Abstract
This review portrays the metabolic consequences of Covid-19 infection at different stages of the clinical syndrome. It also describes how events can change when patients with metabolic problems are infected and the effects that diet and nutrition might play to influence the outcome of infection. We also discuss the types of maneuvers that could be used to reshape metabolic events and question if this approach could be a practical therapy used alone or in combination with other approaches to reduce the burden of Covid-19 infection.
Keywords: SARS-Cov-2, Covid-19, Immunometabolism, Metabolic disease, Nutrition, Anti-metabolite therapy
In the past year, the whole world has become aware of SARS-CoV-2 (Covid-19). Thus perhaps around September 2019, humans became infected by a novel passenger coronavirus of bats, Covid-19 and this has led to a pandemic, which as of early January 2021, has led to 84 million confirmed infections with at least 1.8 million deaths [1]. No country that tests for Covid-19 has escaped infection and currently the virus is spreading dramatically in most places. This state of affairs is likely to continue unless the recently developed vaccines, or other ways to control the infection, prove effective. The main mission of this review is to describe metabolic changes set into play when viruses infect us and to discuss the various ways host metabolism affects the outcome of infection focusing on Covid-19. We also evaluate if manipulating metabolism at various stages of the Covid-19 induced syndrome provides a useful approach to limit the consequences of the infection.
As with all infections, the outcome following exposure to Covid-19 is highly variable with inapparent effects the most frequent, but all too commonly a severe debilitating often lethal consequence. We, and others, have discussed the many variables that impact on the consequences of virus infection with different agents [2,3], but so far there is minimal information with respect to Covid-19. This situation is likely to change rapidly given the astonishing pace of research on Covid-19 (almost 80,000 articles on Covid-19 in PubMed < 9 months after the first report) [4]. However, the topic we cover in this review, namely the interplay between Covid-19 and host metabolism, has received less attention to date although at least one outstanding review has been written on this topic [2]. The topics covered in this review include the changes that Covid-19 infection imposes on cells they infect, changes in metabolism that occurs in host cells that respond to infections, the influence of host metabolism on the outcome of virus infection at different stages of Covid-19 disease, the production of host molecules that impact on infections by changing metabolic events, as well the value of manipulating metabolism by drugs and dietary changes or to speed the recovery and control the outcome of Covid-19 infections.
1. The variable consequences of Covid-19 infection
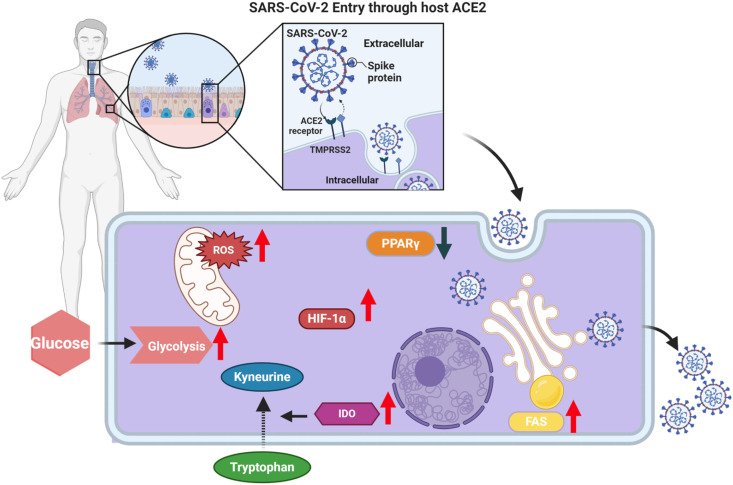
Covid-19 infection normally occurs by exposure of the respiratory tract to virus-containing aerosols and droplets. Depending on the size of these particles, they either become trapped in the upper respiratory tract where they can infect mainly ciliated cells of the nasal epithelium, or can pass deep into the lungs where the main cell types infected are alveolar epithelial type II cells and their massive cell death results in pneumonia [5]. In both situations, cell infection requires binding of the viral spike protein to the angiotensin converting enzyme 2 (ACE2) receptors on target cells (Fig. 1 ). This results in activating the transmembrane cell membrane TMPRSS2 and in some cases the metalloproteinase ADAM17, to cause access to the cell’s cytoplasm, after which the viral replication program begins [6]. New virus is assembled and released and the infected cell dies. Newly formed virus is expelled by coughing and can infect other persons, but also disseminates to other parts of the respiratory tract and in addition can spread to infect ACE2 positive cells in extrapulmonary tissues [7]. These include adipose tissue, the kidneys, the myocardium, the liver, the gastro-intestinal tract and occasionally the brain [6,7].
Fig. 1.
Schematic representation of some changes in cellular metabolism attributed to Covid-19 infection. ACE2; angiotensin-converting enzyme 2 and TMPRSS2; transmembrane protease serine 2 [6], FAS; fatty acid synthesis [15], IDO; indoleamine 2,3-dioxygenase [15,16], HIF-1α; hypoxia inducible factor 1 subunit alpha [18,19], ROS; reactive oxygen species [19], PPARγ; peroxisome proliferator-activated receptor gamma [20].
The majority of infected persons do not show symptoms and some might not even seroconvert, perhaps reflecting infection with a minimal dose that is controlled effectively by innate immune mechanisms [8,9]. The clinical consequences of infection can be divided into several stages with the majority (perhaps 80%) developing mild lesions that quickly resolve. Stage 1 disease is typified by a dry cough, often loss of taste and smell, and general malaise, but usually rapid recovery. During stage one, which largely involves the upper respiratory tract, levels of viral secretion may be high. Stage 2 involves the lower respiratory tract with patients developing pulmonary inflammation and hypoxia that may require hospitalization. Viral secretion levels are usually less than during stage 1, and patients either recover or pass into stage 3 [10]. This stage involves severe inflammatory lesions in the lungs and usually also in several extrapulmonary tissues. The lesions represent reactions by the host immune system, which generate a tissue damaging, so-called cytokine storm [11,12]. The damage to extrapulmonary tissues is likely not the result of viral replication, although ACE2 positive infectible cells occur in many extrapulmonary tissues [7]. Stage 3 patients often die, but improvements in management, particularly the use of dexamethasone and monoclonal antibodies to some cytokines, are permitting recovery for many patients, although long term damage to the lungs and other organs may occur. The recovery phase, where infectious virus has disappeared can be referred to as stage 4 [10].
