Abstract
Aim of the study
To study clinical, laboratory and imaging features correlated with complete response (CR) to transarterial chemoembolization (TACE) in patients with unresectable hepatocellular carcinoma (HCC) through 162 patients collected in Hassan II University Hospital of Fez.
Material and methods
From January 2015 to December 2019, 162 patients diagnosed with 225 HCC were treated by TACE. Among them, 14 showed CR during the follow-up. Imaging response was evaluated using the modified Response Evaluation Criteria in Solid Tumors (mRECIST). A multivariate analysis was performed including demographic parameters, etiology, α-fetoprotein (AFP) rates, hepatic function scores, imaging and TACE features. In cases with complete response and remission, follow-up duration was considered from the first to the last imaging control showing no viable tumor and eventually nodule retraction.
Results
Among the 162 patients with 225 nodules, 14 (9%) of them showed remission and 148 (91%) did not. There was no significant difference between the two groups in age, performance status (PS), AFP, nodularity, size nodule or number of TACE cures. Sex, etiology, Child-Pugh and MELD scores, location, BCLC stage and blush extinction were all found to have a significant impact on therapeutic response.
Conclusions
This study demonstrates that CR of HCC treated by TACE is strongly correlated with male sex, etiology (viral hepatitis C), location (segments VI and VII) and complete blush extinction on digital subtraction angiography (DSA). No significant correlation was found, particularly that of tumor size and segment IV (as a pejorative location).
Keywords: HCC, TACE, complete response
Introduction
Hepatocellular carcinoma (HCC) is the most common primary malignant liver tumor. Incidence varies by age, gender, geographic region, and ethnicity. It is the fifth most common cancer in the world and the second leading cause of cancer death, which makes it a public health problem [1, 2]. It develops in the majority of cases in cirrhosis, rarely in chronic liver disease and exceptionally in a healthy liver [3].
The prognosis of HCC patients is reserved and associated with a high recurrence rate of nearly 80% at 5 years after resection [4].
Surgical or radiofrequency resection and transplantation are the most efficient treatment options for improving survival rates [5]. These therapies are only performed during the early stages of the disease [6, 7].
Since most HCC patients are diagnosed at an advanced stage, transarterial chemoembolization (TACE) is considered to be the best therapeutic option [8]; it is more so when surgery or radiofrequency ablation (RFA) is unsuccessful or unfeasible. The global survival rate of HCC patients treated with palliative TACE is about 26% at 5 years [9]. TACE usage during the early stage of HCC resulted in 52% overall survival at 5 years. Serum albumin, tumor size, tumor number and recurrence interval are described as the mortality impact factor [10].
Main complete response factors are tumor location and size: segments I and IV locations are a pejorative factor whereas tumor size < 5 cm is a positive predictive factor [11].
Material and methods
162 newly diagnosed patients with 225 HCC nodules were treated with TACE as first treatment in our institution between January 2015 and December 2019.
Complete response (CR) was reached in 14 patients. It was considered, according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST), as the total disappearance of any intratumoral arterial enhancement in all target lesions after TACE.
After obtaining an angiographic access through the right femoral artery, we proceeded to perform selective catheterization of segmental feeding arteries.
A stable emulsion was obtained by combining 50 to 75 mg/m2 of doxorubicin and 10 ml of Lipiodol. After that, the doxorubicin-Lipiodol emulsion was mixed with a nonionic water-soluble contrast agent: ULTRAVIST R (Iopromide) at 20 mg of doxorubicin per 1 ml of ULTRAVIST.
The emulsion was then administered into the feeders. Once the flow became sluggish, gelatin sponge particles mixed with contrast material were administered into the feeders until blood flow stopped completely. After every TACE, a multiphasic computed tomography (CT) scan was performed. If the CT scan detected viable tumors after TACE, TACE would be performed again few days later. If no viable tumor was detected on CT scan, hepatic magnetic resonance imaging (MRI) would be indicated. The routine post-TACE follow-up protocol included biochemical liver function tests and serum α-fetoprotein (AFP) level measurements.
