Abstract
Disease registry systems provide a strong information infrastructure for decision-making and research. The purpose of this study is to describe the implementation method and protocol of the COVID-19 registry in Khuzestan province, Iran. We established a steering committee and formulated the purposes of the registry. Then, based on reviewing the literature, and expert panels, the minimum data set, the data collection forms and the web-based software were developed. Data collection is done retrospectively through Hospital Information Systems, Medical Care Monitoring Center system (MCMC), Management of Communicable Disease Prevention and Control system (MCDPC) as well as, patients' records. For prospective data collection, the data collection forms are compiled with patients' medical records by the medical staff and are then entered into the registry system. We collect patients' administrative and demographic data, history and physical examinations, test and imaging results, disease progression, treatment, outcomes, and follow-ups of the confirmed and suspected inpatients and outpatients. From April 20 to December 5, 2020, the data of 4,812 confirmed cases and 7,113 suspected cases were collected from two COVID-19 referral hospitals. Based on our experience, recording information along with providing care for patients and putting patients' data registration in the medical staff's routine, structuring data, having a flexible technical team and rapid software development for multiple and continuous updates, automating data collection by connecting the registry to existing information systems and having different incentives, the registration process can be strengthened.
Keywords: COVID-19, SARS-CoV-2, Disease registry, Iran, Khuzestan
1. Introduction
The COVID-19 pandemic is a global health crisis of our time and the greatest challenge humanity has encountered since World War II [1]. Even though 63 different vaccines are undergoing clinical trials on humans and vaccinations have begun in some countries, it is still a long way from complete control of the pandemic [2]. From the beginning of the COVID-19 pandemic, various countries have realized that access to accurate, complete, actionable, reliable, and timely data plays a vital role to effectively manage the outbreak [3]. Access to information helps policymakers facilitate the prioritization of care, monitoring the disease outbreak situation, trends, progress, and performance of health systems for disease control [[3], [4], [5]]. Furthermore, access to quality information also plays an important role in supporting research [6]. Therefore, all over the world, governments, research centers, universities, medical centers, etc. have collected COVID-19 data through programs such as the development or improvement of national information systems, information dashboards, data hubs, datasets, and COVID-19 -related registration systems [[7], [8], [9], [10], [11], [12], [13]].
Iran was one of the first countries in the world which faced a widespread outbreak of COVID-19 and has one of the highest mortality rates compared to the number of diagnosed cases [14,15]. In Iran, before the COVID-19 pandemics, the SIB and hospital information systems (HIS) were the two major systems for documenting health data in healthcare centers, the first one is used to collect primary health care data in urban and rural health centers and the second one is for collecting outpatients and inpatients data in hospitals, respectively. However, due to the critical need for real-time COVID-19 patient data, from the beginning of the COVID-19 pandemic, the Ministry of Health set up two web-based information systems to report COVID-19 cases. The two systems included the Medical Care Monitoring Center system (MCMC) for reporting COVID-19 cases in hospitals and the Management of Communicable Disease Prevention and Control (MCDPC) system for reporting COVID-19 cases from primary healthcare centers [16].
Although the two systems collect primary data about COVID-19 cases, there are several important issues regarding the adequacy of data for COVID-19 research. First, the key issue with COVID-19 is the unknown and unpredictability of many aspects of the disease. Despite much research on COVID-19, there are still many questions among researchers about the disease [17,18]. Therefore, there is a need for a comprehensive information system to collect all available data on all aspects of this disease. The second issue is data quality. The low quality of COVID-19 data limits its usefulness to conduct studies [19]. Although no specific study has been done on the quality of data in the two mentioned information systems, some studies and reports indicate problems in collecting and reporting COVID-19 data in Iran [20], [21].
A systematic approach to data quality control is not envisaged in the two information systems. The issue of poor data quality in the COVID-19 epidemic crisis is a problem in many countries around the world [22], [23], [24], [25]. One of the approaches that can help systematically collect data on a specific disease considering the data quality control process is a disease registry system. The disease registries and databases constitute key instruments to develop clinical research [26]. In the disease registry systems, patients with a specific disease are systematically recorded and analyzed in a predefined population. Therefore, these systems can create a real-world view of the disease, disease outcomes, safety, and the effectiveness of treatment methods [26]. Therefore, the implementation of the COVID-19 registration system can help to systematically data collection for researches [27].
Khuzestan province in the south of Iran is one of the provinces with the highest number of COVID-19 cases. Therefore, implementing the COVID-19 registry in this province provides the opportunity to identify the prevalence and extent of the outbreak, complications, and treatment outcomes. Hence, from the beginning of the outbreak in this province, a regional COVID-19 registry program has been established. In this study, we describe the measures taken to set up this COVID-19 registry system in Khuzestan in southwestern Iran. Implementing the COVID-19 registry provides the opportunity to identify the prevalence and extent of the outbreak, early and late complications of the disease, and factors affecting response to treatment, and outcomes. Furthermore, according to the COVID-19 vaccination program, registration systems may help investigate the side effects of vaccines and their effectiveness in large populations. In the following sections, we described the purpose and protocol of this registry as well as lessons learned from pilot implementation.
