Abstract
Background
Patients with major depressive disorder (MDD) having active suicidal ideation with intent require immediate treatment.
Methods
This double-blind study (ASPIRE II) randomized adults (aged 18–64 years) with MDD having active suicidal ideation with intent to esketamine 84 mg or placebo nasal spray twice weekly for 4 weeks, given with comprehensive standard of care (hospitalization ≥5 days and newly initiated or optimized oral antidepressant[s]). Change from baseline to 24 hours post-first dose in Montgomery-Asberg Depression Rating Scale total score (primary efficacy endpoint) was analyzed using ANCOVA. Clinical Global Impression–Severity of Suicidality–revised (key secondary endpoint) was analyzed using ANCOVA on ranks of change.
Results
Of 230 patients who were randomized (115 per arm), 227 received study drug and were included in efficacy/safety analyses; 184 (80.0%) completed double-blind treatment. Greater improvement in Montgomery-Asberg Depression Rating Scale total score was observed with esketamine (mean [SD]: –15.7 [11.56]) vs placebo (–12.4 [10.43]), each with standard of care, at 24 hours (least-squares mean difference [SE]: –3.9 [1.39], 95% CI: –6.60, –1.11; 2-sided P = .006). This was also noted at the earlier (4-hour) timepoint (least-squares mean difference –4.2, 95% CI: –6.38, –1.94). Patients in both treatment groups experienced rapid reduction in Clinical Global Impression–Severity of Suicidality–revised score; the between-group difference was not statistically significant. The most common adverse events among esketamine-treated patients were dizziness, dissociation, nausea, dysgeusia, somnolence, headache, and paresthesia.
Conclusion
This study confirmed rapid and robust reduction of depressive symptoms with esketamine nasal spray in severely ill patients with MDD who have active suicidal ideation with intent.
Trial Registration: Clinical Trials.gov identifier: NCT03097133
Keywords: Esketamine, depression, suicidal ideation, suicide risk
Significance Statement.
Until the 2020 approval of esketamine nasal spray for the indication, there was no approved medication for the emergency treatment of patients with major depressive disorder (MDD) who are in need of rapid symptom control. In this phase 3, double-blind study (ASPIRE II) of patients with MDD and active suicide ideation with intent, patients randomized to esketamine nasal spray plus comprehensive standard of care (inpatient psychiatric hospitalization and newly initiated or optimized oral antidepressant therapy) experienced clinically meaningful and statistically significant improvement in depressive symptoms at 24 hours post-first dose compared with those randomized to placebo plus comprehensive standard of care. At 24 hours, patients in both treatment groups experienced similar improvement in severity of suicidality. The safety profile of esketamine was further characterized in this study. Our findings from the ASPIRE II trial suggest that esketamine nasal spray may address the unmet need for a rapid-acting antidepressant in patients with MDD and active suicidal ideation with intent.
Introduction
Depression is a common, disabling, and costly psychiatric illness that can have a devastating impact on all aspects of life; affected individuals are often unable to continue with ordinary family, social, or work activities and may feel that life is not worth living (World Health Organization, 2019). Moreover, suicide-related morbidity and mortality in patients with major depressive disorder (MDD) are major public health concerns. Between 10% and 20% of patients with MDD attempt suicide over their lifetime (Holma et al., 2010; Hasin et al., 2018), with an estimated lifetime risk of 3.4% for completed suicide (Blair-West et al., 1999).
Depression with suicidal ideation is a particularly severe form of MDD; these patients show more severe depressive symptoms (Sokero et al., 2003; van Ballegooijen et al., 2019) and worse response to treatment (Lopez-Castroman et al., 2016; Dold et al., 2018) compared with MDD patients without suicidal ideation. Nearly all patients with MDD who attempt or complete suicide have suicidal ideation prior to the event (Brown et al., 2000; Sokero et al., 2003). Thus, patients with MDD who have active suicidal ideation with intent require prompt intervention to protect them from self-harm. Current standard of care includes initiation or optimization of oral antidepressants and, frequently, hospitalization (American Psychiatric Association, 2003; Wasserman et al., 2012). However, there are no approved pharmacological treatments for the rapid reduction of depressive symptoms in this patient population (van der Feltz-Cornelis et al., 2011; Wasserman et al., 2012), and standard antidepressants often take 4 or more weeks for optimal effect (Simon and Savarino, 2007; Wasserman et al., 2012). Furthermore, the benefits of hospitalization are short-lived; the risks for attempted and completed suicide remain high in the weeks immediately after discharge (Chung et al., 2019).
Esketamine nasal spray was approved in 2019 in the United States and European Union for treatment-resistant depression in adults (Spravato Summary of Product Characteristics 2019; Spravato Prescribing Information 2020). The primary mechanism of esketamine, an N-methyl-D-aspartate receptor antagonist, is distinct from that of conventional monoaminergic antidepressants (Duman and Aghajanian, 2012). Rapid, robust, clinically meaningful reduction in depressive symptoms has been demonstrated in phase 2/3 studies of treatment-resistant depression (Kim et al., 2019) and in a phase 2, proof-of-concept study of depressed patients at imminent risk for suicide (Canuso et al., 2018), suggesting a role for esketamine nasal spray in the urgent care of patients with MDD who have active suicide ideation with intent. The first clinical development program, consisting of 2 identically designed, phase 3 global studies (ASPIRE I and ASPIRE II), was launched to confirm the antidepressant benefits of esketamine in this population. These studies, which were conducted to determine if treatment with esketamine nasal spray plus standard of care would significantly reduce Montgomery-Asberg Depression Rating Scale (MADRS) total score at 24 hours, compared to placebo plus standard of care, formed the basis for esketamine’s approval in 2020 in the United States for the treatment of depressive symptoms in adult MDD patients with acute suicidal ideation or behavior (Spravato Prescribing Information 2020). The results of ASPIRE II are reported herein.
Materials and Methods
Ethical Practices
The study protocol and amendments were approved by an independent review board (in the United States) or ethics committee for each site (listed in the online supplement). Ethical principles of the Declaration of Helsinki, Good Clinical Practices, and applicable regulatory requirements guided the conduct of the study. Written informed consent was secured from all patients before they participated in the study.
