Abstract
Objectives
The authors evaluated the outcome of adult patients with coronavirus disease 2019 (COVID-19)–related acute respiratory distress syndrome (ARDS) requiring the use of extracorporeal membrane oxygenation (ECMO).
Design
Multicenter retrospective, observational study.
Setting
Ten tertiary referral university and community hospitals.
Participants
Patients with confirmed severe COVID-19–related ARDS.
Interventions
Venovenous or venoarterial ECMO.
Measurements and Main Results
One hundred thirty-two patients (mean age 51.1 ± 9.7 years, female 17.4%) were treated with ECMO for confirmed severe COVID-19–related ARDS. Before ECMO, the mean Sequential Organ Failure Assessment score was 10.1 ± 4.4, mean pH was 7.23 ± 0.09, and mean PaO2/fraction of inspired oxygen ratio was 77 ± 50 mmHg. Venovenous ECMO was adopted in 122 patients (92.4%) and venoarterial ECMO in ten patients (7.6%) (mean duration, 14.6 ± 11.0 days). Sixty-three (47.7%) patients died on ECMO and 70 (53.0%) during the index hospitalization. Six-month all-cause mortality was 53.0%. Advanced age (per year, hazard ratio [HR] 1.026, 95% CI 1.000-1-052) and low arterial pH (per unit, HR 0.006, 95% CI 0.000-0.083) before ECMO were the only baseline variables associated with increased risk of six-month mortality.
Conclusions
The present findings suggested that about half of adult patients with severe COVID-19–related ARDS can be managed successfully with ECMO with sustained results at six months. Decreased arterial pH before ECMO was associated significantly with early mortality. Therefore, the authors hypothesized that initiation of ECMO therapy before severe metabolic derangements subset may improve survival rates significantly in these patients. These results should be viewed in the light of a strict patient selection policy and may not be replicated in patients with advanced age or multiple comorbidities. Clinical Trial Registration: identifier, NCT04383678.
Key Words: extracorporeal membrane oxygenation, ECMO, ECLS, coronavirus disease 2019, COVID-19, acute respiratory distress syndrome, ARDS
THE OUTBREAK of coronavirus disease 2019 (COVID-19) has led to a pandemic associated with a mortality ranging from 21% to 28% among hospitalized patients.1, 2, 3 A large study from the New York City area hospitals showed that 14.2% of hospitalized patients were admitted to the intensive care unit (ICU) and 12% received invasive mechanical ventilation.1 Mortality among patients who received mechanical ventilation was 88%.1 A study from Northern Italy showed that, among 3,988 COVID-19 patients admitted to ICUs of the Lombardy region of Italy, about 50% have died.4
Acute respiratory distress syndrome (ARDS) is a common complication of COVID-19, and extracorporeal membrane oxygenation (ECMO) can be a salvage therapy in this dramatic medical context. However, ECMO therapy was not used in significant numbers in the first phase of the COVID-19 infection outbreak,4 and initially the Extracorporeal Life Support Organization (ELSO) has not supported the use of ECMO in these patients.5 Indeed, only 64 (1.7%) of 3,857 severe COVID-19 patients admitted to the ICUs of the Lombardy region of Italy were treated with ECMO, and their ICU mortality was 62.5%.4 Experience with influenza A(H1N1)–related ARDS patients showed that ECMO therapy was associated with a significantly lower hospital mortality compared to no ECMO therapy (24.0% v 46.7%).6 Consonant data also were observed in the Reseau Europeen de Recherche en Ventilation Artificielle Research Network analysis for young patients with influenza A(H1N1)–related ARDS.7 The results of a recent meta-analysis further consolidated the growing body of evidence of the benefits of ECMO in patients with ARDS.8 Initial data on the treatment of patients with severe COVID-19 from the Paris-Sorbonne University Hospital Network documented a 60-day mortality rate of 31%.9 This excellent result was confirmed by a multicenter study by the ELSO, which documented a hospital mortality of 37.4%.10 However, these studies also included patients who were still on treatment during the index hospitalization, and there is a lack of data on the midterm outcome of patients who survived to discharge. In the present study, the authors aimed to evaluate the six-month outcome of patients with COVID-19 who were treated with ECMO for refractory ARDS, from a multicenter study.
Material and Methods
Study Design and Participants
The authors conducted a retrospective cohort study of all patients who underwent ECMO of any configuration for confirmed COVID-19–related ARDS at ten ECMO centers in five European countries (France, Germany, Italy, Sweden, UK) (ClinicalTrials.gov identifier, NCT04383678). All participating centers but one (Lecco, Hospital, Italy) were tertiary care university hospitals and designated COVID-19 ECMO centers. The study protocol was approved by the institutional or national review board of each participating center according to national legislations. Informed consent was waived because of the retrospective nature of this study. The inclusion criteria for the study entry were adults aged 18 years or older with severe hypoxemia with or without hemodynamic instability refractory to conventional therapies associated with laboratory-confirmed COVID-19–related ARDS. ARDS was defined according to the Berlin definition criteria.11
Data were collected retrospectively into a dedicated electronic datasheet with prespecified variables by experienced clinicians, and underwent checking of its completeness and quality. Data on baseline characteristics, ECMO therapy, and adverse events were retrieved from the electronic patient records. Data on intermediate survival were retrieved from national registries and electronic patient records, and by contacting patients and their general practitioners. Six-month follow-up was complete for all but four patients whose follow-up ranged from 3.0-to-5.2 months.
