Abstract
Macrophomina phaseolina is an important necrotrophic phytopathogenic fungus and cause extensive damage in many oilseed crops. Twelve M.phaseolina isolates with diverse biological phenotypes were selected for a high-throughput sequencing-based metatranscriptomic and bioinformatics analysis to identify viruses infecting M.phaseolina. The analysis identified 40 partial or nearly complete viral genome segments, 31 of which were novel viruses. Among these viral sequences, 43% of the viral genomes were double-stranded RNA (dsRNA), 47% were positive single-stranded RNA (ssRNA+), and the remaining 10% were negative sense-stranded RNA (ssRNA−). The 40 viruses showed affinity to 13 distinct viral lineages, including Bunyavirales (four viruses), Totiviridae (three viruses), Chrysoviridae (five viruses), Partitiviridae (four viruses), Hypoviridae (one virus), Endornaviridae (two viruses), Tombusviridae (three viruses), Narnaviridae (one virus), Potyviridae (one virus), Bromoviridae (one virus), Virgaviridae (six viruses), ‘Fusagraviridae’ (five viruses), and Ourmiavirus (four viruses). Two viruses are closely related to two families, Potyviridae and Bromoviridae, which previously contained no mycovirus species. Moreover, nine novel viruses associated with M.phaseolina were identified in the family Totiviridae, Endornaviridae, and Partitiviridae. Coinfection with multiple viruses is prevalent in M.phaseolina, with each isolate harboring different numbers of viruses, ranging from three to eighteen. Furthermore, the effects of the viruses on the fungal host were analyzed according to the biological characteristics of each isolate. The results suggested that M.phaseolina hypovirus 2, M.phaseolina fusagravirus virus 1-5 (MpFV1-5), M.phaseolina endornavirus 1-2 (MpEV1-2), M.phaseolina ourmia-like virus 1-3 (MpOLV1-3), M.phaseolina mitovirus 4 (MpMV4), and M.phaseolina mycobunyavirus 1-4 (MpMBV1-4) were only detected in hypovirulent isolates. Those viruses associated with hypovirulence might be used as biological control agents as an environmentally friendly alternative to chemical fungicides. These findings considerably expand our understanding of mycoviruses in M.phaseolina and unvailed the presence of a huge difference among viruses in isolates from different hosts in distant geographical regions. Together, the present study provides new knowledge about viral evolution and fungus-virus coevolution.
Keywords: Macrophomina phaseolina, mycovirus, hypovirulence, coinfection, diversity, metatranscriptomic
1. Introduction
Macrophomina phaseolina is a destructive Ascomycota fungus belonging to the family Botryosphaeriaceae, which is capable of infecting more than 500 species of plants and causing large losses on a large-scale of oilseed crops throughout the world, including assoybean (Glycine max) and sesame (Sesame indicum) (Wyllie 1988; Kaur et al. 2012). M.phaseolina is primarily soil-borne in nature and is able to survive for up to 2 years as a saprophyte in soybean root residue (Baird, Watson, and Scruggs 2003) and for approximately 15 years as the microsclerotia under stress condition (Short, Wyllie, and Bristow 1980). It has been documented in infected seeds that M.phaseolina is seed-borne and undergoes seed-to-seedling transmission (Pun, Sabitha, and Valluvaparidasan 1998). The typical symptom of soybean infected by M.phaseolina is known as charcoal rot, forming spindle-shaped lesions with a dark border, a light gray center covered with black pinhead-sized pycnidia and microsclerotia (Ammon, Wyllie, and Brown 1974). In the USA, the annual yield of soybean loss due to charcoal rot was estimated to be 1.98, 0.28, and 0.49 million metric tons in 2003, 2004, and 2005, respectively (Wrather and Koenning 2006). In China, the annual yield loss due to sesame charcoal rot was 10–20%, and oil content was reduced by 4.2–12.6% (Li 1989). In addition, M.phaseolina has the potential to infect immunocompromised patients and can be treated by prophylactic antifungal therapy (Srinivasan et al. 2009).
Mycoviruses are viruses that infect fungi and replicate in their host cells. Most mycovirus genomes consist of double-stranded RNA (dsRNA) or positive single-stranded RNA (ssRNA+) (Ghabrial et al. 2015). Moreover, single-stranded circular DNA virus and negative-stranded RNA viruses were recently reported in filamentous fungi (Yu et al. 2010; Liu et al. 2014; Donaire, Pagán, and Ayllón 2016; Varsani and Krupovic 2017; Wang et al. 2018; Li et al. 2020). Most mycoviruses are transmitted horizontally through hyphal anastomosis and vertically through sexual or asexual spores. However, a gemycircularvirus Sclerotinia sclerotiorum hypovirulence-associated DNA virus 1 (SsHADV-1) was discovered to be transmitted extracellularly through a mycophagous insect (Liu et al. 2016). The development of deep sequencing technology (metagenomics) has greatly promoted the discovery of new viruses. Increasing research has supported that mycoviruses are ubiquitous in the kingdom fungi, including medical, endophytic, entomopathogenic, and phytopathogenic fungi (Liu et al. 2009; Nedveckyte, Pečiulyte, and Buda 2014; Xie and Jiang 2014; Rosseto et al. 2016). The discovery of novel viruses by high throughput sequencing has revealed a remarkable diversity (Mokili, Rohwer, and Dutilh 2012; Roossinck 2014; Shi et al. 2016; García-Pedrajas et al. 2019). Furthermore, a single fungal isolate infected by multiple viruses or different dsRNA elements has frequently been reported, such as in S.sclerotiorum, Rosellinia necatrix, Botrytis cinerea, and Rhizoctonia solani (Xie and Ghabrial 2012; Kondo, Kanematsu, and Suzuki 2013; Bartholomäus, Wibberg, and Winkler 2016; Hao et al. 2018).
Mycoviruses are prevalent in fungus, and they usually remain latent and seldom induce symptoms in the host (Pearson et al. 2009). Some viruses have negative effects on hosts, including an abnormal colony morphology, growth reduction, and altered pigmentation and sexual reproduction (Jiang, Fu, and Ghabrial 2013; Ghabrial et al. 2015). Notably, some viruses are responsible for hypovirulence in phytopathogenic fungi and used as potential biological control resources (Nuss 2005; Xie and Jiang 2014). Cryphonectria hypovirus 1 (CHV1) was successfully utilized to control the disastrous chestnut blight disease in Europe (Milgroom and Cortesi 2004). Moreover, S.sclerotiorum hypovirulence-associated DNA virus 1 (SsHADV1) and R.necatrix megabirnavirus 1 (RnMBV1) have been confirmed to effectively control plant pathogenic fungal diseases under field and greenhouse conditions, respectively (Chiba et al. 2009; Yu et al. 2013). Therefore, the application of viruses to control fungal diseases has great potential as biological control strategies, prompting researchers to prioritize the hypovirulence-associated viruses as candidates (Ghabrial and Suzuki, 2009; Xie and Jiang 2014; Ghabrial et al. 2015; García-Pedrajas et al. 2019).
