Abstract
The novel Coronavirus SARS-CoV-2 is the viral pathogen responsible for the ongoing global pandemic, COVID-19 (Coronavirus disease 2019). To date, the data recorded indicate 1.62 Mln deaths and 72.8 Mln people infected (WHO situation report Dec 2020). On December 27, the first anti-COVID-19 vaccinations started in Europe. There are no direct antivirals against SARS-CoV-2. Understanding the pathophysiological and inflammatory/immunological processes of SARS-CoV-2 infection is essential to identify new drug therapies. In the most severe COVID-19 cases, an unregulated immunological/inflammatory system results in organ injury that can be fatal to the host in some cases. Pharmacologic approaches to normalize the unregulated inflammatory/immunologic response is an important therapeutic solution. Evidence associates a non-regulation of the “complement system” as one of the causes of generalized inflammation causing multi-organ dysfunction. Serum levels of a complement cascade mediator, factor “C5a”, have been found in high concentrations in the blood of COVID-19 patients with severe disease. In this article we discuss the correlation between complement system and COVID-19 infection and pharmacological solutions directed to regulate.
Keywords: COVID-19, SARS-CoV-2, Complement system, Inflammatory/immunological, Eculizumab
1. Introduction
1.1. SARS-CoV-2, clinical features
The new Coronavirus SARS-CoV-2 (COVID-19) is the cause of Severe Acute Respiratory Syndrome (SARS), a severe form of viral pneumonia. The virus spread rapidly from China to the rest of the world in a very short time and with considerable intensity and severity creating a “global emergency”. To date the data recorded indicated about 1.62 million deaths and 72.8 million people infected [52]. SARS-CoV-2 is an RNA virus similar for about 80% of the viral genome to SARS-CoV (responsible for the 2003 outbreak) [53]. In vitro studies confirm that the virus penetrates human cells by binding to ACE-2 protein, the angiotensin-converting enzyme 2, which is part of the renin-angiotensin system (RAS) (Guoping et al., 2020) and is considered as a possible receptor protein. Patients infected with this virus are also known to exhibit changes and variations in the concentrations of enzymatic components of the RAS during days of illness [20], [41], [42]. SARS-CoV-2 infection can also have a totally asymptomatic or mildly symptomatic course. In a percentage of cases, the infection has a course consisting of an asymptomatic or mildly symptomatic initial phase and subsequent phases characterized by a generalized inflammatory state causing multi-organ tissue injury and respiratory distress syndrome[50], [3]. According to the observational studies conducted, most of the patients considered as severe cases have bilateral interstitial pneumonia and a hyperactive inflammatory state that is not only localized in the lung tissue, but in all tissues of the body, causing multi-organ dysfunction and high risk of thrombosis [40]. The presence of generalized inflammation in all organs and the resulting increased risk of thrombosis requires timely anti-inflammatory/immunomodulatory and anticoagulant treatment. The generalized inflammatory state responsible for severe injury is caused by hyperactivation of components of the host inflammatory/immune system characterized by a sudden and elevated release of cytokines, an event called a “cytokine storm” that leads to severe and sometimes fatal tissue damage. A major contributor to this dysregulation of the inflammatory/immune system is attributed to an overactive and unregulated complement system [15], [16]. In fact, elevated serum levels of C5a (complement cascade component) have been reported in blood in COVID-19 patients with severe staging, suggesting an important hyperactivation of the complement system.