2. Registry purpose and objectives
The main purpose of this registry is to prepare a database of patients with COVID-19 to determine the prevalence and the incidence of the disease and to perform medical research. In this regard, the steering committee considered the following objectives for this program:
-
1.
Epidemiological studies on the incidence and prevalence of COVID-19
-
2.
Determining the underlying factors affecting the severity of COVID-19
-
3.
Determining the complications and outcomes among patients with COVID-19
-
4.
Identifying the outcomes of interventions and treatment methods used for patients with COVID-19
-
5.
Determining the individual and social factors (age, sex, occupation, education, etc.) in patients with COVID-19
-
6.
Determining the prevalence of complications caused by COVID-19 in patients
-
7.
Determining the long-term complications of COVID-19
-
8.
Determining the effect of underlying diseases on the outcome of the patients with COVID-19
-
9.
Determining the causes of differences in patients' response to treatment
3. Protocol of the registry implementation
3.1. Registry governance structure
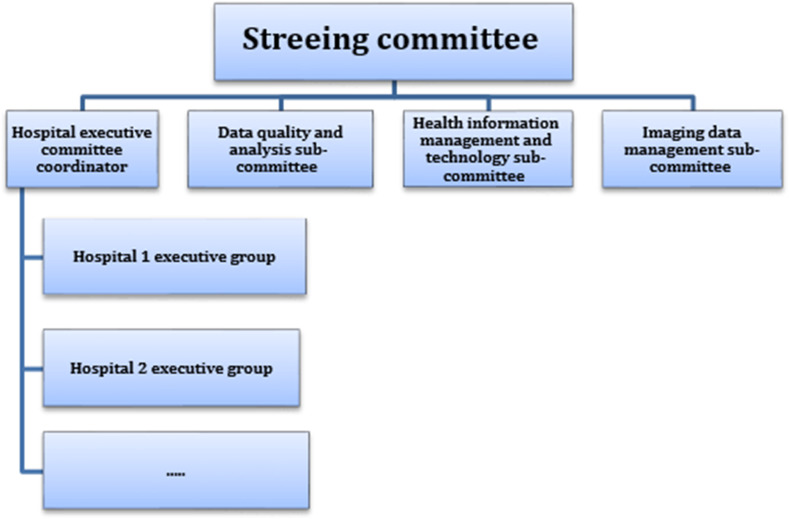
To establish this registry, we firstly identified stakeholders in the province and then formed a steering committee. The steering committee consists of 10 experts with different specialties, including health information management, computer science, epidemiology, infectious disease, lung diseases, emergency medicine, and radiology. This committee is responsible to formulate the registry purposes and develop action plans and the protocol for the implementation of the program, evaluate the implementation of the program and seek supports. Four subcommittees have also been established to carry out the activities of the program. The information management committee is responsible to develop data sets, data dictionaries, data collection forms, and registry software. The data quality assessment and analysis committee was formed for evaluating data quality, providing feedbacks for data collection, and data analysis, finally the imaging data committee is responsible for developing the data needed to manage imaging information, as well as, image interpretation. Besides, the hospital executive committee was established under the supervision of the executive director of the registry with the responsibility of making arrangements with the hospitals for data collection (Fig. 1 ).
Fig. 1.
The governance of the Khuzestan COVID-19 registry.
3.2. Registry design and population
In this observational study, patients' data are recorded both retrospectively and prospectively. Since the establishment of the registry (April 2020), all outpatients and inpatients diagnosed or treated throughout healthcare facilities in Khuzestan province, according to the inclusion and exclusion criteria, are registered. Patients who have been diagnosed or treated before the start of the program were also retrospectively registered.
Khuzestan province (see Fig. 2 ) is located in the southwest of Iran and with a population of 4,700,000 people is the fifth most populated province of the country. Khuzestan province has 27 towns and 54 cities [28]and its health care system is managed by four universities of medical sciences: Ahvaz Jundishapur, Abadan, Behbahan, and Shushtar. The present study was first performed as a pilot in hospitals affiliated to Ahvaz Jundishapur University of Medical Sciences, in Ahvaz city (the capital of Khuzestan province). Then, based on participation agreements, healthcare centers affiliated with other medical universities will also be involved in the registry.
Fig. 2.
Map of Khuzestan province and counties.