Outcomes
The primary outcome of this study was all-cause mortality at six months from the initiation of ECMO therapy. The secondary outcomes included mortality on ECMO, ICU mortality, hospital mortality, lung or pleural complications requiring thoracic surgery, stroke, acute kidney injury, new renal replacement therapy, transfusion of blood products, bloodstream infection, confirmed or suspected pulmonary embolism, duration of invasive mechanical ventilation, and lengths of ICU and hospital stay.
Intensive care unit mortality was defined as all-cause mortality in the ICU where ECMO therapy was performed or in any ICU facility where the patient was transferred after weaning from ECMO. Stroke was defined as any focal or global neurologic syndrome occurring during the ICU stay caused by ischemia and/or hemorrhage not resolving within 24 hours. The diagnosis of stroke was made based on findings by computed tomography and/or magnetic resonance imaging of the brain. Acute kidney injury was defined as an increase in serum creatinine concentration at least 1.5-fold the baseline level or a serum creatinine concentration ≥ 25.6 µmol/L or new renal replacement therapy from the initiation of ECMO therapy during the index hospitalization.
Statistical Analysis
No sample size calculation was performed. Continuous variables are reported as mean and SD, and categorical variables are reported as counts and percentages. Comparisons of baseline and treatment variables among the study groups were performed using the Kaplan-Meier and the Cox proportional hazards methods. Comparisons among the study groups were performed using the Mann-Whitney U test, Fisher exact test, Kruskal-Wallis test, and χ2 tests. Cox proportional hazards analysis was performed to identify risk factors for six-month all-cause mortality by including into the regression model the following covariates with p < 0.2 for six-month mortality in univariate analysis: age, tidal volume, platelet counts before ECMO, arterial pH before ECMO, use of lopinavir/ritonavir, and use of convalescent plasma. The receiver operating characteristic curve analysis was performed to evaluate the predictive ability of continuous variables. Youden's test was used to identify the best cutoff value of continuous covariates in predicting early mortality. A classification and regression tree (CART) analysis was performed to identify risk factors of six-month mortality. The CART model included risk factors identified in the Cox proportional hazards analysis as independent predictors of six-month mortality, considering a minimum number of patients for the parent node of ten patients and for the child node of five patients. The independent predictors were dichotomized according to cutoff values identified by the CART analysis. A p < 0.05 was set for statistical significance. Statistical analyses were performed using Stata version 15.1 (StataCorp LLC, College Station, TX) and SPSS version 25.0 (IBM Corporation, Armonk, NY) statistical software.
Results
Characteristics of the Patients
Between March 2, 2020 and April 30, 2020, 132 patients (mean age 51.1 ± 9.7 years, female 17.4%) were treated at ten centers with ECMO for confirmed severe COVID-19–related ARDS and/or hemodynamic instability (Tables 1 and 2 ). The number of patients treated with ECMO at each center varied from two-to-44 patients (Table 1). Before initiation of ECMO therapy, the mean Sequential Organ Failure Assessment score was 10.1 ± 4.4, mean pH was 7.23 ± 0.09, and mean PaO2/fraction of inspired oxygen ratio was 77 ± 50 mmHg (Table 2). Patients’ age, Sequential Organ Failure Assessment score, and arterial pH before ECMO differed significantly among centers (Table 1).
Table 1.
Participating Centers, Number of ECMO Treated Patients for Severe COVID-19, and Their Main Characteristics
| Centers | No. of Patients | Age (y)* | SOFA Score† | Pre-ECMO Arterial pH‡ | 6-Month Mortality (%)§ |
|---|---|---|---|---|---|
| Paris Créteil, France | 44 | 52.3 ± 7.8 | 8.7 ± 2.1 | 7.22 ± 0.05 | 65.9 |
| Leicester, UK | 26 | 45.8 ± 7.8 | 7.1 ± 3.0 | 7.30 ± 0.08 | 50.0 |
| Nancy, France | 18 | 52.6 ± 11.5 | 9.2 ± 2.0 | 7.34 ± 0.09 | 38.9 |
| Stockholm, Sweden | 15 | 46.7 ± 11.5 | 13.9 ± 2.9 | 7.18 ± 0.09 | 46.7 |
| Reims, France | 7 | 54.6 ± 8.6 | 14.3 ± 5.1 | 7.32 ± 0.11 | 42.9 |
| Hamburg, Germany | 7 | 52.9 ± 11.7 | 10.3 ± 3.3 | 7.19 ± 0.11 | 28.6 |
| Bologna, Italy | 5 | 48.6 ± 8.8 | 8.4 ± 0.5 | 7.29 ± 0.10 | 80.0 |
| Münster, Germany | 5 | 61.2 ± 8.0 | 9.2 ± 1.6 | 7.23 ± 0.08 | 60.0 |
| Besançon, France | 3 | 59.7 ± 4.9 | 8.0 ± 2.6 | 7.34 ± 0.07 | 33.3 |
| Lecco, Italy | 2 | 44.5 ± 0.7 | 8.5 ± 2.1 | 7.27 ± 0.01 | 50.0 |
NOTE. Continuous values are reported as mean and standard deviation.
