Abstract
Immune-mediated diseases and immunotherapeutics can negatively affect normal immune functioning and, consequently, vaccine safety and response. The COVID-19 pandemic has incited research aimed at developing a novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine. As SARS-CoV-2 vaccines are developed and made available, the assessment of anticipated safety and efficacy in patients with immune-mediated dermatologic diseases and requiring immunosuppressive and/or immunomodulatory therapy is particularly important. A review of the literature was conducted by a multidisciplinary committee to provide guidance on the safety and efficacy of SARS-CoV-2 vaccination for dermatologists and other clinicians when prescribing immunotherapeutics. The vaccine platforms being used to develop SARS-CoV-2 vaccines are expected to be safe and potentially effective for dermatology patients on immunotherapeutics. Current guidelines for the vaccination of an immunocompromised host remain appropriate when considering future administration of SARS-CoV-2 vaccines.
Key words: COVID-19, immunomodulatory therapy, immunosuppressive therapy, SARS-CoV-2, vaccine
Capsule Summary.
-
•
The safety and efficacy of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines in patients with immune-mediated dermatologic diseases requiring immunotherapeutics is unknown.
-
•
The SARS-CoV-2 vaccines approved and distributed are expected to be safe for patients on immunotherapeutics with some variability in efficacy, depending on the degree of immunosuppression and type of vaccine given.
Patients with immune-mediated dermatologic diseases can require treatment with short-term and long-term immunosuppressive and/or immunomodulatory therapy. Immune-mediated diseases and immunotherapeutics can negatively affect normal immune functioning, placing these patients at increased risk of infection.1, 2, 3 However, patients on immunotherapies for dermatologic and rheumatologic disease do not appear to be more susceptible to COVID-19.4
Vaccines protect against infection by provoking a protective humoral and cellular immune response.5 , 6 Assessment of vaccine safety is largely derived from observational studies,7 whereas the efficacy of vaccination is commonly investigated by using postimmunization antibody titers as correlates of protection.6 , 8, 9, 10 For patients on immunotherapeutics, clinical decision making regarding vaccination must weigh the anticipated disease protection achieved by immunization against the risk of vaccine-induced adverse events. Meanwhile, the risk of discontinuation or temporary withdrawal of therapy must also be considered because some immunotherapies can carry the risk of increased disease activity, relapse, or loss of response.3 , 11
The COVID-19 pandemic has included a rapid increase in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) research around the globe, particularly research aimed at developing a SARS-CoV-2 vaccine. SARS-CoV-2 vaccination research has resulted in the development of novel vaccine platforms (ie, RNA, DNA, nonreplicating viral vectors, etc).12 , 13 Furthermore, SARS-CoV-2 is a novel vaccine target. As SARS-CoV-2 vaccines are developed and made available, the assessment of potential safety and efficacy in this population is particularly important. The launch of SARS-CoV-2 vaccines creates a unique clinical challenge for dermatologists and other clinicians when prescribing immunotherapeutics. We aim to provide guidance on the safety and efficacy of SARS-CoV-2 vaccination for dermatology patients on immunotherapeutics as an adjunct to existing guidelines, including the Infectious Diseases Society of America “Clinical Practice Guideline for Vaccination of the Immunocompromised Host.”14 Specifically, this review is intended to serve as a point of reference to assist dermatologists and clinicians when approaching SARS-CoV-2 vaccination and their patients receiving immunotherapeutics through (1) a review of the SARS-CoV-2 vaccines now authorized for distribution (Moderna messenger RNA [mRNA] and Pfizer-BioNTech mRNA) as well as those under development and an outline of the potential risks to patients receiving immunotherapeutics, (2) a summary of current evidence pertaining to the safety and efficacy of nonviral vaccines in patients receiving immunotherapeutics, and (3) an extrapolation of these data to comment on the anticipated safety and efficacy outcomes with the novel SARS-CoV-2 vaccines.
Methods
A review of the literature was conducted by a multidisciplinary committee comprising dermatologists (MGK, JD), immunologists (MGK, JD), a rheumatologist (JD), dermatology residents (LMG, BM) and a specialist in virology and vaccination (MS). Studies were identified by performing a search across electronic databases (MEDLINE, Embase, PubMed) and divided into 3 areas of focus based on major search terms in addition to advanced searching within these databases using the following Medical Subject Headings terms: (1) “SARS-CoV-2” or “COVID-19” and “vaccine” or “vaccination”; (2) “vaccine” or “vaccination” and “glucocorticoid” or “prednisone” or “corticosteroid,” as well as “vaccine” or “vaccination” and specific systemic immunotherapy (“apremilast,” “azathioprine,” “cyclosporine,” “methotrexate,” “mycophenolate mofetil,” and “JAK inhibitors”); (3) “vaccine” or “vaccination” and specific biologic agent (“adalimumab,” “certolizumab,” “etanercept,” “infliximab,” “ustekinumab,” “brodalumab,” “ixekizumab,” “secukinumab,” “guselkumab,” “risankizumab,” “tildrakizumab,” “rituximab,” “anakinra,” “dupilumab,” “omalizumab,” and “IVIG”). Additional relevant studies were identified from the reference lists of primary studies and reviews and included based on relevance to these major search terms. Published studies including clinical trials, meta-analyses, systematic reviews, case series, and case reports were reviewed and assessed for content and grading of quality of evidence adapted from Robinson et al15 to support recommendations. Data were extracted from individual studies and synthesized into tables.
Results and discussion
Review of SARS-CoV-2 vaccines under development
To properly assess risks of vaccines against SARS-CoV-2 to patients on immunotherapeutics, it is important to understand the basic mechanisms of the vaccines' platforms. There are more than 90 vaccines against SARS-CoV-2 in development; the wide range of strategies used to stimulate the immune system to develop protective antibodies is summarized in Table I .5 Live attenuated vaccines are weakened wild-type viruses that have accumulated mutations to diminish their ability to cause disease and therefore pose the highest risk to dermatology patients on immunotherapeutics because of the rare risk of reversion to the original pathogenic infectious agent.3 , 16 However, currently, there are no live attenuated SARS-CoV-2 vaccines in phase 2 or phase 3 trials.
Table I.
Review of COVID-19 vaccines in development
| Type of vaccine (approved examples) | Description | Example companies and phase of development | Anticipated risk to patients on immunotherapeutics |
|---|---|---|---|
| Inactivated virus | SARS-CoV-2 is allowed to replicate in cells and then killed by using chemicals, heat, or radiation |
|
None |
| Live, attenuated virus | SARS-CoV-2 is genetically engineered to limit infection and reproduction |
|
Low |
| Protein subunit | SARS-CoV-2 protein is engineered and produced to stimulate antiviral antibodies |
|
None |
| Virus-like particles | Virus-like structures enter cells like virus to deliver SARS-CoV-2 protein subunit to stimulate immune response |
|
None |
| Nonreplicating viral vectors | Nonreplicating engineered viruses, such as adenovirus or vaccinia, that carry genetic code for proteins of the SARS-CoV-2 virus to stimulate an immune response |
|
None to minimal |
| Replicating viral vectors | Weakened versions of carrier viruses, like influenza or measles, that can replicate in the body and carry genetic code for a protein of SARS-CoV-2. Do not usually cause symptoms. |
|
Minimal |
| RNA | RNA is injected into the body that codes for a SARS-CoV-2 protein that is then produced and leads to antibody development. |
|
None |
| DNA | DNA is injected into the body, often in the form of a plasmid, that codes for a SARS-CoV-2 protein that is then produced and leads to antibody development. |
|
None |
SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2.
Otherwise, there are 3 principal vaccine platforms that have been used to develop already approved vaccines and are considered safe for patients on immunotherapeutics: inactivated vaccines, protein subunit vaccines, and virus-like particle vaccines. These platforms have been used to develop pertussis vaccines, hepatitis B vaccines, and human papilloma virus vaccines. With regard to developmental SARS-CoV-2 vaccines, there is currently 1 protein subunit vaccine in phase 3 trials (NVX-CoV2373, Novavax), which, based on phase 1 and 2 data, appears to be safe, and elicits a strong antibody response.17 Nonreplicating viral vectors and RNA/DNA vaccines are in phase 3 trials or have completed phase 3 trials and represent novel methods of vaccination.18, 19, 20 Results suggest that these vaccines are safe and have the ability to produce protective antibody responses.18, 19, 20, 21, 22, 23, 24 The data from phase 2 and 3 trials of ChAdOx1/AZD1222 (Oxford-AstraZeneca) (nonreplicating viral vector) and phase 3 trials of mRNA-1273 (Moderna) (mRNA vaccine) and BNT162 (Pfizer-BioNTech) (mRNA vaccine) indicate that these vaccines are safe, with mild to moderate adverse events and the development of antibody responses that are above convalescent serum controls.18, 19, 20 The US Government has prepurchased mRNA-1273 (mRNA vaccine), BNT162 (mRNA vaccine), JNJ-78436735 (Johnson & Johnson) (nonreplicating viral vector), ChAdOx1/AZD1222 (nonreplicating viral vector), NVX-CoV2373 (Novavax) (protein subunit vaccine), and a protein subunit vaccine from Sanofi/GlaxoSmithKline.
Systemic immunotherapies and vaccines
The following dermatology-relevant immune-targeting therapies were reviewed in the setting of studies evaluating the safety and efficacy of nonviral and live vaccines: apremilast, azathioprine, cyclosporine, methotrexate, mycophenolate mofetil, systemic corticosteroids, and JAK inhibitors. No studies evaluated vaccination in patients receiving thalidomide or apremilast; safety has been addressed in the literature on the basis of expert opinion only.25
Safety of vaccines in patients receiving nonbiologic systemic immunotherapy
Based on available studies, detailed in Table II ,26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69 the majority of vaccines are safe in patients receiving nonbiologic immunotherapy. There is ultimately good evidence for the safety of nonviral vaccines in patients with dermatologic, autoimmune, or inflammatory disease treated with standard dermatologic doses of immunosuppressive agents, and these are generally well tolerated (Table II). These findings are aligned with current guideline recommendations.3 , 70 , 71
Table II.