3.3. The registry inclusion and exclusion criteria
In this registry, all confirmed suspected inpatients and outpatients with COVID-19 are registered according to the following inclusion criteria:
-
•
Outpatients or hospitalized patients referred in one of the healthcare facilities in Khuzestan province or the residents of Khuzestan province who diagnosed or treated in other provinces;
-
•
Positive results for Polymerase Chain Reaction (PCR) or high clinical suspicion for COVID-19 based on signs and symptoms based on physicians' diagnosis
The exclusion criteria included a negative test for COVID-19 (negative PCR) and having no clinical symptoms of COVID-19.
3.4. Data set and data collection forms
To prepare the initial data set, we reviewed electronic or paper-based COVID-19 data collection and reporting forms [29], [30], [31], patients’ medical record forms, COVID-19 diagnosis guidelines of the Ministry of Health in Iran, other guidelines on acute respiratory infections caused by corona viruses, available studies on COVID-19, structure, protocol and minimum data set of similar disease registration systems in Iran, as well as data elements of related health information systems in Iran, mentioned in Table 1 . Table 2 shows the information sources used to develop the data set.
Table 1.
The specifications of two information systems of the Ministry of Health for recording and reporting of COVID-19 data.
| Health information system | Deputy in charge of system and place of recording and reporting of data | Data elements related to COVID-19 |
|---|---|---|
| Medical Care Monitoring Center system (MCMC) | Vice-Chancellor for Treatment Hospitals |
Patient identification data, Phone, and address,- Admission data, The name of the hospital, Early signs and symptoms of the disease, CT Scan, PaO2(Saturation, Underlying conditions, and comorbidity, COVID-19 test result, Intubation, Hospitalization in intensive care unit (ICU), Outcome, Date of discharge or death |
| Management of Communicable Disease Prevention and Control system (MCDPC) | Vice-Chancellor for Health Health centers |
Patient identification data, Healthcare center identification data, Phone and address, Occupation, Direct contact with an infected person, Admission data, Signs and symptoms of the disease, Underlying conditions, and comorbidity- Covid19 screening result, drugs, Disease complications (Pneumonia and respiratory distress syndrome, Outcome, Date of discharge or death |
Table 2.
The main information sources used to design the data set.
| Information resources | Details |
|---|---|
| Articles | We searched PubMed and Google using the terms “COVID-19″ and “SARS-CoV-2″ to retrieve related articles |
| COVID-19 data collection and reporting forms | To retrieve COVID-19 data collection and reporting forms, we searched Google using the following terms in Persian and English: “COVID-19″, “SARS-COV-2″ and “Coronavirus disease” in combination with the terms “data collection form”, “data gathering form”, “minimum data set”, “data set”, “data dictionary”, “questionnaire” and “form”. Besides, we requested information from other hospitals, universities, or researchers in Iran for forms that were not available by Internet |
| Patient medical record | Some COVID-19 patients; medical records in one hospital in Ahvaz were reviewed. The structure and content (data elements) of paper-based forms of medical records and data recorded in these forms were considered |
| Health information systems | Content (data elements and their values) were examined in the following health information systems in Iran: MCMC system, MCDPC, SIB, HIS |
| Disease registry documents | Data sets, software, protocols and guidelines of other current registration systems in Ahvaz University of Medical Sciences were examined |
| Guidelines for other acute respiratory infections in Iran | The previous instructions and forms of the Ministry of Health of Iran were reviewed for other acute respiratory infections caused by corona viruses such as severe acute respiratory syndrome (SARS) and the Middle East respiratory syndrome (MERS), and Influenza |
To finalize the data set, we considered the opinions of the experts in the field. To this end, we conducted several expert panel discussion sessions. Table 3 shows the characteristics of the experts involved in designing the minimum data set. In these meetings, the clinical experts and the members of the steering committee discussed the initial version of the data set. For each data element, the feasibility of data collection (based on the indicators, such as the availability or accessibility of the data), the standard definition, the data format (for example, free text, multiple-choice, etc.), reliable data sources for collecting data, method of data collection (patient interview, review of test results, images, etc.) and the time of data collection (at the time of admission, or during the hospitalization period) were discussed. All data items with more than 50% of agreement between panelists were included in the final data set.
Table 3.
Characteristics of participants in the expert panel.
| Demographic characteristics | Frequency | Percent | |
|---|---|---|---|
| Sex | Male | 5 | 50 |
| Female | 5 | 50 | |
| Education | PhD | 3 | 30 |
| Medical Specialist | 3 | 30 | |
| Fellowship | 4 | 40 | |
| Specialty | Pulmonology | 3 | 30 |
| Infectious disease | 1 | 10 | |
| Internal medicine | 1 | 10 | |
| Cardiology | 1 | 10 | |
| Health information management | 1 | 10 | |
| Epidemiology | 1 | 10 | |
| Laboratory Science | 1 | 10 | |
| Emergency medicine | 1 | 10 | |
We, based on the results of the experts' opinions, developed the first version of the COVID-19 data set, data dictionary, data collection forms, and registry software. In the next step, we piloted the program for three weeks in one of the COVID-19 referral hospitals in Ahvaz. The purpose of the pilot was to evaluate the structure and the content of the data collection forms, determine required data elements, identify new information requirements, evaluate the feasibility of data collection in the real environment, and determine the appropriate approach for data collection.
