Abstract
Background
We previously showed the efficacy of bi-anodal transcranial direct current stimulation (tDCS) over the prefrontal cortex (PFC) regions with extracephalic reference placement in improving negative symptoms in schizophrenia. In this ancillary investigation, the effects of this intervention on insight levels, other clinical outcomes, and cardio-respiratory and autonomic functions were examined and the potential of biomarkers for treatment response was explored.
Methods
Schizophrenia patients were randomly allocated to receive 10 sessions of bi-anodal tDCS over the PFC regions with extracephalic reference placement (2 mA, 20 minutes, twice daily for 5 weeks) or sham stimulation. We examined, in 60 patients at baseline, immediately after stimulation and at follow-up visits, the insight levels, other clinical outcomes, blood pressure, respiratory rate, heart rate, and heart rate variability.
Results
Insight levels as assessed by the abbreviated version of the Scale to Assess Unawareness in Mental Disorder in schizophrenia awareness of the disease, positive and negative symptoms dimensions, and beliefs about medication compliance as assessed by Medication Adherence Rating Scale were significantly enhanced by active stimulation relative to sham. No effects were observed on cognitive insight, other clinical outcomes, or cardio-respiratory and autonomic functions. Heart rate variability indices as biomarkers were not associated with the clinical response to the intervention.
Conclusions
Our results provide evidence for bi-anodal tDCS over the PFC regions with extracephalic reference placement in heightening the levels of insight into the disease and symptoms, as well as beliefs about medication compliance in schizophrenia, without impacting other clinical outcomes and cardio-respiratory/autonomic functions.
Keywords: Schizophrenia, impaired insight, transcranial direct current stimulation, extracephalic montage, prefrontal cortex
Significance Statement.
Bi-anodal transcranial direct current stimulation targeting the prefrontal cortex coupled with bilateral extracephalic reference placement has been shown to reduce negative symptoms in schizophrenia. Dysfunctional prefrontal cortex may represent shared pathogenic etiology common to both negative symptoms and insight impairment of schizophrenia, but little is known about the effects of the intervention on insight levels of schizophrenia patients. Extracephalic tDCS raises a safety concern related to cardio-respiratory/autonomic functions due to the potential shunting of current flowing through the heart and brainstem. Therefore, we aimed to investigate these issues and further perform exploratory biomarker analyses for treatment response to aid patient selection for this intervention. Our main findings indicated that bi-anodal tDCS with extracephalic references enhanced awareness of the disease/symptoms, as well as beliefs about medication compliance, without impacting other clinical outcomes and cardio-respiratory/autonomic functions. Another finding was that physiological biomarkers were not associated with the treatment response.
Introduction
Lack of insight in schizophrenia creates barriers to treatment compliance and contributes to a longer duration of untreated psychosis and poorer clinical outcomes (Lysaker et al., 2018). Many lines of evidence provide links between poor insight and symptom severity and support the view that poor insight may root in having difficulties in understanding anomalous self-experience of positive and negative symptoms (Lysaker et al., 2018). Researchers conceptualized insight and developed measures to quantify its distinct dimensions (Konsztowicz et al., 2018). A rapid-onset, effective therapy for impaired insight is still an unmet need for the management of schizophrenia.
Evidence links poor insight to abnormalities in brain function such as volumetric gray matter reductions across different brain regions (frontal/prefrontal, parietal, temporal, and occipital regions and cerebellum), reduced white matter integrity of the fronto-temporo-parietal regions, abnormal activity in midline cortical/subcortical structures, and aberrant functional connectivity of resting-state networks (Antonius et al., 2011; Lee et al., 2015; Sapara et al., 2016; Xavier and Vorderstrasse, 2016; Lysaker et al., 2018; Tordesillas-Gutierrez et al., 2018). These anatomical/functional deficits have thus far served as targets for treating insight impairment in schizophrenia. In recent non-invasive brain stimulation studies, transcranial direct current stimulation (tDCS) showed promising results in improving insight impairment of schizophrenia patients. In single-session tDCS, 2 electrodes are placed over the targeted cortical sites, and low-intensity direct current is passed through the brain, which leads to polarity-dependent effects on cortical excitability: potentiating depolarization (anode area) and hyperpolarization (cathode area), respectively (Nitsche et al., 2003). Repeated tDCS can produce long-lasting effects on cortical excitability through enhancing N-methyl-d-aspartate receptor-dependent long-term potentiation and synaptic plasticity (Nitsche et al., 2008). The effects of tDCS on cortical excitability are variable between individuals (mainly due to inter-individual differences in skull thickness and gyral/sulcal geometry that determine the electric field and current modeling) (Datta et al., 2009; Opitz et al., 2015) and between parameter choices (e.g., different interelectrode distance and cephalic/extracephalic montages). For example, the electrical field peaks midway between anode and cathode (Datta et al., 2009; Opitz et al., 2015), while interelectrode distance is negatively related to the after-effects of tDCS (Moliadze et al., 2010). Cephalic electrode montage is the most widely used in tDCS with both anode and cathode attached to the scalp surface. When the anode is attached to the target area to upregulate the cortical excitability, the inhibitory effect on neural activity under the cathode cannot be discounted. If the cathode is moved to a location outside the scalp (being renamed as an extracephalic reference electrode), the unwanted modulation of the cerebral activity can be eluded (Noetscher et al., 2014).
Fronto-temporal tDCS (anode over the left dorsolateral prefrontal cortex (PFC) and cathode over the left temporo-parietal junction) was initially proposed as a novel treatment for medication-refractory auditory hallucinations of schizophrenia (Brunelin et al., 2012) and subsequently found to facilitate patients’ insight (Chang et al., 2018). These effects may arise as a result of the combination of anodal-excitatory and cathodal-inhibitory effects on the primary stimulation sites and the distant impact of the 2 active electrodes on other brain regions, which may functionally compensate for the abnormalities in prefrontal and fronto-temporal cortical regions (Mondino et al., 2016).
Distinct clinical insight (awareness of clinical state) and cognitive insight (awareness of cognitive deficits) comprise the multidimensional construct of insight in schizophrenia, and their specific neural correlates have been identified. For example, structural and functional deficits in bilateral dorsolateral PFC (DLPFC) play an especially vital role in clinical insight of schizophrenia (Xavier and Vorderstrasse, 2016). It has not been examined whether bi-anodal tDCS over bilateral DLPFC exerts a differential effect on clinical insight and cognitive insight (i.e., improving clinical insight but being unable to change cognitive insight) of schizophrenia patients. Our recent trial evaluating bi-anodal tDCS over bilateral DLPFC with extracephalic references for the treatment of negative symptoms of schizophrenia achieved the primary efficacy endpoint (Chang et al., 2020). Given that impaired clinical insight and negative symptoms in schizophrenia share the common pathogenic feature of dysfunctional DLPFC (Zhou et al., 2015; Xavier and Vorderstrasse, 2016), the present study further explored the differential effect of this stimulation mode on clinical/cognitive insight. The clinical outcomes that are subjective to the variations in insight levels were also measured.
Research indicated that 20 minutes of 1-mA tDCS with an anode on the midline Fz and a reference electrode over the right tibia delivered a rather limited current flow through the brainstem by computational estimation of current density distribution in realistic human models (Parazzini et al., 2013b) and showed no impact on the brainstem autonomic centers by physiological measures in healthy individuals (Vandermeeren et al., 2010). Little is known about whether more intense, prolonged tDCS with an extracephalic montage interferes with the cardio-respiratory/autonomic functions at the level of the brainstem in schizophrenia patients. As secondary aims, we examined the changes over time in the participants’ blood pressure, respiratory frequency, heart rate, and heart rate variability (HRV).
Reduced cardiac vagal activity, indexed by low HRV, represents a potential biomarker for negative symptoms (Huang et al., 2020) and poor insight (Ma et al., 2020). The frontal-vagal network theory posits that non-invasive brain stimulation over the PFC regions may increase HRV through transynaptically enhancing parasympathetic signaling (Iseger et al., 2020). Thus, we performed exploratory analyses to assess the predictive and surrogate characteristics of HRV indices as biomarkers for the treatment response to aid patient selection for this promising intervention.
Methods
Participants
We previously performed a double-blind, randomized, sham-controlled trial of bi-anodal tDCS over the PFC regions with extracephalic reference placement for negative symptoms of schizophrenia (NCT03701100) and reported the study design, completed CONSORT flow chart, safety profiles, and psychopathological and neuropsychological findings of the participants elsewhere (Chang et al., 2020). All procedures performed in the participants were in line with the ethical standards of a local ethics committee, and written informed consent was obtained from all individual participants. The aspects relevant to the present work are detailed here. Patients who met the DSM-IV-TR criteria for schizophrenia or schizoaffective disorder were included. All participants had illness duration ≥1 year, were at non-acute phase of illness (clinically stable but symptomatic), defined as Clinical Global Impression-Severity of Illness scale score ≤4, and had been treated with adequate doses of antipsychotics ≥4 weeks before enrolment. Patients were excluded if they were found to have a pregnancy, psychiatric comorbidity/active substance use disorder, contraindications for tDCS, a history of seizure, intracranial neoplasms/surgery, or severe head injuries/cerebrovascular diseases at enrollment. The primary outcome measure was the change over time in the Positive and Negative Syndrome Scale (PANSS) negative dimension score. Here we report data regarding insight levels, other clinical outcomes, and physiological parameters.