Review of data on systemic immune targeting therapies and vaccines (see Table 2 in van Riel and de Wit12)
| Drug | Type of vaccination | Adverse events | Effects on immunity | Level of evidence |
|---|---|---|---|---|
| Systemic corticosteroids (prednisone) | Influenza26, 27, 28, 29, 30, 31, 32, 33, 34,73,75,82,113,116,121 PPSV2335,36,101,117,119,120 Hepatitis B37 HPV38 Herpes/varicella zoster (LZV)39,40,145 Yellow fever41 |
Safe, generally well tolerated. Increased frequency of moderate/severe local reactions compared to healthy control individuals have been observed; as well as a few reports of increased incidence of clinical and/or biochemical parameters of disease flare30 or increased herpes zoster risk observed in patients on immune-suppressive therapy39 | Variable effect on immunity: adequate seroprotection and/or no significant suppression of response in several studies and associated with doses up to <10-20 mg/day.37,38 Reduced seroconversion rates and/or impaired immune response/humoral response noted in a number of studies and, in particular, associated with a high-dose regimen of >20 mg/day.27,29,35,116 In VZV, long-term seroprotection for VZV at the 2-year follow-up was also observed.40,145 |
A-B |
| Methotrexate | Influenza: trivalent,42,43,79,80,146 pandemic (A/H1N1)44,45,73,76,78,82, 83, 84 PPSV2343,111 PCV7/1346,120,143 HAV86,99 HBV100 Tetanus/diphtheria102 MMR1,47, 48, 49,74 Herpes/varicella zoster (LZV,39,50, 51, 52,85,93,145 RZV92) Yellow fever53, 54, 55, 56,129 |
Safe, generally well tolerated with both nonviral and live-attenuated/live vaccines7,56,57,∗,† Rare risk of systemic rash and fever with live-attenuated/live vaccine (ie, MMR48,49 and HZV39,145) |
Variable effect on immunity: Most studies involving live-virus vaccines showed no significant effect on children and adult populations and satisfactory vaccine response/adequate seroprotection with a methotrexate dose of 10-25 mg/week. There is some support for improved response with temporary discontinuation and/or second dose. Nonviral vaccine is overall associated with a negative effect on immunogenicity, including reduced humoral response and insufficient protection with a single dose, with the exception of HBV (no significant effect).86,99,100 |
A-B |
| Azathioprine | Influenza: trivalent,32,58, 59, 60 pandemic (A/H1N1)61,62,82,84 PPSV23110,118 PCV1363,118 HAV131 HBV97,100 Tetanus, pertussis107 Herpes/varicella zoster (LZV)39,64, 65, 66,92 RZV92 Yellow fever61 |
Safe, consistently well tolerated with nonviral vaccines and live-attenuated/live vaccines | Variable effects on immune response for nonviral and live-attenuated/live vaccines described. Most studies report blunted to impaired immunogenicity for nonviral and live vaccines (eg, reduced humoral response). Comparable response to healthy control individuals also has been observed in pandemic influenza strains61,82 and HAV131 | B |
| Cyclosporine | Influenza: trivalent62 Pandemic (A/H1N1)44,61,84 Herpes/varicella zoster (LZV)39,145 Yellow fever61 PPSV2372 HAV67 Tetanus toxoid72 |
Safe, consistently well tolerated with nonviral vaccines and live-attenuated/live vaccines. | Consistent findings describing overall negative effect on immune response with nonviral and live-attenuated/live vaccines (ie, reduced recall humoral response, reduced rates of seroconversion, in vitro cellular immune response). | A-B |
| Mycophenolate mofetil | Influenza: trivalent,59,87 pandemic (A/H1N1)68,84,88 PPSV2372 Tetanus toxoid72 Yellow fever61 |
Safe, generally well tolerated (few reports of mild adverse effects) | Variable effects on immune response described in the literature. Most studies describe reduced immunogenicity/reduced humoral response with nonviral vaccines and worse with doses >2 g/day. Some support for antibody response comparable to healthy control individuals or nonsignificantly reduced/improved response with second dose. No studies evaluating immunogenicity in live-attenuated or live vaccines. | A-B |
| JAK inhibitors | Influenza (trivalent)95 PPSV2389,94,95 Tetanus toxoid89,95 Herpes/varicella zoster69,91,92 |
No reports of clinically significant adverse effects | Evidence is limited. Overall consistently preserved immunogenicity with nonviral and live-attenuated/live vaccine (ie, LZV†); sustained/long-term seroprotection may be inadequate.‡ | B |
HAV, Hepatitis A vaccine; LZV, live zoster vaccine; MMR, measles, mumps, rubella; PPSV, pneumococcal polysaccharide vaccine; RZV, recombinant zoster vaccine; VZV, Varicella zoster virus.
No significant adverse effects and no reports of increased clinical or laboratory index of disease activity. No exacerbation of disease activity in a number for autoimmune/inflammatory diseases. No adverse effects in function or graft failure in solid organ transplant recipients. One case report of fatal vaccine-associated viscerotropic disease.74,83
In a cohort of patients vaccinated 2 to 3 weeks before starting tofacitinib treatment.
Diminished humoral response to tetanus toxoid vaccine at week 12 and only 60% mounting 4-fold response to tetanus toxoid vaccine in patients with psoriasis on JAK inhibitors.
Efficacy of vaccines in patients receiving systemic immunotherapies
There is a trend toward a decreased immune response and vaccine immunogenicity in patients on systemic immunotherapies, particularly patients receiving azathioprine, cyclosporine, methotrexate, mycophenolate mofetil, or JAK inhibitors (Table II). Studies evaluating vaccine efficacy in patients receiving mycophenolate mofetil and cyclosporine have been primarily conducted in kidney transplant recipients and/or solid organ transplant recipients, in whom the immunosuppressive regimens result in severely disturbed primary and secondary humoral responses and, therefore, an impaired ability to mount a protective immune response.72 This may not be generalized to patients with dermatology immune disease on dermatologic doses of immunotherapies. The efficacy of inactivated, attenuated, and recombinant vaccines (ie, trivalent [A/H1N1, H3N2, B strain] and pandemic [A/H1N1] influenza vaccine) has been evaluated in patients receiving methotrexate.73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83 A significant reduction of inactivated and subunit vaccine antibody titers84 and inadequate sustained response or nonprotective titers on follow-up (at 4 to 12 weeks) has been reported in patients treated with methotrexate.85 On the other hand, the response appears to improve with second vaccination in studies evaluating influenza85 and hepatitis A86 vaccines in patients receiving methotrexate (15-20 mg per week),85 , 86 azathioprine, or cyclosporine.85 , 87 , 88 Satisfactory immune responses to influenza vaccine and nonviral vaccine (PPSV23, tetanus toxoid) in JAK inhibitor–treated patients with rheumatoid arthritis89 and inflammatory bowel disease have been observed when vaccines were administered either before the initiation of therapy90, 91, 92, 93 or after temporary withdrawal of JAK inhibitors 2 to 3 weeks before vaccination,89 , 94 , 95 which is consistent with most consensus guideline recommendations.3 , 70 , 71 Overall, vaccine efficacy may be reduced in patients receiving systemic immune-targeting therapies because of the impaired immune response in these patients; however, temporary withdrawal and/or additional vaccinations may be considered to achieve adequate protection.
Vaccines and biologics
The majority of primary data on the safety and efficacy of vaccines in patients exposed to biologics focuses on tumor necrosis factor (TNF) alpha inhibitors (primarily infliximab and etanercept) and the anti-CD20 monoclonal antibody rituximab.74 , 76 , 77 , 79 , 80 , 83 , 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 137, 138, 139, 140, 141, 142, 143, 144, 145 Patients with rheumatoid arthritis and inflammatory bowel disease were the most frequently studied populations.76 , 77 , 83 , 97, 98, 99, 100, 101 , 103 , 105 , 108, 109, 110 , 113 , 114 , 116 , 117 , 120, 121, 122 , 124 , 126 , 127 , 129, 130, 131 , 133, 134, 135, 136, 137 , 139 , 140 , 142, 143, 144, 145, 146 No studies on the safety or efficacy of vaccination in patients exposed to the following biologics were identified: brodalumab, anakinra, omalizumab, guselkumab, risankizumab, or tildrakizumab (Table III ).147, 148, 149, 150, 151, 152
Table III.
| Drug | Type of vaccination | Adverse events | Effects on immunity | Level of evidence |
|---|---|---|---|---|
| Adalimumab (TNF inhibitor) | PPSV23117 Influenza117,156 HBV114,133 |
Safe, generally well tolerated | Variable; some studies show no significant effect on humoral response,117,133 while others show reduced humoral response.114,117,156 | A-B |
| Certolizumab (TNF inhibitor)121 | Influenza PPSV23 |
Safe, generally well tolerated | No significant effect on humoral response | A |
| Etanercept (TNF inhibitor) | MMR74 PPSV2396,125 PCV13134 Influenza106 HBV114,136 |
Safe, generally well tolerated No increase in disease activity |
Variable; most studies showed no significant effect on humoral response,96,125,134 while some showed reduced humoral response.73,106,114 | A-B |
| Infliximab (TNF inhibitor) | Influenza76,105,109,113,124 HBV97,114,133,136 Yellow fever129,139 PPSV23110,143 |
Safe, generally well tolerated No increase in disease activity |
Variable efficacy for trivalent influenza and PPSV23 vaccination. Some studies show no significant effect on humoral response,76,109,124 while others show reduced humoral response.105,110,113,124 Most studies showed reduced humoral response to HBV vaccination.97,114,133 Adequate humoral response to yellow fever vaccination. | A-B C∗ |
| TNF inhibitors grouped | HBV100,133,138 HAV99,103,131 HZ145 PPSV23103,120,123,138 PCV13115,118,126,128,147 Tdap102,107 Influenza79,80,115,116,122,137,140,154 Pandemic (A/H1N1)77,83,103 |
Safe, well tolerated No increase in disease activity | Variable; some studies show no significant effect on humoral response, while others show reduced humoral response. Vaccine possibly associated with lower HZ incidence 2 years after vaccination.145 No significant difference in humoral response to PPSV23 vaccine between infliximab or etanercept treated patients.138 |
A-B |
| Ustekinumab (IL-12/23 inhibitor) | Influenza156 PPSV23155 Tetanus toxoid155 HBV114 |
N/A | Nonimpaired immune response and efficacy of inactivated influenza vaccine. No significant effect on humoral response to PPSV23 and tetanus vaccination. Possible reduced humoral response to HBV vaccination | A-B |
| Ixekizumab (IL-17 inhibitor) | PPSV23148 Tetanus toxoid148 |
Well tolerated | No significant effect to humoral response | A |
| Secukinumab (IL-17 inhibitor) | Meningococcal C Conjugate149 Trivalent influenza136,149,150 |
Well tolerated No increase in disease activity |
No significant effect to humoral response | A-B |
| Rituximab (anti–CD-20) | Influenza98,104,108,111,115,116,122,130,135,142,144 PPSV23101,115,135 PCV13112,126, 127, 128 PCV7157 TdaP102 Yellow fever129 HBV136 HZ145 |
Well tolerated No increase in disease activity |
The majority of studies found a reduced humoral response to influenza, pneumococcal, HBV, and TdaP vaccine. Vaccination possibly associated with significantly lower HZ incidence 2 years after vaccination.145 No significant effect on humoral response to yellow fever vaccination. |
A-B C∗ |
| Dupilumab (IL-4/13 inhibitor) | TdaP MPSV4153 |
Safe, well tolerated | No significant effect on humoral response | A |
| IVIG | MMR151 Influenza152 |
N/A | No significant effect on humoral response when vaccination occurs before IVIG administration. Decreased humoral response when vaccination occurs after IVIG administration. | B |
HAV, Hepatitis A vaccine; HBV, hepatitis B virus; HZ, herpes zoster; IL, interleukin; IVIG, intravenous immunoglobulin; MMR, measles, mumps, rubella; MPSV, meningococcal polysaccharide vaccine; N/A, not applicable; PCV, pneumococcal conjugate vaccine; PPSV, pneumococcal polysaccharide vaccine; TdaP, tetanus/diphtheria/pertussis; TNF, tumor necrosis factor.
