Abstract
The latest pandemic, coronavirus disease-2019 (COVID-19), is associated with high prevalence and easy transmission, which is expanding globally with no conventional treatment or vaccine. The new virus revealed 79% and 50% genomic similarities with severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV), respectively. Accordingly, since the disease resists testing and adopting new therapeutics, repositioning pre-existing drugs may present a fast and attractive strategy with known safety, characteristics, and dosage used. However, they are not specific and targeted. Therefore, several drugs have been investigated for their efficacy and safety in the treatment of COVID-19; most of them are undergoing clinical trials. This article summarizes clinical investigations of potential therapeutic drugs used as COVID-19 therapy. Subsequently, it prepares a pattern of results and therapeutic targets to help further experiment designs. We have investigated drugs as classified in the following three groups; 1) The drugs which computationally showed effectiveness (in silico) but needed further lab confirmations; 2) Emetine, Teicoplanin, and Nelfinavir have shown effectiveness in vitro; 3) The drugs currently under clinical trial.
Keywords: COVID-19, SARS-CoV-2, Drug repositioning, Outbreak, 2019-nCoV, Review
1. Introduction
In late December 2019, novel pneumonia began in Wuhan, China, and has since spread worldwide, being established as the global pandemic health emergency. The causative pathogen has been introduced as the 2019 novel coronavirus (2019-nCoV) described by the International Committee on Taxonomy of Viruses as the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). On 11 February 2020, the disease was announced coronavirus disease-2019 (COVID-2019) by the World Health Organization (WHO) (Hassan et al., 2020; Lai et al., 2020).
SARS-CoV-2 is a member of an enveloped single-strand RNA virus family named coronaviruses, which belong to the Coronaviridae family of the order Nidovirales. There are four genera in this subfamily: Alpha, Beta, Delta, and Gamma-Coronaviruses (CoVs) that cause mild to severe lower respiratory tract disease (Ko et al., 2020). Since the beginning of the 21st century, outbreaks of fatal human pneumonia have occurred via CoVs, such as severe acute respiratory syndrome coronavirus (SARS-CoV) and the Middle East Respiratory Syndrome Coronavirus (MERS-CoV). SARS-CoV spread to five countries in 2003 after its emergence in Guangdong province of China, infecting 8098 people and leaving 774 deaths. MERS-CoV appeared in Arabian Peninsula in 2012, was isolated in 27 countries, and infected 2494 people with 858 related deaths. The fatality rate was 10% and 35% for SARS-CoV and MERS-CoV, respectively (Wu et al., 2020a), belonging to the genus Beta-CoV. Of note, sequencing studies have identified SARS-CoV-2 as a new member of Beta-CoVs with 80% similarities, which have the most extended genome, containing 27 to 37 thousand bases among RNA viruses (Payne, 2017).
Coronaviruses generally have four structural proteins: Spike (S), envelope (E), membrane (M), and nucleocapsid (N) (Wu et al., 2020a). Notably, the virus can enter the host cell through the S protein. It is cleaved by the host protease into two functional subunits, S1 and S2, which are in charge of the host cell binding and the viral-cellular membrane fusion, respectively. Different CoVs recognize a variety of proteases and entry receptors, where SARS-CoV and SARS-CoV-2 process their S protein via employing the cellular serine protease TMPRSS2 and subsequent interaction with angiotensin-converting enzyme two cellular (ACE2) receptors (Hoffmann et al., 2020a; Walls et al., 2020; Wang et al., 2020c). These viral components can be targeted as potential sites for drug therapy against COVID-19 (Fig. 1 ). However, the pathophysiology for SARS-CoV-2 has not been well spotted, but similar to SARS-CoV, viral replication leads to aggressive inflammation and causes acute lung injury. After that, the leading cause of SARS-CoV-2 fatality is uncontrolled pulmonary inflammation (Fu et al., 2020). Remarkably, COVID-19 is clinically manifested as fever accompanied by chills or headache, fatigue, myalgia, shortness of breath, and dry coughing. Gastrointestinal disorders and lymphopenia have also been reported in severe cases. Besides, lung imaging shows viral lung involvement even in asymptomatic individuals (Huang et al., 2020a; Liu et al., 2020b).
Fig. 1.
The infection cycle of SARS-COV-2 and the effecting pints of potential drugs against COVID-19.1) SARS-COV-2 binds to the cell surface through ACE-II and TMPRSS2 cell membrane proteins (Entrance blocking drugs). 2) Virus enters to the cells through receptor mediated endocytosis and formation of endosomes, 3) Fusion of virus to endosomes leads to the genome releasing to the cytoplasm (Endosome fusion blocking agents), 4) Replication of the genome (Replication inhibitors), 5) Transcription of viral genes, 6) Translation of virus proteins (Virus proteins inhibitors), 7) Packaging the virus particles and, 8) releasing the newly formed virus particles to the extracellular environment. Cytokine storm and inflammatory mediators in the infection site leads to tissue damage (Inhibitors of inflammatory mediators). Clot formation, as a secondary effect of COVID-19, is inhibited by heparins. Bradykinin receptor repressor inhibits plasma leakage to the lung tissue. BK: Bradykinin, ACE-II angiotensin converting enzyme 2, IFN: Interferon, IL-6: Interleukin 6, IL-1, Interleukin 1, TNFa: Tumor necrosis factor α, EGFR: Epidermal growth factor receptor, TMPRSS2: Transmembrane serine protease 2.
There has been a wide prevalence of this infectious disease, with 15,961,099 cases and 643,118 related deaths as of 25 July 2020, and there is still no specific medication for it. In this regard, the most convenient way to develop medicines is to use pre-existing and marketed drugs whose mechanisms, characteristics, potential efficacy, cytotoxicity, and dosages have been approved. However, being too broad-spectrum, these therapies cannot kill CoVs specifically (Ko et al., 2020; Wu et al., 2020a).
In this review, we studied the clinical trials published on the treatment of COVID-19 and summarized recent clinical experiences and treatment outcomes to provide a view of potentially useful drugs.
2. Antiviral drugs
2.1. Darunavir
Darunavir is a nonpeptidyl HIV-1 protease inhibitor with a bimodal mechanism of action including, inhibition of HIV protease dimerization and protease enzymatic activity. It selectively inhibits Gag-Pol polyprotein cleavage leading to immature and non-infectious viral particles (Spagnuolo et al., 2018; Aok et al., 2018). One of the best targets for SARS-COV-2 is its main protease, so its inhibition may block the virus. Several in silico studies have introduced Darunavir with a high score for binding to SARS-COV-2 protease, which may be useful in the battle with the COVID-19 disease after further testing (Beck et al., 2020; Khan et al., 2020; Pant et al., 2020).
Although Darunavir was thought to be an effective candidate, De Meyer et al. showed this drug does not have an antiviral effect for treatment of covid-19. (De Meyer et al., 2020).
Reportedly, Darunavir's co-administration with other antivirals has had positive effects on SARS-CoV-2 patients (Costanzo et al., 2020; Spezzani et al., 2020). Darunavir has also been used for a married couple in which the wife was partially immunocompromised because of starting chemotherapy. They received 200 mg Darunavir/Cobicistat and Hydroxychloroquine along with antiviral therapy, twice daily. Both patients were recovered (Spezzani et al., 2020). There is a report of HIV positive patients who received Darunavir-based antiretroviral treatment (800 mg), which were also admitted as SARS-CoV-2 positive. This study suggests that despite Darunavir's potential effectiveness, it did not protect people living with HIV from SARS-CoV-2 infection, at least 800 mg, the currently given dosage (Riva et al., 2020).
2.2. Oseltamivir
Oseltamivir is an antiviral drug that inhibits Neuraminidase. It blocks the activity of various types of influenza A and B viruses. Neuraminidase enzyme, expressed on the viral surface, plays an essential role in viral entry to host cells, viral release from infected cells, and further spread in the body. Oseltamivir, as the Neuraminidase inhibitor, prevents the release of virions, keeps them attached to the membrane of previously infected cells and subsequently hinders their expansion in the body (Gubareva et al., 2000; Ward et al., 2005). A report from Rajavithi Hospital in Bangkok, prescribing Oseltamivir, in combination with Lopinavir/Ritonavir, alleviated several patients' symptoms (Offord, 2020). Subsequently, a group has explored the synergistic effects of these three drugs in silico, and they suggested that their combination is highly effective against SARS-CoV-2 protease (Muralidharan et al., 2020). However, case report studies using 75 mg twice a day have suggested that Oseltamivir is ineffective against COVID-19, possibly because the SARS-COV-2 virus lacks Neuraminidase (Li et al., 2020b; Rosa and Santos, 2020; Wang et al., 2020a). Recently, through in-silico and in-vitro study and clinical case analysis, some researchers demonstrated that Oseltamivir is not useful for patients suffering from covid-19 (Qi et al., 2020).
2.3. Umifenovir
Umifenovir is a broad-spectrum antiviral compound, licensed as an anti-influenza drug. This antiviral agent was developed at the Russian Research Chemical and Pharmaceutical Institute about 25 years ago to treat influenza A and B in Russia and China. It is also patented for its medicinal use against SARS-CoV since 2004 (Blaising et al., 2014). Viral glycoproteins, responsible for fusion and cellular recognition, are affected by Umifenovir, which interacts with their aromatic residues. Subsequently, Umifenovir interferes with clathrin-mediated exocytosis through the plasma membrane interaction or directly intercalate into membrane lipids, specifically through hemagglutinin inhibition (HA) (Polyak et al., 2019; Kadam et al., 2017; Blaising et al., 2013).
It was observed that Umifenovir binds directly to influenza HA and increases its stability blocking its transition to the functional form. As a result, the viral-host fusion is inhibited, and the host cell entry is blocked (Haviernik et al., 2018; Hulseberg et al., 2019). Meanwhile, it has an immune-stimulating effect with interferons' induction, enhancement of phagocytosis, and activation of natural killer cells. Fortunately, reports have revealed a favorable safety profile (Li et al., 2020c). Due to such broad antiviral activities, Umifenovir has been proposed as a potential treatment for COVID-19. In a clinical study on 50 cases at ELACOI hospital, Umifenovir monotherapy at the dose of 100 mg did not significantly improve patients compared to control (Li et al., 2020c). In another study in non- Intensive Care Unit (ICU) patients who received Umifenovir, compared to control, no improvement in outcome was observed (Lian et al., 2020). The other group compared the Favipiravir and Umifenovir on 120 cases in each group. The clinical recovery rate was 51.67% and 61.21% for Umifenovir and Favipiravir group, respectively (Chen et al., 2020a).
2.4. Favipiravir
Favipiravir, an anti-RNA virus drug, has been introduced in Japan for novel or re-emerging influenza viruses in 2014. It undergoes ribosylation and phosphorylation intracellularly to become activated and incorporates into the virus RNA through substitution with purine nucleosides. Subsequently, the RNA dependent RNA polymerase (RdRp) of viruses will be inhibited and prevented RNA strand elongation and viral proliferation. In addition to influenza, Favipiravir works against a broad range of RNA viruses, including Rhinovirus, Arenavirus, Bunyavirus, Flavivirus, and filoviruses, causing hemorrhagic fever as well as the Ebola virus. Noticeably, the RNA structure of SARS-CoV-2 and its similarities to SARS-CoV makes Favipiravir a potential candidate for COVID-19 treatment (Du and Chen, 2020; Shiraki and Daikoku, 2020).
Consequently, a clinical trial was conducted in Shenzhen, with 80 patients recruited. The results demonstrated that viral clearance time became significantly shorter, and chest X-ray imaging improved at a higher rate in the Favipiravir group (91.43% and 62%, respectively) (Cai et al., 2020). Another study on 120 patients confirmed Favipiravir's efficacy with a 7-day clinical recovery rate of 71.43% and reduction of fever and cough (Chen et al., 2020a). Based on a systematic review and meta-analysis by Shrestha et al., the use of Favipiravir improves clinical symptoms (Shrestha et al., 2020). Another systematic review study demonstrated that Favipiravir has faster viral clearance than Lopinavir/Ritonavir and Umifenovir (Siordia et al., 2020).
2.5. Remdesivir
Remdesivir is a small-molecule monophosphoramidate prodrug. It is an adenosine analog that blocks the RNA-dependent RNA-polymerase through its nucleoside component. It works after the virus entry into the host cell. After entering the cells, Remdesivir is cleaved to the nucleoside monophosphate analog and subsequently goes into further phosphorylation to yield its active triphosphate form (RDV-TP), which resembles Adenosine triphosphate (ATP). Competing with ATP, RDV-TP incorporates by the RdRp and viral RNA chain complex leading to pre-mature termination of viral RNA transcription and subsequent RNA synthesis inhibition (Khanal et al., 2020; Saha et al., 2020).
Gilead Science synthesized this medication in 2017 for Ebola virus infection treatment, and further studies considered it a right candidate against coronaviruses. In vitro and in vivo experiments on SARS-CoV and MERS-CoV showed that Remdesivir could inhibit the viruses’ replication and reduces viral lung load and SARS-CoV-induced lung pathology such as denuding bronchiolitis (Al-Tawfiq et al., 2020; Ko et al., 2020). Therefore, on the outbreak of SARS-CoV-2, a study using molecular dynamic simulation examined the inhibitory effect of Remdesivir on RNA-dependent RNA polymerase of SARS-CoV-2. They reported that the relative free energy of Remdesivir is −8.28 ± 0.65 kcal/mol, which is too strong compared to the natural substrate ATP (−4.14 ± 0.89 kcal/mol) (Zhang and Zhou, 2020). Thus, further experiments were designed on Vero E6 cells (a lineage of kidney epithelial) for in vitro examinations. One study working on Remdesivir and Chloroquine suggested the half-maximal effective concentration (EC50) and the half-cytotoxic concentration (CC50) values of Remdesivir as 0.77 μM and 100 μM, respectively, confirming its inhibition activity (Wang et al., 2020b). Another experiment carried out on the same cell line estimated its EC50 at 23.15 μM (Choy et al., 2020). Specifically, a human study was designed on patients hospitalized with confirmed COVID-19. Fifty-three patients, including the study, received 200 mg of Remdesivir intravenously on day 1, followed by 100 mg daily for nine days. A total of 47% of patients were discharged, 68% improved significantly, and 13% died. The most common adverse events were diarrhea, rash, renal impairment, hypotension, and increased hepatic enzymes (Grein et al., 2020). There is a report on a COVID-19 patient that is declaring the delayed effectiveness of Remdesivir. The drug was administered 13 days after the onset of symptoms. Sixty hours after initiating Remdesivir, the patient was extubated and continued the recovery into a stable condition (Hillaker et al., 2020).
Moreover, a double-blind trial was carried out on 453 patients in the two groups of Remdesivir and placebo, designed for 28 days. The loading dose of Remdisivir is considered 200 mg in 350 ml normal saline infused intravenously over approximately 30–60 min for the first day and 100 mg in 250 ml normal saline for nine days. Serious considerations have been assumed for this study (Cao et al., 2020a). However, in a clinical trial in China, the use of Remdesivir for severe covid-19 patients did not show remarkable advantages (Wang et al., 2020).
On May 1, 2020, the FDA provided an Emergency Use Authorization (EUA) for Remdesivir as the treatment of hospitalized COVID-19 patients (Rhoades, 2020). Recently it was noted that for the mild or moderate covid-19 patients, Remdesivir is not recommended. Still, for the ones who require respiratory support, the use of Remdesivir decreases the improvement time and reduces the hazard of progression (Young et al., 2020).
Remdesivir is the only medication that has been approved for COVID-19 infection by the U.S. Food and Drug Administration so far.
2.6. Ribavirin
As a guanosine analog, Ribavirin prevents RNA and DNA virus's replication and inhibits RNA capping, leading to RNA degradation. It also inhibits inosine monophosphate dehydrogenase, which results in the prevention of natural guanosine generation. In general, Ribavirin acts in several mechanisms of action. Ribavirin triphosphate, one of its predominant metabolites, binds to the nucleotide-binding site of mRNA polymerase enzyme instead of correct nucleotide, resulting in defective production virions and viral replication reduction. It can also incorporate at the 5՛-end of viral mRNA and disrupt the posttranslational capping. The next target of Ribavirin is inosine monophosphate dehydrogenase (IMPDH), which provides the intracellular guanosine triphosphate (GTP). Ribavirin mimics the endogenous substrate of IMPDH, inosine-5-monophosphate, occupying the substrate-binding site leading to the depletion of GTP pools and subsequent viral limitation genome replication. Furthermore, it acts as a mutagen in some viruses, causing an error catastrophe. In this regard, Ribavirin triphosphate is substituted for GTP and pairs with cytidine triphosphate or uridine triphosphate through which can block the RNA elongation and produce defective virions (Khalili et al., 2020; Nyström et al., 2019; Patterson et al., 1990; Song et al., 2020; Te et al., 2007).
Ribavirin has been active as an antiviral agent against the respiratory syncytial virus (RSV), SARS-CoV, and MERS-CoV. Now in the outbreak of COVID-19, virtual analysis has suggested it as a potential treatment (Elfiky, 2020). In an in vitro study on Vero E6 cells infected by COVID-19, Ribavirin has confirmed antiviral activity. They reported its EC50 as 109.50 μM and CC50 > 400 μM (Wang et al., 2020b). Although no definitive clinical study has yet registered the benefits of Ribavirin on COVID-19, there is a study that has worked on 3 Chines hospital, where Ribavirin was prescribed intravenously for 3–12 days along with other drugs. There was no death among the 80 cases, 21 patients (23.75%) had an average length of hospital stay of 8 days, and others were still in hospital after 12 days (Wu et al., 2020b). Also, a retrospective cohort study reported that Ribavirin could not decrease the mortality rate; 16/1% of patients with severe coronavirus disease treated by Ribavirin died compared to 24.6% in the control group (Tong et al., 2020). However, most of the previous studies on SARS CoV and MERS CoV suggest Ribavirin as an add-on therapy, which is also indicated by China's NHC for COVID-19 in combination with Lopinavir/Ritonavir or interferon, reportedly (Song et al., 2020).
2.7. Nafamostat and Camostat
Nafamostat mesylate and Camostat mesylate belong to synthetic serine protease inhibitors; Nafamostat mesylate, also named FUT-175 and 6′-amidino-2-naphthyl-4 -guanidinobenzoate dihydrochloride. At the first phase of infection with SARS-CoV-2 Virus, Nafamostat can inhibit the S-mediated membrane fusion. This drug is used for acute pancreatitis, and intracellular coagulation has been revealed to be useful in the first phase of infection with the SARS-CoV-2 virus. Nafamostat prevents cell entry of virus by inhibiting enzyme transmembrane protease serine 2 (TMPRSS2), blocking the S-protein mediated membrane fusion. These inhibitors form a close interaction to Asp435 in the S1 pocket and close contact with catalytic serine in TMPRSS2 and produce a reactive complex, resulting in enzyme hampering (Hempel et al., 2020; Hoffmann et al., 2020b).