Interventions
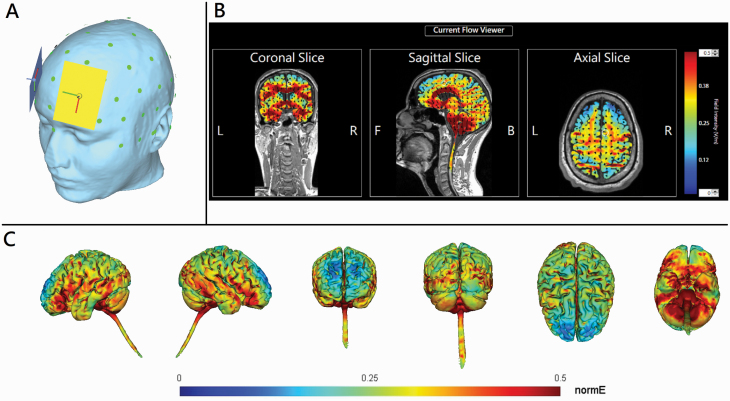
The participants, stimulation operators, and outcome assessors/raters were all blinded to the treatment group. Two Eldith direct current (DC) stimulators (Neuroconn DC Stimulator Plus, GmbH, Ilmenau, Germany) were used, with four 7 × 5 cm2 sponge electrodes soaked in a 0.9% NaCl saline solution. Two anodes were placed over the points midway between International 10–20 electrode positions F3 and Fp1 and between F4 and Fp2 (Figure 1A), respectively, while 2 extracephalic reference electrodes were placed over bilateral forearms. The stimulation parameter was set up as 2 mA, 20 minutes per session, twice per day (with 2 sessions separated by a break for 2 hours) for 5 consecutive weekdays. The numerical computation of electric field was simulated (Figure 1B-C). In sham stimulation, the chosen stimulation mode was identical to the real stimulation in the beginning, but after 30 second of 2-mA stimulation, only a small current pulse occurred every 550 ms (110 μA over 15 milliseconds) instead of the real stimulation throughout the remainder of the 20-minute period. All patients were asked whether they received active or sham tDCS treatment 1 hour after the first stimulation session. Throughout the trial, any change in patients’ original antipsychotic regimes was prohibited. Appropriate doses of sedative hypnotics were allowed; however, daily diazepam equivalent doses were registered for the future exploration of the potential effects of benzodiazepine doses on treatment response given that benzodiazepines might have interactions with tDCS (McLaren et al., 2018).
Figure 1.
(A) The head model of bi-anodal transcranial direct current stimulation (tDCS) over bilateral dorsolateral prefrontal cortex coupled with bilateral extracephalic references. The light green dots represent the international 10–20 electroencephalogram system of electrodes placement. The rectangles indicate 7- × 5-cm sponge electrodes. Yellow and blue colors represent first and second anodes, respectively. (B) 2D and (C) 3D representation of electric field simulation (HD-Explore, Soterix Medical, New York, NY). The red color indicates the strongest electrical field. Black arrows indicate the vectors of electrical current flow.
Study Measures
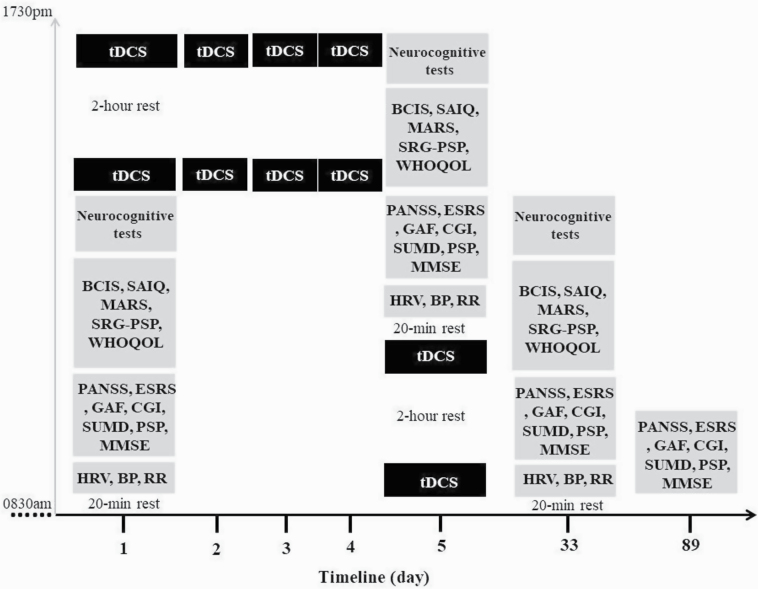
The outcome measures were the changes over time in the scores of researcher- and self-administered rating scales for the intention-to-treat (ITT) sample and the changes over time in the objectively measured physiological indices for the per-protocol (PP) sample. The Chinese version of the PANSS was administered to assess the severity of psychopathology (Lindenmayer et al., 1995). The abbreviated version of the Scale to Assess Unawareness in Mental Disorder (SUMD) in schizophrenia is an interviewer-rating scale to assess patients’ current states of awareness (Michel et al., 2013). The Taiwanese version of the Self-Appraisal of Illness Questionnaire (SAIQ) (Kao and Liu, 2010c) was used to assess subjective experiences and attitudes toward mental illness and experience of psychiatric treatment. The Taiwanese version of the Beck Cognitive Insight Scale (BCIS) (Kao and Liu, 2010b; Kao et al., 2011) was used to assess patients’ cognitive insight. The Extrapyramidal Symptoms Rating Scale (ESRS) total score was used to quantitatively measure antipsychotic-induced movement disorders, including Parkinsonism, akathisia, dystonia, and tardive dyskinesia (Chouinard and Margolese, 2005). The Taiwanese version of Medication Adherence Rating Scale (MARS) (Kao and Liu, 2010a) was used to assess patients’ beliefs about medication adherence. The self-reported version of the graphic personal and social performance scale (SRG-PSP) was used to assess psychosocial function outcomes (Bai et al., 2014). The self-administered Taiwanese version of World Health Organization Questionnaire on Quality of Life: Short Form was used to assess patients’ quality of life (Yao et al., 2002). The patients’ cardio-respiratory and autonomic functions were assessed by measuring their blood pressure, respiratory rate, heart rate, and HRV. The detailed description of the study measures and the overview of their measurement time points are provided in the supplementary Appendix and Figure 2, respectively. The PANSS, SUMD, and ESRS were measured at baseline, shortly after the intervention, and at 1- and 3-month follow-ups. The BCIS, SAIQ, MARS, SRG-PSP, and physiological indices were measured at baseline, shortly after the 10-session stimulation, and at 1-month follow-up.
Figure 2.
The overview of all the measurements conducted. Interviewer-rated assessments were administered by Hsin-An Chang at baseline, shortly after the intervention and the 1- and 3-month follow-ups. The self-rated questionnaires and the measurement of physiological parameters were undertaken at baseline, shortly after the 10-session stimulation and at 1-month follow-up. BCIS, Taiwanese version of Beck’s Cognitive Insight Scale; BP, blood pressure; CGI, Clinical Global Impression; ESRS, Extrapyramidal Symptoms Rating Scale; GAF, Global Assessment of Functioning; HRV, heart rate variability; MARS, Taiwanese version of Medication Adherence Rating Scale; MMSE, Mini-Mental State Examination; PANSS, Positive and Negative Syndrome Scale; PSP, Taiwanese version of Personal and Social Performance Scale; RR, respiratory rate; SAIQ, Taiwanese version of Self-Appraisal of Illness Questionnaire; SRG-PSP, self-reported version of Graphic Personal and Social Performance Scale; SUMD, abbreviated version of Scale to Assess Unawareness in Mental Disorder in schizophrenia; tDCS, bi-anodal transcranial direct current stimulation over bilateral dorsolateral prefrontal cortex coupled with bilateral extracephalic references; WHOQOL, World Health Organization Quality of Life-BREF.
Biomarkers
Peripheral biomarkers (i.e., HRV indices) associated with outcomes of negative symptoms (Huang et al., 2020) and insight levels (Ma et al., 2020) were investigated as predictors and mediators of the treatment response (see the supplementary Appendix).
Statistical Analyses
SPSS Statistics 21.0 software (IBM SPSS Inc., Chicago, IL, USA) was applied for analyses. To compare the between-group differences in characteristics at baseline, Pearson chi-square test or Fisher’s test was used for qualitative variables and Student’s t test or Mann-Whitney test for quantitative variables. In the ITT and PP sample, linear mixed effect model analyses, which can handle missing data, were used to assess the effects of intervention on outcome measures with fixed factors of “time” (baseline, immediately after intervention, and follow-up visits) and “treatment group” (active vs sham stimulation), with a random factor “participant” to account for repeated measures within participants. The interaction between time and treatment group was defined as the effects of time on treatment group. The adjustment for any imbalance in the covariates at baseline was undertaken. For each factor and their interaction in the mixed model, Kenward–Roger approximations were used to perform F-tests and calculate P values. When significant time × treatment group interactions were found, the post-hoc analyses were used to compare the between-group differences. Eta-squared (η 2) values were calculated as measures of effect sizes between 2 groups, and the value of 0.01, 0.06, and 0.14 represented a small, medium, and large effect, respectively. Statistical significance was corrected for the multiple tests (false discovery rate method). Exploratory analyses were conducted to assess HRV as a predictor or mediator for the changes in negative symptoms and insight levels by running separate general linear models to estimate the main and interaction effects in terms of slopes (coefficients) and their 95% confidence interval. Multiple comparisons were not corrected due to the nature of exploratory testing. A total of 120 analyses were performed, and 6 positive results were expected by chance. Furthermore, Spearman’s rank correlation coefficients were calculated to examine the correlations between the changes in insight levels and the corresponding changes in the beliefs about medication adherence. Partial correlations were adjusted using the change in psychopathological symptom severity as a covariate. A total 6 analyses were performed and at least 0–1 positive result was expected by chance.
Results
Sample Characteristics
Sixty patients comprised the ITT sample and all of them completed 10 sessions of stimulation. Compared with the sham group, the active stimulation group had fewer female participants and less impairment in insight levels as measured by the SUMD (Table 1). The other demographic and clinical data did not show any significant between-group differences.
Table 1.
