those aged 8–13 years (600 mg CQ base on day 1, and 150 mg each on day 2 and 3), and those aged 14 years and above (900 mg CQ base on day 1, and 300 mg each on day 2 and 3). All patients received a 14-day PQ treatment (0.25 mg/kg/day). Participants were asked to return for follow-up evaluations on days 1, 2, 3, 7, 14, and 28. They were also encouraged to come back to the clinics for a check in case they were feeling sick at any time during the follow-up period. At each visit, axillary temperature was measured and finger-prick blood sample was collected to make thin and thick blood smears for microscopic examinations and filter paper blood spots for molecular confirmation of the parasites.
2.3. Parasite identification and genotyping
Parasite DNA was isolated from the filter paper blood by using a QIAamp DNA mircrokit (Qiagen, Germany). Malaria parasite species at the times of recruitment and recurrence were confirmed nested PCR analysis of the 18S rRNA genes [26]. PCR was performed in 20 μl with 3 μl of DNA template, 1 μl of each primer, and 10 μl 2× Taq. For both primary and nested reactions, the following cycling parameters were used: 3 min at 94 °C, 35 cycles at 94 °C for 30 s, 58 °C for 30 s, 72 °C for 1 min, and final extension for 5 min at 72 °C. The PCR product was separated by electrophoresis on a 1.5% agarose gel.
For the parasites of the day 0 and day of recurrence, they were also genotyped at the P. vivax merozoite protein 1 (Pvmsp1) gene [27]. The pvmsp1 gene fragment was amplified by nested PCR using Premix Taq™ (TaKaRa) with outer primers A5 (5’-TACTACTTGATGGTCCT-3’) and A6 (5’-CCTTCTGGTACAGCTCAATG-3’), and nested primers N1 (5’-TTCATCCCCAAAATCGAGAG-3’) and N2 (5’-TAGGAGGTCCAATTCATCGC-3’). PCR was performed in 20 μl with 2 μl of DNA template, 0.5 μl of each primer, 10 μl 2× Taq and 7 μl water. For both primary and nested PCR, the following cycling parameters were used: 5 min at 94 °C, 34 cycles at 94 °C for 60 s, 60 °C for 60 s, 72 °C for 3 min, and final extension for 5 min at 72 °C. The PCR products were purified and sequenced using the BigDye Terminator v3.1. Sequences were assembled using the SeqMan program of Lasergene software (DNASTAR, Madison, WI, USA) with manual editing, and aligned with the Sal I reference sequence (PVX_099980).
2.4. Data analysis
Statistical analysis was performed using the Graphpad Prism 6 (GraphPad Software, California). Asexual parasite densities were compared between the two groups by using the Mann-Whitney test. Kaplan-Meier survival analysis was done to capture the hazard of recrudescence during the follow-up period. Cumulative hazard between the two age groups was compared using the Log-rank (Mantel-Cox) test. To summarize the estimated risk of treatment failure, we used standard life table calculations to assess the cumulative incidence of recurrent parasitemia, corrected for dropouts. To determine the similarity between parasites at day 0 and at the day of recurrence, we performed a phylogenetic analysis using a maximum likelihood method implemented in MEGA6.0 with 500 bootstrap replicates.
3. Results
During the 33-month study period of DOT of vivax patients with CQ/PQ, 281 of the 322 vivax malaria patients fulfilled the inclusion criteria and were enrolled in the study (Fig. 1). These participants had uncomplicated P. vivax malaria by microscopy, which were subsequently confirmed by PCR targeting the 18S rRNA genes. Both males and females presented about equally, and they ranged in age from 3 to 59 years (median 17) (Table 1). At the time of recruitment, 90% (253/281) of the patients had fever with auxiliary temperature higher than 37.5 °C. Based on the treatment regimens, we categorized the patients into three age groups: 3 – 7 years, 8 – 13 years, and ≥14 years (Table 1). Children below 14 years made up 47% (132/281) of the patient population. Asexual stage parasite density differed among the three age groups with the mean parasite density inversely correlated with age, but the difference was not statistically significant (Table 1). The majority of the patients (~92%) had detectable gametocytemia at the time of recruitment.
Fig. 1.