2. Metabolic changes set into play by Covid-19 infection
All viruses lack metabolic processes of their own and depend on the cells they infect to generate the components needed to assemble new virions. Many viruses markedly change the metabolism of infected cells and at the end some shut down all cellular metabolism and the cell dies. This is the usual fate of cells infected with Covid-19 and cells such as alveolar epithelial cells must be replaced or their function, which involves gas exchange and production of surfactants needed for lung function, will cease [13]. Recording the metabolic consequences of Covid-19 infection in vivo is beginning to be assembled. Some studies have compared the metabolic profile of Covid-19 patients with uninfected persons, but these studies are limited in value since they often do not clarify what stage of infection, or its severity, is being studied [14]. What is still needed are longitudinal studies that record metabolic events in infected patients at multiple times after they become Covid-19 positive and to ascertain if metabolic changes correlate with the severity and outcome of the disease process.
From so far published studies on in vivo effects on metabolism, it appears that Covid-19 infected patients may have elevated blood glucose and fatty acid levels and also show changes in amino acid metabolism. With regard to the latter, genes involved in tryptophan metabolism, such those encoding kyneurine and indoleamine 2,3-dioxygenase (IDO) were upregulated [15,16]. Additional intermediates involved in arginine, aspartate, tyrosine and lysine may also be changed in infected patients [15,17]. These patient studies indicate that several aspects of normal metabolism can be affected during the disease process presenting a challenge for any therapy used to restore metabolic equilibrium. The topic of targeting metabolic events to control the outcome of Covid-19 infection is covered in the final section.
Another approach used to record the metabolic consequences of Covid-19 infection has been to perform ex vivo studies on bronchial lavage cells and blood monocytes from Covid-19 patients comparing any metabolic changes to normal controls using a range of measurements that include single cell RNA-seq, metabolomics and transcriptomic. One such study using single cell RNA-seq on bronchial alveolar cells revealed significant increases in metabolites involved in glycolysis that included HIF-1α and genes involved in the induction of oxidative stress (Fig. 1) [18,19]. This indicates that the infected cells need to elevate cellular energy levels which is accomplished via glycolysis. Other studies have reported changes in lipid metabolism, as well as changes in tryptophan metabolism similar to those referred to previously in patient studies also occur [15].
One notable change reported in one ex vivo study was reduced expression of the enzyme PPAR involved in both glucose and lipid metabolism. PPARγ deregulation was demonstrated using single cell transcriptomics on bronchial lavage monocytes (Fig. 1) [20]. Curiously, in a model study where PPARy expression was ablated in mouse adipocytes, animals developed fatty livers and several metabolic changes. These included elevated levels of blood glucose, insulin resistance, as well as increased plasma free fatty acids (FFAs) and triglyceride levels [21,22]. In another mouse study, when PPARγ gene expression was deleted in mononuclear cells, mice became more susceptible to influenza virus infection (Covid-19 was not studied) and this outcome could be rescued by therapy with a PPARγ agonist [22]. We anticipate that switching on and enhancing PPAR function might be a useful approach to reduce the burden of Covid-19 disease.
It is evident that our understanding of the metabolic consequences of Covid-19 infection from ex vivo studies is advancing, but with most studies it was not clear if the cells being evaluated were actively infected with virus or not. Moreover, the relevant issue of the stage of disease being studied was usually not mentioned.
To record the direct effects that Covid-19 infection has on metabolic events in target cells, studies have been done using permissive or semi-permissive cells infected in vitro, then comparing the metabolic consequences to events in uninfected cells. One study with peripheral blood monocytes reported multiple metabolic differences. These included increases in HIF-1α protein levels, and increased transcriptional activity of molecules such as GLUT-1, PFKFB3, PKM2, and LDH-A [19]. However, with this study, all cells would not have been virus infected. Moreover, more potentially informative information might derive from longitudinal studies that measure metabolic changes at different times after synchronized infection in a system where all cells were shown to be infected. Accordingly, it is still not clear what direct effects Covid-19 infection has on cellular metabolism, and if this information would be useful to reshape events to achieve a more favorable outcome once the infection has been initiated.
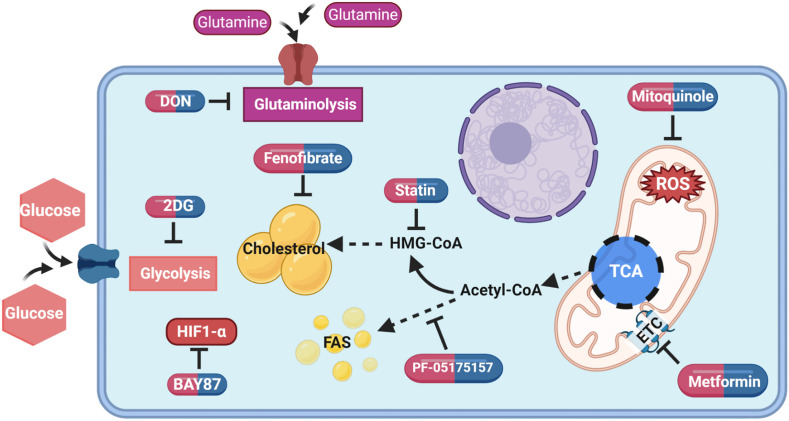
A further approach being used to comprehend how Covid-19 infection interferes with metabolism is to infect susceptible cells in vitro and to measure the influence of manipulating metabolic events on the outcome of infection. Several studies have evaluated the consequences of interfering with glucose utilization using the drug 2-Deoxy-d-glucose (2DG) [19,23]. This approach produces a marked reduction in viral replication and the infected cells survived. A similar outcome was obtained using drugs that targeted cholesterol metabolism (fenofibrate) or mitochondrial oxidative phosphorylation (mitoquinole) events (Fig. 2 ) [19,24]. Usually, the design of experiments was to add the metabolism modulating drugs before, or from the onset of infection, but perhaps of more therapeutic interest will be to discover procedures that can change the outcome of infection when given at a later time period when replication events are underway after infection. This experimental design might better simulate what would be useful during active infections in patients to limit the severity of their infection.