Abbreviations: COVID-19, coronavirus disease 2019; ECMO, extracorporeal membrane oxygenation; SOFA, Sequential Organ Failure Assessment.
Kruskal-Wallis's test, p = 0.002.
Kruskal-Wallis's test, p ≤ 0.0001.
Kruskal-Wallis's test, p ≤ 0.0001.
Log-rank test, p = 0.347.
Table 2.
Characteristics of the Patients With Severe COVID-19 Before ECMO Initiation
| Characteristics | Overall Series (N = 132) | 6-Month Survivors (N = 62) | 6-Month Deaths (N = 70) | p Value |
|---|---|---|---|---|
| Patients’ characteristics | ||||
| Age, y | 51.1 (9.7) | 49.5 (9.4) | 52.4 (9.9) | 0.131 |
| Age > 60 y | 22 (16.7) | 7 (11.3) | 15 (21.4) | 0.119 |
| Female | 23 (17.4) | 12 (19.4) | 11 (15.7) | 0.582 |
| Pregnancy or postpartum | 5 (21.7) | 3 (25.0) | 2 (18.2) | 1.000 |
| Body mass index, kg/m2 | 30.9 (6.6) | 31.4 (7.1) | 30.4 (6.0) | 0.428 |
| Body mass index >30 kg/m2 | 56 (43.8) | 30 (49.2) | (38.8) | 0.237 |
| Coexisting comorbidities | ||||
| Dialysis | 0.473 | |||
| Chronic dialysis | 1 (0.8) | 0 | 1 (1.4) | |
| New dialysis | 14 (10.6) | 8 (12.9) | 6 (8.6) | |
| Diabetes on drug treatment | 29 (22.0) | 13 (21.0) | 16 (22.9) | 0.794 |
| Cancer | 4 (3.0) | 1 (1.6) | 3 (4.3) | 0.622 |
| Hypertension | 38 (28.8) | 16 (25.8) | 22 (31.4) | 0.565 |
| Smoking habit | 23 (17.4) | 12 (19.4) | 11 (15.7) | 0.585 |
| Asthma/chronic obstructive pulmonary disease | 12 (9.2) | 6 (9.7) | 6 (8.7) | 1.000 |
| Congestive heart failure | 1 (0.8) | 0 | 1 (1.4) | 1.000 |
| Coronary artery disease | 4 (3.0) | 3 (4.8) | 1 (1.4) | 0.254 |
| Peripheral arterial disease | 0 | 0 | 0 | - |
| Stroke/transient ischemic attack | 2 (1.5) | 1 (1.6) | 1 (1.4) | 1.000 |
| No. of comorbidities* | 0.851 | |||
| None | 69 (52.3) | 34 (54.8) | 35 (50.0) | |
| 1 | 40 (30.3) | 18 (29.0) | 22 (31.4) | |
| ≥2 | 23 (17.4) | 10 (16.1) | 13 (18.6) | |
| Conditions at presentation | ||||
| Increased cardiac troponin level | 31 (23.8) | 16 (25.8) | 15 (22.1) | 0.616 |
| Pneumothorax | 3 (4.3) | 0 | 3 (4.3) | 0.247 |
| Pleural effusion | 17 (12.9) | 6 (9.7) | 11 (15.7) | 0.436 |
| 4C mortality score | 10.4 (2.4) | 10.2 (2.2) | 10.6 (2.6) | 0.593 |
| SIRS score | 2.5 (1.0) | 2.6 (1.0) | 2.5 (1.0) | 0.456 |
| SOFA score | 10.1 (4.4) | 9.7 (4.2) | 10.4 (4.6) | 0.550 |
| Ventilation parameters | ||||
| Duration of invasive mechanical ventilation, d | 5.5 (5.3) | 5.6 (5.6) | 5.3 (5.1) | 0.926 |
| PaO2/FIO2 ratio, mmHg | 71 (23) | 74 (27) | 68 (18) | 0.380 |
| PaCO2, mmHg | 61 (17) | 58.2 (15.8) | 62.6 (18.1) | 0.068 |
| Tidal volume, mL per kg | 6.1 (2.3) | 5.6 (2.4) | 6.5 (2.2) | 0.025 |
| PEEP, cmH2O | 12.4 (3.7) | 12.0 (3.6) | 12.7 (3.8) | 0.601 |
| Median laboratory values | ||||
| Hemoglobin, mg/L | 113 (18) | 113 (17) | 114 (19) | 0.814 |
| eGFR, mL/min/ 1.73 m2 | 93 (58) | 96 (61) | 89 (56) | 0.606 |
| Leukocytes, 109/L | 12.1 (9.0) | 13.0 (12.5) | 11.3 (4.1) | 0.720 |
| Platelets, 109/L | 271 (111) | 285 (119) | 259 (103) | 0.116 |
| Arterial pH | 7.23 (0.09) | 7.29 (0.09) | 7.23 (0.09) | <0.0001 |
| Arterial lactate, mmol/L | 2.5 (2.2) | 2.3 (1.7) | 2.6 (2.5) | 0.640 |
| Antiviral drugs prior or during ECMO | ||||
| Lopinavir/ritonavir | 27 (20.5) | 16 (25.8) | 11 (15.7) | 0.151 |
| Oseltamivir | 5 (3.8) | 1 (1.6) | 4 (5.7) | 0.370 |
| Ganciclovir | 5 (3.8) | 3 (4.8) | 2 (2.9) | 0.552 |
| Acyclovir | 5 (3.8) | 2 (3.2) | 3 (4.3) | 0.750 |
| Emtricitabile/tenofovir | 1 (0.8) | 1 (1.6) | 0 | 0.470 |
| Rescue therapies prior or during ECMO | ||||
| Prone positioning | 123 (93.2) | 58 (93.5) | 65 (92.9) | 1.000 |