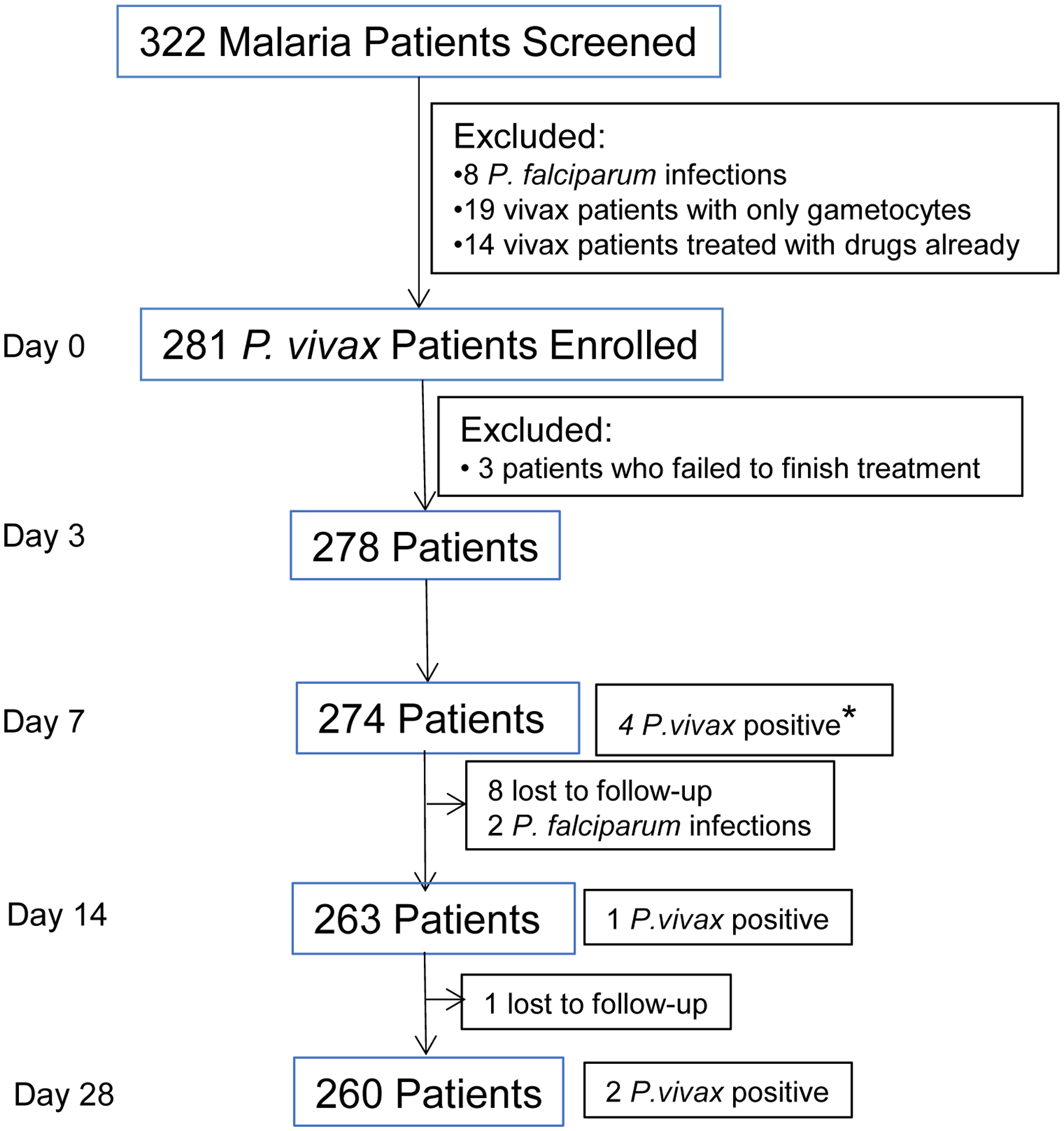
Flow chart of the follow-up of the directly observed chloroquine/primaquine (CQ/PQ) treatment in P. vivax patients during 2014–2016. * Of the four P. vivax positive patients, two had cleared parasitemias within three days of CQ/PQ treatment, whereas two had never cleared their parasitemias within 7 days of treatment.
Table 1.
Demographic and clinical features of the P. vivax patients at the time of enrollment.
| Patient characteristics | Values |
|---|---|
| Total no. of patients (% male) | 281 (53.7) |
| Age in years [median (range)] | 17 (3 – 59) |
| Febrile patients on day 0 (axillary temperature > 37.5 °C) [% (n)] | 90.0 (253/281) |
| Mean temperature (°C) (range) | 38.7 (36.0 – 41.0) |
| Asexual parasite density (/μl) on day 0 [geometric mean (range)] in age group* | |
| 3 – 7 (n =21) | 5422 (138 – 40040) |
| 8 – 13 (n = 111) | 3021 (22–57860) |
| ≥14 (n =149) | 2308 (22–58314) |
| Gametocytemia patients on day 0 [% (n)]* | 91.81 (258/281) |
| Gametocyte density (/μl) on day 0 [geometric mean (range)] | 1000 (0 – 10450) |
Asexual parasite densities compared between two groups. The P values between 3–7 and 8–13 groups, between 3–7 and ≥14 groups, and between 8–13 and ≥14 groups are 0.46, 0.11, and 0.14, respectively (Mann Whitney U test).