Baseline Demographic and Clinical Characteristics of Participants
| Characteristics | Active tDCS (n = 30) |
Sham tDCS (n = 30) |
t/U or χ 2/Fisher’s | P value |
|---|---|---|---|---|
| Sociodemographic characteristics | ||||
| Females (%) | 11 (36.70%) | 19 (63.30%) | 4.27 | .039 |
| Age, y | 44.70 ± 10.70 | 45.03 ± 10.91 | −0.12 | .91 |
| Education level, y | 11.93 ± 2.08 | 12.30 ± 3.19 | 449.00 | .99 |
| Handedness (right/left) | 28/2 | 27/3 | 0.22 | 1.00 |
| Cardiovascular characteristics | ||||
| BMI, kg/m2 | 27.09 ± 5.23 | 25.54 ± 5.74 | 370.00 | .24 |
| Smokers (%) | 11 (36.70%) | 9 (30.00%) | 0.30 | .58 |
| Weekly regular exercise, h | 1.95 ± 2.29 | 1.72 ± 1.82 | 429.50 | .76 |
| Alcohol use, drinks/da | 1.23 ± 0.63 | 1.23 ± 0.63 | 439.00 | .79 |
| Hypertension, n (%) | 6 (20.0%) | 5 (16.7%) | 0.11 | .74 |
| Diabetes mellitus, n (%) | 4 (13.3%) | 1 (3.3%) | 1.96 | .35 |
| Hyperlipidemia, n (%) | 3 (10%) | 1 (3.3%) | 1.07 | .61 |
| Coronary artery disease, n (%) | 1 (3.3%) | 1 (3.3%) | 0.00 | 1.00 |
| Clinical characteristics | ||||
| Schizophrenia/schizoaffective disorder | 24/6 | 26/4 | 0.48 | .73 |
| Onset age, y | 30.07 ± 10.39 | 29.13 ± 10.42 | 0.35 | .73 |
| Length of illness, y | 14.73 ± 9.53 | 15.77 ± 10.60 | 421.50 | .67 |
| Antipsychotic dosage, mg/db | 18.81 ± 10.40 | 18.81 ± 10.50 | 0.001 | 1.00 |
| Anticholinergic dosage, mg/dc | 0.93 ± 1.72 | 1.02 ± 1.69 | 476.50 | .64 |
| Sedative-hypnotic dosage, mg/dd | 27.40 ± 30.04 | 38.85 ± 31.75 | 547 | .15 |
| On clozapine, n (%) | 8 (26.7%) | 5 (16.7%) | 0.88 | .35 |
| On LAI, n (%) | 5 (16.7%) | 5 (16.7%) | 0.00 | 1.00 |
| On mood stabilizer, n (%) | 5 (16.7%) | 5 (16.7%) | 0.00 | 1.00 |
| On antidepressant, n (%) | 8 (26.7%) | 7 (23.3%) | 0.09 | .77 |
| On anticholinergic agent, n (%) | 10 (33.3%) | 10 (33.3%) | 0.00 | 1.00 |
| On propranolol, n (%) | 6 (20%) | 8 (26.7%) | 0.37 | .54 |
| Use of sedative-hypnotic, n (%) | 24 (80%) | 25 (83.3%) | 0.11 | .74 |
| On anti-hypertensive drug, n (%) | 6 (20%) | 5 (16.7%) | 0.11 | .74 |
| On anti-diabetic medication, n (%) | 4 (13.3%) | 1 (3.3%) | 1.96 | .35 |
| On lipid-lowering medication, n (%) | 3 (10%) | 1 (3.3%) | 1.07 | .61 |
| On medication of CAD, n (%) | 1 (3.3%) | 1 (3.3%) | 0.00 | 1.00 |
| PANSS total score | 67.43 ± 13.02 | 73.30 ± 10.30 | −1.94 | .06 |
| PANSS-positive | 12.37 ± 4.51 | 13.40 ± 2.77 | 328.50 | .07 |
| PANSS-negative | 20.43 ± 4.33 | 22.57 ± 4.41 | −1.89 | .06 |
| PANSS-grandiosity/excitement | 5.53 ± 1.63 | 5.83 ± 1.97 | 419.00 | .64 |
| PANSS-disorganization | 11.30 ± 2.53 | 12.13 ± 2.46 | 374.50 | .26 |
| PANSS-depression | 5.40 ± 1.77 | 5.90 ± 1.79 | 355.50 | .15 |
| PANSS-cognitive component | 8.87 ± 2.15 | 9.57 ± 1.81 | −1.37 | .18 |
| SUMD | ||||
| Awareness of disease | 78.52 ± 17.00 | 91.11 ± 14.71 | 265.00 | .003 |
| Awareness of positive symptoms | 55.93 ± 21.93 | 65.56 ± 19.21 | 323.00 | .047 |
| Awareness of negative symptoms | 81.48 ± 19.21 | 92.59 ± 12.49 | 307.00 | .017 |
| BCIS-R | 24.4 ± 3.60 | 23.33 ± 4.96 | 0.95 | .35 |
| BCIS-C | 16.20 ± 3.50 | 15.77 ± 3.75 | 0.46 | .65 |
| BCIS R-C index | 7.87 ± 4.24 | 7.57 ± 7.38 | 445.00 | .94 |
| SAIQ total score | 51.30 ± 7.23 | 47.73 ± 9.52 | 1.63 | .11 |
| SAIQ presence/outcome | 14.53 ± 2.79 | 13.47 ± 3.32 | 362.50 | .19 |
| SAIQ need treatment | 15.67 ± 1.92 | 15.57 ± 2.39 | 0.18 | .86 |
| SAIQ worry | 20.77 ± 5.39 | 19.70 ± 5.24 | 0.78 | .44 |
| MARS total score | 3.27 ± 1.95 | 3.27 ± 1.46 | 448.50 | .98 |
| MARS-SR | 2.73 ± 1.55 | 2.87 ± 1.11 | 465.50 | .81 |
| MARS-MA | 0.53 ± 0.51 | 0.40 ± 0.50 | 390.00 | .31 |
| ESRS | 4.33 ± 1.03 | 5.07 ± 2.07 | 540.00 | .17 |
| SRG-PSP | ||||
| Global score | 30.07 ± 7.49 | 29.40 ± 8.84 | 0.32 | .75 |
| Social useful activities | 8.07 ± 2.69 | 8.53 ± 3.19 | 421.00 | .67 |
| Personal and social relationships | 12.07 ± 3.45 | 12.00 ± 3.69 | 0.07 | .94 |
| Self-care | 15.60 ± 2.98 | 15.13 ± 3.13 | 421.50 | .67 |
| Disturbing and aggressive behavior | 6.13 ± 2.62 | 6.37 ± 1.96 | 377.50 | .23 |
| WHOQOL—total score | 75.20 ± 14.22 | 79.07 ± 14.22 | −1.00 | .32 |
| WHOQOL—physical health | 27.63 ± 5.79 | 28.87 ± 4.95 | −1.05 | .30 |
| WHOQOL—psychology | 19.87 ± 4.43 | 21.10 ± 5.11 | 431.00 | .78 |
| WHOQOL—social relationships | 17.17 ± 4.44 | 17.37 ± 3.96 | 367.50 | .22 |
| WHOQOL—environment | 10.87 ± 2.43 | 11.83 ± 2.45 | 372.00 | .25 |
Abbreviations: BCIS, Taiwanese version of Beck Cognitive Insight Scale; BMI, body mass index; CAD, coronary artery disease; ESRS, Extrapyramidal Symptoms Rating Scale; LAI, long-acting antipsychotics injectable; MARS, Taiwanese version of Medication Adherence Rating Scale; PANSS, Positive and Negative Syndrome Scale;
SAIQ, Taiwanese version of Self-Appraisal of Illness Questionnaire; SRG-PSP, self-reported version of the Graphic Personal and Social Performance Scale subjective response to taking medication; SUMD, abbreviated version of Scale to Assess Unawareness in Mental Disorder in schizophrenia; WHOQOL, self-administered Taiwanese version of World Health Organization Questionnaire on Quality of Life: Short Form.
aAlcohol use is assessed with 2 items of the Alcohol Use Disorder Identification Test questionnaire (AUDIT) and is defined by the average frequency of drinking and number of drinks consumed on a typical drinking day in the past year. From these items, we derived the average amount of alcoholic drinks per day, with 1 drink defined as a standard drink.
bOlanzapine equivalent.
cBiperidin equivalent.
dDiazepam equivalent.
Data are presented as means ± SDs, unless otherwise stated.
Data are presented as means ± SDs, unless otherwise stated. Significant P values are presented in bold.
Integrity of Blinding
The effectiveness of blinding in this trial was satisfactory because 80.0% of patients in the active tDCS and 73.3% in the sham group guessed that they had received active tDCS (P = .54), suggesting that patients failed to correctly identify their actual treatment beyond chance.
Influence of tDCS on Insight Levels
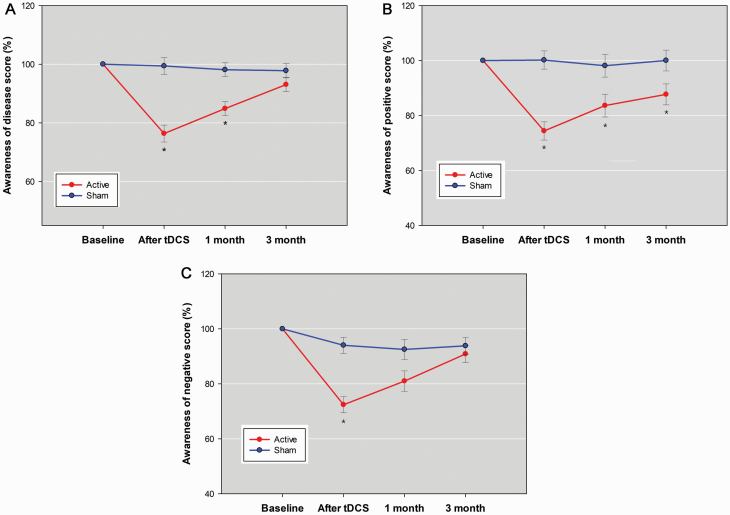
There were significant between-group differences in the changes over time for researcher-rated measures of insight levels, including the SUMD dimension scores of awareness of the disease, positive symptoms, and negative symptoms (supplementary Table 1), but not for subjective attitudes toward mental illness (SAIQ total and each dimension) or self-report measures of cognitive insight (BCIS-R, BCIS-C, and R-C index). Real stimulation rapidly reduced all SUMD dimensions scores compared with sham (see Table 2 for the acute effects). The after-effect of tDCS was maintained at 1-month follow-up for SUMD awareness of the disease dimension (Figure 3A) and was maintained from end of treatment to 3-month follow-up for SUMD awareness of positive symptoms dimension (Figure 3B). There was no maintenance effect for SUMD awareness of negative symptoms dimension (Figure 3C).