The final response was evaluated after the last cure because large tumors might need more than 1 session to reach CR. The tumor response was assessed 6 weeks after treatment and every 3 or 6 months until the end of the study.
The baseline characteristics of the 14 patients with CR after TACE were evaluated including clinical features: age, gender, performance status (PS), etiology of cirrhosis, biological parameters: AFP, imaging features: number, localization and lesions’ size and their extension to main vessels, the presence or absence of a capsule were also described. TACE characteristics were defined: number of sessions, technique and blush extinction. Liver function was evaluated using the Model for End-Stage Liver Disease (MELD) and Child-Pugh scores. Staging was established using the Barcelona Clinic Liver Cancer (BCLC) system.
Between the 162 patients treated with TACE, 14 patients achieved continuous CR and remission (during the 5 years of the study) after one or more sessions. The baseline characteristics of all patients are summarized in the Table 1.
Table 1.
Data from patients treated for HCC by TACE. A comparison between the two groups (with and without remission) is included
| Characteristics | Factors | With remission | Without remission | P-value (correlation with CR) | |
|---|---|---|---|---|---|
| Age (years) | Average | 60 (19-80) | 62 (6-87) | 1 (CR is not correlated with age) |
|
| ≥ 55 | 12 (86%) | 120 (81%) | |||
| < 55 | 2 (14%) | 28 (19 %) | |||
| Gender | Males | 11 (78%) | 67 (45%) | 0.023 (CR is correlated with male gender) |
|
| Females | 3 (22%) | 81 (55%) | |||
| PS | PS 0 | 7 (50%) | 73 (50%) | 0.538 (CR is not correlated with PS) |
|
| PS 1 | 6 (43%) | 53 (36%) | |||
| PS 2 | 1 (7%) | 14 (10%) | |||
| PS 3 | 0 | 6 (4%) | |||
| Cause | VHC | 9 (65%) | 69 (48%) | 0.0001 (CR is correlated with VHC) |
|
| VHB | 2 (14%) | 32 (21%) | |||
| PBC | 1 (7%) | 1 (7%) | |||
| Alagille syndrome | 1 (7%) | 1 (7%) | |||
| Wilson syndrome | 0 | 1 (7%) | |||
| Not identified | 1 (7%) | 0 | |||
| AFP (ng/ml) | Average | 3158 (1-22069) | 2884 (50-120560) | 0.35 (CR is not correlated with AFP) |
|
| < 20 | 6 (43%) | 60 (40%) | |||
| < 400 | 9 (64%) | 112 (76%) | |||
| Child-Pugh score | A | 11 (79%) | 83 (61%) | 0.001 (CR is correlated with Child-Pugh score A) |
|
| B | 3 (21%) | 50 (37%) | |||
| C | 0 | 3 (2%) | |||
| MELD score | Average | 10 (8-12) | 5 (1-86) | 0.0001 (CR is correlated with MELD score ≤ 19) |
|
| ≤ 9 | 11 (78%) | 105 (86%) | |||
| 10-19 | 3 (22%) | 9 (7%) | |||
| 20-29 | 0 | 4 (3%) | |||
| 30-39 | 0 | 2 (2%) | |||
| ≥ 40 | 0 | 2 (2%) | |||
| Number of lesions | 1 | 11 (78%) | 100 (61%) | 0.96 (CR is not correlated with number of lesions) |
|
| 2 | 3 (22%) | 52 (31%) | |||
| 3 | 0 | 4 (2%) | |||