CQ/PQ treatment resulted in rapid fever clearance; from 90% of the patient being febrile at the initiation of the treatment, only 7.2% and 0.36% remained febrile at 24 h and 48 h after treatment. All patients were free of malaria symptoms on day 3 after treatment. Parasite clearance was also rapid, with 98.6% (277/281) of patients having cleared asexual blood parasitemia in 3 days (Fig. 2A). Out of the initial 258 patients with gametocytemia at enrollment, 76% (196/258) cleared gametocytemia by day 1, whereas 13.04% (3/23) patients without gametocytemia on admission developed gametocytemia on day 1 and 3 (Fig. 2B). On day 2, nine patients remained gametocytemic, whereas one without gametocytes at enrollment developed gametocytemia. Only three individuals did not clear gametocytemia on day 3. Between day 7 and 28, a total of 11 patients had patent gametocytemia, and one had detectable gametocytes throughout the follow-up period.
Fig. 2.
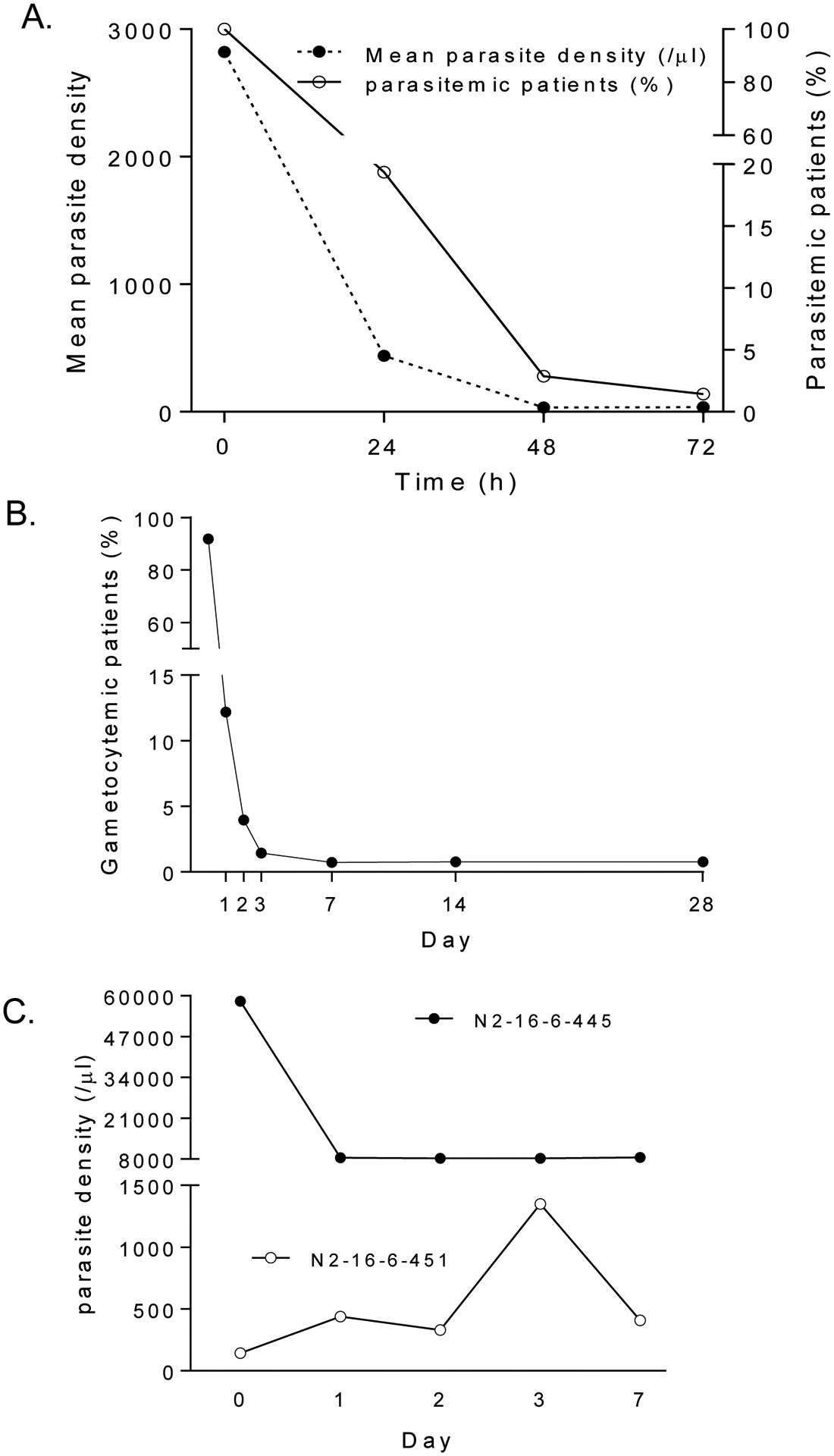
A. Mean parasite density (/μl) (dotted line and left y axis) for 281 P. vivax patients and percent of patients remaining parasitemic (solid line and right y axis) at the time of enrollment and after chloroquine/primaquine treatment. B. Percent of P. vivax patients that were gametocyte carriers at the time of enrollment and during the 28 day follow-up. C. Parasite densities in two patients (N2-16-6-445, filled circle; and N2-16-6-451, open circle) during the first 7 days after chloroquine/primaquine treatment.