Table 2.
Mean Changes in Levels of Psychopathological Symptoms, Insight Levels, Medication Adherence, Extrapyramidal Symptoms, Psychosocial Functioning, and Life Quality After 5 Days of Bi-Anodal tDCS With Extracephalic References or Sham Treatment in Participants
| Variablesa | Active tDCS (n = 30) Mean ± SE |
Sham tDCS (n = 30) Mean ± SE |
F | P value | Partial η 2 |
|---|---|---|---|---|---|
| PANSS | |||||
| Negative score (primary endpoint) | −3.70 ± 0.46 | −0.67 ± 0.46 | 20.53 | <.001 | 0.268 |
| Total score | −11.19 ± 1.08 | −0.98 ± 1.08 | 42.12 | <.001 | 0.429 |
| Positive score | −0.55 ± 0.15 | −0.12 ± 0.15 | 3.64 | .06 | 0.060 |
| Grandiosity/excitement score | −0.30 ± 0.19 | 0.36 ± 0.19 | 5.60 | .03 | 0.091 |
| Disorganization score | −1.96 ± 0.24 | −0.24 ± 0.24 | 25.47 | <.001 | 0.313 |
| Depression score | −0.63 ± 0.13 | 0.03 ± 0.13 | 3.62 | .062 | 0.061 |
| Cognitive component score | −1.78 ± 0.20 | −0.15 ± 0.20 | 32.20 | <.001 | 0.365 |
| SUMD | |||||
| Awareness of diseaseb | −21.06 ± 2.39 | 0.32 ± 2.39 | 36.66 | <.001 | 0.400 |
| Awareness of positive symptomsb | −18.57 ± 2.41 | 1.16 ± 2.41 | 30.62 | <.001 | 0.358 |
| Awareness of negative symptomsb | −23.46 ± 2.48 | −4.32 ± 2.48 | 27.33 | <.001 | 0.332 |
| SAIQ | |||||
| Total score | −0.95 ± 1.08 | 1.98 ± 1.08 | 3.45 | .07 | 0.058 |
| Worry | −0.69 ± 0.73 | 0.69 ± 0.73 | 1.65 | .20 | 0.029 |
| Need treatment | −0.01 ± 0.44 | −0.26 ± 0.44 | 0.16 | .69 | 0.003 |
| Presence/outcome | −0.24 ± 0.47 | 0.87 ± 0.47 | 2.60 | .11 | 0.044 |
| BCIS | |||||
| BCIS-R | 0.46 ± 0.71 | 0.27 ± 0.71 | 0.03 | .86 | 0.001 |
| BCIS-C | −0.07 ± 0.70 | 0.54 ± 0.70 | 0.37 | .55 | 0.006 |
| R-C index | 0.92 ± 0.94 | −0.32 ± 0.94 | 0.83 | .37 | 0.015 |
| MARS | |||||
| Total score | 1.64 ± 0.31 | 0.06 ± 0.31 | 12.03 | .001 | 0.177 |
| Subjective response to taking medication | 1.39 ± 0.28 | −0.12 ± 0.28 | 14.27 | <.001 | 0.203 |
| Medication adherence | 0.25 ± 0.08 | 0.19 ± 0.08 | 0.24 | .62 | 0.004 |
| ESRS | −0.33 ± 0.28 | 0.23 ± 0.28 | 1.95 | .17 | 0.034 |
| SRG-PSP | |||||
| Social useful activities | 1.06 ± 0.41 | 0.51 ± 0.41 | 0.85 | .36 | 0.015 |
| Personal and social relationships | 0.18 ± 0.58 | 0.56 ± 0.58 | 0.21 | .65 | 0.004 |
| Self-care | 0.84 ± 0.41 | 0.36 ± 0.41 | 0.65 | .42 | 0.011 |
| Disturbing and aggressive behavior | −0.78 ± 0.39 | −0.35 ± 0.39 | 0.58 | .45 | 0.010 |
| Global score | 2.38 ± 0.98 | 1.56 ± 0.98 | 0.33 | .57 | 0.006 |
| WHOQOL | |||||
| Physical health | 1.25 ± 0.71 | −0.68 ± 0.71 | 3.48 | .07 | 0.058 |
| Psychology | 0.25 ± 0.75 | 1.02 ± 0.75 | 0.49 | .49 | 0.009 |
| Social relationships | 0.69 ± 0.43 | 0.28 ± 0.43 | 0.44 | .51 | 0.008 |
| Environment | 1.19 ± 0.95 | −0.75 ± 0.95 | 1.97 | .17 | 0.034 |
| Total score | 3.81 ± 2.21 | −0.15 ± 2.21 | 1.51 | .22 | 0.026 |
Abbreviations: BCIS, Beck’s Cognitive Insight Scale; BCIS-C, Self-certainty subscale of BCIS; BCIS-R, Self-reflectiveness subscale of BCIS; ESRS, Extrapyramidal Symptoms Rating Scale; MARS, Taiwanese version of Medication Adherence Rating Scale; PANSS, Positive and Negative Syndrome Scale; SAIQ, Self-Appraisal of Illness Questionnaire; SRG-PSP, self-reported version of the Graphic Personal and Social Performance Scale; SUMD, abbreviated version of Scale to Assess Unawareness in Mental Disorder; tDCS, transcranial direct current stimulation; WHOQOL, World Health Organization Quality of Life-BREF.
aValues were adjusted for the covariates of gender and baseline PANSS total score.
bAdditionally adjusted for the covariate of baseline SUMD subscale score of awareness of disease.
P values are in bold if the primary endpoint reaches the significance level of <.05 or the secondary endpoints reach the corrected significance level of <.0129 (false discovery rate method).
Figure 3.
Score as percentage of baseline in (A) the “awareness of the disease” dimension score, (B) the “awareness of positive symptoms” dimension score, and (C) the “awareness of negative symptoms” dimension score of the abbreviated version of the Scale to Assess Unawareness in Mental Disorder in schizophrenia (SUMD) between the active stimulation group and sham group across the 4 assessments. Error bars indicated the SE. Post-hoc analyses were undertaken to examine between-group differences at each post-baseline assessment with P < .0129 considered reaching the corrected significance level (false discovery rate method). *P < .0129.
Influence of tDCS on Other Clinical Outcomes
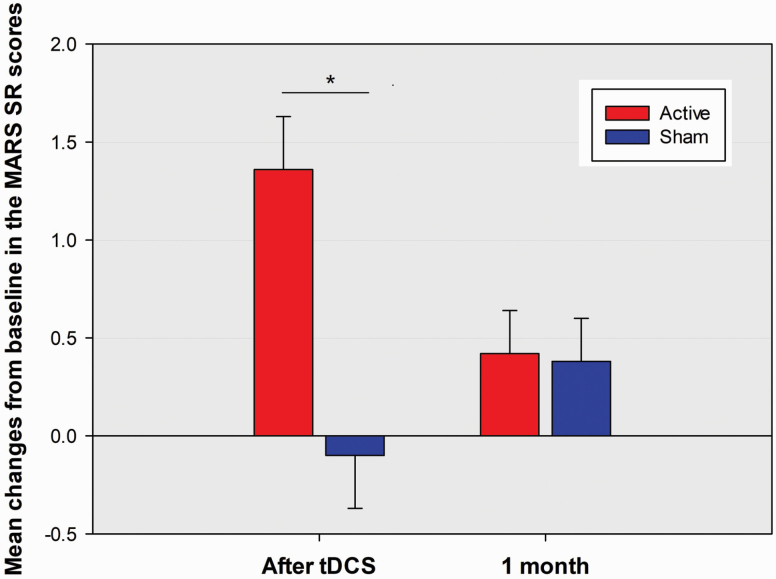
There were significant between-group differences in the changes over time for PANSS total score and cognitive component score, negative and disorganization dimension scores, but not for PANSS positive, grandiosity/excitement, or depression dimension score (supplementary Table 1). Post-hoc analyses showed tDCS rapidly reduced PANSS total score and the PANSS cognitive component and negative and disorganization dimension scores compared with sham treatment (Table 2). The effects were maintained at 1- and 3-month follow-ups for PANSS total score and negative dimension score but only at 1-month follow-up for PANSS cognitive component and disorganization dimension scores. However, there were no significant between-group differences in the changes over time for global and subscale scores of SRG-PSP and WHOQOL (supplementary Table 1). There were significant between-group differences in the changes over time for MARS total scores and MARS subjective response to taking medication subscale scores, but not for MARS medication adherence subscale scores and ESRS scores (supplementary Table 1). Compared with the sham group, the active group had significant increases in MARS total scores and subjective response to taking medication subscale scores from baseline to immediately after stimulation (Table 2; Figure 4). In the real stimulation group, the changes in MARS total scores and MARS subjective response to taking medication, subscale scores from baseline to immediately after stimulation did not correlate with that in all SUMD dimensions scores regardless of whether the corresponding change in overall psychopathological symptoms severity (PANSS total score) was controlled or not (Table 3).
Figure 4.
