Abstract
Background
Neuropathic pain is a multifaceted and ubiquitous disease across the globe. Mood disorders, such as anxiety and depression, are frequently observed in patients suffering from neuropathic pain. Both neuropathic pain and comorbid mood disorders seriously impact quality of life. Accumulated evidence shows that activation of the NOD-like receptor protein 3 (NLRP3) inflammasome is involved in the neuroinflammatory pathogenesis of neuropathic pain, anxiety, and depression. However, the role of the NLRP3 inflammasome in the pathological process of anxiety and depression under the neuropathic pain state has not been fully described. Albiflorin, a monoterpene glycoside, may be a potential regulator of the NLRP3 inflammasome, but it is not clear whether albiflorin relates to NLRP3 inflammasome activation.
Methods
We used a systematic pharmacological method to confirm whether the activation of the NLRP3 inflammasome in the hippocampus was involved in the development of neuropathic pain associated with mood disorders and whether albiflorin could be an effective treatment for these symptoms.
Results
The NLRP3 inflammasome contributed to the neuropathic pain and comorbid anxiety and depression-like behaviors induced by chronic constriction injury of the sciatic nerve, and albiflorin may relieve these symptoms via inhibition of the NLRP3 inflammasome activity. Moreover, albiflorin enhanced the translocation of the nuclear factor erythroid 2-related factor 2 into the nucleus and suppressed nuclear factor-kappa B activity in the hippocampus.
Conclusions
Albiflorin, as a potential therapeutic agent, might greatly improve the overall symptoms of neuropathic pain.
Keywords: Albiflorin, neuropathic pain, anxiety and depression, NLRP3 inflammasome
Significance Statement.
Neuropathic pain is a severe condition with several comorbidities, such as mood disorders, affecting many individuals worldwide. This study examines the effect of albiflorin on the NLRP3 inflammasome, which is involved in the neuroinflammatory pathogenesis of neuropathic pain, anxiety, and depression. We found that the NLRP3 inflammasome contributed to neuropathic pain and comorbid anxiety and depression-like behaviors induced by chronic constriction injury of the sciatic nerve and that albiflorin may relieve these symptoms via inhibition of the NLRP3 inflammasome activity. Moreover, albiflorin enhanced the translocation of Nrf2 into the nucleus and suppressed nuclear factor-kappa B (NF-κB) activity in the hippocampus. We believe that our study makes a significant contribution to the literature because it strongly suggests that albiflorin directly regulates the NLRP3 inflammasome, proposing albiflorin as a potential therapeutic agent for neuropathic pain and related mood disorders.
Introduction
Neuropathic pain is defined as pain caused by a lesion or disease of the somatosensory system and is a multifaceted and ubiquitous disease across the globe (Jensen et al., 2011). Mood disorders, such as anxiety and depression, are frequently observed in patients who are suffering from chronic pain (Davis et al., 2011; Radat et al., 2013). These mental comorbidities increase neuropathic hypersensitivity (Yalcin et al., 2014; Doan et al., 2015; Humo et al., 2019). Thus, both neuropathic pain and comorbid mood disorders constitute a severe burden for patients, which can seriously impact quality of life (Yalcin et al., 2014; Barthas et al., 2015; Humo et al., 2019). Current drugs for neuropathic pain exhibit both poor efficacy and tolerability due to the lack of knowledge regarding the mechanisms of pathological pain (Cornelius et al., 2013; Peng et al., 2017; Inoue and Tsuda, 2018; Calvo et al., 2019; Meyer et al., 2019). Therefore, it is urgent to further clarify the mechanisms of neuropathic pain and identify more effective and non-addictive pharmacological therapies.
Accumulated studies have verified that neuroinflammation is involved in the pathogenesis of various neurogenic diseases and the overproduction of inflammatory cytokines are essential molecules responsible for neuroinflammation (Capuron and Miller, 2011; Fleshner et al., 2017). Among various inflammatory cytokines, a wide array of evidence supports the vital role of interleukin-(IL)-1β in the pathogenesis of multiple neuroinflammatory symptoms (Koo and Duman, 2008; Heneka et al., 2013; Clausen et al., 2016; Chen et al., 2018; Gordon et al., 2018; Li et al., 2019b). Nevertheless, IL-1β is only active after proteolysis by cysteine proteases (Afonina et al., 2015). Inflammasomes as cytosolic multiprotein complexes serve as platforms for the activation of cysteinyl aspartate specific proteinase (caspase)-1, which is the main mediator promoting the processing and secretion of IL-1β (Swanson et al., 2019). Nucleotide binding and oligomerization domain-like receptor family pyrin domain-containing 3 (NLRP3) is the most widely implicated regulator of caspase-1 activation. The NLRP3 inflammasome consists of NLRP3, apoptosis-associated speck-like protein, and caspase-1. When activated by exogenous irritants or endogenous danger signals, the NLRP3 inflammasome generates and activates caspase-1, which subsequently cleaves pro-IL-1β to its mature form, IL-1β (Swanson et al., 2019). Various studies have reported that the NLRP3 inflammasome is involved in the pathogenesis of neuropathic pain (Jia et al., 2017; Pan et al., 2018; Tonkin et al., 2018; Xu et al., 2019a), or anxiety and depression symptoms (Xu et al., 2016; Alcocer-Gómez and Cordero, 2017; Carney and Freedland, 2017; Yue et al., 2017; Wang et al., 2018; Feng et al., 2019; Zhang et al., 2019). Importantly, inhibition of the NLRP3 inflammasome could independently relieve each of these symptoms. However, to our knowledge, NLRP3 inflammasome has not been reported to be involved in the pathological process of anxiety and depression under the neuropathic pain state.
In addition to NLRP3, the Kelch-like ECH associated protein 1(Keap1)/nuclear factor erythroid 2-related factor 2 (Nrf2) signaling pathway is also involved in neuropathic pain and depression in preclinical and clinical studies (Braidy et al., 2018; Li et al., 2018; Bansal et al., 2019; Chu et al., 2019; Dang et al., 2019; Swanson et al., 2019; Zborowski et al., 2020; Zhou et al., 2020a, 2020b). Keap1 suppresses Nrf2 activity under physiological conditions, whereas Nrf2 is liberated from Keap1-mediated suppression on exposure to stresses. When the Keap1-Nrf2 signaling pathway is activated, Keap1 is chemically modified and releases Nrf2, which results in Nrf2 translocating into the nucleus. Nuclear Nrf2-driven gene transcription regulates the levels of antioxidant genes limiting reactive oxygen species (ROS) levels. Therefore, the significant increase of Nrf2 in nucleus (Nuclear Nrf2) is a good marker indicating the activation of the Keap1-Nrf2 pathway (Sies et al., 2017; Yamamoto et al., 2018). Moreover, the Keap1-Nrf2 signaling pathway has been reported to regulate the levels of antioxidant genes limiting ROS levels and attenuating the activation of NF-κB, thus inhibiting NLRP3 activation (Li et al., 2008; Liu et al., 2017; Chu et al., 2019; Dang et al., 2019), which implies that regulating the Keap1-NRF2/NLRP3 pathway might be a potential therapy against neuropathic pain and depression.
Paeoniae Alba Radix, the dried roots of P. lactiflora Pallas or P. veitchii Lynch, possesses various medicinal properties, including activating blood and alleviating pain (Ma et al., 2015). It has been widely used in traditional Chinese prescriptions to alleviate depression-like symptom for hundreds of years (Mao et al., 2012; Li et al., 2013; Zhu et al., 2015). Albiflorin (Figure 1) is a major constituent among the glucosides contained in P. alba Radix. Albiflorin has been reported to exert powerful anti-inflammatory effects and antidepressant activity in clinical reports and preclinical studies (Song et al., 2015; Wang et al., 2016; Han et al., 2018; Zhao et al., 2018; Cai et al., 2019; Wang et al., 2019; Zhang and Wei, 2020). Our previous study (Zhou et al., 2016) has demonstrated that albiflorin displays a significant analgesic effect on peripheral nerve injury-induced neuropathic pain rats. We also found that albiflorin efficiently reduced the elevated spinal IL-1β levels in neuropathic pain rats (Zhou et al., 2016). Previous studies have reported that albiflorin exerted antioxidant effects via reducing ROS levels (Suh et al., 2013; Xu et al., 2019b). Moreover, some Chinese herbal formulas containing albiflorin have been reported to ameliorate oxidative stress via the activation of the Keap1-Nrf2 signaling pathways to limit ROS (Ma et al., 2015; Yang et al., 2015; Hong et al., 2017). According to the above evidence, we hypothesized that albiflorin might reduce mood disorders accompanied with neuropathic pain through inhibition of the NLRP3 inflammasome, which is related to the regulation of the Keap1-Nrf2 pathway. It should be noted that data regarding the biological activities of albiflorin are scarce, and no report to our knowledge has examined the effect of albiflorin on the NLRP3 inflammasome.
