Dear editor,
We read with great interest the recent article published by Lansbury et al. in this Journal. They found that only 7% of hospitalized COVID-19 patients had a bacterial co-infection, increasing to 14% in studies that only included ICU patients, a percentage very low when compared to bacterial coinfections associated with previous influenza pandemics1. The Authors underline that testing for co-infecting pathogens during the course of a pandemic is important but most of the studies they screened did not report on this.
Then, few works reported coinfection with atypical pathogens such as Mycoplasma pneumoniae 2, 3, 4, 5, 6, 7 and Chlamydia pneumoniae 8, even if previous studies on severe coronavirus infections have shown a serological evidence among SARS patients that the incidence of acute or recent Chlamydia pneumoniae and Mycoplasma pneumoniae infection was about 30% and 9%, respectively 9.
Here, we present a retrospective study with the aim to evaluate the prevalence of co-infections with atypical pathogens in SARS-CoV-2 infected patients compared to non-infected patients.
We included 721 hospitalized patients who underwent testing for SARS-CoV-2, Mycoplasma pneumoniae, Chlamydia pneumoniae and Legionella pneumophyla over a two months period between 6 March 2020 and 12 May 2020 to the Spedali Civili's Hospital, Montichiari's Hospital and Gardone Val-Trompia's Hospital, Brescia, Italy. A real time reverse polymerase chain reaction was used to detect SARS-CoV-2. Chemiluminescence immunoassays and immunoenzymatic assays were used to detect IgM antibodies for Mycoplasma pneumoniae and IgM/IgA/IgG for Chlamydia pneumoniae in the serum of patients. Legionella diagnosis was made by urinary antigen testing.
Definition of pneumonia or severe pneumonia was done according to the WHO guidelines and included clinical signs of pneumonia (fever, cough, dyspnea, fast breathing).
Categorical variables are the number (percentage), and continuous variables are the median (interquartile range [IQR]). Categorical data were compared by using the χ2 test or the Fisher exact test, as appropriate. The data in different groups were compared with the ANOVA or independent t-test for normally distributed variables.
Logistic regression was used in order to perform univariate and multivariate analysis for determination of risk factors [odds ratios (ORs) and 95% CIs] associated with the presence of Chlamydia pneumoniae and Mycoplasma pneumoniae infection in SARS-CoV-2 positive patients after adjusting for confounders.
Multivariate logistic regression was used to account for differences in-hospital mortality for Chlamydia pneumoniae and Mycoplasma pneumoniae infection in SARS-CoV-2 positive patients. A P value <0.05 was considered statistically significant.
Out of 721 subjects, 443 individuals were infected with SARS-CoV-2 and 278 subjects were negative. All individuals were negative for Legionella antigen. Of the 443 patients with a SARS-CoV-2 infection, 242 individuals had an antibody positivity against Mycoplasma and/or Chlamydia, while of the 278 negative for SARS-CoV-2, only 97 showed Mycoplasma and/or Chlamydia pneumoniae antibody positivity (p < 0.0001). Of the 242 seropositive subjects, 179 individuals were positive only for Chlamydia pneumoniae and 63 exhibited a positive result both for Chlamydia and for Mycoplasma. Our analyses were performed on the total of patients seropositive for both Chlamydia and Mycoplasma pneumoniae without distinction between the two pathogens.
Demographic and clinical characteristics of the patients on admission are listed in Table 1 . The median age was 70 (IQR, 20), 294 (66.3%) were male and 149 (34%) were female. Most of them (339, 76.5%) had one or more pre-existing comorbidities, mostly hypertension (224, 50.5%), cardiovascular disease (11, 25%), diabetes (83, 18.7%) and hyperlipidemia (32, 7.2%). The most common signs were fever (338, 76.2%), cough (223, 50.3%) and dyspnea (186, 41.9%).
Table 1.