Mean percentage changes from baseline in the subjective response to taking medication subscale score of Medication Adherence Rating Scale (MARS SR) between the active stimulation group and sham group at post-baseline assessments of immediately after stimulation and 1 month after stimulation. Other descriptions are as in Figure 3. *P < .0129.
Table 3.
Correlations of Changes in MARS Total Score, MARS Subjective Response to Taking Medication Subscale Score, and SUMD Dimensions Scores From Baseline to Immediately After tDCS Among Patients Treated With Active Bi-Anodal tDCS With Extracephalic References (Zero-Order And Partial Correlations [Controlling for the Change in Psychopathological Symptom Severity])
| ΔMARS total score | ΔMARS_SR score | ΔPANSS total score | ||||
|---|---|---|---|---|---|---|
| Zero-order correlations | ||||||
| r | P | r | P | r | P | |
| ΔSUMD_AOD | −0.37 | .047 | −0.29 | .12 | 0.73 | <.001 |
| ΔSUMD_AOP | −0.42 | .02 | −0.33 | .08 | 0.68 | <.001 |
| ΔSUMD_AON | −0.29 | .12 | −0.21 | .27 | 0.54 | .002 |
| Partial correlations controlled for ΔPANSS total score | ||||||
| r | P | r | P | r | P | |
| △SUMD_AOD | −0.23 | .24 | −0.19 | .32 | — | — |
| △SUMD_AOP | −0.31 | .10 | −0.25 | .19 | — | — |
| △SUMD_AON | −0.16 | .41 | −0.11 | .56 | — | — |
Abbreviations: MARS, Medication Adherence Rating Scale; MARS_SR, subjective response to taking medication subscale of MARS; PANSS, Positive and Negative Syndrome Scale; SUMD_AOD, awareness of the disease dimension score of abbreviated version of Scale to Assess Unawareness in Mental Disorder in schizophrenia; SUMD_AON, awareness of negative symptoms dimension score of abbreviated version of Scale to Assess Unawareness in Mental Disorder in schizophrenia; UMD_AOP, awareness of positive symptoms dimension score of abbreviated version of Scale to Assess Unawareness in Mental Disorder in schizophrenia.
Δ indicates the change from baseline to immediately after 10-session tDCS. A total of 6 analyses were performed and at least 0–1 positive result was expected by chance.
Effects of tDCS on Cardio-Respiratory and Autonomic Functions
Of the ITT sample (n = 60), 54 completed all study visits for physiological indices measurement (PP sample, Table 4). There were no significant between-group differences in the changes over time in blood pressure, pulse pressure, respiratory rate, heart rate, and HRV (supplementary Tables 2 and 3).
Table 4.
Physiological Measures Over Time
| HRV values | Baseline | After tDCS | 1-month follow-up | ||||
|---|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | ||
| SBP | Active | 30 | 119.73 (16.88) | 30 | 119.57 (16.36) | 28 | 118.75 (15.58) |
| Sham | 30 | 119.57 (16.81) | 30 | 119.37 (16.20) | 26 | 117.15 (16.43) | |
| DBP | Active | 30 | 74.53 (9.78) | 30 | 75.10 (9.35) | 28 | 75.14 (8.92) |
| Sham | 30 | 78.13 (10.93) | 30 | 78.27 (10.25) | 26 | 77.19 (10.61) | |
| PP | Active | 30 | 45.20 (11.71) | 30 | 44.47 (12.11) | 28 | 43.61 (12.25) |
| Sham | 30 | 41.43 (10.84) | 30 | 41.10 (10.69) | 26 | 39.96 (11.75) | |
| RR | Active | 30 | 13.75 (2.41) | 30 | 12.83 (2.36) | 28 | 13.49 (1.72) |
| Sham | 30 | 13.31 (2.49) | 30 | 13.30 (2.83) | 26 | 13.91 (1.67) | |
| RR interval | Active | 30 | 759.43 (131.98) | 30 | 788.44 (176.73) | 28 | 725.36 (152.94) |
| Sham | 30 | 702.83 (160.73) | 30 | 743.67 (139.31) | 26 | 714.98 (76.73) | |
| Var (total HRV) | Active | 30 | 6.44 (2.33) | 30 | 6.57 (1.30) | 28 | 6.09 (1.34) |
| Sham | 30 | 6.29 (1.59) | 30 | 6.67 (1.71) | 26 | 6.19 (1.45) | |
| VLF | Active | 30 | 5.32 (1.30) | 30 | 5.48 (1.30) | 28 | 5.05 (1.34) |
| Sham | 30 | 5.36 (1.73) | 30 | 5.48 (1.78) | 26 | 5.05 (1.32) | |
| LF | Active | 30 | 4.44 (1.70) | 30 | 4.86 (1.76) | 28 | 4.40 (1.64) |
| Sham | 30 | 4.62 (1.83) | 30 | 4.91 (2.11) | 26 | 4.14 (1.96) | |
| HF | Active | 30 | 3.88 (1.36) | 30 | 4.52 (1.67) | 28 | 3.84 (1.46) |
| Sham | 30 | 4.10 (2.03) | 30 | 4.66 (1.89) | 26 | 3.75 (1.46) | |
| LF/HF ratio | Active | 30 | 0.57 (1.17) | 30 | 0.34 (0.85) | 28 | 0.56 (1.17) |
| Sham | 30 | 0.52 (1.16) | 30 | 0.25 (1.07) | 26 | 0.39 (1.39) |
Abbreviations: DBP, diastolic blood pressure (mmHg); HF, high-frequency power (ln[ms2]); LF, low-frequency power (ln[ms2]); PP, pulse pressure (mmHg); RR interval, time elapsing between 2 consecutive R waves in electrocardiogram (ms); Var, total variance (ln[ms2]); RR, respiratory rate (breaths per minute); SBP, systolic blood pressure (mmHg); tDCS, transcranial direct current stimulation; VLF, very low-frequency power (ln[ms2]).
Exploratory Biomarker Analyses for Treatment Response
Neither HRV indices at baseline nor the changes in HRV measures were associated with further improvement in negative symptoms (supplementary Tables 4–6) and awareness of the disease (supplementary Tables 7–9), positive symptoms (supplementary Tables 10–12), and negative symptoms (supplementary Tables 13–15).
Discussion
The current work provides evidence for bi-anodal tDCS over bilateral DLPFC with extracephalic reference placement in heightening patients’ awareness of the disease/symptoms and beliefs about medication compliance without changing their cognitive insight, psychosocial function, life quality, or cardio-respiratory/autonomic functions. HRV indices were neither suitable predictors nor mediators of the response to treatment to the intervention.
Our results showed that the current stimulation mode rapidly improved patients’ clinical insight (awareness of illness/symptoms). Evidence has correlated poor insight with positive, negative, disorganization, and overall symptoms severity (Lysaker et al., 2018). We cannot exclude the possibility that the gain in clinical insight may come from the reductions in psychopathological symptoms because the intervention was associated with significant improvements in negative, cognitive, disorganization, and overall symptoms. For active tDCS compared with the sham condition, the improvements in unawareness of illness, positive and negative symptoms, and overall symptom level from baseline to shortly after 10-session tDCS were 20%, 21%, 23%, and 14%, respectively. It means that the effects of add-on tDCS in causing improved insight gains were not completely explained by the reduction in overall symptoms.
Several potential mechanisms underlying the beneficial effects of current stimulation mode on unawareness of illness/symptoms are proposed. The first is the simultaneous bilateral application of tDCS targeted to DLPFC. Impaired illness awareness is proposed to arise from interhemispheric imbalance (i.e., left hemisphere overactivation, primarily in the posterior parietal area) (van der Meer et al., 2013). Recent research supports this model by demonstrating the improvement in impaired illness awareness with the use of cathodal tDCS inhibition of left temporo-parietal junction area activity in schizophrenia patients (Chang et al., 2018; Sreeraj et al., 2018). Emerging evidence suggests right-sided or bilateral DLPFC involvement in impaired insight of schizophrenia. For example, neuroimaging studies indicates that right hemisphere deficits in fronto-temporo-parietal brain regions (Gerretsen et al., 2013; Emami et al., 2016) and anatomical deficits in bi-hemispheric PFC regions (Berge et al., 2011; Parellada et al., 2011) in schizophrenia patients are associated with unawareness of illness and symptoms. However, these areas have not yet been thoroughly investigated for tDCS treatment. Previous studies indicate that anodal tDCS over left DLPFC may restore the ipsilateral DLPFC functioning through producing bi-hemispheric changes in its resting-state functional connectivity within frontal- thalamic-temporo-parietal networks (Palm et al., 2016), thereby increasing its top-down control on temporo-parietal areas (Mondino et al., 2016). Our study demonstrates for the first time, to our knowledge, that simultaneous bilateral tDCS over DLPFC is a novel, promising approach for the treatment of impaired clinical insight in schizophrenia.
The second is the unique electric field produced in the brain during bilateral tDCS using extracephalic references (Figure 1). In this trial, the placement of extracephalic references was originally used to avoid potential confounding effects from inhibitory stimulation of other cortical sites. However, computer-based modeling research has indicated that lengthening the inter-electrode distance (i.e., between the PFC regions and the forearms) facilitates the increase in the proportion of electric current into the brain by lowering current shunting across the scalp (Miranda et al., 2006). Compared with bifrontal tDCS (anode over F3 and cathode over F4 or Fp2) used in most studies, anodal tDCS over the frontal cortex coupled with an extracephalic reference leads to much more widespread activation of brain regions (Miranda et al., 2006; Wagner et al., 2007). A growing body of evidence supports a role for altered functioning of the brain regions other than fronto-temporo-parietal networks (e.g., occipital regions, basal ganglia, thalamus, and cerebellum) in impaired clinical insight of schizophrenia patients (Xavier and Vorderstrasse, 2016; Lysaker et al., 2018). Evidence indicates that tDCS can exert intracortical effects at the stimulation sites and subcortical effects at the areas distal to the electrodes (Polania et al., 2012). Specifically, anodal tDCS over left DLPFC with a cephalic cathode positioned over the contralateral supraorbital area or right DLPFC has been reported to increase healthy individuals’ regional electrical activity (Keeser et al., 2011) and neuronal metabolism activity (Hone-Blanchet et al., 2016) in the ipsilateral PFC as well as enhance glutamate transmission of ipsilateral striatum (Hone-Blanchet et al., 2016). Thus, anodal tDCS over DLPFC with an extracephalic reference might be superior to that with a cephalic cathode for producing subcortical effects, which play a vital role in effectively modulating functional connectivity in distinct functional networks that underlie the pathophysiology of unawareness of illness/symptoms.