| 4 | 0 | 2 (1%) | |||
| 5 | 0 | 5 (3%) | |||
| 6 | 0 | 1 (1%) | |||
| 7 | 0 | 1 (1%) | |||
| Location | Segment I | 0 | 2 | 0.00003 (CR is correlated with segments VI and VII) |
|
| Right lateral sector (RLS) | Total: | 9 (53%) | 32 (15%) | ||
| Segment VI | 3 (18%) | 9 (4%) | |||
| Segment VII | 5 (29%) | 17 (8%) | |||
| Both or between | 1 (6%) | 6 (3%) | |||
| Right paramedian sector (RPMS) | Total: | 3 (18%) | 35 (17%) | ||
| Segment V | 2 (12%) | 12 (6%) | |||
| Segment VIII | 1 (6%) | 21 (10%) | |||
| Both or between | 0 | 2 (1%) | |||
| Location | Left paramedian sector (LPMS) | Segment IV | 1 (6%) | 2 (1%) | |
| Left lateral sector | Total: | 1 (6%) | 14 (7%) | ||
| Segment II | 0 | 3 (14%) | |||
| Segment III | 0 | 9 (4%) | |||
| Both or between | 1 (6%) | 2 (1%) | |||
| Tumor size per nodule (mm) | Average | 81 (17-145) | 56 (12-136) | 0.9 (CR is not correlated with size) |
|
| < 50 | 10 (54%) | 112 (54%) | |||
| 50-100 | 6 (38%) | 75 (36%) | |||
| > 100 | 1 (8%) | 21 (10%) | |||
| Capsule | Present | 2 (15%) | N/A | N/A | |
| Absent | 15 (85%) | N/A | |||
| BCLC stage | A | 1 (7%) | 117 (57%) | 0.0001 (CR is correlated with BCLC stage B and C) |
|
| B | 5 (36%) | 5 (2%) | |||
| C | 8 (57%) | 73 (35%) | |||
| D | 0 | 13 (6%) | |||
| Number of TACE sessions | 1 | 7 (50%) | 89 (60%) | 0.245 (CR is not correlated with number of TACE sessions) |
|
| 2 | 5 (36%) | 37 (25%) | |||
| 3 | 1 (7%) | 12 (14%) | |||
| 4 | 1 (7%) | 0 | |||
| 5 | 0 | 0 | |||
| 6 | 0 | 0 | |||
| Duration of follow-up with a complete response | 396 (197-694) | No CR | |||
Results
General features
Demographic features and etiology
Ages of the 14 patients were between 19 and 80 years old with an average of 50 years old. Most of them (12; 86%) were older than 55 years old. 11 were male and 3 were female. Only 1 (7%) patient had a level 2 of PS, 6 (43%) patients had a level 1 of PS, and the other 7 (50%) patients had no clinical symptoms (PS 0). The etiology was considered as viral hepatitis C (VHC) in 9 (65%) patients, while 2 (14%) had viral hepatitis B (VHB). One patient (7%) was followed up for Alagille syndrome while another one (7%) had a history of primary biliary cirrhosis (PBC). No cause was identified in one case (7%).
AFP
α-fetoprotein value ranged significantly from 1 to 22 069 ng/ml with an average of 3158 ng/ml. Only 6 (43%) patients had < 20 ng/ml and 9 (64%) patients had < 400 ng/ml.
Hepatic function
Child-Pugh class A was identified in 11 (79%) cases while class B was identified in 3 (21%) patients. The MELD score ranged from 8 to 12 with an average of 10.
Imaging features
Nodularity
Eleven (78%) patients had a single nodule while 3 (22%) others had 2 nodules (Fig. 1).
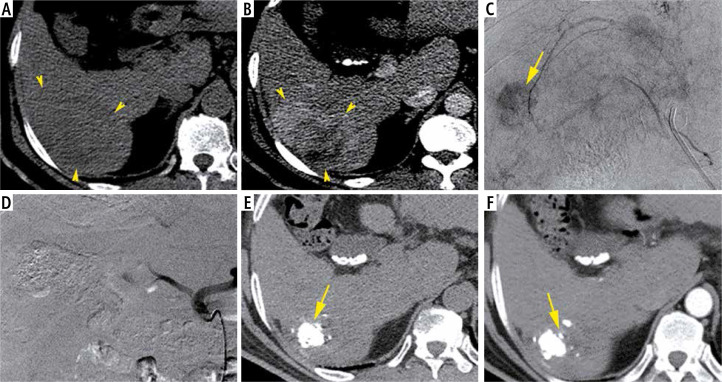
Fig. 1.
