Abstract
The obesity epidemic continues to rise as a global health challenge. Thermogenic brown and beige adipocytes dissipate chemical energy as heat, providing an opportunity for developing new therapeutics for obesity and related metabolic diseases. Anatomically, brown adipose tissue is distributed as discrete depots, while beige adipocytes exist within certain depots of white adipose tissue. Developmentally, brown and beige adipocytes arise from multiple embryonic progenitor populations that are distinct and overlapping. Functionally, they respond to a plethora of stimuli to engage uncoupling protein 1-dependent and independent thermogenic programs, thus improving systemic glucose homeostasis, lipid metabolism, and the clearance of branched-chain amino acids. In this review, we highlight recent advances in our understanding of the molecular and cellular mechanisms that contribute to the developmental and functional heterogeneity of thermogenic adipose tissue.
Keywords: brown adipose tissue, beige adipocyte, BAT involution, lineage tracing, browning, UCP1
Introduction
Brown adipose tissue (BAT) was first described by Conrad Gesner in hibernators as ‘neither fat, nor flesh [nec pinguitudo, nec caro]’ (Gessner, 1551). Hibernating mammals use BAT, also once known as the ‘hibernating gland’, to rewarm from hypothermic torpor (Ballinger and Andrews, 2018). Later, a little over 100 years ago, BAT was also recognized in non-hibernating mammals including humans (Hatai, 1902; Bonnot, 1908). In early 1960s, the thermogenic role of BAT during cold acclimation was experimentally established (Smith and Roberts, 1964). It was not until late 1970s that uncoupling protein 1 (UCP1) was identified as a functional marker of BAT (Heaton et al., 1978; Aquila et al., 1985) and BAT was suggested to be involved in metabolic efficiency and resistance to obesity (Rothwell and Stock, 1979). In addition, beige, brite (brown-in-white), inducible, or recruitable brown adipocyte-like cells that are multilocular and UCP1-positive were discovered in predominantly white adipose tissue (WAT) depots (Young et al., 1984; Loncar, 1991; Guerra et al., 1998; Vitali et al., 2012). Thanks to recent advances in gene targeting, lineage tracing, and single-cell sequencing, there has been an exponential growth of our knowledge in the development and function of thermogenic brown and beige adipocytes (Wang et al., 2013; Wu et al., 2013; Inagaki et al., 2016; Wang and Seale, 2016; Shao et al., 2019). In this review, I will discuss the current understanding of molecular and cellular heterogeneity of thermogenic adipose tissue between species, through life, and during metabolic stress.
The development and age-related involution of BAT
Human BAT develops during gestation and the main depots can be found in interscapular, neck, axillary, and perirenal areas, while smaller deposits are behind the sternum and along the spine (Lidell, 2019). Cellular heterogeneity already exists in fetal BAT, as interscapular, perirenal, and suprailiac depots are predominantly multilocular adipocytes, while other depots contain mixed multilocular and unilocular fat cells (Merklin, 1974). However, molecular characterization of these BAT depots that contain unilocular white-like adipocytes is still lacking. It would be informative to determine whether they represent beige fat that has been extensively studied in mice.
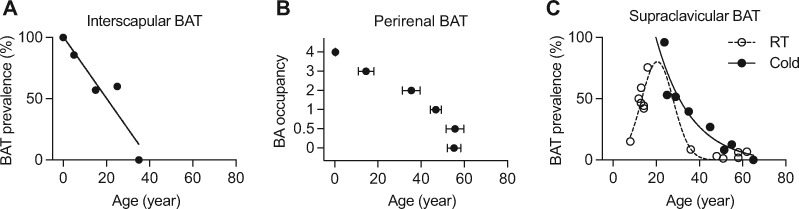
BAT is maximally recruited at birth as infants do not possess sufficient muscle for shivering thermogenesis. However, as we grow, the prevalence and activity of BAT steadily decline throughout the body. The interscapular BAT (iBAT) can be found in all infants, but it rapidly atrophies in adolescents and young adults (Heaton, 1972; Sidossis and Kajimura, 2015; Figure 1A). Perirenal BAT in newborns contains exclusively brown adipocytes, but they are gradually replaced by unilocular white adipocytes (Tanuma et al., 1975; Figure 1B).
Figure 1.
Progressive BAT involution with age in humans. (A) Re-plotting of the prevalence of interscapular BAT in humans, described in Heaton (1972). (B) Occupancy of brown adipocytes (BA) in human perirenal BAT with age. Data from Tanuma et al. (1975). BA occupancy: 0, absent of brown adipocytes; 0.5, only a few multilocular fat cells; 1, small accumulation of brown adipocytes; 2, medium accumulation of brown adipocytes; 3, large accumulation of brown adipocytes; 4, almost fully occupied by brown adipocytes. (C) Summary of the prevalence of supraclavicular BAT in humans at room temperature (RT) or cold acclimated, determined by 18F-PET/CT. Raw data collected from previous publications (Truong et al., 2004; Gelfand et al., 2005; Lee et al., 2010; Pfannenberg et al., 2010; Drubach et al., 2011; Gilsanz et al., 2011, 2012; Jacene et al., 2011; Ouellet et al., 2011; Zhang et al., 2014).
In adult humans, metabolically active, anatomically dispersed BAT exists in cervical, supraclavicular, and axillary areas, shown by [18F]-fluorodeoxyglucose-positron emission tomography/computed tomography (PET/CT) imaging and subsequent histological and molecular characterization (Cypess et al., 2009; Saito et al., 2009; van Marken Lichtenbelt et al., 2009; Virtanen et al., 2009; Zingaretti et al., 2009). These BAT depots around the neck appear to develop postnatally and their prevalence peaks around the age of 20 years and then reduces to <5% in middle-aged men and women (Figure 1C; Truong et al., 2004; Gelfand et al., 2005; Lee et al., 2010; Pfannenberg et al., 2010; Drubach et al., 2011; Gilsanz et al., 2011, 2012; Jacene et al., 2011; Ouellet et al., 2011; Zhang et al., 2014). Cold acclimation increases the activity of BAT and recruits brown fat even in subjects with previously undetectable metabolically active BAT (Saito et al., 2009; van Marken Lichtenbelt and Schrauwen, 2011; Yoneshiro et al., 2011; Figure 1C). However, the recruitment largely happens in young people and there is little evidence that brown fat can be recruited by cold in aged subjects with no detectable BAT (Rogers, 2015). It is worth noting that most of these studies relied only on [18F]-fluorodeoxyglucose-PET/CT assessment of glucose uptake in BAT, thus possibly underestimating the prevalence and activity of BAT (Lee et al., 2013).
The mechanisms underlying age-related BAT involution remain enigmatic, but can be multi-fold: (i) neuronal and hormonal regulation as a result of reduced need for BAT to produce heat (e.g. thermal insulation, muscle shivering, lower ratio of body surface area to body volume, etc.); (ii) brown adipocyte ‘whitening’ or transdifferentiation to white adipocytes; and (iii) the defect in browning ability of adipose progenitors and/or the loss of their population. Although mice display human-like brown/beige fat depots in anatomically comparable regions (Cypess et al., 2013; Shinoda et al., 2015; Zhang et al., 2018), they have constitutively active interscapular BAT throughout life. In mice, aging is associated with thermogenic dysfunction, but brown adipocytes largely preserve their ‘browning’ ability to external cues (Sellayah and Sikder, 2014; Goncalves et al., 2017; Tajima et al., 2019). At the thermoneutral zone, brown adipocytes in mice also remain their cellular identity, despite decreased thermogenic program (Cui et al., 2016; Roh et al., 2018). Therefore, complementary animal models that possess characteristics of human BAT involution are immediately needed.