Out of 281 enrolled patients, 274, 263 and 260 patients finished the 7-, 14-, and 28-day follow-up, respectively (Fig. 1). Four patients had parasitemia on day 7, giving a day-7 cumulative incidence of treatment failure of 1.46% (Table 2, Supplementary Table 1). Surprisingly, of the four patients who were parasitemic on day 7, two never cleared parasitemia within 7 days, although both became afebrile in two days. A 17-year old female (N2-16-6-445) had an initial drop of parasitemia upon treatment, but the parasite density remained at ~8,000/μl from day 1 through 7. The other patient (N2-16-6-451), a 19-year old male, did not seem to respond to the treatment and parasite density continually increased during the first 3 days, and day 7 parasite density was higher than that at the time of enrollment (Fig. 2C). On day 14, there was one additional patient who developed recurrent P. vivax parasitemia. Subsequently, two more patients were recorded with recurrent parasites, and the cumulative incidence rate of recurrence within 28 days reached 2.6% (Table 2). All the seven patients with recurrent parasites were treated with dihydroartemisinin-piperaquine, and all were responsive to the treatment. Since patients in the three age groups received different CQ treatment regimens, we wondered whether this might result in different drug dosages and lead to the difference in recurrence rate. We found no statistically significant difference in the cumulative hazard of recurrence between the 3–13 years and ≥14 years groups (Fig. 3).
Table 2.
Life table for cumulative incidence of recurrent parasitemia in P. vivax patients treated with chloroquine/primaquine.