Patients
Eligible patients were between 18 and 64 years of age, met the Diagnostic and Statistical Manual of Mental Disorders–5th edition (DSM-5) criteria (American Psychiatric Association, 2013) for MDD (without psychosis) based on diagnostic assessment with the Mini International Neuropsychiatric Interview, and had a MADRS (Williams and Kobak, 2008) total score >28 at baseline. Enrollees were also required to present with suicidal ideation with intent based on affirmative responses to Mini International Neuropsychiatric Interview (for DSM-5) questions B3 (Currently think about harming/hurting/injuring yourself or about suicide?) and B10 (Intend to act on thoughts of killing yourself?) assessed within 24 hours of randomization. Additionally, eligible patients agreed to undergo comprehensive standard-of-care treatment, including initial hospitalization and having an antidepressant(s) initiated or optimized during double-blind treatment.
Key exclusion criteria were selected concurrent psychiatric illnesses (e.g., current diagnosis of bipolar disorder, obsessive compulsive disorder, antisocial personality disorder, borderline personality disorder); moderate to severe substance or alcohol use disorder within 6 months (12 months in some countries) prior to screening; current or prior diagnosis of psychotic disorder; and positive urine test result(s) for phencyclidine, cocaine, or amphetamines unless patients were on prescription psychostimulant(s) treatment. A complete list of inclusion and exclusion criteria is presented in the online supplement.
Study Design
This study (NCT03097133) was a double-blind, randomized, placebo-controlled, multicenter study conducted between June 2017 and April 2019 at 47 research sites in Argentina, Austria, Belgium, Brazil, Canada, Czech Republic, France, Lithuania, Poland, Spain, Turkey, and the United States.
The study comprised 3 phases: (1) a screening phase/visit performed within 48 hours before the first dose of intranasal study drug to assess patients’ eligibility for study enrollment, (2) a 25-day double-blind treatment phase, and (3) a 9-week follow-up phase (days 26–90).
Randomization and Blinding
Eligible patients were randomized (1:1) to 84 mg esketamine nasal spray or matching placebo nasal spray according to a computer-generated schedule. Randomization was balanced using randomly permuted blocks (4 patients per block) and stratified by study site and standard-of-care antidepressant type (i.e., monotherapy or antidepressant plus augmentation therapy).
To maintain blinding, a bittering agent was added to the placebo solution, and patients used the same number of intranasal study drug devices (3 devices, described below) at all dosing sessions.
The raters for efficacy assessments were different from the raters for safety assessments. Efficacy raters were not privy to safety data and were not allowed to provide patient care during the study. All raters were trained and certified.
Intranasal Study Drug and Standard-of-Care Oral Antidepressant Therapy
The intranasal study drugs were administered via identical, disposable nasal spray devices. Each device delivered 2 sprays of either esketamine (total of 28 mg of esketamine base) or placebo.
Patients self-administered intranasal study drug twice weekly for 4 weeks (days 1, 4, 8, 11, 15, 18, 22, and 25); intranasal study drug dosing occurred at the study site and was supervised by a site healthcare professional. After the first dose, a protocol-permitted 1-time dose reduction of esketamine (or corresponding placebo) from 84 mg to 56 mg was allowed for patients who experienced intolerance; patients who required a dose reduction continued 56 mg dosing thereafter.
Standard-of-care oral antidepressant(s) (monotherapy or antidepressant plus an augmenting agent [e.g., second antidepressant, an atypical antipsychotic, or a mood stabilizer]) was initiated or optimized at randomization by investigators based on their clinical judgment and practice guidelines. The dose of standard-of-care antidepressant(s) could be titrated/adjusted, if needed, during the first 2 weeks of double-blind treatment but not thereafter. Patients also received standard-of-care antidepressant(s) during the follow-up phase; antidepressant drug and dosage were based on the clinical judgment of investigators.
The first dose of study drug was administered in an emergency department or in an inpatient psychiatric unit, where study patients were to remain for a recommended 5 days (14 days in 7 countries in the European Union based on health authority request during the clinical trial approval); shorter or longer periods of hospitalization were permitted, if clinically necessary, per local standard practice.
Benzodiazepines at an equivalent dosage to lorazepam ≤6 mg/d were allowed during the study but prohibited within 8 hours before each intranasal study drug dose, and none were to be used within 4 hours after the first dose of intranasal study drug and within 8 hours of day 2 assessments. Psychotherapy was permitted, but electroconvulsive therapy was restricted during the study.
Efficacy and Safety Assessments
Using the Structured Interview Guide for MADRS, efficacy raters assessed depressive symptom severity before and 4 and 24 hours after the first dose of intranasal study drug (days 1–2), pre-dose at all subsequent double-blind treatment visits, and 4 hours post-dose on day 25. The severity of depressive symptoms was assessed at all post-treatment follow-up phase visits: twice weekly days 26–39, weekly days 40–53, and biweekly days 54–90.
Suicidal ideation and behavior were assessed using the Suicide Ideation and Behavior Assessment Tool (supplementary Figure 1) at all in-person visits during the double-blind treatment phase (pre-dose; 4 hours post-first dose) and follow-up phase. The Suicide Ideation and Behavior Assessment Tool, a computerized instrument (Alphs et al., 2020), contains both patient- and clinician-reported modules, including assessments of Clinical Global Impression–Severity of Suicidality–revised (CGI-SS-r), Clinical Global Impression of Imminent Suicide Risk (CGI-SR-I), and clinician- and patient-reported Frequency of Suicidal Thinking (FoST).
Safety assessments included monitoring of adverse events throughout the study and the evaluation of vital signs, Clinician Administered Dissociative States Scale (Bremner et al., 1998) (CADSS), and Modified Observer’s Assessment of Alertness/Sedation (Chernik et al., 1990) (MOAA/S) at all dosing visits.
Statistical Methods
The safety analysis set included all randomized patients who received at least 1 dose of study drug. The full efficacy analysis set included all randomized patients who received at least 1 dose of study drug and had baseline and at least 1 post-baseline evaluation for MADRS or CGI-SS-r. The follow-up analysis set included all patients who completed the double-blind treatment phase and either entered the follow-up phase or had adverse events evaluated after the double-blind treatment phase.
Efficacy Endpoints and Analyses
Statistical analyses were conducted at a 2-sided .05 level of significance. Multiplicity with regard to testing multiple endpoints (primary and key secondary) was controlled by a fixed sequence testing procedure (i.e., the key secondary efficacy endpoint was to be tested only after the null hypothesis for the primary endpoint was rejected).