A 59-year-old man with chronic hepatitis C and raised AFP (8513 ng/ml), Child-Pugh class A6, MELD score of 12 and BCLC stage C. Axial non-enhanced CT image (A) show a slightly hypodense lesion of the segment VI with heterogeneous enhancement on the arterial phase (B). The HCC size is about 80 mm. Hyperselective TACE demonstrate a nodular blush (C) which completely disappeared after chemoembolization (D). CT control in axial plan performed 6 weeks later shows retraction of the HCC and intense Lipiodol fixation clearly identified on the non-enhanced CT (E) without any enhancement on the arterial phase (F)
Location
Among the 17 embolized HCC nodules, all the locations were unilobar. The most frequently noted location was the right lateral sector with 9 (47%) nodules: 3 (18%) in segment VI, 5 (29%) in segment VII (Fig. 2). The second most frequent location observed was the right paramedian sector with 2 nodules.
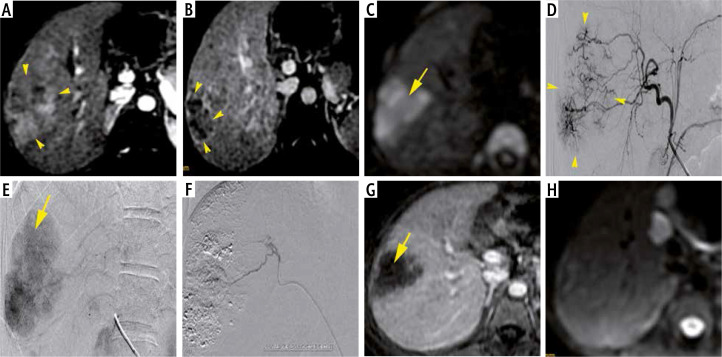
Fig. 2.
A 19-year-old women with Alagille syndrome and raised AFP (282 ng/ml), Child-Pugh class A5, MELD score of 10 and BCLC stage B. Axial MRI T1 FS images demonstrate an ill-defined HCC (yellow arrows) of segment VII with heterogeneous wash-in at the arterial phase (A), wash-out on portal venous phase (B) with restrictive diffusion (C). The HCC size is about 50 mm. Hyperselective TACE illustrates tumoral blush (arrow-heads) (D, E) which completely disappeared after chemoembolization (arrow-heads) (F). MRI control performed 1 year later shows no enhancement on late arterial phase (G) and no hypersignal on diffusion image (H)
Size
The size of different tumors ranged from 17 to 145 mm, with an average of 81 mm. 10 (54%) among the 14 nodules had a size < 50 mm and 16 (92%) had a size ≤ 100 mm, whereas 1 (8%) nodule had a size larger than 100 mm.
Hypervascularization and capsule
All tumors were hypervascularized in the arterial phase, 2 (15%) of which had a capsule on delayed enhancement (Figs. 3 and 4).
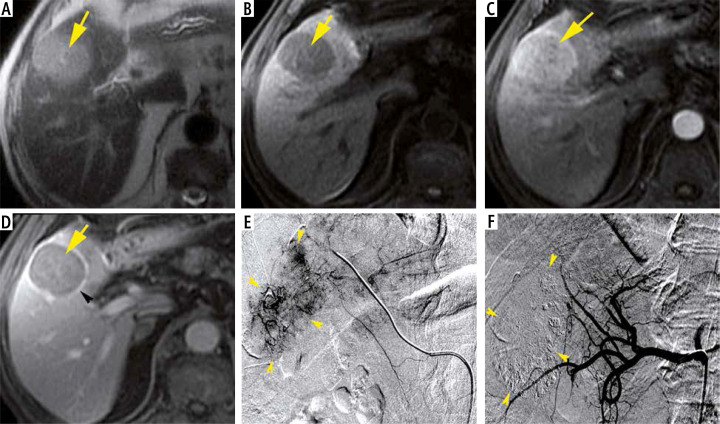
Fig. 3.