Fig. 2.
Cellular metabolism map showing pathways that could be targeted with metabolic drugs and inhibitors to reduce the burden of infection. Mitoquinol; ROS (reactive oxygen species) inhibitor [19], BAY87; HIF-1α (hypoxia inducible factor 1 alpha) inhibitor [19], 2DG (2-Deoxy-d-glucose); inhibits glycolysis [19,23], fenofibrate; reduce lipid level and accumulation [24], statin; HMG-CoA reductase (3-hydroxy-3-methyl-glutaryl-CoA) inhibitor [73], PF-05175157; acetyl-CoA carboxylase 1 and 2 inhibitor [78], DON (6-Diazo-5-oxo-l-norleucine); inhibits glutaminolysis [79], metformin; ETC (electron transport chain) complex I inhibitor [85], TCA; tricarboxylic acid cycle, FAS; fatty acid synthesis.
3. Metabolic events during severe disease and chronic sequelae
As mentioned previously, the severe tissue damaging phase of Covid-19 infection (Stage 3) is attributed mainly to the host response to infection and at this stage viral levels are usually low or even undetectable [10]. However, the driving force for the dramatic hyperinflammatory response, which usually involves a cytokine storm, remains uncertain. Thus, the process could be orchestrated largely by innate immune recognition and activation events, either responding directly or indirectly to the infection. Alternatively, the hyperinfammation could represent a process mainly orchestrated by T cells recognizing viral or conceivable host derived components [25]. Several reports have described events that occur in the Covid-19 associated cytokine storm and the value of several therapeutic procedures that counteract event that occur [12,26]. Nevertheless, currently there is minimal information about the metabolic consequences of the hyperinflammatory stage of Covid-19 infection.
Patients with the hyperinflammatory syndrome usually recover, especially if treated with dexamethasone and/or mAb to inflammatory cytokines, and receive supplemental oxygen [27,28]. However, many patients die or develop a chronic multisystem syndrome that involves the respiratory tract and several other extrapulmonary organs [7]. The extent of damage and its duration, which includes major effects on tissue metabolic events and on metabolic health in general, was elegantly discussed by Ayres [2]. This article also elaborated on the various therapeutic modalities that could be used to potentially correct the metabolic problems, although this information was largely derived not from Covid-19 patients with chronic multisystem inflammatory syndrome, but from animal model studies.
Damage to several extrapulmonary organs, which can be chronic, is not uncommon, especially in those that suffer severe clinical signs. The organs involved include the heart and other cardiovascular components, skeletal muscle, the brain and kidney, as well as the pancreas and gastrointestinal tissues [6]. The cause of the damage is still not clear, but many of these organs contain ACE2 positive cells so could be infected and damaged by virus. However, a more likely mechanism is that the damage results from direct effects of inflammatory reactions, although the actual molecular mechanisms are yet to be explained. Some long term consequences can result from damage to the affected organs. For example, damage to the respiratory tract itself can result in hypoxia with widespread consequences. The damaged respiratory tract may also undergo fibrosis and diminished surfactant production, issues difficult to reverse by therapy [25,29]. Damage to the kidney can lead to accumulation of toxic metabolites such as ketone bodies which cause systemic problems [30]. Damage to the pancreas, the source of insulin, can result in diabetes and hyperglycemia as well as hyperlipidemia [31]. Both have long term pathological consequences and in one study of patients that succumbed to fatal severe Covid-19 infection, 22% showed damage to the pancreas [32]. The liver, brain and cardiovascular tissues can also manifest chronic damage that provide a challenge to therapeutic control. It has also been observed that some patients are left with chronic functional problems with their immune systems [33]. The widespread damage to respiratory and extrapulmonary organs likely involves changes in their metabolic activities such as changes in mitochondrial metabolism [2]. However, reversing these effects with future therapies could prove to be very challenging. The issue merits far more study.
4. Influence of nutrition on outcome of infection
There is abundant evidence from studies done on several viral infections that the nutritional status of a person can affect the susceptibility and course of events following infection [3]. The effects are most evident when the diet is deficient in certain nutrients, such as vitamin A. For example, children receiving a vitamin A deficient diet are more susceptible to measles infection [34] and the same consequence also might apply to Covid-19 infection [28,34]. Dietary effects, such as fiber and fat content, can also impact on susceptibility to some virus infections. These effects may be mediated largely by influencing the balance of microbial species in the gastrointestinal tract, that in turn influence the quality of the immune response generated. Excellent reviews have been written on this topic [35,36]. Currently, there is minimal information as to whether or not diet affects either the susceptibility to, or course of events following Covid-19 infection, but such information is likely to accrue with time. Some studies on nutritional effects have reported that patients fed a diet supplemented with a variety of substances, that include the amino acid l-glutamine, vitamins such as A, D and C, derivatives of omega 3 fatty acid such as icosapent ethyl, as well as minerals such as zinc can favorably influence the outcome [[37], [38], [39], [40], [41], [42]]. Some clinical trials are in progress, such as exploring the value of omega 3 fatty acid and its derivative on the outcome of Covid-19 infection [43].
To date trials on the value of diet supplements have been limited in scope and are usually unconfirmed. Nevertheless, from what we know from studies with other viruses, it seems likely that modest effects of nutrition, particularly fiber content, fat composition and calorie intake will influence the outcome of Covid-19 infection and perhaps also responses to vaccines. Curiously, there are reports that the presence of Covid-19 in a community may cause persons to change their dietary habits such as adopting a Mediterranean diet or changing the quantities of different dietary components they consume [44]. Lockdown and boredom also can cause many changes in behavior! One common consequence is becoming more obese and its consequences [45,46], which, as discussed subsequently, increases susceptibility to the dire consequences of Covid-19 infection.