Heterogeneity in the browning capacity of WAT depots
Cold exposure recruits BAT and induces WAT ‘browning’. When housing mice at thermoneutral conditions, UCP1 is only well expressed in bona-fide BAT including the interscapular, cervical, axillary, and mediastinal depots. Cold-induced Ucp1 mRNA expression increases 2−3 folds in BAT depots whereas 10−200 folds in cardiac, inguinal, and retroperitoneal WAT depots that contain significant numbers of ‘dormant’ beige adipocytes and progenitors (Walden et al., 2012). Among beige fat, the inguinal subcutaneous WAT appears to have the highest Ucp1, while retroperitoneal WAT shows the most induction of Ucp1 by cold (Walden et al., 2012). Visceral WAT including the mesenteric and epididymal depots lacks beige adipocytes, thus expressing very little Ucp1 under thermoneutral or cold conditions (Walden et al., 2012).
On the other hand, fasting could preferentially downregulate the thermogenic program in retroperitoneal WAT, but to a much lesser extent in other WAT depots and not in BAT (Ruan et al., 2014). Although the mechanisms of differential responses to thermogenic regulators among WAT depots are largely unclear, the diversity in neuronal innervation and sympathetic activity may be one contributing factor (Ruan et al., 2014; Yang and Ruan, 2015). Retroperitoneal WAT shows the most robust responses to diverse metabolic stimuli, such as cold (Walden et al., 2012), β3-adrenergic receptor agonism (Poher et al., 2015), fasting (Ruan et al., 2014), and calorie restriction (Narita et al., 2018), pointing to the possibility that retroperitoneal WAT could function as a first line of defense to maintain energy and lipid homeostasis when environmental temperature and food are fluctuant.
Genetic background is another determinant of the heterogeneity in WAT browning. The Kozak group elegantly demonstrated that the obesity-resistant A/J mice have significantly higher thermogenic gene expression preferentially in retroperitoneal WAT when compared with obesity-prone C57BL/6 mice (Guerra et al., 1998; Koza et al., 2000; Coulter et al., 2003). Later, they identified nine quantitative trait loci that may regulate the browning process in retroperitoneal WAT (Xue et al., 2005). A recent study found that obesity resistance in 129/Sv and FVB/NJ mice is associated with adipose browning in epididymal WAT and inguinal WAT, respectively (Ferrannini et al., 2016). Systemically defining genetic underpinnings of depot-specific browning capacity will be an important avenue of future research. We urge researchers to analyze as many beige adipose depots as possible in their studies on WAT browning.
Similar to that in mice, subcutaneous WAT in humans also undergoes the thermogenic activation in response to cold (Finlin et al., 2018), adrenergic stress associated with burn trauma (Patsouris et al., 2015; Sidossis et al., 2015), and exercise (Otero-Diaz et al., 2018). However, there is also evidence that humans, being opposite to what is observed in mice, demonstrate higher thermogenic gene expression in visceral WAT than subcutaneous WAT (Zuriaga et al., 2017). Caution should be taken when extrapolating data from mice to humans.
Lineage heterogeneity of thermogenic adipocytes
Brown, beige, and white adipocytes arise from a heterogeneous group of progenitor cells during development and in pathophysiological conditions (Sanchez-Gurmaches et al., 2016). Early studies had hinted the shared features between BAT and muscle (Timmons et al., 2007). During early embryogenesis, interscapular BAT and muscle, along with others, are derived from the En1-expressing dermomyotome (Atit et al., 2006). Later, several independent fate-mapping experiments demonstrated that brown adipocytes in mice originate from myogenic precursor cells that express Myf5 (Seale et al., 2008), Pax3 (Sanchez-Gurmaches and Guertin, 2014), and Pax7 (Lepper and Fan, 2010). However, recent studies have revealed that the composition of thermogenic adipose lineage is more complex than we previously thought of (Table 1).
Table 1.
Lineage tracing of BAT and WAT depots.
| Cre line | En1-CreER | Myf5-Cre | Pax3-Cre | Pax7-Cre | Meox1-Cre | HoxB6-Cre | MyoD1-Cre | WT1-CreER | Prx1-Cre | Pdgfra-Cre | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BAT | Interscapular | + | All | All | <50% | >85% | – | – | – | – | + |
| Subscapular | All | All | – | – | |||||||
| Cervical | 50% | 80% | – | – | |||||||
| Perirenal | – | 70% | – | + | – | ||||||
| Periaortic | – | – | – | – | |||||||
| WAT | Interscapular | Most | Most | <50% | >85% | – | |||||
| Anterior subQ | All | All | – | – | +1 | ||||||
| Retroperitoneal | All2 | All | – | >85% | – | – | + | – | All | ||
| Inguinal | – | – | – | – | All | –3 | – | All | All | ||
| Mesenteric | – | – | – | – | All | – | + | – | All | ||
| Gonadal | – | 58%4 | – | 60%5 | All6 | – | + | – | All |
–, none from the corresponding lineage; blank, not determined.
Approximately half of the ventral region but none of the dorsal region of the anterior subcutaneous adipocytes are positive (Sanchez-Gurmaches et al., 2015).
Decreases with age, in short-term HFD, and after β3-adrenergic receptor activation (Sanchez-Gurmaches and Guertin, 2014).
MyoD1 + lineage can give rise to the so-called ‘glycolytic beige fat’ during chronic cold adaptation in the absence of β-adrenergic receptor signaling (Chen et al., 2019).
Percentage in males. No gonadal adipocytes trace to Pax3-lineage in females (Sanchez-Gurmaches and Guertin, 2014).
Percentage in males. Only 4% of gonadal adipocytes trace to Meox1-lineage in females (Sebo et al., 2018).
Percentage in females. Only 5% of gonadal adipocytes trace to HoxB6-lineage in males (Sebo et al., 2018).
The Guertin group systemically evaluated the Myf5+ and Pax3+ lineages and found that most dorsal-anterior adipocytes, in both BAT and WAT depots, are derived from the largely overlapping Myf5-Cre- and Pax3-Cre-expressing precursors (Sanchez-Gurmaches et al., 2012). In contrast, Myf5-Cre and Pax3-Cre do not label any inguinal and mesenteric WAT adipocytes, though Pax3-Cre progenitors do contribute to about half of the gonadal adipocytes in male mice (Sanchez-Gurmaches and Guertin, 2014). Pax7, the expression of which is restricted to the central dermomyotome, defines progenitors of a minority of interscapular brown and white adipocytes (Sebo et al., 2018). While Meox1-Cre, which is broadly expressed in the dermomyotome as Pax3-Cre, traces to most adipocytes in the dorsal−anterior and male gonadal depots (Sebo et al., 2018). On the other hand, HoxB6-Cre, with expression restricted to the posterior domain of lateral plate mesoderm, complements the tracing patterns of Meox1-Cre and labels most adipocytes in the inguinal, mesenteric, and female gonadal depots (Sebo et al., 2018). Based on these data, Sebo and Rodeheffer (2019) proposed that the dorsal-anterior-located interscapular and retroperitoneal adipose depots predominately arise from a Pax3+/Meox1+/Myf5+ lineage in the epaxial dermomyotome (Table 1), with a minor contribution from the Pax7+ central dermomyotome. Intriguingly, MyoD1, a classic myogenic transcription factor that acts downstream of Pax3 and Myf5, does not label any adipocytes at physiological conditions (Sanchez-Gurmaches and Guertin, 2014), suggesting that the adipose and muscle lineages diverge before the MyoD1 gene is expressed.