The primary efficacy endpoint was change in MADRS total score from baseline (day 1, pre-dose) to 24 hours after the first dose (day 2). The primary endpoint was analyzed on last observation carried forward (LOCF) data using ANCOVA with treatment, analysis center, and standard-of-care antidepressant as randomized (monotherapy or antidepressant plus augmentation therapy) as factors, and baseline MADRS total score as a continuous covariate. Five patients (3 patients in the esketamine plus standard-of-care group and 2 patients in the placebo plus standard-of-care group) did not have a day 2 MADRS total score for analysis of the primary endpoint; the MADRS total score from 4 hours post-first dose was carried forward to day 2 (i.e., LOCF) for these 5 patients. One additional patient in the esketamine plus standard-of-care group was missing day 1, 4 hours post-dose, and day 2 values and was not included in the primary analysis on LOCF data using ANCOVA.
Treatment effect over time for MADRS total score during the double-blind and follow-up phases was analyzed using a mixed model for repeated measures based on observed case data. The model included baseline MADRS total score as a continuous covariate; day, treatment, analysis center, standard-of-care antidepressant treatment as randomized, and day-by-treatment interaction as fixed effects, and a random patient effect.
The key secondary efficacy endpoint, change in CGI-SS-r from baseline to 24 hours after the first dose, was analyzed using an ANCOVA model on the ranks of change with the same factors used for the primary endpoint and unranked baseline score as a covariate.
Subgroup analyses were performed according to the ANCOVA model used for the primary efficacy endpoint and key secondary efficacy endpoint (unranked data); the subgroups are shown in supplementary Figure 2 and supplementary Figure 5, respectively.
The percentage of patients in remission (defined as MADRS total score ≤12) and response rate (≥50% improvement in MADRS total score) over time were summarized, and estimates of the treatment difference in proportions and 95% CIs were calculated. Differences in least square (LS) means and 95% CIs were calculated for other indices of suicidality (MADRS suicide item, CGI-SR-I, clinician- and patient-rated FoST) based on ANCOVA modeling similar to that described for the primary endpoint analysis.
Frequency distributions or descriptive statistics were provided for adverse events, vital signs, CADSS, and MOAA/S. Adverse events potentially related to suicidality (MedDRA preferred terms presented in the online supplement) were summarized.
Sample Size Determination
Sample size was calculated based on a standardized effect size of 0.45 for the change in MADRS total score between esketamine and placebo, a 2-sided significance level of 0.05, and a drop-out rate of 5% at 24 hours. Randomization of approximately 112 patients to each treatment group would result in 90% power.
Results
Patients and Treatment
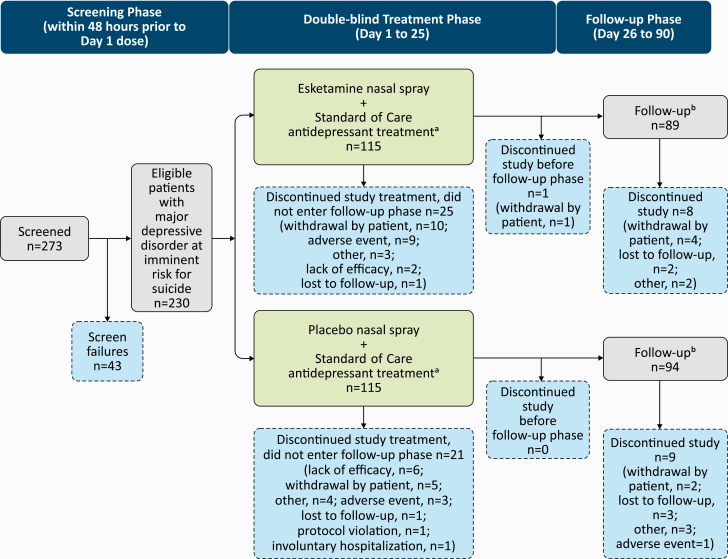
Of 273 patients screened, 230 were randomized (115 to each treatment arm), 3 of whom were excluded from all analyses because they did not receive a dose of intranasal study drug. The majority of randomized patients (esketamine: 90/115, 78.3%; placebo: 94/115, 81.7%) completed the 4-week double-blind treatment phase; 183 patients entered the follow-up phase, and 166 completed the day 90 follow-up visit (Figure 1).
Figure 1.
Study design and disposition of patients. aStandard antidepressant treatment was initiated or optimized on day 1. bPatients who completed the double-blind phase and either entered the follow-up phase or had adverse events evaluated after the double-blind treatment phase. Note: Three patients were not included in the efficacy analysis dataset due to discontinuing prior to receiving study drug or not providing postbaseline efficacy data.
The treatment groups were similar, in general, based on demographics, baseline clinical ratings, psychiatric histories (Table 1), and standard-of-care antidepressant use (supplementary Table 1). The mean (SD) baseline MADRS total score was 39.7 (5.48).
Table 1.