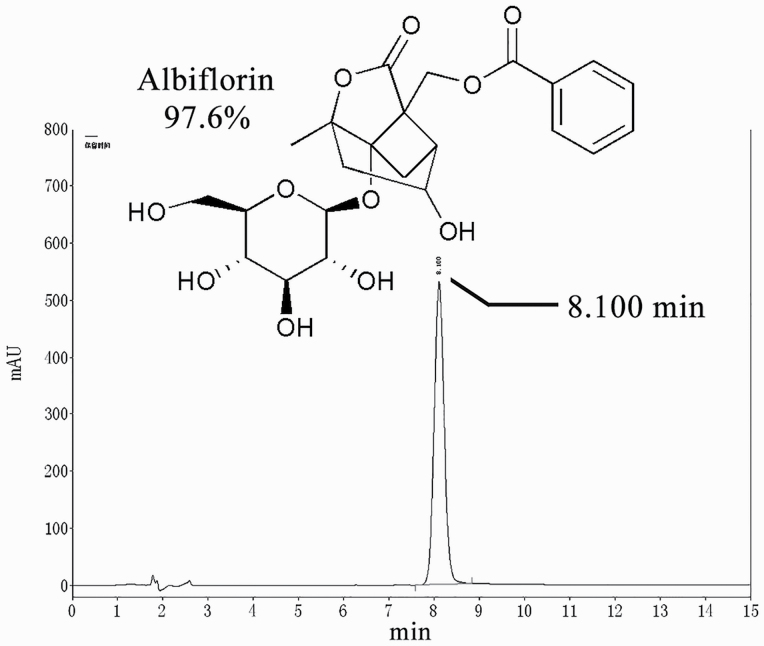
Figure 1.
Chemical structure and high-performance liquid chromatography chromatogram of albiflorin.
In the present study, we confirmed that activation of the NLRP3 inflammasome was involved in the pathogenesis of comorbid mood disorders (anxiety and depression) associated with neuropathic pain. Blocking the activation of the inflammasome constituted an ideal approach to improve anxiety and depression-like behaviors under the neuropathic pain state. Moreover, the present study showed a novel role for albiflorin, which exerted anti-anxiety and antidepressant effects highly associated with the inhibition of hippocampal inflammasome activation. Finally, we found that albiflorin inhibits the activation of NLRP3 inflammasome involved in enhancing Nrf2 translocation into the nucleus.
METHODS
Drugs
MCC950 (a selective NLRP3 inhibitor) was obtained from MedChemExpress (Monmouth Junction, NJ). Albiflorin was purchased from Chengdu Refmedic (Chengdu, China). The purity of albiflorin, as determined by high-performance liquid chromatography (Figure 1), was greater than 97%.
Animals
Adult male Sprague–Dawley rats (180–200 g) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd (Beijing, China). The rats were housed under a scheduled 12-h-light/-dark cycle at a constant temperature of 23°C ± 1°C. This study was carried out in accordance with the principles of the Basel Declaration and recommendations of the Guide for the Care and Use of Laboratory Animals (Ministry of Science and Technology of China, 2006). The protocol was approved by the Chengde Medical University Animal Care Committee.
Chronic Constriction Injury (CCI) of the Sciatic Nerve
The Sprague–Dawley rats were anesthetized by i.p. injection of chloral hydrate (300 mg/kg) and i.m. injection of ketorolac tromethamine (1 mg/kg). The operation was performed as described by Bennett and Xie (Bennett and Xie, 1988). Briefly, a small incision was made in the right thigh and the sciatic nerve was exposed. Then, 4 ligatures (4.0 chromic catgut) were tied loosely at the mid-thigh level of the sciatic nerve. Sham surgery comprised of exposure of the right sciatic nerve without ligation.
Drug Administration
Albiflorin and MCC950 were dissolved in sterile normal saline solution before use. The optimal administration doses of albiflorin (50 mg/kg) and MCC950 (10 mg/kg) were selected based on previous studies (Zhou et al., 2016; Khan et al., 2018; Chen et al., 2019b) and the results of preliminary experiments. MCC950 and albiflorin or vehicle were administered by i.p. injection daily for 15 days, starting on day 15 after CCI.
Behavioral Measurements
Before initiating the behavioral tests, rats were acclimated to experimental room conditions for at least 30 minutes. All tests were carried out according to the schedules depicted in Figure 2A and Figure 4A. Each of the individual behavioral tests was performed by the same observers blind to the grouping scheme.
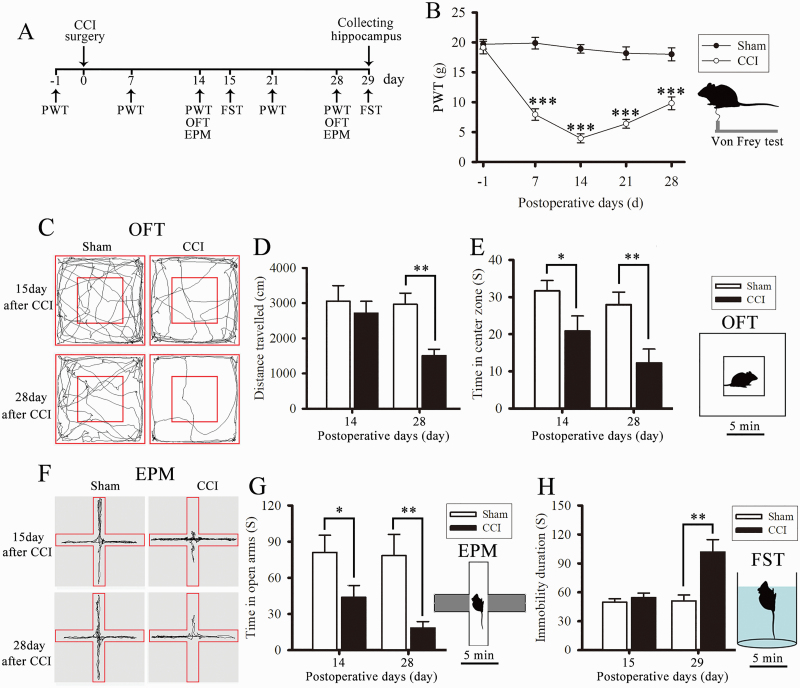
Figure 2.
Chronic constriction injury (CCI) surgery induced neuropathic pain and anxiety and depression-like behaviors in model rats. The scheme of this animal experiment is presented in panel A. (B) Paw withdrawal threshold (PWT) was measured using an electronic Von Frey Aesthesiometer. (C) Representative tracks showed the horizontal movement traces in the open field test (OFT) of Sham and CCI rats. (D) Total distance traveled (cm) in the OFT by sham and CCI rats. (E) Time (seconds) spent in the central zone. (F) Representative tracks showed the horizontal movement traces in the elevated plus maze test (EPM) of Sham and CCI rats. (G) Time (seconds) spent in the open arms. (H) Time (seconds) of immobility in the forced swim test (FST). Data are expressed as the mean ± SE for n = 10 rats/group. *P < .05, **P < .01, and ***P < .001 compared with sham group.
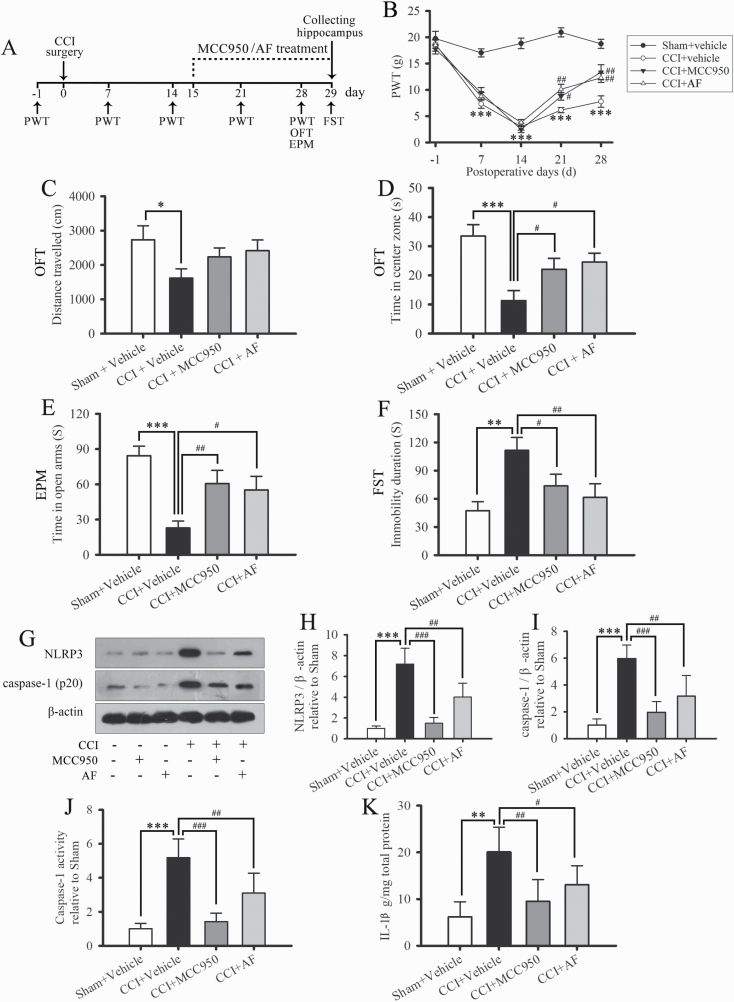
Figure 4.