The final explanation is the potential effect of the currently used stimulation mode on white matter integrity. Accumulating evidence linked impaired awareness of illness and/or symptoms to compromised integrity of white matter tracts associated with cortical midline structures (Asmal et al., 2017) and in several frontal, temporal, and parietal areas (Antonius et al., 2011) and the splenium of corpus callosum (Gerretsen et al., 2019). These areas of white matter disruption have been therapeutic targets for impaired awareness of illness in patients with schizophrenia or other neuropsychiatric disorders (Lehrer and Lorenz, 2014; Gerretsen et al., 2015). Specifically, repetitive transcranial magnetic stimulation that enhanced the integrity of transcallosal interhemispheric white matter tracts linking the bilateral posterior parietal areas (i.e., splenium) resulted in sustained improvements in illness awareness of patients with post-stroke aphasia (Allendorfer et al., 2012; Gerretsen et al., 2015). There is also evidence for the structural effects of the longitudinal delivery of cephalic tDCS on white matter integrity (Lindenberg et al., 2013; Hirtz et al., 2018). Recently, researchers have speculated that the observed improvement in clinical insight following cephalic tDCS treatment could be associated with counteracting the interhemispheric imbalance related to white matter disintegrity (Chang et al., 2018). Relative to cephalic tDCS, its equivalent extracephalic montage has been shown to create larger average vertical and total current densities in deeper brain regions, specifically in white matter (Noetscher et al., 2014). Future replication studies choosing the enhancement of white matter integrity as an outcome may provide evidence to support our speculation.
Notably, the patients’ self-appraisal of mental illness did not significantly change following stimulation. Low correlations and discrepancies between patient-rated and clinician-rated measures of insight are frequently reported, which may be partially attributed to the differences in standpoints between the raters due to personal experience, stigma, or cognitive deficits (Tranulis et al., 2008). Schizophrenia patients might have an unrealistic appreciation of self and environment (So et al., 2010), which may compromise their ability to validly experience, feel, and report their variations in self-awareness of illness/symptoms over time and thus interfere with the correspondence between self- and interviewer-rated measures. It deserves attention that the intervention did not improve the patients’ cognitive insight. Clinical and cognitive insight may be independent of each other, capturing different dimensions of insight and providing unique perspectives and non-redundant information relevant for clinical outcomes (Bayard et al., 2009; Donohoe et al., 2009). The intervention targeting bilateral DLPFC regions especially implicated in clinical insight (Xavier and Vorderstrasse, 2016) may be accountable for its differential effects on clinical and cognitive insight. Further neuroimaging studies are required to prove our perspectives.
Our results proved that the increased awareness of illness/symptoms following neuro-stimulation was accompanied by the improvement in patients’ beliefs about medication compliance. Patients’ ability to recognize their illness/symptoms as psychopathology requiring treatment influences their attitudes toward medication treatment (David, 1990; Larkin and Hutton, 2017). Previous reports have shown that impaired insight into mental illness predicts poor medication adherence and medication discontinuation (Kao and Liu, 2010c; Velligan et al., 2017; Lysaker et al., 2018). However, we failed to find significant correlations between the improvement in medication adherence and the enhancement of awareness of the illness/symptoms regardless of whether the change in psychopathological severity was controlled. It implies that tDCS may improve the beliefs about medication adherence through other mechanisms. Notably, the gain in the beliefs about medication adherence came from the improvement in subjective response to taking medication, which reflects the experience of how antipsychotic medication affects patients’ perception of well-being (Kao and Liu, 2010a). Following tDCS, the patients gave less negative and/or more positive appraisals of the antipsychotic medications they were receiving. Schizophrenia patients may report a variety of negative and unpleasant subjective responses anytime during their course of antipsychotic drug treatment. Extrapyramidal side effects caused by antipsychotic medication that antagonizes dopamine D2 receptors in the nigrostriatal system are one of the negative subjective responses that may contribute to medication noncompliance (Kao and Liu, 2010c). Neuroleptic dysphoria, which relates to low dopamine functioning in the ventral striatum region of the brain, is another negative subjective response that predicts medication non-adherence and poorer life quality (Wu and Okusaga, 2015; Awad, 2019). Recent studies on healthy individuals reported that the release of endogenous dopamine in ventral/dorsal striatum and putamen significantly increased following anodal tDCS over DLPFC (Fonteneau et al., 2018; Fukai et al., 2019). Kamp et al. (2019) reported that left prefrontal high-frequency repetitive transcranial magnetic stimulation improved neuroleptic-induced motor symptoms of schizophrenia patients, possibly through its dopaminergic effect on the ipsilateral caudate. However, our results did not show significant effects of tDCS on extrapyramidal side effects as indexed by the change in ESRS score. Further research using reliable tools to measure neuroleptic dysphoria before and after tDCS is warranted to verify whether tDCS improves beliefs about medication adherence through reducing neuroleptic dysphoria.
Extracephalic tDCS raises a cardiac safety concern due to the potential shunting of electrical current flowing through the heart. The concern has been addressed by previous research reporting that 2-mA tDCS with an extracephalic reference electrode demonstrated far lower levels of current intensities/densities in the heart muscle than the induction thresholds of cardiac fibrillation (Parazzini et al., 2013a). Our results further establish the cardiac safety of bi-anodal tDCS with extracephalic references. Another concern is the potential shunting of electrical current flowing through the brainstem. Vandermeeren et al. (2010) reported that 20 minutes of 1-mA tDCS with anode on the midline Fz and an extracephalic reference electrode over the right tibia did not interfere with the activity of the brainstem autonomic centers. Parazzini et al. (2013b) further proved a quite limited interference of tDCS with an extracephalic reference electrode at the level of the brainstem. However, the use of higher intensity tDCS with an extracephalic reference electrode has not been without controversy. Lippold and collaborators (Lippold and Redfearn, 1964; Redfearn et al., 1964) reported an episode of transient respiratory depression in a healthy participant under 16 minutes of 3-mA bi-frontal cathodal tDCS with an extracephalic reference electrode. One proposed mechanism underlying the potential impact of lateralized tDCS with an extracephalic electrode on cardio-respiratory homeostasis may result from an asymmetrical distribution of the DC within cortical (e.g., insula, ventral medial prefrontal gyri, and the cingulate cortex) or subcortical (e.g., hippocampus, hypothalamus, amygdala, thalamus, and basal ganglia) areas or cerebellar structures involved in the control of autonomic nervous functions (Harper et al., 2015; Macey et al., 2015; Shoemaker et al., 2015) or a specific part of the brainstem respiratory and autonomic centers (Vandermeeren et al., 2010; Chouchou et al., 2019). Consistent with this concept, it was found that tDCS and TMS that reduced heart rate or increased HRV usually aimed at either left or right DLPFC (Iseger et al., 2020). Asymmetrical DC stimulation of vagus nerve is another possible mechanism given that the DC flowing through the lateral aspect of the neck can modulate the excitability of peripheral nerve (e.g., vagus nerve) (Ardolino et al., 2005) and the right vagus nerve might give greater outflow toward the heart relative to the left (Milby et al., 2008). Preferential activation of the right vagus nerve seems to convey elevated risks for bradycardia, bronchoconstriction, and breathing difficulties (McGregor et al., 2005; Milby et al., 2008). Taken together, bilaterally and symmetrically delivered DC flow could be a key element for the slight impact of bi-anodal tDCS with extracephalic references on cardio-pulmonary and autonomic functioning shown in our study. It may also be a possible reason why HRV failed to serve as a biomarker for treatment response to this intervention.
Our study has several limitations. First, sham tDCS could be a hidden confounding factor given its undetermined biological effects beyond the intended transient sensations, and novel research avenues in future studies should be applied to minimize the influence of sham tDCS (Fonteneau et al., 2019). Second, the mean insight score on the PANSS G12 item of the participants at baseline was 4.63 ± 0.98, suggesting the inclusion of a sample with impaired illness awareness (≥3 PANSS G12) (Kim et al., 2019). Caution should be taken in interpreting the results of self-report tests because their validity may have been reduced (Bell et al., 2007; Jung et al., 2010). Finally, the stimulation mode of this trial failed to produce long-lasting after-effects for improving disorganization/cognitive symptoms, unawareness of the disease/negative symptoms, and beliefs about medication adherence. Further studies are required to confirm whether follow-up tDCS “top-up” sessions can maintain the positive effects of 5-day, 10-session tDCS on these outcomes.
Conclusion
In summary, our results suggest that bi-anodal tDCS over bilateral DLPFC with extracephalic references is effective and safe for improving schizophrenia patients’ awareness of the disease/symptoms as well as their beliefs about medication compliance, opening a new area of research on brain stimulation targeting the putative neural circuits of impaired insight into illness, thereby improving medication adherence.
Supplementary Material
Acknowledgments
This study was supported in part by grants from the Medical Affairs Bureau, Ministry of National Defense (MAB-109-078) and the Ministry of Science and Technology of the Taiwanese Government (MOST 106-2314-B-016-021-MY3 and 109-2628-B-016-003) and the Tri-Service General Hospital (TSGH-C109-152).
Statement of Interest
None.