Demographics, Baseline Clinical Ratings, and Psychiatric History
| Parameter | Placebo + standard of care n = 113 | Esketamine 84 mg + standard of care n = 114 | Total n = 227 |
|---|---|---|---|
| Age, y | |||
| Mean (SD) |
41.4 (13.43) |
40.2 (12.73) |
40.8 (13.07) |
| Sex, n (%) | |||
| Female |
67 (59.3) |
69 (60.5) |
136 (59.9) |
| Male |
46 (40.7) |
45 (39.5) |
91 (40.1) |
| Race, n (%) | |||
| White |
87 (77.0) |
92 (80.7) |
179 (78.9) |
| Black or African American |
8 (7.1) |
7 (6.1) |
15 (6.6) |
| Asian |
2 (1.8) |
1 (0.9) |
3 (1.3) |
| Other |
16 (14.2) |
14 (12.3) |
30 (13.2) |
| MADRS total score, mean (SD) |
39.9 (5.76) |
39.5 (5.19) |
39.7 (5.48) |
| CGI-SS-r, n (%) | |||
| Normal, not at all suicidal |
0 0 |
0 0 |
0 0 |
| Questionably suicidal |
3 (2.7) |
1 (0.9) |
4 (1.8) |
| Mildly suicidal |
6 (5.3) |
10 (8.8) |
16 (7.0) |
| Moderately suicidal |
33 (29.2) |
35 (30.7) |
68 (30.0) |
| Markedly suicidal |
42 (37.2) |
48 (42.1) |
90 (39.6) |
| Severely suicidal |
28 (24.8) |
17 (14.9) |
45 (19.8) |
| Among the most extremely suicidal patients |
1 (0.9) |
3 (2.6) |
4 (1.8) |
| Prior suicide attempt, n (%) | |||
| Yes |
72 (63.7) |
78 (68.4) |
150 (66.1) |
| No |
41 (36.3) |
36 (31.6) |
77 (33.9) |
| Suicide attempt in the last month, n (%) | |||
| Yes |
24 (21.2) |
36 (31.6) |
60 (26.4) |
| No |
89 (78.8) |
78 (68.4) |
167 (73.6) |
| Standard-of-care antidepressanta, n (%) | |||
| Antidepressant monotherapy |
43 (38.1) |
45 (39.5) |
88 (38.8) |
| Antidepressant plus augmentation therapy |
70 (61.9) |
69 (60.5) |
139 (61.2) |
Abbreviations: CGI-SS-r, clinical global impression of severity of suicidality revised version; MADRS, Montgomery-Asberg Depression Rating Scale.
aAs randomized.
Note: CGI-SS-r (score ranges from 0–6; a higher score indicates a more severe condition): “Considering your total clinical experience with suicidal patients and all information now available to you, how suicidal is this patient at this time?”
Most patients (91.2%) were moderately to extremely suicidal, according to the investigator-rated CGI-SS-r. Two-thirds (66.1%) of patients reported a prior suicide attempt. A higher proportion of patients in the esketamine plus standard-of-care group had a recent (in the last month) suicide attempt than those in the placebo plus standard-of-care group (31.6% vs 21.2%).
Mean (SD) length of hospitalization was 21.6 (20.58) days for patients in the esketamine plus standard-of-care group and 19.1 (19.60) days for patients in the placebo plus standard-of-care group. Duration of hospitalization varied across countries due to differences in local practice for these types of patient. The 5 most frequently used standard-of-care antidepressants were quetiapine (28.2%), venlafaxine (28.2%), escitalopram (17.2%), duloxetine (14.5%), and sertraline (13.7%) (supplementary Table 1). Two-thirds of patients (153/227, 67.4%; esketamine plus standard of care: 83/114, 72.8%; placebo plus standard of care: 70/113, 61.9%) received concomitant benzodiazepine(s) during the double-blind treatment (supplementary Table 2). A minority of patients (6 [5.3%] in the esketamine plus standard-of-care group and 5 [4.4%] in the placebo plus standard-of-care group) received psychotherapy during the double-blind treatment phase.
Efficacy Results
MADRS total score decreased from baseline to 24 hours after the first dose (day 2) in both the esketamine plus standard-of-care group (mean [SD]: –15.7 [11.56]) and the placebo plus standard-of-care group (–12.4 [10.43]), with significantly greater improvement in depressive symptoms with esketamine (LS mean difference [SE]: –3.9 [1.39], 95% CI: –6.60, –1.11; 2-sided P = .006). The treatment effect of esketamine was observed in most subgroups (supplementary Figure 2), importantly among patients who had attempted suicide (–4.26 [–7.66, –0.86]) and patients with more severe depressive symptoms (i.e., MADRS total score > median) (–4.84 [–8.85, –0.83]).
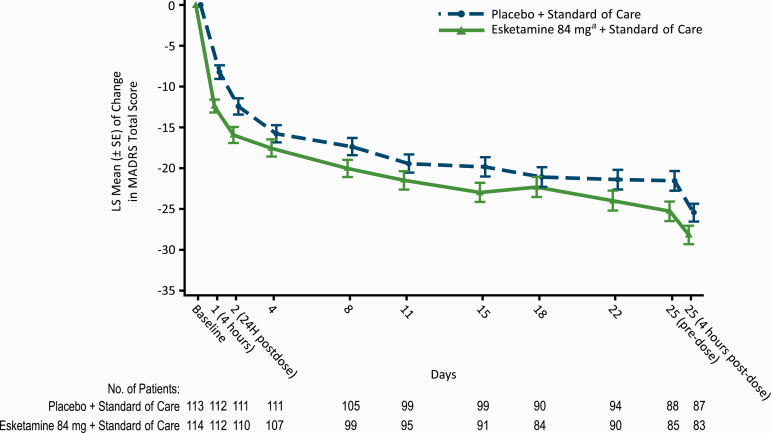
The difference between treatment groups was evident at 4 hours (LS mean difference –4.2, 95% CI: –6.38; –1.94) and generally remained over the double-blind treatment phase (after the 24-hour assessment, significant only at day 25 pre-dose [–3.7, 95% CI: –7.09; –0.38]; Figure 2). Among the patients who completed double-blind treatment and entered the follow-up phase, MADRS total scores remained low throughout follow-up (supplementary Figure 3).
Figure 2.
Least-square mean changes (±SE) from baseline for Montgomery-Asberg Depression Rating Scale (MADRS) total score during the double-blind treatment phase (mixed-effects model using repeated measures [MMRM]; observed cases). aIncludes patients who had their dose reduced due to tolerability issues. Note: Negative change in score indicates improvement.