The final data set of our COVID-19 registry system consists of seven top data classes including administrative data (39 data elements), disease and encounter data (65 data elements), medical history and physical examination (121 data elements), findings of clinical diagnostic tests (95 data elements), disease progression and outcome of treatment (39 data elements), medical diagnosis and cause of death (14 data elements), and follow-up data (23 data elements). Most of these data items are coded (to select from a drop-down menu) and only nine of them are free text. Furthermore, 45 data elements as minimum data sets are compulsory.
We organized these data items in five data collection forms including basic data, medical history and physical examination, laboratory and imaging findings at the time of admission, disease progression and outcome as well as follow-up forms.
3.5. Registry software
Based on our COVID-19 data set, we developed web-based registry software using the Rapid Application Development (RAD) technique. In this system, an electronic record containing administrative and clinical data about the admission, diagnosis, treatment, and follow-ups of the patients is created for each patient. This software was developed using the NET programming language and is available at Covid.ajums.ac.ir (Fig. 3, Fig. 4 ).
Fig. 3.
The Login page of the COVID-19 registry software.
Fig. 4.
A sample screenshot of the COVID-19 registry software that shows the laboratory data section. The right panel of this figure shows the other groups of data such as demographics, encounter data, comorbidities, Chest X-ray, CT scan, EKG findings, outcomes, Follow-ups, ICD codes, medications, and attachments.
3.6. Data collection methods
In Khuzestan province, similar to the rest of Iran, depending on the population and COVID-19 epidemic situation, one or more hospitals (mainly affiliated to the Universities of Medical Sciences) are considered as COVID-19 referral centers in each city. In addition to hospitals, there are some health centers (known as 16-h centers) for diagnosing and PCR-testing for ambulatory and mild cases of the disease. In hospitals, the data of admitted patients are recorded in paper-based medical records and the HIS. Furthermore, according to the instruction of the Ministry of Health, all confirmed and suspected cases of COVID-19 should be recorded and reported in the MCMC system. Data of patients referred to 16-h centers are recorded in the MCDPC system.
Therefore, case finding is done retrospectively and prospectively. The retrospective method is for patients who are admitted to the participant healthcare centers before the implementation of the registry. For retrospective case finding and data collection, since the basic data about these patients was available in both MCMC and MCDPC systems, we received a report of patients registered in these two systems. To control the quality of this data, we compared the recorded data for each patient in both systems to detect and correct any inconsistencies and missing data. Then, we imported the demographic data (such as the address and the telephone), signs and symptoms of the disease, information about the contacts, date of symptom onset, date of admission, comorbidities, type of admission (outpatient, hospitalized), method of testing and PCR test results into the COVID-19 registry software. To complete the data, we actively collect information related to the medications, laboratory findings, disease progression using HIS, and patients' medical records.
In the prospective method, all data collection forms are provided for the participant facilities. These forms are completed like other forms of the patients' medical records during hospitalization. Test results, medications, and ICD-10 codes are also imported from HIS. To facilitate data collection and increase data quality, the people in charge of data entry into the registry software were selected from the staff of the same facility. The criteria for selecting the staff were having at least a bachelor's degree in one of the fields of medical or paramedical sciences, interest in medical studies, and full-time employment in the relevant facility. A financial contract was signed with all these individuals to participate in the registry program.
3.7. Data quality and quality assurance
We developed several preventive, detective, and corrective actions adopted from literature to ensure data quality [32]. Table 4 shows our measures for these three-controls. The purpose of these measures is to minimize data errors and ensure that data collection is in accordance with the objectives of the registry.
Table 4.
The data quality control measures in the COVID-19 registry system.
| The general approach to data quality control | Measures are taken to control the quality of data |
|---|---|
| Preventive actions |
|
| Detective actions |
|
| Corrective actions |
|
Until December 5, 2020, as a result of the data quality control process, 158 duplicate cases were identified and excluded. Additionally, 1955 data inconsistencies in the recorded data (such as first and last name, father's name, age, address, mobile, and telephone number, admission and discharge data, admission ward, signs and symptoms at the time of admission, underlying conditions and comorbidity, admission date, discharge date, length of stay, and outcome), 9189 missing data (such as signs and symptoms at the time of admission, underlying conditions, and comorbidity, discharge date, PCR test result, outcome) and 1102 data errors (such as ICD-10 miscoding, misspelling, invalid national ID, mobile and phone number) were recognized and corrected.