It has been previously reported that approximately 21.7% of M.phaseolina strains isolated from cluster bean (Cyamopsis tetragonoloba) harbor diverse dsRNA elements, but their sequence dates were not obtained (Arora, Dilbaghi, and Chaudhury 2012). A high-throughput-sequencing-based metatranscriptomics approach was utilized to detect viral sequences from 48 M.phaseolina isolates from soybeans (G.max), and 11 novel viruses with partial or complete genome segments were identified. Those viruses were grouped into six distinct lineages: Bunyavirales, Hypoviridae, Narnaviridae, Virgaviridae, Tombusviridae, and Chrysoviridae (Marzano et al. 2016). Here, we report a metatransciptomic virus survey of M.phaseolina strains isolated from sesame (S.indicum) in different geographical regions of China. We describe thirty-one novel RNA viruses representing twelve distinct virus lineages and confirm their presence in each isolate using reverse transcription PCR (RT-PCR). In a previous study, the complete genome sequences of the novel viruses M.phaseolina victorivirus 1 and M.phaseolina fusagravirus 1 were reported (Wang et al. 2019, 2020). In the present study, the virus diversity of M.phaseolina strains isolated from sesame were analyzed and illustrated that viruses associated with hypovirulence might serve as biological control resources.
2. Materials and methods
2.1 Fungal isolates and culture conditions
For obtaining the field isolates of the M.phaseolina strains, the diseased stems of sesames were cut into 0.5 cm2 samples and soaked for 30 s in 75% ethanol. Then, they were rinsed three times with sterilized water, dried on sterilized absorbent paper, and finally placed in the center of potato dextrose agar (PDA) and cultured at 30°C in the dark for 2 days. A small amount of mycelia was scraped into a centrifuge tube (2 ml), followed by the addition of two sterilized steel balls and 500 μl sterilized water, and ground by the tissue homogenizer (Retsch MM301) for 30 s at 30 times/s. Then, 200 μl of the hyphal suspension was spread on a fresh PDA plate and incubated overnight at 30°C. The next day, a single colony was selected and recultured on a new PDA plate, and the new colony was considered to be a purified isolate. Next, overnight cultures of the purified isolates were stocked on PDA slants at 4°C. All M.phaseolina isolates were grown on PDA at 28–30°C.
2.2 Biological properties and microscopic observation of fungal isolates
Mycelial growth and colony morphology were evaluated according to the procedures described by Wu et al. (2007). The morphology of hyphal tips and fresh weight of the mycelia were recorded following the procedures of Liu et al. (2014).
2.2.1 Pathogenicity assay.
Seedlings of sesame (S.indicum, Cultivar Yuzhi No.13) were used for the pathogenicity tests of M.phaseolina isolates. Seeds were disinfected with sodium hypochlorite for 20 min, followed by rinsing three times with sterilized water and air-drying. Ten seeds were sown in one bowl (10 × 7 × 9 cm, top width × bottom width × height) and then cultured at 30°C under fluorescent light (16 h light/8 h darkness) for 5 weeks until the six-leaf stage. Finally, three seedlings in one bowl with same growth status were selected to test the virulence of M.phaseolina strains. The cut-stem inoculation method was performed with minor modifications (Twizeyimana et al. 2012). The linear extent of stem necrosis (mm) was recorded every two days, four times in total. The significant differences (P < 0.05) in relative area under the disease progress curve (RAUDPC) among the twelve isolates were calculated. The assay treatments were repeated three times with three seedlings each.
2.3 Total RNA extraction and RNA sequencing
Mycelial agar plugs of twelve isolates of M.phaseolina were inoculated on a PDA plate cover with cellophane membranes and cultured at 30°C in the dark for 2–4 days. Mycelial in each dish were harvested using a medicine spoon and stored at −70°C until use. Subsequently, total RNA was extracted from 1.0 g of mycelium using an RNAiso kit (TaKaRa, Dalian, China). Total RNA of twelve isolates was mixed into one sample and sent to Shanghai Bohao Biotechnology Co., Ltd. for high-throughput sequencing on an Illumina HiSeq 2500 platform. The rRNA was depleted using a Ribo-Zero™ rRNA Removal Kit (Illumina, CA, USA).
A sequencing library was prepared from rRNA-depleted total RNA of twelve M.phaseolina isolates. The raw reads were filtered based on default parameters, and clean reads were then matched against genome sequences of M.phaseolina using Bowtie (1.0) software. Now these clean reads were assembled de novo in CLC Genomics Workbench (version: 6.0.4) with scaffolding contig algorithm, word-size = 45, and minimum contig length ≥200. Primary UniGenes were obtained and then CAP3 EST used to splice it to construct the final unigenes. The final UniGenes that annotated with ‘virus’ or ‘viral’ were retrieved and compared to the non-redundant protein sequences (nr) database using BLASTX (https://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastx&PAGE_TYPE=BlastSearch&LINK_LOC=blasthome). Contigs that were identical or complementary to the viral genomic sequence were extracted and identified as potential viral sequences (Wang et al. 2019).
2.4 RT-PCR detection and RACE
The cDNA of M.phaseolina isolates was synthesized following the instructions supplied with the PrimerScriptII™ 1st Strand cDNA Synthesis Kit (TaKaRa, Dalian, China). To confirm the presence of the newly discovered viruses in each isolate, the virus-specific primers were used (Designed by Primer Premier 5.0) (Supplementary Table S1) for RT-PCR to detect specific amplicons of each virus. The amplicons included part of the RdRp gene. RT-PCR was performed in a total volume of 25 μl, containing 9.5 μl deionized water, 12.5 μl Premix Taq (TaKaRa Taq Version 2.0), 1 μl of 100 mM reverse and forward primer of each contig, and 1 μl cDNA template. The annealing temperature and extension time was based on each primer and product size. The RT-PCR products were subjected to agarose gel electrophoresis. Products showing the target band were sent to Sangon Biotech for sequencing and verification. To complete the sequences of the M.phaseolina hypovirus 1 (MpHV1) and M.phaseolina ilar-like virus (MpILV) genomes, the 5′- and 3′-terminal sequences were determined using a SMARTer RACE cDNA Amplification Kit (Clontech, CA). The gene-specific primers (GSPs) GSP-215F1 (5′-GCCACGGGTTGAAACGCCTAA-3′) and GSP-215F2 (5′-GTTGCGTTATGATGGGCATGTACC-3′) were used for 5′-RACE of MpILV as outer and inner primers based on the number of sequences, respectively. The GSPs GSP-215R3 (5′-ACCGACGCATAGTCTCCGCCG-3′) and GSP-215R4 (5′-AGTCTCCGCCGTAGAATCAT-3′) were used for 3′-RACE of MpILV as outer and inner primers, respectively. For MpHV1, GSP-216R2 (5′-ATGTTTCAGATGCGAGTAGGC-3′) and GSP-216R4 (5′-CGAAGTTTCAACACATCATT-3′) were used for 5′-RACE as outer and inner primers based on the number of sequences, respectively.