A German group introduced Nafamostat as an inhibitor of SARS-CoV-2 infection. They stated that Nafmostat is more effective than Camostat to prevent membrane fusion and host cell entry (Hoffmann et al., 2020b). In a comparative analysis, covid-19 antiviral drugs' efficacy was determined in human lung cells and revealed Nafamostat is the most potent drug for blocking virus entry (Ko et al., 2020). Moreover, a case report on three old patients with COVID-19 pneumonia showed that Nafamostat's use could improve clinical symptoms. Disseminated intravascular coagulation (DIC) dose (0.06–0.2 mg/kg/hour) was used for these patients (Jang and Rhee, 2020). However, harmful incidents such as bleeding should be considered after using this drug (Hifumi et al., 2020).
2.8. Lopinavir/Ritonavir
Lopinavir is a protease inhibitor approved against the human immunodeficiency virus 1 (HIV-1), which is usually administered in combination with Ritonavir. Ritonavir, a cytochrome P450 3A inhibitor, increases the plasma half-life of Lopinavir. These protease inhibitors mimic the normal peptide linkage and bind to the substrate-binding pockets of viral enzymes such as papain-like cysteine proteinase (PLpro) and 3C-like proteinase (3CLpro). By inhibiting the enzyme activity, drugs prevent the proteolysis of Gag polyprotein precursors, which causes the formation of immature, non-infectious viral particles (Uzunova et al., 2020).
Previous in vitro studies and trials on coronaviruses (SARS and MERS) have reported that Lopinavir/Ritonavir conferred clinical benefits, either in single administrations or combined with other agents as ribavirin, corticosteroids, etc. (Li et al., 2020c; Song et al., 2020; Yao et al., 2020a). They could lower the viral load, mortality rates, and adverse respiratory distress syndrome (ARDS). Of note, the in vitro EC50 of Lopinavir ranged from 4.0 to 10.7 μg/ml for SARS-CoV to 5.0–7.0 μg/ml for MERS-CoV (Cao et al., 2020b). There is a report on a patient in Korea prescribed to use Lopinavir/Ritonavir (200 mg/50 mg) on day 10 of the contraction. They observed a decrease in the viral load since then (Lim et al., 2020). besides, a group of researchers conducted a trial on 199 COVID-19 patients in two groups; 1- Lopinavir/Ritonavir along with standard care and 2- standard-care alone (Comprising supplemental oxygen, ventilation, antibiotic therapy, and vasopressor support, if necessary). Patients received 400 mg Lopinavir and 100 mg Ritonavir two times a day for up to 14 days. Approximately 45% and 40% of the patients were detected with positive viral RNA on day 14 and 28, respectively. Indeed, treatment with Lopinavir/Ritonavir had no benefit beyond standard care. Furthermore, the treated group encountered commonly gastrointestinal adverse events, including nausea, vomiting, and diarrhea, although the overall reported adverse events were 48.4% in the Lopinavir/Ritonavir group and 49.5% in the standard care group (Cao et al., 2020b). Another study enrolled 86 patients in 3 groups: i) 34 patients receiving Lopinavir/Ritonavir (200 mg/50 mg) twice daily ii) 35 patients receiving Umifenovir (100 mg) three times daily iii) 17 patients with no antiviral.
Repeatedly, groups didn't acquire significant differences in improvement rates (Li et al., 2020c). Despite the lack of statistically significant difference, the lopinavir-ritonavir group had a numerical decrease in mortality rate, and less stayed in an intensive care unit. Based on these findings, some researchers suggested the earlier usage of Lopinavir/Ritonavir in the course of the disease (Owa et al., 2020).
2.9. Nelfinavir
Nelfinavir is a viral protease inhibitor, approved as an HIV-1 protease inhibitor by the FDA in 1997. HIV protease activity is essential for the cleavage of viral polyproteins leading to subsequent assembly of immature virus proteins into infectious virions. Nelfinavir prevents proteolytic cleavage of viral polyproteins by occupying the enzyme active site— results in the formation of un-developed non-infectious viral particles. Moreover, the main protease or chymotrypsin-like protease of COVID-19 has been suggested as a potential drug target (Kaldor et al., 1997; Xu et al., 2020b; Yamamoto et al., 2004). In silico studies modeling the main protease of SARS-CoV-2 have introduced Nelfinavir as the best candidate with the most binding free energy (Xu et al., 2020b).
Furthermore, studies on SARS-CoV have revealed that Nelfinavir has strongly inhibited SARS-CoV replication, lowering viral antigens significantly. Nelfinavir is also very safe, with mild diarrhea as a side effect in 15–20% of patients (Yamamoto et al., 2004). Therefore, an in vitro study evaluated its efficacy on SARS-CoV-2. The findings revealed that Nelfinavir could effectively inhibit SARS-CoC-2 replication in vitro. This study suggested Nelfinavir concentration of 1.13 μM and 1.76 μM as the sufficient concentrations for 50% and 90% inhibition (EC50 and EC90), respectively (Yamamoto et al., 2020). The results suggest that Nelfinavair can be clinically assessed. Researchers showed that Nelfinavir is a potent inhibitor of cell membrane fusion resulting from covid-19 spike glycoprotein based on an in-vitro study. So they suggested subsequent and more evaluation of the potential of Nelfinavir to prevent virus spread when the first symptoms of SARS-CoV-2 appear in patients (Musarrat et al., 2020).
3. Antibacterials
3.1. Teicoplanin
Teicoplanin, a glycopeptide antibiotic, is currently applied as a therapy for gram-positive bacterial infections such as Methicillin-resistant Staphylococcus aureus, septicemia, endocarditis, and lower respiratory tract infections. It has already shown benefits against various viruses, including Ebola, influenza virus, flavivirus, hepatitis C virus, HIV, MERS-CoV, and SARS-CoV (Baron et al., 2020; Brogden and Peters, 1994).
Teicoplanin, a glycopeptide antibiotic, blocks the cathepsin L, located in the late endosome. Cathepsin L mediates the cleavages of viral S protein, which leads to the virus-host cell fusion and viral genome released into the cytoplasm. Therefore, blocking this mechanism would prevent the viral replication cycle (Jean et al., 2020; Zhang et al., 2020b). Reportedly, the cathepsin L cleavage site is conserved among coronaviruses, SARS-CoV, and SARS-CoV-2. In an in vitro study on SARS-CoV-2, Teicoplanin could prevent viruses' entrance into the cytoplasm. They determined its half-maximal inhibitory concentration (IC50) as 1.66 μM (Zhang et al., 2020b). Recently in Italy, a cohort study on 21 COVID-19 patients hospitalized in ICU received Teicoplanin 6 mg/kg every 24 h for 7–12 days. The findings showed a viral clearance rate of 40% and suggested that Teicoplanin might be potentially appropriate for the treatment of SARS-Cov-2 infection (Ceccarelli et al., 2020).
3.2. Azithromycin
Azithromycin, a macrolide antimicrobial agent, acts against a broad range of Gram-positive and Gram-negative bacteria and plays an immunomodulator. Azithromycin targets bacterial 50s ribosomal subunit through binding to its 23s rRNA, leading to the inhibition of its assembly, which results in bacterial protein synthesis blockade. Prevention of the transpeptidation/translocation step of protein synthesis, Azithromycin controls various bacterial infections (Champney et al., 2002; Parnham et al., 2014). It has anti-inflammatory and direct antiviral effects. Previous in vitro reports suggested it combined with Hydroxychloroquine as an active agent against Zika and Ebola viruses. Azithromycin is a common therapy for many chronic lung diseases, including chronic obstructive pulmonary disease (COPD), asthma, interstitial lung diseases, bronchiectasis, and cystic fibrosis (La Scola et al.; Martinez et al., 2008). Thus, in studies, it has been suggested as the right candidate for co-treatment with Hydroxychloroquine for COVID-19. In an in vitro study on Vero E6 cells, the concentration of 1, 2, and 5 μM of Hydroxychloroquine and 2, 5, and 10 μM for Azithromycin were used. They observed viral replication inhibition for 5 μM of Hydroxychloroquine in combination with Azithromycin at 10 and 5 μM (Andreani et al., 2020). Another study, following 80 patients for at least six days, suggested 200 mg of oral Hydroxychloroquine sulfate, three times per day combined with 500 mg of Azithromycin on the first day, then 250 mg daily for the following four days. The viral RNA load was decreased rapidly. All the combinational group participants were virologically cured on day 6. The majority of patients, 81.3%, were favorably discharged from their unit, and only 15% required oxygen therapy (Gautret et al., 2020b). There is also an open-label non-randomized clinical trial conducted on 36 patients. All patients were treated with oral Hydroxychloroquine sulfate 200 mg, three times per day, while six patients received Azithromycin (500 mg on day one followed by 250 mg per day). 100% of patients treated with Hydroxychloroquine and Azithromycin were cured virologically compared with single-drug therapy. In comparison, 57.1% of patients received single Hydroxychloroquine and 12.5% in the control group (Gautret et al., 2020a).
However, there is a preliminary work on QT interval (electrical disturbance of the cardiovascular system, seen on an electrocardiogram) of this combination therapy. They reported the changes in the QT interval of 84 adult patients, of which 11% demonstrated an increased risk of malignant arrhythmia and sudden cardiac death (Chorin et al., 2020). In this regard, they suggest there should be vital considerations to prescribe this compound. Further, in a randomized clinical trial in Brazil, 447 patients with severe covid-19 received Azithromycin (500 mg for ten days) in addition to standard of care, including Hydroxychloroquine or standard of care alone. The findings revealed Azithromycin has no beneficial effect on clinical outcome (Furtado et al., 2020; Oldenburg et al., 2020).
4. Antimalarial agents
4.1. Chloroquine & hydroxychloroquine
Chloroquine and Hydroxychloroquine are antimalarial drugs that inhibit lysosomes' vital functions by increasing pH, which results in blocking endosome-mediated entry (Al-Bari, 2017). They can also interfere with nucleic acid replication, viral protein glycosylation, virus assembly, and release. Notably, Chloroquine has an immune-modulatory activity, which may enhance its antiviral effects synergistically (Ponticelli and Moroni, 2017). However, its utilization is restricted because of its potential overdose, acute poisoning, and death. Hydroxychloroquine is a derivative of Chloroquine, which has been demonstrated to be much less toxic (~40%) (Liu et al., 2020a). It is known for its clinical safety profile. Meanwhile, both effectively treat many inflammatory diseases such as rheumatoid arthritis and lupus by inhibiting cytokine (IL-1 and IL-6) generation, phospholipase A2 and matrix metalloproteinases, and modulating B and T cell function (Singh et al., 2020). However, they can cause some side effects, such as gastrointestinal disorders, headaches, retinopathy, and arrhythmia.
In an in vitro study on SARS-CoV, Chloroquine was introduced as a potent prophylactic and therapeutic treatment that interferes with the glycosylation of viral cellular receptors (Vincent et al., 2005). By the outbreak of SARS-CoV2, a team examining Chloroquine on Vero E6 cells indicated that it could potently block virus infection with an EC90 value of 6.90 μM (Wang et al., 2020b). Besides, another in vitro study was designed to compare the antiviral effects of Hydroxychloroquine and Chloroquine. The 50% cytotoxic concentration (CC50) values were evaluated as 273.20 and 249.50 μM for Chloroquine and Hydroxychloroquine, respectively. The total data suggested that although Hydroxychloroquine can efficiently inhibit SARS-CoV-2, its anti-SARS-CoV-2 activity is less potent than Chloroquine (Liu et al., 2020a). In contrast, Dongyang Liu and his colleagues found Hydroxychloroquine more potent than Chloroquine based on their half-maximal effective concentration (EC50), evaluated as 0.72 μM and 5.47 μM for Hydroxychloroquine and Chloroquine, respectively (Yao et al., 2020b).
An open-label non-randomized clinical trial targeted 36 COVID-19 patients and prescribed 600 mg/day Hydroxychloroquine for six days. Seventy percent of patients were virologically cured compared with 12.5% in the control group on day 6. They reported the mean Hydroxychloroquine serum concentration as 0.46 μg/ml+0.2 (Gautret et al., 2020a).
A combination of Hydroxychloroquine and Azithromycin has also been evaluated. A total of 80 patients with COVID-19 were enrolled and received 200 mg Hydroxychloroquine three times per day for ten days combined with 500 mg Azithromycin on day 1 and 250 mg/day the next four days. The results showed a significant reduction in the nasopharynx viral load (virus-negative rate of 93% on day 8). (Gautret et al., 2020b). Contrary to this finding, In a clinical trial on 504 patients with mild to moderate covid-19, compared to the standard care group, improvement in the clinical condition of the ones who received Hydroxychloroquine (400 mg, twice daily) alone or with Azithromycin (500 mg, once daily) was not observed (Fatima et al., 2020; Cavalcanti et al., 2020). A clinical study by a Chinese team of 62 patients found that taking 400 mg of Hydroxychloroquine for five days significantly reduced the fever recovery time and cough duration compared to standard treatments (Chen et al., 2020d).
However, in a systematic review and meta-analysis, a study on 12 observational and randomized trials revealed that the use of Hydroxychloroquine and Chloroquine does not improve clinical outcomes in patients with covid-19 (Elavarasi et al., 2020). A large randomized clinical trial confirmed Hydroxychloroquine and Chloroquine are not recommended to treat hospitalized patients with COVID-19. These medications didn't associate with mortality rate reduction, nor did these drugs related to recovery rate improvement (Horby et al., 2020a, Horby et al., 2020b). On 15th June 2020, Food and drug administration (FDA) defined that Hydroxychloroquine and Chloroquine were not beneficial for the treatment of covid-19 (https://www.fda.gov/media/138945/download).
5. Immunomodulators
5.1. Anakinra
Anakinra is a 17 kD biological recombinant, non-glycosylated human interleukin-1 receptor antagonist with a short half-life of approximately 3–4 h and an acceptable safety profile to neutralize hyperinflammatory-related to COVID-19 with the severe respiratory syndrome. IL-1 plays a significant role in stimulating the production of inflammatory cytokines and TNFα. Anakinra blocks the action of IL-1, which leads to inhibit the inflammatory responses (King et al., 2020). A cohort study evaluated the effect of Anakinra on the severe respiratory syndrome of COVID-19. Patients received a dose of 100 mg subcutaneously twice daily for 72 h, followed by 100 mg once daily for seven days along with a standard treatment regimen consist of oral agents (10 days course of Hydroxychloroquine 600 mg/day, five days course of Azithromycin 250 mg/day), and intravenous antibiotics (Ceftriaxone 1 g/day or Amoxicillin 3 g/day) for seven days. Prophylaxis of thromboembolism was considered for all cases. Some patients were a candidate for an intravenous bolus 500 mg dose of methylprednisolone. This study determined a notable decrease in the demand for admission to the Intensive Care Unit, invasive mechanical ventilation, and mortality compared with standard of care. More patients experienced elevated liver enzymes in the Anakinra group than the control group (Huet et al., 2020). A previous study exhibited improved respiratory system and reduced serum C-reactive protein in 72% of patients with a high-dose of Anakinra (IV) in severe COVID-19, ARDS, and hyper inflammation (Cavalli et al., 2020). An open-label study recruited nine patients with moderate to severe pneumonia results from COVID-19. Only an old patient exhibited an acute respiratory failure after Anakinra that led to discontinuing the treatment and ICU admission. Other patients revealed good clinical and biological outcomes, in which C reactive protein (CRP) levels reduced at day 6 in all cases and controlled in 5 at day 11. Chest CT scan showed cessation of lesions development. Patients who received Anakinra were alive at the latest follow-up (Aouba et al., 2020).
5.2. Bamlanivimab
Bamlanivimab, known as LY-CoV555 and LY3819253, is a neutralizing IgG1 monoclonal antibody against the receptor-binding domain of SARS-CoV-2 spike protein. So, this monoclonal antibody prevents viral attachment and entry of SARS-CoV-2 to the host cells and viral replication as a result. Within November 2020, the US Food and Drug Administration (FDA) provided authorization for emergency use of Bamlanivimab therapy for mild-to-moderate COVID-19. This unapproved agent is only indicated for use in non-hospitalized adults and pediatrics 12 years of age or older with COVID-19 test positive, weight at least 40 kg (88 pounds), and who have increased risk for disease progressing to severe COVID-19 and/or hospitalization, including ≥65 years of age, underlying chronic medical condition. It has been emphasized on prompt administration of Bamlanivimab within ten days of symptom onset or following a positive test. It should be noticed that this medication has not an authorization of use in patients with COVID-19 related hospital admission or need for oxygen therapy. Bamlanivimab is manufactured as a single dose aqueous solution vials in 700 mg/20 ml for intravenous infusion over 60 min (https://www.fda.gov/media/143603/download; https://www.clinicaltrialsarena.com/projects/bamlanivimab-ly-cov555-for-the-treatment-of-covid-19/).
Emergency use authorization of this investigational monoclonal antibody is based on the results of an interim analysis of an ongoing randomized clinical phase 2 trial involved 452 patients with mild-moderate COVID-19 in outpatient settings. Of 452 patients were a candidate for a single infusion of LY-CoV555, 101 received a dose of 700 mg, 107 received a dose of 2800 mg, 101 received a dose of 7000 mg and 143 received placebo. Viral load significantly decreased with Bamlanivimab at a dose of 2800 mg vs. placebo. Hospitalization, emergency department visits, or death within 28 days of treatment was significantly lower than those in placebo (Chen et al., 2020). Any benefit has not been found in hospital admitted patients (https://www.niaid.nih.gov/news-events/statement-nih-sponsored-activ-3-trial-closes-ly-cov555-sub-study).
5.3. Bevacizumab
As a monoclonal antibody, Bevacizumab acts against Vascular Endothelial Growth Factor (VEGF) and is indicated for cancer therapy (Wang et al., 2004). VEGF is discussed as the most potent vascular penetrance inducers. Bevacizumab binds to VEGF and inhibits the constitution of neovascularization, thereby reduces tumor growth (Garcia et al., 2020). The last evidence has displayed a high level of VEGF in patients with coronavirus disease (COVID-19) compared with healthy controls. Factors such as hypoxia, severe inflammation, and upregulation of the infected respiratory tract epithelium cause increased VEGF levels. Numerous investigations have supported VEGF's fundamental role as a potential clinical goal in acute lung injury (ALI) and acute respiratory distress syndrome (ARDS). So that, Bevacizumab, an anti-VEGF therapy, may suggest a new approach to the treatment of ALI/ARDS due COVID-19.