The only study with level of evidence C is Oliveira et al.129
There were no studies identified evaluating vaccine safety and/or efficacy with following biologics: brodalumab (IL-17 inhibitor), guselkumab (IL-23 inhibitor), risankizumab (IL-23 inhibitor), tildrakizumab (IL-23 inhibitor), anakinra (IL-1 inhibitor), omalizumab.
Vaccination safety in patients on biologics
There have been few serious adverse events reported with vaccination and patients on biologic therapies, and the majority of reported adverse events were unrelated to vaccination.96 , 117 , 121 , 153 Aikawa et al96 reported 1 serious adverse event in a patient on TNF inhibitor therapy who developed invasive pneumococcal disease with bacterial pneumonia 5 months after vaccination, despite seroconverting 6 out of 7 polysaccharide serotypes analyzed. Blauvelt et al153 reported 1 treatment-related serious event in their dupilumab treatment group: a serum sickness-like reaction that resolved without sequelae.
Vaccine efficacy in patients on biologics
Data pertaining to vaccine efficacy are heterogeneous. Good antibody levels are observed after vaccination for patients on interleukin (IL) 17 (brodalumab, ixekizumab, secukinumab) and IL-4/13 inhibitors (dupilumab). Anti-TNF (adalimumab, certolizumab, etanercept) and anti–IL-12/23 (ustekinumab) biologics have been associated with a significant decrease in antibody levels. Variable data are observed for rituximab. Exposure to TNF inhibitors did not have a significant effect on humoral responses to pneumococcal (PPS23 and PCV13) or influenza vaccination in patients with rheumatoid arthritis.76 , 79 , 83 , 96 , 109 , 115 , 117 , 120 , 121 , 125 , 134 , 137 , 143 , 154 Curiously, TNF inhibitor exposure was associated with a reduced humoral response to pneumococcal and influenza vaccination in patients with inflammatory bowel disease.103 , 105 , 110 , 113 , 123 , 124 Belle et al100 found that treatment with immunomodulators and TNF inhibitors in patients with inflammatory bowel disease did not influence humoral response to hepatitis B vaccination compared to healthy control individuals.100 Patients with moderate to severe psoriasis treated with ustekinumab did not experience a change in humoral response to PPSV23 and tetanus toxoid vaccination.155 This is further supported by a recent study showing decreased efficacy of influenza vaccination in patients treated with adalimumab but not ustekinumab.156 In patients exposed to rituximab, most studies found a reduced humoral response to pneumococcal,101 , 112 , 115 , 126, 127, 128 , 135 , 157 hepatitis B,136 and influenza vaccination.73 , 108 , 111 , 116 , 122 , 130 , 142 , 144 Rituximab exposure did not significantly affect humoral response to seasonal influenza vaccination in patients with autoimmune blistering disease.104 Blauvelt et al153 found that patients with moderate to severe atopic dermatitis treated with dupilumab did not have a decreased humoral response to meningococcal and tetanus/diphtheria/pertussis vaccination.
SARS-CoV-2 candidate vaccines and immunotherapeutics: estimating risk and response
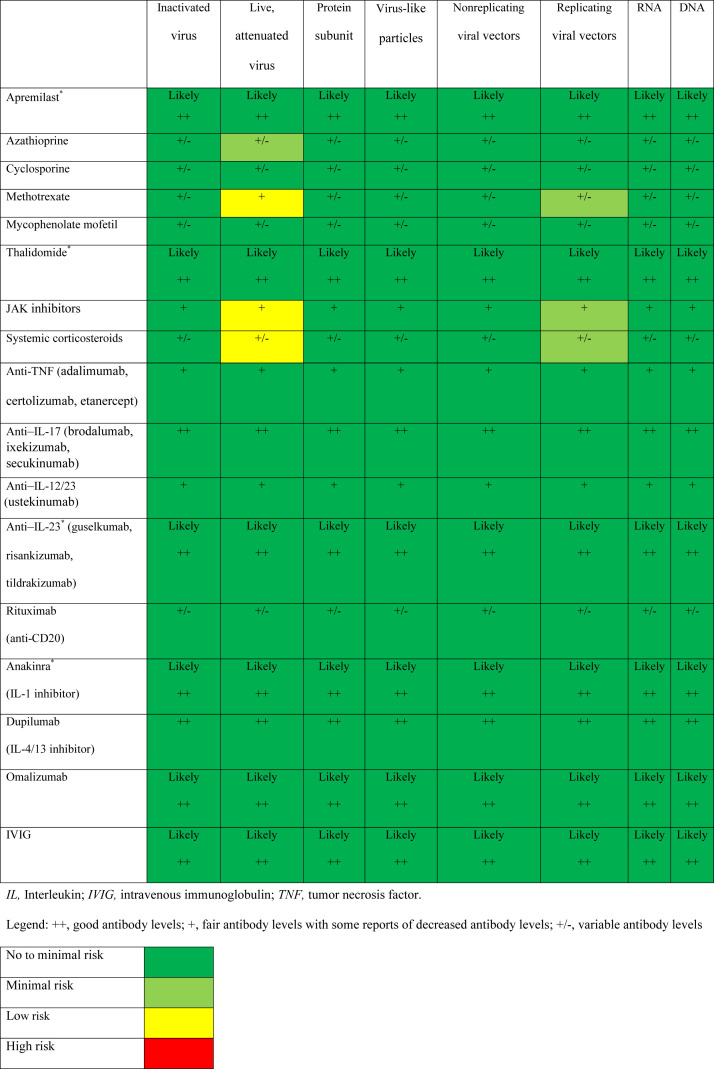
It is not possible to determine the true risk associated with any potential SARS-CoV-2 vaccine until it has gone through all phases of clinical trials and real-world evidence has been gathered from a widely distributed and adopted vaccination program. Nonetheless, we are able to estimate risk from the limited trial data for the SARS-CoV-2 vaccines and from a review of the literature for patients on immunotherapeutics and established vaccines (Fig 1 ). Considering the immunologic basis of the SARS-CoV-2 vaccine platforms in late-stage development, the estimated risk to patients on immunotherapies is low. From the review of the literature, patients on biologics have no abnormal immune responses leading to detrimental outcomes (Table III). The safety of a potential SARS-CoV-2 vaccine can be estimated based on the mechanism of action of the biologic or on inferences from the limited data on other biologics. For instance, there are no safety or efficacy data for vaccination of patients on anti–IL-23 biologics, but we can infer the safety profile from vaccination of patients on anti–IL-17 biologics and anti–IL-12/23 biologics. Omalizumab, which blocks immunoglobulin E, is also regarded as safe based on the mechanism of action. For the systemic immunotherapeutics, systemic corticosteroids, methotrexate, and JAK inhibitors appear to have the highest risk of reduced antibody production. However, it should be noted that in previous reviews, methotrexate and JAK inhibitors were considered safe therapies during the COVID-19 pandemic and, in fact, are being studied as potential treatments for COVID-19.158 , 159
Fig 1.
Summary of the safety and efficacy for potential SARS-CoV-2 vaccines for patients on immunotherapeutics. ∗Insufficient data. There were no studies evaluating the safety and/or efficacy of vaccination in patients receiving thalidomide, apremilast, IVIg, or the following biologics: brodalumab, anakinra, omalizumab, guselkumab, risankizumab, or tildrakizumab. Data on apremilast has been addressed in the literature on the basis of expert opinion only.
With regard to vaccine-generated antibody response, data generally support a possible decrease in antibody titers with the TNF-αbiologics, rituximab, ustekinumab, and many of the oral immunotherapies.3 Given the possibility of decreased antibody titers to vaccination with some of these treatments, there have been suggestions for withholding immunotherapeutics at the time of vaccination to promote a better vaccine response.157 For instance, a 2-week temporary withdrawal of methotrexate after vaccination for influenza has been shown to result in higher antibody titers in patients with rheumatoid arthritis.160 It would thus be prudent to check the titers after vaccination for any patients on a immunotherapeutic because they might require a booster to establish or maintain protective antibody titers. If protective antibody titers are inadequate and skewed to a T helper type 2 phenotype, vaccine-associated enhanced respiratory disease (VAERD) can develop.161 VAERD is a condition in which vaccination makes subsequent infections with the same virus worse. VAERD has been noted with vaccines to respiratory syncytial virus162 and measles,163 as well as vaccination in animal models of Middle East respiratory syndrome coronavirus (MERS-CoV).164 Based on the data from the current SARS-CoV-2 vaccines, the risk of VAERD appears to be low in the absence of immune modulatory therapy,22, 23, 24 , 165 but the possibility of T helper type 2 deviation may need to be considered. Otherwise, general considerations of vaccine safety need to be considered, such as allergic or anaphylactic reactions and exuberant inflammatory responses with fever and systemic symptoms. The benefit-to-risk ratio for vaccinating patients for SARS-CoV-2 is ultimately a discussion that needs to involve informed clinicians and patients.