2. Discussion
2.1. Complement system and SARS-CoV-2
The pathophysiology of COVID-19 infection and the mechanisms underlying the severe tissue damage is still not fully understood and clear. In the early stages of infection, an adequate inflammatory/immunological response is of paramount importance for the host organism to fight the virus and avoid more severe stages of infection. In the more severe stages of infection, an inadequate and dysregulated inflammatory/immunological response to the infection may be responsible for elevated accumulation of immune cells and inflammatory mediators (cytokine storm) in tissues which may lead to damage of the lung architecture and multi-organ damage. In the early stages of viral infection, a proper initial inflammatory response attracts T cells to the site of infection where they can fight and eliminate virus-infected cells. In the more severe stages of infection, if the immune response has not been sufficient to completely eliminate the viral load, a hyperactive and unregulated inflammatory/immunologic response, induced by a cytokine storm, can lead to severe lung injury, generalized inflammation, and multi-organ dysfunction [39]. Evidence has shown that in severe cases of COVID-19, the high concentration of proinflammatory mediators (particularly IL-2, IL-6, and TNF-α) is responsible for tissue injury. A key contributor to the state of excessive inflammatory activation in severe cases is the complement system. The complement system is part of the immune system that provides an innate defense against pathogens and mediates inflammatory responses. It consists of numerous plasma proteins that, when activated, interact with various cells and mediators of the immune system [30]. These activations and interactions vary throughout the stages of a viral infection, the complement system is responsible for a dual action, innate immunity response, as well as in the events that occur later during the adaptive immune reaction. Thus, the complement response can lead to an acute inflammatory reaction that, by the immune system, aims to eliminate host pathogens. In some cases, however, as was demonstrated during COVID-19 infection, an overactive and unregulated complement system can be deleterious to the host itself and contribute to the state of systemic inflammation, however, a lack of an inadequate contribution of the complement system can promote viral replication and infection and a failure of the host's defenses to respond. Adequate and regulated activation of the complement system is therefore essential in combating COVID-19 infection. From a molecular perspective, complement is well known to promote immune cell activation and pro-inflammatory states; complement mediators, particularly C3a and C5a are able to activate neutrophils, mast cells, monocytes/macrophages, T cells, and B cells [28]. As noted above, the mediator C5a, in high concentrations in patients with moderate and severe COVID-19 along with elevated IL-6 [4], has been shown in plasma, suggesting that complement plays a key role. The SARS-CoV-2 genome encodes for spike protein (S), which is essential for entry into cells through the ACE-2 membrane. The complement system can be activated through three pathways, the classical, alternative, and lectin pathway, SARS-CoV-2 spike protein (S) is detected by mannan-binding lectin (MBL) which induces complement activation in lectin-mediated SARS-CoV infection [22], [54]. The classical pathway is activated by binding of IgM- or IgG-induced natural antibodies, which form immunocomplexes with viral antigens (Fig. 1 ).
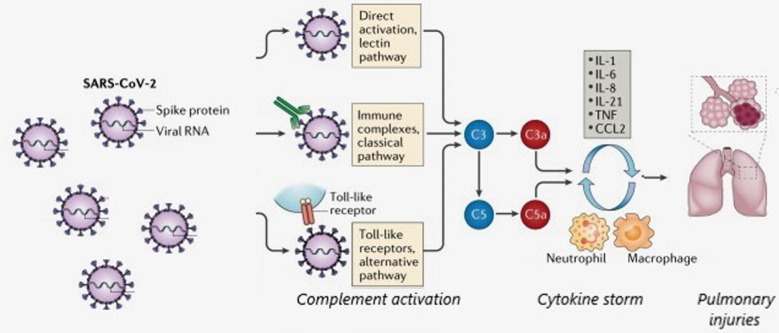
Fig. 1.
SARS-CoV-2 and activation of complement system. Schematic representation of complement activation by SARS-CoV-2. SARS-CoV-2 infection mediates complement system activation via the classical, lectin and alternative pathways. The classical pathway is activated by the binding of IgM or IgG-induced antibodies, which form immunocomplexes with viral antigens, the lectin pathway is activated by the binding of SARS-CoV-2 proteins to an MBL protein (Mannan-binding lectin). Complement activation leads to the formation of a membrane attachment complex (MAC) on the surface of the target cells. C3a and C5a mediate complement actions such as inflammation, coagulation, platelet activation, leukocyte recruitment and endothelial cell activation, which are observed in COVID-19 infection. If hyperactivated, the complement leads to an inflammatory pulmonary complement-mediated lesion. The C5 or C3 inhibition, blocking the generation of proinflammatory mediators.