In this regard, the efficacy and safety of Bevacizumab have been assessed in a single-arm trial. Researchers enrolled 26 patients with severe Covid-19 from China and Italy and followed them for 28 days. Patients received a single 500 mg dose of Bevacizumab as an intravenous infusion over 90 min with a standard of care. Patients experienced considerable increases of PaO2/FiO2 values at day one and day seven after Bevacizumab therapy. More than half of the patients discharged. Oxygen-support status did not worsen in any patients, and there was no mortality report during the follow-up period. Chest computerized tomography (CT) or X-ray findings exhibited a substantial decrease in lesion areas and ratios within Seven days. Fever disappeared in 3 days in patients. Results expressed meaningful increased peripheral lymphocyte counts with a significant reduction in CRP levels. Significant improvement occurred in oxygen support in most of the patients. Elevation of liver function test was the most common adverse event (Pang et al., 2020). Thus, researchers described clinical trials for the evaluation of Bevacizumab on COVID-19.
An open-label clinical trial targeted 27 COVID-19 patients and prescribed Bevacizumab 500 mg + 0.9% sodium chloride for 7 days (https://clinicaltrials.gov/ct2/show/NCT04275414?term=NCT04275414&draw=2&rank=1). Another one is a randomized clinical trial on Bevacizumab's efficacy in Severe Patient with COVID-19 (https://clinicaltrials.gov/ct2/show/NCT04305106).
5.4. Sarilumab
Sarilumab, as a humanized monoclonal antibody, inhibits the interleukin-6 receptor. An open-label study assessed Sarilumab's safety and efficacy in patients with severe COVID-19 pneumonia and hyper inflammation. Twenty-eight patients received an intravenous infusion of Sarilumab at the single dose of 400 mg in addition to standard treatment, and 28 patients, as a control group, treated with standard of care. Based on the local institutional standard of care, all patients received oral lopinavir/ritonavir, hydroxychloroquine, and a course of azithromycin on admission. Supportive care with supplemental oxygen and/or ventilation support with continuous positive airway pressure was provided based on the physician's decision. Sarilumab associated with non-significant clinical improvement and die compared to control at day 28. There was no correlation between IL-6 serum concentration and the other clinical outcome predictors. Time to the normalization of C reactive protein levels was significantly shorter with Sarilumab. Considering adverse events, the infection and pulmonary embolism rate were not different between groups (Della-Torre et al., 2020).
Sanofi and Regeneron conducted a multicenter, double-blind, and phase 3 trial to evaluate the clinical benefit of intravenous Sarilumab in three arms, including 161 patients in 200 mg dose, 173 patients in 400 mg dose, and 86 patients in placebo. Unfortunately, ill patients with severe COVID-19 did not improve outcomes throughout evaluation than placebo added to standard care. 24–29% of treated patients with Sarilumab and 24% of patients who received placebo expressed severe adverse effects. Serious infection occurred in 11–13% of treated cases and 12% of the placebo group (https://clinicaltrials.gov/ct2/show/NCT04327388). An observational clinical study has reported Sarilumab therapy's outcome in 53 patients with SARS-CoV-2 associated with severe pneumonia. All patients received an intravenous infusion of Sarilumab at a dose of 400 mg on day 1and followed for 14 days. Additional administration was at the clinical decision. All patients who were positive for SARS-CoV-2 concomitantly received lopinavir/ritonavir or darunavir/ritonavir; Hydroxychloroquine; Azithromycin, and a prophylactic dose of heparin. Glucocorticoids could be used for patients who were admitted to the ICU. Of 53 cases, 39 were treated in medical wards and 14 in ICU. 7 (17.9%) medical ward admitted patients needed to ICU admission, and 4 of whom were readmitted to the ward within 5–8 days. Most of the medical inpatients showed considerable improvement in medical outcome at 19 days median follow-up, and most of the cases no longer required oxygen support. More than half of the patients were discharged to the ward (Gremese et al., 2020).
5.5. Thalidomide
Thalidomide, an anti-inflammatory and immune-modulating drug, has been used to treat multiple inflammatory diseases, such as Crohn's disease, Behcet's disease, and myeloma (Tu et al., 2020). It induces the degradation of messenger RNA in blood cells and reduces tumor necrosis factor-α (TNFα). The secretion of interleukins (IL), such as IL-12, and activation of natural killer cells can also be increased under the influence of Thalidomide (Rosa and Santos, 2020). Other mechanisms include suppressing angiogenesis, preventing DNA damage caused by free radicals, and altering the cellular adhesive molecules expression (Khalil et al., 2020). Fortunately, its side effects are limited, including drowsiness, dizziness, and rash (Franks et al., 2004). Thalidomide has been considered to reduce inflammation related to COVID-19. Meanwhile, in a case study, a 45-year-old woman positive covid-19 was prescribed Thalidomide at a dose of 100 mg/day and low-dose methylprednisolone (40 mg every 12 h). The patient's clinical conditions as oxygen index, anxiety, nausea, vomiting, improved during three days, and cytokine levels returned to normal after a week (Chen et al., 2020b). There are also two clinical trials working on Thalidomide, with the results still not published. Both are planned to use 100 mg/d for 14 days and are in phase 2 (https://clinicaltrials.gov/ct2/show/NCT04273581?term=NCT04273581&draw=2&rank=1) (https://clinicaltrials.gov/ct2/show/NCT04273529?term=NCT04273529&draw=2&rank=1).
5.6. Tocilizumab
Tocilizumab is an FDA-approved immunosuppressive drug that is effective against Rheumatoid arthritis, systemic juvenile idiopathic arthritis, and chimeric antigen receptor (CAR)-cell-induced cytokine release syndrome (CRS) (Genentech, 2017). It is a monoclonal antibody against the cytokine Interleukin 6 receptor. Tocilizumab is a competitive inhibitor of IL-6-mediated signaling. Interleukin 6 plays a crucial role in inflammation and immune responses, and its overexpression has pathological effects on chronic inflammation and autoimmunity (Tanaka et al., 2014). Notably, headache, dizziness, upper abdominal pain, mouth ulcers, neutropenia, thrombocytopenia, increased liver enzymes, increased total cholesterol, and triglycerides are common complications of Tocilizumab (Zhang et al., 2020a).
Reportedly, in COVID-19, inflammatory cytokines, including interleukin 6, 10, and TNFα increase, leading to cytokine storms and acute symptoms in patients, progressing to cardiovascular collapse, multiple organ dysfunction, and death. Tocilizumab binds to interleukin-6 receptors, interrupts cellular signals transduction pathway, and subsequently decreases inflammatory responses (Zhang et al., 2020a).
In a study on 15 COVID-19 patients, eight were given Tocilizumab combined with prednisolone, and five patients received Tocilizumab alone, twice or more. In all patients, serum interleukin-6 levels decreased significantly after Tocilizumab treatment. Although the CRP rate returned to normal rapidly, it was not significant for four critically-ill patients who took only one dose of Tocilizumab (Luo et al., 2020). However, in a cautionary case report working on two patients, despite a decrease in CRP post-Tociizumab therapy, the disease developed. It should be noted that Tocilizumab may worsen the clinical course of patients by adding to immunosuppression. Elevated Interleukin 6 levels may be a compensatory mechanism for impaired viral responses; therefore, Tocilizumab-induced decrease in Interleukin 6 can promote increased viral replication (Radbel et al., 2020). Another group working on 21 COVID-19 patients prescribed 400 mg (up to 800 mg) Tocilizumab along with routine treatments. Symptoms improved dramatically within a few days. Seventy-five percent of patients needed low oxygen therapy. The rate of lymphocytes returned to normal at 52.6%. Furthermore, interleukin 6 and CRP levels were significantly reduced in 90% of patients, and all cases were discharged on average 15.1 d after Tocilizumab therapy (Xu et al., 2020a). Additionally, Zhang et al. reported the first case of COVID-19 in a Multiple Myeloma, which has been successfully treated after Tocilizumab therapy (8 mg/kg administered IV, one time). Of note, after Tocilizumab administration, a fluctuation in Interleukin 6 levels appeared. It decreased gradually and then increased to the peak, followed by another decrease to a low level, which can be attributed to the recovery of the normal T cells (Zhang et al., 2020c). A 45-year old COVID-19 positive patient with Sickle cell disease was also treated with Tocilizumab and other standard treatments in France. They prescribed 8 mg/kg Tocilizumab; on day three, an improvement of the patient's general condition was observed, and on day five, he was discharged (De Luna et al., 2020). The effect of Tocilizuman on mortality and/or invasive mechanical ventilation in 30 severe COVID-19 patients compared with those in 176 patients who were not treated with Tocilizumab. This retrospective case-control study revealed that treatment with Tocilizumab results in a considerably lower mortality rate and/or invasive mechanical ventilation (Klopfenstein et al., 2020).
Generally, there are other cases where favorable changes were observed in CT findings, CRP and Interleukin 6 levels, fever, and lymphocyte rate after taking Tocilizumab (Di Giambenedetto et al., 2020; Michot et al., 2020). However, several clinical trials are actively inspecting Tocilizumab to determine its safety and efficacy in treating severe COVID-19 pneumonia (https://clinicaltrials.gov/ct2/show/NCT04315480, https://clinicaltrials.gov/ct2/show/NCT04317092; https://clinicaltrials.gov/ct2/show/NCT04320615).
5.7. Interferon
Interferons (IFN) discovered by virologists in 1957 are essential components of the immune system against viral infections. Most cells produce type I IFNs (α and β) as a direct response to viruses, while type II IFN (λ) is produced by activated natural killer (NK) cells and T cells. They hinder viral replication, including viral entry, uncoating, mRNA synthesis, protein synthesis, and subsequently reduce the viral load (Meng et al., 2020; Sainz Jr et al., 2004). Clinically, IFN therapy has already been approved for cancers, autoimmune diseases, and hepatitis B and C (Mantlo et al., 2020). Moreover, numerous studies have been conducted on MERS-CoV and SARS-CoV, in vitro and in vivo. An in vitro study in 2003 assessed recombinant IFN against SARS-CoV and showed their inhibitory and prophylactic protection, reporting the 18 IU/ml as EC50 (Cinatl et al., 2003). Furthermore, another group has suggested a synergistic antiviral relationship between type I and type II IFNs. They demonstrated a potent 105-fold inhibition on SARS-CoV replication by IFN-β and λ, at a low concentration of 100 u/ml each (Sainz Jr et al., 2004). Meanwhile, there are retrospective studies on SARS-CoV and MERS-CoV, which have used IFNs in combination with different agents (Song et al., 2020).
Although IFNs were suggested to be systemically efficient in SARS and MERS-CoV, for example, improving pulmonary function or delaying mortality, they generally failed to significantly improve the disease in humans (Sallard et al., 2020). Despite similarities between coronaviruses, in a report, researchers have demonstrated that IFN production is not induced efficiently in response to SARS-CoV-2, which leads to the prevention of innate immune response and higher viral levels. Therefore, exogenous IFN might be more efficient for treating SARS-CoV-2 infection (Meng et al., 2020; O'Brien et al., 2020).
An experiment on SARS-CoV-2, suggesting IFN pre-treatment, incubated Vero E6 cells with 1000 units/ml of recombinant IFN-α 18 h before infection. The results showed a significant reduction in viral replication, massive drops in viral titer, and a considerable deficit in viral nucleocapsid protein production (Lokugamage et al., 2020). There is also a medical staff trial indicating that recombinant IFN-α nasal drops can protect susceptible healthy people. They included 2944 participants in two high- and low-risk groups. After 28 days of following up new COVID-19 cases, positive pulmonary images, and fever/respiratory symptoms were zero, confirming IFN-α′s protective effects (Meng et al., 2020). Furthermore, a group in America investigated antiviral activities of type I Interferons on SARS-CoV-2 in vitro. They treated infected Vero cells with IFN-α and β at different concentrations (0.49–250 IU/ml). The results indicated no detectable virus titers and determined the EC50 of IFN-α and β as 1.35 IU/ml and 0.75 IU/ml, respectively (Mantlo et al., 2020).
A double-blind placebo-controlled phase 2 trial studied the safety and efficacy of inhaled nebulized interferon beta-1a in COVID-19 infection-related hospitalized patients. Forty-eight patients were randomized to receive inhaled nebulized interferon beta-1a (6 Million units/day) and 50 to inhale placebo for 14 days. Clinical improvement, on the WHO Ordinal Scale for Clinical Improvement (OSCI), was more than two times in inhaled interferon beta-1a compare to placebo on day 15 or 16 (odds ratio (OR) 2.32; 95% confidence interval (CI), 1.07–5.04; p = 0.033) and three-times greater on day 28 (OR 3.15, 95% CI 1.39–7.14, p = 0.006). Also, inhaled interferon beta-1a leads to more recovery of activities with no limitation (hazard ratio (HR) = 2.19; 95% CI, 1.03–4.69; p = 0.043) vs. placebo. Inhaled interferon beta-1a resulted in a reduction of severe disease development (OR 0·21; 95% CI 0·04–0·97]; p = 0·046). Headache was more commonly experienced by patients in the interferon and placebo group. The mortality report belonged to the placebo group (three patients) (Monk et al., 2020). A randomized open-label, Phase 2 trial enrolled 127 COVID-19 related hospitalized patients to treat a combination antiviral regimen or lopinavir/ritonavir as a control. Depend on hospital admission time from onset of symptoms, which was <7 days or ≥7 days, and hospitalized patients were a candidate for 14 days triple or dual therapy, respectively. Fifty-two cases received subcutaneous interferon beta-1b 8 million units every second day, lopinavir/ritonavir, and ribavirin; 34 cases received lopinavir/ritonavir and ribavirin. Forty-one in the control group received lopinavir/ritonavir unrelated to time from onset of symptom to hospitalization. The median time to negative nasopharyngeal swab was substantially shorter in combination therapy. The combination group revealed meaningful clinical improvement, according to the National Early Warning Score 2 (NEWS2) and Sequential Organ Failure Assessment (SOFA) score, and considerably shorter hospital stay. Prominent antiviral activity and clinical effect resulting from treatment with a combination antiviral regimen less than seven days from the onset of symptom indicate that interferon beta-1b was a crucial component in combination therapy (Hung et al., 2020).
A cohort study of 77 positive COVID-19 patients in China assessed patients treated with IFN-a2b 5 million international units twice a day, Umifenovir 200 mg three times a day, as well as a combination of IFN-a2b and Umifenovir. Outcomes recommended IFN-a2b therapy with or without Umifenovir resulted in a faster rate of viral clearance from the respiratory tract, decreased inflammatory cytokine and biomarker levels, including IL-6 and CRP (Zhou et al., 2020a, Zhou et al., 2020b).
6. Angiotensin II receptor blocker
6.1. Losartan
Losartan, an angiotensin II (Ang-II) receptor antagonist, is used to treat heart failure, high blood pressure, and diabetic kidney diseases. In competition with angiotensin II, Losartan inhibits its binding to the AT1 receptor, thereby counteracts the physiological effects of Ang-II, which consequently dilates smooth blood vessels and lowers blood pressure (Zeinalian et al., 2020). Analysis of clinical characteristics of covid-19 patients suggesting that hypertension has been responsible for 60.9% of deaths. Otherwise, coronaviruses transfer their genetic materials through fusing to the host cell, mediated by binding to the ACE2 receptor (Li et al., 2020a). In the outbreak of SARS-CoV in 2003, it was identified that ACE2 knockout mice had significantly lower viral loads in the lung following infection. They showed that treatment with Losartan 15 mg/kg attenuated lung injuries (Kuba et al., 2005).
Therefore, there is a perspective if ACE2 blockade act as a viable approach to attenuate COVID-19. In this regard, there are two double-blinded clinical studies actively working on Losartan in hospitalized and non-hospitalized COVID-19 patients. Both are in phase 2. About 200 hospitalized patients randomly are assigned to receive Losartan 50 mg daily or placebo for seven days. The primary outcome is the evaluation and measurement of PaO2 or SaO2/FiO2 ratio after seven days (https://clinicaltrials.gov/ct2/show/NCT04312009). The other one is conducted in 580 non-hospitalized COVID-19 cases to intake Losartan 25 mg per day or placebo for ten days with the primary outcome of hospital admission within 15 days of treatment (https://www.clinicaltrials.gov/ct2/show/NCT04311177).
7. Bradykinin B2 receptor antagonist
7.1. Icatibant
Icatibant is a bradykinin B2 receptor inhibitor and available in the US and Europe as a therapy for hereditary angioedema. Bradykinin, as a potent inflammatory mediator, causes more dilation and permeability of blood vessels, leading to fluid accumulation in the interstitial tissue. Icatibant binds to B2 receptors and hinders bradykinin functionality (Farkas, 2016).
It is revealed that COVID-19 enters the host cells through binding to the ACE2 receptor, highly expressed on pulmonary cells. Following virus activity, the ACE2 receptors will be occupied, and active ACE2 will reduce in the body, subsequently, and of note, ACE2 is responsible for hydrolyzing the active bradykinin metabolite [des-Arg973] (DABK). Therefore, as a side effect, COVD-19 activates this bradykinin system, which leads to fluid extravasation and leukocyte recruitment to the lung, which persists in pulmonary edema subsequently. This will deteriorate the lung damages caused by the virus. Thus, it seems that targeting the bradykinin system may be a new therapeutic approach for patients with COVID-19. Exploratory research investigated the effect of Icatibant in 9 CVID-19 infected patients. Icatibant 30 mg was injected subcutaneously every 6 h for three doses and lead to a substantial reduction in oxygen supplementation with no serious adverse events. (Tolouian et al., 2020; van de Veerdonk et al., 2020). Although there is no randomized clinical evaluation of Icatibant yet, it has been introduced as a potential candidate in purpose.
8. Corticosteroids
Corticosteroids, including glucocorticoids and mineralocorticoids, are produced by the adrenal cortex. They have been proved as immunosuppressive and anti-inflammatory drugs for the treatment of conditions such as asthma, allergy, septic shock, multiple sclerosis, and lung tissue disorders. Corticosteroids alter gene transcription through binding to a particular receptor in target cells. However, their use is limited by their massive probable side effects as hyperglycemia, hypertension, infection, osteoporosis, growth retardation, skin atrophy, glaucoma, and cataract (Ramamoorthy and Cidlowski, 2016; Song et al., 2020; Wang et al., 2020d). Systemic inflammation is an adverse outcome caused by coronaviruses, which persists after viral clearance. So, theoretically, corticosteroids can be potential candidates for suppressing lung inflammations. There are some reviews summarizing reports on SARS and MERS, revealing no benefits of corticosteroids. In general, the studies suggest associations between corticosteroid administrations and disease deterioration (worsening pulmonary conditions) and mechanical ventilation requirements, delayed viral clearance, avascular necrosis, and diabetes. They have called it a double-edged sword (Nasim et al., 2020; Russell et al., 2020). Since the outbreak of COVID-19, new studies have been designed on Corticosteroids. In an in vitro study on VeroE6 cells, Ciclesonide has been introduced as a safe corticosteroid to reduce viral replication and host inflammation by EC90 = 6.3 μM (Matsuyama et al., 2020). A clinical study reviewed 46 patients with severe COVID-19, in which 26 patients received 1–2 mg/kg/d methylprednisolone intravenously for 5–7 days. Results revealed faster improvement of oxygen saturation, better absorption degree of the focus in chest CT, and shorter time to overcome hyperthermia (Wang et al., 2020d).