Study limitations
This article provides an overview of current evidence on the administration of existing approved vaccines in patients receiving immunotherapy. Consequently, information is subject to process bias secondary to the methodology of the review. Existing evidence is frequently of low/limited quality with a lack of control groups, insufficient sample size and therefore limited power, and/or inconsistent findings. There is a paucity of data pertaining to vaccination in patient populations on immunosuppressive and immunomodulatory therapies, especially patients with dermatologic disease. Moreover, there is variability of underlying disease or treatment in study populations, which reflects the current diversity of immunosuppressive and immunomodulatory medications and the range of combinations in treatment regimens.
Recommendations and conclusions
The data reviewed in this article support the safety and potential efficacy of SARS-CoV-2 vaccines for our dermatology patients on immunotherapies (Box 1 ). The SARS-CoV-2 vaccines currently approved (Moderna/NIAID mRNA-1273, Pfizer/BioNTech/Fosun Pharma BNT162) and most likely to be approved (Astra-Zeneca/University of Oxford ChAdOx1/AZD1222, Johnson & Johnson JNJ-78436735, Novavax NVX-CoV2373) in North America are vaccine platforms (ie, RNA, protein subunit, and nonreplicating viral vectors) that are expected to be safe for patients on immunotherapeutics. The anticipated efficacy is variable in the setting of systemic immunotherapies. Although most biologics are associated with good (anti-IL-17, anti-IL-4/13) to fair (anti-TNF, anti-IL-12/23) antibody response to all vaccine subtypes, there is paucity in data for a number of agents. The current Infectious Diseases Society of America “Clinical Practice Guideline for Vaccination of the Immunocompromised Host” remains appropriate when considering future administration of a SARS-CoV-2 vaccine,14 although additional vaccinations and monitoring antibody titers can be considered.
Box 1. Summary of the recommendations for vaccination as applied to a potential SARS-CoV-2 vaccine.
-
1.Nonviral or inactivated SARS-CoV-2 vaccine subtypes may be considered before, during, or after immunosuppressive therapy in patients receiving systemic immunosuppressant or immune-targeting therapy without significant modification of ongoing treatments.
-
1.1.Safety: minimal to no risk of adverse events
-
1.2.Efficacy: variable antibody levels expected depending on vaccine and immunotherapy
-
1.1.
-
2.Nonviral SARS-CoV-2 vaccine subtypes may be considered in patients receiving biologic therapy without significant modification of ongoing immune therapy.
-
2.1.Safety: minimal to no risk of adverse events
-
2.2.Efficacy: at least fair to good antibody response for most biologics
-
2.1.
-
3.
The risk-to-benefit ratio may favor immunization if immunosuppression is low and there is significant risk of disease development.
-
4.
Consider checking antibody titers after vaccination and using additional vaccinations, if needed, to boost the level of protective antibodies.
SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2.
Conflicts of interest
None disclosed.
Acknowledgments
We would like to acknowledge the help of Dr Manish Sadarangani, director, Vaccine Evaluation Center, BC Children's Hospital, Sauder Family Chair in Pediatric Infectious Diseases, University of British Columbia. He reviewed the manuscript and provided helpful comments and suggestions.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
References
- 1.Huber F., Ehrensperger B., Hatz C., Chappuis F., Bühler S., Eperon G. Safety of live vaccines on immunosuppressive or immunomodulatory therapy—a retrospective study in three Swiss travel clinics. J Travel Med. 2018;25(1):1–8. doi: 10.1093/jtm/tax082. [DOI] [PubMed] [Google Scholar]
- 2.McKinnon J.E., Maksimowicz-McKinnon K. Autoimmune disease and vaccination: impact on infectious disease prevention and a look at future applications. Transl Res. 2016;167(1):46–60. doi: 10.1016/j.trsl.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Papp K.A., Haraoui B., Kumar D., et al. Vaccination guidelines for patients with immune-mediated disorders on immunosuppressive therapies. J Cutan Med Surg. 2019;23(1):50–74. doi: 10.1177/1203475418811335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jessica Chang T.Y., Pope J.E. How COVID-19 affects patients receiving anticytokine and JAK inhibitors in rheumatology and dermatology. Immunotherapy. 2020;12:1115–1119. doi: 10.2217/imt-2020-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Callaway E. The race for coronavirus vaccines: a graphical guide. Nature. 2020;580(7805):576–577. doi: 10.1038/d41586-020-01221-y. [DOI] [PubMed] [Google Scholar]
- 6.Plotkin S.A. Correlates of protection induced by vaccination. Clin Vaccine Immunol. 2010;17(7):1055–1065. doi: 10.1128/CVI.00131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Croce E., Hatz C., Jonker E.F., Visser L.G., Jaeger V.K., Bühler S. Safety of live vaccinations on immunosuppressive therapy in patients with immune-mediated inflammatory diseases, solid organ transplantation or after bone-marrow transplantation—a systematic review of randomized trials, observational studies and case reports. Vaccine. 2017;35(9):1216–1226. doi: 10.1016/j.vaccine.2017.01.048. [DOI] [PubMed] [Google Scholar]
- 8.Amanna I.J., Messaoudi I., Slifka M.K. Protective immunity following vaccination: how is it defined? Hum Vaccin. 2008;4(4):316–319. doi: 10.4161/hv.4.4.5751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andrews N.J., Waight P.A., Burbidge P., et al. Serotype-specific effectiveness and correlates of protection for the 13-valent pneumococcal conjugate vaccine: a postlicensure indirect cohort study. Lancet Infect Dis. 2014;14(9):839–846. doi: 10.1016/S1473-3099(14)70822-9. [DOI] [PubMed] [Google Scholar]
- 10.Taranger J., Trollfors B., Lagergård T., et al. Correlation between pertussis toxin IgG antibodies in postvaccination sera and subsequent protection against pertussis. J Infect Dis. 2000;181(3):1010–1013. doi: 10.1086/315318. [DOI] [PubMed] [Google Scholar]
- 11.Blauvelt A., Papp K.A., Sofen H., et al. Continuous dosing versus interrupted therapy with ixekizumab: an integrated analysis of two phase 3 trials in psoriasis. J Eur Acad Dermatol Venereol. 2017;31(6):1004–1013. doi: 10.1111/jdv.14163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Riel D., de Wit E. Next-generation vaccine platforms for COVID-19. Nat Mater. 2020;19(8):810–812. doi: 10.1038/s41563-020-0746-0. [DOI] [PubMed] [Google Scholar]
- 13.Abbasi J. COVID-19 and mRNA vaccines—first large test for a new approach. JAMA. 2020;324(12):1125–1127. doi: 10.1001/jama.2020.16866. [DOI] [PubMed] [Google Scholar]
- 14.Rubin L.G., Levin M.J., Ljungman P., et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58(3):e44–e100. doi: 10.1093/cid/cit684. [DOI] [PubMed] [Google Scholar]
- 15.Robinson J.K., Dellavalle R.P., Bigby M., Callen J.P. Systematic reviews: grading recommendations and evidence quality. Arch Dermatol. 2008;144(1):97–99. doi: 10.1001/archdermatol.2007.28. [DOI] [PubMed] [Google Scholar]
- 16.Introduction of inactivated poliovirus vaccine into oral poliovirus vaccine-using countries. Wkly Epidemiol Rec. 2003;78(28):241–250. [PubMed] [Google Scholar]
- 17.Keech C., Albert G., Cho I., et al. Phase 1–2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020;383:2320–2332. doi: 10.1056/NEJMoa2026920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baden L.R., El Sahly H.M., Essink B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramasamy M.N., Minassian A.M., Ewer K.J., et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396(10267):1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bar-Zeev N., Moss W.J. Encouraging results from phase 1/2 COVID-19 vaccine trials. Lancet. 2020;396:448–449. doi: 10.1016/S0140-6736(20)31611-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Callaway E. Coronavirus vaccine trials have delivered their first results—but their promise is still unclear. Nature. 2020;581(7809):363–364. doi: 10.1038/d41586-020-01092-3. [DOI] [PubMed] [Google Scholar]
- 23.Folegatti P.M., Ewer K.J., Aley P.K., et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corbett K.S., Flynn B., Foulds K.E., et al. Evaluation of the mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. N Engl J Med. 2020;383:1544–1555. doi: 10.1056/NEJMoa2024671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baumrin E., Van Voorhees A., Garg A., Feldman S.R., Merola J.F. A systematic review of herpes zoster incidence and consensus recommendations on vaccination in adult patients on systemic therapy for psoriasis or psoriatic arthritis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2019;81(1):102–110. doi: 10.1016/j.jaad.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 26.Abu-Shakra M., Press J., Varsano N., et al. Specific antibody response after influenza immunization in systemic lupus erythematosus. J Rheumatol. 2002;29(12):2555–2557. [PubMed] [Google Scholar]
- 27.Borba E.F., Saad C.G., Pasoto S.G., et al. Influenza A/H1N1 vaccination of patients with SLE: can antimalarial drugs restore diminished response under immunosuppressive therapy? Rheumatology (Oxford) 2012;51(6):1061–1069. doi: 10.1093/rheumatology/ker427. [DOI] [PubMed] [Google Scholar]
- 28.Chalmers A., Scheifele D., Patterson C., et al. Immunization of patients with rheumatoid arthritis against influenza: a study of vaccine safety and immunogenicity. J Rheumatol. 1994;21(7):1203–1206. [PubMed] [Google Scholar]