Clinical characteristics of COVID 19 patients according to their Chlamydia and Mycoplasma serology status.
| Chlamydia and Mycoplasma serology status |
P value | ||
| Positive (n = 242) | Negative (n = 201) | ||
| Age, mean (IQR) | 71 (19) | 68 (20) | 0.11 |
| Age group, n°/total (%) | |||
| 0–20 | 0 | 1 (0.4) | / |
| 21–40 | 5 (2) | 9 (4.4) | 0.2 |
| 41–60 | 58 (23.9) | 51 (25.3) | 0.8 |
| 61–80 | 127 (52.4) | 100 (49.7) | 0.6 |
| >80 | 52 (21.4) | 40 (19.9) | 0.7 |
| Gender, n°/total (%) | |||
| Male | 173 (71.4) | 121 (60.1) | 0.01 |
| Number of comorbidities, n (%) | |||
| None | 50 (20.6) | 54 (26.8) | 0.15 |
| 1 or 2 | 99 (40.9) | 89 (44.2) | 0.53 |
| ≥3 | 93 (38.4) | 58 (28.8) | 0.04 |
| Comorbidities | |||
| Diabetes | 49 (20.2) | 34 (16.9) | 0.4 |
| Hypertension | 130 (53.7) | 94 (46.7) | 0.15 |
| Cardiovascular disease | 69 (28.5) | 42 (20.8) | 0.08 |
| Chronic pulmonary disease | 17 (7) | 18 (8.9) | 0.5 |
| Chronic kidney disease | 13 (5.3) | 3 (1.4) | 0.03 |
| Hyperlipidemia | 25 (10.3) | 7 (3.4) | 0.005 |
| Malignancy | 18 (7) | 16 (7.9) | 0.87 |
| Hematologic disease | 8 (3.3) | 8 (3.4) | 0.9 |
| Neurologic disease | 11 (4.5) | 5 (2.4) | 0.3 |
| Symptoms, n (%) | |||
| Fever (temperature >37.5 °C) | 174 (71.9) | 164 (81.5) | 0.02 |
| Cough | 127 (52.4) | 96 (47.7) | 0.37 |
| Dyspnea | 116 (47.9) | 70 (34.8) | 0.005 |
| Chest pain | 4 (1.6) | 3 (1.4) | 0.80 |
| Nausea | 2 (0.82) | 3(1.4) | 0.83 |
| Vomiting | 13 (5.3) | 11 (5.4) | 0.86 |
| Diarrhea | 19 (7.8) | 21 (10.4) | 0.43 |
| Myalgia | 15 (6.1) | 10 (4.9) | 0.72 |
| Loss of smell and taste | 2 (0.82) | 0 | / |
Compared with those without atypical pathogen antibody positivity, patients seropositive for Mycoplasma and/or Chlamydia had a larger proportion of male, had dyspnea (47.9% vs 34.8%, p = 0.005) and were more likely to have more than three comorbidities (p = 0.04), in particular chronic renal disease (5.3% vs 1.4%, p = 0.03) and hyperlipidemia (10.3% vs 3.4%, p = 0.005) (Table 1).
Patients with SARS-CoV-2 and antibody positivity for Mycoplasma and/or Chlamydia had a higher median leukocyte count (median 6.6 [IQR, 3.6] x109/L vs median 5.7 [IQR, 3.8] x109/L, p = 0.01) and a lower level of lymphocytes (71.9% vs 62%, p = 0.003) (Table S1). An increased ALT serum level (29.1% vs 19.8%, p = 0.02) and also a higher median serum level (median 33 [IQR, 31] U/L vs 26 [IQR, 26] U/L, p = 0.02) was found in a higher percentage of patients with SARS-CoV-2 and antibody positivity for Mycoplasma and/or Chlamydia pneumoniae (Table S1). Higher median serum level of PCR was observed in patients with SARS-CoV-2 and antibody positivity for Mycoplasma and/or Chlamydia pneumoniae (median 100.4 [IQR, 108.5] mg/L vs 64.45 [IQR, 89] mg/L, p = 0.006).
More than three quarters of patients received antibiotic treatment and more than half patients (266, 60%) received antiviral treatment. The percentage of patients receiving azithromycin was significantly higher in those with SARS-CoV-2 and antibody positivity for Mycoplasma and/or Chlamydia pneumoniae (57% vs 46.2%, p = 0.02) (Table S2).
The proportions of critical COVID-19 patients with atypical pathogens coinfection were higher than those of patients infected only with SARS-CoV-2 (13.2% vs 5.9%, p = 0.01). Furthermore, requirement and use of a nasal cannula, high flow oxygen support and non- invasive ventilation was significantly higher in co-infected patients than in only SARS-CoV-2 positive patients (18.1% vs 3.6%, p<0.0001; 45% vs 23.3%, p < 0.0001; 14.7% vs 4.6%, p = 0.001, respectively) (Table S2).