5. Metabolic diseases and Covid-19 infection
When Covid-19 infection emerged, it soon became evident that a risk factor was the presence of a metabolic disease [47,48]. The most common comorbidities were diabetes and obesity along with hypertension and cardiovascular also involved [49]. In the case of diabetes, it is still not clear if diabetics are more susceptible to initial infection, but it is evident those who are infected are likely to experience more severe disease consequences. Thus, 20% or more of patients admitted to the ICU can be diabetics [50] and one third or more of those dying from Covid-19 suffer from diabetes type 1 or 2 [51]. In fact, in the UK, serious complications were 50% more prevalent in diabetics than in healthy infected persons [52]. These complications include a more severe cytokine storm, death and more frequent longtime consequences. The hyperglycemia, characteristic of diabetes, is made worse by Covid-19 infection and many patients require more insulin to control their disease [53]. In some instances, Covid-19 infection may actually trigger the onset of diabetes [54], although whether or not this is a direct consequence of virus infection, or a secondary consequence of inflammatory events, is not clear [54]. Obesity, which is a common clinical problem in diabetes, is discussed in a different section of this review.
Whereas both diabetes type 1 and 2 are definite risk factors to develop lesions following Covid-19 infection, understanding at a mechanistic level how the effects of diabetes are mediated needs clarification. Several suggestions have been advocated and it is likely that reasons for the heightened susceptibility are multifactorial. A favored idea is that the deterioration of innate and adaptive immunity that occurs in diabetics contributes to the problem [55,56]. It is also evident that changes in expression levels of the ACE2 receptor on pancreatic beta cells could make those cells more susceptible to virus infection [57].
With regard to diminished innate immune activity during diabetes, reports have described reduced NK cell responses [58] and reduction in neutrophil functions caused by the hyperglycemia. The latter effects include reduced neutrophil extracellular trap formation, diminished superoxide responses and reduced production of elastase, myeloperoxidase and beta defensins [55,[59], [60], [61], [62]]. Additional effects include reduction in several proteins involved in innate immunity that include interferon-γ-induced protein-10, monocyte chemoattractant protein-1 and macrophage inflammatory proteins MIP-1α and MIP-1β [63]. Since innate defenses are those first encountered by infection and can function alone to control infection one might expect diabetics to be more susceptible to Covid-19 infection. However, currently there is no clear evidence for this, although we would suspect that the dose of virus needed to establish infection could be less than in non-diabetics.
Diabetics also show changes in the efficiency of adaptive aspects of immunity. For instance, the largely T cell mediated hyperinflammatory reaction that results in the cytokine storm during severe disease, is more intense in diabetics with high levels of circulating inflammatory cytokines such as IL-17, IFN-γ, TNF-α and GM-CSF present in plasma. Diabetics may also have less CD39+ Treg cells (responsible for suppression of Th17 cell) and their CD4 and CD8 T cells may show phenotypic changes such as chemokine receptor expression, so reducing their responses to inflammatory mediators [63,64].
6. Effect of obesity on susceptibility and outcome of Covid-19 infection-
Being obese, especially when morbidly so, is not good news if infected with Covid-19. Several hospital-based studies in different locations have shown that persons with a body mass index that equals or exceeds 30 account for 40% or more of those who contract Covid-19 [65]. Moreover, compared to non-obese persons, obese individuals are more likely to develop severe disease and mortality [66]. One study observed a direct correlation between progression of disease with obesity and ICU admittance for ventilation [67,68]. Moreover, compared to non-obese persons, obese people had more symptoms, such as cough and fever, and had a 3.4-fold greater chance of developing severe Covid-19 disease that included pneumonia [69].
The fact that obesity is a susceptibility factor for Covid-19 morbidity and mortality is well accepted, but why this is the case is not fully understood. However, it is well known that adipocytes can express ACE2 receptors making them potential targets for Covid-19 infection, acting perhaps to increase the amount of virus produced [70]. Against this idea, the severe consequences of Covid-19 infection during obesity occur mainly in non-adipose tissues, especially the respiratory tract. Conceivably greater susceptibility could be explained by fat deposits causing mechanical problems that interfere with breathing and other organ functions [71]. Alternatively, obesity could result in metabolic changes that interfere with the development or activity of antiviral defense mechanisms. Support for this notion comes from studies on human influenza virus infections where immune responses are diminished in obese persons and poor immunity results from vaccination [72]. The balance of immune responsiveness may also be different in obese persons with them often responding with a pattern that is proinflammatory rather than protective. Further support for the detrimental effects of obesity also comes from experimental influenza virus infections in mice. For example, obese mice, compared to lean controls, show several changes and these are listed in Table 1 .
Table 1.
Immunological changes reported in obese mice.
| Mice strain studied | Effect induced by | Outcome of respective diet |
|---|---|---|
| C57BL/6 | High-fat diet (45% of calories from fat) | Adipose tissue increases M1 macrophages and higher expression of TNF-α, IL-6 and iNOS. |
| Standard diet (4.5% fat) | Adipose tissue mainly has M2 macrophages that express arginase-1, and IL-10. | |
| High fat diet (60 Kcal% fat) | Lower level of hematopoiesis, higher level of oxidative stress and overexpression of Growth factor independence 1. | |
| Normal chow diet (13 Kcal% fat) | Expresses higher level of hematopoiesis, reduced oxidative stress and normal expression of Gfi1. | |
| C57BL/6 J and RAG mice | High fat diet (60% Kcal from fat) | Reduced lymphatic flow and migration of dendritic cell to local lymph nodes. Low level of CD4 and CD8 T cells and higher level of B and macrophages in lymph nodes. |
| Normal chow diet (13% kcal from fat) | Normal lymphatic flow and migration of dendritic cell to local lymph nodes. High level of CD4 and CD8 T cells and low level of B and macrophages in lymph node. | |
| C57BL/6 | High fat diet (60% calories from fat) | Low level of naive and CD4 T cells, reduced levels of IFN-γ, IL-6, TNF and TGF-β1. |
| Low fat diet (4.5% fat) | High levels of naive and CD4 T cells, higher levels of IFN-γ, IL-6, TNF and TGF-beta1. | |
| High fat diet (60% calorie) | More prone to reinfection by influenza due to lower level of memory T cells in lungs. |
As regards obesity and Covid-19 infection, the issue arises as to how patients should be managed therapeutically. Accordingly, many obese patients take medications to control obesity, but responses to them might change during some stages of Covid-19 infection. Curiously, the use statins, commonly used in obese persons to lower cholesterol levels, might be beneficial since cholesterol is part of the cell entry system for the virus and lowering cholesterol could increase resistance to infection [73].