| Convalescent plasma | 6 (4.5) | 5 (8.1) | 1 (1.4) | 0.099 |
| Hydroxychloroquine/choloroquine | 33 (25.0) | 14 (22.6) | 19 (27.1) | 0.546 |
| Extracorporeal cytokine absorber | 9 (6.8) | 4 (6.5) | 5 (7.1) | 1.000 |
| Tocilizumab | 9 (6.8) | 4 (6.5) | 5 (7.1) | 1.000 |
| Corticosteroids | 47 (35.6) | 23 (37.1) | 24 (34.3) | 0.736 |
NOTE. Continuous values are reported as mean and standard deviation (in parentheses) and categorical variables as counts and percentages (in parentheses).
Abbreviations: COVID-19, coronavirus disease 2019; ECMO, extracorporeal membrane oxygenation; eGFR, estimated glomerular filtration rate according to the Modification of Diet in Renal Disease equation; ICU, intensive care unit; PEEP, positive end-expiratory pressure; SIRS, Systemic inflammatory Response Syndrome; SOFA, Sequential Organ Failure Assessment.
Includes diabetes, cancer, hypertension, pulmonary disease, chronic heart failure, coronary artery disease, peripheral vascular disease, and stroke or transient ischemic attack.
Primary venovenous ECMO (VV ECMO) was used in 122 patients (92.4%), while venoarterial ECMO (VA ECMO) was used in ten patients (7.6%) because of coexistent hemodynamic instability (Table 3 ). Eight (6.6%) patients on primary VV ECMO had a second run of ECMO (five VV ECMO, two VA ECMO and one veno-venoarterial-ECMO) and a third run of ECMO (one VV ECMO and one venoarterial-venous ECMO). Six (60.0%) patients on primary VA ECMO had a second run of ECMO (four venoarterial-venous ECMO, one VV ECMO, and one VA ECMO). A double-lumen cannula was used for VV ECMO in 23 patients (17.4%). Other ECMO configurations are summarized in Table 3. Fourteen (10.6%) patients required conversion to another ECMO configuration, and two (1.5%) patients required a third different ECMO configuration. Heparin was used for anticoagulation during ECMO to achieve an activated clotting time varying from 160-to-300 seconds or an activated partial thromboplastin time of 40-to-60 seconds according to different institutional policies. The mean overall duration of ECMO therapy was 14.6 ± 11.0 days.
Table 3.
ECMO-Related Data in Patients With Severe COVID-19
| Variables | Overall Series (N = 132) | 6-Month Survivors (N = 62) | 6-Month Deaths (N = 70) | p Value |
|---|---|---|---|---|
| Time from ICU admission to ECMO, days | 5.8 (5.3) | 5.3 (5.5) | 6.2 (5.2) | 0.182 |
| Primary ECMO inflow vessel | 0.395 | |||
| Femoral vein | 90 (68.2) | 40 (64.5) | 50 (71.4) | |
| Jugular vein | 42 (31.8) | 22 (35.5) | 20 (28.6) | |
| Primary ECMO outflow vessel | 0.286 | |||
| Femoral vein | 21 (15.9) | 11 (17.7) | 10 (14.3) | |
| Jugular vein | 102 (77.3) | 49 (79.0) | 53 (75.7) | |
| Femoral artery | 9 (6.8) | 2 (3.2) | 7 (10.0) | |
| Primary ECMO double-lumen cannula | 23 (17.4) | 11 (17.7) | 12 (17.1) | 1.000 |
| Primary ECMO configuration | 0.102 | |||
| VV ECMO | 122 (92.4) | 60 (96.8) | 62 (88.6) | |
| VA ECMO | 10 (7.6) | 2 (3.2) | 8 (11.4) | |
| Second run ECMO configuration | 0.513 | |||
| VV ECMO | 6 (4.5) | 4 (6.5) | 2 (2.9) | |
| VA ECMO | 3 (2.3) | 1 (1.6) | 2 (2.9) | |
| VAV ECMO | 4 (3.0) | 1 (1.6) | 3 (4.3) | |
| VVA ECMO | 1 (0.8) | 0 | 1 (1.4) | |
| Third run ECMO configuration | 0.407 | |||
| VV ECMO | 1 (0.8) | 0 | 1 (1.4) | |
| VAV ECMO | 1 (0.8) | 0 | 1 (1.4) | |
| Mean duration of ECMO, days | 14.6 (11.0) | 15.1 (9.8) | 14.1 (12.0) | 0.295 |
NOTE. Continuous values are reported as mean and standard deviation (in parentheses) and categorical variables as counts and percentages (in parentheses).