2.5 Sequence and phylogenetic analyses
The assembled 40 contigs were compared to the NCBI nonredundant database by BLASTX and BLASTn analyses based on the translated amino sequences and nucleotide sequences, respectively (https://blast.ncbi.nlm.nih.gov/Blast.cgi). Sequences were aligned using the CLUSTALX program (Version 2.1), and phylogenetic trees were constructed using the neighbor-joining method with a bootstrap value of 1,000 replicates through the MEGA program (Version 7). Some contigs had incomplete sequences or incomplete RdRp sequences and could not be used for phylogenetic analysis, so the relationships were analyzed based on the BLASTn results. The selected viruses and their accession numbers for phylogenetic analysis in this study are listed in Supplementary Table S2.
2.6 Statistical analysis
Data for the growth rate and RAUDPC of each isolate were subjected to statistical analysis of significant variance among replicates using SAS Version 8.0 (SAS Institute, Cary, NC, 1999). Treatment means for each of these parameters for the tested isolates were compared using the least-significant-difference test at P < 0.05.
3. Results
3.1 Metatranscriptomic identification of mycoviruses infecting isolates of M.phaseolina
Twelve M.phaseolina isolates from the main sesame planting areas of China, including Henan province, Anhui province, Hubei province, and Jiangxi province were screened using a metatranscriptomic approach (Supplementary Table S3). After filtering unqualified reads, 7.6 × 107 paired-end reads with lengths >20 nt were obtained. After, these reads were assembled into longer contigs and blasted against the NCBI nonredundant database. Forty-one putative viral sequences were identified that represented partial or complete genome segments. The provisional names and most similar viruses are listed in Table 1. RT-PCR amplification further confirmed that these putative viruses were originated from ten isolates, while two isolates harbored no viruses (Fig. 1). More than three viruses have been found to infect a single isolate, whereas none of the isolates were infected by only a single virus. The forty putative viral sequences had identities ranging from 26% to 99% to previously reported viruses. Interestingly, thirty-one putative viral sequences among them were novel viruses, rather than known viruses reported in M.phaseolina isolates from North America (Supplementary Table S4). The majority (47%) of the putative viruses were predicted to present ssRNA+ genomes and to be related to the viruses in Hypoviridae (one novel virus sequence), Endornaviridae (two novel virus sequences), Virgaviridae (seven virus sequences), Narnaviridae (one novel virus sequence), Potyviridae (one novel virus sequence), Bromoviridae (one novel virus sequence), Ourmiavirus (four novel virus sequences), and Tombusviridae (three novel virus sequences). Additionally, 43% of the total viruses were predicted to have dsRNA genomes and related to the viruses in Totiviridae (three novel virus sequences), Chrysoviridae (five novel virus sequences), Partitiviridae (four novel virus sequences), and ‘Fusagraviridae’ (five novel sequences); 10% of the total viruses were predicted to have ssRNA− genomes and related to the viruses in order Bunyavirales (four novel virus sequences) (Table 1). Among the 31 putative viral sequences, two showed affiliations to two families (Potyviridae and Bromoviridae) that previously contained no mycovirus species.
Table 1.
Assembled sequences with similarity to those of previously described viruses.
| Contig number | Accession No. | Contig length | Name of putative viruses | Best match | % aa identity | Genome type | Family/genus | Reference |
|---|---|---|---|---|---|---|---|---|
| Contig 634CCG | MT023007 | 5,194 |
Macrophomina phaseolina victorivirus 2 |
Sphaeropsis sapinea RNA virus 2(NC_001964.1) | 68 | dsRNA | Totiviridae | Preisig et al. (1998) |
| Contig3764PG | MT024302 | 515 |
Macrophomina phaseolina victorivirus 3 |
Alternaria arborescens victorivirus 1 (BAU71113.1) | 70 | dsRNA | Totiviridae | Komatsu et al. (2016) |
| Contig 11CCG | MT035905 | 3,780 |
Macrophomina phaseolina chrysovirus 2 RNA1 |
Aspergillus fumigatus chrysovirus |
48 | dsRNA | Chrysoviridae | Ejmal et al. (2018) |
| Contig2262PG | MT035906 | 1,463 |
Macrophomina phaseolina chrysovirus 3 |
Aspergillus thermomutatus chrysovirus 1(MF045841.1) | 42 | dsRNA | Chrysoviridae | – |
| Contig 566PG | MT035907 | 1,682 |
Macrophomina phaseolina chrysovirus 4 RNA1 |
Colletotrichum fructicola chrysovirus 1 |
45 | dsRNA | Chrysoviridae | – |
| Contig1222PG | MT035908 | 458 |
Macrophomina phaseolina chrysovirus 4 RNA2 |
Botryosphaeria dothidea chrysovirus 1 (KF688738.1) | 35 | dsRNA | Chrysoviridae | – |
| Contig 3CCG | MT035909 | 2,271 | Macrophomina phaseolina chrysovirus 2 RNA2 |
Coniothyrium diplodiella chrysovirus 1 (QDB74973.1 ) |
27 | dsRNA | Chrysoviridae | – |
| Contig 168CCG | MT035910 | 1,650 |
Macrophomina phaseolina partitivirus 1 RNA1 |
Ustilaginoidea virens partitivirus 3(KC469948) | 69 | dsRNA | Partitiviridae | Zhong et al. (2014) |
| Contig 382CCG | MT035911 | 1,367 |
Macrophomina phaseolina partitivirus 1 RNA2 |
Ustilaginoidea virens partitivirus 2 (NC_021873.1) | 60 | dsRNA | Partitiviridae | Zhong et al. (2014) |
| Contig 397CCG | MT035912 | 1,741 |
Macrophomina phaseolina partitivirus 2 RNA1 |
Fusarium solani virus 1 (NP_624350.1) | 55 | dsRNA | Partitiviridae | Nogawa et al. (1996) |