7. Are there practical approaches to manipulate metabolism and constrain Covid-19 lesions?
This question currently has no simple answer and needs far more exploration before solutions can be advocated. Moreover, counteracting metabolic events in any disease syndrome is subject to Faustian consequences to bystander tissues if the therapy is not highly focused on the problem lesions. The concept of targeting metabolism to shape the outcome of any viral infection is in its infancy and almost unexplored in the case of Covid-19. As we have documented, Covid-19 infection does cause a range of metabolic changes in infected cells and tissues and is more of a problem when the host’s metabolism is compromised in some way, such as occurs in diabetes. Furthermore, Covid-19 infection can result in long term damage to several organs where metabolic changes are characteristic [2]. Even though Covid-19 disease does involve short term and long term changes in metabolism, the question remains whether it is practical to change the metabolic environment and if so how might such manipulations best be achieved.
Although it recently became evident that Covid-19 may be effectively controlled by vaccines, any approach that increases the resistance of persons to infection would be welcome, particularly in societies that have many citizens who refuse to use vaccines or are opposed to social distancing and wearing masks. With regard to changing metabolic events to increase resistance to the untoward effect of virus infections, a simple way may be dietary manipulation. Thus, as we discussed in the section on nutrition diet composition can impact on resistance to many virus infections acting in some cases by causing changes in the bacterial composition at mucosal surfaces. High fiber diets and diets rich in some types of fatty acids favor the induction of responses to infections that are less tissue-damaging and encourage lesion resolution [74,75]. In support of this concept, we recently showed in an inflammatory viral disease model that feeding mice extra short chain fatty acids resulted in markedly reduced ocular lesions in response to herpes simplex virus (HSV) infection [76]. Other studies showed that diets enriched in fiber led to reduced inflammatory lesions caused by infections [74]. However, the nutritional modification approach is only useful for minimizing problems if the diet change is used some time before infection. To deal with ongoing viral induced inflammatory reactions by manipulating metabolism provides more of a challenge. Some have achieved this objective in model systems, but few if any studies have reported success using such strategies in virus infected patients. For example, working with an inflammatory lesion in eyes set off by HSV infection, our group showed that therapy from around the time of lesion onset with 2DG, which compromises glucose utilization and energy generation by the main cell types responsible for tissue damage, resulted in mild lesions [77]. Other model studies showed that the impact of West Nile fever virus infection in mice was minimized using drugs to reduce fatty acid synthesis and in another model study using Sindbis virus infection, blocking glutamine metabolism protected mice against inflammatory brain lesions [78,79]. Currently, the approach of shaping the outcome of viral infections by manipulating some component of metabolism is very much in its infancy and has seen little or no use in clinical situations, particularly against Covid-19 infections. We expect, this scenario to change with manipulating one or even multiple aspects of metabolism becoming part of the therapeutic armamentarium to control Covid-19 and other infections (see Fig. 2). However, lest we become too enthusiastic, there are many caveats to consider. Metabolic pathways are very much intertwined and are involved in many other physiological events that would be hazardous to unsettle. With infections, for example controlling one agent such as Covid-19 could result in heightened susceptibility to another such as bacterial and fungal infections. Undoubtedly timing the use of metabolic changes will be especially critical as we observed in our studies on controlled HSV ocular infections in mice. Thus, if 2DG therapy was used early after infection when abundant viral replication was still occurring, the virus could readily access the nervous system and cause a lethal encephalitis [77]. There would likely be problems too of systemic toxicity if metabolic reshaping was used for prolonged time periods.
8. Conclusions
In this review, the metabolic changes that occur in response to Covid-19 infection at different stages in the host are summarized. We also discuss differences that can occur if the infected host has abnormal metabolism when infected. The effects of nutrition on the outcome of infection was also summarized. Finally, we evaluate how reshaping metabolism might be used as a therapeutic approach to help reduce the consequences of Covid-19 infection, as well as mention some limitations.
Declaration of competing interest
There is no conflict of interest to declare.
Acknowledgments
This work was supported by the NIH 2020R21 AI (Grant no: 5R21AI142862-02) and NIH 2020 R01 (Grant no: EY5R01EY005093-35).