- 29.Crowe S.R., Merrill J.T., Vista E.S., et al. Influenza vaccination responses in human systemic lupus erythematosus: impact of clinical and demographic features. Arthritis Rheum. 2011;63(8):2396–2406. doi: 10.1002/art.30388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Del Porto F., Laganà B., Biselli R., et al. Influenza vaccine administration in patients with systemic lupus erythematosus and rheumatoid arthritis. Safety and immunogenicity. Vaccine. 2006;24(16):3217–3223. doi: 10.1016/j.vaccine.2006.01.028. [DOI] [PubMed] [Google Scholar]
- 31.Herron A., Dettleff G., Hixon B., et al. Influenza vaccination in patients with rheumatic diseases. Safety and efficacy. JAMA. 1979;242(1):53–56. [PubMed] [Google Scholar]
- 32.Holvast A., Stegeman C.A., Benne C.A., et al. Wegener's granulomatosis patients show an adequate antibody response to influenza vaccination. Ann Rheum Dis. 2009;68(6):873–878. doi: 10.1136/ard.2008.092924. [DOI] [PubMed] [Google Scholar]
- 33.Louie J.S., Nies K.M., Shoji K.T., et al. Clinical and antibody responses after influenza immunization in systemic lupus erythematosus. Ann Intern Med. 1978;88(6):790–792. doi: 10.7326/0003-4819-88-6-790. [DOI] [PubMed] [Google Scholar]
- 34.Williams G.W., Steinberg A.D., Reinertsen J.L., Klassen L.W., Decker J.L., Dolin R. Influenza immunization in systemic lupus erythematosus. A double-blind trial. Ann Intern Med. 1978;88(6):729–734. doi: 10.7326/0003-4819-88-6-729. [DOI] [PubMed] [Google Scholar]
- 35.Fischer L., Gerstel P.F., Poncet A., et al. Pneumococcal polysaccharide vaccination in adults undergoing immunosuppressive treatment for inflammatory diseases—a longitudinal study. Arthritis Res Ther. 2015;17(1):151. doi: 10.1186/s13075-015-0663-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ulinski T., Leroy S., Dubrel M., Danon S., Bensman A. High serological response to pneumococcal vaccine in nephrotic children at disease onset on high-dose prednisone. Pediatr Nephrol. 2008;23(7):1107–1113. doi: 10.1007/s00467-008-0782-5. [DOI] [PubMed] [Google Scholar]
- 37.Sempere L., Almenta I., Barrenengoa J., et al. Factors predicting response to hepatitis B vaccination in patients with inflammatory bowel disease. Vaccine. 2013;31(30):3065–3071. doi: 10.1016/j.vaccine.2013.04.059. [DOI] [PubMed] [Google Scholar]
- 38.Mok C.C., Ho L.Y., Fong L.S., To C.H. Immunogenicity and safety of a quadrivalent human papillomavirus vaccine in patients with systemic lupus erythematosus: a case-control study. Ann Rheum Dis. 2013;72(5):659–664. doi: 10.1136/annrheumdis-2012-201393. [DOI] [PubMed] [Google Scholar]
- 39.Cheetham T.C., Marcy S.M., Tseng H.F., et al. Risk of herpes zoster and disseminated varicella zoster in patients taking immunosuppressant drugs at the time of zoster vaccination. Mayo Clin Proc. 2015;90(7):865–873. doi: 10.1016/j.mayocp.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 40.Russell A.F., Parrino J., Fisher C.L., Jr., et al. Safety, tolerability, and immunogenicity of zoster vaccine in subjects on chronic/maintenance corticosteroids. Vaccine. 2015;33(27):3129–3134. doi: 10.1016/j.vaccine.2015.04.090. [DOI] [PubMed] [Google Scholar]
- 41.Kernéis S., Launay O., Ancelle T., et al. Safety and immunogenicity of yellow fever 17D vaccine in adults receiving systemic corticosteroid therapy: an observational cohort study. Arthritis Care Res (Hoboken) 2013;65(9):1522–1528. doi: 10.1002/acr.22021. [DOI] [PubMed] [Google Scholar]
- 42.Park J.K., Lee Y.J., Shin K., et al. Impact of temporary methotrexate discontinuation for 2 weeks on immunogenicity of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis. 2018;77(6):898–904. doi: 10.1136/annrheumdis-2018-213222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mori S., Ueki Y., Akeda Y., et al. Pneumococcal polysaccharide vaccination in rheumatoid arthritis patients receiving tocilizumab therapy. Ann Rheum Dis. 2013;72(8):1362–1366. doi: 10.1136/annrheumdis-2012-202658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guissa V.R., Pereira R.M., Sallum A.M., et al. Influenza A H1N1/2009 vaccine in juvenile dermatomyositis: reduced immunogenicity in patients under immunosuppressive therapy. Clin Exp Rheumatol. 2012;30(4):583–588. [PubMed] [Google Scholar]
- 45.Elkayam O., Caspi D., Reitblatt T., Charboneau D., Rubins J.B. The effect of tumor necrosis factor blockade on the response to pneumococcal vaccination in patients with rheumatoid arthritis and ankylosing spondylitis. Semin Arthritis Rheum. 2004;33(4):283–288. doi: 10.1053/j.semarthrit.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 46.Kapetanovic M.C., Nagel J., Nordström I., Saxne T., Geborek P., Rudin A. Methotrexate reduces vaccine-specific immunoglobulin levels but not numbers of circulating antibody-producing B cells in rheumatoid arthritis after vaccination with a conjugate pneumococcal vaccine. Vaccine. 2017;35(6):903–908. doi: 10.1016/j.vaccine.2016.12.068. [DOI] [PubMed] [Google Scholar]
- 47.Heijstek M.W., Kamphuis S., Armbrust W., et al. Effects of the live attenuated measles-mumps-rubella booster vaccination on disease activity in patients with juvenile idiopathic arthritis: a randomized trial. JAMA. 2013;309(23):2449–2456. doi: 10.1001/jama.2013.6768. [DOI] [PubMed] [Google Scholar]
- 48.Heijstek M.W., Pileggi G.C., Zonneveld-Huijssoon E., et al. Safety of measles, mumps and rubella vaccination in juvenile idiopathic arthritis. Ann Rheum Dis. 2007;66(10):1384–1387. doi: 10.1136/ard.2006.063586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Uziel Y., Moshe V., Onozo B., et al. Live attenuated MMR/V booster vaccines in children with rheumatic diseases on immunosuppressive therapy are safe: multicenter, retrospective data collection. Vaccine. 2020;38(9):2198–2201. doi: 10.1016/j.vaccine.2020.01.037. [DOI] [PubMed] [Google Scholar]
- 50.Groot N., Pileggi G., Sandoval C.B., et al. Varicella vaccination elicits a humoral and cellular response in children with rheumatic diseases using immune suppressive treatment. Vaccine. 2017;35(21):2818–2822. doi: 10.1016/j.vaccine.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 51.El-Darouti M.A., Hegazy R.A., Abdel Hay R.M., Abdel Halim D.M. Live attenuated varicella vaccine: a new effective adjuvant weapon in the battlefield against severe resistant psoriasis, a pilot randomized controlled trial. J Am Acad Dermatol. 2012;66(3):511–513. doi: 10.1016/j.jaad.2011.07.032. [DOI] [PubMed] [Google Scholar]
- 52.El-Darouti M.A., Hegazy R.A., Abdel Hay R.M., Rashed L.A. Study of T helper (17) and T regulatory cells in psoriatic patients receiving live attenuated varicella vaccine therapy in a randomized controlled trial. Eur J Dermatol. 2014;24(4):464–469. doi: 10.1684/ejd.2014.2377. [DOI] [PubMed] [Google Scholar]
- 53.Wieten R.W., Jonker E.F., Pieren D.K., et al. Comparison of the PRNT and an immune fluorescence assay in yellow fever vaccinees receiving immunosuppressive medication. Vaccine. 2016;34(10):1247–1251. doi: 10.1016/j.vaccine.2016.01.037. [DOI] [PubMed] [Google Scholar]
- 54.Wieten R.W., Goorhuis A., Jonker E.F.F., et al. 17D yellow fever vaccine elicits comparable long-term immune responses in healthy individuals and immune-compromised patients. J Infect. 2016;72(6):713–722. doi: 10.1016/j.jinf.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 55.Stuhec M. Yellow fever vaccine used in a psoriatic arthritis patient treated with methotrexate: a case report. Acta Dermatovenerol Alp Pannonica Adriat. 2014;23(3):63–64. [PubMed] [Google Scholar]
- 56.Whittembury A., Ramirez G., Hernández H., et al. Viscerotropic disease following yellow fever vaccination in Peru. Vaccine. 2009;27(43):5974–5981. doi: 10.1016/j.vaccine.2009.07.082. [DOI] [PubMed] [Google Scholar]
- 57.Winthrop K.L., Yamanaka H., Valdez H., et al. Herpes zoster and tofacitinib therapy in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66(10):2675–2684. doi: 10.1002/art.38745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holvast A., Huckriede A., Wilschut J., et al. Safety and efficacy of influenza vaccination in systemic lupus erythematosus patients with quiescent disease. Ann Rheum Dis. 2006;65(7):913–918. doi: 10.1136/ard.2005.043943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Keshtkar-Jahromi M., Argani H., Rahnavardi M., et al. Antibody response to influenza immunization in kidney transplant recipients receiving either azathioprine or mycophenolate: a controlled trial. Am J Nephrol. 2008;28(4):654–660. doi: 10.1159/000119742. [DOI] [PubMed] [Google Scholar]
- 60.Salles M.J., Sens Y.A., Boas L.S., Machado C.M. Influenza virus vaccination in kidney transplant recipients: serum antibody response to different immunosuppressive drugs. Clin Transplant. 2010;24(1):E17–E23. doi: 10.1111/j.1399-0012.2009.01095.x. [DOI] [PubMed] [Google Scholar]
- 61.Azevedo L.S., Gerhard J., Miraglia J.L., et al. Seroconversion of 2009 pandemic influenza A (H1N1) vaccination in kidney transplant patients and the influence of different risk factors. Transpl Infect Dis. 2013;15(6):612–618. doi: 10.1111/tid.12140. [DOI] [PubMed] [Google Scholar]
- 62.Versluis D.J., Beyer W.E., Masurel N., Wenting G.J., Weimar W. Impairment of the immune response to influenza vaccination in renal transplant recipients by cyclosporine, but not azathioprine. Transplantation. 1986;42(4):376–379. doi: 10.1097/00007890-198610000-00009. [DOI] [PubMed] [Google Scholar]
- 63.Dotan I., Werner L., Vigodman S., et al. Normal response to vaccines in inflammatory bowel disease patients treated with thiopurines. Inflamm Bowel Dis. 2012;18(2):261–268. doi: 10.1002/ibd.21688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wasan S.K., Zullow S., Berg A., Cheifetz A.S., Ganley-Leal L., Farraye F.A. Herpes zoster vaccine response in inflammatory bowel disease patients on low-dose immunosuppression. Inflamm Bowel Dis. 2016;22(6):1391–1396. doi: 10.1097/MIB.0000000000000743. [DOI] [PubMed] [Google Scholar]