| Contig2423CCG | MT035913 | 1,636 |
Macrophomina phaseolina partitivirus 2 RNA2 |
Verticillium albo-atrum partitivirus-1 |
50 | dsRNA | Partitiviridae | Cañizares et al. (2014) |
| Contig 22CCG | MT035914 | 9,024 |
Macrophomina phaseolina fusagravirus 2 |
Macrophomina phaseolina double-stranded RNA virus 2 (ALD89097.1) |
63 | dsRNA | Fusagraviridae | Marzano et al. (2016) |
| Contig116CCG | MT035915 | 9,328 |
Macrophomina phaseolina fusagravirus 3 |
Macrophomina phaseolina double-stranded RNA virus 2 (ALD89097.1) |
89 | dsRNA | Fusagraviridae | Marzano et al. (2016) |
| Contig23CCG | MT035916 | 8,930 |
Macrophomina phaseolina fusagravirus 4 |
Macrophomina phaseolina double-stranded RNA virus 2 (ALD89097.1) |
96 | dsRNA | Fusagraviridae | Marzano et al. (2016) |
| Contig110CCG | MT035917 | 8,954 |
Macrophomina phaseolina fusagravirus 5 |
Macrophomina phaseolina double-stranded RNA virus 2 (ALD89097.1) |
96 | dsRNA | Fusagraviridae | Marzano et al. (2016) |
| Contig 598CCG | MT062421 | 9,933 |
Macrophomina phaseolina mycobunyavirus 1 |
Botrytis cinerea negative-stranded RNA virus 1(NC_028466) | 29 | ssRNA− | Bunyavirales | Donaire et al. (2016) |
| Contig 156PG | MT062422 | 2,143 |
Macrophomina phaseolina mycobunyavirus 2 |
Botrytis cinerea negative-stranded RNA virus 1(NC_028466) | 35 | ssRNA− | Bunyavirales | Donaire et al. (2016) |
| Contig 80CCG | MT062423 | 10,651 |
Macrophomina phaseolina mycobunyavirus 3 |
Macrophomina phaseolina negative-stranded RNA virus 1 (ALD89106.2) |
92 | ssRNA− | Bunyavirales | Marzano et al. (2016) |
| Contig2249PG | MT062424 | 3,034 |
Macrophomina phaseolina mycobunyavirus 4 |
Macrophomina phaseolina negative-stranded RNA virus 1 (ALD89106.2) |
99 | ssRNA− | Bunyavirales | Marzano et al. (2016) |
| Contig216CG | MT062425 | 1,3364 |
Macrophomina phaseolina hypovirus 2 |
Macrophomina phaseolina hypovirus 1 (ALD89099.1) | 89 | ssRNA+ | Hypoviridae | Marzano et al. (2016) |
| Contig1085PG | MT062426 | 8,310 |
Macrophomina phaseolina endornavirus 1 |
Hordeum vulgare alphaendornavirus (MN_ 107383.1) |
33 | ssRNA+ | Endornaviridae | Candresse et al. (2016) |
| Contig371PG | MT062427 | 8,245 |
Macrophomina phaseolina endornavirus 2 |
Hordeum vulgare alphaendornavirus (MN_ 107383.1) |
31 | ssRNA+ | Endornaviridae | Candresse et al. (2016) |
| Contig101PG | MT062435 | 3,796 |
Macrophomina phaseolina umbra-like virus 1 |
Sclerotinia sclerotiorum umbra-like virus 1 (NC_030203.1) | 41 | ssRNA+ | Tombusviridae | Marzano et al. (2016) |
| Contig 103PG | MT062436 | 388 |
Macrophomina phaseolina umbra-like virus 2 |
Sclerotinia sclerotiorum umbra-like virus 1 (NC_030203.1) | 45 | ssRNA+ | Tombusviridae | Marzano et al. (2016) |
| Contig 1PG | MT062437 | 3,518 |
Macrophomina phaseolina umbra-like virus 3 |
Sclerotinia sclerotiorum umbra-like virus 1 (NC_030203.1) | 36 | ssRNA+ | Tombusviridae | Marzano et al. (2016) |
| Contig 7CCG | MT062428 | 1,884 |
Macrophomina phaseolina ourmia-like virus 1 |
Erysiphe necator associated ourmia-like virus 8 |
44 | ssRNA+ | Ourmiavirus | Nerva et al. (2019) |
| Contig 179CCG | MT062429 | 2,218 |
Macrophomina phaseolina ourmia-like virus 2 |
Acremonium sclerotigenum ourmia-like virus 1 |
41 | ssRNA+ | Ourmiavirus | Nerva et al. (2019) |
| Contig 18CCG | MT062430 | 2,745 |
Macrophomina phaseolina ourmia-like virus 2-A |
Acremonium sclerotigenum ourmia-like virus 1 |
40 | ssRNA+ | Ourmiavirus | Nerva et al. (2019) |
| Contig 12CCG | MT062431 | 2,833 |
Macrophomina phaseolina ourmia-like virus 3 |
Neofusicoccum parvum ourmia-like virus 1 |
69 | ssRNA+ | Ourmiavirus | Nerva et al. (2019) |
| Contig 51CCG | MT062432 | 2,567 |
Macrophomina phaseolina mitovirus 4 |
Rhizoctonia solani mitovirus 10 (KP900896) | 94 | ssRNA+ | Narnaviridae | Marzano et al. (2016) |
| Contig 69PG | MT062433 | 7,296 |
Macrophomina phaseolina poty-like virus |
Watermellon mosaic virus (KU240107) | 26 | ssRNA+ | Potyviridae | – |
| Contig 215CG | MT062434 | 3,450 |
Macrophomina phaseolina ilar-like virus |
Tomato necrotic streak virus (KT779204) | 37 | ssRNA+ | Bromoviridae | Badillo-Vargas et al. (2016) |
| Contig 334PG | MT062438 | 3,671 | Macrophomina phaseolina tobamo-like virus 2 |
Botryosphaeria dothidea tobamo-like virus |
78 | ssRNA+ | Virgaviridae | – |
| Contig 66PG | MT062439 | 2,073 |
Macrophomina phaseolina tobamo-like virus 1a–A |
Macrophomina phaseolina tobamo-like virus 1a(KP900897) | 99 | ssRNA+ | Virgaviridae | Marzano et al. (2016) |
| Contig 76PG | MT062440 | 3,417 |
Macrophomina phaseolina tobamo-like virus –A |
Macrophomina phaseolina tobamo-like virus (YP_009109559.1) | 88 | ssRNA+ | Virgaviridae | Marzano et al. (2016) |
| Contig 49PG | MT062441 | 3,155 |
Macrophomina phaseolina tobamo-like virus -B |
Macrophomina phaseolina tobamo-like virus (YP_009109559.1) | 92 | ssRNA+ | Virgaviridae | Marzano et al. (2016) |
| Contig 15PG | MT062442 | 4,677 |
Macrophomina phaseolina tobamo-like virus C |
Macrophomina phaseolina tobamo-like virus (YP_009109559.1) | 93 | ssRNA+ | Virgaviridae | Marzano et al. (2016) |
| Contig 47PG | MT062443 | 2,381 |
Macrophomina phaseolina tobamo-like virus -D |
Macrophomina phaseolina tobamo-like virus(YP_009109559.1) | 94 | ssRNA+ | Virgaviridae | Marzano et al. (2016) |
The sequences are complete genomes.
The sequences are complete coding sequence.
The sequences are partial genome.
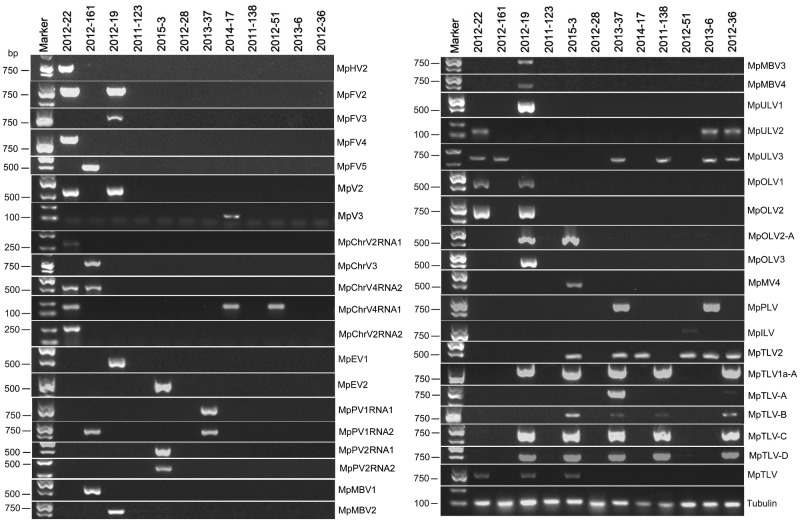
Figure 1.