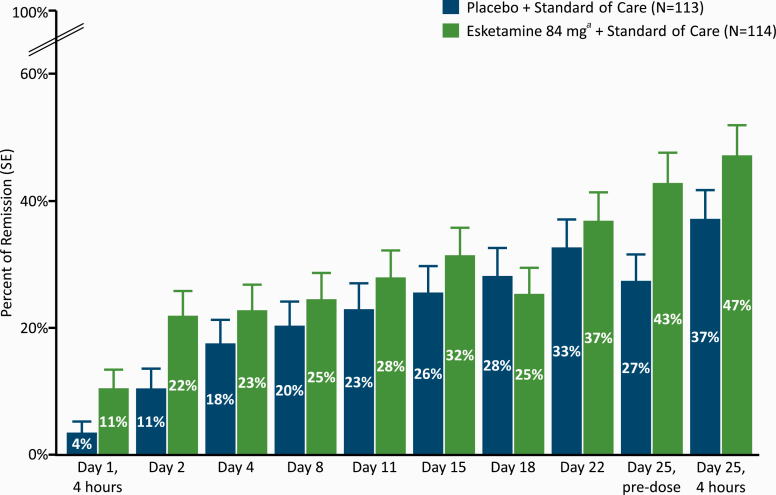
The percentage of patients who achieved remission (MADRS total score ≤12) was numerically greater for the esketamine plus standard-of-care group than the placebo plus standard-of-care group at all time points during double-blind treatment, except on day 18 (Figure 3). The treatment difference (95% CI) in remission between study arms was 11.3% (1.83, 20.80) 24 hours post-first dose on day 2 and 10.2% (–2.58, 22.98) 4 hours post-dose on day 25. Response rates directionally favored esketamine plus standard of care over placebo plus standard of care at all timepoints but the day 25, 4-hour post-dose timepoint (supplementary Figure 4).
Figure 3.
Montgomery-Asberg Depression Rating Scale (MADRS) remission rate over time during the double-blind treatment phase. aIncludes patients who had their dose reduced due to tolerability issues. Note: Remission was based on a MADRS total score of ≤12. Patients who did not meet such criterion or discontinued prior to the time point for any reason were not be considered to be in remission.
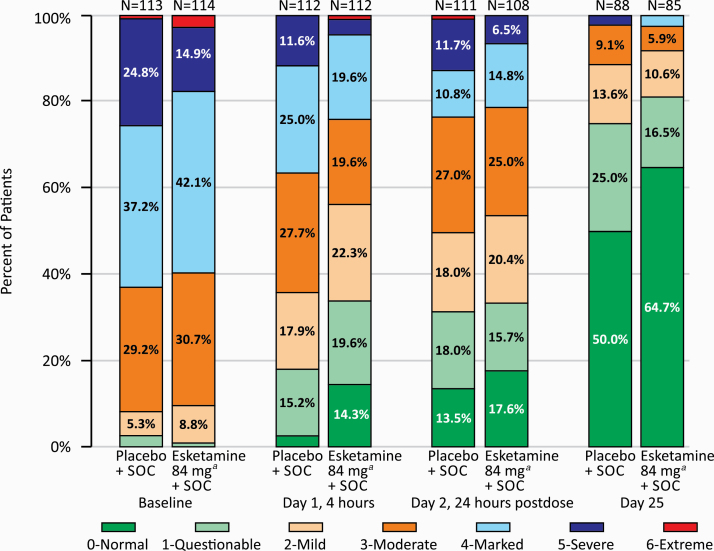
Patients in both treatment groups experienced rapid reduction in the severity of their suicidality as measured by the CGI-SS-r (median change from baseline at 24 hours post-first dose: –1.0 point); the between-group difference was not statistically significant (2-sided P = .379). At the 24-hour time point, the treatment difference (LS mean difference, 95% CI) observed in the subgroup of patients with a history of prior suicide attempt was –0.22 (–0.64, 0.19) and in the subgroup of patients with more severe depressive symptoms was –0.31 (–0.79, –0.18) (supplementary Figure 5). Severity of suicidality also improved in both treatment groups at the end of double-blind treatment (Figure 4).
Figure 4.
Frequency distribution of Clinical Global Impression-Severity of Suicidality-Revised (CGI-SS-r) score at baseline, 4 and 24 hours post-first dose, and day 25 (observed cases). SOC, standard of care. aIncludes patients who had their dose reduced due to tolerability issues.
Severity of suicidality was low at the end of the follow-up phase for most patients in both treatment groups. Among patients who completed the double-blind treatment and entered the follow-up phase (observed cases), 86.3% (69/80) of patients in the esketamine plus standard-of-care group and 76.7% (66/86) of patients in the placebo plus standard-of-care group had a CGI-SS-r score of 0 or 1 at day 90, indicating the patient was normal/questionably suicidal.
Results for other indices of suicidality are presented in supplementary Figure 6.
Safety Results
The most frequently reported adverse events are listed in Table 2 (reported during the double-blind treatment phase) and supplementary Table 3 (reported during the follow-up phase). The majority of events in the esketamine plus standard-of-care group (89.1%) and placebo plus standard-of-care group (68.0%) occurred on intranasal dosing days, and most of these events (94.9% and 84.9%, respectively) resolved on the same day they began. Thirteen esketamine-treated patients (11.4%) had a dose reduction to 56 mg due to intolerance.
Table 2.
Summary of Most Frequently Reporteda Treatment-Emergent Adverse Events During Double-Blind Phases
| Number (%) of patients | ||||
|---|---|---|---|---|
| Placebo + standard of care n = 113 | Esketamine 84 mg + standard of care n = 114 | |||
| Dizziness | 21 | (18.6) | 47 | (41.2) |
| Dissociation | 9 | (8.0) | 44 | (38.6) |
| Nausea | 16 | (14.2) | 38 | (33.3) |
| Dysgeusia | 18 | (15.9) | 29 | (25.4) |
| Somnolence | 12 | (10.6) | 26 | (22.8) |
| Headache | 26 | (23.0) | 25 | (21.9) |
| Paresthesia | 7 | (6.2) | 23 | (20.2) |
| Vomiting | 5 | (4.4) | 18 | (15.8) |
| Anxiety | 7 | (6.2) | 17 | (14.9) |
| Vision blurred | 6 | (5.3) | 17 | (14.9) |
| Sedation | 3 | (2.7) | 16 | (14.0) |
| Paresthesia oral | 3 | (2.7) | 14 | (12.3) |
| Euphoric mood | 1 | (0.9) | 13 | (11.4) |
| Hypoesthesia | 1 | (0.9) | 12 | (10.5) |
| Nasal discomfort | 9 | (8.0) | 10 | (8.8) |
| Constipation | 1 | (0.9) | 9 | (7.9) |
| Depersonalization/derealization disorder | 0 | 9 | (7.9) | |
| Insomnia | 11 | (9.7) | 9 | (7.9) |
| Dry mouth | 5 | (4.4) | 8 | (7.0) |
| Blood pressure increased | 3 | (2.7) | 7 | (6.1) |
| Constipation | 9 | (8.0) | 7 | (6.1) |
| Hypoesthesia oral | 2 | (1.8) | 7 | (6.1) |
| Vertigo | 0 | 7 | (6.1) | |
| Diplopia | 0 | 6 | (5.3) | |
| Hyperhidrosis | 3 | (2.7) | 6 | (5.3) |
| Oropharyngeal pain | 3 | (2.7) | 6 | (5.3) |
| Feeling drunk | 1 | (0.9) | 6 | (5.3) |
| Throat irritation | 4 | (3.5) | 6 | (5.3) |
| Suicide ideation | 6 | (5.3) | 5 | (4.4) |
aMost frequently reported is defined as ≥5% of patients in either treatment group during the double-blind phase. Events are presented in descending order in the esketamine group for the double-blind phase.