| Day | Na | No. P. vivax failure | LFUb | Interval riskc | CIF% (95% CI)d |
|---|---|---|---|---|---|
| 3 | 278 | 0 | 0 | 0 | 0 |
| 7 | 274 | 4 | 0 | 0.0146 | 1.46 (0.05 – 2.87) |
| 14 | 263 | 1 | 10 | 0.0039 | 1.84 (0.23 – 3.45) |
| 28 | 260 | 2 | 1 | 0.0077 | 2.60 (0.68 – 4.51) |
The number of patients remaining at risk on the indicated day post-study enrollment.
LFU, number of patients lost to follow-up, including two P. falciparum patients on day 14.
Calculated as i[N – (LFU/2)]−1.
CIF, the cumulative incidence of failures (recurrent parasitemia), calculated as follows:
1 –{[1 – IRn] × [1 – CIF(n – 1)]}, where n is the day of the test and n – 1 is the prior interval.
Fig. 3.
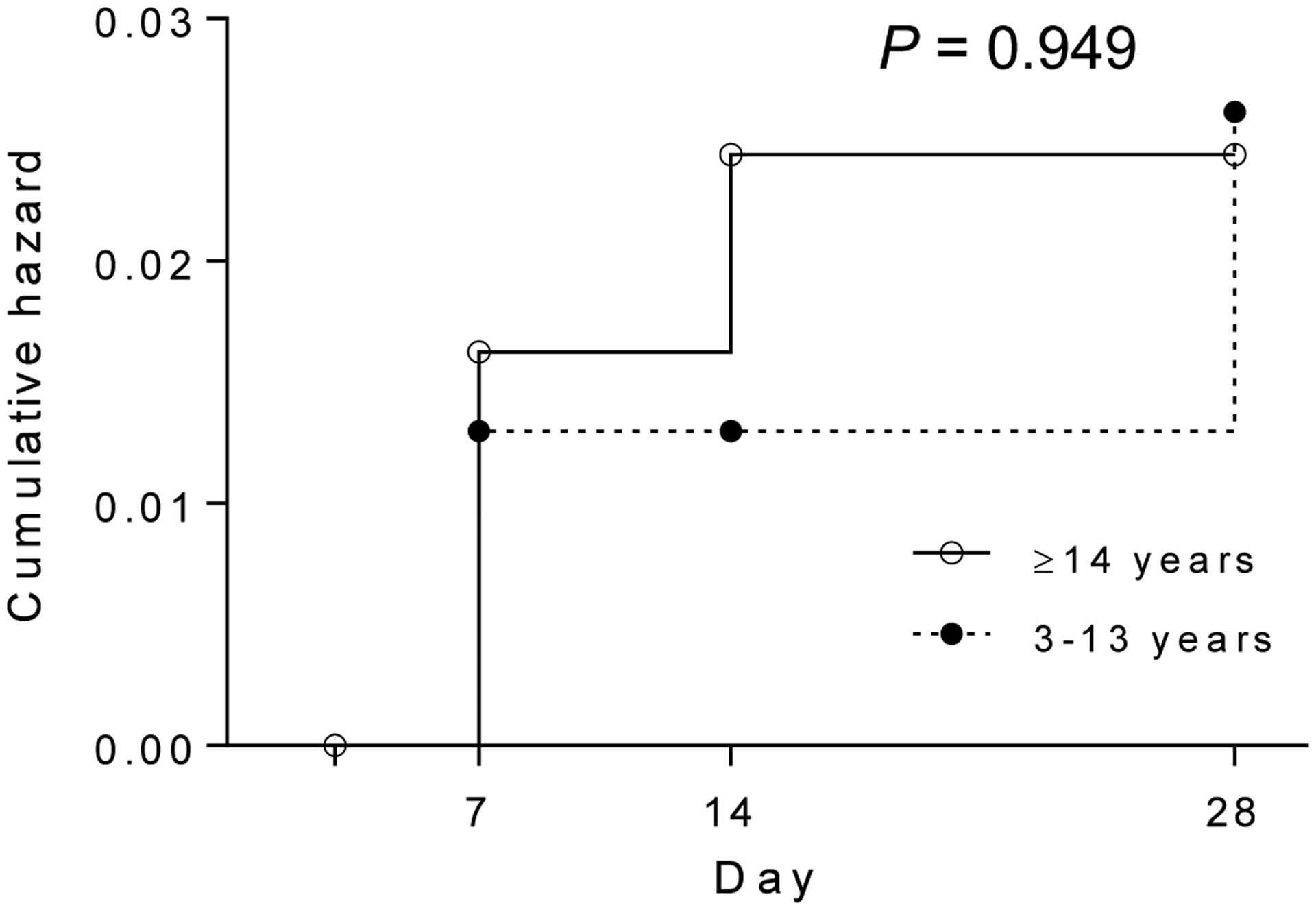
Cumulative hazard of recurrence between the 3–13 years and ≥14 years groups. P = 0.9494 (Log-rank Mantel-Cox test).
To determine whether the parasites responsible for the initial infection and recurrence were genetically similar, we genotyped the pvmsp1 gene in paired samples (day 0 and day of recurrence). PCR amplification and sequencing were successful for five patients out of the seven with recurrent parasitemia. For the five pairs of parasite isolates, the pvmsp1 genotypes at day 0 and the day of recurrence were the same in two patients (Fig. 4). The other three patients had different pvmsp1 genotypes at day 0 and the day of recurrence (Fig. 4).
Fig. 4.
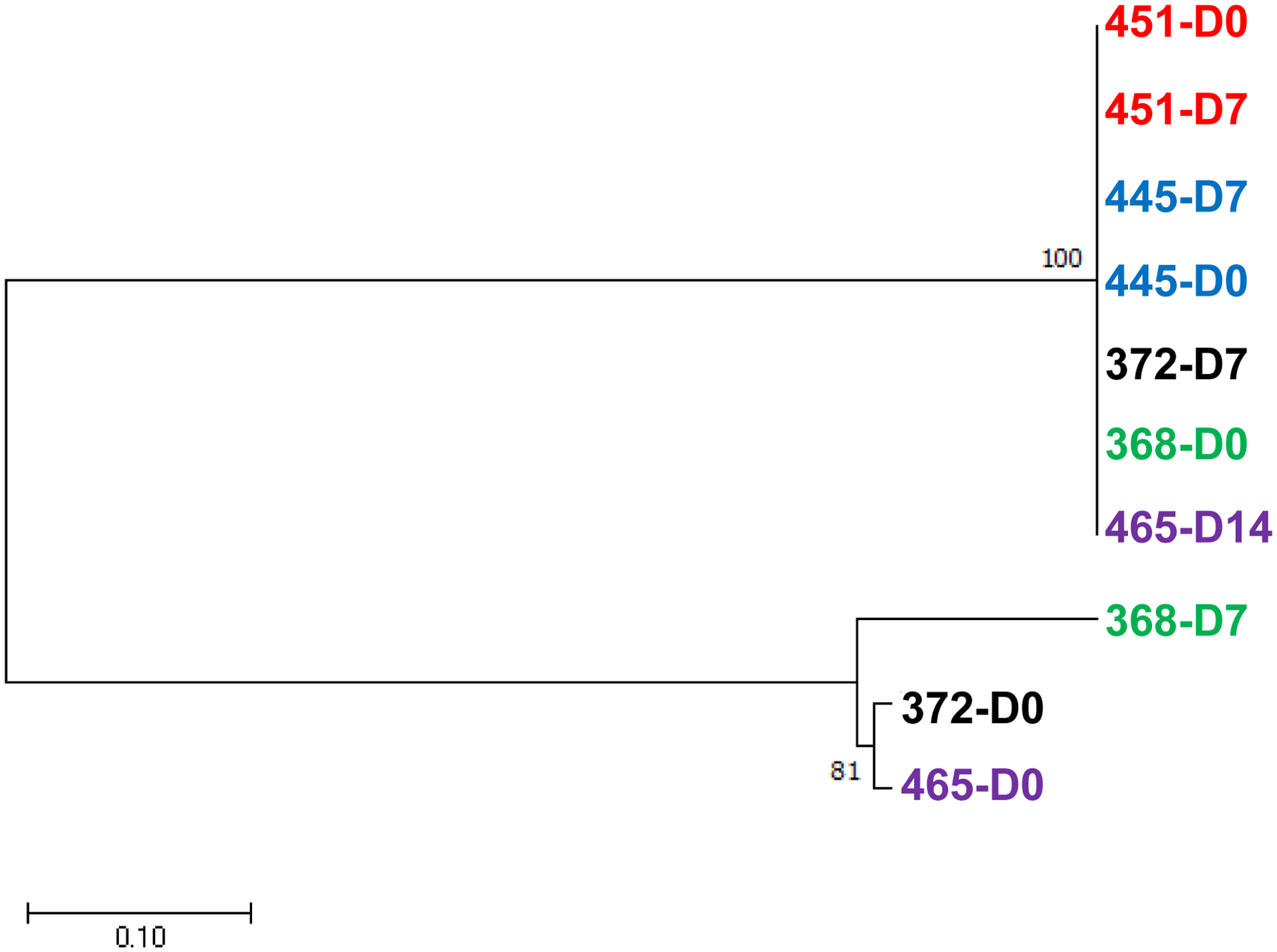
Phylogenetic tree of pvmsp1 genes in five pairs of parasites at the time of enrollment (D0) and recurrence (D7 or D14). The tree was constructed using the maximum likelihood method with 500 bootstraps. Two pvmsp1 sequences from the same patients were marked as the same colors.