MCC950 and Albiflorin (AF) relieve chronic constriction injury (CCI)-induced pain, anxiety, and depression-like behavior and suppress hippocampal NLRP3 inflammasome activation. (A) The scheme of this animal experiment. (B) Paw withdrawal threshold (PWT) was detected 1 day before CCI and on days 7, 14, 21, and 28 after CCI surgery. (C) Total distance traveled in the open field test (OFT) of sham and CCI rats. (D) Time spent in the central area of the OF. (E) Time spent in the open arms of the elevated plus maze (EPM). (F) Time of immobility in the forced swim test (FST). Data are expressed as the mean ± SE for n = 8 to 12 rats/group; *P < .05, **P < .01, and ***P < .001 compared with the sham group; #P < .05, ##P < .01 compared with the CCI group. (G) Representative western-blot bands of NLRP3 and caspase-1 (p20). Quantitative analysis of western-blot expression of NLRP3 (H) and caspase-1 (I) in sham-operated and CCI-operated rats. The fold change in the protein band density was normalized to that of β-actin. The activity of caspase-1 (J) and levels of interleukin (IL)-1β (K) in the hippocampus. Data are expressed as the mean ± SD of n = 4 rats/group; **P < .01, ***P < .001 compared with the sham group; #P < .05, ##P < .01, and ###P < .001 compared with the CCI group.
Mechanical Allodynia
Mechanical allodynia was measured using an electronic Von Frey Aesthesiometer (BIO-EVF4, Bioseb, Vitrolles, France). Briefly, the rats were placed on an elevated mesh platform in acrylic cages to acclimate for 15 minutes before the test. The elastic spring-type tip was fitted on the hand-held force transducer and stimulated the plantar surface of the right hind paw. The tests were repeated 3 times, with an interval of 5 minutes between each test, and the mechanical threshold was defined as the force (in g) initiating a withdrawal response averaged from the 3 measurements. Then the paw withdrawal threshold was captured as the mechanical allodynia threshold.
Open Field Test
Rats were placed in the center of an non-transparent arena (100 cm × 100 cm × 40 cm) individually; then, total distance traveled and duration of central zone visits were recorded for 5 minutes and analyzed using a behavior analysis system (SMART 3.0, Panlab, Barcelona, Spain). The method was modified from that used in a previous study (Bambico et al., 2007; Hu et al., 2009).
Elevated Plus Maze Test (EPM)
The test was conducted in a cross-shaped elevated maze consisting of 2 closed arms (50 cm × 10 cm × 40 cm) and 2 open arms (50 cm × 10 cm). Rats were singly placed in the middle of the maze, facing one of the open arms; then, their behavior was recorded for 5 minutes. The method is modified from that described in a previous study (Pellow et al., 1985; Bambico et al., 2010). The open arm entries and the duration of visiting the open arms were detected and analyzed using the SMART 3.0 software.
Forced Swim Test (FST)
The test was conducted in a glass cylinder (diameter, 22 cm; height, 50 cm) containing 30 cm of water at 23°C ± 1°C. The test was examined using the SMART 3.0 software according to a previously described protocol (Porsolt et al., 1978; Bambico et al., 2007). First, the rats were placed in the water for a 15-minute training exposure, and 24 hours later, they were re-exposed for a 5-minute test session, during which the duration of immobility was analyzed.
Immunofluorescence Labeling
On day 29 after CCI surgery, rats were deeply anesthetized by injections of chloral hydrate (350 mg/kg, i.p.) and ketorolac tromethamine (1 mg/kg, i.m.) after finishing the behavioral tests. The hippocampus was collected, post-fixed, embedded in paraffin, and cut into 5-μm thick sections. The sections were deparaffinized and incubated in citrate repair buffer (pH 6.0); sections were incubated in 3% bovine serum albumin for 30 minutes at 25°C to block non-specific proteins. The hippocampal sections were incubated with a mixture of mouse anti-neuronal nuclear antigen (NeuN) (1:200, Millipore, Billerica, MA), and rabbit anti-NLRP3 antibody (1:50, Novus, Littleton, CO) or rabbit anti-caspase-1 (1:500, servicebio, Wuhan, China) overnight at 4°C. After 3 washes in phosphate-buffered solution (PBS; pH 7.4), sections were incubated with Cy3-conjugated goat anti-rabbit (1:300, Servicebio) or 488-conjugated goat anti-mouse (1:400, Servicebio) secondary antibodies. Next, the sections were washed in PBS for 15 minutes, mounted on slides, and covered with coverslips. For control, primary antibodies were omitted in the process. Sections were imaged with a fluorescence microscope (BX53, Olympus, Tokyo, Japan). Images were taken using a digital camera system (DP74, Olympus). Analysis was performed by the same observer who was blinded to the grouping scheme.
Western-Blot Analysis
On day 29 after CCI, the hippocampus was collected, frozen in liquid nitrogen, and stored at −80°C until further processing. The total protein was supplemented with protease inhibitors and extracted using radioimmunoprecipitation assay lysis buffer (Beyotime Biotechnology, Shanghai, China). The nuclear protein fraction was extracted using the Nuclear Extraction Reagents kit (Beyotime Biotechnology). Proteins were separated using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and the resolved proteins were transferred onto nitrocellulose membranes (Millipore, Billerica, MA). Membranes were incubated in 5% nonfat dry milk for 2 hours at 25°C and overnight at 4°C with primary antibodies. Primary antibodies used in this study were: a rabbit anti-NLRP3 (1:500, Abcam, Cambridge, MA); a mouse anti-caspase-1 p20 (1:500, Santa Cruz, CA); a rabbit anti-Nrf2 (1:1000, Abcam); a rabbit anti-Keap1 (1:1000, Abcam); a rabbit anti-β-actin (1:1000, Cell Signaling Technology, Danvers, MA); and a rabbit anti-histone H3 (1:2000, Cell Signaling Technology, Danvers, MA). The blots were then incubated with goat anti-rabbit IgG H&L (horseradish peroxidase) (1:10 000, Abcam) or goat anti-mouse H&L (horseradish peroxidase) (1:1000, Beyotime, Shanghai, China) for 1 hour at 25°C. The membranes were visualized using an enhanced chemiluminescence western-blot kit (eECL, Beijing ComWin Biotech Co., Ltd., Beijing, China). The band analysis was performed using the ImageJ software (National Institutes of Health, Bethesda, MD).
Caspase-1 Activity Assay
On day 29 after CCI surgery, after finishing the final behavior test, the hippocampus was removed and homogenized in cold lysis buffer. The supernatant was collected after centrifugation at 20 000 × g for 15 minutes. The activity of caspase-1 in the hippocampus was detected using a caspase-1 activity assay kit (Beyotime Biotechnology, Shanghai, China) according to the manufacturer’s instruction. Absorbance measurements were performed using a microplate reader (1510, ThermoFisher, Vantaa, Finland) at 405 nm.
Enzyme-Linked Immunosorbent Assay
The frozen hippocampus was homogenized in cold PBS. Next, the supernatant was collected after centrifugation at 20 000 g for 10 minutes. The concentration of IL-1β was measured using an enzyme-linked immunosorbent assay kit (RLB00, R&D Systems, Minneapolis, MN) according to the manufacturer’s instruction.
Detection of RO) Generation in Hippocampus Tissue
After finishing the final behavioral test, the hippocampus was collected and frozen in liquid nitrogen using Tissue-Tek O.C.T. Compound (Sakura Finetek, VWR, Radnor, PA). The hippocampus tissue was cut into 8-μm-thick sections and rewarmed at 25°C for 30 minutes and then incubated in ROS dye solution (1:200, Dihydroethidium, Servicebio) at 37°C for 30 minutes. The sections were placed in PBS (pH 7.4) and washed 3 times, with shaking, for 5 minutes. After washing in PBS (pH 7.4), the sections were incubated with DAPI (Servicebio) at 25°C for 10 minutes, followed by a 15-minute wash in PBS, mounting on slides, and covered with coverslips. The hippocampal slices were imaged using a fluorescence microscope (BX53, Olympus). Images were captured with a digital camera (DP74, Olympus). The fluorescence intensity was examined to determine the ROS levels. Data are presented as fold-changes of the fluorescence signal.