There were significantly more patients need mechanical ventilation support in SARS-CoV-2 and antibody positivity for Mycoplasma and/or Chlamydia pneumoniae than in the other group of patients (6.8% vs 2.5%, p = 0.04) (Table S2). Furthermore, seropositive patients for Mycoplasma and/or Chlamydia were more likely to develop more complications (8.3% vs 2.5%, p = 0.01), including acute respiratory distress (8.6% vs 3.4%, p = 0.03), renal disease (2.8% vs 0.9%, p = 0.1), pulmonary embolism and thrombotic events (2.8% vs 1.9%, p = 0.7) and acute cardiac injury (2.4% vs 0, p = 0.03).
Patients with SARS-CoV-2 and antibody positivity for Mycoplasma and/or Chlamydia pneumoniae had a slight higher fatality rate compared to the other group of patients, even if not statistically significant (24.2% vs 21.8%, p = 0.63). However, multivariate logistic regression showed that age >65 years was an independent risk factor for death in patients with SARS-CoV-2 infection and seropositive for Mycoplasma and/or Chlamydia pneumoniae (OR=4.02, 95%CI=1.7–11, p = 0.003).
SARS-CoV-2 infected patients were associated with an increased probability of both Chlamydia/Mycoplasma pneumoniae positivity by serology (OR=2.2, 95%CI=1.6–3.0, p<0.0001). A logistic regression for univariate and multivariate analyses was performed in order to identify risk factors associated with Chlamydia/Mycoplasma infection in SARS-CoV-2 positive patients.
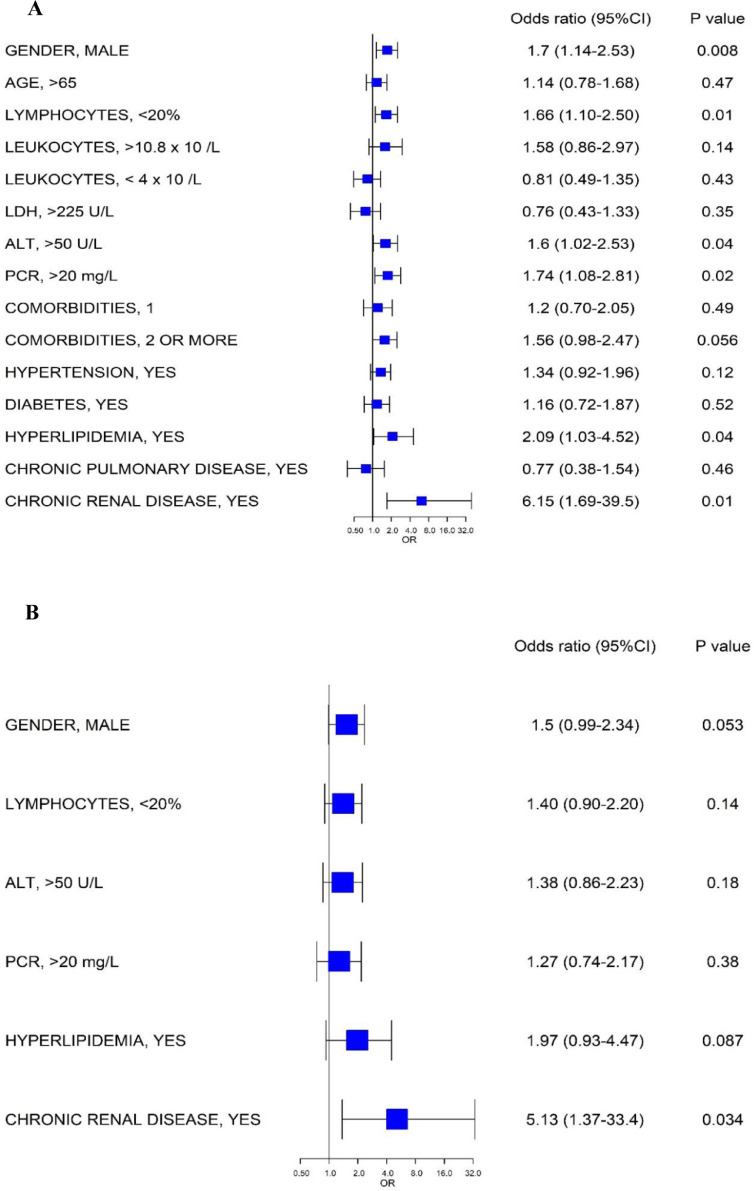
Six categorical variables were identified in univariate logistic regression analysis, namely: male sex, lymphopenia, ALT >50 U/L, PCR>20 mg/L, hyperlipidemia and chronic renal disease (Fig. 1 A). Only chronic renal disease was demonstrated as independent risk factor based on the multivariate logistic regression model (Fig. 1B).