4. Discussion
Monitoring therapeutic efficacy of drugs is very important for detecting emergence of resistance to existing antimalarial regimens and implementing timely drug policy changes. We acknowledge that the WHO and other authorities recommend withholding PQ therapy until day 28 after starting CQ therapy in order to directly measure CQ efficacy without interference by the known blood schizontocidal effects of PQ. That proved impractical at our study site because CQ/PQ is the routinely practiced standard of care. We thus instead estimated the efficacy of CQ combined with PQ against acute vivax malaria. This approach mirrors that taken in our earlier report from the same study site in northeastern Myanmar [21]. Efforts have been undertaken to also measure the efficacy of this regimen of radical cure against potential relapses with prolonged follow up.
The 28-day in vivo test of CQ efficacy cannot discriminate between recurrences due to recrudescence, relapse, or reinfection. The rationale at work supposes that normally effective levels of CQ persist in blood for at least 28 days in most people, therefore any parasitemia that develops within that period is resistant to CQ no matter where it originated [6, 28]. That rationale also supposes that parasitemias that fail to clear or those that recur relatively early (< 14 days) very probably derive from the parasitemia appearing at diagnosis at the start of the test. The same rationale is applied to the testing we performed, except that concurrent PQ therapy is very probably diminishing the rate of treatment failures that may occur without the PQ adjunct to CQ. The two infections we treated with CQ/PQ which failed to clear by day 7 appear to be resistant to the blood schizontocidal effects of both CQ and PQ. Similarly, the other three cases with recurrent parasitemias on days 7 and 14 may also be recrudenscences due to resistance to CQ despite the parasites on day 0 and the day of recurrence were genotypically different. P. vivax infections are very often highly complex consisting of multiple parasite strains [29, 30] the genotyping applied in this study had limited ability to detect minority strains that could have later recrudesced. Nonetheless, even if those new parasitemias had been derived from new infections, they had penetrated normally lethal levels of chloroquine which typically persist until day 35 after treatment [28].
In our earlier 2012 – 2013 study period we observed a 28-day cumulative incidence rate of therapeutic failure of CQ/PQ of 5.2% [21], whereas the present study measured that at 2.6%. We wondered if DOT in the current study would in part explain the higher efficacy. That may be a reasonable explanation given the normally efficacious blood schizontocidal activity of PQ for P. vivax [31]. A comparison of DOT and non-DOT CQ/PQ treatment at the Thai-Myanmar border showed that patient compliance with the 14-day PQ treatment under DOT improves the outcome of vivax malaria treatment [32].
Though the efficacy of CQ/PQ for treating uncomplicated acute vivax malaria remains relatively high in northeast Myanmar, this study confirmed the emergence of P. vivax parasites resistant to both CQ and PQ. Of the seven patients who had recurrent parasitemia during the follow-up period, five were within two weeks of initiating CQ/PQ therapy. Although we did not measure the blood CQ levels at the time of parasite recurrence, parasites within two weeks of the treatment were unlikely due to low CQ levels in the blood. There seems to be little doubt that CQ/PQ-resistant strains of P. vivax have been occurring in northeastern Myanmar for at least the past decade. This deserves close attention as this region has experienced persistent vivax malaria transmission and even vivax outbreaks in recent years [23], despite intensified malaria control during the elimination phase. This study accords with another recent study conducted in southern Myanmar, which also detected low levels of CQ resistance [22], indicating CQ resistance might be emerging in multiple sites of this country. It is noteworthy that the observed CQ resistance in P. vivax conforms to the “antimicrobial resistance” definition adopted by the World Health Organization [33], which requires case management with another antimalarial drug such as an ACT.