An 80-year-old man with chronic hepatitis C and raised AFP (845 ng/ml), Child-Pugh class A6, MELD score of 9 and BCLC stage C. Axial MRI T1 FS images demonstrate a rounded HCC (yellow arrows) of segment V, showing hyposignal on the non-enhanced image (A), with homogeneous wash-in at arterial phase compared to adjacent normal liver (B) and wash-out on portal venous phase (C). Note the enhancing capsule (arrow-head) in portal venous phase (C). The HCC size is about 38 mm. DSA images demonstrate tumoral blush (arrow-heads) (D, E). Note the total extinction of tumoral blush after Lipiodol-doxorubicin injection (arrow-heads) (F)
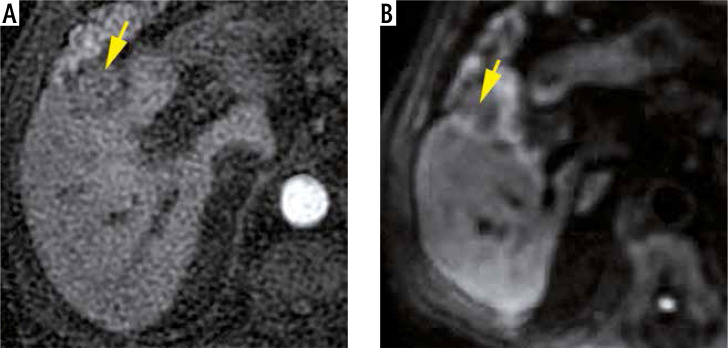
Fig. 4.
The same last patient: Axial MRI images performed 18 months later show the retraction of the embolized HCC (yellow arrows) located in segment V and measuring about 25 mm vs. 38 mm, with no enhancement on the arterial phase (A) or diffusion restriction (B)
BCLC stage
There was no invasion of the main hepatic vessels and their immediate branches. One (7%) patient had an early stage of HCC (A stage), 5 (36%) patients had an intermediate stage (B stage), and 8 (57%) patients with an advanced stage (C stage).
TACE features
All of the 14 patients received one or more TACE: 7 (50%) patients received 1 session, 5 (36%) patients benefited from 2 sessions, 3 sessions were performed for 1 (7%) patient, while 1 (7%) patient received 4 sessions (with the largest 145 mm HCC). After every TACE, complete blush extinction was observed (Figs. 1-4).
Response evaluation
The complete response was considered as the disappearance of any arterial enhancement on control imaging. In cases with a complete response, follow-up duration was considered from the first to the last imaging control showing no viable tumor and eventually nodule retraction.
In cases with a complete response, follow-up duration with CR ranged from 197 to 694 days with an average of 396 days (Figs. 1-4).
Discussion
TACE in one of the most frequently used treatment options for HCC worldwide, especially when the HCC is at an early or intermediate stage. Curative therapies such as hepatic resection, RFA or liver transplantation are possible in only 20% to 40% of cases. In fact, and due to the particular circumstances of our working environment, liver transplantation is not available. Hence, eligible patients are referred for either surgical resection or percutaneous destruction. However, both these treatments face frequent contraindications and technical difficulties. As a result, TACE remains an interesting alternative option for those patients. It has proved beneficial to patients with unresectable HCC in a number of non-randomized case-control studies [12, 13].
General features
Demographic features, PS and etiology
In our institution, 283 TACE was performed for 162 patients with 225 nodules; 14 (9%) of them showed CR and 148 (91%) did not. None of the demographic parameters (age and sex) modified the probability of CR according to the study conducted by Vesselle et al. [10]. In our cases, age was not related to CR whereas male gender was (p = 0.023). PS in this study was not correlated with CR, but etiology was: VHC was most prevalent in the CR group (65% vs. 47%), while VHB was less prevalent in the CR group: 14% vs. 21% in the group without CR (p = 0.0001).
AFP
Studies showed that high AFP serum (> 20 ng/ml) level was associated with recurrence after CR [14]. AFP serum level after locoregional therapy can predict tumor response and survival [15], but 10% to 30% of patients are negative for AFP expression [16, 17].
In this study, 6 (43%) patients had a serum AFP < 20 ng/ml and 9 (64%) had rates < 400 ng/ml. As for the control group without CR, 76% had AFP rates lower than 400 ng/ml. We deduce that an AFP rate < 400 ng/ml is not correlated with CR in this study (p = 0.35).