The reverse transcription (RT)-PCR detection of mycovirus contigs in Macrophomina phaseolina strains with primer pairs and predicted sizes of amplicons are listed in Supplementary Table S1. Lane M, DL2000 DNA Marker (Takara Bio Inc., Japan); Lane 1 to 12, 12 M.phaseolina strains tested in this study (see Supplementary Table S1 for details); abbreviates of viruses are on the far right side of the lane.
3.2 Predicted four viruses characterized in the order Bunyavirales
Four sequences, contig 598, contig 80, contig 156, and contig 2249, showed similarity to ssRNA− viruses in the order Bunyavirales, which contains twelve families of viral genome with three unique molecules. Contig 598 was identified from strain 2012-161, and the other three contigs were identified form isolate 2012-019 by RT-PCR amplification (Fig. 1). The assembled sequences ranged in lengths from 2,143 to 10,651 nt, and all of them contained one large open reading frame (ORF).
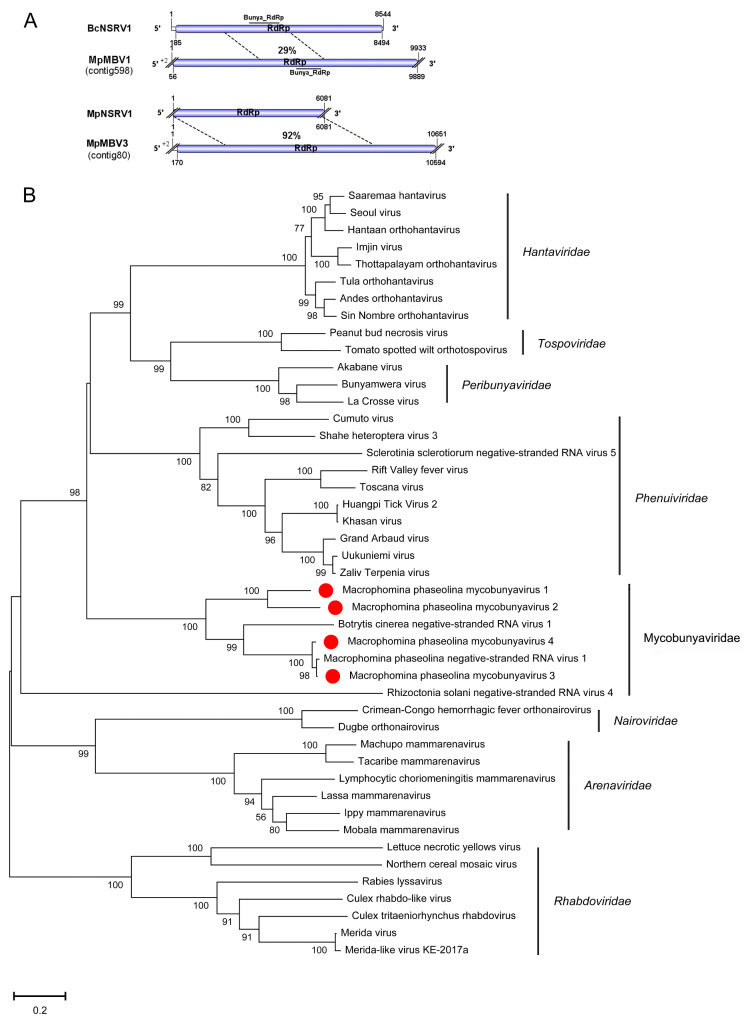
The genomes of contig 598 and contig 80 were nearly complete and longer than the most similar viruses in the NCBI database. Contig 598 consisted of 9,889 nt and had a large ORF encoding putative RNA-dependent RNA polymerase (RdRp) of 3,277 aa, and this RdRp included domains for Bunya_RdRp (pfam04196). Blastp analysis revealed the putative protein encoded by contig 598 was similar to the RdRp of B.cinerea negative-stranded RNA virus 1 with 29% identity (Table 1). Contig 80 was 10,651 nt and had a large ORF encoding a putative RdRp of 3,525 aa. The Blastp analysis results showed that this putative protein of contig 80 was similar to the RdRp of M.phaseolina negative-stranded RNA virus 1 with 92% identity (Table 1). The other two viral sequences, contig 156 and contig 2249, were 2,143 nt and 3,034 nt and showed similarity to B.cinerea negative-stranded RNA virus 1 and M.phaseolina negative-stranded RNA virus 1 with 35% and 98% identity, respectively (Table 1). Moreover, these two contigs had one incomplete ORF encoding a putative RdRp that all included the domain Bunya_RdRp (pfam04196). Both BcNSRV1 and MpNSRV1 were phylogenetically related to members of the order Bunyavirales (Donaire, Pagán, and Ayllón 2016; Marzano et al. 2016). These results suggest that contig 598, contig156, contig 80, and contig 2249 likely represented novel viruses in the order Bunyavirales, which we named M.phaseolina mycobunyvirus 1 (MpMBV1), M.phaseolina mycobunyvirus 2 (MpMBV2), M.phaseolina mycobunyvirus 3 (MpMBV3), and M.phaseolina mycobunyvirus 4 (MpMBV4), respectively. The MpMBV4 likely represent one isolate of MpNSRV1. Furthermore, a phylogenetic analysis of the conserved RdRp domain among MpMBV1, MpMBV2, MpMBV3, MpMBV4, and other selected nsRNA viruses, was conducted, and a phylogenetic tree was constructed (Fig. 2). The tree suggested that MpMBV1-3 was a new virus in the order Bunyavirales. Furthermore, Picarelli et al. (2019) proposed a new family name ‘Mycobunyaviridae’ that included mycoviruses B.cinerea negative-stranded RNA virus and M.phaseolina negative-stranded RNA virus. According to phylogenetic analysis the mycoviruses MpMBV1-4 also belong to this family ‘Mycobunyaviridae’.
Figure 2.
Genome organizations and phylogenetic analysis of the putative negative-stranded RNA virus genomes detected from Macrophomina phaseolina. (A) Comparison of the organizations of putative negative-stranded RNA viruses M.phaseolina mycobunyavirus 1 (MpMBV1) and M.phaseolina mycobunyavirus 3 (MpMBV3) to Botrytis cinerea negative-stranded RNA virus 1 (BcNSRV1), and M.phaseolina negative-stranded RNA virus 1 (MpNSRV1), respectively. Open reading frame (ORF) is shown as colored box. The horizontal line represent conserved domain. The numbers in the dotted line represent identity between viruses. (B) Neighbor joining tree depicting the relationships of the predicted RdRp amino acid sequences were aligned with CLUSTALX, and trees were inferred using MEGA-X. The viruses marked with red dot are found in M.phaseolina.