3.8. Patient follow-up
All outpatients and hospitalized patients discharged from the healthcare facilities are followed-up during a period of three months for the outcomes of the disease. The aim is to identify patients with the reoccurrence of the disease, deaths after discharge, and subsequent consequences of the disease. Using the unique national ID of the patients, the A feature for reporting the patients' readmission and death in subsequent admissions were developed in the software. Our mechanism to follow-up patients is to review the reports of MCDPC and MCMC systems to identify the subsequent admissions of patients. The data quality assessment and analysis committee reviews the registered patients in the reports of MCDPC and MCMC systems so that in case of a referral to other healthcare facilities or death, this information is updated in the registry software.
3.9. Implementation of the registry
To implement the registry, we first developed the registry protocol, which includes instructions and the method of data collection, follow-ups, exclusion of the patients, and the quality control of the information. Then, data collection staff in one of the hospitals were trained. The registration process was piloted for three weeks in this COVID-19 referral hospital in Ahvaz. Based on the feedback and experiences gained from the pilot, paper forms, the electronic systems, and registration protocols were updated based on the new problems and needs. It should be noted that in October, based on the feedback from the initial data analysis and pilot study, we reviewed the data set and removed 26 data elements, and added 12 new data elements to the forms and software.
After the initial phase of the pilot, the implementation phase of the registration program was performed. At this stage, the registration program was implemented in two hospitals in Ahvaz, which are considered as the main hospitals for COVID-19 patients. To expand the registry, the steering committee decided to officially communicate with four universities of medical sciences in Khuzestan province, as well as, healthcare facilities and invite them to participate. So that after the initial data sharing and confidentiality agreement, and signing the contract of cooperation, these centers and physicians will participate in the registry and receive the forms, privileged access to the electronic system, and the registration protocol. Implementation of the program began on April 20, and on December 5, 4,812 confirmed and 7,113 suspected cases were registered.
3.10. Data analysis plans
Following the objectives of the registry program, we implemented various features for reporting information in the electronic system. Data analysis will be done following the objectives of the registry and based on the conventional methods of descriptive and inferential statistics. Registry outputs will be provided to managers, policymakers, and researchers in the form of reports and other written outputs.
3.11. Ethics approval
This study received ethical approvals from the Ethics Research Committee of Ahvaz Jundishapur University of Medical Sciences (IR.AJUMS.REC.1399.061).
4. Lessons learned from the implementation
Khuzestan is one of the biggest provinces in Iran with most cases of COVID-19. Due to its diverse geographical and cultural conditions, the province faces many problems in fighting with COVID-19 [33], [34]. Implementation of the COVID-19 registry under the supervision of the educational and health authorities of this province can provide access to quality information to deal with COVID-19 and perform medical studies on this disease.
The implementation of the pilot program has resulted in lessons that can be learned as a model for starting other similar registry programs. One of the primary goals of this program was to record the patients' lifestyle and nutrition and its effect on the complications and outcomes of the disease. However, pilot implementation of the program based on the designed forms and data set showed that it was not possible to collect patients’ lifestyle, and nutrition data in the real environment, because, in the routines of taking information from the patients, collecting these data is not a usual practice of physicians. Studies have shown that the separation of the data collection process or data entry from the daily routine processes of the clinical teams is one of the serious hindrances in the implementation of registry programs [35]. Also, concerning the appearance of the data collection forms and the method of arranging data elements, the pilot experiences showed that these forms should be based on the actual process of patient care (from admission to discharge) in healthcare facilities as much as possible, and this structure should be similar to paper medical record forms so that the clinical team does not need to worry about making mistakes in completing them. Studies have shown that considering the routine clinical procedures of clinical teams and considering it in designing the data collection method in registry programs is effective in their success [36]. Therefore, the lifestyle and nutrition data elements were excluded by the decision of the steering committee, and the necessary modifications were made in the design of the forms to make the registration process easier.
Pilot implementation experiences have shown that in the prospective data collection methods, the best approach to ensure the correct and complete data collection is to record information at the same time as providing care for patients. Secondary use of available data may result in missing data. For this reason, prospective data collection was integrated into the patients' care process and data collection forms were placed in the patients’ medical records so that the clinical teams could complete these forms like other medical reports.
The pilot implementation showed that some important data, such as saturation percentage, were not considered in our data set or the values of some fields in the software needed to be standardized and the data should be structured as much as possible to increase the common understanding of the data and improve the quality of information. More importantly, the pilot implementation showed that based on new scientific findings and clinical team experiences, the data set and data values need constant updating. Lack of support for the required changes in the data based on the available scientific evidence [37] can make the registry invalid for the clinical team and reduce their cooperation. Therefore, during the pilot, several changes were made to the paper forms and the registry software.