NF-κB Activity Assay
The nuclear protein of hippocampus was extracted using a nuclear extraction kit (Cayman Chemical, Ann Arbor, MI), and the NF-κB activity of the hippocampus tissue was measured using an NF-κB transcription factor assay kit (Cayman Chemical) according to the manufacturer’s instruction.
Molecular Docking Between Albiflorin and Keap1
The protein-ligand docking studies were performed using Discovery Studio Client software 4.0 (Omaha, NE) to calculate the possible interaction of albiflorin with KEAP1. Chemical structure of albiflorin (CID—51346141) was available from PubChem database (https://pubchem.ncbi.nlm.nih.gov/). The crystal structures of Keap1 were available in the RCSB Protein Data Bank (http://www.rcsb.org/, PDB ID: 6TYP), and the protein structure is further processed by the Prepare Protein protocol of Macromolecules function module. CDOCKER protocol was used to analyze the binding affinity between albiflorin and the protein.
Statistical Analysis
Data were analyzed using the SPSS software (17.0, IBM, Chicago, IL). The behavioral data from different time points were analyzed using 2-way ANOVA analysis (time courses with repeated measures). Statistical comparisons between the 2 groups were performed using the Student’s t test. One-way ANOVA was used to compare the results of behavioral and biochemical data from multiple groups followed by the least-significant difference post-hoc test or Dunnett’s T3 test, where appropriate. A P < .05 was considered statistically significant.
RESULTS
Chronic Constriction Injury Surgery Induced Neuropathic Pain and Anxiety and Depression-Like Behaviors in Model Rats
Mechanical allodynia (Figure 2B) was significantly induced by CCI from day 7 to 28 after surgery (2-way ANOVA, main effect group: F1,18 = 318.21, P < .001; main effect time: F4,72 = 21.096, P < .001; group × time interaction: F4,72 = 16.762, P < .001; Figure 2B). The open field test was used to assess locomotor activity and anxiety-like behavior in model rats. The time CCI rats spent in the center zone of the open field was significantly reduced on days 14 and 28 after CCI surgery compared with that of the sham group (2-way ANOVA, main effect group: F1,18 = 13.949, P = .002; main effect time: F1,18 = 3.055, P = .098; group × time interaction: F1,18 = 0.486, P = .495; Figure 2E). In addition, the total distance traveled by the CCI rats in the open field did not significantly differ from that of the sham group on day 15 but significantly decreased on day 28 after CCI surgery (2-way ANOVA, main effect group: F1,18 = 10.12, P = .005; main effect time: F1,18 = 2.92, P = .105; group × time interaction: F1,18 = 2.176, P = .157; Figure 2C–D). This result suggests that the nerve damage did not affect the locomotor activity of rats on day 14, while the decreased total distance on day 28 after surgery was a manifestation of a depression-like behavior. The EPM test was used to assess anxiety-like behavior. The time CCI rats spent in the open arms of the EPM was significantly decreased on days 14 and 28 after surgery compared with the sham group (2-way ANOVA, main effect group: F1,18 = 14.442, P = .001; main effect time: F1,18 = 1.256, P = .277; group × time interaction: F1,18 = 0.837, P = .372; Figure 2F–G). The FST was used to assess depression-like behavior. FST results showed that the duration of immobility of CCI rats was significantly extended on day 29 after surgery compared with the sham group (2-way ANOVA, main effect group: F1,18 = 13.458, P = .002; main effect time: F1,18 = 9.31, P = .007; group × time interaction: F1,18 = 8.27, P = .01; Figure 2H). Altogether, these results indicate that CCI surgery not only induces neuropathic pain but also anxiety and depression-like behaviors in model rats.
CCI Surgery Induced Activation of the Hippocampal NLRP3 Inflammasome
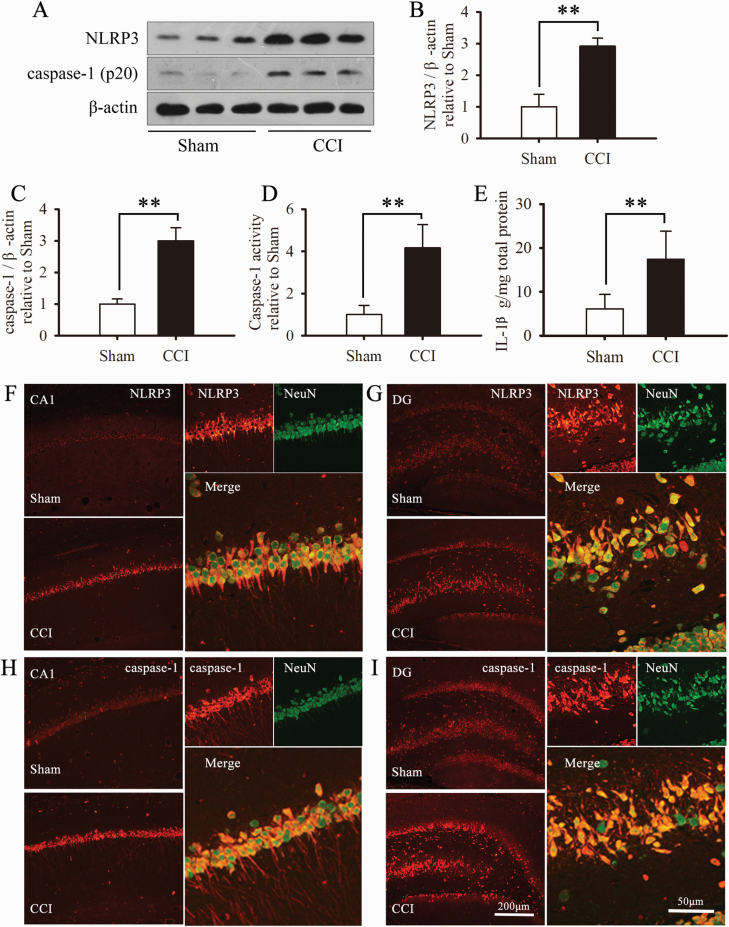
To test whether the NLRP3 inflammasome was activated in mood disorders associated with neuropathic pain, we examined activation of NLRP3 inflammasome in the hippocampus. NLRP3 (but not NLRP1; supplementary Figure 1) and caspase-1 expression were significantly increased in hippocampal neurons in CCI rats compared with the sham group (Figure 3A–C). Moreover, CCI surgery significantly enhanced caspase-1 activity and increased IL-1β levels in the hippocampus on day 29 after surgery (Figure 3D–E). To further confirm the cellular localization of the NLRP3 inflammasome after CCI surgery, we used double immunofluorescence for NLRP3 and the neuronal marker NeuN, and caspase-1 and NeuN. NLRP3 and caspase-1 were mainly expressed in the CA1 layer and dentate gyrus regions of the hippocampus and predominantly colocalized with NeuN (Figure 3F–I). These results indicate that hippocampal NLRP3 inflammasomes are activated in the state of neuropathic pain associated with anxiety/depression-like behaviors.
Figure 3.
Chronic constriction injury (CCI) surgery induced activation of the hippocampal NLRP3 inflammasome. (A) Representative Western blot bands of NLRP3 and caspase-1 (p20). Quantitative analysis of western-blot expression of NLRP3 (B) and caspase-1 (C) in sham-operated and CCI-operated rats. The fold change in the protein band density was normalized to that of β-actin, and the results are presented as fold change from the sham group (n = 3 rats/group). Activity of caspase-1 (D) and levels of interleukin (IL)-1β (E) in the hippocampus (n = 5 rats/group). Data are expressed as the mean ± SD; *P < .05, **P < .01, and ***P < .001 compared with sham group. Double-immunofluorescence representative photomicrographs displaying the cellular localization of NLRP3 (red) and NeuN (green) (F, G) and caspase-1 (red) and NeuN (green) (H, I) on day 29 after CCI. Photomicrographs are of the hippocampus of rats with CCI.
MCC950 and Albiflorin Relieved CCI-Induced Pain and Anxiety and Depression-Like Behavior and Suppressed Hippocampal NLRP3 Inflammasome Activation
To investigate the role of the NLRP3 inflammasome in CCI-induced mood disorders, MCC950, a selective NLRP3 inflammasome inhibitor, was administered by i.p. injection to CCI rats (Figure 4A). The behavioral tests were conducted after MCC950 treatment following the experimental scheme shown in Figure 4A. As illustrated in Figure 4B, administration of MCC950 relieved CCI-induced mechanical allodynia during days 21 to 28 after surgery (2-way ANOVA, main effect group: F5,48 = 164.649, P < .001; main effect time: F4,192 = 41.702, P < .001; group × time interaction: F20,192 = 10.755, P < .001). Meanwhile, MCC950 significantly reduced the anxiety and depression-like behavior induced by CCI surgery on days 28 and 29 after CCI (Figure 4D–F). In addition, MCC950 significantly reduced the expression of NLRP3 and caspase-1 in the hippocampus on day 29 after CCI (Figure 4G–I). Furthermore, MCC950 treatment decreased the CCI-elevated activity of caspase-1 and the content of IL-1β in the hippocampus (Figure 4J–K). Broadly analogous to that of MCC950, administration of albiflorin significantly relieved CCI-induced pain, anxiety, and depression-like behavior and suppressed hippocampal NLRP3 inflammasome activation (Figure 4B–K). Moreover, both the administration of albiflorin and MCC950 had no significant effects on the behavior or hippocampal NLRP3 inflammasome activation of sham-operated rats (supplementary Figure 2).