Hepatic function
Child-Pugh class A was identified in 11 (79%) cases while class B was identified in 3 (21%) patients. Comparison with the control group (61% with class A) shows a correlation between Child-Pugh class A and CR (p = 0.001). MELD score of 10 to 19 is correlated with CR (p = 0.001).
Imaging features
Nodularity
Single nodularity can be treated effectively by occluding tumor feeding arteries. The tumor recurrence is higher in multinodular HCC. Thus, closer follow-up should be considered in patients with multinodular HCC even if CT or MRI shows remission after TACE [18].
In this study, all patients with CR had 1 or 2 nodules vs. 91% of patients without CR. Nodularity is not significantly correlated with CR (p = 0.96).
Location
Vesselle et al. showed that tumor location wholly or partially in the 4th and 1st Couinaud’s segments was a significant pejorative factor, whereas segments VI and VII locations are correlated with CR [10]. Those results were also observed by Kwan et al. [18]. Bryant et al. proposed another parameter, which is the distance to the portal bifurcation, and they even demonstrated that this location is associated with a lower response rate [19].
Our findings confirmed those of the literature; segments VI and VII are strongly associated with CR: 47% vs. 15% in the group control (p = 0.00003).
Size
Tumor size is commonly described as the factor most strongly correlated with CR. Ebied et al. reported response rates of 70% of HCCs < 3 cm, 56% of HCCs of 3 to 5 cm, and in 43% of HCCs > 10 cm [11]. Golfieri et al. reported that a diameter ≤ 5 cm was the best size factor associated with CR whereas HCCs > 5 cm showed only 25% of CR rates [20]. In another study conducted by Jeong et al. the CR rate was 87.7% if the size was ≤ 5 cm and 12.3% if the size was > 5 cm [14].
Tumor size in our cases was ≤ 50 mm in 10 nodules (54%), and ≤ 100 mm in 16 nodules (92%) whereas 1 nodule (8%) had a size larger than 100 mm. Comparison with patients without CR showed no correlation between size and CR (p = 0.9).
Hypervascularization is reported as a predictive factor for CR but our study showed no significant impact on response outcome.
TACE features
Complete blush extinction on DSA is linked to CR according to the findings of Vesselle et al. [10] and Loffroy et al. [15]. This finding is confirmed here, where all patients with CR showed total extinction of tumoral blush on DSA vs. 73% of patients without CR (p = 0.00004).
Conclusions
In this study, CR of HCC treated by TACE is strongly correlated with male sex, etiology (VHC), location (segments VI and VII) and complete blush extinction on DSA. No significant correlation was found, particularly that of size tumor and segment IV as a pejorative location. Child-Pugh score A, MELD score ≤ 19, BCLC B and C stage were also correlated with CR in this study. These criteria should be used to select, more accurately, patients who could benefit from TACE and identify those for whom ablative treatment, radioembolization, or a combined approach may be considered.
Disclosure
The authors declare no conflict of interest.
References
- 1.Kim JU, Shariff MIF, Crossey MME, et al. Hepatocellular carcinoma: Review of disease and tumor biomarkers. World J Hepatol. 2016;8:471–484. doi: 10.4254/wjh.v8.i10.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Société Nationale Française de Gastro-Entérologie Carcinome hépatocellulaire (cancer primitif du foie) (Last updated: 19.03.2019) Available from: https://www.snfge.org/content/7-carcinome-hepatocellulaire-cancer-primitif-du-foie.