3.3 One novel virus in the family Hypoviridae
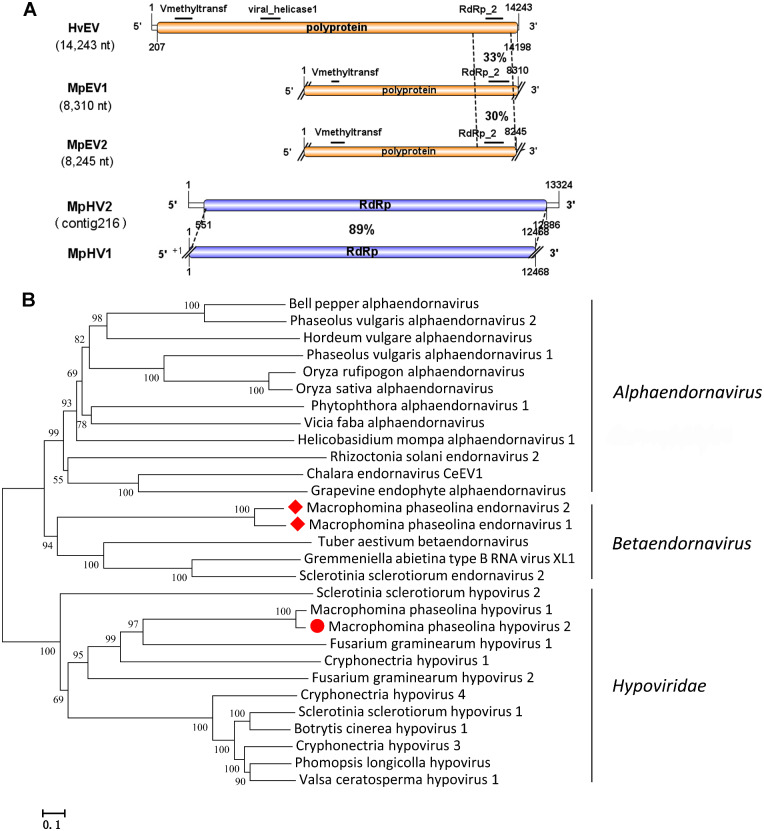
Viruses in the family Hypoviridae typically have positive single-stranded RNA genomes of 9–13 kb with one or two ORFs (Suzuki et al. 2018). One hypovirus has been reported in M.phaseolina named M.phaseolina hypovirus 1 (MpHV1; KP900893; 12,468 nt) (Marzano et al. 2016). In this study, one viral genome sequence was similar to the members of the family Hypoviridae. Contig 216 was identified in isolate 2012-022 (Fig. 1). Contig 216 (13,324 nt) contained one large ORF (551–12,886 nt) encoding a RdRp of 4,111 aa and a 5′-UTR and 3′-UTR of 550 nt and 438 nt, respectively. The predicted amino acid sequence of RdRp was most similar to the RdRp of the MpHV1with 89% identity (Table 1). Both contig 216 and MpHV1 contained domains DUF3525 (pfam12039) and DEXDc (smart00487). In addition, contig216 also included domains for DEXHc_RE_I_III_res (cd18032), ResIII (pfam04851), DEAH_box_HrpB (TIGR1970), and SSL2 (COG1061). According to the species demarcation criteria of ICTV that two species share less than 60% aa sequence identity (Suzuki et al. 2018). Contig 216 represents strain of MpHV1 originally reported from North America. However, contig 216 also contains four different domains which MpHV1 does not. Thus, contig 216 might represented a novel virus in the family Hypoviridae, and we named this contig M.phaseolina hypovirus 2 (MpHV2). The phylogenetic tree of the MpHV2 and other related hypoviruses were constructed, and the results showed that MpHV2 was a novel virus in the family Hypoviridae (Fig. 3).
Figure 3.
Genome organizations and phylogenetic analysis of the putative viruses in family Endornaviridae and Hypoviridae. (A) Comparison of the organizations of putative viruses Macrophomina phaseolina endornavirus 1 (MpEV1) and M.phaseolina endornavirus 2 (MpEV2) to Hordeum vulgare endornavirus (HvEV1), and M.phaseolina Hypovirus 2 (MpHV2) to M.phaseolina Hypovirus 1 (MpHV1). (B) Predicted RdRp amino acid sequences of endornaviruses and hypovirus were aligned and phylogenetic tree were constructed as described in Fig. 1. The viruses marked with red dot or red diamonds are found in M.phaseolina.
3.4 Four novel viruses characterized in the family Chrysoviridae
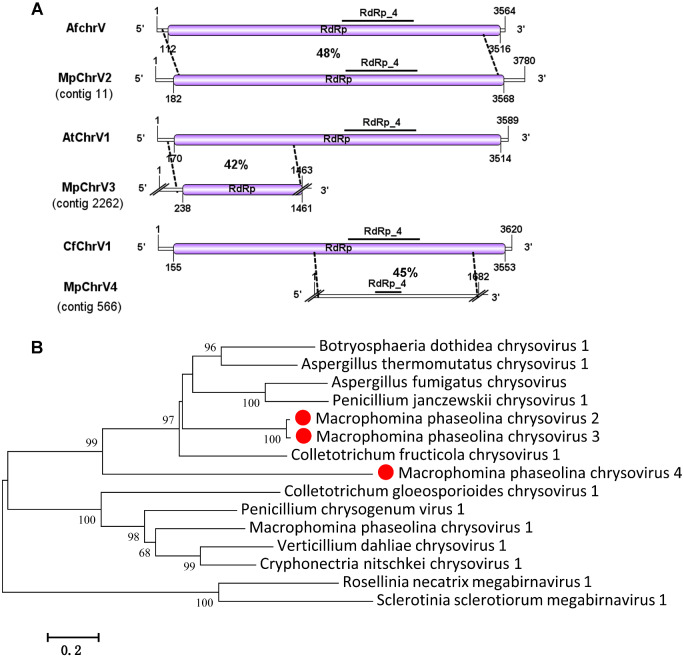
The family Chrysoviridae contained only one genus, Chrysovirus, of which the typical characteristic is inclusion in the genome of four unrelated linear, separately encapsidated dsRNA segments of approximately 2.4-3.6 kb in size. The largest segment dsRNA-1 of chrysovirus encodes a virion-associated RNA polymerase and dsRNA-2 encodes major coat protein (CP), but dsRNA-3 and dsRNA-4 encode unknown functional protein (Ghabrial et al. 2015). Previously, a chrysovirus has been discovered in M.phaseolina that was named as M.phaseolina chrysovirus 1 (MpChrV1) (Marzano et al. 2016). In this study, five viral sequences contig 11, contig 2622, contig 566, contig 1222, and contig 3, showed similarity to members of the family Chrysoviridae. Contig 11 (3,780 nt) contained a large ORF predicted to encoded a RdRp of 1,128 aa and that had 48% identity to the RdRp encoded by Aspergillus fumigatus chrysovirus 1. Moreover, Contig11 also showed 26% identity to the RdRp encoded by MpChrV1. This putative RdRp contained the RdRp_4 (pfam02123) domain, and we named this novel virus M.phaseolina chrysovirus 2 RNA1 (MpChrV2RNA1). Contig 2622 (1,463 nt) contained an incomplete ORF encoding a putative protein of 407 aa and showed similarly to the protein of Aspergillus thermomutatus chrysovirus 1 with 42% identity (Table 1). Thus, we named it M.phaseolina chrysovirus3 (MpChrV3). Contig 566 (1,682 nt) contained an incomplete ORF encoding a putative RdRp of 317 aa, which contained the RdRp_4 domain. Blastp analysis showed that the putative protein was similar to that of Colletotrichum fructicola chrysovirus 1 with 45% identity (Table 1). Contig 566 was similar to the RdRp of MpChrV1 with 31% identity match. We named it M.phaseolina chrysovirus 4 RNA1 (MpChrV4RNA1). The putative viral sequence of contig 1222 (458 nt) was not complete but showed 35% identity to a hypothetical protein encoded by Botryosphaeria dothidea chrysovirus 1 (Table 1), and we named it M.phaseolina chrysovirus 4 RNA2 (MpChrV4RNA2). Contig 3 (2,271 nt) contained one large ORF encoding a hypothetical protein of 666 aa. The predicted amino sequence of protein was 27% identical to the protein encoded by Coniothyrium diplodiella chrysovirus 1 (Table 1), and we named it M.phaseolina chrysovirus 2 RNA2 (MpChrV2RNA2).