Fig. 1.
A) Univariate logistic regression showing the categorical variables associated with Chlamydia and Mycoplasma pneumoniae antibody positivity in SARS-CoV-2 infected patients, B) Factors showing significantly independent association with Chlamydia and Mycoplasma pneumoniae antibody positivity in SARS-CoV-2 infected patients. Odds ratio, 95%CI and P values are obtained from logistic regression modeling.
To the best of our knowledge, this study was the first in Europe to report a high rate of detection of antibody positivity for these atypical pathogens in SARS-CoV-2 infected patients, with 26% of the patients positive for Mycoplasma IgM, 18% of the patients positive for Chlamydia IgM and 81% of the patients positive for both Chlamydia IgA and IgG.
So far, in the management of COVID-19 patients, we have to keep in mind the possibility of the presence of other respiratory pathogens causing coinfections. This is important to guide the clinicians for the use of targeted therapies, aimed at treating this potential micro-organisms in order to avoid more severe outcomes of patients.
Declaration of Competing Interest
All the authors declare no competing interest.
Acknowledgments
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki and national standards, and was approved by the Brescia ethical committee (EvoSCO NP 4369).
Author contributions
M.A.D.F. and C. P. conceived and designed the study. M.A.D.F. analyzed the data and wrote the first draft of the manuscript. F.C., C.B., P.P, S.F., F.C. (Francesca Caccuri), V.C, and L.M. performed and analyzed microbiological tests and participated in manuscript revision. S.P. analyzed the data and performed statistical analyses. D.R., P.M., M.S., L.M., F.A., F.S., A.P. (Andrea Pilotto), A. P. (Alessandro Padovani), M.B., R.C., C.R., M.C., M.B., G.S., G.M., M.R., S.B., E.F., L.T., F.C. (Francesco Castelli), A.R., R.I., and M.M. were directly involved in the patient care, collected data and participated in manuscript revision. A.C. provided study oversight and participated in manuscript revision. All the Authors approved manuscript submission
Acknowledgements
We thank all medical and healthcare workers of Spedali Civili's Hospital, Montichiari's Hospital and Gardone Val Trompia's Hospital who are fighting against this pandemic and are directly involved in the care of patients. We also thank all the medical and technical staff of the Microbiology section for the performance of all microbiological tests.
Supplementary material
Supplementary material associated with this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jinf.2021.01.009.
Appendix. Supplementary materials
References
- 1.Lansbury L., Lim B., Baskaran V., Lim W.S. Co-infections in people with COVID-19: a Systematic review and meta-analysis. J Infect. 2020;81:266–275. doi: 10.1016/j.jinf.2020.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang J.J., Dong X., Cao Y.Y. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan China. Allergy. 2020;75:1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 3.Gayam V., Konala V.M., Naramala S. Presenting characteristics, comorbidities, and outcomes of patients coinfected with COVID-19 and Mycoplasma pneumoniae in the USA. J Med Virol. 2020 doi: 10.1002/jmv.26026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Q., Xing Y., Shi L. Coinfection and other clinical characteristics of COVID-19 in children. Pediatrics. 2020;146 doi: 10.1542/peds.2020-0961. [DOI] [PubMed] [Google Scholar]
- 5.Fan B.E., Lim K., Chong Lian. COVID-19 and Mycoplasma pneumoniae coinfection. Am J Hematol. 2020;95:723–724. doi: 10.1002/ajh.25785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen H.R., Zou H., Xue M., Chen Z.B., Chen W.X. A case of childhood COVID 19 infection with pleural effusion complicated by possible secondary Mycoplasma pneumoniae infection. Pediatr Infect Dis J. 2020;39:e135–e137. doi: 10.1097/INF.0000000000002721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plebani A., Meini A., Cattalini M. Mycoplasma infection may complicate the clinical course of SARS-CoV-2 associated Kawasaki-like disease in children. Clin Immunol. 2020;221 doi: 10.1016/j.clim.2020.108613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richardson S., Hirsch J.S., Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zahariadis G., Gooley T.A., Ryall P. Risk of ruling out severe acute respiratory syndrome by ruling in another diagnosis: variable incidence of atypical bacteria coinfection based on diagnostic assays. Canc Res J. 2006;3:17–22. doi: 10.1155/2006/862797. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.