Abbreviations: COVID-19, coronavirus disease 2019; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; VA, venoarterial; VAV, venoarterio-venous; VV, venovenous; VVA, veno-venoarterial.
Outcomes
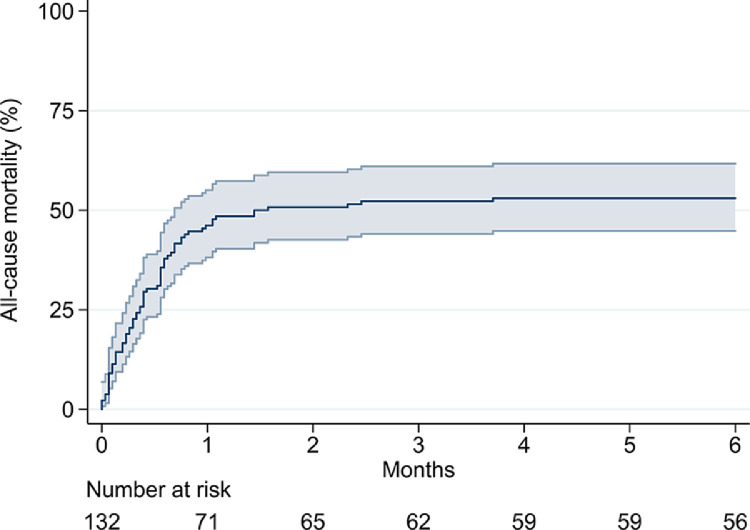
Sixty-three (47.7%) patients died on ECMO, and 70 (53.0%) patients died during the index hospitalization. Overall, six-month all-cause mortality was 53.0% (Fig 1 ) and did not differ among centers (log-rank, p = 0.347) (Table 1). Ten (7.6%) patients required VA ECMO as primary configuration and had higher risk of death on ECMO (80.0% v 45.1%, p = 0.034) as well as during index hospitalization (80.0% v 50.8%, p = 0.075) and at six months (80.0% v 50.8%%, log-rank test: p = 0.008). Fourteen patients had their ECMO configuration changed, but they did not have significantly increased risk of death on ECMO (50.0% v 47.5%, p = 0.857) as well as during index hospitalization (57.1% v 52.5%, p = 0.744) and at six months (57.1% v 52.6%, log-rank test: p = 0.679).
Fig 1.
Kaplan-Meier estimate of six-month all-cause mortality in patients treated with extracorporeal membrane oxygenation for severe COVID-19. COVID-19, coronavirus disease 2019.
The most frequent complications occurring during or immediately after ECMO therapy were acute kidney injury (64.1%), bloodstream infection (31.8%), stroke (14.4%), confirmed or suspected pulmonary embolism (13.6%), and deep vein thrombosis (11.4%) (Table 4 ). One hundred five (79.5%) patients required red blood cell transfusion (mean 7.8 ± 13.5 units) (Table 4).
Table 4.