A large body of literature has documented significant differences between visceral and subcutaneous WAT depots, in terms of their morphological and molecular characteristics, thermogenic capacity, and metabolic contributions. Apparently, these two fat subtypes have different origins and developmental timing (Wang et al., 2013, 2015). The postnatally differentiated visceral fat, including perirenal, epicardial, retroperitoneal, omental, mesenteric, and gonadal depots, receives significant contributions from Wt1+ progenitors in the intermediate mesoderm and splanchnic lateral plate mesoderm (Chau et al., 2014). On the other hand, limb-associated subcutaneous fat initiates its differentiation during embryonic days 14‒18 and is labelled by Prx1-Cre that traces the somatic lateral plate mesoderm (Krueger et al., 2014; Sanchez-Gurmaches et al., 2015; Table 1).
The lineage distribution of thermogenic adipose tissue is dynamic during obesity, aging, and thermal stress. WAT browning induced by cold exposure or β-adrenergic receptor activation involves the transdifferentiation of white to beige adipocytes (Barbatelli et al., 2010; Lee et al., 2015), the activation of existing ‘dormant’ beige cells (Rosenwald et al., 2013), and the de novo adipogenesis from beige adipocyte progenitors (Wang et al., 2013; Berry et al., 2016). These beige adipocyte progenitors may share the common embryonic origin with the white lineage, as they can be both tracing-labelled by constitutive Prx1-Cre (Sanchez-Gurmaches et al., 2015), Pdgfra-Cre (Lee et al., 2012, 2015; Berry and Rodeheffer, 2013; Krueger et al., 2014), and Pdgfrb-Cre (Vishvanath et al., 2016; Figure 2). However, in adult mice, the contribution of Pdgfra- and Pdgfrb-expressing cells to WAT browning is relatively limited, revealed by temporal fate-mapping experiments (see below).
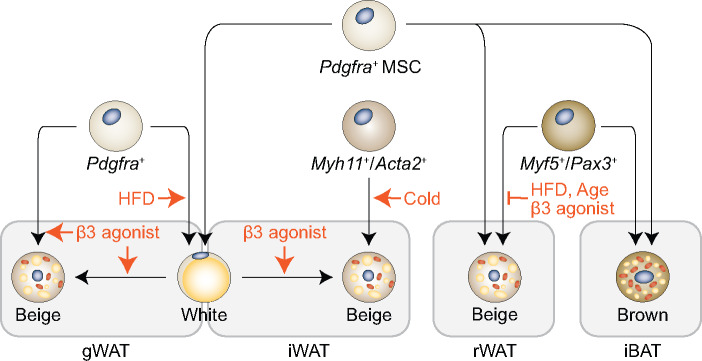
Figure 2.
Dynamic lineage contribution within WAT. During embryonic development, Pdgfra-labelled mesenchymal stem cells (MSCs) give rise to all adipocytes in WAT and some brown adipocytes in interscapular BAT (iBAT). In gonadal WAT (gWAT), HFD and β-adrenergic receptor activation stimulate the differentiation of Pdgfra+ precursors. It is unclear whether beige adipocytes differentiate directly from Pdgfra+ precursors or via an intermediate white phenotype (these two processes are not necessarily exclusive). In inguinal WAT (iWAT), Myh11+/Acta2+ mural cells give rise to new beige adipocytes upon cold stimulation. However, β-adrenergic receptor activation does not stimulate de novo adipogenesis, rather it promotes transdifferentiation of existing white adipocytes. Retroperitoneal WAT (rWAT) and iBAT both arise from Myf5+/Pax3+ precursor cells. While the lineage distribution within iBAT is constitutive, the percentage of Myf5-derived beige adipocytes decreases with age, in short-term HFD feeding, and after β3-adrenergic receptor activation in rWAT.
Cold and β-adrenergic receptor activation induce distinct mechanisms for adaptive thermogenesis (Jiang et al., 2017; Chen et al., 2019). Exposing animals to cold activates beige adipogenesis in inguinal WAT from smooth muscle-like perivascular mural cells that express Myh11 (Long et al., 2014), Tagln (Sm22), Cspg4 (Ng2), and Acta2 (Sma) (Berry et al., 2016). None of these newly differentiated beige cells can be labelled by temporally expressed Pdgfra-CreER. On the other hand, β3-adrenergic receptor activation stimulates the conversion of mature white adipocytes into beige adipocytes in both inguinal and gonadal WAT (Lee et al., 2012; Jiang et al., 2017). Similar to high-fat diet (HFD)-stimulated adipogenic differentiation in gonadal WAT (Jeffery et al., 2015), beige adipocytes induced by β-adrenergic receptor activation arise from Pdgfra+ precursor cell (Lee et al., 2012; Cattaneo et al., 2020). Nonetheless, it is not fully resolved whether an intermediate transition is required for the fully differentiation of Pdgfra+ precursors into mature adipocytes. Moreover, future works will be required to determine whether the Pdgfra+ precursors are homogeneous or subpopulations of precursors exist for white and beige adipocyte lineages, respectively. Indeed, Pdgfra+ and Pdgfrb+ progenitors have lineage plasticity and adopt a fibrogenic phenotype in obesity (Marcelin et al., 2017).
The percentage of Myf5-derived adipocytes decreases with age, in short-term HFD feeding, and after β3-adrenergic receptor activation in retroperitoneal WAT, but not in classic BAT (Sanchez-Gurmaches and Guertin, 2014; Figure 2). Similarly, changes in insulin signaling by deleting PTEN or IRβ can alter the Myf5+ lineage composition within anterior subcutaneous and retroperitoneal WAT, not within BAT (Sanchez-Gurmaches and Guertin, 2014). Thermal stress in ‘β-less’ mice that lack β-adrenergic receptor signaling induces a group of non-canonical beige adipocytes that display enhanced glycolysis and originated from the MyoD1+ progenitors (Chen et al., 2019). A thorough understanding of adipose lineage plasticity will provide new insights into the pathogenesis of obesity and obesity-associated complications.
Cellular heterogeneity within the thermogenic adipose tissue
Originally considered as a connective tissue, the adipose is now regarded as an organ. It contains several cell types that interact to coordinate its metabolic function. In addition to adipocytes, there are various immune cells that control adipose inflammation and insulin sensitivity (Lu et al., 2019), fibroblasts that mediate adipose fibrosis in obesity (Crewe et al., 2017), endothelial cells that constitute the adipose vasculature, and sympathetic and sensory nerve cells. Moreover, there are adipocyte precursors, including stem/progenitor cells and more committed preadipocytes, which reside in the stromal vascular fraction (SVF). These lineage negative adipocyte precursors normally express PDGFRα, PREF1, CD34, SCA1, CD29, and CD24 (Rodeheffer et al., 2008; Schulz et al., 2011; Berry and Rodeheffer, 2013). However, most of these markers label more than just adipocyte precursors in the SVF and are largely established from studies on WAT. Recently, the transcription factor EBF2 was identified as a highly selective marker of brown and beige adipocytes that also controls the thermogenic program (Wang et al., 2014). Nonetheless, EBF2 also expresses in non-adipose tissues. Truly unique markers of thermogenic adipocyte precursors and corresponding Cre-driver mouse lines are still lacking.