Nevertheless, a report on 31 patients with 11 administrated corticosteroids indicated no statistically significant differences in treated patients and the non-treated. They investigated the virus clearance time, hospital length of stay, and duration of symptoms, and there was no improvement compared with the control patients (Zha et al., 2020). Moreover, an open-labeled, randomized controlled trial enrolled 48 cases from Chongqing Public Health Medical Center, China. The subjects are assigned in two groups, the intervention group, which receives an intravenous injection of 1–2 mg/kg/day methylprednisolone for three days, and the control group. The study is examined the timing of clinical improvement, duration of mechanical ventilation and hospitalization, rate of adverse effects, and mortality. The results have not yet been revealed (Zhou et al., 2020a, Zhou et al., 2020b). As of now, the use of corticosteroids in patients with COVID-19 is controversial since the WHO and the Centers for Disease Control and Prevention (CDC) generally recommend that glucocorticoids not be used in COVID-19 pneumonia unless in specific comorbid clinical conditions, e.g., exacerbation of chronic obstructive pulmonary disease (Song et al., 2020).
The RECOVERY trial is an ongoing, open-label, controlled trial conducted on hospitalized patients with COVID 19 in the UK. The primary endpoint was mortality rate at 28 days. The study assigned 2104 patients to receive Dexamethasone, oral or intravenous, at the dose of 6 mg daily for ten days, plus standard care, and 4321 patients to receive usual care alone. According to the preliminary analysis report, of 6425 patients, 22.9% of patients (n = 482) in the dexamethasone group and 25.7% (n = 1110) in the standard care group died within 28 days of randomization (P < 0.001). Compared to the usual care group, the dexamethasone group had a lower incidence rate of mortality among patients who required invasive mechanical ventilation (29.3% vs. 41.4%) and oxygen supplement (23.3% vs. 26.2%). A significant survival benefit was not found in patients who did not receive respiratory support (Horby et al., 2020a, Horby et al., 2020b).
The World Health Organization (WHO) rapid evidence appraisal for COVID-19 therapies (REACT) assessed the association between corticosteroids and mortality rate in critically ill patients infected with COVID-19. This meta-analysis includes seven trials (DEXA-COVID 19, RECOVERY, REMAP-CAP, CoDEX, CAP COVID, COVID STEROID, and Steroids-SARI) pooled data. Of 1703 patients who had participated in the analysis, 678 received corticosteroids (3 trials Dexamethasone, three trials Hydrocortisone, one trial Methylprednisolone) 1025 had received standard of care or placebo. 222 patients (32%) in the corticosteroid group and 425 patients (40%) in the standard care or placebo group died (p < 0.001) within 28 days. Dexamethasone and hydrocortisone were the same in mortality rate reduction (Sterne et al., 2020).
At the same time, WHO released the guideline of corticosteroids for COVID-19 in which systemic administration of Dexamethasone 6 mg daily or Hydrocortisone 150 mg (e.g., 50 mg every 8 h), or Prednisone 40 mg, or Methylprednisolone 32 mg (e.g., 8 mg every 6 h or 16 mg every 12 h) for 7–10 days has been recommended for patients with severe and critical COVID-19 (https://www.who.int/publications/i/item/WHO-2019-nCoV-Corticosteroids-2020.1).
Early administration of corticosteroids at a low dose for the short term has been evaluated in 475 hospitalized patients with non-severe COVID 19 related pneumonia. Methylprednisolone 20 mg per day or 40 mg per day for 3–5 days was prescribed intravenously for 50 patients, and five patients received prednisone 20 mg per day for three days. 420 patients did not receive corticosteroid therapy. The length of fever, virus clearance, and hospital stay were significantly prolonged in the corticosteroid group. More patents in the corticosteroid group progressed to severe disease. Antimicrobial therapy was more remarkable in the corticosteroid group. So worse outcomes are expected from corticosteroid therapy in non-severe COVID-19 pneumonia (Li et al., 2020).
Another retrospective study evaluated the effect of early administration of corticosteroids on mortality rate and mechanical ventilation in 1806 hospitalized patients with COVID 19. Of 1806 patients, 140 received corticosteroids within the first 48 h of admission. Corticosteroid therapy in patients with initial C-reactive protein (CRP) 20 mg/dL or more significantly decreased mortality or mechanical ventilation. In contrast, mortality or mechanical ventilation became more significant in patients with a CRP level of less than 10 mg/dL (Keller et al., 2020).
Careful interpretation of these findings needs to perform further randomized clinical studies.
9. Anthelmintic
9.1. Ivermectin
Studies on SARS-CoV proteins have revealed an important role for importin (IMP) α/β1 during infection, impacting host cell division. There is an FDA-approved drug termed Ivermectin that has been licensed as an anti-parasite and antiviral agent (Caly et al., 2020). Ivermectin inhibits IMP α/β1-mediated nuclear import, specifically nuclear transport of viral proteins. Subsequently, it can suppress several RNA virus replication, including HIV, the chikungunya virus, and the yellow fever virus (Patrì and Fabbrocini, 2020). Based on the similarity of SARS-CoV and SARS-CoV2, Ivermectin could be a potential drug candidate for controlling COVID-19. An in vitro study has been designed towards Ivermectin antiviral activity on Vero/hSLAM cells infected with COVID-19. They proved its efficacy in a single dose for 48 h, observing 93% reduction in viral RNA presented in the supernatant at 24 h and 99% reduction in cell-associated viral RNA equivalent to a ~5000-fold reduction by 48 h (Caly et al., 2020). Ivermectin is also a safe drug, which is a study of phase 3 clinical trials on dengue patients in Thailand; it was introduced to be secure enough (Caly et al., 2020). Also, in a meta-analysis working on its high doses, its safety was strongly confirmed even beyond its prescribed doses (Navarro et al., 2020). Nonetheless, the use of Ivermectin to combat COVID-19 would depend on further pre-clinical and clinical trials. There is even a hypothesis suggesting its combined use with Hydroxychloroquine (Patrì and Fabbrocini, 2020). A study retrospectively analyzed the data of 280 patients with COVID-19 related infection admitted to four hospitals in Florida, 173 received Ivermectin (200 mcg/kg single dose plus usual care, the dose could be repeated at the physician's discretion on day 7 after treatment initiation) and 107 received usual treatment. All-cause mortality was substantially lower in Ivermectin patients (OR, 0.27; 95% CI, 0.09–0.80; P, 0.03), also patients with severe pulmonary disease treated with Ivermectin had lower mortality rate (38.8% vs. 80.7%; OR, 0.15; 95% CI, 0.05–0.47; P, 0.001). Data analysis didn't demonstrate any significant differences in extubation rate (36.1% vs. 15.4%; OR, 3.11; 95% CI, 0.88–11.00; P, 0.07) and hospital stay (Cepelowicz Rajter et al., 2020). A trial of 116 patients with mild to moderate COVID-19 infection compared combination therapy of Ivermectin and Doxycycline with Hydroxychloroquine plus Azithromycin. Sixty patients treated with Ivermectin and Doxycycline had a better recovery to symptom-free and shorter time to recovery.
Additionally, Ivermectin-Doxycycline was better tolerated vs. Hydroxychloroquine plus Azithromycin therapy. Based on these results, the authors believe Ivermectin can be considered an acceptable choice for infected patients with mild to moderate COVID-19 (Taiub et al., 2020). One hundred forty patients with COVID-19 have been investigated in a randomized control study. 70 Participants received oral Ivermectin 200 mcg daily for two to three days plus oral doxycycline 100 mg twice daily for 5–10 days in addition to standard treatment, and 70 participants in the control group received standard therapy. Ivermectin group had less progression to more advanced disease and mortality rate, also significantly decreased time to recovery (Hashim et al., 2020). Currently, clinical trials at various stages of completion have been registered in the European Union Clinical Trials Register and the US clinical trials registry about Ivermectin in COVID-19 infection (https://www.clinicaltrialsregister.eu/ctr-search/search?query=ivermectin+AND+covid-19) (https://clinicaltrials.gov/ct2/results?term=ivermectin&recrs=abcdefghl&cond=covid19).
9.2. Nitazoxanide
Nitazoxanide has a broad-spectrum antiviral and anti-parasitic activity. Nitazoxanide targets regulatory mechanisms involved in virus replication (Mahmoud et al., 2020). This thiazole derivative has been clinically developed for the treatment of patients with viral respiratory infections. In in vitro studies, Nitazoxanide inhibited viral N protein expression in the MERS-CoV and other human coronaviruses. Furthermore, Nitazoxanide inhibits the pro-inflammatory cytokines and interleukin 6 in peripheral blood mononuclear cells in mice.
Lately, Kelleni has suggested combination therapy of Nitazoxanide with azithromycin to treat COVID-19 (Kelleni, 2020). Besides, Pepperrell et al. summarize the reported clinical investigations about Nitazoxanide to define the safety of this drug for the treatment of COVID-19. Presently, 14 clinical trials are being investigated for using Nitazoxanide alone or in combination with Ivermectin or Hydroxychloroquine to manage patients with COVID-19 (Mahmoud et al., 2020; Pepperrell et al., 2020).
In a placebo-controlled trial, 392 patients were randomly allocated to receive Nitazoxanide 500 mg three times a day for five days or placebo group. On day 5, symptom relief in 194 patients treated with Nitazoxanide did not significantly differ from 198 patients in the placebo group, although, after seven days of therapy, symptom relief in the Nitazoxanide group was considerably better than the control group. Viral load was reduced substantially with Nitazoxanide following five days of treatment. Nitazoxanide did not associate with serious adverse events or death, and its early use is suggested by the authors (Rocco et al., 2020). Another double-blind phase 3 clinical study is related to the efficacy evaluation of Nitazoxanide extended-release tablet in mild or moderate cases of COVID-19. In this undergoing research, participants will receive Nitazoxanide or placebo bidaily for five days. Recovery time and progression to severe disease will be assessed (https://clinicaltrials.gov/ct2/show/NCT04359680).
10. Antiprotozoal
10.1. Emetine
Emetine is a protein inhibitor approved for the treatment of amoebiasis as anti-protozoan. It also inhibits malaria, blocking its protein synthesis by binding to the Plasmodium falciparum's ribosomal E site. Besides, Emetine processes antiviral activity against a broad range of RNA and DNA viruses, specifically coronaviruses as SARS-CoV and MERS (Choy et al., 2020). A report against four strains of coronaviruses declared EC50 values ranging from 0.12 to 1.43 μM, with the MERS being 0.34 μM (Bleasel and Peterson, 2020). Thus, a study has examined Emetine on SARS-CoV2 in vitro, working on Vero E6 cells infected by the virus. They estimated its EC50 at 0.46 μM. Furthermore, this study observed its synergism with Remdesivir, achieving 64% inhibition of viral yield with the subsequent reduction in the effective concentration of compounds and consequent side effects (Choy et al., 2020). Noticeably, there is no in vivo or clinical trial of Emetine on COVID-19.
11. H2 blocker
11.1. Famotidine
Famotidine is an H2 receptor antagonist, which inhibits the secretion of gastric acid. There is a hypothesis that Famotidine binds to papain-like protease, and the SARS-CoV-2 genome may encode protease. Although no evidence supports this hypothesis, Famotidine was administrated because of low side effects and bioavailability (Janowitz et al., 2020). A systematic review assessed the results of 5 types of research consisting of 2 case series and three cohort studies in a clinical outcome case following Famotidine therapy. Patients in 3 cohort studies and 1 case series were hospitalized with COVID-19 and in 1 case series did not. A different daily dose of Famotidine was used in the range of 20 mg–320 mg for 5–28 days. Famotidine significantly decreased in-hospital mortality, rate of mortality/intubation, progression to severe disease, and progressively improved radiographic findings (Sethia et al., 2020).
12. Anticoagulant
12.1. Heparin
Heparin is a glycosaminoglycan, as an anticoagulant, prevents blood clot formation. Heparin inhibits the activation of the fibrin stabilizing factor through a trombone, which prevents the fibrin clot formation. Anticoagulants, in particular heparin, are suggested for patients with severe COVID-19 (Driggin et al., 2020; Gozzo et al., 2020). Since severe hypercoagulability occurs in these patients, early treatment with anticoagulation may decrease coagulopathy and reduce the risk of organ damages. The effect of heparin in COVID-19 is determined by lots of investigations describing its pleiotropic activity. The acute lung injury has a high level of D-dimer and fibrinogen, associated with the hypercoagulable phase. Besides, patients with severe disease and prolonged immobility are exposed to a high risk of venous thromboembolism (VTE), and acute pulmonary embolism (PE) or deep vein thrombosis (DVT) may be experienced in patients with mechanical ventilation. Numerous randomized controlled trials have been designed to assess anticoagulation risks and efficacy in patients with COVID-19 (Buijsers et al., 2020; Fletcher-Sandersjöö and Bellander, 2020). By its spike (S) protein, SARS-CoV-2 binds to the TMPRSS2 receptor, and this viral entry is facilitated by the ACE-2 receptor (Hoffmann et al., 2020a). In a similar mechanism, SARS-CoV-1 enhances the expression of fibroblast growth factor (FGF), fibrinogen gamma chain (FGG), and serine protease genes (SERPIN), and eventually upregulation of coagulation cascade factors as a result (Giannis et al., 2020).
Moreover, a cohort study on 184 hospitalized patients with COVID-10 pneumonia evaluated the thrombotic event. All patients received standard doses of thromboprophylaxis. The researchers suggest thrombosis prophylaxis and high-prophylactic doses for all patients admitted to the intensive care unit (Klok et al., 2020).
13. Conclusion
The emergence of novel viruses during the last two decades and their pandemic has called for a need for massive experiments in a short time. As an essential step, drugs can be developed through three strategies:
-
1.
Directly developing a new viral-specific drug based on the genomic and pathological information. Theoretically, these drugs would exhibit targeted effects, but the procedure may last several years, which is not appropriate for a pandemic.
-
2.
Screening databases for potential molecules with therapeutic effects that introduce good candidates for virtual functions for further investigations.
-
3
Using pre-existing components. That would be the fastest way with known safety and side effects, the dosage used, absorption, and metabolic characteristics (de Wilde et al., 2014; Dyall et al., 2014; Wu et al., 2020a). The novel coronavirus, SARS-CoV-2, is the latest outbreak with a serious threat to the global public, and to date, there is no approved therapeutic drug or vaccine against it. Many investigations have been designed on broad-spectrum inhibitors, in vitro, in vivo, and clinical. The drugs described here belong to 12 different pharmaceutical drug classes where antivirals are the most used, which all are summarized in Table 1 . Table 2 summarized the physical and chemical properties and structure of these agents.
Table 1.
Potential therapies for COVID-19 treatment as drug repositioning.