References
- 1.WHO . 2021. Coronavirus disease (COVID-19) dashboard.https://covid19.who.int/ Available at: [Google Scholar]
- 2.Ayres J.S. A metabolic handbook for the COVID-19 pandemic. Nat Metab. 2020;2:572–585. doi: 10.1038/s42255-020-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sumbria D., Berber E., Rouse T.B. Virus infections and host metabolism-can we manage the interactions? Front Immunol. 2021 doi: 10.3389/fimmu.2020.594963. [Ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.U.S . 2021. NLoM. PubMed.https://pubmed.ncbi.nlm.nih.gov/ Available at: [Google Scholar]
- 5.Hou Y.J., Okuda K., Edwards C.E., Martinez D.R., Asakura T., Dinnon K.H., 3rd SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell. 2020;182:429–446. doi: 10.1016/j.cell.2020.05.042. e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zipeto D., Palmeira J.D.F., Argañaraz G.A., Argañaraz E.R. ACE2/ADAM17/TMPRSS2 Interplay may be the main risk factor for COVID-19. Front Immunol. 2020;11:576745. doi: 10.3389/fimmu.2020.576745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta A., Madhavan M.V., Sehgal K., Nair N., Mahajan S., Sehrawat T.S. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26:1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PubMed] [Google Scholar]
- 8.Van Damme W., Dahake R., van de Pas R., Vanham G., Assefa Y. COVID-19: does the infectious inoculum dose-response relationship contribute to understanding heterogeneity in disease severity and transmission dynamics? Med Hypotheses. 2020:110431. doi: 10.1016/j.mehy.2020.110431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sehrawat S., Rouse B.T. COVID-19: disease, or no disease? –that is the question. It’s the dose stupid! Microb Infect. 2021;23(1):104779. doi: 10.1016/j.micinf.2021.104779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant : the official publication of the International Society for Heart Transplantation. 2020;39:405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mangalmurti N., Hunter C.A. Cytokine storms: understanding COVID-19. Immunity. 2020;53:19–25. doi: 10.1016/j.immuni.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fajgenbaum D.C., June C.H. Cytokine storm. N Engl J Med. 2020;383:2255–2273. doi: 10.1056/NEJMra2026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas-Rüddel D., Winning J., Dickmann P., Ouart D., Kortgen A., Janssens U. Coronavirus disease 2019 (COVID-19): update for anesthesiologists and intensivists March 2020. Anaesthesist. 2020:1–10. doi: 10.1007/s00101-020-00758-x. [DOI] [PubMed] [Google Scholar]
- 14.Gardinassi L.G., Souza C.O.S., Sales-Campos H., Fonseca S.G. Immune and metabolic signatures of COVID-19 revealed by transcriptomics data reuse. Front Immunol. 2020:11. doi: 10.3389/fimmu.2020.01636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas T., Stefanoni D., Reisz J.A., Nemkov T., Bertolone L., Francis R.O. COVID-19 infection alters kynurenine and fatty acid metabolism, correlating with IL-6 levels and renal status. JCI insight. 2020;5 doi: 10.1172/jci.insight.140327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blasco H., Bessy C., Plantier L., Lefevre A., Piver E., Bernard L. The specific metabolome profiling of patients infected by SARS-COV-2 supports the key role of tryptophan-nicotinamide pathway and cytosine metabolism. Sci Rep. 2020;10:16824. doi: 10.1038/s41598-020-73966-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moolamalla S.T.R., Chauhan R., Deva Priyakumar U., Vinod P.K. Host metabolic reprogramming in response to SARS-Cov-2 infection. bioRxiv. 2020 doi: 10.1101/2020.08.02.232645. [Preprint] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bost P., Giladi A., Liu Y., Bendjelal Y., Xu G., David E. Host-viral infection maps reveal signatures of severe COVID-19 patients. Cell. 2020;181:1475–1488. doi: 10.1016/j.cell.2020.05.006. e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Codo A.C., Davanzo G.G., Monteiro LdB., de Souza G.F., Muraro S.P., Virgilio-da-Silva J.V. Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1α/glycolysis-dependent axis. Cell Metabol. 2020;32:437–446. doi: 10.1016/j.cmet.2020.07.007. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desterke C., Turhan A.G., Bennaceur-Griscelli A., Griscelli F. PPARγ cistrome repression during activation of lung monocyte-macrophages in severe COVID-19. iScience. 2020;23:101611. doi: 10.1016/j.isci.2020.101611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He W., Barak Y., Hevener A., Olson P., Liao D., Le J. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci USA. 2003;100:15712–15717. doi: 10.1073/pnas.2536828100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang F., Mullican S.E., DiSpirito J.R., Peed L.C., Lazar M.A. Lipoatrophy and severe metabolic disturbance in mice with fat-specific deletion of PPARγ. Proc Natl Acad Sci USA. 2013;110:18656–18661. doi: 10.1073/pnas.1314863110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bojkova D., Klann K., Koch B., Widera M., Krause D., Ciesek S. Proteomics of SARS-CoV-2-infected host cells reveals therapy targets. Nature. 2020;583:469–472. doi: 10.1038/s41586-020-2332-7. [DOI] [PubMed] [Google Scholar]
- 24.Ehrlich A., Uhl S., Ioannidis K., Hofree M., tenOever B.R., Nahmias Y. 2020. The SARS-CoV-2 transcriptional metabolic signature in lung epithelium. Available at: SSRN 3650499. [Google Scholar]
- 25.Melenotte C., Silvin A., Goubet A.-G., Lahmar I., Dubuisson A., Zumla A. Immune responses during COVID-19 infection. OncoImmunology. 2020;9:1807836. doi: 10.1080/2162402X.2020.1807836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ye Q., Wang B., Mao J. The pathogenesis and treatment of the `cytokine storm’ in COVID-19. J Infect. 2020;80:607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Group A-TL-CS A neutralizing monoclonal antibody for hospitalized patients with COVID-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2033130. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen C., Yue X., Wang J., Shi C., Li W. Nocturnal oxygen therapy as an option for early COVID-19. Int J Infect Dis. 2020;98:176–179. doi: 10.1016/j.ijid.2020.06.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mirastschijski U., Dembinski R., Maedler K. Lung surfactant for pulmonary barrier restoration in patients with COVID-19 pneumonia. Front Med. 2020;7:254. doi: 10.3389/fmed.2020.