Activated components of the complement system are important effector molecules that attract, activate, and regulate innate and adaptive immune cells [25]. Studies have shown that there is a correlation between complement and the coagulation cascade (Riedl et al., 2020; [34]). Several evidences show that patients with severe COVID-19 have a hypercoagulable state [6]. The hypothesis that dysregulated complement activation may also be responsible for hypercoagulability in severe COVID-19 patients is not fully understood [18]. Exposure of the endothelium to C5b-9 leads to the release of VWF (Von Willembrand factor) [2], [8], [9] which leads to platelet activation and aggregation, contributing to a prothrombotic state with thrombus formation and arteriolar occlusion and thrombotic microangiopathy (TMA) [33]. However, although the role of complement in acute respiratory distress syndrome caused by SARS-CoV (2003 outbreak) is known, its role in COVID-19 infection has not yet been fully investigated and elucidated [17]. The complement system is constantly active and thus requires tight regulation [48], [49]. Therefore, complement blockade in a hyperactive state caused by viral infection might play an important role in halting the progression of TMA. Based on the above, the pharmacological hypothesis of terminal complement pathway blockade represents a potential therapeutic option to reduce the inflammatory state and the risk of thrombosis in the most severe stages of COVID-19 infection. Examples of pathological situations with unregulated complement proteins and subsequent tissue injury include atypical hemolytic uremic syndrome (HUS), a rare disorder characterized by a damaged endothelial surface of small vessels with fibrin deposition and platelet aggregation resulting in microangiopathy.
2.2. Pharmacological approach with a “terminal complement inhibitor”
On December 27, 2020, the first COVID-19 vaccinations began in Europe. Currently, drug therapy management of the COVID-19 positive patient is limited to symptomatic and palliative treatment. To date, there are no antivirals directed against SARS-CoV-2. A potential pharmacological therapeutic approach against COVID-19 is modifying and regulating agents of the complement cascade. Clinical signs of severely ill patients with COVID-19 showed significant complement activation [15] suggesting that blocking the complement cascade in the most severe stages could be an effective therapeutic target to avoid severe severe complications. Evidence has shown rapid clinical improvement in patients with COVID-19 mediated by complement cascade inhibitory agents [29], [5]. In this direction, a pharmacological solution to prevent the multi-organ inflammatory alteration and the risk of thrombosis is the blockade of the complement protein C5, thus inhibiting the activation of the terminal portion of the cascade with the antibody Eculizumab [35] or Ravulizumab. Drug therapy with Eculizumab in particular has already been shown to be effective in thrombotic, hematologic and inflammatory diseases [51]. Through its mechanism of action, it inhibits cleavage into C5a and C5b and prevents the formation of the C5b-9 complex of the terminal portion of the complement cascade by maintaining the first components of complement activation that are essential for opsonization of microorganisms and clearance of immune complexes [37], [38]. The use of Eculizumab could exert a favorable effect by blocking the proinflammatory and prothrombotic actions of the end products of the complement cascade activated by SARS-CoV-2, while maintaining the activity of the first components of complement activation that are critical and essential for the activation of the adaptive immune response. Several clinical trials are currently underway (https://clinicaltrials.gov/) to test the off-label use of Eculizumab for the treatment of patients with COVID-19. Importantly, it should be added that therapeutic treatment with an anti-C5 antibody has resulted in important improvements in lung function and decreased systemic inflammation [15]. However, inhibition of the complement system by these agents acting on terminal C5 may be partial, allowing the activity of other complement components to be altered and uncontrolled. Other complement inhibitors acting on other targets are currently in clinical development. Inhibition of the complement system has the therapeutic potential to stop systemic inflammation affecting vital organs, in the most severe COVID-19 cases, however, the time window of administration, dosing, and patient population that may benefit from it must be fully defined, considering that drugs are not free of adverse reactions [26], including Eculizumab or Ravulizumab. (e.g., respiratory tract infections with common frequency). However, the use of Eculizumab or Ravulizumab might be effective in severe patients with COVID-19, to reduce the hyperactive inflammatory state caused by the cytokine storm, but not effective in the early stages of COVID-19 infection when the inflammatory/immune system needs to fight viral replication and be fully functional.
The identification of new effective and safe therapeutic solutions for COVID-19 positive patients is a great challenge for the whole scientific world [10], [11], [12], [13], [14], [41], [42], [43], [44], [45], [46].