References
- Allendorfer JB, Storrs JM, Szaflarski JP (2012) Changes in white matter integrity follow excitatory rTMS treatment of post-stroke aphasia. Restor Neurol Neurosci 30:103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonius D, Prudent V, Rebani Y, D’Angelo D, Ardekani BA, Malaspina D, Hoptman MJ (2011) White matter integrity and lack of insight in schizophrenia and schizoaffective disorder. Schizophr Res 128:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardolino G, Bossi B, Barbieri S, Priori A (2005) Non-synaptic mechanisms underlie the after-effects of cathodal transcutaneous direct current stimulation of the human brain. J Physiol 568:653–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmal L, du Plessis S, Vink M, Fouche JP, Chiliza B, Emsley R (2017) Insight and white matter fractional anisotropy in first-episode schizophrenia. Schizophr Res 183:88–94. [DOI] [PubMed] [Google Scholar]
- Awad AG. (2019) Revisiting the concept of subjective tolerability to antipsychotic medications in schizophrenia and its clinical and research implications: 30 years later. CNS Drugs 33:1–8. [DOI] [PubMed] [Google Scholar]
- Bai YM, Hsiao CY, Chen KC, Huang KL, Lee IH, Hsu JW, Chen PS, Yang YK (2014) The development of a self-reported scale for measuring functionality in patients with schizophrenia–self-reported version of the graphic Personal and Social Performance (SRG-PSP) scale. Schizophr Res 159:546–551. [DOI] [PubMed] [Google Scholar]
- Bayard S, Capdevielle D, Boulenger JP, Raffard S (2009) Dissociating self-reported cognitive complaint from clinical insight in schizophrenia. Eur Psychiatry 24:251–258. [DOI] [PubMed] [Google Scholar]
- Bell M, Fiszdon J, Richardson R, Lysaker P, Bryson G (2007) Are self-reports valid for schizophrenia patients with poor insight? Relationship of unawareness of illness to psychological self-report instruments. Psychiatry Res 151:37–46. [DOI] [PubMed] [Google Scholar]
- Bergé D, Carmona S, Rovira M, Bulbena A, Salgado P, Vilarroya O (2011) Gray matter volume deficits and correlation with insight and negative symptoms in first-psychotic-episode subjects. Acta Psychiatr Scand 123:431–439. [DOI] [PubMed] [Google Scholar]
- Brunelin J, Mondino M, Gassab L, Haesebaert F, Gaha L, Suaud-Chagny MF, Saoud M, Mechri A, Poulet E (2012) Examining transcranial direct-current stimulation (tDCS) as a treatment for hallucinations in schizophrenia. Am J Psychiatry 169:719–724. [DOI] [PubMed] [Google Scholar]
- Chang CC, Tzeng NS, Chao CY, Yeh CB, Chang HA (2018) The effects of add-on fronto-temporal transcranial direct current stimulation (tdcs) on auditory verbal hallucinations, other psychopathological symptoms, and insight in schizophrenia: a randomized, double-blind, sham-controlled trial. Int J Neuropsychopharmacol 21:979–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CC, Kao YC, Chao CY, Tzeng NS, Chang HA (2020) Examining bi-anodal transcranial direct current stimulation (tDCS) over bilateral dorsolateral prefrontal cortex coupled with bilateral extracephalic references as a treatment for negative symptoms in non-acute schizophrenia patients: a randomized, double-blind, sham-controlled trial. Prog Neuropsychopharmacol Biol Psychiatry 96:109715. [DOI] [PubMed] [Google Scholar]
- Chouchou F, Mauguière F, Vallayer O, Catenoix H, Isnard J, Montavont A, Jung J, Pichot V, Rheims S, Mazzola L (2019) How the insula speaks to the heart: cardiac responses to insular stimulation in humans. Hum Brain Mapp 40:2611–2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouinard G, Margolese HC (2005) Manual for the Extrapyramidal Symptom Rating Scale (ESRS). Schizophr Res 76:247–265. [DOI] [PubMed] [Google Scholar]
- Datta A, Bansal V, Diaz J, Patel J, Reato D, Bikson M (2009) Gyri-precise head model of transcranial direct current stimulation: improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimul 2:201–7, 207.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David AS. (1990) Insight and psychosis. Br J Psychiatry 156:798–808. [DOI] [PubMed] [Google Scholar]
- Donohoe G, Hayden J, McGlade N, O’Gráda C, Burke T, Barry S, Behan C, Dinan TG, O’Callaghan E, Gill M, Corvin AP (2009) Is “clinical” insight the same as “cognitive” insight in schizophrenia? J Int Neuropsychol Soc 15:471–475. [DOI] [PubMed] [Google Scholar]
- Emami S, Guimond S, Mallar Chakravarty M, Lepage M (2016) Cortical thickness and low insight into symptoms in enduring schizophrenia. Schizophr Res 170:66–72. [DOI] [PubMed] [Google Scholar]
- Fonteneau C, Redoute J, Haesebaert F, Le Bars D, Costes N, Suaud-Chagny MF, Brunelin J (2018) Frontal transcranial direct current stimulation induces dopamine release in the ventral striatum in human. Cereb Cortex 28:2636–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonteneau C, Mondino M, Arns M, Baeken C, Bikson M, Brunoni AR, Burke MJ, Neuvonen T, Padberg F, Pascual-Leone A, Poulet E, Ruffini G, Santarnecchi E, Sauvaget A, Schellhorn K, Suaud-Chagny MF, Palm U, Brunelin J (2019) Sham tDCS: a hidden source of variability? Reflections for further blinded, controlled trials. Brain Stimul 12:668–673. [DOI] [PubMed] [Google Scholar]
- Fukai M, Bunai T, Hirosawa T, Kikuchi M, Ito S, Minabe Y, Ouchi Y (2019) Endogenous dopamine release under transcranial direct-current stimulation governs enhanced attention: a study with positron emission tomography. Transl Psychiatry 9:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerretsen P, Chakravarty MM, Mamo D, Menon M, Pollock BG, Rajji TK, Graff-Guerrero A (2013) Frontotemporoparietal asymmetry and lack of illness awareness in schizophrenia. Hum Brain Mapp 34:1035–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerretsen P, Menon M, Chakravarty MM, Lerch JP, Mamo DC, Remington G, Pollock BG, Graff-Guerrero A (2015) Illness denial in schizophrenia spectrum disorders: a function of left hemisphere dominance. Hum Brain Mapp 36:213–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerretsen P, Rajji TK, Shah P, Shahab S, Sanches M, Graff-Guerrero A, Menon M, Pollock BG, Mamo DC, Mulsant BH, Voineskos AN (2019) Impaired illness awareness in schizophrenia and posterior corpus callosal white matter tract integrity. NPJ Schizophr 5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper RM, Kumar R, Macey PM, Harper RK, Ogren JA (2015) Impaired neural structure and function contributing to autonomic symptoms in congenital central hypoventilation syndrome. Front Neurosci 9:415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirtz R, Weiss T, Huonker R, Witte OW (2018) Impact of transcranial direct current stimulation on structural plasticity of the somatosensory system. J Neurosci Res 96:1367–1379. [DOI] [PubMed] [Google Scholar]
- Hone-Blanchet A, Edden RA, Fecteau S (2016) Online effects of transcranial direct current stimulation in real time on human prefrontal and striatal metabolites. Biol Psychiatry 80:432–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang WC, Liu WS, Chen TT, Chen WH, Huang WL (2020) Parasympathetic activity as a potential biomarker of negative symptoms in patients with schizophrenia. Asia Pac Psychiatry 12:e12392. [DOI] [PubMed] [Google Scholar]
- Iseger TA, van Bueren NER, Kenemans JL, Gevirtz R, Arns M (2020) A frontal-vagal network theory for major depressive disorder: implications for optimizing neuromodulation techniques. Brain Stimul 13:1–9. [DOI] [PubMed] [Google Scholar]
- Jung HY, Hwang SS, Yi JS, Kim Y, Kim YS (2010) Clinician-rated functioning and patient-rated quality of life in schizophrenia: implications of their correspondence for psychopathology and side effects. Prog Neuropsychopharmacol Biol Psychiatry 34:225–230. [DOI] [PubMed] [Google Scholar]
- Kamp D, et al. (2019) Left prefrontal high-frequency rTMS may improve movement disorder in schizophrenia patients with predominant negative symptoms - a secondary analysis of a sham-controlled, randomized multicenter trial. Schizophr Res 204:445–447. [DOI] [PubMed] [Google Scholar]
- Kao YC, Liu YP (2010a) Compliance and schizophrenia: the predictive potential of insight into illness, symptoms, and side effects. Compr Psychiatry 51:557–565. [DOI] [PubMed] [Google Scholar]
- Kao YC, Liu YP (2010b) The Beck Cognitive Insight Scale (BCIS): translation and validation of the Taiwanese version. BMC Psychiatry 10:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao YC, Liu YP (2010c) The clinical applicability of the Self-Appraisal of Illness Questionnaire (SAIQ) to chronic schizophrenic patients in Taiwan. Psychiatr Q 81:215–225. [DOI] [PubMed] [Google Scholar]
- Kao YC, Wang TS, Lu CW, Liu YP (2011) Assessing cognitive insight in nonpsychiatric individuals and outpatients with schizophrenia in Taiwan: an investigation using the Beck Cognitive Insight Scale. BMC Psychiatry 11:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeser D, Padberg F, Reisinger E, Pogarell O, Kirsch V, Palm U, Karch S, Möller HJ, Nitsche MA, Mulert C (2011) Prefrontal direct current stimulation modulates resting EEG and event-related potentials in healthy subjects: a standardized low resolution tomography (sLORETA) study. Neuroimage 55:644–657. [DOI] [PubMed] [Google Scholar]
- Kim J, Plitman E, Nakajima S, Alshehri Y, Iwata Y, Chung JK, Caravaggio F, Menon M, Blumberger DM, Pollock BG, Remington G, De Luca V, Graff-Guerrero A, Gerretsen P (2019) Modulation of brain activity with transcranial direct current stimulation: targeting regions implicated in impaired illness awareness in schizophrenia. Eur Psychiatry 61:63–71. [DOI] [PubMed] [Google Scholar]