Another problem in the implementation of the pilot was the lack of proper cooperation of medical staff in completing the information. Specifically from the mid of May to the end of June, due to the sudden increase in the number of cases and the critical conditions of the hospitals, a severe overload of work was on the medical staff. The reluctance of the people to the registry due to the lack of time and increased workload [38] is one of the most common problems in registries that require appropriate and creative solutions.
The steering committee used four solutions to solve this problem. First of all, we differentiated the role of staff in completing the forms and data entry into the software. In this regard, the clinical team only completes the forms and other staffs are responsible to enter the data into the software. Also, the people in charge of entering the data in the software were selected from among the employees of the same hospital. This made other hospital staff more cooperative with these staff than the outsiders. The second solution was to provide various incentives for the medical staff. These incentives include financial payment and participation in the scientific outputs resulted from the data. Studies have shown that financial and non-financial incentives are very effective in the successful implementation of registry programs [39]. Our third solution was to get the full support of the senior management of the pilot hospitals (director, manager, deputy director of education and research department of the hospital and the hospital matrons). Supports of the senior managers of the hospitals resulted in less resistance of the medical staff to the implementation of the registry. Previous studies have also shown that the support of the leaders of an organization in solving problems [39] plays a significant role in the success of the registration programs.
The fourth solution was to minimize the manual data collection by receiving some data from HIS, MCMC, and MCDPC systems. According to previous studies, automatic data entry in a registry system by using electronic tools through integrating the registry system with the available information systems, despite having some complexities and problems, if implemented properly can increase the speed of data collection and reduce the cost of data collection to a great extent [40], [41], [42], [43]. Our pilot implementation showed that for some data, such as the results of tests performed for patients and the medications prescribed, the secondary data entry of this information from the patient records is very time-consuming and error-prone. Therefore, we automatically import laboratory data, medications, and ICD codes from HIS into the registry software using web services. Some data, such as demographic data, address, and telephone, the date of first symptoms, the date of referral, the method of treatment, and the symptoms of the disease, comorbidities, PCR test results, and treatment outcomes are documented in MCDPC and MCMC systems. With the cooperation of the IT Management of the university, we obtain different outputs from these systems in different periods and import these data into the registry software after quality control and data correction. Although receiving these outputs increases the speed of data collection, due to the manual process, it can lead to errors in the data; therefore, it requires data quality assessment.
5. Conclusion
In conclusion, disease registry systems can provide valuable information about COVID-19 disease to track the prevalence and incidence of the disease and conduct the research. In this study, the process of implementing the COVID-19 registry in Khuzestan, its pilot implementation, as well as the challenges and strategies adopted to address these challenges were described. If other provinces establish the COVID-19 registry, they can start or upgrade their registry based on our experiences gained from this registry. We are planned to continue this registry by the end of the pandemic and the Ahvaz Jundishapur University of Medical Sciences will support financially this registry. Further, according to the COVID-19 vaccination program and the use of vaccines, it is recommended to expand the registry to include the side effects of vaccines and their effectiveness.
Authors’ contributions
Javad Zarei: Conceptualization; Data curation; Investigation; Methodology; Software; Project administration; Resources; Writing -review and editing.
Maryam Dastoorpoor: Conceptualization; Data curation; Investigation; Methodology; Writing-review and editing.
Amir Jamshidnezhad: Conceptualization; Data curation; Investigation; Methodology; Software; Writing-review and editing.
Maria Cheraghi: Conceptualization; Data curation; Investigation; Methodology; Writing - review and editing.
Abbas Sheikhtaheri: Conceptualization; Methodology; Writing-the original draft; Writing-review and editing.
All authors reviewed the final version of the manuscript and approved it to submit.
Funding
This study was supported by Ahvaz Jundishapur University of Medical Sciences. The funder had no role in study design; data collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank all of the participants, especially those who help us in data gathering.