Using RT-PCR amplification to detect the four viruses in each isolate, we showed that MpChrV2 was harbored in isolate 2012-022; MpChrV3 was in isolate 2012-161; MpChrV4 was in isolate 2012-022, 2012-161, 2014-017, and 2012-051 (Fig. 1). A comprehensive and phylogenetic analysis also confirmed that MpChrV2 and MpChrV3 are new virus species in the genus Chrysovirus, family Chrysoviridae (Fig. 4).
Figure 4.
Genome organizations and phylogenetic analysis of the putative viruses in family Chrysoviridae detected from Macrophomina phaseolina. (A) Comparison of the organizations of putative viruses M.phaseolina chrysovirus 2 (MpChrV2), M.phaseolina chrysovirus 3 (MpChrV3), and M.phaseolina chrysovirus 4 (MpChrV4) to Aspergillus fumigatus chrysoviurs, Aspergillus thermomutatus chrysoviurs 1, and Colletotrichum fructicola chrysoviurs 1, respectively. (B) Predicted RdRp amino acid sequences of chrysovirus were aligned and phylogenetic tree were constructed as described in Fig. 1. The viruses marked with red dot were found in M.phaseolina.
3.5 Two novel viruses characterized in the family Totiviridae
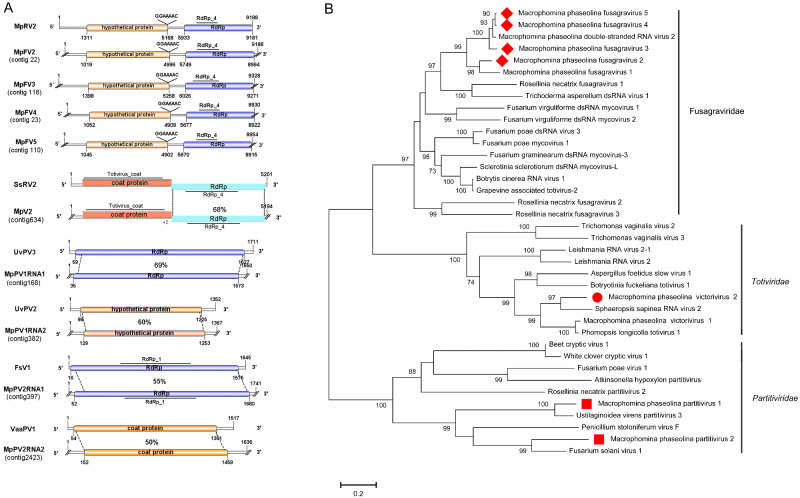
The family Totiviridae contains four genera of viruses with double-stranded RNA genomes of 4.6-7.0 kbp. The two large ORFs of Totiviridae viruses were usually overlapping, and the 5′-proximal ORF encoded the major CP, while the 3′-proximal ORF encoded RdRp (Wickner, 2012). Two viral sequences, contig 634 (5,194 nt) and contig 3764 (515 nt), were similar to members of the family Totiviridae. RT-PCR results suggested that contig 634 was harbored in isolate 2012-019 and 2012-022, and contig 3764 was harbored in isolate 2014-017 (Fig. 1). Contig 634 had two complete ORFs and overlapped at the tetranucleotide AUGA; those ORFs encoded CP and RdRp of 766 aa and 826 aa, respectively. Both CP and RdRp showed similarly to those encoded by Sphaeropsis sapinea RNA virus 2, with 78% and 68% identity, respectively (Table 1). Furthermore, the putative CP contained the Totivirus_coat (pfam05518) domain, and the putative RdRp contained the RdRp_4 domain. Contig 634 represented a novel victorivirus that we named M.phaseolina victorivirus 2 (MpV2).
Because the putative viral sequence of contig3764 was incomplete and only 515 nt, Blastp analysis showed a predicted protein similar to that of Alternaria arborescens victorivirus 1 with 70% identity (Table 1). The predicted protein contained a Totivirus_coat domain. Thus, we named it M.phaseolina victorivirus 3 (MpV3).
Furthermore, M.phaseolina victorivirus 1 was also identified in isolate 2012-019 (Wang et al. 2020). We performed pairwise comparisons and phylogenic analysis of RdRp among MpV2, MpV1 and other selected viruses and constructed a phylogenetic tree (Fig. 5B).The tree highlighted that MpV2 was a new victorivirus in the family Totiviridae.
Figure 5.
Genome organizations and phylogenetic analysis of the putative viruses in family ‘Fusagraviridae’, Totiviridae, and Partitiviridae detected from Macrophomina phaseolina. (A) Comparison of the organizations of putative viruses M.phaseolina fusagravirus 2 (MpFV2), M.phaseolina fusagravirus 3 (MpFV3), M.phaseolina fusagravirus 4 (MpFV4), and M.phaseolina fusagravirus 5 (MpFV5) to M.phaseolina double-stranded RNA virus 2 (MpRV2). Comparison of the organizations of putative viruses M.phaseolina victorivirus 2 (MpV2) to Sphaeropsis sapinea RNA virus 2 (SsRV2). Comparison of the organizations of putative viruses M.phaseolina partitivirus 1 (MpPV1) and M.phaseolina partitivirus 2 (MpPV2) to Ustilaginoidea virens partitivirus 3 (UvPV3) and Fusarium solani virus 1 (FsV1), respectively. (B) Predicted RdRp amino acid sequences of MpFV2, MpFV3, MpFV4, MpFV5, MpV2, MpPV1, and MpPV2 were aligned and phylogenetic tree were constructed as described in Fig. 1. The viruses marked with red diamonds, red dots, and red squares were found in M.phaseolina.