Owing to the recent advance in single-cell RNA sequencing (scRNA-seq) technologies and bioinformatics, we are at the beginning of a new era for understanding the adipose tissue heterogeneity (Burl et al., 2018; Hepler et al., 2018; Schwalie et al., 2018; Cho et al., 2019; Merrick et al., 2019; Rajbhandari et al., 2019). Generally, there are one or more clusters of stem-like adipocyte progenitors (e.g. Dpp4+ and Pi16+ cells) and committed/differentiating preadipocytes (e.g. Icam1+ and Cd142+ cells) in the SVF fraction. Schwalie et al. (2018) demonstrated that the Cd142+ cell population suppresses adipogenesis in a paracrine manner, while Merrick et al. (2019) proposed these Cd142+ cells as a distinct adipogenic population that are poised to differentiate into mature adipocytes. The reason for this discrepancy is not clear but might stem from differences in cell purification strategies. Meta-analyses of data from all these scRNA-seq studies using computational integration are highly desired. In addition, all these published studies focused on visceral or subcutaneous WAT depots, while the cellular heterogeneity of the SVF cells within the classic BAT has not been revealed yet.
Using single nuclei adipocyte RNA sequencing (SNAP-seq) of mature adipocytes isolated from subcutaneous adipose tissue, Rajbhandari et al. (2019) defined more than a dozen clusters of lipid-laden adipocytes, among which was thermogenic beige adipocytes that were responsive to cold, β3-adrenergic receptor activation, and IL10Rα deletion. However, distinct progenitor populations specifically committed for beige adipogenesis have not been reported so far.
Functional heterogeneity of thermogenic adipocytes
Since its discovery, UCP1 has been wildly used as a functional marker of both brown and beige adipocytes. Indeed, UCP1 mediates most, if not all, of the non-shivering thermogenesis induced by norepinephrine (NE), and loss of UCP1 renders mice to become sensitive to acute cold exposure (Cannon and Nedergaard, 2004). In addition to NE, the sympathetic nerve system also provides purine nucleotides and nucleosides to activate the purinergic receptors on thermogenic adipocytes (Gnad et al., 2014; Ussar et al., 2014; Razzoli et al., 2016). Moreover, many hormones and cytokines derived from a whole range of tissues and cells can act independently or in parallel with the adrenergic signaling to activate and/or recruit brown/beige adipocytes (Figure 3; Wang and Yang, 2017).
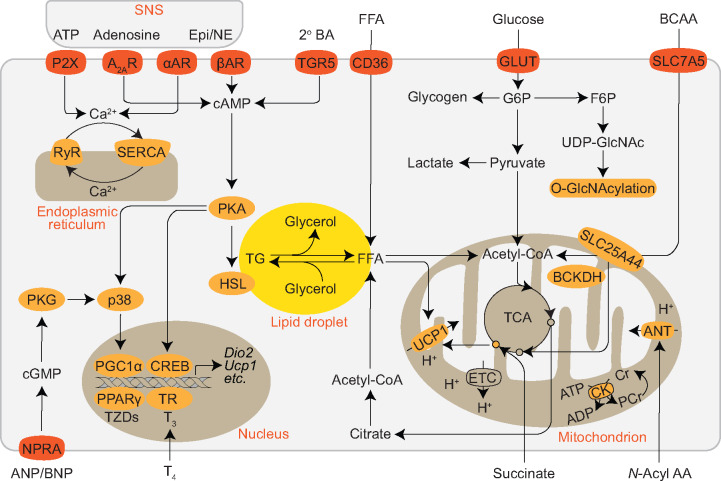
Figure 3.
Diversity in stimulus sensing, fuel sources, and thermogenic mechanisms of brown and beige adipocytes. Thermogenic adipocytes receive signals from the sympathetic nerve terminals, including catecholamines, ATP, and adenosine. Together with secondary bile acid (2° BA), atrial/brain natriuretic peptide, thyroid hormone, and others not shown, adrenergic and purinergic signaling stimulates lipolysis that generates free fatty acids (FFAs) for mitochondrial fuel and UCP1 activation, promotes thermogenic gene expression, and initiates the futile calcium cycle in the endoplasmic reticulum. FFA and triacylglycerol-rich lipoproteins are taken up into adipocytes by CD36. Glucose can be stored as glycogen, undergo glycolysis, enter the hexosamine biosynthetic pathway for protein O-GlcNAcylation, be used for de novo lipogenesis, or fuel the thermogenic program. Activated BAT also clears BCAAs from the circulation and utilizes them in the mitochondria for thermogenesis via solute carrier family 25 member 44 (SLC25A44)-mediated transportation and branched-chain α-keto acid dehydrogenase (BCKDH)-dependent catabolism. Succinate is accumulated in activated BAT mitochondria to drive UCP1-dependent thermogenesis via reactive oxygen species and protein succinylation. The creatine futile cycle mediated by the creatine kinase (CK) triggers mitochondrial ATP turnover and UCP1-independent thermogenesis. N-Acyl amino acid is proposed to engage members of the SLC25 family, such as adenine nucleotide translocase (ANT) to uncouple mitochondrial respiration. A2AR, adenosine A2A receptor; AR, adrenergic receptor; Epi, epinephrine; ETC, electron transport chain; F6P, fructose-6-phosphate; G6P, glucose-6-phosphate; Gpbar1; GLUT, glucose transporter; HSL, hormone-sensitive lipase; NE, norepinephrine; NPRA, natriuretic peptide receptor A; P2X, P2X receptor; PKA, protein kinase A; PKG, protein kinase G; RyR, ryanodine receptor; SERCA, sarco/endoplasmic reticulum Ca2+-ATPase; SNS, sympathetic nerve system; TCA, the citrate cycle; TGR5, G-protein-coupled bile acid receptor; TR, thyroid hormone receptor; TZDs, thiazolidinediones; UDP-GlcNAc, uridine diphosphate N-acetylglucosamine.
When gradually acclimated to cold, UCP1-deficient mice are still able to maintain tolerance to cold (Ukropec et al., 2006). Intriguingly, or rather paradoxically, UCP1-deficient mice are resistant to diet-induced obesity when housed at ambient temperature that evokes mild cold stress in mice (Enerback et al., 1997; Liu et al., 2003). Since these early findings indicating the functional compensation for the loss of UCP1, several UCP1-independent thermogenic mechanisms have been discovered (Figure 3; Chang et al., 2019). These include the phosphocreatine/creatine futile cycle in brown and beige adipocytes (Kazak et al., 2015), Ca2+ cycling mediated by sarco/endoplasmic reticulum Ca2+-ATPase in muscle and beige adipocytes (Bal et al., 2012; Ikeda et al., 2017), mitochondrial uncoupling by N-acyl amino acids that are generated by PM20D1 in brown and beige fat cells (Long et al., 2016), the futile cycle between triglyceride breakdown and re-esterification in adipocytes (Guan et al., 2002), and the futile cycle between lipid oxidation and de novo lipogenesis in muscle and adipocytes (Solinas et al., 2004; Mottillo et al., 2014). A better understanding of these non-canonical mechanisms of thermogenic fat cells will potentially lead to therapeutic interventions for the treatment of obesity.
Even the classic BAT contains heterogeneous groups of brown adipocytes. An early study by Cinti et al. (2002) presented the non-homogeneous pattern of UCP1 expression after cold stimulus and β3-adrenoceptor agonist treatment. Very recently, Song et al. (2020), taking advantage of the Adipo-Chaser system and SNAP-seq, identified Adipoqhi/Ucp1hi high-thermogenic and Adipoqlow/Ucp1low low-thermogenic brown adipocytes within the BAT. Compared to classic high-thermogenic brown adipocytes, these low-thermogenic cells have larger lipid droplets but lower mitochondrial content and respiration, are specialized in fatty acid uptake, and engage the creatine futile cycle. Importantly, cold exposure can convert low-thermogenic brown adipocytes into high-thermogenic cells, the efficiency of which declines with age (Song et al., 2020). However, it is unclear whether this postnatally established functional heterogeneity is due to the developmental heterogeneity of brown adipocyte precursors.