References
- 1.Coronavirus disease COVID-19 pandemic. United Nations Development Programme; 2020. [Google Scholar]
- 2.Coronavirus vaccine tracker. The New York Times; 2020. [Google Scholar]
- 3.Azzopardi-Muscat N., Kluge H.H.P., Asma S., Novillo-Ortiz D. A call to strengthen data in response to COVID-19 and beyond. J Am Med Inf Assoc. 2020 doi: 10.1093/jamia/ocaa308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan American Health Organization . 2020. COVID-19 and the importance of strengthening Information Systems. [Google Scholar]
- 5.Davenport T.H., Godfrey A.B., Redman T.C. To fight pandemics, we need better data. MIT Sloan Manag Rev. 2020;62:1–4. [Google Scholar]
- 6.Moore J.H., Barnett I., Boland M.R., Chen Y., Demiris G., Gonzalez-Hernandez G., Herman D.S., Himes B.E., Hubbard R.A., Kim D. Springer; 2020. Ideas for how informaticians can get involved with COVID-19 research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Q., Liu W., Sha D., Kumar S., Chang E., Arora V., Lan H., Li Y., Wang Z., Zhang Y. An environmental data collection for COVID-19 pandemic research. Data. 2020;5:68. [Google Scholar]
- 8.Guidotti E., Ardia D. COVID-19 data hub. J Open Source Software. 2020;5:2376. [Google Scholar]
- 9.Freeman E.E., McMahon D.E., Fitzgerald M.E., Fox L.P., Rosenbach M., Takeshita J., French L.E., Thiers B.H., Hruza G.J. The American Academy of Dermatology COVID-19 registry: crowdsourcing dermatology in the age of COVID-19. J Am Acad Dermatol. 2020;83:509–510. doi: 10.1016/j.jaad.2020.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caillard S., Anglicheau D., Matignon M., Durrbach A., Greze C., Frimat L., Thaunat O., Legris T., Moal V., Westeel P.F. Kidney international; 2020. An initial report from the French SOT COVID Registry suggests high mortality due to Covid-19 in recipients of kidney transplants. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.von Stillfried S., Bülow R.D., Röhrig R., Knüchel‐Clarke R., Boor P., DeRegCOVID, Tholen P., Nöthel B., Wienströer J., Majeed R. Autopsy registry can facilitate COVID‐19 research. EMBO Mol Med. 2020;12 doi: 10.15252/emmm.202012885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hasell J., Mathieu E., Beltekian D., Macdonald B., Giattino C., Ortiz-Ospina E., Roser M., Ritchie H. Vol. 7. 2020. A cross-country database of COVID-19 testing, Scientific data; pp. 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu J., Wang J., Nicholas S., Maitland E., Fan Q. Application of big data technology for COVID-19 prevention and control in China: lessons and recommendations. J Med Internet Res. 2020;22 doi: 10.2196/21980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raoofi A., Takian A., Sari A.A., Olyaeemanesh A., Haghighi H., Aarabi M. COVID-19 pandemic and comparative health policy learning in Iran. Arch Iran Med. 2020;23:220–234. doi: 10.34172/aim.2020.02. [DOI] [PubMed] [Google Scholar]
- 15.Daneshpazhooh M., Mahmoudi H. COVID-19: the experience from Iran. Clin Dermatol. 2020 doi: 10.1016/j.clindermatol.2020.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guide to diagnosis and treatment of Covid 19. Ministry of Health and Medical Education; Iran,Tehran: 2020. [Google Scholar]
- 17.S. Pagiotti, M. Mazzoni, R. Mincigrucci, A. Stanziano, S. Verza, The role of the press in times of pandemics: old features in the face of a new risk. The Italian case.
- 18.Ansell C., Sørensen E., Torfing J. The COVID-19 pandemic as a game changer for public administration and leadership? The need for robust governance responses to turbulent problems. Publ Manag Rev. 2020:1–12. [Google Scholar]
- 19.Costa-Santos C., Luísa Neves A., Correia R., Santos P., Monteiro-Soares M., Freitas A., Ribeiro-Vaz I., Henriques T., Rodrigues P.P., Costa-Pereira A., Pereira A.M., Fonseca J. 2020. COVID-19 surveillance - a descriptive study on data quality issues. medRxiv. 2020.2011.2003.20225565. [Google Scholar]
- 20.Mounesan L., Eybpoosh S., Haghdoost A., Moradi G., Mostafavi E. Is reporting many cases of COVID-19 in Iran due to strength or weakness of Iran's health system? Iran J Microbiol. 2020;12:73–76. [PMC free article] [PubMed] [Google Scholar]
- 21.Ghafari M., Hejazi B., Karshenas A., Dascalu S., Ferretti L., Ledda A., Khosravi M.A., Abbasalipour M., Zeinali S., Katzourakis A. 2020. Ongoing outbreak of COVID-19 in Iran: challenges and signs of concern with under-reporting of prevalence and deaths. [Google Scholar]
- 22.Vasudevan V., Gnanasekaran A., Sankar V., Vasudevan S.A., Zou J. Journal of the Indian Institute of Science; 2020. Variation in COVID-19 data reporting across India: 6 Months into the pandemic; pp. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silva L., Figueiredo Filho D. Using Benford's law to assess the quality of COVID-19 register data in Brazil. J Publ Health. 2020 doi: 10.1093/pubmed/fdaa193. (Oxford, England) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Costa-Santos C., Neves A.L., Correia R., Santos P., Monteiro-Soares M., Freitas A., Ribeiro-Vaz I., Henriques T., Rodrigues P.P., Costa-Pereira A. 2020. COVID-19 surveillance-a descriptive study on data quality issues. medRxiv. [Google Scholar]