No deaths were reported during the study. Serious adverse events (supplementary Tables 4 and 5) and events leading to discontinuation of study drug are summarized in the online supplement.
The numbers of patients who reported adverse events potentially related to suicidality during the double-blind treatment phase were balanced between the treatment groups (10 patients in each treatment group; 3 patients per group who reported a suicide attempt). During the follow-up phase when patients were only receiving standard of care and not study medication, 9 patients in each treatment group reported events related to suicidality. Four patients previously treated with esketamine plus standard of care and 1 patient previously treated with placebo plus standard of care attempted suicide in the follow-up phase. The occurrence of these suicide attempts in the follow-up phase was dispersed over the follow-up phase without an apparent pattern suggestive of a rebound effect. Six of the 11 patients who attempted suicide during the study had a history of a prior suicide attempt.
Dissociative symptoms and transient perceptual effects measured by the CADSS total score (supplementary Figure 7) began shortly after the start of esketamine dosing, peaked at the 40-minute post-dose assessment, and generally resolved by 1.5 hours post-dose. The highest (mean [SD]) CADSS total score post-first dose was 15.9 [12.8] in the esketamine plus standard-of-care group and 1.9 [4.4] in the placebo plus standard-of-care group (supplemental Figure 7). No symptoms or adverse events of psychosis were reported during treatment with intranasal study drug. No symptoms or adverse events consistent with withdrawal or abuse were reported during the follow-up period.
Blood pressure increased following esketamine dosing, peaking at 40 minutes post dose, and generally returning to baseline by 1.5 hours post dose (supplementary Figure 8).
More patients in the esketamine plus standard-of-care group (21/114 [18.4%]) had a MOAA/S score ≤3 (indicating moderate or greater sedation) at any time during the double-blind phase vs patients in the placebo plus standard-of-care group (3/113 [2.7%]). None of these patients required medical intervention or manifested respiratory depression.
Discussion
In this study of patients with MDD with suicidal ideation and intent—a particularly vulnerable patient population—treatment with esketamine plus standard of care resulted in a significantly greater improvement of depressive symptoms compared with placebo plus standard of care at the primary endpoint (24 hours post-first dose). Given the substantial benefit of standard of care (Leuchter et al., 2014), consisting of initial inpatient psychiatric hospitalization and newly initiated or optimized oral antidepressant therapy, the additional treatment effects with esketamine are noteworthy. Rapid improvement in depressive symptoms was observed at 4 hours after the first dose. The advantage with esketamine continued until the end of 4-week double-blind treatment (LS mean treatment difference ranging from –1.2 to –3.7, statistically significant at day 25 predose), at which time oral antidepressant therapy putatively has sufficient time to exert its full effect. The findings of this phase 3 study confirm those from the identically designed study reported by Fu et al. (2020), both showing that esketamine nasal spray rapidly reduces depressive symptoms in MDD patients having suicidal ideation with intent.
The difference in the key secondary outcome, as measured by CGI-SS-r, did not reach the level of statistical significance at 24 hours between the 2 treatment groups. Several factors may have contributed to this finding. Psychiatric hospitalization may have played a role in mitigating the suicidal crisis among patients in both treatment groups (American Psychiatric Association, 2003). In addition, the rapid reduction of suicidality observed in both treatment groups may be at least partly related to the enhanced clinical contact associated with extensive study assessments and the use of benzodiazepines (as described in the Methods section) in both treatment arms.
The adverse events observed in this study are consistent with the established safety profile of esketamine nasal spray (Spravato Summary of Product Characteristics 2019; Spravato Prescribing Information 2020). Dissociative symptoms (based on CADSS score), sedation (based on MOAA/S score), and blood pressure elevation associated with esketamine administration were, in general, confined to the immediate (1.5 hour) post-dosing period.
No completed suicides were observed in either treatment group during the duration of the double-blind and follow-up phases. Overall, the number of patients who reported adverse events potentially related to suicidality was balanced between the treatment groups in both the double-blind treatment and follow-up phases and appear low in the context of published literature. For example, in a study of the emergence of suicidal ideation during post-discharge treatment of depressed patients, 70% of those with severe suicidality at index hospitalization exhibited recurrence of suicidal ideation after discharge and nearly 80% of patients with post-discharge suicidal ideation experienced suicidal thinking within 2 months of the hospitalization (Gaudiano et al., 2008).
Specifically, with regard to suicide attempts, 3 patients in each treatment group attempted suicide during the double-blind treatment. During the follow-up, among the 4 patients previously treated with esketamine who attempted suicide, 3 had a recent prior attempt. The 1 patient formerly treated with placebo who attempted suicide had a history of prior suicide attempt, though not within the past month. The fact that more patients randomized to esketamine had a history of recent (in the last month) suicide attempts compared with placebo (31.6% vs 21.2%), a known risk factor for subsequent suicidal behavior (Gramaglia et al., 2016), may relate to the difference in the observed number of events in the follow-up phase. Indeed, in the phase 2 study (Canuso et al., 2018), 3 patients in the group previously treated with placebo attempted suicide in the follow-up phase compared with 0 suicide attempts in the patients previously treated with esketamine plus standard of care; all 3 patients who attempted suicide in that study had a prior suicide attempt, 2 within the most recent month. Nonetheless, given the severity of suicidality of the studied population, the number of suicide attempts was low overall. Furthermore, severity of suicidality, based on CGI-SS-r scores, remained low during the follow-up phase of ASPIRE II.