In addition to maintaining body temperature via thermogenesis, BAT and beige fat also serve as metabolic sinks for glucose, fatty acids, and branched-chain amino acids (BCAAs) to improve metabolic health (Figure 3; Harms and Seale, 2013; Wallace et al., 2018; Yoneshiro et al., 2019; Maurer et al., 2020). Cold exposure dramatically increases the uptake of glucose, fatty acids, lipoproteins, and BCAAs into the BAT, which can be reversed when animals are returned to a warm environment (Bartelt et al., 2011; Labbe et al., 2015; Yoneshiro et al., 2019). Recently, the tricarboxylic acid cycle intermediate succinate was shown to be accumulated in thermogenic adipose tissue upon cold exposure (Mills et al., 2018; Wang et al., 2019). Circulating succinate may be derived from muscle and active UCP1-dependent thermogenesis via reactive oxygen species and mitochondrial protein succinylation. Of note, fatty acids are traditionally considered as the main fuel for thermogenesis in BAT (Trayhurn, 1995). Glucose uptake does not necessarily reflect the thermogenic activity in BAT (Olsen et al., 2017; Skorobogatko et al., 2018), and the relative fates of glucose metabolism (glycolysis, glycogenesis, lipogenesis, oxidation, and thermogenesis) are still unclear (Townsend and Tseng, 2014). On the other side, a portion of glucose can enter the hexosamine biosynthetic pathway to generate uridine diphosphate N-acetylglucosamine (UDP-GlcNAc), which is then used for O-linked β-N-acetylglucosamine modification (O-GlcNAcylation) of intracellular proteins (Ruan et al., 2013). Protein O-GlcNAcylation has been also shown to modulate adipose tissue thermogenesis via cell autonomous and non-autonomous mechanisms (Ruan et al., 2014; Ohashi et al., 2017). In spite of these knowledge, it is desired to determine whether there are heterogenous adipocyte populations that prefer to use certain nutrient substrate(s) than others.
Conclusion
Obesity is a major risk factor for many diseases, including type 2 diabetes, cardiovascular disease, and some types of cancers (Taksler et al., 2017). The activity of BAT in adult humans is inversely associated with body mass index (Betz and Enerback, 2011; Tam et al., 2012), indicating its potential involvement in obesity development and therapeutic function. There are many outstanding questions regarding the heterogeneity of lineage development and metabolic function within thermogenic adipose tissue. Supraclavicular, cervical, and axillary BAT in adult humans appears to contain mixed brown and beige adipocytes; however, their developmental origins, even in mouse models, have not been identified. What are the cellular and molecular mechanisms governing the age-dependent involution of BAT? Does the diversity in fuel utilization, gene expression, and thermogenic activity observed in brown and beige adipocytes stem from the lineage heterogeneity of adipocyte precursors during development? Do beige and white adipocytes arise from distinct precursor populations and do these precursors still possess plasticity in their cellular identity? What is the ‘master’ switch that regulates metabolic flexibility of thermogenic adipocytes and whether the fuel inflexibility within the brown and beige fat contributes to systemic metabolic dysfunction? How do thermogenic adipocytes utilize the two-way communication with tissue-resident immune, fibrogenic, and nerve cells to maintain tissue homeostasis and control whole-body metabolism? Answering these questions will pave the way for activating, rejuvenating, and regenerating BAT to combat obesity, diabetes, and related chronic conditions.
Funding
This work was supported by the American Diabetes Association (1-18-IBS-167) and National Institute of Health (R01AI139420 and R21AI140109) to H.-B.R.
Conflict of interest: none declared.
References
- Aquila H., Link T.A., Klingenberg M. (1985). The uncoupling protein from brown fat mitochondria is related to the mitochondrial ADP/ATP carrier. Analysis of sequence homologies and of folding of the protein in the membrane. EMBO J. 4, 2369–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atit R., Sgaier S.K., Mohamed O.A., et al. (2006). β-catenin activation is necessary and sufficient to specify the dorsal dermal fate in the mouse. Dev. Biol. 296, 164–176. [DOI] [PubMed] [Google Scholar]
- Bal N.C., Maurya S.K., Sopariwala D.H., et al. (2012). Sarcolipin is a newly identified regulator of muscle-based thermogenesis in mammals. Nat. Med. 18, 1575–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballinger M.A., Andrews M.T. (2018). Nature’s fat-burning machine: brown adipose tissue in a hibernating mammal. J. Exp. Biol. 221, jeb162586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbatelli G., Murano I., Madsen L., et al. (2010). The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am. J. Physiol. Endocrinol. Metab. 298, E1244–E1253. [DOI] [PubMed] [Google Scholar]
- Bartelt A., Bruns O.T., Reimer R., et al. (2011). Brown adipose tissue activity controls triglyceride clearance. Nat. Med. 17, 200–205. [DOI] [PubMed] [Google Scholar]
- Berry D.C., Jiang Y., Graff J.M. (2016). Mouse strains to study cold-inducible beige progenitors and beige adipocyte formation and function. Nat. Commun. 7, 10184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry R., Rodeheffer M.S. (2013). Characterization of the adipocyte cellular lineage in vivo. Nat. Cell Biol. 15, 302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz M.J., Enerback S. (2011). Therapeutic prospects of metabolically active brown adipose tissue in humans. Front. Endocrinol. 2, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnot E. (1908). The interscapular gland. J. Anat. Physiol. 43, 43–58. [PMC free article] [PubMed] [Google Scholar]
- Burl R.B., Ramseyer V.D., Rondini E.A., et al. (2018). Deconstructing adipogenesis induced by β3-adrenergic receptor activation with single-cell expression profiling. Cell Metab. 28, 300–309.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon B., Nedergaard J. (2004). Brown adipose tissue: function and physiological significance. Physiol. Rev. 84, 277–359. [DOI] [PubMed] [Google Scholar]
- Cattaneo P., Mukherjee D., Spinozzi S., et al. (2020). Parallel lineage-tracing studies establish fibroblasts as the prevailing in vivo adipocyte progenitor. Cell Rep. 30, 571–582.e2. [DOI] [PubMed] [Google Scholar]
- Chang S.H., Song N.J., Choi J.H., et al. (2019). Mechanisms underlying UCP1 dependent and independent adipocyte thermogenesis. Obes. Rev. 20, 241–251. [DOI] [PubMed] [Google Scholar]
- Chau Y.Y., Bandiera R., Serrels A., et al. (2014). Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. Nat. Cell Biol. 16, 367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Ikeda K., Yoneshiro T., et al. (2019). Thermal stress induces glycolytic beige fat formation via a myogenic state. Nature 565, 180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho D.S., Lee B., Doles J.D. (2019). Refining the adipose progenitor cell landscape in healthy and obese visceral adipose tissue using single-cell gene expression profiling. Life Sci. Alliance 2, e201900561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinti S., Cancello R., Zingaretti M.C., et al. (2002). CL316,243 and cold stress induce heterogeneous expression of UCP1 mRNA and protein in rodent brown adipocytes. J. Histochem. Cytochem. 50, 21–31. [DOI] [PubMed] [Google Scholar]
- Coulter A.A., Bearden C.M., Liu X., et al. (2003). Dietary fat interacts with QTLs controlling induction of Pgc-1α and Ucp1 during conversion of white to brown fat. Physiol. Genomics 14, 139–147. [DOI] [PubMed] [Google Scholar]