Altogether, these results demonstrated that NLRP3 inflammasome activation was involved in the pathological process of neuropathic pain associated with anxiety and depression-like behavior. In addition, the effect of albiflorin in attenuating the mood disorder under neuropathic pain state was related to the inhibition of the NLRP3 inflammasome.
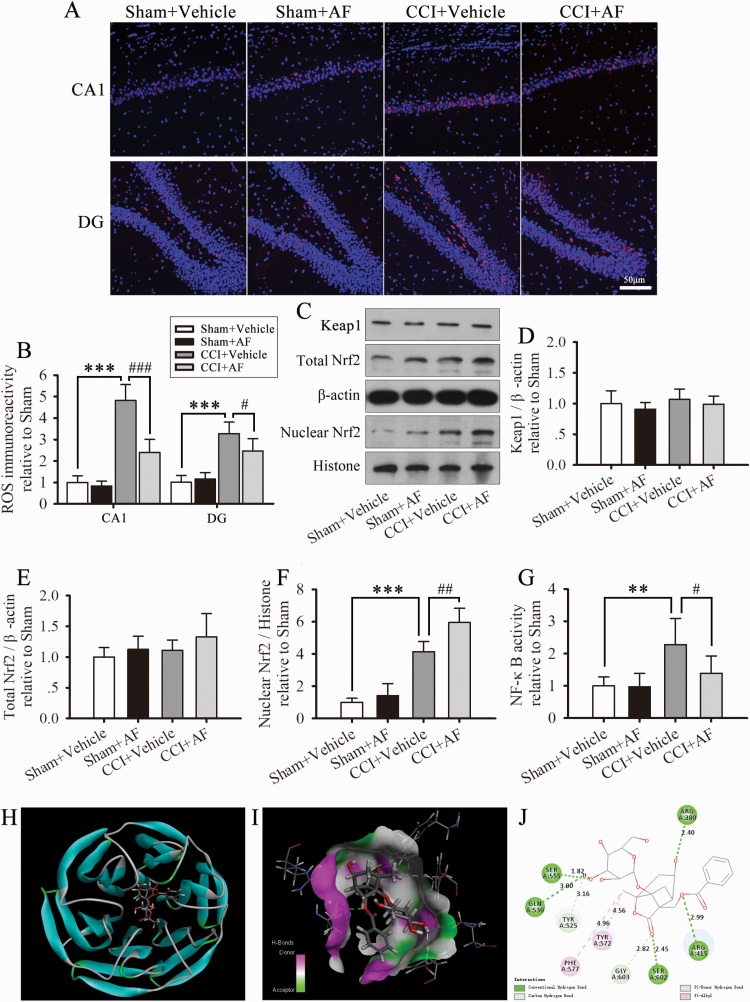
Albiflorin Promotes Nrf2 Translocation Into the Nucleus and Limits the Elevated Level of ROS and Activity of NF-κB in Hippocampus
To investigate the role of albiflorin in the inhibition of the NLRP3 inflammasome, we examined the effect of albiflorin on the Keap1-Nrf2 signaling pathway. As illustrated in (Figure 5A–B), albiflorin treatment significantly limited the increased levels of ROS induced by CCI in the hippocampus. In addition, albiflorin administration clearly enhanced the translocation of Nrf2 into the nucleus but did not affect the total expression levels of Nrf2 and Keap1. These results suggest that albiflorin activates the Keap1-Nrf2 signaling pathway, thus attenuating the ROS levels after CCI. In addition, albiflorin significantly limited the activity of hippocampal NF-κB (Figure 5G). Furthermore, albiflorin had no significant effect on the sham group (Figure 5A–G).
Figure 5.
Albiflorin (AF) promotes Nrf2 translocation into the nucleus and limits the elevated level of reactive oxygen species (ROS) and activity of NF-κB in the hippocampus. (A) Representative photographs of hippocampal sections of ROS. (B) Quantification of ROS immunoreactivity in the CA1 layer and dentate gyrus (DG) regions of the hippocampus. (C–F) Western-blot analysis showing the expression of total and nuclear Nrf2 and Keap1 in the hippocampus on day 29 after CCI. The fold changes in the density of total Nrf2 and Keap1 are normalized to that of β-actin, and that of nuclear Nrf2 is normalized to that of histone H3. (G) NF-κB activity. The results are presented as fold changes compared with the sham group. Data are expressed as the mean ± SD for n = 4 rats/group; **P < .01, ***P < .001 compared with sham group; #P < .01, ##P < .01 compared with the CCI group. (H–J) Molecular docking of AF to Keap1. (H) KEAP1 Kelch domain in complex with AF (CDOCKER_INTERACTION_ENERGY: -50.142 kcal/mol). (I) Magnified portion of 3D interaction map between AF and 6TYP binding site. (J) 2D diagram of docking structure of AF with 6TYP.
Molecular Docking of Albiflorin to Keap1
To further investigate the possible mechanism of albiflorin in the regulation of the Keap1-Nrf2 signaling pathway, we used molecular docking to examine the binding modes of albiflorin with Keap1. As shown in Figure 5H, Albiflorin is predicted to dock with KEAP1 Kelch domain, a recognition module for Nrf2 (CDOCKER_INTERACTION_ENERGY: -50.142 kcal/mol). Figure 5I and J show the predicted binding sites of albiflorin to Keap1. These data suggest that albiflorin displays non-covalent binds to Keap1.
Discussion
The NLRP3 inflammasome is the most widely implicated regulator of caspase-1 activation. Excessive NLPR3 inflammasome activity leads to systemic “sterile” inflammation in both periphery and brain (Youm et al., 2013). The hippocampus is an important brain region in stress response and adjustment for emotion and memory (Liu et al., 2013; Pan et al., 2014). Some animal studies reported that harmful chemicals (including lipopolysaccharides) or chronic stress can lead to NLRP3 inflammasome activation in the hippocampus. Meanwhile, animal models displayed anxiety and depression-like behavior and memory loss symptoms. Suppressing the activation of the NLRP3 inflammasome exerted neuroprotective effects and improved the aforementioned neurological symptoms (Lu et al., 2014; Zhang et al., 2014; Xu et al., 2016; Liu et al., 2019; Fu et al., 2020; Li et al., 2020). In addition, several recent studies have shown that the NLPR3 inflammasome is involved in the pathogenesis of neuropathic pain, and inhibition of NLPR3 inflammasome can effectively alleviate nociceptive hypersensitivity (Jia et al., 2017; Zhang et al., 2017; Grace et al., 2018; Khan et al., 2018; Pan et al., 2018; Chen et al., 2019a, 2019b). Although many studies have been conducted on NLRP3 inflammasome’s involvement in the pathological process of neuropathic pain, anxiety, and depression, so far there are no studies, to our knowledge, on NLPR3 inflammasome’s involvement in neuropathic pain associated with anxiety and depression-like behaviors. A recent study indicated that the hippocampal NLRP1 inflammasome pathway plays a pivotal role in the development of anxiety and depressive behaviors after chronic neuropathic pain (Li et al., 2019a). To our surprise, our present data showed that CCI significantly induced elevated NLPR3, instead of NLRP1 (supplementary Figure 1), expression in hippocampal neurons, contributing to anxiety and depression-like behaviors. To further confirm that the activation of NLRP3 inflammasome contributes to these mood disorders, we treated CCI rats with a selective NLRP3 inhibitor. We found that suppressing the activation of the NLRP3 inflammasome not only significantly decreased the hyperalgesia but also attenuated the anxiety and depression-like behaviors in CCI rats. These results indicate that the activation of NLRP3 inflammasome is involved in the pathological process of anxiety and depression-like behaviors associated with neuropathic pain. Moreover, NLPR3 inflammasome might be a potential therapeutic target for neuropathic pain-associated anxious and depressive disorders.
Albiflorin and paeoniflorin are the main monoterpene glycosides among the glucosides contained in P. alba Radix. The anti-inflammatory and antidepressant effects of albiflorin and paeoniflorin have been well documented in existing reports (Wang et al., 2019; Zhang and Wei, 2020). Albiflorin displays more powerful antidepressant activity than its isomer, paeoniflorin (Wang et al., 2016). However, there has been no study, to our knowledge, on albiflorin’s role in the regulation of NLRP3 inflammasome. Our results show that albiflorin effectively alleviates neuropathic pain induced by CCI surgery, which is consistent with results of a previous study (Zhou et al., 2016). Importantly, albiflorin significantly inhibited the activation of NLRP3 inflammasome in the hippocampus and relieved the anxiety-depressive behavior of model rats. These results suggest that albiflorin produces these effects by inhibiting the activation of NLPR3, similar to the action of the selective NLRP3 inhibitor MCC950.