Inhibiting terminal complement factor could be an important strategy to reduce the inflammatory state and the risk of thrombosis that can cause severe injury and in some cases death. Well-structured clinical studies will provide us with the clinical evidence needed to investigate this important scientific hypothesis. Evidence shows that the acute respiratory syndrome caused by SARS-CoV-2, is closely associated with activation of the C3 component of complement [19]. This suggests that C3 inhibition may alleviate the inflammatory pulmonary complications of SARS-CoV-2 infection [24]. Furthermore, the upstream placement of C3 signaling in the innate immune cascade further supports the broader anti-inflammatory potential of C3 blockade with pharmacological agents [7]. C3 inhibition could simultaneously block C3a and C5a generation, as well as intrapulmonary C3 activation and IL-6 release from alveolar macrophages, or other cells expressing C3a receptors (C3aRs) and/or C5a receptors (C5aRs), thereby ameliorating lung injury [23], Proximal complement inhibitors (which target C3 or its upstream activators) might be more effective in combating severe COVID-19 stages than C5 inhibitors, but these hypotheses have yet to be demonstrated by clinical evidence. Several clinical trials are underway to test complement system-modifying agents by different targets (Fig. 2 ). Important information such as the right time window of administration for optimal intervention, the patient populations that could benefit from pharmacological modulation of complement have yet to be established.
Fig. 2.
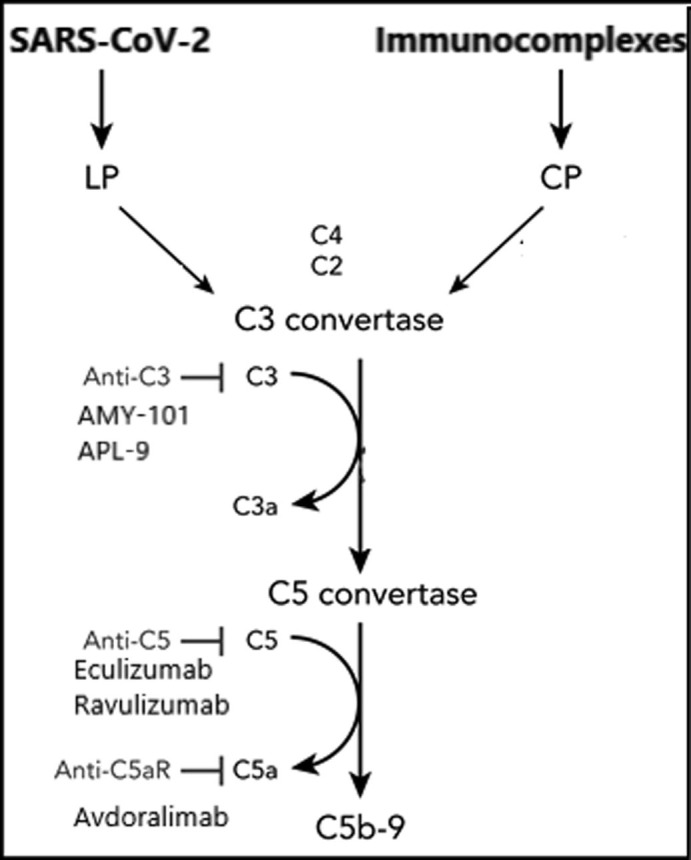
Schematic representation summarizing the different targets of the agents acting on the complement system.
2.3. Trials clinic on going
To date, many clinical trials are underway to investigate the efficacy of pharmacological approaches that act on different molecular targets of the complement system. Specifically, clinical trial NCT04395456 investigates the efficacy and safety of AMY-101, a potent C3 inhibitor, for the management of patients with ARDS caused by SARS-CoV-2 infection, clinical trial NCT04402060 evaluates the safety and efficacy of APL-9, a potent C3 inhibitor in adults with mild to moderate ARDS (acute respiratory distress syndrome) caused by COVID-19 who are hospitalized and require supplemental oxygen therapy with or without mechanical ventilation. Clinical trial NCT04346797 evaluates the efficacy and safety of Eculizumab in patients with COVID-19 infection, clinical trial NCT04369469 evaluates the efficacy, safety, pharmacokinetics and pharmacodynamics of ravulizumab administered in adult patients with coronavirus 2019 disease (COVID-19), severe pneumonia, acute lung injury or acute respiratory distress syndrome. Clinical trial NCT04382755 evaluates the efficacy of a potent C5 inhibitor; Zilucoplan, in COVID-19 patients, clinical trial NCT04371367 investigates the efficacy of Avdoralimab an Anti-C5aR antibody, in patients with COVID-19 severe pneumonia. Finally, also under clinical investigation are C1 esterase inhibitors, which block the classical complement pathway. Specifically, clinical trial NCT04414631 aims to analyze whether administration of conestat alfa for 72 h beyond standard of care (SOC) in patients hospitalized with non-critical SARS-CoV-2 pneumonia reduces the risk of disease progression to Acute Lung Injury (ALI) and Acute Respiratory Distress Syndrome (ARDS). Clinical trial NCT04530136 is designed to evaluate whether the addition of recombinant human C1 esterase inhibitor (rhC1INH) (Ruconest) to standard of care (SOC) in patients hospitalized for COVID-19 stage II infection can reduce the risk of disease progression (Table 1 ).