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu C., Zia U. Recovery from acute kidney injury with diabetic ketoacidosis following SARS-CoV-2 infection: a case report and literature review. Cureus. 2020;12 doi: 10.7759/cureus.11702. e11702-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shemesh E., Zafrir B. Hypertriglyceridemia-related pancreatitis in patients with type 2 diabetes: links and risks. Diabetes Metab Syndr Obes. 2019;12:2041–2052. doi: 10.2147/DMSO.S188856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanley B., Naresh K.N., Roufosse C., Nicholson A.G., Weir J., Cooke G.S. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. The Lancet. Microbe. 2020;1:e245–e253. doi: 10.1016/S2666-5247(20)30115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Files J.K., Boppana S., Perez M.D., Sarkar S., Lowman K.E., Qin K. Sustained cellular immune dysregulation in individuals recovering from SARS-CoV-2 infection. J Clin Invest. 2021:131. doi: 10.1172/JCI140491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hussey G.D., Klein M. A randomized, controlled trial of vitamin A in children with severe measles. N Engl J Med. 1990;323:160–164. doi: 10.1056/NEJM199007193230304. [DOI] [PubMed] [Google Scholar]
- 35.den Besten G., van Eunen K., Groen A.K., Venema K., Reijngoud D.J., Bakker B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54:2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Makki K., Deehan E.C., Walter J., Bäckhed F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe. 2018;23:705–715. doi: 10.1016/j.chom.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 37.Cengiz M., Borku Uysal B., Ikitimur H., Ozcan E., Islamoğlu M.S., Aktepe E. Effect of oral l-Glutamine supplementation on COVID-19 treatment. Clin Nutr Exp. 2020;33:24–31. doi: 10.1016/j.yclnex.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Panagiotou G., Tee S.A., Ihsan Y., Athar W., Marchitelli G., Kelly D. Low serum 25-hydroxyvitamin D (25[OH]D) levels in patients hospitalized with COVID-19 are associated with greater disease severity. Clin Endocrinol. 2020;93:508–511. doi: 10.1111/cen.14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hiedra R., Lo K.B., Elbashabsheh M., Gul F., Wright R.M., Albano J. The use of IV vitamin C for patients with COVID-19: a case series. Expert Rev Anti Infect Ther. 2020;18:1259–1261. doi: 10.1080/14787210.2020.1794819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Finzi E. Treatment of SARS-CoV-2 with high dose oral zinc salts: a report on four patients. Int J Infect Dis : IJID : official publication of the International Society for Infectious Diseases. 2020;99:307–309. doi: 10.1016/j.ijid.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berger A.A., Sherburne R., Urits I., Patel H., Eskander J. Icosapent ethyl - a successful treatment for symptomatic COVID-19 infection. Cureus. 2020;12:e10211. doi: 10.7759/cureus.10211. e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Daneshkhah A., Agrawal V., Eshein A., Subramanian H., Roy H.K., Backman V. The possible role of vitamin D in suppressing cytokine storm and associated mortality in COVID-19 patients. MedRxiv. 2020 doi: 10.1101/2020.04.08.20058578. [Preprint] [DOI] [Google Scholar]
- 43.U.S. NLoM.Clinical trial . 2020. Prevention of COVID19 with EPA in healthcare providers at risk - intervention trial (PREPARE-IT)https://clinicaltrials.gov/ct2/show/NCT04460651 Identifier NCT04460651. Available at: [Google Scholar]
- 44.Di Renzo L., Gualtieri P., Pivari F., Soldati L., Attinà A., Cinelli G. Eating habits and lifestyle changes during COVID-19 lockdown: an Italian survey. J Transl Med. 2020;18:229. doi: 10.1186/s12967-020-02399-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pietrobelli A., Pecoraro L., Ferruzzi A., Heo M., Faith M., Zoller T. Effects of COVID-19 lockdown on lifestyle behaviors in children with obesity living in verona, Italy: a longitudinal study. Obesity. 2020;28:1382–1385. doi: 10.1002/oby.22861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jia P., Zhang L., Yu W., Yu B., Liu M., Zhang D. Impact of COVID-19 lockdown on activity patterns and weight status among youths in China: the COVID-19 Impact on Lifestyle Change Survey (COINLICS) IJO. 2020 doi: 10.1038/s41366-020-00710-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xie J., Zu Y., Alkhatib A., Pham T.T., Gill F., Jang A. Metabolic syndrome and COVID-19 mortality among adult black patients in new orleans. Diabetes Care. 2020 doi: 10.2337/dc20-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.le Roux C.W. COVID-19 alters thinking and management in metabolic diseases. Nat Rev Endocrinol. 2021;17(2):71–72. doi: 10.1038/s41574-020-00449-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Apicella M., Campopiano M.C., Mantuano M., Mazoni L., Coppelli A., Del Prato S. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020;8:782–792. doi: 10.1016/S2213-8587(20)30238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Korytkowski M., Antinori-Lent K., Drincic A., Hirsch I.B., McDonnell M.E., Rushakoff R. A pragmatic approach to inpatient diabetes management during the COVID-19 pandemic. J Clin Endocrinol Metab. 2020;105:3076–3087. doi: 10.1210/clinem/dgaa342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hillson R. COVID-19: diabetes and death. A call to action. Practical Diabetes Int. 2020;37:76–78. [Google Scholar]
- 52.Muniangi-Muhitu H., Akalestou E., Salem V., Misra S., Oliver N.S., Rutter G.A. COVID-19 and diabetes: a complex bidirectional relationship. Front Endocrinol. 2020:11. doi: 10.3389/fendo.2020.582936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu L., Girgis C.M., Cheung N.W. COVID-19 and diabetes: insulin requirements parallel illness severity in critically unwell patients. J Clin Endocrinol Metab. 2020;93:390–393. doi: 10.1111/cen.14288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lim S., Bae J.H., Kwon H.-S., Nauck M.A. COVID-19 and diabetes mellitus: from pathophysiology to clinical management. Nat Rev Endocrinol. 2021;17:11–30. doi: 10.1038/s41574-020-00435-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jafar N., Edriss H., Nugent K. The effect of short-term hyperglycemia on the innate immune system. Am J Med Sci. 2016;351:201–211. doi: 10.1016/j.amjms.