It is well documented that ROS are pivotal upstream events implicated in the activation of NLRP3 (Dostert et al., 2008; Zhou et al., 2011; Groß et al., 2016). ROS are continuously released by mitochondria as byproducts of oxidative phosphorylation. Under physiological conditions, cellular antioxidants reduce ROS, maintaining the redox level balance. During the inflammatory response, excessive production of ROS causes oxidative stress, which usually leads to NLRP3 activation (Dostert et al., 2008; Groß et al., 2016). In addition to ROS, the Keap1-Nrf2 pathway participates in the regulation of the inflammasome. Under physiological conditions, Nrf2 is sequestered in the cytosol and maintained at a low level through a Keap1-Nrf2 protein to protein interaction. Under oxidative stress conditions, Keap1 is chemically modified and releases Nrf2, which results in Nrf2 translocating into the nucleus. Nrf2-driven gene transcription regulates the levels of antioxidant genes limiting ROS levels, thus inhibiting NLRP3 activation (Liu et al., 2017; Chu et al., 2019; Dang et al., 2019). Moreover, Nrf2 attenuates the activation of NF-κB; this leads to suppression of the inflammasome activity by downregulating the expression of the inflammasome components NLRP3, caspase-1, and pro-IL-1β (Li et al., 2008; Chu et al., 2019; Dang et al., 2019). In addition to its role in NLRP3 inflammasome, the Kea1-Nrf2 pathway is also involved in depression in preclinical and clinical studies (Bansal et al., 2019; Chu et al., 2019; Dang et al., 2019; Swanson et al., 2019; Zborowski et al., 2020), which implies that interrupting the Keap1-NRF2 protein–protein interaction might be a potential therapy against depression. Furthermore, a recent animal study indicated that the Nrf2-NLRP3 signaling pathway, which is responsible for adjusting inflammation levels, plays a vital role in the depression induced by environmental factors (Chu et al., 2019). Albiflorin has shown strong antioxidant activity in in vitro and in vivo studies (Suh et al., 2013; Ho et al., 2015; Hong et al., 2017; Xu et al., 2019b). However, to our knowledge, no previous reports have shown that albiflorin activates the Keap1-Nrf2 pathway to regulate the NLRP3 inflammasome. In the present study, albiflorin treatment significantly promoted the Nrf2 translocation to the nucleus and reduced the levels of hippocampal ROS and caspase-1 activity. To our surprise, albiflorin had no significant effect on Keap1 or total Nrf2 expression. However, molecular docking revealed that albiflorin displayed affinity for Keap1. These data suggest that albiflorin may disrupt Keap1-Nrf2 interaction through a non-covalent binding, which deserves further investigation. In addition, consistent with previous studies (Zhou et al., 2016; Cai et al., 2019), our present results indicate that albiflorin also markedly reduces the elevated NF-κB activity induced by CCI surgery. Moreover, the current results indicate that the regulation of antioxidant levels may be one of the mechanisms through which albiflorin inhibits the activation of the NLRP3 inflammasome.
Conclusions
In conclusion, our study confirms that activation of the NLRP3 inflammasome is involved in CCI-induced anxiety and depression. The administration at analgesic dose of albiflorin simultaneously exerted an antidepressant and anxiolytic-like activity. Our present data also demonstrated that inhibiting the activation of the NLRP3 inflammasome in the hippocampus is highly associated with the therapeutic effects of albiflorin. Albiflorin represents a potential therapeutic agent in the treatment of neuropathic pain conditions, which might greatly improve the overall symptoms of neuropathic pain with relevant clinical benefit.
Supplementary Material
Acknowledgments
This study was supported by the National Natural Science Foundation of China (no. 81703736), Key Discipline Construction Projects of Higher School, Hebei Province Natural Science Foundation of China (no. H2018406038), Science and Technology Research Youth Fund Project of Higher School in Hebei Province (no. QN2019167), and Hebei Province Medical Science Research Key Project (no. 20181140).
Statement of Interest
None.
References
- Afonina IS, Müller C, Martin SJ, Beyaert R (2015) Proteolytic processing of Interleukin-1 family cytokines: variations on a common theme. Immunity 42:991–1004. [DOI] [PubMed] [Google Scholar]
- Alcocer-Gómez E, Cordero MD (2017) NLRP3 inflammasome: common nexus between depression and cardiovascular diseases. Nat Rev Cardiol 14:124. [DOI] [PubMed] [Google Scholar]
- Bambico FR, Katz N, Debonnel G, Gobbi G (2007) Cannabinoids elicit antidepressant-like behavior and activate serotonergic neurons through the medial prefrontal cortex. J Neurosci 27:11700–11711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bambico FR, Nguyen NT, Katz N, Gobbi G (2010) Chronic exposure to cannabinoids during adolescence but not during adulthood impairs emotional behaviour and monoaminergic neurotransmission. Neurobiol Dis 37:641–655. [DOI] [PubMed] [Google Scholar]
- Bansal Y, Singh R, Parhar I, Kuhad A, Soga T (2019) Quinolinic acid and nuclear factor erythroid 2-related factor 2 in depression: role in neuroprogression. Front Pharmacol 10:452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthas F, Sellmeijer J, Hugel S, Waltisperger E, Barrot M, Yalcin I (2015) The anterior cingulate cortex is a critical hub for pain-induced depression. Biol Psychiatry 77:236–245. [DOI] [PubMed] [Google Scholar]
- Bennett GJ, Xie YK (1988) A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 33:87–107. [DOI] [PubMed] [Google Scholar]
- Braidy N, Izadi M, Sureda A, Jonaidi-Jafari N, Banki A, Nabavi SF, Nabavi SM (2018) Therapeutic relevance of ozone therapy in degenerative diseases: focus on diabetes and spinal pain. J Cell Physiol 233:2705–2714. [DOI] [PubMed] [Google Scholar]
- Cai Z, Liu J, Bian H, Cai J (2019) Albiflorin alleviates ovalbumin (OVA)-induced pulmonary inflammation in asthmatic mice. Am J Transl Res 11:7300–7309. [PMC free article] [PubMed] [Google Scholar]
- Calvo M, Davies AJ, Hébert HL, Weir GA, Chesler EJ, Finnerup NB, Levitt RC, Smith BH, Neely GG, Costigan M, Bennett DL (2019) The genetics of neuropathic pain from model organisms to clinical application. Neuron 104:637–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Miller AH (2011) Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther 130:226–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney RM, Freedland KE (2017) Reply: NLRP3 inflammasome as a mechanism linking depression and cardiovascular diseases. Nat Rev Cardiol 14:124. [DOI] [PubMed] [Google Scholar]
- Chen G, Zhang YQ, Qadri YJ, Serhan CN, Ji RR (2018) Microglia in pain: detrimental and protective roles in pathogenesis and resolution of pain. Neuron 100:1292–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Zhou C, Xie K, Meng X, Wang Y, Yu Y (2019a) Hydrogen-rich saline alleviated the hyperpathia and microglia activation via autophagy mediated inflammasome inactivation in neuropathic pain rats. Neuroscience 421:17–30. [DOI] [PubMed] [Google Scholar]
- Chen SP, Zhou YQ, Wang XM, Sun J, Cao F, HaiSam S, Ye DW, Tian YK (2019b) Pharmacological inhibition of the NLRP3 inflammasome as a potential target for cancer-induced bone pain. Pharmacol Res 147:104339. [DOI] [PubMed] [Google Scholar]
- Chu C, Zhang H, Cui S, Han B, Zhou L, Zhang N, Su X, Niu Y, Chen W, Chen R, Zhang R, Zheng Y (2019) Ambient PM2.5 caused depressive-like responses through Nrf2/NLRP3 signaling pathway modulating inflammation. J Hazard Mater 369:180–190. [DOI] [PubMed] [Google Scholar]
- Clausen BH, Lambertsen KL, Dagnæs-Hansen F, Babcock AA, von Linstow CU, Meldgaard M, Kristensen BW, Deierborg T, Finsen B (2016) Cell therapy centered on IL-1Ra is neuroprotective in experimental stroke. Acta Neuropathol 131:775–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius VR, Sauzet O, Williams JE, Ayis S, Farquhar-Smith P, Ross JR, Branford RA, Peacock JL (2013) Adverse event reporting in randomised controlled trials of neuropathic pain: considerations for future practice. Pain 154:213–220. [DOI] [PubMed] [Google Scholar]