Table 1.
Clinical trials on going of complement-modifying agents in COVID-19 patients.
| Clinical trials | ||
|---|---|---|
| NCT number | Drug | Mechanism of action |
| NCT04395456 | AMY-101 | C3 inhibitor |
| NCT04402060 | APL-9 | C3 inhibitor |
| NCT04346797 | Eculizumab | C5 inhibitor |
| NCT04369469 | Ravulizumab | C5 inhibitor |
| NCT04382755 | Zilucoplan | C5 inhibitor |
| NCT04371367 | Avdoralimab | Anti-C5aR |
| NCT04414631 | conestat alfa | C1 esterase inhibitors |
| NCT04530136 | Ruconest | C1 esterase inhibitors |
2.4. Considerations
Several complement inhibitory agents are being tested in COVID-19 patients, aimed at regulating targets such as C3, C5, C5a, and C5aR. However, the various pharmacological agents may have differences and potential advantages in targeted inhibition of various targets. For example, blockade of C5a leaves intact the formation of C5b-9 (the so-called membrane attack complex), which is a crucial component of host defense, particularly bacterial lysis. In fact, an upstream blockade of complement pathways will inevitably affect the formation of the membrane attack complex, such pharmacological intervention could put COVID-19 patients at risk of infection. Studies have shown that targeted blockade of C5a is necessary to completely inhibit inflammation [36]. In a phase 2 study [47] a significant temporary increase in D-dimer was observed at the start of Anti-C5a antibody therapy, this suggests a direct or indirect profibrinolytic effect and is consistent with the lower pulmonary embolism rate observed in the treatment group versus the control group [27], [1]. Activation of coagulation in COVID-19 could be initiated by direct virus-induced endothelial injury resulting in upregulation of tissue factor, suppressed fibrinolysis and production of other procoagulant proteins [31], [32]. In particular, activation of C5a has been shown to directly induce endothelial tissue factor upregulation [21], activation of neutrophil-mediated coagulation, and to change inflammatory cells from a profibrinolytic (t-PA release) to a prothrombotic phenotype. In these advanced stages of COVID-19, C3 inhibition has the potential to largely control not only ARDS but also systemic inflammation affecting the microvascular beds of the kidney, brain, and other vital organs, which appears to be a complication in severe cases. Complement is a key player in protective immunity against pathogens, but its excessive or deregulated activation can result in collateral tissue injury.
3. Conclusions
3.1. Future prospectives and suggestions
SARS-CoV-2 can cause an abnormal inflammatory/immunologic response responsible for the most severe complications and tissue damage, in some cases fatal. Regulation of the inflammatory/immunologic response in patients with ongoing SARS-CoV-2 infection with specific pharmacologic agents may be of great clinical benefit. In particular, an essential element of the immune system, termed the “complement system,” has been shown to be responsible for much of the inflammatory/immunologic dysregulation and cytokine cascade that occurs in the most severe phases of viral infection. Pharmacological modulation of the complement system (including in combination with other immunomodulants and anticoagulants) may be useful in managing the dysregulated and generalized inflammatory state, endothelial injury, and hypercoagulable state and preventing COVID-19 cases from having severe complications. Too many questions remain unanswered. First, what is the optimal time to initiate anti-complement therapy? Which protein of the complement system is the most effective target against COVID-19? Will treatment with anti-complement therapies increase the risk of complications from other infections? And finally, does complement inhibition reduce the risk of thrombotic events? Ongoing clinical trials will provide the necessary answers.
Founds
None.
Copyright
The authors certify that the manuscript is original, never submitted to other journal for publication before. All authors contributed equally to the manuscript and had the opportunity to revise and approve the final text.
Disclosure statement
Dr. A. Vitiello has nothing to disclose.
Dr. F. Ferrara has nothing to disclose.
Dr. R. La Porta has nothing to disclose.