- 3.Park W, Chung YH, Kim JA, et al. Recurrences of hepatocellular carcinoma following complete remission by transarterial chemoembolization or radiofrequency therapy: Focused on the recurrence patterns: HCC recurrence following CR by TACE or RFA. Hepatol Res. 2013;43:13041312. doi: 10.1111/hepr.12083. [DOI] [PubMed] [Google Scholar]
- 4.Sugioka A, Tsuzuki T, Kanai T. Postresection prognosis of patients with hepatocellular carcinoma. Surgery. 1993;113:612–618. [PubMed] [Google Scholar]
- 5.El Serag HB, Marrero JA, Rudolph L, et al. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology. 2008;134:17521763. doi: 10.1053/j.gastro.2008.02.090. [DOI] [PubMed] [Google Scholar]
- 6.Yu SJ. A concise review of updated guidelines regarding the management of hepatocellular carcinoma around the world: 2010-2016. Clin Mol Hepatol. 2016;22:717. doi: 10.3350/cmh.2016.22.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kohles N, Nagel D, Jüngst D, et al. Prognostic relevance of oncological serum biomarkers in liver cancer patients undergoing transarterial chemoembolization therapy. Tumor Biol. 2012;33:3340. doi: 10.1007/s13277-011-0237-7. [DOI] [PubMed] [Google Scholar]
- 8.Takayasu K, Arii S, Ikai I, et al. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology. 2006;131:461469. doi: 10.1053/j.gastro.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 9.Hsu KF, Chu CH, Chan DC, et al. Superselective transarterial chemoembolization vs hepatic resection for resectable early-stage hepatocellular carcinoma in patients with Child-Pugh class a liver function. Eur J Radiol. 2012;81:466–471. doi: 10.1016/j.ejrad.2010.12.058. [DOI] [PubMed] [Google Scholar]
- 10.Vesselle G, Quirier LC, Velasco S, et al. Predictive factors for complete response of chemoembolization with drug-eluting beads (DEB-TACE) for hepatocellular carcinoma. Eur Radiol. 2016;26:1640–1648. doi: 10.1007/s00330-015-3982-y. [DOI] [PubMed] [Google Scholar]
- 11.Ebied MO, Federle PM, Carr BI, et al. Evaluation of responses to chemoembolization in patients with unresectable hepatocellular carcinoma. Cancer. 2003;97:10421050. doi: 10.1002/cncr.11111. [DOI] [PubMed] [Google Scholar]
- 12.Greten T, Papendorf F, Bleck J, et al. Survival rate in patients with hepatocellular carcinoma: a retrospective analysis of 389 patients. Br J Cancer. 2005;92:1862–1868. doi: 10.1038/sj.bjc.6602590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El Khaddari S, Gaudin JL, Abidi H, et al. Chemoembolization in hepatocellular carcinoma: multivariate analysis of survival prognostic factors after the first session. Gastroenterol Clin Biol. 2002;26:728–734. [PubMed] [Google Scholar]
- 14.Jeong SO, Kim EB, Jeong SW, et al. Predictive factors for complete response and recurrence after transarterial chemoembolization in hepatocellular carcinoma. Gut Liver. 2017;11:409–416. doi: 10.5009/gnl16001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loffroy R, Lin M, Yenokyan G, et al. Intraprocedural C-arm dual-phase cone-beam CT: can it be used to predict short-term response to TACE with drug-eluting beads in patients with hepatocellular carcinoma? Radiology. 2013;266:636–648. doi: 10.1148/radiol.12112316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evdokimova VN, Liu Y, Potter DM, et al. AFP-specific CD4+ helper T-cell responses in healthy donors and HCC patients. J Immunother. 2007;30:425437. doi: 10.1097/CJI.0b013e31802fd8e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riaz A, Ryu RK, Kulik LM, et al. Alpha-fetoprotein response after locoregional therapy for hepatocellular carcinoma: oncologic marker of radiologic response, progression, and survival. J Clin Oncol. 2009;27:5734–5742. doi: 10.1200/JCO.2009.23.1282. [DOI] [PubMed] [Google Scholar]
- 18.Kwan SW, Fidelman N, Ma E, et al. Imaging predictors of the response to transarterial chemoembolization in patients with hepatocellular carcinoma: A radiological-pathological correlation. Liver Transpl. 2012;18:727736. doi: 10.1002/lt.23413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bryant KM, Dorn PD, Zarzour J, et al. Computed tomography predictors of hepatocellular carcinoma tumour necrosis after chemoembolization. HPB. 2014;16:327335. doi: 10.1111/hpb.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golfieri R, Renzulli M, Mosconi C, et al. Hepatocellular carcinoma responding to superselective transarterial chemoembolization: an issue of nodule dimension. J Vasc Interv Radiol. 2013;24:509–517. doi: 10.1016/j.jvir.2012.12.013. [DOI] [PubMed] [Google Scholar]