- 25.de Lusignan S., Williams J. BJGP open; 2020. To monitor the COVID-19 pandemic we need better quality primary care data. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gliklich R.E., Dreyer N.A., Leavy M.B. Government Printing Office; 2014. Registries for evaluating patient outcomes: a user's guide. [PubMed] [Google Scholar]
- 27.Khorrami F., Shahi M., DavariDolatabadi N., Karami N.A., HasaniAzad M., Jafariyan F., Sheikhtaheri A. Implementation of regional COVID-19 registry in Hormozgan (RCovidRH), Iran: rationale and study protocol. Med J Islam Repub Iran. 2020;34:96. doi: 10.34171/mjiri.34.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wikipedia, Khuzestan province. 2020. [Google Scholar]
- 29.CDC . Center for Disease Prevention and Control; USA: 2020. Human infection with 2019 novel coronavirus person under investigation (PUI) and case report form. 2020. [Google Scholar]
- 30.WHO . World Health Organization; 2020. Interim case reporting form for 2019 novel coronavirus (2019-nCoV) of confirmed and probable cases: WHO minimum data set report form. [Google Scholar]
- 31.ISARIC . 2020, international severe acute respiratory and emerging infection consortium. 2020. Novel coronavirus (ncov) acute respiratory infection clinical characterization data tool. [Google Scholar]
- 32.Sheikhtaheri A., Nahvijou A., Sedighi Z., Hadji M., Golmahi M., Roshandel G., Beiki O., Ravankhah Z., Zendehdel K. Development of a tool for comprehensive evaluation of population-based cancer registries. Int J Med Inf. 2018;117:26–32. doi: 10.1016/j.ijmedinf.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 33.Mohammadi A., Valinejadi A., Sakipour S., Hemmat M., Zarei J., Majdabadi H.A. Improving the distribution of rural health houses using elicitation and GIS in Khuzestan province (the southwest of Iran) Int J Health Pol Manag. 2018;7:336. doi: 10.15171/ijhpm.2017.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pourahmad A., Khavarian-Garmsir A.R., Hataminejad H. Social inequality, city shrinkage and city growth in Khuzestan Province, Iran. Area Dev Pol. 2016;1:266–277. [Google Scholar]
- 35.Egholm C.L., Helmark C., Doherty P., Nilsen P., Zwisler A.D., Bunkenborg G. Struggling with practices" - a qualitative study of factors influencing the implementation of clinical quality registries for cardiac rehabilitation in England and Denmark. BMC Health Serv Res. 2019;19 doi: 10.1186/s12913-019-3940-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mandavia R., Knight A., Phillips J., Mossialos E., Littlejohns P., Schilder A. What are the essential features of a successful surgical registry? a systematic review. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2017-017373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bernal-González P.J., Navarro-Alonso J.A., Pérez-Martín J. Computerised vaccination register for the Murcia region, Spain, 1991 to 2011. Euro Surveill. 2012;17 [PubMed] [Google Scholar]
- 38.Sehgal A., Davies E. Lessons from developing and running a clinical database for colorectal cancer. J Eval Clin Pract. 2006;12:94–101. doi: 10.1111/j.1365-2753.2005.00628.x. [DOI] [PubMed] [Google Scholar]
- 39.Holtrop J.S., Hall T.L., Rubinson C., Dickinson L.M., Glasgow R.E. What makes for successful registry implementation: a qualitative comparative analysis. J Am Board Fam Med : JABFM. 2017;30:657–665. doi: 10.3122/jabfm.2017.05.170096. [DOI] [PubMed] [Google Scholar]
- 40.Gabbay R.A., Khan L., Peterson K.L. Critical features for a successful implementation of a diabetes registry. Diabetes Technol Therapeut. 2005;7:958–967. doi: 10.1089/dia.2005.7.958. [DOI] [PubMed] [Google Scholar]
- 41.Aliabadi A., Sheikhtaheri A., Ansari H. EHR-based disease surveillance systems: a systematic literature review on challenges and solutions. J Am Med Inf Assoc. 2020;27:1977–1986. doi: 10.1093/jamia/ocaa186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Surodina S., Lam C., de Cock C., van Velthoven M., Milne-Ives M., Meinert E. Engineering requirements of a Herpes simplex virus patient registry: discovery phase of a real-world evidence platform to advance pharmacogenomics and personalized medicine. Biomedicines. 2019;7 doi: 10.3390/biomedicines7040100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hume K., Crotty C., Simmons C., Neumeister M., Chung K. Medical specialty society-sponsored data registries: opportunities in plastic surgery. Plast Reconstr Surg. 2013;132:159e–167e. doi: 10.1097/PRS.0b013e3182910cf4. [DOI] [PMC free article] [PubMed] [Google Scholar]