Outcomes After ECMO in Patients With Severe COVID-19
| Outcomes | Overall Series (N = 132) | 6-Month Survivors (N = 62) | 6-Month Deaths (N = 70) | p Value |
|---|---|---|---|---|
| Hospital death | 70 (53.0) | - | 70 (100) | - |
| ICU death | 70 (53.0) | - | 70 (100) | - |
| Died on ECMO | 63 (47.7) | - | 63 (90.0) | - |
| Acute kidney injury | 84 (64.1) | 35 (57.4) | 49 (70.0) | 0.133 |
| New dialysis | 50 (37.9) | 21 (33.9) | 29 (41.4) | 0.372 |
| Bloodstream infection | 42 (31.8) | 23 (37.1) | 19 (27.1) | 0.220 |
| Thromboembolic complications | ||||
| Stroke | 19 (14.4) | 6 (9.7) | 13 (18.6) | 0.146 |
| Confirmed/suspected pulmonary embolism | 18 (13.6) | 5 (8.1) | 13 (18.6) | 0.079 |
| Deep vein thrombosis | 15 (11.4) | 8 (12.9) | 7 (10.0) | 0.600 |
| Lung/pleural complications requiring surgery | 5 (3.8) | 1 (1.6) | 4 (5.7) | 0.294 |
| Bleeding complications | ||||
| RBC transfusion | 105 (79.5) | 48 (77.4) | 57 (81.4) | 0.569 |
| RBC transfusion, units | 7.8 (13.5) | 7.5 (12.8) | 8.1 (14.3) | 0.349 |
| Platelets transfusion | 20 (15.2) | 5 (8.1) | 15 (21.4) | 0.033 |
| Fresh frozen plasma transfusion | 30 (22.7) | 11 (17.7) | 19 (27.1) | 0.198 |
| Lowest hemoglobin, mg/liter | 80 (13) | 84 (15) | 78 (11) | 0.045 |
| Duration of mechanical ventilation, d | 25.1 (18.6) | 30.1 (16.7) | 20.6 (19.1) | <0.0001 |
| Length of ICU stay, d | 30.5 (21.4) | 39.7 (19.8) | 22.4 (19.6) | <0.0001 |
| Length of hospital stay, d | 36.6 (28.1) | 49.4 (30.6) | 24.5 (19.1) | <0.0001 |
NOTE. Continuous values are reported as mean and standard deviation (in parentheses) and categorical variables as counts and percentages (in parentheses).
Abbreviations: COVID-19, coronavirus disease 2019; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; RBC, red blood cell.
Cox proportional hazard analysis, including covariates before ECMO, showed that age (per-year hazard ratio [HR] 1.026, 95% CI 1.000-1-052) and arterial pH before ECMO (per-unit HR 0.006, 95% CI 0.000-0.083) were independent predictors of six-month mortality. The VA ECMO configuration was not associated with increased six-month mortality at multivariate analysis (adjusted HR 1.844, 95% CI 0.824-4.128). Youden's test showed that the best cutoff values of arterial pH before ECMO to predict hospital mortality were 7.23 (arterial pH < 7.23 72.9% v arterial pH ≥ 7.23 41.7%, p = 0.001, sensitivity 50.0%, specificity 79.0%, and area under the receiver operating characteristic curve 0.304, 95% CI 0.214-0.394). The CART analysis showed that patients aged ≥60 years had a higher six-month mortality compared to younger patients (68.2% v 50.0%) (Fig 2 ). A pH < 7.23 before ECMO was associated with excessive six-month mortality either in patients ≥60 years old (90.9%) and in those <60 years old (68.4%) (Fig 2). Significantly lower six-month mortality was observed with pH ≥ 7.23 both in patients ≥60 years old (45.5%) and in younger patients (40.3%).
Fig 2.
Classification and regression tree analysis of risk factors predicting six-month all-cause mortality in patients treated with extracorporeal membrane oxygenation for severe COVID-19. COVID-19, coronavirus disease 2019; ECMO, extracorporeal membrane oxygenation.
Discussion
The findings of the present analysis can be summarized as follows: (1) half of relatively young adults with COVID-19–related ARDS can be salvaged using ECMO; (2) all these patients required prolonged ECMO therapy (mean 14.6 days) as well as long ICU (mean 30.5 days) and hospital stays (mean 36.6 days); (3) advanced age and decreased pH before initiation of ECMO were associated with prohibitive six-month mortality; and (4) mortality was <50% when ECMO was started before severe metabolic derangements subsided.
The outbreak of the COVID-19 pandemic resulted in high mortality among the vulnerable elderly population.12 , 13 However, mortality also is significant among younger patients with or without comorbidities. The prospective ISARIC WHO CCP-UK study showed that 26% of patients hospitalized with a diagnosis of COVID-19 died and 34% still were receiving care.13 Similar figures were reported in previous studies.1, 2, 3, 4 In the ISARIC WHO Clinical Characterisation Protocol-UK study,13 patients who were treated with mechanical ventilation had a mean age of 61 years, and 37% of them died; while 46% still were receiving care.
In this scenario with high mortality and prolonged hospital treatment for severe COVID-19, the present analysis, along with two previous studies,9 , 10 documented that ECMO may be beneficial in many patients with severe COVID-19–related ARDS and/or hemodynamic instability. This information is of crucial importance when planning treatment of patients who are unresponsive to conventional medical therapies. Based on the findings of these relatively large series, allocation of resources seems to be justified for ECMO treatment of young adults with severe COVID-19.
The early and small series of patients with ECMO for severe COVID-19 reported on excessive mortality rates with this salvage therapy.14, 15, 16 Furthermore, the interpretation of the results was difficult because of incomplete follow-up of these patients.17 Despite these discouraging initial results, experienced ECMO centers adopted an active approach toward the management of these critically ill patients and documented satisfactory early mortality rates. Schmidt et al.9 reported the results of ECMO therapy in patients with COVID-19 treated at the Paris-Sorbonne University Hospital Network, including five ICUs. Eighty-three patients had a complete follow-up at 60 days, and their mortality rate was 31%.