- Konsztowicz S, Schmitz N, Lepage M (2018) Dimensions of insight in schizophrenia: exploratory factor analysis of items from multiple self- and interviewer-rated measures of insight. Schizophr Res 199:319–325. [DOI] [PubMed] [Google Scholar]
- Larkin A, Hutton P (2017) Systematic review and meta-analysis of factors that help or hinder treatment decision-making capacity in psychosis. Br J Psychiatry 211:205–215. [DOI] [PubMed] [Google Scholar]
- Lee JS, Chun JW, Lee SH, Kim E, Lee SK, Kim JJ (2015) Altered neural basis of the reality processing and its relation to cognitive insight in schizophrenia. Plos One 10:e0120478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer DS, Lorenz J (2014) Anosognosia in schizophrenia: hidden in plain sight. Innov Clin Neurosci 11:10–17. [PMC free article] [PubMed] [Google Scholar]
- Lindenberg R, Nachtigall L, Meinzer M, Sieg MM, Flöel A (2013) Differential effects of dual and unihemispheric motor cortex stimulation in older adults. J Neurosci 33:9176–9183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenmayer JP, Grochowski S, Hyman RB (1995) Five factor model of schizophrenia: replication across samples. Schizophr Res 14:229–234. [DOI] [PubMed] [Google Scholar]
- Lippold OC, Redfearn JW (1964) Mental changes resulting from the passage of small direct currents through the human brain. Br J Psychiatry 110:768–772. [DOI] [PubMed] [Google Scholar]
- Lysaker PH, Pattison ML, Leonhardt BL, Phelps S, Vohs JL (2018) Insight in schizophrenia spectrum disorders: relationship with behavior, mood and perceived quality of life, underlying causes and emerging treatments. World Psychiatry 17:12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma CC, Kao YC, Tzeng NS, Chao CY, Chang CC, Chang HA (2020) A higher degree of insight impairment in stabilized schizophrenia patients is associated with reduced cardiac vagal tone as indexed by resting-state high-frequency heart rate variability. Asian J Psychiatr 53:102171. [DOI] [PubMed] [Google Scholar]
- Macey PM, Ogren JA, Kumar R, Harper RM (2015) Functional imaging of autonomic regulation: methods and key findings. Front Neurosci 9:513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor A, Wheless J, Baumgartner J, Bettis D (2005) Right-sided vagus nerve stimulation as a treatment for refractory epilepsy in humans. Epilepsia 46:91–96. [DOI] [PubMed] [Google Scholar]
- McLaren ME, Nissim NR, Woods AJ (2018) The effects of medication use in transcranial direct current stimulation: a brief review. Brain Stimul 11:52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel P, Baumstarck K, Auquier P, Amador X, Dumas R, Fernandez J, Lancon C, Boyer L (2013) Psychometric properties of the abbreviated version of the scale to assess unawareness in mental disorder in schizophrenia. BMC Psychiatry 13:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milby AH, Halpern CH, Baltuch GH (2008) Vagus nerve stimulation for epilepsy and depression. Neurotherapeutics 5:75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda PC, Lomarev M, Hallett M (2006) Modeling the current distribution during transcranial direct current stimulation. Clin Neurophysiol 117:1623–1629. [DOI] [PubMed] [Google Scholar]
- Moliadze V, Antal A, Paulus W (2010) Electrode-distance dependent after-effects of transcranial direct and random noise stimulation with extracephalic reference electrodes. Clin Neurophysiol 121:2165–2171. [DOI] [PubMed] [Google Scholar]
- Mondino M, Jardri R, Suaud-Chagny MF, Saoud M, Poulet E, Brunelin J (2016) Effects of fronto-temporal transcranial direct current stimulation on auditory verbal hallucinations and resting-state functional connectivity of the left temporo-parietal junction in patients with schizophrenia. Schizophr Bull 42:318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Fricke K, Henschke U, Schlitterlau A, Liebetanz D, Lang N, Henning S, Tergau F, Paulus W (2003) Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol 553:293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, Paulus W, Hummel F, Boggio PS, Fregni F, Pascual-Leone A (2008) Transcranial direct current stimulation: state of the art 2008. Brain Stimul 1:206–223. [DOI] [PubMed] [Google Scholar]
- Noetscher GM, Yanamadala J, Makarov SN, Pascual-Leone A (2014) Comparison of cephalic and extracephalic montages for transcranial direct current stimulation–a numerical study. IEEE Trans Biomed Eng 61:2488–2498. [DOI] [PubMed] [Google Scholar]
- Opitz A, Paulus W, Will S, Antunes A, Thielscher A (2015) Determinants of the electric field during transcranial direct current stimulation. Neuroimage 109:140–150. [DOI] [PubMed] [Google Scholar]
- Palm U, Keeser D, Hasan A, Kupka MJ, Blautzik J, Sarubin N, Kaymakanova F, Unger I, Falkai P, Meindl T, Ertl-Wagner B, Padberg F (2016) Prefrontal transcranial direct current stimulation for treatment of schizophrenia with predominant negative symptoms: a double-blind, sham-controlled proof-of-concept study. Schizophr Bull 42:1253–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parazzini M, Rossi E, Rossi L, Priori A, Ravazzani P (2013a) Evaluation of the current density in the brainstem during transcranial direct current stimulation with extra-cephalic reference electrode. Clin Neurophysiol 124:1039–1040. [DOI] [PubMed] [Google Scholar]
- Parazzini M, Rossi E, Rossi L, Priori A, Ravazzani P (2013b) Numerical estimation of the current density in the heart during transcranial direct current stimulation. Brain Stimul 6:457–459. [DOI] [PubMed] [Google Scholar]
- Parellada M, Boada L, Fraguas D, Reig S, Castro-Fornieles J, Moreno D, Gonzalez-Pinto A, Otero S, Rapado-Castro M, Graell M, Baeza I, Arango C (2011) Trait and state attributes of insight in first episodes of early-onset schizophrenia and other psychoses: a 2-year longitudinal study. Schizophr Bull 37:38–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanía R, Paulus W, Nitsche MA (2012) Modulating cortico-striatal and thalamo-cortical functional connectivity with transcranial direct current stimulation. Hum Brain Mapp 33:2499–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfearn JW, Lippold OC, Costain R (1964) A preliminary account of the clinical effects of polarizing the brain in certain psychiatric disorders. Br J Psychiatry 110:773–785. [DOI] [PubMed] [Google Scholar]
- Sapara A, Ffytche DH, Cooke MA, Williams SC, Kumari V (2016) Voxel-based magnetic resonance imaging investigation of poor and preserved clinical insight in people with schizophrenia. World J Psychiatry 6:311–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker JK, Norton KN, Baker J, Luchyshyn T (2015) Forebrain organization for autonomic cardiovascular control. Auton Neurosci 188:5–9. [DOI] [PubMed] [Google Scholar]
- So SH, Garety PA, Peters ER, Kapur S (2010) Do antipsychotics improve reasoning biases? A review. Psychosom Med 72:681–693. [DOI] [PubMed] [Google Scholar]
- Sreeraj VS, Dinakaran D, Parlikar R, Chhabra H, Selvaraj S, Shivakumar V, Bose A, Narayanaswamy JC, Venkatasubramanian G (2018) High-definition transcranial direct current simulation (HD-tDCS) for persistent auditory hallucinations in schizophrenia. Asian J Psychiatr 37:46–50. [DOI] [PubMed] [Google Scholar]
- Tordesillas-Gutierrez D, Ayesa-Arriola R, Delgado-Alvarado M, Robinson JL, Lopez-Morinigo J, Pujol J, Dominguez-Ballesteros ME, David AS, Crespo-Facorro B (2018) The right occipital lobe and poor insight in first-episode psychosis. Plos One 13:e0197715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranulis C, Lepage M, Malla A (2008) Insight in first episode psychosis: who is measuring what? Early Interv Psychiatry 2:34–41. [DOI] [PubMed] [Google Scholar]
- van der Meer L, de Vos AE, Stiekema AP, Pijnenborg GH, van Tol MJ, Nolen WA, David AS, Aleman A (2013) Insight in schizophrenia: involvement of self-reflection networks? Schizophr Bull 39:1288–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandermeeren Y, Jamart J, Ossemann M (2010) Effect of tDCS with an extracephalic reference electrode on cardio-respiratory and autonomic functions. BMC Neurosci 11:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velligan DI, Sajatovic M, Hatch A, Kramata P, Docherty JP (2017) Why do psychiatric patients stop antipsychotic medication? A systematic review of reasons for nonadherence to medication in patients with serious mental illness. Patient Prefer Adherence 11:449–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner T, Fregni F, Fecteau S, Grodzinsky A, Zahn M, Pascual-Leone A (2007) Transcranial direct current stimulation: a computer-based human model study. Neuroimage 35:1113–1124. [DOI] [PubMed] [Google Scholar]
- Wu HE, Okusaga OO (2015) Antipsychotic medication-induced dysphoria: its meaning, association with typical vs. atypical medications and impact on adherence. Psychiatr Q 86:199–205. [DOI] [PubMed] [Google Scholar]
- Xavier RM, Vorderstrasse A (2016) Neurobiological basis of insight in schizophrenia: a systematic review. Nurs Res 65:224–237. [DOI] [PubMed] [Google Scholar]
- Yao G, Chung CW, Yu CF, Wang JD (2002) Development and verification of validity and reliability of the WHOQOL-BREF Taiwan version. J Formos Med Assoc 101:342–351. [PubMed] [Google Scholar]
- Zhou Y, Fan L, Qiu C, Jiang T (2015) Prefrontal cortex and the dysconnectivity hypothesis of schizophrenia. Neurosci Bull 31:207–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.