- Dang R, Guo YY, Zhang K, Jiang P, Zhao MG (2019) Predictable chronic mild stress promotes recovery from LPS-induced depression. Mol Brain 12:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Robinson RL, Le TK, Xie J (2011) Incidence and impact of pain conditions and comorbid illnesses. J Pain Res 4:331–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doan L, Manders T, Wang J (2015) Neuroplasticity underlying the comorbidity of pain and depression. Neural Plast 2015:504691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostert C, Pétrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J (2008) Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 320:674–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X, Zhao Y, Yang T, Song M, Wang C, Yao Y, Fan H (2019) Glucocorticoid-driven NLRP3 inflammasome activation in hippocampal microglia mediates chronic stress-induced depressive-like behaviors. Front Mol Neurosci 12:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleshner M, Frank M, Maier SF (2017) Danger signals and inflammasomes: stress-evoked sterile inflammation in mood disorders. Neuropsychopharmacology 42:36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Q, Li J, Qiu L, Ruan J, Mao M, Li S, Mao Q (2020) Inhibiting NLRP3 inflammasome with MCC950 ameliorates perioperative neurocognitive disorders, suppressing neuroinflammation in the hippocampus in aged mice. Int Immunopharmacol 82:106317. [DOI] [PubMed] [Google Scholar]
- Gordon R, Albornoz EA, Christie DC, Langley MR, Kumar V, Mantovani S, Robertson A, Butler MS, Rowe DB, O’Neill LA, Kanthasamy AG, Schroder K, Cooper MA, Woodruff TM (2018) Inflammasome inhibition prevents α-synuclein pathology and dopaminergic neurodegeneration in mice. Sci Transl Med 10:eaah4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace PM, Strand KA, Galer EL, Rice KC, Maier SF, Watkins LR (2018) Protraction of neuropathic pain by morphine is mediated by spinal damage associated molecular patterns (DAMPs) in male rats. Brain Behav Immun 72:45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groß CJ, et al. (2016) K+ Efflux-independent NLRP3 inflammasome activation by small molecules targeting mitochondria. Immunity 45:761–773. [DOI] [PubMed] [Google Scholar]
- Han J, Xia Y, Lin L, Zhang Z, Tian H, Li K (2018) Next-generation metabolomics in the development of new antidepressants: using albiflorin as an example. Curr Pharm Des 24:2530–2540. [DOI] [PubMed] [Google Scholar]
- Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, Griep A, Axt D, Remus A, Tzeng TC, Gelpi E, Halle A, Korte M, Latz E, Golenbock DT (2013) NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 493:674–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SL, Poon CY, Lin C, Yan T, Kwong DW, Yung KK, Wong MS, Bian Z, Li HW (2015) Inhibition of β-amyloid aggregation by albiflorin, aloeemodin and neohesperidin and their neuroprotective effect on primary hippocampal cells against β-amyloid induced toxicity. Curr Alzheimer Res 12:424–433. [DOI] [PubMed] [Google Scholar]
- Hong C, Cao J, Wu CF, Kadioglu O, Schüffler A, Kauhl U, Klauck SM, Opatz T, Thines E, Paul NW, Efferth T (2017) The Chinese herbal formula free and easy wanderer ameliorates oxidative stress through KEAP1-NRF2/HO-1 pathway. Sci Rep 7:11551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Doods H, Treede RD, Ceci A (2009) Depression-like behaviour in rats with mononeuropathy is reduced by the CB2-selective agonist GW405833. Pain 143:206–212. [DOI] [PubMed] [Google Scholar]
- Humo M, Lu H, Yalcin I (2019) The molecular neurobiology of chronic pain-induced depression. Cell Tissue Res 377:21–43. [DOI] [PubMed] [Google Scholar]
- Inoue K, Tsuda M (2018) Microglia in neuropathic pain: cellular and molecular mechanisms and therapeutic potential. Nat Rev Neurosci 19:138–152. [DOI] [PubMed] [Google Scholar]
- Jensen TS, Baron R, Haanpää M, Kalso E, Loeser JD, Rice AS, Treede RD (2011) A new definition of neuropathic pain. Pain 152:2204–2205. [DOI] [PubMed] [Google Scholar]
- Jia M, Wu C, Gao F, Xiang H, Sun N, Peng P, Li J, Yuan X, Li H, Meng X, Tian B, Shi J, Li M (2017) Activation of NLRP3 inflammasome in peripheral nerve contributes to paclitaxel-induced neuropathic pain. Mol Pain 13:1744806917719804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N, Kuo A, Brockman DA, Cooper MA, Smith MT (2018) Pharmacological inhibition of the NLRP3 inflammasome as a potential target for multiple sclerosis induced central neuropathic pain. Inflammopharmacology 26:77–86. [DOI] [PubMed] [Google Scholar]
- Koo JW, Duman RS (2008) IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci U S A 105:751–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Liu S, Zhu X, Mi W, Maoying Q, Wang J, Yu J, Wang Y (2019a) Hippocampal PKR/NLRP1 inflammasome pathway is required for the depression-like behaviors in rats with neuropathic pain. Neuroscience 412:16–28. [DOI] [PubMed] [Google Scholar]
- Li S, Yang C, Fang X, Zhan G, Huang N, Gao J, Xu H, Hashimoto K, Luo A (2018) Role of Keap1-Nrf2 signaling in anhedonia symptoms in a rat model of chronic neuropathic pain: improvement with sulforaphane. Front Pharmacol 9:887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Khor TO, Xu C, Shen G, Jeong WS, Yu S, Kong AN (2008) Activation of Nrf2-antioxidant signaling attenuates NFkappaB-inflammatory response and elicits apoptosis. Biochem Pharmacol 76:1485–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhang Y, Li B, Yang H, Cui J, Li X, Zhang X, Sun H, Meng Q, Wu S, Li S, Wang J, Aschner M, Chen R (2020) Activation of NLRP3 in microglia exacerbates diesel exhaust particles-induced impairment in learning and memory in mice. Environ Int 136:105487. [DOI] [PubMed] [Google Scholar]
- Li XQ, Yu Q, Zhang ZL, Sun XJ, Ma H (2019b) MiR-187-3p mimic alleviates ischemia-reperfusion-induced pain hypersensitivity through inhibiting spinal P2X7R and subsequent mature IL-1β release in mice. Brain Behav Immun 79:91–101. [DOI] [PubMed] [Google Scholar]
- Li Y, Sun Y, Ma X, Xue X, Zhang W, Wu Z, Ouyang Y, Chen J, Wang W, Guo S, Wang W (2013) Effects of Sini San used alone and in combination with fluoxetine on central and peripheral 5-HT levels in a rat model of depression. J Tradit Chin Med 33:674–681. [DOI] [PubMed] [Google Scholar]
- Liu P, Bai X, Zhang T, Zhou L, Li J, Zhang L (2019) The protective effect of Lonicera japonica polysaccharide on mice with depression by inhibiting NLRP3 inflammasome. Ann Transl Med 7:811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Sheng H, Xu Y, Liu Y, Lu J, Ni X (2013) Swimming exercise ameliorates depression-like behavior in chronically stressed rats: relevant to proinflammatory cytokines and IDO activation. Behav Brain Res 242:110–116. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhang X, Ding Y, Zhou W, Tao L, Lu P, Wang Y, Hu R (2017) Nuclear factor E2-related Factor-2 negatively regulates NLRP3 inflammasome activity by inhibiting reactive oxygen species-induced NLRP3 priming. Antioxid Redox Signal 26:28–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Yang JZ, Geng F, Ding JH, Hu G (2014) Iptakalim confers an antidepressant effect in a chronic mild stress model of depression through regulating neuro-inflammation and neurogenesis. Int J Neuropsychopharmacol 17:1501–1510. [DOI] [PubMed] [Google Scholar]
- Ma X, Zhao YL, Zhu Y, Chen Z, Wang JB, Li RY, Chen C, Wei SZ, Li JY, Liu B, Wang RL, Li YG, Wang LF, Xiao XH (2015) Paeonia lactiflora Pall. protects against ANIT-induced cholestasis by activating Nrf2 via PI3K/Akt signaling pathway. Drug Des Devel Ther 9:5061–5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao QQ, Ip SP, Xian YF, Hu Z, Che CT (2012) Anti-depressant-like effect of peony: a mini-review. Pharm Biol 50:72–77. [DOI] [PubMed] [Google Scholar]
- Meyer L, Taleb O, Patte-Mensah C, Mensah-Nyagan AG (2019) Neurosteroids and neuropathic pain management: basic evidence and therapeutic perspectives. Front Neuroendocrinol 55:100795. [DOI] [PubMed] [Google Scholar]