A large multicenter study by the ELSO investigators documented an in-hospital mortality at 90 days after ECMO of 37.4%.10 Multivariate analysis of the ELSO registry showed that advanced age, the use of VA ECMO, low PaO2/ fraction of inspired oxygen, acute kidney injury, pulmonary disease, immunocompromised state, and cardiac arrest before ECMO were independent predictors of in-hospital mortality. Importantly, higher adult 2019 hospital ECMO case volume was associated with improved outcome.10
The present series shared similar patients’ age and risk profile of those by Schmidt et al.9 and Barbaro et al.,10 but documented an in-hospital and six-month mortality rate of 53.0%, which was much higher than these previous multicenter studies. The current series included half of patients without any significant comorbidity, and only 17% of patients had two or more comorbidities. Importantly, these risk factors did not have any impact on the early mortality. However, the large ELSO10 report did not include data on arterial pH and arterial lactate before ECMO, which prevents any analysis of the clinical importance of metabolic derangements before ECMO. Schmidt et al.9 reported a median arterial pH of 7.32 and of arterial lactate of 1.6 mmol/L before initiation of ECMO, while in this current series the median arterial pH (7.26) and median arterial lactate (2.0 mmol/L) were poorer than those of the Schimdt's series. These findings suggest that, despite a similar age-based selection of patients, in the present study ECMO often was started when severe metabolic derangements had occurred. Indeed, CART analysis showed that six-month mortality rate was below 45% when, independently of patients’ age, the arterial pH was equal to or higher than 7.23. Therefore, the authors hypothesized that initiation of ECMO therapy before severe metabolic derangements subset might improve survival rates significantly in these patients as documented in prior studies.
This study showed that bleeding and thromboembolism are frequent complications among ECMO-treated patients with COVID-19 (Table 3). Prior studies demonstrated a hypercoagulability state related to COVID-19,18 , 19 which may indicate anticoagulation as a valid preventative therapy in these patients. However, there also is evidence of a tendency for intracranial bleeding in patients with COVID-19, which is associated with poor prognosis.20 , 21 Bleeding complications are frequent during ECMO treatment,4 , 22 and point-of-care management should be considered when anticoagulation is started to reduce the risk of bleeding and to avoid ECMO circuit thrombosis.
Patients included in the present analysis were among the first ones who have been treated with ECMO therapy for COVID-19–related ARDS. At that time, the ELSO did not support the use of this therapy in patients with COVID-19. Therefore, each institution decided on their treatment policy based on a strict patient selection and the availability of resources for this costly therapy. Analysis of the present data showed that ECMO was used in rather young patients (only 5.3% of patients were ≥65 years old) and without severe comorbidities. Some centers treated mostly patients aged younger than 50 years, and there was a significant interinstitutional differences also in arterial pH levels before ECMO (Table 1). Still, ECMO was used in a large number of patients with diabetes and obesity, but only 17% of patients had two or more comorbidities. Therefore, these results should be viewed in light of a strict patient selection policy and may not be replicated in patients with advanced age or multiple comorbidities.
The retrospective nature was one of the main limitations of this study. Second, the lack of standardized selection criteria for starting and managing ECMO treatment was a major limitation of this study. However, at the outbreak of COVID-19 pandemic, there were not internationally recognized and specific criteria for starting ECMO treatment in these patients. Indeed, ECMO therapy was employed in these rather young patients mostly on a compassionate basis. Third, conclusive results on the benefits of ECMO in these critically ill patients cannot be reached without a comparative analysis with conventionally-treated patients. However, a randomized study is hardly feasible in this setting. In fact, the ECMO to Rescue Lung Injury in Severe ARDS study showed that the use of this salvage therapy in critically ill patients may lead to crossover to ECMO in a significant proportion of control patients.23 A previous study8 on patients with influenza A(H1N1)–related ARDS demonstrated that matching ECMO-treated patients with conventionally treated patients is problematic and may not provide conclusive results. Despite this limitation, severe respiratory failure and sepsis conditions, as observed in this series, are expected to be fatal in nearly all patients. Fourth, the limited size of this series prevented conclusive results on the risk factors that may contraindicate the use of ECMO in these critically ill patients. Finally, the multicenter nature of this study may have introduced bias related to the volume and experience of each center, although this was ruled out by the recent ELSO study.10 On the contrary, the multicenter approach may even-out single-center specifics and, thus, increase the generalizability of the results.
Conclusions
In conclusion, the present study showed, in a cohort of relatively young adult patients with severe COVID-19–related ARDS, that almost half of them may be salvaged with ECMO support, with sustained results at six months. Decreased arterial pH before ECMO was associated significantly with early mortality. Therefore, the authors hypothesized that initiation of ECMO therapy before severe metabolic derangements subset may improve survival rates significantly in these patients. These results should be viewed in light of a strict patient selection policy and may not be replicated in patients with advanced age or multiple comorbidities.