| Agent | Classification | Target | Treatment Dosage | Common side effects | Approved for | Clinical trials (Based on Clinicaltrials.gov) | Contraindication | Comments for COVID-19 |
|---|---|---|---|---|---|---|---|---|
| Darunavir (Prezista) | Antiviral | Protease inhibitor: inhibiting Gag-Pol polyprotein cleavage | 800 mg daily | Nausea, Vomiting, Diarrhea, Stomach pain, Headache, Rash, | HIV | (NCT04252274) | Co-administration with CYP3A highly dependent drugs are associated with serious and/or life-threatening events. | Positive effects in combination with other antivirals |
| Oseltamivir (Tamiflu) | Antiviral | Neuraminidase inhibitor | 75 mg twice a day | Nausea, Vomiting, Headache, Pain; Sudden confusion, |
Influenza A and B | (NCT04303299) (NCT04338698) (NCT04255017) (NCT04261270) | Hypersensitivity to Oseltamivir or any component of the formulation. | Ineffective |
| Umifenovir (Arbidol) | Antiviral | Hemagglutinin inhibitor | 200 mg three times daily | Limited allergic reactions | Influenza A and B | (NCT04260594) (NCT04350684) | Increased sensitivity to the medication in children under two years. | No significant improvements |
| Favipiravir (Avigan) | Antiviral | RdRp inhibitor | 1600 mg twice daily on day 1 and 600 mg twice daily on days 2–14 | Decreased RBC production, increases in liver function parameters. | Influenza | (NCT04336904) (NCT04358549) (NCT04349241) | Using in women who might be or are pregnant | Symptoms reduction |
| Remdesivir (Veklury) | Antiviral | RdRp inhibitor | 200 mg/dose | Swelling, Bruising or bleeding around the IV needle, Rash, Diarrhea, Renal impairment, Hypotension and increased hepatic enzymes | Investigational drug | (NCT04365725) (NCT04323761) (NCT04302766) (NCT04410354) | Hypersensitivity to Remdesivir or any component of the formulation | Authorized emergency use |
| Ribavirin (Virazole) | Antiviral | Viral protein synthesis inhibitor | 6 g over 12–18 h daily, Oral inhalation | Anxiety, Cough or hoarseness, Diarrhea, Sleeplessness, Headache, Vomiting, Nausea, Lack of appetite | Hepatitis C, Respiratory Syncytial Virus. | (NCT04392427) (NCT04356677) | Hypersensitivity to ribavirin or any component of the formulation; Pregnant women or may become pregnant | effective as an add-on therapy |
| Nafamostat mesylate | Antiviral | Serine protease inhibitor | 0.1–0.2 mg/kg/h of mixed with 5% DW | Nausea, Vomiting, Sweating, Chest discomfort Agranulocytosis, Hyperkalemia | Chronic pancreatitis, Anticoagulant in Japan | (NCT04352400) (NCT04473053) | Heparin | Improve patient's conditions effectively |
| Camostat mesylate | Antiviral | Serine protease inhibitor | 2 × 100 mg pills 3 times daily for 5 days | Nausea, Vomiting, Rashes | Chronic pancreatitis, Anticoagulant in Japan | (NCT04455815) (NCT04353284) (NCT04470544) (NCT04530617) | Heparin | Less effective than Nafmostat |
| Lopinavir/Ritonavir (Kaletra/Norvir) | Antiretroviral | Protease inhibitor, CYP4503A inhibitor | 400 mg/100 mg; or 200 mg/50 mg | Headache. Stomach pain or Diarrhea, Chest pain or pressure. Dizziness or passing out. |
HIV | (NCT04330690) (NCT04409483) | Hypersensitivity to lopinavir, ritonavir, Pregnancy; hepatic or renal failure; co-administration with disulfiram or metronidazole. | Contradictory results |
| Nelfinavir (Viracept) | Antiretroviral | Protease inhibitor | In vitro dosage: EC50 = 1.13, EC90 = 1.76 | Upset stomach, Diarrhea | HIV | – | Co-administration with drugs that are highly dependent on CYP3A | In vitro effectiveness with the IC50 of 1.3 μM |
| Teicoplanin (Targocid) | Antibiotic | Cathepsin L blocker | In vitro dosage: IC50 = 1.66 | Fever, Chills, Allergic reactions, Headache, dizziness, “Red-man” syndrome. | Treatment of bacterial infections. | – | Hypersensitivity to Teicoplanin or any component of the formulation. | In vitro effectiveness with the IC50 of 1.66 μM |
| Azithromycin (Zithromax) | Antibacterial | RNA-dependent protein synthesis inhibitor | 500 mg on day 1 followed by 250 mg once daily | Stomach pain, Diarrhea, Nausea, Vomiting, Shortness of breath, Sudden dizziness | Treatment or prevention of bacterial infections. | (NCT04381962) (NCT04329832) (NCT04359316) (NCT04332107) | Hypersensitivity to azithromycin. History of cholestatic jaundice/hepatic dysfunction associated with prior azithromycin use | Effective through co-treatment with HCQ |
| Chloroquine (Aralen) | Antimalarial | Lysosome inhibitor | 600 mg base once on day 1 followed by 300 mg base once daily for a total treatment duration | Nausea, Vomiting, Diarrhea, Headache, Hair loss, Increased sensitivity to light | Malaria | (NCT04333628) (NCT04353336) (NCT04331600) (NCT04344951) | Hypersensitivity to chloroquine, the presence of retinal or visual field changes of any etiology (when used for indications other than acute malaria) | Ineffective and clinical trials paused by FDA on 25 May 2020 |
| Hydroxychloroquine (Plaquenil) | Antimalarial | Lysosome inhibitor | 800 mg once on day 1, followed by 400 mg/day as a single dose or in 2 divided doses | Headache, Dizziness, nausea, vomiting, stomach pain, weight loss, feeling irritated, skin rash, hair loss | Malaria, Rheumatoid arthritis, Discoid or Systemic lupus erythematosus. | (NCT04340544) (NCT04351620) (NCT04345692) (NCT04385264) | Known hypersensitivity to HCQ, 4-aminoquinoline derivatives, or any component of the formulation. | Ineffective and clinical trials paused by FDA on 25 May 2020 |
| Thalidomide (Thalomid) | Immunomodulatory agent | TNF-α suppressor | 100 mg/day | Drowsiness, Dizziness, and Rash | Leprosy, Multiple myeloma | (NCT04273529) (NCT04273581) |
Hypersensitivity to thalidomide or any component of the formulation; pregnancy. | Patient's conditions improvement effectively. |
| Bevacizumab | Monoclonal antibody | Anti-VEGF | 7.5 or 15 milligrams per kilogram (mg/kg) IV. | Dry mouth, Cough, Voice changes, loss of appetite, Diarrhea, Nausea, Vomiting, Constipation |
Cancer | (NCT01351415) (NCT01239732) | Hypersensitivity in severe cardiac disease, Thrombosis, Hemorrhage, Stroke, Hemoptysis, or Colon perforation | Second line treatment |
| Tocilizumab (Actemra) | Monoclonal antibody | Interleukin inhibitor | 8 mg/kg, Maximum (800 mg/dose) | Headache, Dizziness, Upper abdominal pain, Mouth ulcers, Neutropenia, Thrombocytopenia, increased liver enzymes, increased total cholesterol and triglycerides | Rheumatoid arthritis, cytokine release syndrome | (NCT04317092) (NCT04345445) (NCT04331795) (NCT04377659) (NCT04359667) |
Known hypersensitivity to Tocilizumab or any component of the formulation | Contradictory results |
| Sarilumab | Monoclonal antibody | IL-6 receptor inhibitor | 200–400 mg/IV/daily | Neutropenia, increased ALT, injection site redness, upper respiratory infections, Nasal congestion, Runny nose |
Moderately to severely active rheumatoid arthritis | (NCT04327388) (NCT04315298) | Active tuberculosis, inactive tuberculosis, opportunistic fungal infection |
No improvement in outcomes |
| Anakinra | Interleukin antagonist | Recombinant IL-1 receptor antagonist | 2 mg/kg/day (Max: 100 mg/day) or 4 mg/kg/day (Max: 200 mg/day) | Redness, Swelling, Bruising, or pain at the site of injection | Rheumatoid arthritis | (NCT03265132) (NCT04443881) (NCT03002974) | E. coli protein hypersensitivity or hypersensitivity to Anakinra or any components of the product. | Effective compared to standard care |
| Interferons (α, β, λ) | Biological response modifier | Hindering the viral replication, the viral load reduction | The EC50 for IFN-α and IFN-β in vitro: 1.35 IU/ml and 0.76 IU/ml, respectively. |
INF-α: Cardiac arrhythmias, Anorexia, Seizures, Retinopathy, Numbness, Palpitation, Paresthesia, and Dizziness. INF-β: Sinus tachycardia, Neutropenia, Hypothyroidism, Depression, Pancytopenia, Hyperthyroidism INF-λ: Bronchospasm, Pancreatitis, Hyponatremia, Interstitial pneumonitis. |
Cancers, Autoimmune diseases, Hepatitis B and C | (NCT04350671) (NCT04343976) |
INF-α: Anemia, Depression, Diabetes mellitus, Hypertension, Hyperthyroidism, Thrombocytopenia, Retinopathy, Leukopenia, Seizures, Coronary artery disease, Autoimmune hepatitis. INF-β: Depression, Lactation. INF-λ: Hypersensitivity. |
Significant reduction in viral replication and titer in combination with other therapies. |
| Losartan (Cozaar) | Angiotensin II receptor antagonists | Angiotensin II receptor blockade | 15 mg/kg | Dry cough, Cramps, Pain in legs or back, Stomach pain, Diarrhea, Headache, Dizziness; Tired feeling; Insomnia |
Heart failure, hypertension | (NCT04335123) (NCT04312009) (NCT04311177) | Hypersensitivity to losartan or any component of the formulation. | Attenuates lung injuries |
| Corticosteroids | Adrenal Cortex hormones. | Anti-inflammation and anti-fibrotic agent | Dexamethasone 6 mg/day Oral/IV Or Equivalent total daily doses of alternative glucocorticoids |
Fluid retention or Swelling of feet and legs, High blood pressure, increase blood sugar levels, increased risk of infection. | Natural corticosteroids, Inflammation, Autoimmune conditions, Allergy symptoms. | (NCT04344288) (NCT04345445) (NCT04359511) (NCT04355247) | Hypersensitivity to active ingredient or any component of the formulation, uncontrolled active infection | Can be used in specific clinical conditions. |
| Ivermectin (Stromectol) | Anthelmintic | IMP α/β1-mediated nuclear import inhibitor | 600 μg/kg once daily | Headache, Muscle aches; Dizziness; Nausea, Diarrhea; Mild skin rash |
Parasitic infections | (NCT04381884) (NCT04360356) (NCT04405843) | Hypersensitivity to Ivermectin or any component of the formulation | Effective in in vitro examinations and safe. |
| Nitazoxanide | Antiviral and anti-parasitic | Antiprotozoal agent | 500 mg every 6 h for 14 days | Nausea, Stomach pain; Headache, Discolored urine |
Treatment of various Helminthic, Protozoal | (NCT04435314) (NCT04552483) (NCT04463264) | Hypersensitivity in hepatic or renal impairment, Diabetes, HIV or other immunodeficiency | Antiviral potential against MERS-CoV and other coronaviruses in in vitro |
| Emetine | Antiprotozoal | Protein synthesis inhibitor: binds to ribosomal E site | In vitro dosage: EC50 = 0.46 μM | Myositis at the injection site, hypotension, tachycardia, chest pain, dyspnea, and abnormalities on electrocardiogram, including T-wave inversion | Amoebiasis | – | Contraindicated in renal, cardiac, and muscular disease and is used cautiously in children and elderly patients | Effective in in vitro with EC50 at 0.46 μM |
| Famotidin | Anti-acid | H2 antagonist | 40 mg–60 mg 8hourly | Constipation, diarrhea, fatigue, dizziness, weakness, mood changes, headache, insomnia | Heartburn, GERD, and Zollinger-Ellison syndrome | (NCT04504240) (NCT04370262) (NCT04545008) | Antimicrobials medicines, Acalabrutinib, Alendronate, Metformin | An empty stomach along with other treatments |
| Heparin (LMWH) | Anticoagulant | Anti-thrombin | UFH 250 U/kg or LMWH 100 U/kg twice daily | Bruising, Bleeding, Irritation, Pain, Redness | Prophylaxis treatment for venous thrombosis, pulmonary embolism, and peripheral arterial embolism | (NCT00182403) (NCT00049777) (NCT03378466) | Hypersensitivity, past or present heparin-induced thrombocytopenia and active bleeding | Effective in combination with other therapies |
AbbreviationsHIV: human immunodeficiency virus; CYP3A: Cytochrome P4503A; RdRp: RNA-dependent RNA-polymerase; RBC: red blood cell; IV: intravenous; FDA: food drug administration; g: gram; mg: milligram; kg: kilogram; DW: dextrose water; EC50: half maximal effective concentration; IC50: half maximal inhibitory concentration; HCQ: hydroxychloroquine; TNF-α; tumor necrosis factor: VEGF: vascular Endothelial Growth Factor; IL-6: Interleukin-6; ALT: alanine aminotransferase; INF-α: interferon-α; INF-β: interferon- β; INF-λ: interferon- λ; IU: international unit; IL-1: Interleukin-6; IMPα/β1: importin α/β1; U: unit; UFH: unfractionated heparin; LMWH: low molecular weight heparin.
Table 2.
Physical and chemical information and structure of drugs
(https://www.usp.org/); (https://pubchem.ncbi.nlm.nih.gov/).
| No | Name | Chemical Formula | Melting Point (°C) | Molecular Weight (g/mol) | Color/Form | Physical Description | Solubility | Identification | IUPAC Name | Structure |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Darunavir | C27H37N3O7S | 74–76 | 547.7 | White, amorphous solid | Solid | In water 0.15 mg/ml at 20 °C | HPLC | [(3aS,4R,6aR)-2,3,3a,4,5,6a-hexahydrofuro [2,3-b]furan-4-yl] N-[(2S,3R)-4-[(4-aminophenyl) sulfonyl-(2-methylpropyl)amino]-3-hydroxy-1-phenylbutan-2-yl]carbamate | 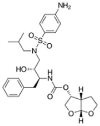 |
| 2 | Oseltamivir | C16H28N2O4 | 190–206 | 312.4 | White, solid | Solid | In water, 1.6 × 10 + 3 mg/L at 25 °C | LC | ethyl (3R,4R,5S)-4-acetamido-5-amino-3-pentan-3-yloxycyclohexene-1-carboxylate | 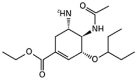 |
| 3 | Favipiravir | C5H4FN3O2 | 187–193 | 157.1 | white to light yellow, Solid | Solid | slightly soluble in water | HPLC | 5-fluoro-2-oxo-1H-pyrazine-3-carboxamide |  |
| 4 | Umifenovir | C22H25BrN2O3S | 133–137 | 477.4 | White, Solid | Solid | “Expected to be poorly soluble | HPLC | ethyl 6-bromo-4-[(dimethylamino)methyl]-5-hydroxy-1-methyl-2-[(phenylsulfanyl)methyl]-1H-indole-3-carboxylate | 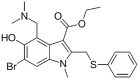 |
| 5 | Remdesivir | C27H35N6O8P | 127 | 602.6 | white to off-white to yellow, Solid | Solid | Insoluble in water and soluble in ethanol | HPLC Mass/Mass | 2-ethylbutyl (2S)-2-[[[(2R,3S,4R,5R)-5-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-5-cyano-3,4-dihydroxyoxolan-2-yl]methoxy-phenoxyphosphoryl]amino]propanoate | 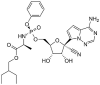 |
| 6 | Ribavirin | C8H12N4O5 | 174–176 | 244.2 | Colorless, Solid | Solid | In water, 142 mg/ml at 25 °C | IR, TLC | 1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-1,2,4-triazole-3-carboxamide |  |
| 7 | Nafamostat | C19H17N5O2 | 217–220° | 347.4 | Colorless, Solid | Solid | In water, 25 mg/ml at 25 °C | HLPC | (6-carbamimidoylnaphthalen-2-yl) 4-(diaminomethylideneamino)benzoate |  |
| 8 | Heparin | C26H42N2O37S5 | >228 | 1134.9 | White or pale-colored amorphous powder | Solid | Soluble in water | Chromatography | 6-[6-[6-[5-acetamido-4,6-dihydroxy-2-(sulfooxymethyl)oxan-3-yl]oxy-2-carboxy-4-hydroxy-5-sulfooxyoxan-3-yl]oxy-2-(hydroxymethyl)-5-(sulfoamino)-4-sulfooxyoxan-3-yl]oxy-3,4-dihydroxy-5-sulfooxyoxane-2-carboxylic acid |  |
| Camostat | C20H22N4O5 | 194–198 | 398.4 | white to tan, Solid | Solid | In water, 24 mg/ml at 25 °C | HPLC | [4-[2-[2-(dimethylamino)-2-oxoethoxy]-2-oxoethyl]phenyl] 4-(diaminomethylideneamino)benzoate |  |
|
| 9 | Lopinavir | C37H48N4O5 | 124–127 | 628.8 | White to light tan powder | Solid | Practically insoluble | reversed phase chromatographic method HPLC | (2S)–N-[(2S,4S,5S)-5-[[2-(2,6-dimethylphenoxy)acetyl]amino]-4-hydroxy-1,6-diphenylhexan-2-yl]-3-methyl-2-(2-oxo-1,3-diazinan-1-yl)butanamide |  |
| 10 | Ritonavir | C37H48N6O5S2 | 126–132 | 720.9 | White to light tan powder | Solid | Practically insoluble | FTIR, LC/MS, HPLC, Polarimetry | 1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[[(2S)-3-methyl-2-[[methyl-[(2-propan-2-yl-1,3-thiazol-4-yl)methyl]carbamoyl]amino]butanoyl]amino]-1,6-diphenylhexan-2-yl]carbamate | 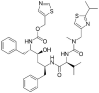 |
| 11 | Nelfinavir | C32H45N3O4S | 349.84 | 567.8 | white to off-white | Solid | Slightly soluble | Liquid chromatography–mass spectrometry | (3S,4aS,8aS)-N-tert-butyl-2-[(2R,3R)-2-hydroxy-3-[(3-hydroxy-2-methylbenzoyl)amino]-4-phenylsulfanylbutyl]-3,4,4a,5,6,7,8,8a-octahydro-1H-isoquinoline-3-carboxamide |  |
| 12 | Teicoplanin | C88H97Cl2N9O33 | 260 | 1879.7 | white to faint yellow | Solid | In water, 10 mg/ml at 25 °C | HPLC | (1S,2R,19R,22R,34S,37R,40R,52S)-2-[(2R,3R,4R,5S,6R)-3-acetamido-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-22-amino-5,15-dichloro-64-[(2S,3R,4R,5S,6R)-3-(decanoylamino)-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-26,31,44,49-tetrahydroxy-21,35,38,54,56,59-hexaoxo-47-[(3S,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-7,13,28-trioxa-20,36,39,53,55,58-hexazaundecacyclo[38.14.2.23,6.214,17.219,34.18,12.123,27.129,33.141,45.010,37.046,51]hexahexaconta-3,5,8,10,12(64),14,16,23(61),24,26,29(60),30,32,41(57),42,44,46(51),47,49,62,65-henicosaene-52-carboxylic acid |  |
| 13 | Azithromycin | C38H72N2O12 | 126 | 749 | Amorphous solid | Solid | In water, 2.37 mg/ml at 25 °C | LC, UV | (2R,3S,4R,5R,8R,10R,11R,12S,13S,14R)-11-[(2S,3R,4S,6R)-4-(dimethylamino)-3-hydroxy-6-methyloxan-2-yl]oxy-2-ethyl-3,4,10-trihydroxy-13-[(2R,4R,5S,6S)-5-hydroxy-4-methoxy-4,6-dimethyloxan-2-yl]oxy-3,5,6,8,10,12,14-heptamethyl-1-oxa-6-azacyclopentadecan-15-one |  |
| 14 | Hydroxychloroquine | C18H26ClN3O | 89–91 | 335.9 | white | Solid | In water,2.61e-02 g/L at 25 °C | LC | 2-[4-[(7-chloroquinolin-4-yl)amino]pentyl-ethylamino]ethanol |  |
| 15 | Thalidomide | C13H10N2O4 | 270 | 258.23 | Needles | Solid | In water, 545 mg/L at 25 °C | HPLC, TLC | 2-(2,6-dioxopiperidin-3-yl)isoindole-1,3-dione | 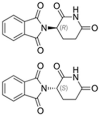 |
| 16 | Losartan | C22H23ClN6O | 178–184 | 422.9 | Light yellow solid | Solid | In water, 8.22 mg/L at 25 °C | HPLC | [2-butyl-5-chloro-3-[[4-[2-(2H-tetrazol-5-yl)phenyl]phenyl]methyl]imidazol-4-yl]methanol | 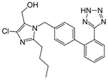 |
| 17 | Icatibant | C59H89N19O13S | 213–218 | 1304.5 | White Solid | Solid | In water, 1 mg/ml at 25 °C | HPLC | (2S)-2-[[(2S,3aS,7aS)-1-[(3R)-2-[(2S)-2-[[(2S)-2-[[2-[[(2S,4R)-1-[(2S)-1-[(2S)-2-[[(2R)-2-amino-5-(diaminomethylideneamino)pentanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]pyrrolidine-2-carbonyl]-4-hydroxypyrrolidine-2-carbonyl]amino]acetyl]amino]-3-thiophen-2-ylpropanoyl]amino]-3-hydroxypropanoyl]-3,4-dihydro-1H-isoquinoline-3-carbonyl]-2,3,3a,4,5,6,7,7a-octahydroindole-2-carbonyl]amino]-5-(diaminomethylideneamino)pentanoic acid | 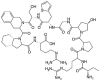 |
| 18 | Ivermectin | C48H74O14 | 155 | 875.1 | colorless,Solid | Solid | In water, 4 mg/L at 25 °C | IR, HPLC | (1R,4S,5′S,6R,6′R,8R,10E,12S,13S,14E,16E,20R,21R,24S)-6'-[(2S)-butan-2-yl]-21,24-dihydroxy-12-[(2R,4S,5S,6S)-5-[(2S,4S,5S,6S)-5-hydroxy-4-methoxy-6-methyloxan-2-yl]oxy-4-methoxy-6-methyloxan-2-yl]oxy-5′,11,13,22-tetramethylspiro[3,7,19-trioxatetracyclo[15.6.1.14,8.020,24]pentacosa- trioxatetracyclo[15.6.1.14,8.020,24]pentacosa10,14,16,22-tetraene-6,2′-oxane]-2-one | 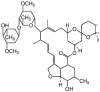 |
| 19 | Nitazoxanide | C12H9N3O5S | 202 | 307.28 | light yellow, Solid | Solid | Insoluble in water. | HPLC | 2-[(5-nitro-1,3-thiazol-2-yl)carbamoyl]phenyl acetate |  |
| 20 | Emetine | C29H40N2O4 | 74.0 | 480.6 | White amorphous powder | Solid | Water soluble | IR, UV | (2S,3R,11bS)-2-[[(1R)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinolin-1-yl]methyl]-3-ethyl-9,10-dimethoxy-2,3,4,6,7,11b-hexahydro-1H-benzo[a]quinolizine |  |
| 21 | Famotidine | C8H15N7O2S3 | 163.5 | 337.5 | White to pale yellow crystals | Solid | In water, 1000 mg/L at 25 °C | Chromatography | 3-[[2-(diaminomethylideneamino)-1,3-thiazol-4-yl]methylsulfanyl]-N′-sulfamoylpropanimidamide |  |
Abbreviations: IUPAC: International Union of Pure and Applied Chemistry; HPLC: High-Performance Liquid Chromatography; UV: Ultra Violet; IR: Infrared; LC: Liquid Chromatography; TLC: Thin-layer chromatography; FTIR: Fourier-transform infrared spectroscopy.