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 56.Zhou T., Hu Z., Yang S., Sun L., Yu Z., Wang G. Role of adaptive and innate immunity in type 2 diabetes mellitus. J Diabetes Res. 2018;2018:7457269. doi: 10.1155/2018/7457269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fignani D., Licata G., Brusco N., Nigi L., Grieco G.E., Marselli L. SARS-CoV-2 receptor angiotensin i-converting enzyme type 2 (ACE2) is expressed in human pancreatic β-cells and in the human pancreas microvasculature. Front Endocrinol. 2020:11. doi: 10.3389/fendo.2020.596898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim J.H., Park K., Lee S.B., Kang S., Park J.S., Ahn C.W. Relationship between natural killer cell activity and glucose control in patients with type 2 diabetes and prediabetes. J Diabetes Investig. 2019;10:1223–1228. doi: 10.1111/jdi.13002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Joshi M.B., Lad A., Bharath Prasad A.S., Balakrishnan A., Ramachandra L., Satyamoorthy K. High glucose modulates IL-6 mediated immune homeostasis through impeding neutrophil extracellular trap formation. FEBS Lett. 2013;587:2241–2246. doi: 10.1016/j.febslet.2013.05.053. [DOI] [PubMed] [Google Scholar]
- 60.Perner A., Nielsen S.E., Rask-Madsen J. High glucose impairs superoxide production from isolated blood neutrophils. Intensive Care Med. 2003;29:642–645. doi: 10.1007/s00134-002-1628-4. [DOI] [PubMed] [Google Scholar]
- 61.Stegenga M.E., van der Crabben S.N., Blümer R.M.E., Levi M., Meijers J.C.M., Serlie M.J. Hyperglycemia enhances coagulation and reduces neutrophil degranulation, whereas hyperinsulinemia inhibits fibrinolysis during human endotoxemia. Blood. 2008;112:82–89. doi: 10.1182/blood-2007-11-121723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kiselar J.G., Wang X., Dubyak G.R., El Sanadi C., Ghosh S.K., Lundberg K. Modification of β-defensin-2 by dicarbonyls methylglyoxal and glyoxal inhibits antibacterial and chemotactic function in vitro. PloS One. 2015;10 doi: 10.1371/journal.pone.0130533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hedman M., Faresjö M., Axelsson S., Ludvigsson J., Casas R. Impaired CD4 and CD8 T cell phenotype and reduced chemokine secretion in recent-onset type 1 diabetic children. Clin Exp Immunol. 2008;153:360–368. doi: 10.1111/j.1365-2249.2008.03720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cortez-Espinosa N., Cortés-Garcia J.D., Martínez-Leija E., Rodríguez-Rivera J.G., Barajas-López C., González-Amaro R. CD39 expression on Treg and Th17 cells is associated with metabolic factors in patients with type 2 diabetes. Hum Immunol. 2015;76:622–630. doi: 10.1016/j.humimm.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 65.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. J Am Med Assoc. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Popkin B.M., Du S., Green W.D., Beck M.A., Algaith T., Herbst C.H. Individuals with obesity and COVID-19: a global perspective on the epidemiology and biological relationships. Obes Rev. 2020;21:e13128. doi: 10.1111/obr.13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Caussy C., Pattou F., Wallet F., Simon C., Chalopin S., Telliam C. Prevalence of obesity among adult inpatients with COVID-19 in France. Lancet Diabetes Endocrinol. 2020;8:562–564. doi: 10.1016/S2213-8587(20)30160-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lighter J., Phillips M., Hochman S., Sterling S., Johnson D., Francois F. Obesity in patients younger than 60 years is a risk factor for COVID-19 hospital admission. Clin Infect Dis : an official publication of the Infectious Diseases Society of America. 2020;71:896–897. doi: 10.1093/cid/ciaa415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cai Q., Chen F., Wang T., Luo F., Liu X., Wu Q. Obesity and COVID-19 severity in a designated hospital in shenzhen, China. Diabetes Care. 2020 doi: 10.2337/dc20-0576. [DOI] [PubMed] [Google Scholar]
- 70.Ryan P.M., Caplice N.M. Is adipose tissue a reservoir for viral spread, immune activation, and cytokine amplification in coronavirus disease 2019? Obesity. 2020;28:1191–1194. doi: 10.1002/oby.22843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sattar N., McInnes Iain B., McMurray John J.V. Obesity is a risk factor for severe COVID-19 infection. Circulation. 2020;142:4–6. doi: 10.1161/CIRCULATIONAHA.120.047659. [DOI] [PubMed] [Google Scholar]
- 72.Sheridan P.A., Paich H.A., Handy J., Karlsson E.A., Hudgens M.G., Sammon A.B. Obesity is associated with impaired immune response to influenza vaccination in humans. IJO. 2012;36:1072–1077. doi: 10.1038/ijo.2011.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rossi R., Talarico M., Coppi F., Boriani G. Protective role of statins in COVID 19 patients: importance of pharmacokinetic characteristics rather than intensity of action. Internal and emergency medicine. 2020;15:1573–1576. doi: 10.1007/s11739-020-02504-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Trompette A., Gollwitzer E.S., Pattaroni C., Lopez-Mejia I.C., Riva E., Pernot J. Dietary fiber confers protection against Flu by shaping Ly6c(-) patrolling monocyte hematopoiesis and CD8(+) T Cell metabolism. Immunity. 2018;48:992–1005.e8. doi: 10.1016/j.immuni.2018.04.022. [DOI] [PubMed] [Google Scholar]
- 75.Kim J.Y., Lim K., Kim K.H., Kim J.H., Choi J.S., Shim S.-C. N-3 polyunsaturated fatty acids restore Th17 and Treg balance in collagen antibody-induced arthritis. PloS One. 2018;13 doi: 10.1371/journal.pone.0194331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sumbria D., Berber E., Rouse B.T. Supplementing the diet with sodium propionate suppresses the severity of viral immuno-inflammatory lesions. J Virol. 2020 doi: 10.1128/JVI.02056-20. [Ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Varanasi S.K., Donohoe D., Jaggi U., Rouse B.T. Manipulating glucose metabolism during different stages of viral pathogenesis can have either detrimental or beneficial effects. J Immunol. 2017;199:1748. doi: 10.4049/jimmunol.1700472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Manivannan S., Baxter V.K., Schultz K.L.W., Slusher B.S., Griffin D.E. Protective effects of glutamine antagonist 6-diazo-5-oxo-l-norleucine in mice with alphavirus encephalomyelitis. Virol J. 2016;90:9251. doi: 10.1128/JVI.01045-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jiménez de Oya N., Esler W.P., Huard K., El-Kattan A.F., Karamanlidis G., Blázquez A.-B. Targeting host metabolism by inhibition of acetyl-Coenzyme A carboxylase reduces flavivirus infection in mouse models. Emerg Microb Infect. 2019;8:624–636. doi: 10.1080/22221751.2019.1604084. [DOI] [PMC free article] [PubMed] [Google Scholar]