- 65.Winthrop K.L., Baddley J.W., Chen L., et al. Association between the initiation of anti-tumor necrosis factor therapy and the risk of herpes zoster. JAMA. 2013;309(9):887–895. doi: 10.1001/jama.2013.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guthridge J.M., Cogman A., Merrill J.T., et al. Herpes zoster vaccination in SLE: a pilot study of immunogenicity. J Rheumatol. 2013;40(11):1875–1880. doi: 10.3899/jrheum.130170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jeon H.J., Ro H., Jeong J.C., et al. Efficacy and safety of hepatitis A vaccination in kidney transplant recipients. Transpl Infect Dis. 2014;16(3):511–515. doi: 10.1111/tid.12217. [DOI] [PubMed] [Google Scholar]
- 68.Egli A., Humar A., Widmer L.A., et al. Effect of immunosuppression on T-helper 2 and B-cell responses to influenza vaccination. J Infect Dis. 2015;212(1):137–146. doi: 10.1093/infdis/jiv015. [DOI] [PubMed] [Google Scholar]
- 69.Winthrop K.L., Curtis J.R., Lindsey S., et al. Herpes zoster and tofacitinib: clinical outcomes and the risk of concomitant therapy. Arthritis Rheumatol. 2017;69(10):1960–1968. doi: 10.1002/art.40189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Canadian immunization guide: part 3—vaccination of specific populations; immunization of immunocompromised persons. Government of Canada. www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-3-vaccination-specific-populations/page-8-immunization-immunocompromised-persons.html Published 2017. Updated 2018. Accessed September 12, 2020. Available at:
- 71.Poelman S.M., Keeling C.P., Metelitsa A.I. Practical guidelines for managing patients with psoriasis on biologics: an update. J Cutan Med Surg. 2019;23(1_suppl):3S–12S. doi: 10.1177/1203475418811347. [DOI] [PubMed] [Google Scholar]
- 72.Struijk G.H., Minnee R.C., Koch S.D., et al. Maintenance immunosuppressive therapy with everolimus preserves humoral immune responses. Kidney Int. 2010;78(9):934–940. doi: 10.1038/ki.2010.269. [DOI] [PubMed] [Google Scholar]
- 73.Adler S., Krivine A., Weix J., et al. Protective effect of A/H1N1 vaccination in immune-mediated disease—a prospectively controlled vaccination study. Rheumatology (Oxford) 2012;51(4):695–700. doi: 10.1093/rheumatology/ker389. [DOI] [PubMed] [Google Scholar]
- 74.Borte S., Liebert U.G., Borte M., Sack U. Efficacy of measles, mumps and rubella revaccination in children with juvenile idiopathic arthritis treated with methotrexate and etanercept. Rheumatology (Oxford) 2009;48(2):144–148. doi: 10.1093/rheumatology/ken436. [DOI] [PubMed] [Google Scholar]
- 75.Elkayam O., Amir S., Mendelson E., et al. Efficacy and safety of vaccination against pandemic 2009 influenza A (H1N1) virus among patients with rheumatic diseases. Arthritis Care Res (Hoboken) 2011;63(7):1062–1067. doi: 10.1002/acr.20465. [DOI] [PubMed] [Google Scholar]
- 76.Fomin I., Caspi D., Levy V., et al. Vaccination against influenza in rheumatoid arthritis: the effect of disease modifying drugs, including TNF alpha blockers. Ann Rheum Dis. 2006;65(2):191–194. doi: 10.1136/ard.2005.036434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.França I.L., Ribeiro A.C., Aikawa N.E., et al. TNF blockers show distinct patterns of immune response to the pandemic influenza A H1N1 vaccine in inflammatory arthritis patients. Rheumatology (Oxford) 2012;51(11):2091–2098. doi: 10.1093/rheumatology/kes202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Iwamoto M., Homma S., Onishi S., et al. Low level of seroconversion after a novel influenza A/H1N1/2009 vaccination in Japanese patients with rheumatoid arthritis in the 2009 season. Rheumatol Int. 2012;32(11):3691–3694. doi: 10.1007/s00296-011-2118-1. [DOI] [PubMed] [Google Scholar]
- 79.Kapetanovic M.C., Saxne T., Nilsson J.A., Geborek P. Influenza vaccination as model for testing immune modulation induced by anti-TNF and methotrexate therapy in rheumatoid arthritis patients. Rheumatology (Oxford) 2007;46(4):608–611. doi: 10.1093/rheumatology/kel366. [DOI] [PubMed] [Google Scholar]
- 80.Kobie J.J., Zheng B., Bryk P., et al. Decreased influenza-specific B cell responses in rheumatoid arthritis patients treated with anti-tumor necrosis factor. Arthritis Res Ther. 2011;13(6):R209. doi: 10.1186/ar3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mori S., Ueki Y., Hirakata N., Oribe M., Hidaka T., Oishi K. Impact of tocilizumab therapy on antibody response to influenza vaccine in patients with rheumatoid arthritis. Ann Rheum Dis. 2012;71(12):2006–2010. doi: 10.1136/annrheumdis-2012-201950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pasoto S.G., Ribeiro A.C., Viana V.S., et al. Short and long-term effects of pandemic unadjuvanted influenza A(H1N1)pdm09 vaccine on clinical manifestations and autoantibody profile in primary Sjögren's syndrome. Vaccine. 2013;31(14):1793–1798. doi: 10.1016/j.vaccine.2013.01.057. [DOI] [PubMed] [Google Scholar]
- 83.Ribeiro A.C., Guedes L.K., Moraes J.C., et al. Reduced seroprotection after pandemic H1N1 influenza adjuvant-free vaccination in patients with rheumatoid arthritis: implications for clinical practice. Ann Rheum Dis. 2011;70(12):2144–2147. doi: 10.1136/ard.2011.152983. [DOI] [PubMed] [Google Scholar]
- 84.Gabay C., Bel M., Combescure C., et al. Impact of synthetic and biologic disease-modifying antirheumatic drugs on antibody responses to the AS03-adjuvanted pandemic influenza vaccine: a prospective, open-label, parallel-cohort, single-center study. Arthritis Rheum. 2011;63(6):1486–1496. doi: 10.1002/art.30325. [DOI] [PubMed] [Google Scholar]
- 85.Pileggi G.S., de Souza C.B., Ferriani V.P. Safety and immunogenicity of varicella vaccine in patients with juvenile rheumatic diseases receiving methotrexate and corticosteroids. Arthritis Care Res (Hoboken) 2010;62(7):1034–1039. doi: 10.1002/acr.20183. [DOI] [PubMed] [Google Scholar]
- 86.Rosdahl A., Herzog C., Frösner G., Norén T., Rombo L., Askling H.H. An extra priming dose of hepatitis A vaccine to adult patients with rheumatoid arthritis and drug induced immunosuppression—a prospective, open-label, multi-center study. Trav Med Infect Dis. 2018;21:43–50. doi: 10.1016/j.tmaid.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 87.Baluch A., Humar A., Eurich D., et al. Randomized controlled trial of high-dose intradermal versus standard-dose intramuscular influenza vaccine in organ transplant recipients. Am J Transplant. 2013;13(4):1026–1033. doi: 10.1111/ajt.12149. [DOI] [PubMed] [Google Scholar]
- 88.Siegrist C.A., Ambrosioni J., Bel M., et al. Responses of solid organ transplant recipients to the AS03-adjuvanted pandemic influenza vaccine. Antivir Ther. 2012;17(5):893–903. doi: 10.3851/IMP2103. [DOI] [PubMed] [Google Scholar]
- 89.Winthrop K.L., Bingham C.O., 3rd, Komocsar W.J., et al. Evaluation of pneumococcal and tetanus vaccine responses in patients with rheumatoid arthritis receiving baricitinib: results from a long-term extension trial substudy. Arthritis Res Ther. 2019;21(1):102. doi: 10.1186/s13075-019-1883-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Measles pneumonitis following measles-mumps-rubella vaccination of a patient with HIV infection, 1993. MMWR Morb Mortal Wkly Rep. 1996;45(28):603–606. [PubMed] [Google Scholar]
- 91.Calabrese L.H., Abud-Mendoza C., Lindsey S.M., et al. Live zoster vaccine in patients with rheumatoid arthritis treated with tofacitinib with or without methotrexate, or adalimumab with methotrexate: a post hoc analysis of data from a phase IIIb/IV randomized study. Arthritis Care Res. 2020;72(3):353–359. doi: 10.1002/acr.24010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Satyam V.R., Li P.H., Reich J., et al. Safety of recombinant zoster vaccine in patients with inflammatory bowel disease. Dig Dis Sci. 2020;65:2986–2991. doi: 10.1007/s10620-019-06016-4. [DOI] [PubMed] [Google Scholar]
- 93.Winthrop K.L., Wouters A.G., Choy E.H., et al. The safety and immunogenicity of live zoster vaccination in patients with rheumatoid arthritis before starting tofacitinib: a randomized phase II trial. Arthritis Rheumatol. 2017;69(10):1969–1977. doi: 10.1002/art.40187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Winthrop K.L., Korman N., Abramovits W., et al. T-cell-mediated immune response to pneumococcal conjugate vaccine (PCV-13) and tetanus toxoid vaccine in patients with moderate-to-severe psoriasis during tofacitinib treatment. J Am Acad Dermatol. 2018;78(6):1149–1155.e1. doi: 10.1016/j.jaad.2017.09.076. [DOI] [PubMed] [Google Scholar]
- 95.Winthrop K.L., Silverfield J., Racewicz A., et al. The effect of tofacitinib on pneumococcal and influenza vaccine responses in rheumatoid arthritis. Ann Rheum Dis. 2016;75(4):687–695. doi: 10.1136/annrheumdis-2014-207191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Aikawa N.E., França I.L., Ribeiro A.C., Sallum A.M., Bonfa E., Silva C.A. Short and long-term immunogenicity and safety following the 23-valent polysaccharide pneumococcal vaccine in juvenile idiopathic arthritis patients under conventional DMARDs with or without anti-TNF therapy. Vaccine. 2015;33(5):604–609. doi: 10.1016/j.vaccine.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 97.Andrade P., Santos-Antunes J., Rodrigues S., Lopes S., Macedo G. Treatment with infliximab or azathioprine negatively impact the efficacy of hepatitis B vaccine in inflammatory bowel disease patients. J Gastroenterol Hepatol. 2015;30(11):1591–1595. doi: 10.1111/jgh.13001. [DOI] [PubMed] [Google Scholar]