- Crewe C., An Y.A., Scherer P.E. (2017). The ominous triad of adipose tissue dysfunction: inflammation, fibrosis, and impaired angiogenesis. J. Clin. Invest. 127, 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X., Nguyen N.L., Zarebidaki E., et al. (2016). Thermoneutrality decreases thermogenic program and promotes adiposity in high-fat diet-fed mice. Physiol. Rep. 4, e12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess A.M., Lehman S., Williams G., et al. (2009). Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 360, 1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess A.M., White A.P., Vernochet C., et al. (2013). Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat. Med. 19, 635–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drubach L.A., Palmer E.L., 3rd, Connolly L.P., et al. (2011). Pediatric brown adipose tissue: detection, epidemiology, and differences from adults. J. Pediatr. 159, 939–944. [DOI] [PubMed] [Google Scholar]
- Enerback S., Jacobsson A., Simpson E.M., et al. (1997). Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature 387, 90–94. [DOI] [PubMed] [Google Scholar]
- Ferrannini G., Namwanje M., Fang B., et al. (2016). Genetic backgrounds determine brown remodeling of white fat in rodents. Mol. Metab. 5, 948–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlin B.S., Memetimin H., Confides A.L., et al. (2018). Human adipose beiging in response to cold and mirabegron. JCI Insight 3, e121510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand M.J., O’Hara S, M., Curtwright L.A., et al. (2005). Pre-medication to block [18F]FDG uptake in the brown adipose tissue of pediatric and adolescent patients. Pediatr. Radiol. 35, 984–990. [DOI] [PubMed] [Google Scholar]
- Gessner K. (1551). Conradi Gesneri medici Tigurini Historiae Animalium Lib. I. De quadrupedibus viviparis 842, I.6–I.9. [Google Scholar]
- Gilsanz V., Chung S.A., Jackson H., et al. (2011). Functional brown adipose tissue is related to muscle volume in children and adolescents. J. Pediatr. 158, 722–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilsanz V., Smith M.L., Goodarzian F., et al. (2012). Changes in brown adipose tissue in boys and girls during childhood and puberty. J. Pediatr. 160, 604–609.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnad T., Scheibler S., von Kugelgen I., et al. (2014). Adenosine activates brown adipose tissue and recruits beige adipocytes via A2A receptors. Nature 516, 395–399. [DOI] [PubMed] [Google Scholar]
- Goncalves L.F., Machado T.Q., Castro-Pinheiro C., et al. (2017). Ageing is associated with brown adipose tissue remodelling and loss of white fat browning in female C57BL/6 mice. Int. J. Exp. Pathol. 98, 100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan H.P., Li Y., Jensen M.V., et al. (2002). A futile metabolic cycle activated in adipocytes by antidiabetic agents. Nat. Med. 8, 1122–1128. [DOI] [PubMed] [Google Scholar]
- Guerra C., Koza R.A., Yamashita H., et al. (1998). Emergence of brown adipocytes in white fat in mice is under genetic control. Effects on body weight and adiposity. J. Clin. Invest. 102, 412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms M., Seale P. (2013). Brown and beige fat: development, function and therapeutic potential. Nat. Med. 19, 1252–1263. [DOI] [PubMed] [Google Scholar]
- Hatai S. (1902). On the presence in human embryos of an interscapular gland corresponding to the so-called hibernating gland of lower mammals. Anat. Anz. 21, 369–373. [Google Scholar]
- Heaton G.M., Wagenvoord R.J., Kemp A., Jr, et al. (1978). Brown-adipose-tissue mitochondria: photoaffinity labelling of the regulatory site of energy dissipation. Eur. J. Biochem. 82, 515–521. [DOI] [PubMed] [Google Scholar]
- Heaton J.M. (1972). The distribution of brown adipose tissue in the human. J. Anat. 112, 35–39. [PMC free article] [PubMed] [Google Scholar]
- Hepler C., Shan B., Zhang Q., et al. (2018). Identification of functionally distinct fibro-inflammatory and adipogenic stromal subpopulations in visceral adipose tissue of adult mice. eLife 7, e39636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K., Kang Q., Yoneshiro T., et al. (2017). UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nat. Med. 23, 1454–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki T., Sakai J., Kajimura S. (2016). Transcriptional and epigenetic control of brown and beige adipose cell fate and function. Nat. Rev. Mol. Cell Biol. 17, 480–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacene H.A., Cohade C.C., Zhang Z., et al. (2011). The relationship between patients’ serum glucose levels and metabolically active brown adipose tissue detected by PET/CT. Mol. Imaging Biol. 13, 1278–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery E., Church C.D., Holtrup B., et al. (2015). Rapid depot-specific activation of adipocyte precursor cells at the onset of obesity. Nat. Cell Biol. 17, 376–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Berry D.C., Graff J.M. (2017). Distinct cellular and molecular mechanisms for β3 adrenergic receptor-induced beige adipocyte formation. eLife 6, e30329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazak L., Chouchani E.T., Jedrychowski M.P., et al. (2015). A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell 163, 643–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koza R.A., Hohmann S.M., Guerra C., et al. (2000). Synergistic gene interactions control the induction of the mitochondrial uncoupling protein (Ucp1) gene in white fat tissue. J. Biol. Chem. 275, 34486–34492. [DOI] [PubMed] [Google Scholar]
- Krueger K.C., Costa M.J., Du H., et al. (2014). Characterization of Cre recombinase activity for in vivo targeting of adipocyte precursor cells. Stem Cell Rep. 3, 1147–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbe S.M., Caron A., Bakan I., et al. (2015). In vivo measurement of energy substrate contribution to cold-induced brown adipose tissue thermogenesis. FASEB J. 29, 2046–2058. [DOI] [PubMed] [Google Scholar]
- Lee P., Greenfield J.R., Ho K.K., et al. (2010). A critical appraisal of the prevalence and metabolic significance of brown adipose tissue in adult humans. Am. J. Physiol. Endocrinol. Metab. 299, E601–E606. [DOI] [PubMed] [Google Scholar]
- Lee P., Swarbrick M.M., Ho K.K. (2013). Brown adipose tissue in adult humans: a metabolic renaissance. Endocr. Rev. 34, 413–438. [DOI] [PubMed] [Google Scholar]
- Lee Y.H., Petkova A.P., Konkar A.A., et al. (2015). Cellular origins of cold-induced brown adipocytes in adult mice. FASEB J. 29, 286–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.H., Petkova A.P., Mottillo E.P., et al. (2012). In vivo identification of bipotential adipocyte progenitors recruited by β3-adrenoceptor activation and high-fat feeding. Cell Metab. 15, 480–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepper C., Fan C.M. (2010). Inducible lineage tracing of Pax7-descendant cells reveals embryonic origin of adult satellite cells. Genesis 48, 424–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidell M.E. (2019). Brown adipose tissue in human infants. Handb. Exp. Pharmacol. 251, 107–123. [DOI] [PubMed] [Google Scholar]
- Liu X., Rossmeisl M., McClaine J., et al. (2003). Paradoxical resistance to diet-induced obesity in UCP1-deficient mice. J. Clin. Invest. 111, 399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loncar D. (1991). Convertible adipose tissue in mice. Cell Tissue Res. 266, 149–161. [DOI] [PubMed] [Google Scholar]