A population-based retrospective cohort study of US health claims was conducted to better understand suicide attempts in the period immediately following a psychiatric hospitalization (Cepeda et al., 2020). Among 121 065 adults with depression who were hospitalized for suicide ideation or attempt, the rate of re-hospitalizations due to suicidal ideation or suicidal attempt ranged from 8.0% to 11.2% (2.4%–3.3% for suicide attempt) within a year of discharge; the highest risk was reported during the first month, and nearly one-half of re-hospitalizations occurred within 3 months after initial hospitalization. Moreover, in a meta-analysis of 29 studies, Chung and associates reported a pooled suicide rate of 2060 per 100 000 person-years in the first month following discharge from a psychiatric hospital (Chung et al., 2019). Therefore, despite the severity of illness in our studied population, the rate of suicide attempts in the period immediately following psychiatric hospitalization is relatively low in this high-risk population and consistent with the literature.
One of the study limitations relates to the unique methodological challenges of conducting clinical trials in a population of patients with MDD who have active suicidal ideation and intent. To study these patients in a safe and ethical manner, the study was conducted in the context of comprehensive standard of care, inclusive of initial hospitalization and optimized oral antidepressant therapy. Given difficulties in masking the dissociative side effects of esketamine, functional unblinding may have biased clinical decisions on patient care; however, potential bias on efficacy outcomes was mitigated by the protocol requirements that efficacy raters could not be involved in patient care and must have been different from safety raters. There is a well-known and substantial placebo response in antidepressant clinical trials due in large part to patient expectations and the intensity of therapeutic contacts (Rutherford and Roose, 2013; Leuchter et al., 2014). Potential regional differences in the standard-of-care treatment provided in this global study, particularly the regional differences in length of inpatient hospitalization, may also impact the study findings. Moreover, hospital duration was influenced by protocol requirements, and further research is needed to understand the impact of hospitalization in real-world practice.
There is a clear unmet medical need for a novel treatment that can rapidly improve the depressive symptoms in patients with MDD who have active suicidal ideation with intent and bridge the efficacy gap created by the delayed onset of action of standard antidepressants. This study and another identically designed, phase 3 study (Fu et al., 2020) are pivotal to the first global clinical development program, to our knowledge, for this severely ill and vulnerable patient population and formed the basis for the FDA’s recent approval of esketamine for these patients (Spravato Prescribing Information 2020). Evidence from this study suggests esketamine nasal spray may fulfill the unmet need for a rapid-acting antidepressant in patients undergoing an acute crisis of suicidal ideation and intent, addressing the limitation of delayed onset of antidepressant efficacy observed with existing pharmacological treatments.
Data Sharing and Accessibility
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. Requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
Supplementary Material
Acknowledgments
We acknowledge Sandra Norris, PharmD, of the Norris Communications Group LLC for medical writing assistance and Ellen Baum, PhD (Janssen Global Services, LLC), for additional editorial support.
We thank the study patients for their participation in this study. The following principal investigators participated in the study:
Argentina: Hernán Alessandria, Eduardo Amado Cattaneo, Salvador Guinjoan, Carlos Morra; Austria: Siegfried Kasper, Elmar Windhager; Belgium: Geert De Bruecker, Gunter Heylens; Brazil: Humberto Correa, Acioly Lacerda, Cintia Perico, Lucas Quarantini, Fabio Souza, Teng Chei Tung; Canada: Sidney Kennedy; Czech Republic: Karolina Czeczotkova, Vlastimil Tichy; France: Mocrane Abbar, Raphaël Gaillard, Emilie Olie, Simon Taib, Chloe Traisnel; Lithuania: Virginija Adomaitiene, Dalia Gudeikiene; Poland: Wieslaw Cubala, Dominik Strzelecki, Agata Szulc, Piotr Zalitacz; SPAIN: Salvador Ros Montalbán, Josep Antoni Ramos Quiroga, Patricio Molero Santos; Turkey: Cengiz Akkaya, Oguz Karamustafalioglu, Tuba Oger; Ibrahim Taymur, United States: California: Michael Plopper, Mounir Soliman; Connecticut: Jayesh Kamath; Florida: Olga Lapeyra; Georgia: Suneel Katragadda; Illinois: Danesh Alam, Jeffrey Bennett; Louisiana: Kashinath Yadalam; Maryland: Adam Kaplin; Nevada: Jelena Kunovac; New York: Raphael Braga, Lucian Manu; North Carolina: Robert Mcclure; Ohio: Caleb Matthew Adler; Wisconsin: Steven Garlow.
The open access fee was paid by Janssen Research and Development, LLC, Titusville, NJ, which also provided funding for the study reported in this article.
Statement of Interest
Drs Ionescu, Fu, Qiu, Lim, Hough, Drevets, Manji, and Canuso and Ms Lane are employees of Janssen Research & Development, LLC. Dr Manji is an inventor on patents that are directed to this technology, are assigned to Icahn School of Medicine at Mount Sinai, Yale University, and NIH, and are exclusively licensed to Janssen; however, he does not receive any direct financial benefit therefrom. Dr Kasper received grants/research support, consulting fees and/or honoraria within the last 3 years from Angelini, AOP Orphan Pharmaceuticals AG, Celgene GmbH, Janssen-Cilag Pharma GmbH, KRKA-Pharma, Lundbeck A/S, Mundipharma, Neuraxpharm, Pfizer, Sage, Sanofi, Schwabe, Servier, Shire, Sumitomo Dainippon Pharma Co. Ltd., Sun Pharmaceutical Industries Ltd., and Takeda.