- 98.Arad U., Tzadok S., Amir S., et al. The cellular immune response to influenza vaccination is preserved in rheumatoid arthritis patients treated with rituximab. Vaccine. 2011;29(8):1643–1648. doi: 10.1016/j.vaccine.2010.12.072. [DOI] [PubMed] [Google Scholar]
- 99.Askling H.H., Rombo L., van Vollenhoven R., et al. Hepatitis A vaccine for immunosuppressed patients with rheumatoid arthritis: a prospective, open-label, multi-centre study. Travel Med Infect Dis. 2014;12(2):134–142. doi: 10.1016/j.tmaid.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 100.Belle A., Baumann C., Bigard M.A., et al. Impact of immunosuppressive therapy on hepatitis B vaccination in inflammatory bowel diseases. Eur J Gastroenterol Hepatol. 2015;27(8):877–881. doi: 10.1097/MEG.0000000000000370. [DOI] [PubMed] [Google Scholar]
- 101.Bingham C.O., 3rd, Looney R.J., Deodhar A., et al. Immunization responses in rheumatoid arthritis patients treated with rituximab: results from a controlled clinical trial. Arthritis Rheum. 2010;62(1):64–74. doi: 10.1002/art.25034. [DOI] [PubMed] [Google Scholar]
- 102.Bühler S., Jaeger V.K., Adler S., et al. Safety and immunogenicity of tetanus/diphtheria vaccination in patients with rheumatic diseases-a prospective multi-centre cohort study. Rheumatology (Oxford) 2019;58(9):1585–1596. doi: 10.1093/rheumatology/kez045. [DOI] [PubMed] [Google Scholar]
- 103.Cao Y., Zhao D., Xu A.T., Shen J., Ran Z.H. Effects of immunosuppressants on immune response to vaccine in inflammatory bowel disease. Chin Med J (Engl) 2015;128(6):835–838. doi: 10.4103/0366-6999.152683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cho A., Bradley B., Kauffman R., et al. Robust memory responses against influenza vaccination in pemphigus patients previously treated with rituximab. JCI Insight. 2017;2(12):e93222. doi: 10.1172/jci.insight.93222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.deBruyn J., Fonseca K., Ghosh S., et al. Immunogenicity of influenza vaccine for patients with inflammatory bowel disease on maintenance infliximab therapy: a randomized trial. Inflamm Bowel Dis. 2016;22(3):638–647. doi: 10.1097/MIB.0000000000000615. [DOI] [PubMed] [Google Scholar]
- 106.Dell' Era L., Esposito S., Corona F., Principi N. Vaccination of children and adolescents with rheumatic diseases. Rheumatology (Oxford) 2011;50(8):1358–1365. doi: 10.1093/rheumatology/ker102. [DOI] [PubMed] [Google Scholar]
- 107.Dezfoli S., Horton H.A., Thepyasuwan N., et al. Combined immunosuppression impairs immunogenicity to tetanus and pertussis vaccination among patients with inflammatory bowel disease. Inflamm Bowel Dis. 2015;21(8):1754–1760. doi: 10.1097/MIB.0000000000000448. [DOI] [PubMed] [Google Scholar]
- 108.Eisenberg R.A., Jawad A.F., Boyer J., et al. Rituximab-treated patients have a poor response to influenza vaccination. J Clin Immunol. 2013;33(2):388–396. doi: 10.1007/s10875-012-9813-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Elkayam O., Bashkin A., Mandelboim M., et al. The effect of infliximab and timing of vaccination on the humoral response to influenza vaccination in patients with rheumatoid arthritis and ankylosing spondylitis. Semin Arthritis Rheum. 2010;39(6):442–447. doi: 10.1016/j.semarthrit.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 110.Fiorino G., Peyrin-Biroulet L., Naccarato P., et al. Effects of immunosuppression on immune response to pneumococcal vaccine in inflammatory bowel disease: a prospective study. Inflamm Bowel Dis. 2012;18(6):1042–1047. doi: 10.1002/ibd.21800. [DOI] [PubMed] [Google Scholar]
- 111.Gelinck L.B., van der Bijl A.E., Visser L.G., et al. Synergistic immunosuppressive effect of anti-TNF combined with methotrexate on antibody responses to the 23 valent pneumococcal polysaccharide vaccine. Vaccine. 2008;26(27-28):3528–3533. doi: 10.1016/j.vaccine.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 112.Gorelik M., Elizalde A., Wong Williams K., Gonzalez E., Cole J.L. Immunogenicity of sequential 13-valent conjugated and 23-valent unconjugated pneumococcal vaccines in a population of children with lupus. Lupus. 2018;27(14):2228–2235. doi: 10.1177/0961203318808589. [DOI] [PubMed] [Google Scholar]
- 113.Hagihara Y., Ohfuji S., Watanabe K., et al. Infliximab and/or immunomodulators inhibit immune responses to trivalent influenza vaccination in adults with inflammatory bowel disease. J Crohns Colitis. 2014;8(3):223–233. doi: 10.1016/j.crohns.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 114.Haykir Solay A., Eser F. High dose hepatitis B vaccine is not effective in patients using immunomodulatory drugs: a pilot study. Hum Vaccin Immunother. 2019;15(5):1177–1182. doi: 10.1080/21645515.2019.1574151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hua C., Barnetche T., Combe B., Morel J. Effect of methotrexate, anti-tumor necrosis factor α, and rituximab on the immune response to influenza and pneumococcal vaccines in patients with rheumatoid arthritis: a systematic review and meta-analysis. Arthritis Care Res (Hoboken) 2014;66(7):1016–1026. doi: 10.1002/acr.22246. [DOI] [PubMed] [Google Scholar]
- 116.Huang Y., Wang H., Wan L., Lu X., Tam W.W. Is systemic lupus erythematosus associated with a declined immunogenicity and poor safety of influenza vaccination? A systematic review and meta-analysis. Medicine (Baltimore) 2016;95(19):e3637. doi: 10.1097/MD.0000000000003637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kaine J.L., Kivitz A.J., Birbara C., Luo A.Y. Immune responses following administration of influenza and pneumococcal vaccines to patients with rheumatoid arthritis receiving adalimumab. J Rheumatol. 2007;34(2):272–279. [PubMed] [Google Scholar]
- 118.Kantsø B., Halkjær S.I., Thomsen O., et al. Immunosuppressive drugs impairs antibody response of the polysaccharide and conjugated pneumococcal vaccines in patients with Crohn's disease. Vaccine. 2015;33(41):5464–5469. doi: 10.1016/j.vaccine.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 119.Kapetanovic M.C., Roseman C., Jönsson G., Truedsson L., Saxne T., Geborek P. Antibody response is reduced following vaccination with 7-valent conjugate pneumococcal vaccine in adult methotrexate-treated patients with established arthritis, but not those treated with tumor necrosis factor inhibitors. Arthritis Rheum. 2011;63(12):3723–3732. doi: 10.1002/art.30580. [DOI] [PubMed] [Google Scholar]
- 120.Kapetanovic M.C., Saxne T., Sjöholm A., Truedsson L., Jönsson G., Geborek P. Influence of methotrexate, TNF blockers and prednisolone on antibody responses to pneumococcal polysaccharide vaccine in patients with rheumatoid arthritis. Rheumatology (Oxford) 2006;45(1):106–111. doi: 10.1093/rheumatology/kei193. [DOI] [PubMed] [Google Scholar]
- 121.Kivitz A.J., Schechtman J., Texter M., Fichtner A., de Longueville M., Chartash E.K. Vaccine responses in patients with rheumatoid arthritis treated with certolizumab pegol: results from a single-blind randomized phase IV trial. J Rheumatol. 2014;41(4):648–657. doi: 10.3899/jrheum.130945. [DOI] [PubMed] [Google Scholar]
- 122.Lakota K., Perdan-Pirkmajer K., Sodin-Šemrl S., et al. The immunogenicity of seasonal and pandemic influenza vaccination in autoimmune inflammatory rheumatic patients—a 6-month follow-up prospective study. Clin Rheumatol. 2019;38(5):1277–1292. doi: 10.1007/s10067-019-04439-y. [DOI] [PubMed] [Google Scholar]
- 123.Lee C.K., Kim H.S., Ye B.D., et al. Patients with Crohn's disease on anti-tumor necrosis factor therapy are at significant risk of inadequate response to the 23-valent pneumococcal polysaccharide vaccine. J Crohns Colitis. 2014;8(5):384–391. doi: 10.1016/j.crohns.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 124.Mamula P., Markowitz J.E., Piccoli D.A., Klimov A., Cohen L., Baldassano R.N. Immune response to influenza vaccine in pediatric patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2007;5(7):851–856. doi: 10.1016/j.cgh.2007.02.035. [DOI] [PubMed] [Google Scholar]
- 125.Mease P.J., Ritchlin C.T., Martin R.W., et al. Pneumococcal vaccine response in psoriatic arthritis patients during treatment with etanercept. J Rheumatol. 2004;31(7):1356–1361. [PubMed] [Google Scholar]
- 126.Nguyen M.T.T., Lindegaard H., Hendricks O., Jørgensen C.S., Kantsø B., Friis-Møller N. Initial serological response after prime-boost pneumococcal vaccination in rheumatoid arthritis patients: results of a randomized controlled trial. J Rheumatol. 2017;44(12):1794–1803. doi: 10.3899/jrheum.161407. [DOI] [PubMed] [Google Scholar]
- 127.Nived P., Jönsson G., Settergren B., et al. Prime-boost vaccination strategy enhances immunogenicity compared to single pneumococcal conjugate vaccination in patients receiving conventional DMARDs, to some extent in abatacept but not in rituximab-treated patients. Arthritis Res Ther. 2020;22(1):36. doi: 10.1186/s13075-020-2124-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nived P., Nagel J., Saxne T., et al. Immune response to pneumococcal conjugate vaccine in patients with systemic vasculitis receiving standard of care therapy. Vaccine. 2017;35(29):3639–3646. doi: 10.1016/j.vaccine.2017.05.044. [DOI] [PubMed] [Google Scholar]
- 129.Oliveira A.C., Mota L.M., Santos-Neto L.L., Simões M., Martins-Filho O.A., Tauil P.L. Seroconversion in patients with rheumatic diseases treated with immunomodulators or immunosuppressants, who were inadvertently revaccinated against yellow fever. Arthritis Rheumatol. 2015;67(2):582–583. doi: 10.1002/art.38960. [DOI] [PubMed] [Google Scholar]
- 130.Oren S., Mandelboim M., Braun-Moscovici Y., et al. Vaccination against influenza in patients with rheumatoid arthritis: the effect of rituximab on the humoral response. Ann Rheum Dis. 2008;67(7):937–941. doi: 10.1136/ard.2007.077461. [DOI] [PubMed] [Google Scholar]