- Long J.Z., Svensson K.J., Bateman L.A., et al. (2016). The secreted enzyme PM20D1 regulates lipidated amino acid uncouplers of mitochondria. Cell 166, 424–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long J.Z., Svensson K.J., Tsai L., et al. (2014). A smooth muscle-like origin for beige adipocytes. Cell Metab. 19, 810–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., Zhao J., Meng H., et al. (2019). Adipose tissue-resident immune cells in obesity and type 2 diabetes. Front. Immunol. 10, 1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcelin G., Ferreira A., Liu Y., et al. (2017). A PDGFRα-mediated switch toward CD9high adipocyte progenitors controls obesity-induced adipose tissue fibrosis. Cell Metab. 25, 673–685. [DOI] [PubMed] [Google Scholar]
- Maurer S.F., Fromme T., Mocek S., et al. (2020). Uncoupling protein 1 and the capacity for nonshivering thermogenesis are components of the glucose homeostatic system. Am. J. Physiol. Endocrinol. Metab. 318, E198–E215. [DOI] [PubMed] [Google Scholar]
- Merklin R.J. (1974). Growth and distribution of human fetal brown fat. Anat. Rec. 178, 637–645. [DOI] [PubMed] [Google Scholar]
- Merrick D., Sakers A., Irgebay Z., et al. (2019). Identification of a mesenchymal progenitor cell hierarchy in adipose tissue. Science 364, eaav2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills E.L., Pierce K.A., Jedrychowski M.P., et al. (2018). Accumulation of succinate controls activation of adipose tissue thermogenesis. Nature 560, 102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottillo E.P., Balasubramanian P., Lee Y.H., et al. (2014). Coupling of lipolysis and de novo lipogenesis in brown, beige, and white adipose tissues during chronic β3-adrenergic receptor activation. J. Lipid Res. 55, 2276–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita T., Kobayashi M., Itakura K., et al. (2018). Differential response to caloric restriction of retroperitoneal, epididymal, and subcutaneous adipose tissue depots in rats. Exp. Gerontol. 104, 127–137. [DOI] [PubMed] [Google Scholar]
- Ohashi N., Morino K., Ida S., et al. (2017). Pivotal role of O-GlcNAc modification in cold-induced thermogenesis by brown adipose tissue through mitochondrial biogenesis. Diabetes 66, 2351–2362. [DOI] [PubMed] [Google Scholar]
- Olsen J.M., Csikasz R.I., Dehvari N., et al. (2017). β3-adrenergically induced glucose uptake in brown adipose tissue is independent of UCP1 presence or activity: mediation through the mTOR pathway. Mol. Metab. 6, 611–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero-Diaz B., Rodriguez-Flores M., Sanchez-Munoz V., et al. (2018). Exercise induces white adipose tissue browning across the weight spectrum in humans. Front. Physiol. 9, 1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellet V., Routhier-Labadie A., Bellemare W., et al. (2011). Outdoor temperature, age, sex, body mass index, and diabetic status determine the prevalence, mass, and glucose-uptake activity of 18F-FDG-detected BAT in humans. J. Clin. Endocrinol. Metab. 96, 192–199. [DOI] [PubMed] [Google Scholar]
- Patsouris D., Qi P., Abdullahi A., et al. (2015). Burn induces browning of the subcutaneous white adipose tissue in mice and humans. Cell Rep. 13, 1538–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfannenberg C., Werner M.K., Ripkens S., et al. (2010). Impact of age on the relationships of brown adipose tissue with sex and adiposity in humans. Diabetes 59, 1789–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poher A.L., Veyrat-Durebex C., Altirriba J., et al. (2015). Ectopic UCP1 overexpression in white adipose tissue improves insulin sensitivity in Lou/C rats, a model of obesity resistance. Diabetes 64, 3700–3712. [DOI] [PubMed] [Google Scholar]
- Rajbhandari P., Arneson D., Hart S.K., et al. (2019). Single cell analysis reveals immune cell-adipocyte crosstalk regulating the transcription of thermogenic adipocytes. eLife 8, e49501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzoli M., Frontini A., Gurney A., et al. (2016). Stress-induced activation of brown adipose tissue prevents obesity in conditions of low adaptive thermogenesis. Mol. Metab. 5, 19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodeheffer M.S., Birsoy K., Friedman J.M. (2008). Identification of white adipocyte progenitor cells in vivo. Cell 135, 240–249. [DOI] [PubMed] [Google Scholar]
- Rogers N.H. (2015). Brown adipose tissue during puberty and with aging. Ann. Med. 47, 142–149. [DOI] [PubMed] [Google Scholar]
- Roh H.C., Tsai L.T.Y., Shao M., et al. (2018). Warming induces significant reprogramming of beige, but not brown, adipocyte cellular identity. Cell Metab. 27, 1121–1137.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwald M., Perdikari A., Rulicke T., et al. (2013). Bi-directional interconversion of brite and white adipocytes. Nat. Cell Biol. 15, 659–667. [DOI] [PubMed] [Google Scholar]
- Rothwell N.J., Stock M.J. (1979). A role for brown adipose tissue in diet-induced thermogenesis. Nature 281, 31–35. [DOI] [PubMed] [Google Scholar]
- Ruan H.B., Dietrich M.O., Liu Z.W., et al. (2014). O-GlcNAc transferase enables AgRP neurons to suppress browning of white fat. Cell 159, 306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan H.B., Singh J.P., Li M.D., et al. (2013). Cracking the O-GlcNAc code in metabolism. Trends Endocrinol. Metab. 24, 301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M., Okamatsu-Ogura Y., Matsushita M., et al. (2009). High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 58, 1526–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Gurmaches J., Guertin D.A. (2014). Adipocytes arise from multiple lineages that are heterogeneously and dynamically distributed. Nat. Commun. 5, 4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Gurmaches J., Hsiao W.Y., Guertin D.A. (2015). Highly selective in vivo labeling of subcutaneous white adipocyte precursors with Prx1-Cre. Stem Cell Rep. 4, 541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Gurmaches J., Hung C.M., Guertin D.A. (2016). Emerging complexities in adipocyte origins and identity. Trends Cell Biol. 26, 313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Gurmaches J., Hung C.M., Sparks C.A., et al. (2012). PTEN loss in the Myf5 lineage redistributes body fat and reveals subsets of white adipocytes that arise from Myf5 precursors. Cell Metab. 16, 348–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz T.J., Huang T.L., Tran T.T., et al. (2011). Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proc. Natl Acad. Sci. USA 108, 143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwalie P.C., Dong H., Zachara M., et al. (2018). A stromal cell population that inhibits adipogenesis in mammalian fat depots. Nature 559, 103–108. [DOI] [PubMed] [Google Scholar]
- Seale P., Bjork B., Yang W., et al. (2008). PRDM16 controls a brown fat/skeletal muscle switch. Nature 454, 961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebo Z.L., Jeffery E., Holtrup B., et al. (2018). A mesodermal fate map for adipose tissue. Development 145, dev166801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebo Z.L., Rodeheffer M.S. (2019). Assembling the adipose organ: adipocyte lineage segregation and adipogenesis in vivo. Development 146, dev172098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellayah D., Sikder D. (2014). Orexin restores aging-related brown adipose tissue dysfunction in male mice. Endocrinology 155, 485–501. [DOI] [PubMed] [Google Scholar]