Remdesivir is the only drug that WHO has issue its emergency use authorization. Darunavir, Oseltamivir, and Arbidol showed no improvement and was introduced as ineffective. Notably, despite positive therapeutic effects, Corticosteroids, the double-edge sward group, are limited by WHO for specific comorbid conditions, and the FDA also stops the use of Chloroquine and Hydroxychloroquine. Their clinical trials are paused on 25 May 2020. Meanwhile, Ribavirin and Azithromycin are introduced as promising candidates for co-treatment and other therapies. This simultaneous combination is an excellent approach to employ different pathways in a short time.
Beclabuvir, Saquinqvir, Ledipasivir, Velpatasivir, Galdesivir, Nitazoxanide, and Indinavir are other drugs shown to be efficient in silico and are potential candidates for further in vitro and clinical investigations.
At this time, there are no approved, safe, and effective pharmacologic agents to treat COVID-19 infected patients, and research of all potential medications is essential to fight the virus. Consequently, various pharmacologic agents are now ordered, targeting different phases of virus activity. In this respect, combination therapy may be useful to inhibit virus activity and complications as a consequence. There are inconsistent reports about the efficacy of candidate medications. Currently, further clinical studies should be urgently designed to evaluate the pharmacotherapy agents that seem to be promising and determine the most appropriate modality to diminish the spread of this infection and avoid the burden of any upcoming outbreak.
Regarding preliminary reports in clinical practices, the combined usage of potential antiviral drugs with a different mechanism of action may be a better therapeutic practice for patients with Covid-19 infection.
Funding sources
None.
CRediT author statement
Parastoo Tarighi: Conceptualization, Investigation, Writing- Reviewing and Editing. Samane Eftekhari: Investigation, Writing- Original draft preparation. Mahsa Sabernavaei: Investigation, Writing- Original draft preparation. Milad Chizari: Investigation, Writing- Original draft preparation. Davod Jafari: Visualization, Editing. Parastoo Mirzabeigi: Conceptualization, Investigation, Writing- Reviewing and Editing, Supervision.
Declaration of competing interest
The authors have no conflict of interest.
References
- A Study to Evaluate the Safety and Efficacy of Tocilizumab in Patients With Severe COVID-19 Pneumonia (COVACTA). https://clinicaltrials.gov/ct2/show/NCT04320615.
- Al-Tawfiq J.A., Al-Homoud A.H., Memish Z.A. Remdesivir as a possible therapeutic option for the COVID-19. Trav. Med. Infect. Dis. 2020;34:101615. doi: 10.1016/j.tmaid.2020.101615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al‐Bari M.A.A. Targeting endosomal acidification by chloroquine analogs as a promising strategy for the treatment of emerging viral diseases. Pharma Res Per. 2017;5(1) doi: 10.1002/prp2.293. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreani J., Le Bideau M., Duflot I., Jardot P., Rolland C., Boxberger M., Wurtza N., Rolain J.-M., Colson Ph, La Scola B., Didier Raoult D. In vitro testing of combined hydroxychloroquine and azithromycin on SARS-CoV-2 shows synergistic effect. Microb. Pathog. 2020;145:21–22. doi: 10.1016/j.micpath.2020.104228. 104228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki M., Das D., Hayashi H., Aoki-Ogata H., Takamatsu Y., Ghosh A.K., Mitsuya H. Mechanism of darunavir (DRV)’s high genetic barrier to HIV-1 resistance: a key V32I substitution in protease rarely occurs, but once it occurs, it predisposes HIV-1 to develop DRV resistance. mBio. 2018;9(2) doi: 10.1128/mBio.02425-17. 6. e02425-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aouba A., Baldolli A., Geffray L., Verdon R., Bergot E., Martin-Silva N., Justet A. Targeting the inflammatory cascade with anakinra in moderate to severe COVID-19 pneumonia: case series. Ann. Rheum. Dis. 2020;79:1381–1382. doi: 10.1136/annrheumdis-2020-217706. [DOI] [PubMed] [Google Scholar]
- Baron S.A., Devaux C., Colson P., Raoult D., Rolain J.-M. Teicoplanin: an alternative drug for the treatment of coronavirus COVID-19. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck B.R., Shin B., Choi Y., Park S., Kang K. Predicting commercially available antiviral drugs that may act on the novel coronavirus (SARS-CoV-2) through a drug-target interaction deep learning model. Comput Sstruct Biotechnol. 2020;18:784–790. doi: 10.1016/j.csbj.2020.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevacizumab in Severe or Critically Severe Patients with COVID-19 Pneumonia-RCT (BEST-RCT). https://clinicaltrials.gov/ct2/show/NCT04305106.
- Bevacizumab in severe or critical patients with CoVID-19 pneumonia. https://clinicaltrials.gov/ct2/show/NCT04275414?term=NCT04275414&draw=2&rank=1.
- Blaising J., Lévy P.L., Polyak S.J., Stanifer M., Boulant S., Pécheur E.-I. Arbidol inhibits viral entry by interfering with clathrin-dependent trafficking. Antivir. Ther. 2013;100:215–219. doi: 10.1016/j.antiviral.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Blaising J., Polyak S.J., Pécheur E.-I. Arbidol as a broad-spectrum antiviral: an update. Antivir. Res. 2014;107:84–94. doi: 10.1016/j.antiviral.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleasel M.D., Peterson G.M. Emetine, Ipecac, Ipecac alkaloids and analogues as potential antiviral agents for coronaviruses. Pharmaceuticals. 2020;13:51. doi: 10.3390/ph13030051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogden R.N., Peters D.H. Teicoplanin: a reappraisal of its antimicrobial activity, pharmacokinetic properties and therapeutic efficacy. Drugs. 1994;47:823–854. doi: 10.2165/00003495-199447050-00008. [DOI] [PubMed] [Google Scholar]
- Buijsers B., Yanginlar C., Maciej-Hulme M.L., de Mast Q., van der Vlag J. Beneficial non-anticoagulant mechanisms underlying heparin treatment of COVID-19 patients. EBioMedicine. 2020;59:102969. doi: 10.1016/j.ebiom.2020.102969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q., Yang M., Liu D., Chen J., Shu D., Xia J., Liao X., Gu Y., Cai Q., Yang Y. Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering. 2020 doi: 10.1016/j.eng.2020.03.007. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caly L., Druce J.D., Catton M.G., Jans D.A., Wagstaff K.M. The FDA-approved Drug Ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020;178 doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B., Wang C., Wang Y., Zhou F., Zhang D., Zhao J., Du R., Hu Y., Cheng Z., Gao L. Evaluation of the efficacy and safety of intravenous remdesivir in adult patients with severe pneumonia caused by COVID-19 virus infection: study protocol for a phase 3 randomized, double-blind, placebo-controlled, multicentre trial. Trials. 2020;21:422. doi: 10.1186/s13063-020-04352-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., Ruan L., Song B., Cai Y., Wei M. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N. Engl. J. Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalcanti A.B., Zampieri F.G., Rosa R.G., Azevedo L.C.P., Veiga V.C., Avezum A., Damiani L.P., Marcadenti A., Kawano-Dourado L., Lisboa T., Junqueira D.L.M., de Barros e Silva P.G.M., Tramujas L., Abreu-Silva E.O., Laranjeira L.N., Soares A.T., Echenique L.S., Pereira A.J., Freitas F.G.R., Gebara O.C.E., Dantas V.C.S., Furtado R.H.M., Milan E.P., Golin N.A., Cardoso F.F., Maia I.S., Hoffmann Filho C.R., Kormann A.P.M., Amazonas R.B., Bocchi de Oliveira M.F., Serpa-Neto A., Falavigna M., Lopes R.D., Machado F.R., Berwanger O. Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2019014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalli G., De Luca G., Campochiaro C., Della-Torre E., Ripa M., Canetti D., Oltolini C., Castiglioni B., Din C.T., Boffini N. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. The Lancet Rheumatol. 2020;2:e325–e331. doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli G., Alessandri F., d'Ettorre G., Borrazzo C., Spagnolello O., Oliva A., Ruberto F., Mastroianni C.M., Francesco Pugliese F., Venditti M. Is Teicoplanin A complementary treatment option for Covid-19? The question remains. Int J Antimicrob. 2020;56(2):106029. doi: 10.1016/j.ijantimicag.2020.106029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepelowicz Rajter J., Sherman M., Fatteh N., Vogel F., Sacks J., Rajter J. Use of ivermectin is associated with lower mortality in hospitalized patients with coronavirus disease 2019. CHEST J. 2020;159:85–92. doi: 10.1016/j.chest.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champney W.S., Miller M. Inhibition of 50S ribosomal subunit assembly in Haemophilus influenzae cells by azithromycin and erythromycin. Curr. Microbiol. 2002;44:418–424. doi: 10.1007/s00284-001-0016-6. [DOI] [PubMed] [Google Scholar]
- Chen P., Nirula A., Heller B., Gottlieb R.L., Boscia J., Jason Morris J., Huhn G., Cardona J., Mocherla B., Stosor V., Shawa I., Adams A.C., Naarden J.V., Custer K.L., Shen L., Durante M., Oakley G., Schade A.E., Sabo J., Patel D.R., Klekotka P., Skovronsky D.M. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with COVID-19. N. Engl. J. Med. 2020;384(3):229–237. doi: 10.1056/NEJMoa2029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Huang J., Cheng Z., Wu J., Chen S., Zhang Y., Chen B., Lu M., Luo Y., Zhang J. 2020. Favipiravir Versus Arbidol for COVID-19: A Randomized Clinical Trial. medRxiv. Preprint. [DOI] [Google Scholar]
- Chen C., Qi F., Shi K., Li Y., Li J., Chen Y., Pan J., Zhou T., Lin X., Zhang J. 2020. Thalidomide combined with Low-dose Glucocorticoid in the Treatment of COVID-19 Pneumonia. Preprints. 2020020395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Hu J., Zhang Z., Jiang S., Han S., Yan D., Zhuang R., Hu B., Zhang Z. 2020. Efficacy of Hydroxychloroquine in Patients with COVID-19: results of a Randomized Clinical Trial. MedRxiv. Preprint. [DOI] [Google Scholar]
- Chorin E., Dai M., Shulman E., Wadhwani L., Cohen R.B., Barbhaiya C., Aizer A., Holmes D., Bernstein S., Soinelli M. 2020. The QT Interval in Patients with SARS-CoV-2 Infection treated with Hydroxychloroquine/Azithromycin. MedRxiv. Preprint. [DOI] [PubMed] [Google Scholar]
- Choy K.-T., Wong A.Y.-L., Kaewpreedee P., Sia S.-F., Chen D., Hui K.P.Y., Chu D.K.W., Chan M.C.W., Cheung P.P.-H., Huang X. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antivir. Res. 2020;178:104786. doi: 10.1016/j.antiviral.2020.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinatl J., Morgenstern B., Bauer G., Chandra P., Rabenau H., Doerr H. Treatment of SARS with human interferons. Lancet. 2003;362:293–294. doi: 10.1016/S0140-6736(03)13973-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical trials for ivermectin AND covid-19. https://www.clinicaltrialsregister.eu/ctr-search/search?query=ivermectin+AND+covid-19.
- Costanzo M., De Giglio M., Roviello G. SARS CoV-2: recent reports on antiviral therapies based on lopinavir/ritonavir, darunavir/umifenovir, hydroxychloroquine, remdesivir, favipiravir and other drugs for the treatment of the new coronavirus. Curr. Med. Chem. 2020;27:4536–4541. doi: 10.2174/0929867327666200416131117. [DOI] [PubMed] [Google Scholar]
- Covid 19 Ivermectin. 2020. https://clinicaltrials.gov/ct2/results?term=ivermectin&recrs=abcdefghl&cond=covid19..
- De Luna G., Habibi A., Deux J.F., Colard M., d'Alexandry d.O.A., Schlemmer F., Joher N., Kassasseya C., Pawlotsky J.M., Ourghanlian C. Rapid and severe covid-19 pneumonia with severe acute chest syndrome in a Sickle cell patient successfully treated with tocilizumab. Am Jur Hematol. 2020;95:786–788. doi: 10.1002/ajh.25833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Meyer S., Bojcova D., Cinat J., Van damme E., Van Loock M., Buyck C., Woodfal B., Siecek S. Lack of antiviral activity of darunavir against SARS-CoV-2. Int. J. Infect. Dis. 2020;97:7–10. doi: 10.1016/j.ijid.2020.05.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wilde A.H., Jochmans D., Posthuma C.C., Zevenhoven-Dobbe J.C., van Nieuwkoop S., Bestebroer T.M., van den Hoogen B.G., Neyts J., Snijder E.J. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob. Agents Chemother. 2014;58:4875–4884. doi: 10.1128/AAC.03011-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della-Torre E., Campochiaro C., Cavalli G., De Luca G., Napolitano A., La Marca S., Boffini N., Da Prat V., Di Terlizzi G., Lanzillotta M. Interleukin-6 blockade with sarilumab in severe COVID-19 pneumonia with systemic hyperinflammation: an open-label cohort study. Ann. Rheum. Dis. 2020;79:1277–1285. doi: 10.1136/annrheumdis-2020-218122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giambenedetto S., Ciccullo A., Borghetti A., Gambassi G., Landi F., Visconti E., Zileri Dal Verme L., Bernabei R., Tamburrini E., Cauda R. Off‐label use of tocilizumab in patients with SARS‐CoV‐2 infection. J. Med. Virol. 2020;92:1787–1788. doi: 10.1002/jmv.25897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driggin E., Madhavan M.V., Bikdeli B., Chuich T., Laracy J., Biondi-Zoccai G., Brown T.S., Der Nigoghossian C., Zidar D.A., Haythe J. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J Am. Col. Cardiol. 2020;75:2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y.X., Chen X.P. Favipiravir: pharmacokinetics and concerns about clinical trials for 2019‐nCoV infection. Clin. Pharmacol. Ther. 2020;108:242–248. doi: 10.1002/cpt.1878. [DOI] [PubMed] [Google Scholar]
- Dyall J., Coleman C.M., Hart B.J., Venkataraman T., Holbrook M.R., Kindrachuk J., Johnson R.F., Olinger G.G., Jahrling P.B., Laidlaw M. Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection. Antimicrob. Agents Chemother. 2014;58:4885–4893. doi: 10.1128/AAC.03036-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elavarasi A., Prasad M., Seth T., Sahoo R.K., Madan K., Nischal N., Manish Soneja M., Atul Sharma A., Maulik S.K., Shalimar M.D., Garg P. Chloroquine and hydroxychloroquine for the treatment of COVID-19: a systematic review and meta-analysis. J. Gen. Intern. Med. 2020;35:3308–3314. doi: 10.1007/s11606-020-06146-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfiky A.A. Anti-HCV, nucleotide inhibitors, repurposing against COVID-19. Life Sci. 2020;248:117477. doi: 10.1016/j.lfs.2020.117477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas H. Icatibant as acute treatment for hereditary angioedema in adults. Expet Rev. Clin. Pharmacol. 2016;9:779–788. doi: 10.1080/17512433.2016.1182425. [DOI] [PubMed] [Google Scholar]
- Fatima U., Rizvi S.S.A., Fatima S., Hassan MdI. Impact of hydroxychloroquine/chloroquine in COVID-19 therapy: two sides of the coin. Interferon Cytokine Res. 2020;40(10) doi: 10.1089/jir.2020.0105. [DOI] [PubMed] [Google Scholar]
- Fletcher-Sandersjöö A., Bellander B.-M. Is COVID-19 associated thrombosis caused by overactivation of the complement cascade? A literature review. Tromb Res. 2020;194:36–41. doi: 10.1016/j.lfs.2020.117477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Drug Administration . 2020. Fact sheet for Healthcare Providers: emergency Use Authorization (EUA) of Bamlanivimab.https://www.fda.gov/media/143603/download [Google Scholar]
- Franks M.E., Macpherson G.R., Figg W.D. Thalidomide. The Lancet. 2004;363:1802–1811. doi: 10.1016/S0140-6736(04)16308-3. [DOI] [PubMed] [Google Scholar]
- Fu Y., Cheng Y., Wu Y. Understanding SARS-CoV-2-mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol. Sin. 2020;35:266–271. doi: 10.1007/s12250-020-00207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furtado R.H.M., Berwanger O., Fonseca H.A., Corrêa T.D., Ferraz L.R., Maura G Lapa M.G., Zampieri F.G., Veiga V.C., Azevedo L.C.P., Rosa R.G., Lopes R.D., Avezum A., Manoel A.L.O., Piza F.M.T., Martins P.A., Lisboa T.C., Pereira A.J., Olivato G.B., Dantas V.C.S., Milan E.P., Gebara O.C.E., Amazonas R.B., Oliveira M.B., Soares R.V.P., Moia D.D.F., Piano L.P.A., Castilho K., Roberta G.R.A.P., Momesso R.G.R.A.P., Schettino G.P.P., Rizzo L.V., Neto A.S., Machado F.R., Cavalcanti A.B. Azithromycin in addition to standard of care versus standard of care alone in the treatment of patients admitted to the hospital with severe COVID-19 in Brazil (COALITION II): a randomised clinical trial. Lancet. 2020;396:959–967. doi: 10.1016/S0140-6736(20)31862-6. 10256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J., Hurwitz H.I., Sandler A.B., Miles D., Coleman R.L., Deurloo R., Chinot O.L. Bevacizumab (Avastin®) in cancer treatment: a review of 15 years of clinical experience and future outlook. Canc. Treat Rev. 2020 doi: 10.1016/j.ctrv.2020.102017. [DOI] [PubMed] [Google Scholar]
- Gautret P., Lagier J.-C., Parola P., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Dupont H.T. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020;56:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gautret P., Lagier J.-C., Parola P., Meddeb L., Sevestre J., Mailhe M., Doudier B., Aubry C., Amrane S., Seng P. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: a pilot observational study. Trav. Med. Infect. Dis. 2020;34:101663. doi: 10.1016/j.tmaid.2020.101663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genentech I. 2017. Actemra (tocilizumab) Prescribing Information. [Google Scholar]