References
- Alphs L, Fu D-J, Williamson D, Turkoz I, Jamieson C, Revicki D, Canuso CM (2020) Suicide ideation and behavior assessment tool (SIBAT): evaluation of intra- and inter-rater reliability, validity, and mapping to Columbia Classification Algorithm of Suicide Assessment. Psychiatry Research; (in press), 10.1016/j.psychres.2020.113495. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (2003) Practice guideline for the assessment and treatment of patients with suicidal behaviors. Am J Psychiatry 160 (11Suppl):1–60. [PubMed] [Google Scholar]
- American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders (DSM-5) 5th ed. Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Blair-West GW, Cantor CH, Mellsop GW, Eyeson-Annan ML (1999) Lifetime suicide risk in major depression: sex and age determinants. J Affect Disord 55:171–178. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS, Mazure CM (1998) Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS). J Trauma Stress 11:125–136. [DOI] [PubMed] [Google Scholar]
- Brown GK, Beck AT, Steer RA, Grisham JR (2000) Risk factors for suicide in psychiatric outpatients: a 20-year prospective study. J Consult Clin Psychol 68:371–377. [PubMed] [Google Scholar]
- Canuso CM, Singh JB, Fedgchin M, Alphs L, Lane R, Lim P, Pinter C, Hough D, Sanacora G, Manji H, Drevets WC (2018) Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: results of a double-blind, randomized, placebo-controlled study. Am J Psychiatry 175:620–630. [DOI] [PubMed] [Google Scholar]
- Cepeda MS, Schuemie M, Kern DM, Reps J, Canuso C (2020) Frequency of rehospitalization after hospitalization for suicidal ideation or suicidal behavior in patients with depression. Psychiatry Res 285:112810. [DOI] [PubMed] [Google Scholar]
- Chernik DA, Gillings D, Laine H, Hendler J, Silver JM, Davidson AB, Schwam EM, Siegel JL (1990) Validity and reliability of the observer’s assessment of alertness/sedation scale: study with intravenous midazolam. J Clin Psychopharmacol 10:244–251. [PubMed] [Google Scholar]
- Chung D, Hadzi-Pavlovic D, Wang M, Swaraj S, Olfson M, Large M (2019) Meta-analysis of suicide rates in the first week and the first month after psychiatric hospitalisation. BMJ Open 9:e023883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dold M, Bartova L, Fugger G, Kautzky A, Souery D, Mendlewicz J, Papadimitriou GN, Dikeos D, Ferentinos P, Porcelli S, Serretti A, Zohar J, Montgomery S, Kasper S (2018) Major depression and the degree of suicidality: results of the European Group for the Study of Resistant Depression (GSRD). Int J Neuropsychopharmacol 21:539–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK (2012) Synaptic dysfunction in depression: potential therapeutic targets. Science 338:68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu D-J, Ionescu DF, Li X, Lane R, Lim P, Sanacora G, Hough D, Manji H, Drevets WC, Canuso CM (2020) Esketamine nasal spray for rapid reduction of major depression symptoms in patients who have active suicidal ideation with intent: double-blind, randomized study (ASPIRE I). J Clin Psychiatry 81:19m13191. doi: 10.4088/JCP.19m13191. [DOI] [PubMed] [Google Scholar]
- Gaudiano BA, Andover MS, Miller IW (2008) The emergence of suicidal ideation during the post-hospital treatment of depressed patients. Suicide Life Threat Behav 38:539–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramaglia C, Feggi A, Bergamasco P, Bert F, Gattoni E, Marangon D, Siliquini R, Torre E, Zeppegno P (2016) Clinical characteristics associated with suicide attempts in clinical settings: a comparison of suicidal and non-suicidal depressed inpatients. Front Psychiatry 7:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Sarvet AL, Meyers JL, Saha TD, Ruan WJ, Stohl M, Grant BF (2018) Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiatry 75:336–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holma KM, Melartin TK, Haukka J, Holma IA, Sokero TP, Isometsä ET (2010) Incidence and predictors of suicide attempts in DSM-IV major depressive disorder: a five-year prospective study. Am J Psychiatry 167:801–808. [DOI] [PubMed] [Google Scholar]
- Kim J, Farchione T, Potter A, Chen Q, Temple R (2019) Esketamine for treatment-resistant depression - first FDA-approved antidepressant in a new class. N Engl J Med 381:1–4. [DOI] [PubMed] [Google Scholar]
- Leuchter AF, Hunter AM, Tartter M, Cook IA (2014) Role of pill-taking, expectation and therapeutic alliance in the placebo response in clinical trials for major depression. Br J Psychiatry 205:443–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Castroman J, Jaussent I, Gorwood P, Courtet P (2016) Suicidal depressed patients respond less well to antidepressants in the short term. Depress Anxiety 33:483–494. [DOI] [PubMed] [Google Scholar]
- Rutherford BR, Roose SP (2013) A model of placebo response in antidepressant clinical trials. Am J Psychiatry 170:723–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon GE, Savarino J (2007) Suicide attempts among patients starting depression treatment with medications or psychotherapy. Am J Psychiatry 164:1029–1034. [DOI] [PubMed] [Google Scholar]
- Sokero TP, Melartin TK, Rytsälä HJ, Leskelä US, Lestelä-Mielonen PS, Isometsä ET (2003) Suicidal ideation and attempts among psychiatric patients with major depressive disorder. J Clin Psychiatry 64:1094–1100. [DOI] [PubMed] [Google Scholar]
- Spravato (esketamine) nasal spray. Summary of product characteristics. 2019 https://www.medicines.org.uk/emc/product/10977/smpc. Accessed March 31, 2020.
- Spravato (esketamine) nasal spray. Prescribing information. 2020. Janssen Pharmaceutical Companies. [Google Scholar]
- van Ballegooijen W, Eikelenboom M, Fokkema M, Riper H, van Hemert AM, Kerkhof AJFM, Penninx BWJH, Smit JH (2019) Comparing factor structures of depressed patients with and without suicidal ideation, a measurement invariance analysis. J Affect Disord 245:180–187. [DOI] [PubMed] [Google Scholar]
- van der Feltz-Cornelis CM, Sarchiapone M, Postuvan V, Volker D, Roskar S, Grum AT, Carli V, McDaid D, O’Connor R, Maxwell M, Ibelshäuser A, Van Audenhove C, Scheerder G, Sisask M, Gusmão R, Hegerl U (2011) Best practice elements of multilevel suicide prevention strategies: a review of systematic reviews. Crisis 32:319–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman D, Rihmer Z, Rujescu D, Sarchiapone M, Sokolowski M, Titelman D, Zalsman G, Zemishlany Z, Carli V; European Psychiatric Association (2012) The European Psychiatric Association (EPA) guidance on suicide treatment and prevention. Eur Psychiatry 27:129–141. [DOI] [PubMed] [Google Scholar]
- Williams JB, Kobak KA (2008) Development and reliability of a structured interview guide for the Montgomery Asberg Depression Rating Scale (SIGMA). Br J Psychiatry 192:52–58. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2019) Depression. Available at: https://www.who.int/news-room/fact-sheets/detail/depression. Accessed March 31, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.