- 131.Park S.H., Yang S.K., Park S.K., et al. Efficacy of hepatitis A vaccination and factors impacting on seroconversion in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2014;20(1):69–74. doi: 10.1097/01.MIB.0000437736.91712.a1. [DOI] [PubMed] [Google Scholar]
- 132.Polachek A., Korobko U., Mader-Balakirski N., et al. Immunogenicity and safety of vaccination against seasonal 2012 influenza virus among patients with psoriatic arthritis and psoriasis. Clin Exp Rheumatol. 2015;33(2):181–186. [PubMed] [Google Scholar]
- 133.Pratt P.K., Jr., David N., Weber H.C., et al. Antibody response to hepatitis B virus vaccine is impaired in patients with inflammatory bowel disease on infliximab therapy. Inflamm Bowel Dis. 2018;24(2):380–386. doi: 10.1093/ibd/izx001. [DOI] [PubMed] [Google Scholar]
- 134.Rákóczi É., Perge B., Végh E., et al. Evaluation of the immunogenicity of the 13-valent conjugated pneumococcal vaccine in rheumatoid arthritis patients treated with etanercept. Joint Bone Spine. 2016;83(6):675–679. doi: 10.1016/j.jbspin.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 135.Rehnberg M., Brisslert M., Amu S., Zendjanchi K., Håwi G., Bokarewa M.I. Vaccination response to protein and carbohydrate antigens in patients with rheumatoid arthritis after rituximab treatment. Arthritis Res Ther. 2010;12(3):R111. doi: 10.1186/ar3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Richi P., Alonso O., Martín M.D., et al. Evaluation of the immune response to hepatitis B vaccine in patients on biological therapy: results of the RIER cohort study. Clin Rheumatol. 2020;39:2751–2756. doi: 10.1007/s10067-020-05042-2. [DOI] [PubMed] [Google Scholar]
- 137.Salemi S., Picchianti-Diamanti A., Germano V., et al. Influenza vaccine administration in rheumatoid arthritis patients under treatment with TNFα blockers: safety and immunogenicity. Clin Immunol. 2010;134(2):113–120. doi: 10.1016/j.clim.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 138.Salinas G.F., De Rycke L., Barendregt B., et al. Anti-TNF treatment blocks the induction of T cell-dependent humoral responses. Ann Rheum Dis. 2013;72(6):1037–1043. doi: 10.1136/annrheumdis-2011-201270. [DOI] [PubMed] [Google Scholar]
- 139.Scheinberg M., Guedes-Barbosa L.S., Mangueira C., et al. Yellow fever revaccination during infliximab therapy. Arthritis Care Res (Hoboken) 2010;62(6):896–898. doi: 10.1002/acr.20045. [DOI] [PubMed] [Google Scholar]
- 140.Shirai S., Hara M., Sakata Y., et al. Immunogenicity of quadrivalent influenza vaccine for patients with inflammatory bowel disease undergoing immunosuppressive therapy. Inflamm Bowel Dis. 2018;24(5):1082–1091. doi: 10.1093/ibd/izx101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.van Aalst M., Lötsch F., Spijker R., et al. Incidence of invasive pneumococcal disease in immunocompromised patients: a systematic review and meta-analysis. Travel Med Infect Dis. 2018;24:89–100. doi: 10.1016/j.tmaid.2018.05.016. [DOI] [PubMed] [Google Scholar]
- 142.van Assen S., Holvast A., Benne C.A., et al. Humoral responses after influenza vaccination are severely reduced in patients with rheumatoid arthritis treated with rituximab. Arthritis Rheum. 2010;62(1):75–81. doi: 10.1002/art.25033. [DOI] [PubMed] [Google Scholar]
- 143.Visvanathan S., Keenan G.F., Baker D.G., Levinson A.I., Wagner C.L. Response to pneumococcal vaccine in patients with early rheumatoid arthritis receiving infliximab plus methotrexate or methotrexate alone. J Rheumatol. 2007;34(5):952–957. [PubMed] [Google Scholar]
- 144.Westra J., van Assen S., Wilting K.R., et al. Rituximab impairs immunoglobulin (Ig)M and IgG (subclass) responses after influenza vaccination in rheumatoid arthritis patients. Clin Exp Immunol. 2014;178(1):40–47. doi: 10.1111/cei.12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhang J., Xie F., Delzell E., et al. Association between vaccination for herpes zoster and risk of herpes zoster infection among older patients with selected immune-mediated diseases. JAMA. 2012;308(1):43–49. doi: 10.1001/jama.2012.7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kapetanovic M.C., Kristensen L.E., Saxne T., Aktas T., Mörner A., Geborek P. Impact of anti-rheumatic treatment on immunogenicity of pandemic H1N1 influenza vaccine in patients with arthritis. Arthritis Res Ther. 2014;16(1):R2. doi: 10.1186/ar4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.van Aalst M., Langedijk A.C., Spijker R., de Bree G.J., Grobusch M.P., Goorhuis A. The effect of immunosuppressive agents on immunogenicity of pneumococcal vaccination: a systematic review and meta-analysis. Vaccine. 2018;36(39):5832–5845. doi: 10.1016/j.vaccine.2018.07.039. [DOI] [PubMed] [Google Scholar]
- 148.Gomez E.V., Bishop J.L., Jackson K., Muram T.M., Phillips D. Response to tetanus and pneumococcal vaccination following administration of ixekizumab in healthy participants. BioDrugs. 2017;31(6):545–554. doi: 10.1007/s40259-017-0249-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chioato A., Noseda E., Stevens M., Gaitatzis N., Kleinschmidt A., Picaud H. Treatment with the interleukin-17A-blocking antibody secukinumab does not interfere with the efficacy of influenza and meningococcal vaccinations in healthy subjects: results of an open-label, parallel-group, randomized single-center study. Clin Vaccine Immunol. 2012;19(10):1597–1602. doi: 10.1128/CVI.00386-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Furer V., Zisman D., Kaufman I., et al. Immunogenicity and safety of vaccination against seasonal influenza vaccine in patients with psoriatic arthritis treated with secukinumab. Vaccine. 2020;38(4):847–851. doi: 10.1016/j.vaccine.2019.10.081. [DOI] [PubMed] [Google Scholar]
- 151.Tacke C.E., Smits G.P., van der Klis F.R., Kuipers I.M., Zaaijer H.L., Kuijpers T.W. Reduced serologic response to mumps, measles, and rubella vaccination in patients treated with intravenous immunoglobulin for Kawasaki disease. J Allergy Clin Immunol. 2013;131(6):1701–1703. doi: 10.1016/j.jaci.2013.01.045. [DOI] [PubMed] [Google Scholar]
- 152.Tamez R.L., Tan W.V., O’Malley J.T., et al. Influenza B virus infection and Stevens-Johnson syndrome. Pediatr Dermatol. 2018;35(1):e45–e48. doi: 10.1111/pde.13370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Blauvelt A., Simpson E.L., Tyring S.K., et al. Dupilumab does not affect correlates of vaccine-induced immunity: a randomized, placebo-controlled trial in adults with moderate-to-severe atopic dermatitis. J Am Acad Dermatol. 2019;80(1):158–167. doi: 10.1016/j.jaad.2018.07.048. [DOI] [PubMed] [Google Scholar]
- 154.Kubota T., Nii T., Nanki T., et al. Anti-tumor necrosis factor therapy does not diminish the immune response to influenza vaccine in Japanese patients with rheumatoid arthritis. Mod Rheumatol. 2007;17(6):531–533. doi: 10.1007/s10165-007-0632-5. [DOI] [PubMed] [Google Scholar]
- 155.Brodmerkel C., Zhu Y., Jiao Q., et al. Effects of ustekinumab administration on primate/human antigen-recall and humoral immune response functions. J Drugs Dermatol. 2010;9(6):677–683. [PubMed] [Google Scholar]
- 156.Doornekamp L., Goetgebuer R.L., Schmitz K.S., et al. High immunogenicity to influenza vaccination in Crohn's disease patients treated with ustekinumab. Vaccines (Basel) 2020;8(3):455. doi: 10.3390/vaccines8030455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Crnkic Kapetanovic M., Saxne T., Jönsson G., Truedsson L., Geborek P. Rituximab and abatacept but not tocilizumab impair antibody response to pneumococcal conjugate vaccine in patients with rheumatoid arthritis. Arthritis Res Ther. 2013;15(5):R171. doi: 10.1186/ar4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ghazawi F.M., Lim M., Dutz J.P., Kirchhof M.G. Infection risk of dermatologic therapeutics during the COVID-19 pandemic: an evidence-based recalibration. Int J Dermatol. 2020;59:1043–1056. doi: 10.1111/ijd.15028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zahedi Niaki O., Anadkat M.J., Chen S.T., et al. Navigating immunosuppression in a pandemic: a guide for the dermatologist from the COVID Task Force of the Medical Dermatology Society and Society of Dermatology Hospitalists. J Am Acad Dermatol. 2020;83:1150–1159. doi: 10.1016/j.jaad.2020.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Park J.K., Choi Y., Winthrop K.L., Song Y.W., Lee E.B. Optimal time between the last methotrexate administration and seasonal influenza vaccination in rheumatoid arthritis: post hoc analysis of a randomised clinical trial. Ann Rheum Dis. 2019;78(9):1283–1284. doi: 10.1136/annrheumdis-2019-215187. [DOI] [PubMed] [Google Scholar]
- 161.Polack F.P. Atypical measles and enhanced respiratory syncytial virus disease (ERD) made simple. Pediatr Res. 2007;62(1):111–115. doi: 10.1203/PDR.0b013e3180686ce0. [DOI] [PubMed] [Google Scholar]
- 162.Kim H.W., Canchola J.G., Brandt C.D., et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89(4):422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 163.Fulginiti V.A., Eller J.J., Downie A.W., Kempe C.H. Altered reactivity to measles virus. Atypical measles in children previously immunized with inactivated measles virus vaccines. JAMA. 1967;202(12):1075–1080. doi: 10.1001/jama.202.12.1075. [DOI] [PubMed] [Google Scholar]
- 164.Scobey T., Yount B.L., Sims A.C., et al. Reverse genetics with a full-length infectious cDNA of the Middle East respiratory syndrome coronavirus. Proc Natl Acad Sci U S A. 2013;110(40):16157–16162. doi: 10.1073/pnas.1311542110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Xia S., Duan K., Zhang Y., et al. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA. 2020;324(10):951–960. doi: 10.1001/jama.2020.15543. [DOI] [PMC free article] [PubMed] [Google Scholar]