Dr.ssa V. D’Aiuto has nothing to disclose.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Ackermann M., Verleden S.E., Kuehnel M. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N. Engl. J. Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bettoni S. Interaction between multimeric von Willebrand factor and complement: a fresh look to the pathophysiology of microvascular thrombosis. J. Immunol. 2017;199(3):1021–1040. doi: 10.4049/jimmunol.1601121. [DOI] [PubMed] [Google Scholar]
- 3.Chen N., Zhou M. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cugno M. Complement activation in patients with COVID-19: A novel therapeutic target. J. Allergy Clin. Immunol. 2020;146(1):215–217. doi: 10.1016/j.jaci.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diurno F., Numis F.G., Porta G., Cirillo F., Maddaluno S., Ragozzino A., Facchini G. Eculizumab treatment in patients with COVID19: Preliminary results from real life ASL Napoli 2 Nord experience. Eur. Rev. Med. Pharmacol. Sci. 2020;24:4040–4047. doi: 10.26355/eurrev_202004_20875. [DOI] [PubMed] [Google Scholar]
- 6.Emert R., Shah P., Zampella J.G. COVID-19 and hypercoagulability in the outpatient setting. Thromb. Res. 2020;192:122–123. doi: 10.1016/j.thromres.2020.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fang S., Wang H., Lu L., Jia Y., Xia Z. Decreased complement C3 levels are associated with poor prognosis in patients with COVID-19: a retrospective cohort study. Int. Immunopharmacol. 2020;89(Pt A) doi: 10.1016/j.intimp.2020.107070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng S., Liang X., Kroll M.H., Chung D.W., Afshar-Kharghan V. von Willebrand factor is a cofactor in complement regulation. Blood. 2015;125(6):1034–1037. doi: 10.1182/blood-2014-06-585430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrara F. Antirheumatic in SARS-CoV-2: benefit or risk? Italian J. Med. 2020;14(2):114–115. doi: 10.4081/itjm.2020.1290. [DOI] [Google Scholar]
- 10.Ferrara F., Granata G., Pelliccia C., La Porta R., Vitiello A. The added value of pirfenidone to fight inflammation and fibrotic state induced by SARS-CoV-2: Anti-inflammatory and anti-fibrotic therapy could solve the lung complications of the infection? Eur. J. Clin. Pharmacol. 2020 Nov;76(11):1615–1618. doi: 10.1007/s00228-020-02947-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrara F., Porta R., Santilli P., D'Aiuto V., Vitiello A. Are multiple sclerosis therapies safe in severe acute respiratory syndrome coronavirus 2 times? Indian J Pharmacol. 2020;52(5):441–442. doi: 10.4103/ijp.IJP_417_20. PMID: 33283779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrara F., Porta R., D'Aiuto V., Vitiello A. Remdesivir and COVID-19. Ir. J. Med. Sci. 2020;17:1–2. doi: 10.1007/s11845-020-02401-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.F. Ferrara, A. Vitiello, Potential pharmacological approach in the regulation of ACE-2 and DPP-IV in diabetic COVID-19 patient. Italian J. Med., (AOP). doi: 10.4081/itjm.2020.1435.
- 14.F. Ferrara, A. Vitiello, Scientific and pharmacological rationale for the treatment of cardiac damage caused by covid 19 (Published on 17 December 2020) Discovery Medicine / No 161 Vol.30 Dec 2020. [PubMed]
- 15.T. Gao, M. Hu, X. Zhang, H. Li, L. Zhu, H. Liu, C. Cao, Highly pathogenic coronavirus N protein aggravates lung injury by MASP2-mediated complement over-activation. 2020. MedRxiv. doi: 10. 1101/2020.03.29.20041962.