- Pan Y, Chen XY, Zhang QY, Kong LD (2014) Microglial NLRP3 inflammasome activation mediates IL-1β-related inflammation in prefrontal cortex of depressive rats. Brain Behav Immun 41:90–100. [DOI] [PubMed] [Google Scholar]
- Pan Z, Shan Q, Gu P, Wang XM, Tai LW, Sun M, Luo X, Sun L, Cheung CW (2018) miRNA-23a/CXCR4 regulates neuropathic pain via directly targeting TXNIP/NLRP3 inflammasome axis. J Neuroinflammation 15:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M (1985) Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods 14:149–167. [DOI] [PubMed] [Google Scholar]
- Peng C, Li L, Zhang MD, Bengtsson Gonzales C, Parisien M, Belfer I, Usoskin D, Abdo H, Furlan A, Häring M, Lallemend F, Harkany T, Diatchenko L, Hökfelt T, Hjerling-Leffler J, Ernfors P (2017) miR-183 cluster scales mechanical pain sensitivity by regulating basal and neuropathic pain genes. Science 356:1168–1171. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Anton G, Blavet N, Jalfre M (1978) Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol 47:379–391. [DOI] [PubMed] [Google Scholar]
- Radat F, Margot-Duclot A, Attal N (2013) Psychiatric co-morbidities in patients with chronic peripheral neuropathic pain: a multicentre cohort study. Eur J Pain 17:1547–1557. [DOI] [PubMed] [Google Scholar]
- Sies H, Berndt C, Jones DP (2017) Oxidative stress. Annu Rev Biochem 86:715–748. [DOI] [PubMed] [Google Scholar]
- Song J, Hou X, Hu X, Lu C, Liu C, Wang J, Liu W, Teng L, Wang D (2015) Not only serotonergic system, but also dopaminergic system involved in albiflorin against chronic unpredictable mild stress-induced depression-like behavior in rats. Chem Biol Interact 242:211–217. [DOI] [PubMed] [Google Scholar]
- Suh KS, Choi EM, Lee YS, Kim YS (2013) Protective effect of albiflorin against oxidative-stress-mediated toxicity in osteoblast-like MC3T3-E1 cells. Fitoterapia 89:33–41. [DOI] [PubMed] [Google Scholar]
- Swanson KV, Deng M, Ting JP (2019) The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol 19:477–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonkin RS, Bowles C, Perera CJ, Keating BA, Makker PGS, Duffy SS, Lees JG, Tran C, Don AS, Fath T, Liu L, O’Carroll SJ, Nicholson LFB, Green CR, Gorrie C, Moalem-Taylor G (2018) Attenuation of mechanical pain hypersensitivity by treatment with Peptide5, a connexin-43 mimetic peptide, involves inhibition of NLRP3 inflammasome in nerve-injured mice. Exp Neurol 300:1–12. [DOI] [PubMed] [Google Scholar]
- Wang YL, Wang JX, Hu XX, Chen L, Qiu ZK, Zhao N, Yu ZD, Sun SZ, Xu YY, Guo Y, Liu C, Zhang YZ, Li YF, Yu CX (2016) Antidepressant-like effects of albiflorin extracted from Radix paeoniae Alba. J Ethnopharmacol 179:9–15. [DOI] [PubMed] [Google Scholar]
- Wang YL, Han QQ, Gong WQ, Pan DH, Wang LZ, Hu W, Yang M, Li B, Yu J, Liu Q (2018) Microglial activation mediates chronic mild stress-induced depressive- and anxiety-like behavior in adult rats. J Neuroinflammation 15:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YS, Shen CY, Jiang JG (2019) Antidepressant active ingredients from herbs and nutraceuticals used in TCM: pharmacological mechanisms and prospects for drug discovery. Pharmacol Res 150:104520. [DOI] [PubMed] [Google Scholar]
- Xu L, Wang Q, Jiang W, Yu S, Zhang S (2019a) MiR-34c ameliorates neuropathic pain by targeting NLRP3 in a mouse model of chronic constriction injury. Neuroscience 399:125–134. [DOI] [PubMed] [Google Scholar]
- Xu Y, Sheng H, Bao Q, Wang Y, Lu J, Ni X (2016) NLRP3 inflammasome activation mediates estrogen deficiency-induced depression- and anxiety-like behavior and hippocampal inflammation in mice. Brain Behav Immun 56:175–186. [DOI] [PubMed] [Google Scholar]
- Xu YJ, Mei Y, Shi XQ, Zhang YF, Wang XY, Guan L, Wang Q, Pan HF (2019b) Albiflorin ameliorates memory deficits in APP/PS1 transgenic mice via ameliorating mitochondrial dysfunction. Brain Res 1719:113–123. [DOI] [PubMed] [Google Scholar]
- Yalcin I, Barthas F, Barrot M (2014) Emotional consequences of neuropathic pain: insight from preclinical studies. Neurosci Biobehav Rev 47:154–164. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Kensler TW, Motohashi H (2018) The KEAP1-NRF2 System: a thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol Rev 98:1169–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Yao W, Li Q, Liu H, Shi H, Gao Y, Xu L (2015) Mechanism of Tang Luo Ning effect on attenuating of oxidative stress in sciatic nerve of STZ-induced diabetic rats. J Ethnopharmacol 174:1–10. [DOI] [PubMed] [Google Scholar]
- Youm YH, Grant RW, McCabe LR, Albarado DC, Nguyen KY, Ravussin A, Pistell P, Newman S, Carter R, Laque A, Münzberg H, Rosen CJ, Ingram DK, Salbaum JM, Dixit VD (2013) Canonical Nlrp3 inflammasome links systemic low-grade inflammation to functional decline in aging. Cell Metab 18:519–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue N, Huang H, Zhu X, Han Q, Wang Y, Li B, Liu Q, Wu G, Zhang Y, Yu J (2017) Activation of P2X7 receptor and NLRP3 inflammasome assembly in hippocampal glial cells mediates chronic stress-induced depressive-like behaviors. J Neuroinflammation 14:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zborowski VA, Heck SO, Vencato M, Pinton S, Marques LS, Nogueira CW (2020) Keap1/Nrf2/HO-1 signaling pathway contributes to p-chlorodiphenyl diselenide antidepressant-like action in diabetic mice. Psychopharmacology (Berl) 237:363–374. [DOI] [PubMed] [Google Scholar]
- Zhang A, Wang K, Ding L, Bao X, Wang X, Qiu X, Liu J (2017) Bay11-7082 attenuates neuropathic pain via inhibition of nuclear factor-kappa B and nucleotide-binding domain-like receptor protein 3 inflammasome activation in dorsal root ganglions in a rat model of lumbar disc herniation. J Pain Res 10:375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Wei W (2020) Anti-inflammatory and immunoregulatory effects of paeoniflorin and total glucosides of paeony. Pharmacol Ther 207:107452. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liu L, Peng YL, Liu YZ, Wu TY, Shen XL, Zhou JR, Sun DY, Huang AJ, Wang X, Wang YX, Jiang CL (2014) Involvement of inflammasome activation in lipopolysaccharide-induced mice depressive-like behaviors. CNS Neurosci Ther 20:119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Huang R, Cheng M, Wang L, Chao J, Li J, Zheng P, Xie P, Zhang Z, Yao H (2019) Gut microbiota from NLRP3-deficient mice ameliorates depressive-like behaviors by regulating astrocyte dysfunction via circHIPK2. Microbiome 7:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZX, Fu J, Ma SR, Peng R, Yu JB, Cong L, Pan LB, Zhang ZG, Tian H, Che CT, Wang Y, Jiang JD (2018) Gut-brain axis metabolic pathway regulates antidepressant efficacy of albiflorin. Theranostics 8:5945–5959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Wang L, Wang J, Wang C, Yang Z, Wang C, Zhu Y, Zhang J (2016) Paeoniflorin and Albiflorin attenuate neuropathic pain via MAPK pathway in chronic constriction injury rats. Evid Based Complement Alternat Med 2016:8082753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Ao L, Yan Y, Li C, Li W, Ye A, Liu J, Hu Y, Fang W, Li Y (2020a) Levo-corydalmine attenuates vincristine-induced neuropathic pain in mice by upregulating the Nrf2/HO-1/CO pathway to inhibit connexin 43 expression. Neurotherapeutics 17:340–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Yazdi AS, Menu P, Tschopp J (2011) A role for mitochondria in NLRP3 inflammasome activation. Nature 469:221–225. [DOI] [PubMed] [Google Scholar]
- Zhou YQ, Liu DQ, Chen SP, Chen N, Sun J, Wang XM, Cao F, Tian YK, Ye DW (2020b) Nrf2 activation ameliorates mechanical allodynia in paclitaxel-induced neuropathic pain. Acta Pharmacol Sin 41:1041–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Jing L, Chen C, Shao M, Fan Q, Diao J, Liu Y, Lv Z, Sun X (2015) Danzhi Xiaoyao San ameliorates depressive-like behavior by shifting toward serotonin via the downregulation of hippocampal indoleamine 2,3-dioxygenase. J Ethnopharmacol 160:86–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.