- Giannis D., Ziogas I.A., Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J. Clin. Virol. 2020;127:104362. doi: 10.1016/j.jcv.2020.104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozzo L., Viale P., Longo L., Vitale D.C., Drago F. The potential role of heparin in patients with COVID-19: beyond the anticoagulant effect. A review. Front Pharmacol. 2020;11 doi: 10.3389/fphar.2020.01307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A., Feldt T., Green G., Green M.L., Lescure F.-X. Compassionate use of remdesivir for patients with severe covid-19. N. Engl. J. Med. 2020;382:2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremese E., Cingolani A., Bosello S.L., Alivernini S., Tolusso B., Perniola S., Landie F., Pompilig M., Murric R., Santoliquidoi A., Garcovichg M., Salij M., Pascalel G.D., Gabriellin M., Biscetti F., Montaltoi M., Tosonig A., Gambassio G., Rapaccinig G.L., Iaconelli A., Vermeg L.Z.D., Petricca L., Fedele A.L., Lizzioa M.M., Tamburrini E., Natalello G., Gigante L., Brunob D., Verardib L., Taddei E., Calabrese A., Lombardi F., Bernabeie R., Caudac R., Franceschin F., Landolfii R., Richeldi L., Sanguinettij M., Fantonic M., Antonellil M., Gasbarrini A. Sarilumab use in severe SARS-CoV-2 pneumonia. Clin. Med. 2020;27 doi: 10.1016/j.eclinm.2020.100553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubareva L.V., Kaiser L., Hayden F.G. Influenza virus neuraminidase inhibitors. Lancet. 2000;355:827–835. doi: 10.1016/S0140-6736(99)11433-8. [DOI] [PubMed] [Google Scholar]
- Hashim H.A., Maulood M.F., Rasheed A.M., Fatak D.F., Kabah K.K., Abdulamir A.S. 2020. Controlled Randomized Clinical Trial on Using Ivermectin with Doxycycline for Treating COVID-19 Patients in Baghdad, Iraq. medRxiv. [DOI] [Google Scholar]
- Hassan S.A., Sheikh F.N., Jamal S., Ezeh J.K., Akhtar A. Coronavirus (COVID-19): a review of clinical features, diagnosis, and treatment. Cureus. 2020;12 doi: 10.7759/cureus.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haviernik J., Štefánik M., Fojtíková M., Kali S., Tordo N., Rudolf I., Hubálek Z., Eyer L., Ruzek D. Arbidol (Umifenovir): a broad-spectrum antiviral drug that inhibits medically important arthropod-borne flaviviruses. Viruses. 2018;10:184. doi: 10.3390/v10040184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempel T., Raich L., Olsson S., Azouz N.P., Klingler A.M., Rothenberg M.E., Noé F. 2020. Molecular Mechanism of SARS-CoV-2 Cell Entry Inhibition via TMPRSS2 by Camostat and Nafamostat Mesylate. BioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hifumi T., Isokawa S., Otani N., Ishimatsu S. Adverse events associated with nafamostat mesylate and favipiravir treatment in COVID-19 patients. Crit. Care. 2020;24:497. doi: 10.1186/s13054-020-03227-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillaker E., Belfer J.J., Bondici A., Murad H., Dumkow L.E. Delayed initiation of remdesivir in a COVID‐19 positive patient. Pharmacother. 2020;40:592–598. doi: 10.1002/phar.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;171:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Schroeder S., Kleine-Weber H., Müller M.A., Drosten C., Pöhlmann S. Nafamostat mesylate blocks activation of SARS-CoV-2: new treatment option for COVID-19. Antimicrob. Agents Chemother. 2020;64 doi: 10.1128/AAC.00754-20. e00754-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horby P., Mafham M., Linsell L., Bell J., Staplin N., Emberson J., Wiselka M., Ustianowski A., Elmahi E., Prudon B., Whitehouse A., Felton T., Williams J., Faccenda J., Underwood J., Baillie K., Chappell L., Faust S., Jaki T., Landray M. 2020. Effect of Hydroxychloroquine in Hospitalized Patients with COVID-19: preliminary Results from a Multi-centre, Randomized, Controlled Trial. medRxiv. [DOI] [Google Scholar]
- Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., Staplin N., Brightling C., Ustianowski A., Elmahi E., Prudon B., Green C., Felton T., Chadwick D., Rege K., Fegan C., Chappell L.C., Faust S.N., Jaki T., Jeffery K., Montgomery A., Kathryn Rowan K., Juszczak E., Baillie J.K., Haynes R., Landray M.J. Dexamethasone in hospitalized patients with COVID-19 - preliminary report. N. Engl. J. Med. 2020:1–11. doi: 10.1056/NEJMoa2021436. [DOI] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huet T., Beaussier H., Voisin O., Jouveshomme S., Dauriat G., Lazareth I., Sacco E., Naccache J.-M., Bézie Y., Laplanche S. Anakinra for severe forms of COVID-19: a cohort study. The Lancet Rheumatol. 2020;2:e393–e400. doi: 10.1016/S2665-9913(20)30164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulseberg C., Fénéant L., Szymańska-de Wijs K., Kessler N., Nelson E., Shoemaker C., Schmaljohn C., Polyak S., White J. Arbidol and other low-molecular-weight drugs that inhibit Lassa and Ebola viruses. J. Virol. 2019;93 doi: 10.1128/JVI.02185-18. e02185-02118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung I.F., Lung K., Tso E.Y., Liu R., Chung T.W., Chu M., Ng Y., Lo J., Chan J., Tam A.R., Shum H., Chan V., Wu A.K., Sin K.M., Leung W.S., Law W., Lung D.C., Sin S., Yeung P., Yip C.C., Zhang R.R., Fung A.Y., Yan E.Y., Leung K., Ip J.D., Chu A.W., Chan W., Ng A.C., Lee R., Fung K., Yeung A., Wu T., Chan J.W., Yan W.W., Chan W.M., Chan J.F., Lie A.K., Tsang O.T., Cheng V.C., Que T., Lau C., Chan K., To K.K., Yuen K. Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 tria. Lancet. 2020;30:1695–1704. doi: 10.1016/S0140-6736(20)31042-4. 395, 10238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S., Rhee J.-Y. Three cases of treatment with Nafamostat in elderly patients with COVID-19 pneumonia who need oxygen therapy. Int. J. Infect. Dis. 2020;96:500–502. doi: 10.1016/j.ijid.2020.05.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowitz T., Gablenz E., Pattinson D., Wang T.C., Conigliaro J., Tracey K., Tuveson D. Famotidine use and quantitative symptom tracking for COVID-19 in non-hospitalised patients: a case series. Gut. 2020;69:1592–1597. doi: 10.1136/gutjnl-2020-321852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean S.-S., Lee P.-I., Hsueh P.-R. Treatment options for COVID-19: the reality and challenges. J. Microbiol. Immunol. Infect. 2020;53:436–442. doi: 10.1016/j.jmii.2020.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadam R.U., Wilson I.A. Structural basis of influenza virus fusion inhibition by the antiviral drug Arbidol. Proc. Natl. Acad. Sci. U. S. A. 2017;114(2):206–214. doi: 10.1073/pnas.1617020114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaldor S.W., Kalish V.J., Davies J.F., Shetty B.V., Fritz J.E., Appelt K., Burgess J.A., Campanale K.M., Chirgadze N.Y., Clawson D.K. Viracept (nelfinavir mesylate, AG1343): a potent, orally bioavailable inhibitor of HIV-1 protease. J. Med. Chem. 1997;40:3979–3985. doi: 10.1021/jm9704098. [DOI] [PubMed] [Google Scholar]
- Kelleni M. Nitazoxanide/Azithromycin combination for COVID-19: a suggested new protocol for COVID-19 early management. Pharmacol. Res. 2020;157:104874. doi: 10.20944/preprints202004.0432.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M.J., Kitsis E.A., Arora S., Jen-Ting Chen J.-T., Shivani Agarwal S., Ross M.J., Tomer Y., Southern W. Effect of systemic glucocorticoids on mortality or mechanical ventilation in patients with COVID-19. J. Hosp. Med. 2020;15(8):489–493. doi: 10.12788/jhm.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil A., Kamar A., Nemer G. Thalidomide-revisited: are COVID-19 patients going to be the latest victims of yet another theoretical drug-repurposing? Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.01248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalili J.S., Zhu H., Mak A., Yan Y., Zhu Y. Novel coronavirus treatment with ribavirin: groundwork for evaluation concerning COVID‐19. J. Med. Virol. 2020;92:740–746. doi: 10.1002/jmv.25798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S.A., Zia K., Ashraf S., Uddin R., Ul-Haq Z. Identification of chymotrypsin-like protease inhibitors of SARS-CoV-2 via integrated computational approach. J. Biomol. Struct. Dyn. 2020;1–10 doi: 10.1080/07391102.2020.1751298. [DOI] [PubMed] [Google Scholar]
- Khanal P. Remdesivir for COVID-19 treatment: mechanism OF action, synthesis, and clinical trials. J Pharm. Pharm. Sci. 2020;9(8):1062–1068. doi: 10.20959/wjpps20208-16808. [DOI] [Google Scholar]
- King A., Vail A., O'Leary C., Hannan C., Brough D., Patel H., Galea J., Ogungbenro K., Wright M., Pathmanaban O. Anakinra in COVID-19: important considerations for clinical trials. Lancet Rheumatol. 2020 doi: 10.1016/S2665-9913(20)30160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klok F., Kruip M., Van der Meer N., Arbous M., Gommers D., Kant K., Kaptein F., van Paassen J., Stals M., Huisman M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopfensteina T., Zayeta S., Anne Lohseb A., Sellesc P., Zahrad H., Kadiane-Oussoua N., Lynda Tokoa L., Royera P., Balblancb J., Gendrina V., Conrozierb T. Impact of tocilizumab on mortality and/or invasive mechanical ventilation requirement in a cohort of 206 COVID-19 patients. Int. J. Infect. Dis. 2020;99:491–495. doi: 10.1016/j.ijid.2020.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko W.-C., Rolain J.-M., Lee N.-Y., Chen P.-L., Huang C.-T., Lee P.-I., Hsueh P.-R. Arguments in favour of remdesivir for treating SARS-CoV-2 infections. Int. J. Antimicrob. Agents. 2020;55:105933. doi: 10.1016/j.ijantimicag.2020.105933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat. Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- iving guidance. 2020. https://www.who.int/publications/i/item/WHO-2019-nCoV-Corticosteroids-2020.1..
- La Scola, B., Le Bideau, M., Andreani, J., Hoang, V.T., Grimaldier, C., Colson, P., Gautret, P., Raoult, D., Viral RNA load as determined by cell culture as a management tool for discharge of SARS-CoV-2 patients from infectious disease wards. Eur. J. Clin. Microbiol. Infect. Dis.. 39, 1059-1061. 10.1007/s10096-020-03913-9. [DOI] [PMC free article] [PubMed]
- Lai C.-C., Shih T.-P., Ko W.-C., Tang H.-J., Hsueh P.-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and corona virus disease-2019 (COVID-19): the epidemic and the challenges. Int. J. Antimicrob. Agents. 2020;55:105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Li W., Jin Y., Xu W., Huang C., Li L., Huang Y., Fu Q., Chen L. Efficacy evaluation of early, low-dose, short-term corticosteroids in adults hospitalized with NonSevere COVID-19 pneumonia: a retrospective cohort study. Infect. Dis. Ther. 2020;9:823–836. doi: 10.1007/s40121-020-00332-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Hu R., Zhang X. Antihypertensive treatment with ACEI/ARB of patients with COVID-19 complicated by hypertension. Hypertens. Res. 2020;43:588–590. doi: 10.1038/s41440-020-0433-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Wang Y., Xu J., Cao B. Potential antiviral therapeutics for 2019 novel coronavirus. Zhonghua Jiehe He Huxi Zazhi. 2020;43 doi: 10.3760/cma.j.issn.1001-0939.2020.0002. E002-E002. Full text in chinese. [DOI] [PubMed] [Google Scholar]
- Li Y., Xie Z., Lin W., Cai W., Wen C., Guan Y., Mo X., Wang J., Wang Y., Peng P., Chen X., Hong W., Xiao G., Liu J., Zhang L., Hu F., Li F., Zhang F., Deng X., Li L. Efficacy and safety of Lopinavir/Ritonavir or Arbidol in adult patients with mild/moderate COVID-19: An exploratory randomized controlled trial. Med (N Y) 2020;1(1):105–113. doi: 10.1016/j.medj.2020.04.001. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian L., Xie H., Lin S., Huang J., Zhao J., Lin Q. Umifenovir treatment is not associated with improved outcomes in patients with coronavirus disease 2019: a retrospective study. Clin. Microbiol. Infect. 2020;26(7):917–921. doi: 10.1016/j.cmi.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J., Jeon S., Shin H.-Y., Kim M.J., Seong Y.M., Lee W.J., Choe K.-W., Kang Y.M., Lee B., Park S.-J. Case of the index patient who caused tertiary transmission of Coronavirus disease 2019 in Korea: the application of lopinavir/ritonavir for the treatment of COVID-19 pneumonia monitored by quantitative RT-PCR. J. Kor. Med. Sci. 2020;35 doi: 10.3346/jkms.2020.35.e79. [DOI] [PubMed] [Google Scholar]
- Liu J., Cao R., Xu M., Wang X., Zhang H., Hu H., Li Y., Hu Z., Zhong W., Wang M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:1–4. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K., Fang Y.-Y., Deng Y., Liu W., Wang M.-F., Ma J.-P., Xiao W., Wang Y.-N., Zhong M.-H., Li C.-H. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin. Med. J. 2020;133 doi: 10.1097/cm9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokugamage K.G., Hage A., de Vries M., Valero-Jimenez A.M., Schindewolf C., Dittmann M., Rajsbaum R., Menachery V.D. Type I interferon susceptibility distinguishes SARSCoV-2 from SARS-CoV. J. Virol. 2020 doi: 10.1128/JVI.01410-20. 94:e01410-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losartan for Patients With COVID-19 Requiring Hospitalization. https://clinicaltrials.gov/ct2/show/NCT04312009.
- Losartan for patients with COVID-19 not requiring hospitalization.https://www.clinicaltrials.gov/ct2/show/NCT04311177.
- Luo P., Liu Y., Qiu L., Liu X., Liu D., Li J. Tocilizumab treatment in COVID‐19: a single center experience. J. Med. Virol. 2020;92:814–818. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud D.B., Shitu Z., Mostafa A. Drug repurposing of nitazoxanide: can it be an effective therapy for COVID-19? J Genet Eng Biotechnol. 2020;18:1–10. doi: 10.1186/s43141-020-00055-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantlo E.K., Bukreyeva N., Maruyama J., Paessler S., Huang C. Potent antiviral activities of Type I interferons to SARS-CoV-2 infection. Antivir. Res. 2020;179:104811. doi: 10.1016/j.antiviral.2020.104811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez F.J., Curtis J.L., Albert R. Role of macrolide therapy in chronic obstructive pulmonary disease. Int. J. Chronic Obstr. Pulm. Dis. 2008;3(3):331–350. doi: 10.2147/copd.s681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama S., Kawase M., Nao N., Shirato K., Ujike M., Kamitani W., Shimojima M., Fukushi S. 2020. The Inhaled Corticosteroid Ciclesonide Blocks Coronavirus RNA Replication by Targeting Viral NSP15. BioRxiv. Preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Z., Wang T., Li C., Chen X., Li L., Qin X., Li H., Luo J. 2020. An Experimental Trial of Recombinant Human Interferon Alpha Nasal Drops to Prevent Coronavirus Disease 2019 in Medical Staff in an Epidemic Area. MedRxiv. Preprint. [DOI] [Google Scholar]
- Michot J.-M., Albiges L., Chaput N., Saada V., Pommeret F., Griscelli F., Balleyguier C., Besse B., Marabelle A., Netzer F. Tocilizumab, an anti-IL6 receptor antibody, to treat Covid-19-related respiratory failure: a case report. Ann. Oncol. 2020;31:961–964. doi: 10.1016/j.annonc.2020.03.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk P.D., Marsden R.J., Tear V.J., Brookes J., Batten T.N., Mankowski M., Gabbay F.J., Davies D.E., Holgate S.T., Ho L., Clark T., Djukanovic R., Wilkinson T.M.A. Safety and efficacy of inhaled nebulised interferon beta-1a (SNG001) for treatment of SARS-CoV-2 infection: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Respir Med. 2020:1–11. doi: 10.1016/S2213-2600(20)30523-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muralidharan N., Sakthivel R., Velmurugan D., Gromiha M.M. Computational studies of drug repurposing and synergism of lopinavir, oseltamivir and ritonavir binding with SARS-CoV-2 Protease against COVID-19. J. Biomol. Struct. Dyn. 2020;1–6 doi: 10.1080/07391102.2020.1752802. [DOI] [PubMed] [Google Scholar]
- Musarrat F., Chouljenko V., Dahal A., Nabi R., Chouljenko T., Jois S.D., Kousoulas K.G. The anti-HIV drug nelfinavir mesylate (Viracept) is a potent inhibitor of cell fusion caused by the SARSCoV-2 spike (S) glycoprotein warranting further evaluation as an antiviral against COVID-19 infections. J. Med. Virol. 2020;6 doi: 10.1002/jmv.25985. 10.1002/jmv.25985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasim S., Kumar S., Azim D., Ashraf Z., Azeem Q. Corticosteroid use for 2019-nCoV infection: a double-edged sword. Infect. Control Hosp. Epidemiol. 2020;1–6 doi: 10.1017/ice.2020.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Allergy and Infectious Diseases. Statement—NIH-sponsored ACTIV-3 trial closes LY-CoV555 sub-study. https://www.niaid.nih.gov/news-events/statement-nih-sponsored-activ-3-trial-closes-ly-cov555-sub-study.
- Navarro M., Camprubí D., Requena-Méndez A., Buonfrate D., Giorli G., Kamgno J., Gardon J., Boussinesq M., Muñoz J., Krolewiecki A. Safety of high-dose ivermectin: a systematic review and meta-analysis. J. Antimicrob. Chemother. 2020;75:827–834. doi: 10.1093/jac/dkz524. [DOI] [PubMed] [Google Scholar]
- nlmhttps://pubchem.ncbi.nlm.nih.gov/.