Footnotes
Conflict of interests: L.M.B. is a member of the EuroELSO (Newcastle upon Tyne, UK) Scientific Committee and Workgroup on Innovation and Technology in ECLS, the Medical Advisory Board of Eurosets Srl., Medolla, Italy, and the Medical Advisory Board of Xenios AG, Heilbronn, Germany. The other coauthors do not have any conflict of interest related to this study.
Funding: M.D. was financially supported by a research grant from The Swedish Heart-Lung Foundation (grant number 20200412).
Clinical trial registration: Identifier, NCT04383678.
References
- 1.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zangrillo A, Beretta L, Scandroglio AM, et al. Characteristics, treatment, outcomes and cause of death of invasively ventilated patients with COVID-19 ARDS in Milan, Italy. Crit Care Resusc. 2020;22:200–211. doi: 10.1016/S1441-2772(23)00387-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grasselli G, Greco M, Zanella A, et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med. 2020;180:1345–1355. doi: 10.1001/jamainternmed.2020.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartlett RH, Ogino MT, Brodie D, et al. Initial ELSO Guidance Document: ECMO for COVID-19 patients with severe cardiopulmonary failure. Asaio J. 2020;66:472–474. doi: 10.1097/MAT.0000000000001173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noah MA, Peek GJ, Finney SJ, et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1) JAMA. 2011;306:1659–1668. doi: 10.1001/jama.2011.1471. [DOI] [PubMed] [Google Scholar]
- 7.Pham T, Combes A, Rozé H, et al. Extracorporeal membrane oxygenation for pandemic influenza A(H1N1)-induced acute respiratory distress syndrome: A cohort study and propensity-matched analysis. Am J Respir Crit Care Med. 2013;187:276–285. doi: 10.1164/rccm.201205-0815OC. [DOI] [PubMed] [Google Scholar]
- 8.Munshi L, Walkey A, Goligher E, et al. Venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: A systematic review and meta-analysis. Lancet Respir Med. 2019;7:163–172. doi: 10.1016/S2213-2600(18)30452-1. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt M, Hajage D, Lebreton G, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID-19: A retrospective cohort study. Lancet Respir Med. 2020;8:1121–1131. doi: 10.1016/S2213-2600(20)30328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barbaro RP, MacLaren G, Boonstra PS, et al. Extracorporeal membrane oxygenation support in COVID-19: An international cohort study of the Extracorporeal Life Support Organization registry. Lancet. 2020;396:1071–1078. doi: 10.1016/S0140-6736(20)32008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The ARDS Definition Task Force Acute respiratory distress syndrome: The Berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 12.McMichael TM, Currie DW, Clark S, et al. Epidemiology of COVID-19 in a long-term care facility in King County, Washington. N Engl J Med. 2020;382:2005–2011. doi: 10.1056/NEJMoa2005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO clinical characterisation protocol: Prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henry BM, Lippi G. Poor survival with extracorporeal membrane oxygenation in acute respiratory distress syndrome (ARDS) due to coronavirus disease 2019 (COVID-19): Pooled analysis of early reports. J Crit Care. 2020;58:27–28. doi: 10.1016/j.jcrc.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alom S, Haiduc AA, Melamed N, et al. Use of ECMO in patients with coronavirus disease 2019: does the evidence suffice? J Cardiothorac Vasc Anesth. 2020 Jul 30 doi: 10.1053/j.jvca.2020.07.070. [e-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kon ZN, Smith DE, Chang SH, et al. Extracorporeal membrane oxygenation support in severe COVID-19. Ann Thorac Surg. 2021;111:537–543. doi: 10.1016/j.athoracsur.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobs JP, Stammers AH, St. Louis J, et al. Extracorporeal membrane oxygenation in the treatment of severe pulmonary and cardiac compromise in coronavirus disease 2019: Experience with 32 patients. ASAIO J. 2020;66:722–730. doi: 10.1097/MAT.0000000000001185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ranucci M, Ballotta A, Di Dedda U, et al. The procoagulant pattern of patients with COVID-19 Acute respiratory distress syndrome. J Thromb Haemost. 2020;18:1747–1751. doi: 10.1111/jth.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Panigada M, Bottino N, Tagliabue P, et al. Hypercoagulability of COVID-19 patients in intensive care unit. A report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18:1738–1742. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radmanesh A, Derman A, Lui YW, et al. COVID-19 -associated diffuse leukoencephalopathy and microhemorrhages. Radiology. 2020;297:E223–E227. doi: 10.1148/radiol.2020202040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morassi M, Bagatto D, Cobelli M, et al. Stroke in patients with SARS-CoV-2 infection: Case series. J Neurol. 2020;267:2185–2192. doi: 10.1007/s00415-020-09885-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kreyer S, Muders T, Theuerkauf N, et al. Hemorrhage under veno-venous extracorporeal membrane oxygenation in acute respiratory distress syndrome patients: A retrospective data analysis. J Thorac Dis. 2017;9:5017–5029. doi: 10.21037/jtd.2017.11.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Combes A, Hajage D, Capellier G, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378:1965–1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]