- 16.Li G., He X., Zhang L., Ran Q., Wang J., Xiong A. Assessing ACE2 expression patterns in lung tissues in the pathogenesis of COVID-19. J. Autoimmun. 2020;112 doi: 10.1016/j.jaut.2020.102463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.L.E. Gralinski, T.P. Sheahan, T.E. Morrison, V.D. Menachery, K. Jensen, S.R. Leist, Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis. mBio. 2018;9 e01753-18. [DOI] [PMC free article] [PubMed]
- 18.Gavriilaki E., Brodsky R.A. Severe COVID-19 infection and thrombotic microangiopathy: success does not come easily. Br. J. Haematol. 2020;189(6):e227–e230. doi: 10.1111/bjh.16783. [DOI] [PubMed] [Google Scholar]
- 19.J.C. Holter, Systemic complement activation is associated with respiratory failure in COVID-19 hospitalized patients, et al Proceedings of the National Academy of Sciences Oct 2020, 117 (40) 25018-25025; 10.1073/pnas.2010540117. [DOI] [PMC free article] [PubMed]
- 20.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ikeda K., Nagasawa K., Horiuchi T., Tsuru T., Nishizaka H., Niho Y. C5a induces tissue factor activity on endothelial cells. Thromb. Haemost. 1997;77:394–398. [PubMed] [Google Scholar]
- 22.Ip W.K., Chan K.H., Law H.K., Tso G.H., Kong E.K., Wong W.H. Mannose-binding lectin in severe acute respiratory syndrome coronavirus infection. J. Infect. Dis. 2005;191(10):1697–1704. doi: 10.1086/429631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim A.H.J., Wu X., Atkinson J.P. The beneficial and pathogenic roles of complement in COVID-19. Cleve Clin. J. Med. 2020 doi: 10.3949/ccjm.87a.ccc065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kulkarni H.S., Atkinson J.P. Targeting complement activation in COVID-19. Blood. 2020;136(18):2000–2001. doi: 10.1182/blood.2020008925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laumonnier Y., Karsten C.M., Köhl J. Novel insights into the expression pattern of anaphylatoxin receptors in mice and men. Mol. Immunol. 2017;89:44–58. doi: 10.1016/j.molimm.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 26.Lombardi N., Crescioli G., Bettiol A., Marconi E., Vitiello A., Bonaiuti R. Characterization of serious adverse drug reactions as cause of emergency department visit in children: a 5-years active pharmacovigilance study. BMC Pharmacol. Toxicol. 2018;19(1):16. doi: 10.1186/s40360-018-0207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magro C., Mulvey J.J., Berlin D. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl. Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Markiewski M.M., Lambris J.D. The role of complement in inflammatory diseases from behind the scenes into the spotlight. Am. J. Pathol. 2007;171(3):715–727. doi: 10.2353/ajpath.2007.070166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mastaglio S., Ruggeri A., Risitano A.M., Angelillo P., Yancopoulou D., Mastellos D.C. The first case of COVID-19 treated with the complement C3 inhibitor AMY-101. Clin. Immunol. 2020;215 doi: 10.1016/j.clim.2020.108450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mastellos D., Morikis D., Isaacs S.N., Holland M.C., Strey C.W., Lambris J.D. Complement: structure, functions, evolution, and viral molecular mimicry. Immunol. Res. 2003;27:367–385. doi: 10.1385/IR:27:2-3:367. [DOI] [PubMed] [Google Scholar]
- 31.Noris M., Benigni A., Remuzzi G. The case of complement activation in COVID-19 multiorgan impact. Kidney Int. 2020;98:314–322. doi: 10.1016/j.kint.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riedl M., Fakhouri F., Le Quintrec M., Noone D.G., Jungraithmayr T.C., Fremeaux-Bacchi V. Spectrum of complement-mediated thrombotic microangiopathies: pathogenetic insights identifying novel treatment approaches. Semin. Thromb. Hemost. 2014;40:444–464. doi: 10.1055/s-0034-1376153. [DOI] [PubMed] [Google Scholar]
- 33.Ota H., Fox-Talbot K., Hu W., Qian Z., Sanfilippo F., Hruban R.H. Terminal complement components mediate release of von Willebrand factor and adhesion of platelets in arteries of allografts. Transplantation. 2005;79(3):276–281. doi: 10.1097/01.TP.0000146195.76904.D3. [DOI] [PubMed] [Google Scholar]