- Nyström K., Waldenström J., Tang K.-W., Lagging M. Ribavirin: pharmacology, multiple modes of action and possible future perspectives. Future Virol. 2019;14:153–160. doi: 10.2217/fvl-2018-0166. [DOI] [Google Scholar]
- Offord C. 2020. Flu and HIV Drugs Show Efficacy against Coronavirus.https://www.the-scientist.com/news-opinion/flu-and-anti-hiv-drugs-show-efficacy-against-coronavirus-67052 [Google Scholar]
- Owa A.B., Owa O.T. Lopinavir/ritonavir use in Covid-19 infection: is it completely non-beneficial? J. Microbiol. Immunol. Infect. 2020;53(5):674–675. doi: 10.1016/j.jmii.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien T.R., Thomas D.L., Jackson S.S., Prokunina-Olsson L., Donnelly R.P., Hartmann R. Weak induction of interferon expression by SARS-CoV-2 supports clinical trials of interferon Lambda to treat early COVID-19. Clin. Infect. Dis. 2020;71:1410–1412. doi: 10.1093/cid/ciaa453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang J., Xu F., Aondio G., Li Yu, Fumagalli A., Lu M., Giuseppe Valmadre G., Wei J., Bian Y., Canesi M., Damiani G., Zhang Y., Dexin Yu D., Chen J., Ji X., Sui W., Wang B., Wu S., Kovacs A., Revera M., Wang H., Zhang Y., Yuguo Chen Y., Cao Y. 2020. Efficacy and Tolerability of Bevacizumab 2 in Patients with Severe Covid -19. medRxiv and bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pant S., Singh M., Ravichandiran V., Murty U., Srivastava H.K. Peptide-like and small-molecule inhibitors against Covid-19. J. Biomol. Struct. Dyn. 2020;1–10 doi: 10.1080/07391102.2020.1757510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnham M.J., Haber V.E., Giamarellos-Bourboulis E.J., Perletti G., Verleden G.M., Vos R. Azithromycin: mechanisms of action and their relevance for clinical applications. Pharmacol. Ther. 2014;143:225–245. doi: 10.1016/j.pharmthera.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Patrì A., Fabbrocini G. Hydroxychloroquine and ivermectin: a synergistic combination for COVID-19 chemoprophylaxis and/or treatment? J. Am. Acad. Dermatol. 2020;82:e221. doi: 10.1016/j.jaad.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson J.L., Fernandez-Larsson R. Molecular mechanisms of action of ribavirin. Rev. Infect. Dis. 1990;12:1139–1146. doi: 10.1093/clinids/12.6.1139. [DOI] [PubMed] [Google Scholar]
- Payne S. Family Coronaviridae. Viruses. 2017:149–158. doi: 10.1016/B978-0-12-803109-4.00017-9. 2017. [DOI] [Google Scholar]
- Pepperrell T., Pilkington V., Owen A., Wang J., Hill A.M. Review of safety and minimum pricing of nitazoxanide for potential treatment of COVID-19. J Vir Erad. 2020;6:52–60. doi: 10.1016/S2055-6640(20)30017-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyak S., White J. Arbidol and other low-molecular-weight drugs that inhibit Lassa and Ebola viruses. J. Virol. 2019;93 doi: 10.1128/JVI.02185-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponticelli C., Moroni G. Hydroxychloroquine in systemic lupus erythematosus (SLE) Expet Opin. Drug Saf. 2017;16:411–419. doi: 10.1080/14740338.2017.1269168. [DOI] [PubMed] [Google Scholar]
- Qi T., Limin D., YanLing M., Feng W., Qi H., Kaimin M., Wenjing X., Hui X., Shujing Z.E.Z., Pei M., Siwei S., YuMei Li, Zilin Z., Yice S., Zeyu L., Wei G., Zengrong Y., Yang J. Is oseltamivir suitable for fighting against COVID-19: in silico assessment, in vitro and retrospective study. Bioorg. Chem. 2020;104:104257. doi: 10.1016/j.bioorg.2020.104257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radbel J., Narayanan N., Bhatt P.J. Use of tocilizumab for COVID-19 infection-induced cytokine release syndrome: a cautionary case report. Chest. 2020;158:e15–e19. doi: 10.1016/j.chest.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthy S., Cidlowski J.A. Corticosteroids: mechanisms of action in health and disease. Rheum. Dis. Clin. 2016;42:15–31. doi: 10.1016/j.rdc.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoades, A., 2020. https://www.fda.gov/media/137564/download.
- Riva A., Conti F., Bernacchia D., Pezzati L., Sollima S., Merli S., Siano M., Lupo A., Rusconi S., Cattaneo D. Darunavir does not prevent SARS-CoV-2 infection in HIV patients. Pharmacol. Res. 2020;157:104826. doi: 10.1016/j.phrs.2020.104826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocco P.R.M., Silva P.L., Cruz F.F., Junior M.A.C.M., Tierno P.F.G.M.M., Moura M.A., Oliveira L.F.G., Lima C.C., Santos E.A.D., Junior W.F., Fernandes A.P.S.M., Franchini K.G., Magri E., Moraes N.F., Gonçalves J.M.J., Carbonieri M.N., Santos I.S.D., Paes N.F., Maciel P.V.M., Rocha R.P., Carvalho A.F.E., Alves P.A., Modena J.L.P., Cordeiro A.T., Trivella D.B.B., Marques R.E., Luiz R.R., Pelosi P., Silva J.R.L. Early use of nitazoxanide in mild Covid-19 disease: randomized, placebo controlled trial. Eur. Respir. J. 2020 doi: 10.1183/13993003.03725-2020. 2003725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romark initiates new phase 3 clinical trial of NT-300 for the treatment of COVID-19. Romark Pharmaceuticals. 2020. https://clinicaltrials.gov/ct2/show/NCT04359680.
- Rosa S.G.V., Santos W.C. Clinical trials on drug repositioning for COVID-19 treatment. Rev. Panam. Salud Públic. 2020;44:e40. doi: 10.26633/RPSP.2020.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha A., Sharma A.R., Bhattacharya M., Sharma G., Lee S.-S., Chakraborty C. Probable molecular mechanism of remdesivir for the treatment of COVID-19: need to know more. Arch. Med. Res. 2020;51(6):585–586. doi: 10.1016/j.arcmed.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainz B., Jr., Mossel E.C., Peters C., Garry R.F. Interferon-beta and interferon-gamma synergistically inhibit the replication of severe acute respiratory syndrome-associated coronavirus (SARS-CoV) Virology. 2004;329:11–17. doi: 10.1016/j.virol.2004.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallard E., Lescure F.-X., Yazdanpanah Y., Mentre F., Peiffer-Smadja N., Florence A., Yazdanpanah Y., Mentre F., Lescure F.-X., Peiffer-Smadja N. Type 1 interferons as a potential treatment against COVID-19. Antivir. Res. 2020;178:104791. doi: 10.1016/j.antiviral.2020.104791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethia R., Manya Prasad M., Mahapatra S.J., Nischal N., Manish Soneja M., Garg P., Shalimar . 2020. Efficacy of Famotidine for COVID-19: A Systematic Review and Meta-Analysis. medRxiv. [DOI] [Google Scholar]
- Shiraki K., Daikoku T. Favipiravir, an anti-influenza drug against life-threatening RNA virus infections. Pharmacol. Ther. 2020;209:107512. doi: 10.1016/j.pharmthera.2020.107512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha D.B., Budhathoki P., Khadka S., Shah P.B., Pokharel N., Rashmi P. Favipiravir versus other antiviral or standard of care for COVID-19 treatment: a rapid systematic review and meta-analysis. Virol. J. 2020;17:141. doi: 10.1186/s12985-020-01412-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H., Chauhan P., Kakkar A.K. Hydroxychloroquine for the treatment and prophylaxis of COVID-19: the journey so far and the road ahead. Eur. J. Pharmacol. 2020;173717 doi: 10.1016/j.ejphar.2020.173717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siordia J.A., Bernaba M., Yoshino K., Ulhaque A., Kumar S., Bernaba M., Bergin E. Systematic and statistical review of coronavirus disease 19 treatment trials. SN Compr. Clin. Med. 2020;2:1120–1131. doi: 10.1007/s42399-020-00399-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y., Zhang M., Yin L., Wang K., Zhou Y., Zhou M., Lu Y. COVID-19 treatment: close to a cure?–A rapid review of pharmacotherapies for the novel coronavirus. Int. J. Antimicrob. Agents. 2020;56:106080. doi: 10.1016/j.ijantimicag.2020.106080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spagnuolo V., Castagna A., Lazzarin A. Darunavir for the treatment of HIV infection. Expet Opin. Pharmacother. 2018;19:1149–1163. doi: 10.1080/14656566.2018.1484901. [DOI] [PubMed] [Google Scholar]
- Spezzani V., Piunno A., Iselin H.-U. Benign COVID-19 in an immunocompromised cancer patient-the case of a married couple. Swiss Med. Wkly. 2020;150:20246. doi: 10.4414/smw.2020.20246. [DOI] [PubMed] [Google Scholar]
- Sterne J.A.C., Murthy S., Diaz J.V., Slutsky A.S., Villar J., Angus D.C., Annane D., Azevedo L.C.P., Berwanger O., Cavalcanti A.B., Dequin P.-F., Bin Du B., Emberson J., Fisher D., Giraudeau B., Gordon A.C., Granholm A., Green C., Haynes R., Heming N., Higgins J.P.T., Horby P., Jüni P., Landray M.J., Gouge A.L., Leclerc M., Lim W.S., Machado F.R., McArthur C., Meziani F., Møller M.H., Perner A., Petersen W.M., Savović J., Tomazini B., Veiga V.C., Webb S., Marshall J.C. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19 A meta-analysis. J. Am. Med. Assoc. 2020;324(13):1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taiub A., Chowdhury M.M., Shahbaz M., Karim R., Islam J., Guo D. 2020. A Randomized Trial of Ivermectin-doxycycline and Hydroxychloroquine-azithromycin Therapy on COVID19 Patients. Research Square. [DOI] [Google Scholar]
- Tanaka T., Narazaki M., Kishimoto T. IL-6 en la inflamación, la inmunidad, y la enfermedad. Cold Spring Harb Perspect Biol. 2014;6:a016295. doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Te H.S., Randall G., Jensen D.M. Mechanism of action of ribavirin in the treatment of chronic hepatitis C. J. Gastroenterol. Hepatol. 2007;3(3):218–225. PMID: 21960835. [PMC free article] [PubMed] [Google Scholar]
- The efficacy and safety of thalidomide combined with low-dose hormones in the treatment of severe Covid-19. https://clinicaltrials.gov/ct2/show/NCT04273581?term=NCT04273581&draw=2&rank=1.
- The efficacy and safety of thalidomide in the adjuvant treatment of moderate new coronavirus (Covid-19) pneumonia. https://clinicaltrials.gov/ct2/show/NCT04273529?term=NCT04273529&draw=2&rank=1..
- To evaluate the clinical efficacy of sarilumab relative to the control arm in adult patients hospitalized with severe or critical COVID-19. https://clinicaltrials.gov/ct2/show/NCT04327388..
- Tocilizumab in COVID-19 pneumonia (TOCIVID-19) (TOCIVID-19). https://clinicaltrials.gov/ct2/show/NCT04317092..
- Tocilizumab for SARS-CoV2 (COVID-19) Severe Pneumonitis. https://clinicaltrials.gov/ct2/show/NCT04315480.
- Tolouian R., Vahed S.Z., Ghiyasvand S., Tolouian A., Ardalan M. COVID-19 interactions with angiotensin-converting enzyme 2 (ACE2) and the kinin system; looking at a potential treatment. J. Ren. Inj. Prev. 2020;9:e19. doi: 10.34172/jrip.2020.19. [DOI] [Google Scholar]
- Tong S., Su Y., Yu Y., Wu C., Chen J., Wang S., Jiang J. Ribavirin therapy for severe COVID-19: a retrospective cohort study. Int. J. Antimicrob. Agents. 2020;56(3) doi: 10.1016/j.ijantimicag.2020.106114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Y.-F., Chien C.-S., Yarmishyn A.A., Lin Y.-Y., Luo Y.-H., Lin Y.-T., Lai W.-Y., Yang D.-M., Chou S.-J., Yang Y.-P. A review of SARS-CoV-2 and the ongoing clinical trials. J. Med. Virol. 2020;21:2657. doi: 10.3390/ijms21072657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- usphttps://www.usp.org/.
- Uzunova K., Filipova E., Pavlova V., Vekov T. Insights into antiviral mechanisms of remdesivir, lopinavir/ritonavir and chloroquine/hydroxychloroquine affecting the new SARS-CoV-2. Biomed. Pharmacother. 2020 doi: 10.1016/j.biopha.2020.110668. 110668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Veerdonk F., Netea M.G., van Deuren M., van der Meer J.W., de Mast Q., Bruggemann R.J., van der Hoeven H. 2020. Kinins and Cytokines in COVID-19: a Comprehensive Pathophysiological Approach. Preprints. 2020040023. [DOI] [Google Scholar]
- Vincent M.J., Bergeron E., Benjannet S., Erickson B.R., Rollin P.E., Ksiazek T.G., Seidah N.G., Nichol S.T. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls A.C., Park Y.-J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–292. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Fei D., Vanderlaan M., Song A. Biological activity of bevacizumab, a humanized anti-VEGF antibody in vitro. Angiogenesis. 2004;7:335–345. doi: 10.1007/s10456-004-8272-2. [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhang D., Du G., Du R., Jianping Zhao J., Jin Y., Shouzhi Fu S., Gao L., Zhenshun Cheng Z., Lu Q., Hu Y., Guangwei Luo G., Wang K., Lu Y., Li H., Wang S., Ruan S., Yang C., Mei C., Wang Yi, Ding D., Wu F., Tang X., Ye X., Ye Y., Liu B., Yang J., Yin W., Wang A., Fan G., Zhou F., Liu Z., Gu X., Xu J., Shang L., Zhang Y., Cao L., Guo T., Wan Y., Qin H., Jiang Y., Jaki T., Hayden F.G., Horby P.W., Bin Cao B., Wang C. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-31029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. Jama. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Zhang Y., Wu L., Niu S., Song C., Zhang Z., Lu G., Qiao C., Hu Y., Yuen K.-Y. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell. 2020;181:894–904. doi: 10.1016/j.cell.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Jiang W., He Q., Wang C., Wang B., Zhou P., Dong N., Tong Q. 2020. Early, Low-dose and Short-term Application of Corticosteroid Treatment in Patients with Severe COVID-19 Pneumonia: single-Center Experience from Wuhan, China. MedRxiv. [DOI] [Google Scholar]
- Ward P., Small I., Smith J., Suter P., Dutkowski R. Oseltamivir (Tamiflu®) and its potential for use in the event of an influenza pandemic. J. Antimicrob. Chemother. 2005;55:i5–i21. doi: 10.1093/jac/dki018. [DOI] [PubMed] [Google Scholar]
- Wu C., Liu Y., Yang Y., Zhang P., Zhong W., Wang Y., Wang Q., Xu Y., Li M., Li X. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B. 2020;10:766–788. doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Liu J., Zhao X., Liu C., Wang W., Wang D., Xu W., Zhang C., Yu J., Jiang B. Clinical characteristics of imported cases of COVID-19 in jiangsu province: a multicenter descriptive study. Clin. Infect. Dis. 2020;71:706–712. doi: 10.1093/cid/ciaa199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Han M., Li T., Sun W., Wang D., Fu B., Zhou Y., Zheng X., Yang Y., Li X. Effective treatment of severe COVID-19 patients with tocilizumab. Pnas. 2020;17 doi: 10.1073/pnas.2005615117. v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Peng C., Shi Y., Zhu Z., Mu K., Wang X., Zhu W. 2020. Nelfinavir was Predicted to be a Potential Inhibitor of 2019-nCov Main Protease by an Integrative Approach combining Homology Modelling, Molecular Docking and Binding Free Energy Calculation. BioRxiv. [DOI] [Google Scholar]
- Yamamoto N., Yang R., Yoshinaka Y., Amari S., Nakano T., Cinatl J., Rabenau H., Doerr H.W., Hunsmann G., Otaka A. HIV protease inhibitor nelfinavir inhibits replication of SARS-associated coronavirus. Biochem. Biophys. Res. Commun. 2004;318:719–725. doi: 10.1016/j.bbrc.2004.04.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N., Matsuyama S., Hoshino T., Yamamoto N. 2020. Nelfinavir Inhibits Replication of Severe Acute Respiratory Syndrome Coronavirus 2 in vitro. BioRxiv. [DOI] [Google Scholar]
- Yao T.T., Qian J.D., Zhu W.Y., Wang Y., Wang G.Q. A systematic review of lopinavir therapy for SARS coronavirus and MERS coronavirus—a possible reference for coronavirus disease‐19 treatment option. J Med. Virol. 2020;92:556–563. doi: 10.1002/jmv.25729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P., Liu X., Zhao L., Dong E., Song C. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin. Infect. Dis. 2020;71:732–739. doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young B., Tan T.T., Leo Y.S. The place for remdesivir in COVID-19 treatment. Lancet Infect. Dis. 2020;21 doi: 10.1016/S1473-3099(20)30911-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeinalian M., Salari-Jazi A., Jannesari A., Khanahmad H. A potential protective role of losartan against coronavirus-induced lung damage. Infect. Control Hosp. Epidemiol. 2020;41:752–753. doi: 10.1017/ice.2020.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha L., Li S., Pan L., Tefsen B., Li Y., French N., Chen L., Yang G., Villanueva E.V. Corticosteroid treatment of patients with coronavirus disease 2019 (COVID‐19) Med. J. Aust. 2020;212(9) doi: 10.5694/mja2.50577. Preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Zhou R. 2020. Binding Mechanism of Remdesivir to SARS-CoV-2 RNA Dependent RNA Polymerase. Preprints. 2020030267. [DOI] [PubMed] [Google Scholar]
- Zhang C., Wu Z., Li J.-W., Zhao H., Wang G.-Q. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Ma X., Yu F., Liu J., Zou F., Pan T., Zhang H. 2020. Teicoplanin Potently Blocks the Cell Entry of 2019-nCoV. BioRxiv. [DOI] [Google Scholar]
- Zhang X., Song K., Tong F., Fei M., Guo H., Lu Z., Wang J., Zheng C. First case of COVID-19 in a patient with multiple myeloma successfully treated with tocilizumab. Blood Adv. 2020;4:1307. doi: 10.1182/bloodadvances.2020001907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y.-H., Qin Y.-Y., Lu Y.-Q., Sun F., Yang S., Harypursat V., Tang S.-Q., Huang Y.-Q., He X.-Q., Zeng Y.-M. Effectiveness of glucocorticoid therapy in patients with severe novel coronavirus pneumonia: protocol of a randomized controlled trial. Chin Med J (Engl). 2020;133:1080–1086. doi: 10.1097/CM9.0000000000000791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q., Chen V., Shannon C.P., Wei X.-S., Xiang X., Wang X., Wang Z.-H., Tebbutt S.J., Kollmann T.R., Fish E.N. Interferon-a2b treatment for COVID-19. Front. Immunol. 2020;11:1061. doi: 10.3389/fimmu.2020.01061. [DOI] [PMC free article] [PubMed] [Google Scholar]