5. Conclusions
High-grade resistance to concurrent CQ and PQ therapy by asexual blood stages of P. vivax occurred at low frequencies in northeastern Myanmar since at least 2012. Continued monitoring of CQ/PQ efficacy in northeast Myanmar is warranted in connection with containing recent outbreaks and efforts to wholly eliminate this infection from the GMS by 2030. Travelers to Myanmar acquiring vivax malaria should be treated with an ACT along with hypnozoitocidal primaquine therapy to ensure a high probability of a good therapeutic response.
Supplementary Material
Appendix. Supplementary Table 1. Characteristics of P. vivax patients with recurrent parasitemias within 28 days after initiation of the directly observed chloroquine/primaquine treatment.
Acknowledgements
We want to thank the medical staff at the malaria clinics serving the IDP settlements for their participations in patient recruitment, malaria diagnosis and treatment.
Funding
This study was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health USA (U19AI089672 and R01AI128940). Z. Yang was supported by the National Natural Science Foundation of China (31860604 and U1802286) and by the major science and technology project of Yunnan (2018ZF0081). Y. Zhang, C. Li and X. Chen were supported by Yunnan Applied Basic Research Projects-Union Foundation (No. 2015FB034/01, 2017FE468-185, and 2018FE001-190).
Abbreviations:
- CQ
chloroquine
- PQ
primaquine
- GMS
Greater Mekong Subregion
- DOT
directly observed therapy
- IDP
internally displaced people
- CQR
CQ-resistant
- ACT
artemisinin-combination therapy
- WBC
white blood cell
Footnotes
Conflicts of interest
The authors affirm that they have no conflict of interest.
References
- [1].WHO. World Malaria Report 2015. 2016.
- [2].Baird JK. Asia-Pacific malaria is singular, pervasive, diverse and invisible. Int J Parasitol 2017;47:371–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Howes RE, Battle KE, Mendis KN, Smith DL, Cibulskis RE, Baird JK, et al. Global Epidemiology of Plasmodium vivax. Am J Trop Med Hyg 2016;95:15–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].WHO. World Malaria Report 2018. 2018.
- [5].WHO. Control and elimination of Plasmodium vivax malaria – A technical brief https://wwwwhoint/malaria/publications/atoz/9789241509244/en/ 2015.
- [6].Baird JK. Resistance to therapies for infection by Plasmodium vivax. Clin Microbiol Rev 2009;22:508–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Price RN, von Seidlein L, Valecha N, Nosten F, Baird JK, White NJ. Global extent of chloroquine-resistant Plasmodium vivax: a systematic review and meta-analysis. Lancet Infect Dis 2014;14:982–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Takala-Harrison S, Laufer MK. Antimalarial drug resistance in Africa: key lessons for the future. Ann N Y Acad Sci 2015;1342:62–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Schuurkamp GJ, Spicer PE, Kereu RK, Bulungol PK, Rieckmann KH. Chloroquine-resistant Plasmodium vivax in Papua New Guinea. Trans R Soc Trop Med Hyg 1992;86:121–2. [DOI] [PubMed] [Google Scholar]
- [10].Guthmann JP, Pittet A, Lesage A, Imwong M, Lindegardh N, Min Lwin M, et al. Plasmodium vivax resistance to chloroquine in Dawei, southern Myanmar. Trop Med Int Health 2008;13:91–8. [DOI] [PubMed] [Google Scholar]
- [11].Yohannes AM, Teklehaimanot A, Bergqvist Y, Ringwald P. Confirmed vivax resistance to chloroquine and effectiveness of artemether-lumefantrine for the treatment of vivax malaria in Ethiopia. Am J Trop Med Hyg 2011;84:137–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Thanh PV, Hong NV, Van NV, Louisa M, Baird K, Xa NX, et al. Confirmed Plasmodium vivax Resistance to Chloroquine in Central Vietnam. Antimicrob Agents Chemother 2015;59:7411–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Saravu K, Kumar R, Ashok H, Kundapura P, Kamath V, Kamath A, et al. Therapeutic Assessment of Chloroquine-Primaquine Combined Regimen in Adult Cohort of Plasmodium vivax Malaria from Primary Care Centres in Southwestern India. PLoS One 2016;11:e0157666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wangchuk S, Drukpa T, Penjor K, Peldon T, Dorjey Y, Dorji K, et al. Where chloroquine still works: the genetic make-up and susceptibility of Plasmodium vivax to chloroquine plus primaquine in Bhutan. Malar J 2016;15:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chu CS, Phyo AP, Lwin KM, Win HH, San T, Aung AA, et al. Comparison of the Cumulative Efficacy and Safety of Chloroquine, Artesunate, and Chloroquine-Primaquine in Plasmodium vivax Malaria. Clin Infect Dis 2018;67:1543–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Delacollette C, D’Souza C, Christophel E, Thimasarn K, Abdur R, Bell D, et al. Malaria trends and challenges in the Greater Mekong Subregion. Southeast Asian J Trop Med Public Health 2009;40:674–91. [PubMed] [Google Scholar]