- 34.Perico L., Benigni A., Casiraghi F., Ng L.F.P., Renia L., Remuzzi G. Immunity, endothelial injury and complement-induced coagulopathy in COVID-19. Nat. Rev. Nephrol. 2021;17(1):46–64. doi: 10.1038/s41581-020-00357-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reis E.S. Safety profile after prolonged C3 inhibition. Clin. Immunol. 2018;197:96–106. doi: 10.1016/j.clim.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riedemann N.C., Habel M., Ziereisen J. Controlling the anaphylatoxin C5a in diseases requires a specifically targeted inhibition. Clin. Immunol. 2017;180:25–32. doi: 10.1016/j.clim.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 37.Risitano A.M., Mastellos D.C., Huber-Lang M., Yancopoulou D., Garlanda C., Ciceri F., Lambris J.D. Author Correction: Complement as a target in COVID-19? Nat. Rev. Immunol. 2020;20(7):448. doi: 10.1038/s41577-020-0366-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stahel P.F., Barnum S.R. Complement inhibition in Coronavirus Disease (COVID)-19: a neglected therapeutic option. Front. Immunol. 2020;11:1661. doi: 10.3389/fimmu.2020.01661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020;20(6):363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toshiaky I., Levy J.H., Levi M., Thachil J. Coagulopathy in COVID-19. J. Thromb. Haemost. 2020;18(9):2103–2109. doi: 10.1111/jth.14975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vitiello A., Ferrara F., Pelliccia C., Granata G., La Porta R. Cytokine storm and colchicine potential role in fighting SARS-CoV-2 pneumonia. Italian J. Med. 2020;14(2):88–94. doi: 10.4081/itjm.2020.1284. [DOI] [Google Scholar]
- 42.A. Vitiello, R. La Porta, Ferrara F. Sacubitril, valsartan and SARS-CoV-2 [published online ahead of print, 2020 Jul 27]. BMJ Evid. Based Med. 2020;bmjebm-2020-111497. doi: 10.1136/bmjebm-2020-111497. [DOI] [PubMed]
- 43.Vitiello A., Ferrara F. Correlation between renin-angiotensin system and Severe Acute Respiratory Syndrome Coronavirus 2 infection: What do we know? Eur. J. Pharmacol. 2020;15(883) doi: 10.1016/j.ejphar.2020.173373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vitiello A., Ferrara F. Remdesivir versus ritonavir/lopinavir in COVID-19 patients. Ir. J. Med. Sci. 2020 doi: 10.1007/s11845-020-02440-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.A. Vitiello, F. Ferrara, Therapeutic Strategies for SARS-CoV-2 acting on ACE-2 [published online ahead of print, 2020 Sep 30]. Eur. J. Pharm. Sci. 2020;156:105579. doi: 10.1016/j.ejps.2020.105579. [DOI] [PMC free article] [PubMed]
- 46.A. Vitiello, F. Ferrara, Pharmacological agents to therapeutic treatment of cardiac injury caused by Covid-19 [published online ahead of print, 2020 Sep 28]. Life Sci. 2020;262:118510. doi: 10.1016/j.lfs.2020.118510. [DOI] [PMC free article] [PubMed]
- 47.Vlaar A.P.J., de Bruin S., Busch M., Timmermans S.A.M.E.G., van Zeggeren I.E. Anti-C5a antibody IFX-1 (vilobelimab) treatment versus best supportive care for patients with severe COVID-19 (PANAMO): an exploratory, open-label, phase 2 randomised controlled trial. Lancet. Rheumatol. 2020;2(12):e764–e773. doi: 10.1016/S2665-9913(20)30341-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.M.J. Walport, Complement. First of two parts. N. Engl. J. Med. 2001; 344(14):1058–1066 49. [DOI] [PubMed]
- 49.M.J. Walport, Complement. Second of two parts. N. Engl. J. Med., 2001;344(15):1140–1144. [DOI] [PubMed]
- 50.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan. China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wijnsma K.L., Ter Heine R., Moes D.J.A.R., Langemeijer S., Schols S.E.M., Volokhina E.B. Pharmacology, pharmacokinetics and pharmacodynamics of eculizumab, and possibilities for an individualized approach to eculizumab. Clin. Pharmacokinet. 2019;58(7):859–874. doi: 10.1007/s40262-019-00742-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.World health organization (WHO) https://www.who.int/emergencies/diseases/novel-coronavirus2019/situation-reports (Situation Reports Dic 2020).
- 53.Wu Y. Compensation of ACE2 Function for Possible Clinical Management of 2019-nCoV-Induced Acute Lung Injury. Virol. Sin. 2020;35:256–258. doi: 10.1007/s12250-020-00205-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou Y., Lu K., Pfefferle S., Bertram S., Glowacka I., Drosten C. A single asparagine-linked glycosylation site of the severe acute respiratory syndrome coronavirus spike glycoprotein facilitates inhibition by mannose-binding lectin through multiple mechanisms. J. Virol. 2010;84(17):8753–8764. doi: 10.1128/JVI.00554-10. [DOI] [PMC free article] [PubMed] [Google Scholar]