- Shao M., Wang Q.A., Song A., et al. (2019). Cellular origins of beige fat cells revisited. Diabetes 68, 1874–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda K., Luijten I.H., Hasegawa Y., et al. (2015). Genetic and functional characterization of clonally derived adult human brown adipocytes. Nat. Med. 21, 389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidossis L., Kajimura S. (2015). Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. J. Clin. Invest. 125, 478–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidossis L.S., Porter C., Saraf M.K., et al. (2015). Browning of subcutaneous white adipose tissue in humans after severe adrenergic stress. Cell Metab. 22, 219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skorobogatko Y., Dragan M., Cordon C., et al. (2018). RalA controls glucose homeostasis by regulating glucose uptake in brown fat. Proc. Natl Acad. Sci. USA 115, 7819–7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R.E., Roberts J.C. (1964). Thermogenesis of brown adipose tissue in cold-acclimated rats. Am. J. Physiol. 206, 143–148. [DOI] [PubMed] [Google Scholar]
- Solinas G., Summermatter S., Mainieri D., et al. (2004). The direct effect of leptin on skeletal muscle thermogenesis is mediated by substrate cycling between de novo lipogenesis and lipid oxidation. FEBS Lett. 577, 539–544. [DOI] [PubMed] [Google Scholar]
- Song A., Dai W., Jang M.J., et al. (2020). Low- and high-thermogenic brown adipocyte subpopulations coexist in murine adipose tissue. J. Clin. Invest. 130, 247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima K., Ikeda K., Chang H.-Y., et al. (2019). Mitochondrial lipoylation integrates age-associated decline in brown fat thermogenesis. Nat. Metab. 1, 886–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taksler G.B., Rothberg M.B., Braithwaite R.S. (2017). Life-years lost to preventable causes-of-death in the US, 2014. J. Gen. Intern. Med. 32, S240–S241. [Google Scholar]
- Tam C.S., Lecoultre V., Ravussin E. (2012). Brown adipose tissue: mechanisms and potential therapeutic targets. Circulation 125, 2782–2791. [DOI] [PubMed] [Google Scholar]
- Tanuma Y., Tamamoto M., Ito T., et al. (1975). The occurrence of brown adipose tissue in perirenal fat in Japanese. Arch. Histol. Jpn. 38, 43–70. [DOI] [PubMed] [Google Scholar]
- Timmons J.A., Wennmalm K., Larsson O., et al. (2007). Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc. Natl Acad. Sci. USA 104, 4401–4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend K.L., Tseng Y.H. (2014). Brown fat fuel utilization and thermogenesis. Trends Endocrinol. Metab. 25, 168–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trayhurn P. (1995). Fuel selection in brown adipose tissue. Proc. Nutr. Soc. 54, 39–47. [DOI] [PubMed] [Google Scholar]
- Truong M.T., Erasmus J.J., Munden R.F., et al. (2004). Focal FDG uptake in mediastinal brown fat mimicking malignancy: a potential pitfall resolved on PET/CT. Am. J. Roentgenol. 183, 1127–1132. [DOI] [PubMed] [Google Scholar]
- Ukropec J., Anunciado R.P., Ravussin Y., et al. (2006). UCP1-independent thermogenesis in white adipose tissue of cold-acclimated Ucp1‒/‒ mice. J. Biol. Chem. 281, 31894–31908. [DOI] [PubMed] [Google Scholar]
- Ussar S., Lee K.Y., Dankel S.N., et al. (2014). ASC-1, PAT2, and P2RX5 are cell surface markers for white, beige, and brown adipocytes. Sci. Transl. Med. 6, 247ra103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Marken Lichtenbelt W.D., Schrauwen P. (2011). Implications of nonshivering thermogenesis for energy balance regulation in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 301, R285–R296. [DOI] [PubMed] [Google Scholar]
- van Marken Lichtenbelt W.D., Vanhommerig J.W., Smulders N.M., et al. (2009). Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 360, 1500–1508. [DOI] [PubMed] [Google Scholar]
- Virtanen K.A., Lidell M.E., Orava J., et al. (2009). Functional brown adipose tissue in healthy adults. N. Engl. J. Med. 360, 1518–1525. [DOI] [PubMed] [Google Scholar]
- Vishvanath L., MacPherson K.A., Hepler C., et al. (2016). Pdgfrβ+ mural preadipocytes contribute to adipocyte hyperplasia induced by high-fat-diet feeding and prolonged cold exposure in adult mice. Cell Metab. 23, 350–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitali A., Murano I., Zingaretti M.C., et al. (2012). The adipose organ of obesity-prone C57BL/6J mice is composed of mixed white and brown adipocytes. J. Lipid Res. 53, 619–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walden T.B., Hansen I.R., Timmons J.A., et al. (2012). Recruited vs. nonrecruited molecular signatures of brown, "brite," and white adipose tissues. Am. J. Physiol. Endocrinol. Metab. 302, E19–E31. [DOI] [PubMed] [Google Scholar]
- Wallace M., Green C.R., Roberts L.S., et al. (2018). Enzyme promiscuity drives branched-chain fatty acid synthesis in adipose tissues. Nat. Chem. Biol. 14, 1021–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Meyer J.G., Cai W., et al. (2019). Regulation of UCP1 and mitochondrial metabolism in brown adipose tissue by reversible succinylation. Mol. Cell 74, 844–857.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q.A., Tao C., Gupta R.K., et al. (2013). Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat. Med. 19, 1338–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q.A., Tao C., Jiang L., et al. (2015). Distinct regulatory mechanisms governing embryonic versus adult adipocyte maturation. Nat. Cell Biol. 17, 1099–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Yang X. (2017). Inter-organ regulation of adipose tissue browning. Cell. Mol. Life Sci. 74, 1765–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Kissig M., Rajakumari S., et al. (2014). Ebf2 is a selective marker of brown and beige adipogenic precursor cells. Proc. Natl Acad. Sci. USA 111, 14466–14471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Seale P. (2016). Control of brown and beige fat development. Nat. Rev. Mol. Cell Biol. 17, 691–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Cohen P., Spiegelman B.M. (2013). Adaptive thermogenesis in adipocytes: is beige the new brown? Genes Dev. 27, 234–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue B., Coulter A., Rim J.S., et al. (2005). Transcriptional synergy and the regulation of Ucp1 during brown adipocyte induction in white fat depots. Mol. Cell. Biol. 25, 8311–8322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Ruan H.B. (2015). Neuronal control of adaptive thermogenesis. Front. Endocrinol. 6, 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneshiro T., Aita S., Matsushita M., et al. (2011). Age-related decrease in cold-activated brown adipose tissue and accumulation of body fat in healthy humans. Obesity 19, 1755–1760. [DOI] [PubMed] [Google Scholar]
- Yoneshiro T., Wang Q., Tajima K., et al. (2019). BCAA catabolism in brown fat controls energy homeostasis through SLC25A44. Nature 572, 614–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young P., Arch J.R., Ashwell M. (1984). Brown adipose tissue in the parametrial fat pad of the mouse. FEBS Lett. 167, 10–14. [DOI] [PubMed] [Google Scholar]
- Zhang F., Hao G., Shao M., et al. (2018). An adipose tissue atlas: an image-guided identification of human-like BAT and beige depots in rodents. Cell Metab. 27, 252–262.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Cypess A.M., Miao Q., et al. (2014). The prevalence and predictors of active brown adipose tissue in Chinese adults. Eur. J. Endocrinol. 170, 359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingaretti M.C., Crosta F., Vitali A., et al. (2009). The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J. 23, 3113–3120. [DOI] [PubMed] [Google Scholar]
- Zuriaga M.A., Fuster J.J., Gokce N., et al. (2017). Humans and mice display opposing patterns of "browning" gene expression in visceral and subcutaneous white adipose tissue depots. Front. Cardiovasc. Med. 4, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]