- [17].Cui L, Yan G, Sattabongkot J, Cao Y, Chen B, Chen X, et al. Malaria in the Greater Mekong Subregion: heterogeneity and complexity. Acta Trop 2012;121:227–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Liu H, Yang HL, Tang LH, Li XL, Huang F, Wang JZ, et al. Monitoring Plasmodium vivax chloroquine sensitivity along China-Myanmar border of Yunnan Province, China during 2008–2013. Malar J 2014;13:364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Myat Phone K, Myint O, Myint L, Thaw Z, Kyin Hla A, Nwe Nwe Y. Emergence of chloroquine-resistant Plasmodium vivax in Myanmar (Burma). Trans R Soc Trop Med Hyg 1993;87:687. [DOI] [PubMed] [Google Scholar]
- [20].Marlar T, Myat Phone K, Aye Yu S, Khaing Khaing G, Ma S, Myint O. Development of resistance to chloroquine by Plasmodium vivax in Myanmar. Trans R Soc Trop Med Hyg 1995;89:307–8. [DOI] [PubMed] [Google Scholar]
- [21].Yuan L, Wang Y, Parker DM, Gupta B, Yang Z, Liu H, et al. Therapeutic responses of Plasmodium vivax malaria to chloroquine and primaquine treatment in northeastern Myanmar. Antimicrob Agents Chemother 2015;59:1230–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Htun MW, Mon NCN, Aye KM, Hlaing CM, Kyaw MP, Handayuni I, et al. Chloroquine efficacy for Plasmodium vivax in Myanmar in populations with high genetic diversity and moderate parasite gene flow. Malar J 2017;16:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhou G, Lo E, Zhong D, Wang X, Wang Y, Malla S, et al. Impact of interventions on malaria in internally displaced persons along the China-Myanmar border: 2011–2014. Malar J 2016;15:471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Li P, Zhao Z, Xing H, Li W, Zhu X, Cao Y, et al. Plasmodium malariae and Plasmodium ovale infections in the China-Myanmar border area. Malar J 2016;15:557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Liu H, Feng G, Zeng W, Li X, Bai Y, Deng S, et al. A more appropriate white blood cell count for estimating malaria parasite density in Plasmodium vivax patients in northeastern Myanmar. Acta Trop 2016;156:152–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Singh B, Bobogare A, Cox-Singh J, Snounou G, Abdullah MS, Rahman HA. A genus- and species-specific nested polymerase chain reaction malaria detection assay for epidemiologic studies. Am J Trop Med Hyg 1999;60:687–92. [DOI] [PubMed] [Google Scholar]
- [27].Leclerc MC, Gauthier C, Villegas L, Urdaneta L. Genetic diversity of merozoite surface protein-1 gene of Plasmodium vivax isolates in mining villages of Venezuela (Bolivar State). Acta Trop 2005;95:26–32. [DOI] [PubMed] [Google Scholar]
- [28].Baird JK, Leksana B, Masbar S, Fryauff DJ, Sutanihardja MA, Suradi, et al. Diagnosis of resistance to chloroquine by Plasmodium vivax: timing of recurrence and whole blood chloroquine levels. Am J Trop Med Hyg 1997;56:621–6. [DOI] [PubMed] [Google Scholar]
- [29].Lin JT, Hathaway NJ, Saunders DL, Lon C, Balasubramanian S, Kharabora O, et al. Using Amplicon Deep Sequencing to Detect Genetic Signatures of Plasmodium vivax Relapse. J Infect Dis 2015;212:999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zhong D, Lo E, Wang X, Yewhalaw D, Zhou G, Atieli HE, et al. Multiplicity and molecular epidemiology of Plasmodium vivax and Plasmodium falciparum infections in East Africa. Malar J 2018;17:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Pukrittayakamee S, Chantra A, Simpson JA, Vanijanonta S, Clemens R, Looareesuwan S, et al. Therapeutic responses to different antimalarial drugs in vivax malaria. Antimicrob Agents Chemother 2000;44:1680–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Maneeboonyang W, Lawpoolsri S, Puangsa-Art S, Yimsamran S, Thanyavanich N, Wuthisen P, et al. Directly observed therapy with primaquine to reduce the recurrence rate of plasmodium vivax infection along the Thai-Myanmar border. Southeast Asian J Trop Med Public Health 2011;42:9–18. [PubMed] [Google Scholar]
- [33].Hanscheid T, Hardisty DW. How “resistant” is artemisinin resistant malaria? - The risks of ambiguity using the term “resistant” malaria. Travel Med Infect Dis 2018;24:23–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix. Supplementary Table 1. Characteristics of P. vivax patients with recurrent parasitemias within 28 days after initiation of the directly observed chloroquine/primaquine treatment.