3.6 Three novel viruses characterized in the family Partitiviridae
The family Partitiviridae is divided into five genera, Alphacryptovirus, Betacruptovirus, Gammapartitivirus, Deltapartitivirus, and Cryspovirus. The members of the Partitiviridae have two linear dsRNA segments ranging from 1.4 to 2.4 kbp in size; the larger dsRNA segment (dsRNA 1) usually encodes virion-associated RNA polymerase, whereas the smaller one (dsRNA 2) encodes CP. In this study, four contigs were predicted to encode a single protein related to the structural or nonstructural proteins of viruses in the family Partitiviridae (Table 1). Contig 168 was 1,650 nt, with one complete ORF encoding a RdRp of 512 aa. The Blastp analysis showed that the putative RdRp was similar to RdRp of Ustilaginoidea virens partitivirus 3 with 69% identity (Fig. 5A). Contig 382 was 1,367 nt, with a complete ORF encoding a 374 aa hypothetical protein. Blastp analysis suggested that this hypothetical protein was similar to the hypothetical protein of Ustilaginoidea virens partitivirus 2 with 60% identity (Fig. 5A). Furthermore, contig168 and contig 382 were identified in isolates 2012-161 and 2013-037 (Fig. 1). The results showed that contigs 168 and 382 represented two new partitiviruses, and we designated them M.phaseolina partitivirus 1 RNA 1 (MpPV1RNA1) and M.phaseolina partitivirus 1 RNA 2 (MpPV1RNA2), respectively.
Contig 397 was 1,741 nt, with a large ORF encoding an RdRp of 542 aa. The predicted amino acid sequence of RdRp showed 55% identical to the RdRp of Fusarium solani virus 1 (Fig. 5A). This RdRp contained the RdRp_1 (pfam00680) domain. Contig 2423 was 1,636 nt, with one complete ORF encoding a CP of 435 aa. This predicted CP was 50% identical to the CP of Verticillium albo-atrum partitivirus-1 (Fig. 5A). Furthermore, contig 397 and contig 2423 were identified in isolate 2015-003 (Fig. 1). The results suggested that contig 397 and contig 2423 represented two new partitiviruses of M.phaseolina, and we named them M.phaseolina partitivirus 2 RNA 1 (MpPV2RNA1) and M.phaseolina partitivirus 2 RNA 2 (MpPV2RNA2), respectively.
The results suggested that M.phaseolina hosted various partitiviruses. A phylogeneic analysis of the conserved RdRp domain of MpPV1, MpPV2, and other related viruses was conducted, and a phylogenetic tree was constructed. The tree further supported that MpPV1 and MpPV2 were new virus species in the family Partitiviridae (Fig. 5B).
3.7 Characterization of four double-stranded RNA viruses in the family ‘Fusagraviridae’
Wang et al. (2016) proposed to establish a new family ‘Fusagraviridae’, it contain the unassigned dsRNA virus infecting fungus. The genome size ranges from 8,112 nt to 9,518 nt, and the genomic structure exhibits a putative -1 PRF translational recoding mechanism. Some reported viruses have been assigned to this family, such as Fusarium poae dsRNA virus 2 (Wang et al. 2016), S.sclerotiorum dsRNA mycovirus-L (Liu et al. 2009), B.cinerea RNA virus 1 (Yu et al. 2015), M.phaseolina dsRNA virus 2 (MpRV2) (Marzano et al. 2016), M.phaseolina fusagravirus 1 (Wang et al. 2019), Fusarium virguliforme dsRNA mycovirus 1, and F.virguliforme dsRNA mycovirus 2 (Marvelli et al. 2014). In our study, five contigs showed high identity to members of the family ‘Fusagraviridae’ (Table 1).
Contig 22 was 9,024 nt and had two complete ORFs encoding a hypothetical protein and RdRp of 1,326 aa and 1,270 aa, respectively. Blastp analysis suggested that the hypothetical protein and RdRp were similar to those of M.phaseolina dsRNA virus 2 with 57% and 62% identity, respectively (Fig. 5A). Thus, contig 22 represented a new fusagravirus, and we named it M.phaseolina fusagravirus 2 (MpFV2).
Contig 116 was 9,328 nt and had two complete ORFs encoding a hypothetical protein and RdRp of 1,287 aa and 1,316 aa, respectively. Blastp analysis suggested that the hypothetical protein and RdRp were similar to those of M.phaseolina dsRNA virus 2 with 88% and 87% identity, respectively (Fig. 5A). We named this new fusagravirus M.phaseolina fusagravirus 3 (MpFV3).
Contig 23 (8,930 nt) had two complete ORFs encoding a hypothetical protein and RdRp of 1,300 aa and 1,082 aa, respectively. The putative amino acid sequence of the hypothetical protein and RdRp were similar to the hypothetical protein and RdRp of M.phaseolina dsRNA virus 2 with 92% and 95% identity, respectively (Fig. 5A). Thus, we named it M.phaseolina fusagravirus 4 (MpFV4). Contig 110 (8,954 nt) contained two complete ORFs encoding a hypothetical protein and RdRp of 1,300 aa and 1,208 aa, respectively. The hypothetical protein and RdRp were similar to those of M.phaseolina dsRNA virus 2 with 91% and 96% identity, respectively (Fig. 5A). We named it M.phaseolina fusagravirus 5 (MpFV5). The Blastp results revealed that MpFV4 and MpFV5 both represented isolates of MpRV2.
Moreover, the four contigs all showed a putative shifting heptamer sequence ‘GGAAAAC’ located upstream of the stop codon of the hypothetical protein. The putative amino acid of RdRp of those four fusagraviruses all contained the RdRp_4 (pfam02123) domain. RT-PCR amplification suggested that MpFV2 was harbored in isolates 2012-022 and 2012-019. MpFV3 was harbored in 2012-019. MpFV4 was harbored in isolate 2012-022. And MpFV5 was harbored in isolate 2012-161 (Fig. 1). Moreover, the MpFV 2 and MpFV4 were both harbored in isolate 2012-022. The amino acid sequence identity of hypothetical protein and RdRp between MpFV 2 and MpFV4 were 59.56% and 60.43% (Supplementary Table S5), respectively. Similarly, the MpFV 2 and MpFV3 were both harbored in isolate 2012-019, the amino acid sequence identity of hypothetical protein and RdRp between MpFV 2 and MpFV3 were 57.39% and 60.18% (Supplementary Table S5), respectively. This result revealed that M.phaseolina hosted various fusagraviruses.
Furthermore, a phylogenetic analysis was conducted based on the conserved RdRp domains of MpFV2, MpFV3, MpFV4, MpFV5, MpFV1, MpRV1, and other selected unassigned dsRNA viruses (Fig. 5B). The phylogenetic tree highlighted that MpFV2 and MpFV3 were new virus species in the family ‘Fusagraviridae’, and MpFV4 and MpFV5 represented strains of M.phaseolina-infecting fusagraviruses originally reported from North America (Marzano et al. 2016).
3.8 Characterization of two novel viruses in the family Endornaviridae
To date, numerous endornavirus have been identified across various timescales and from diverse organisms, including organisms of plant, fungal and oomycetes origin (Aiewsakun and Katzourakis 2015). The family consists of two genera, Alphaendornavirus and Betaendornavirus. The members in Betaendornavirus infect ascomycete fungi (Rodrigo et al, 2019). The typical features of endornavirus are a linear ssRNA ranging from 9.7 to 17.6 kbp in size, which encodes a single long polypeptide includes of viral RNA helicases, UDP-glucosyltransferases and RdRps (Ghabrial et al. 2015). In our study, two viral genome sequences, contig 1085 (8,310 nt) and contig 371 (8,245 nt), showed similarity to members of the family Endornaviridae. RT-PCR amplification suggested that contig 1085 and contig 371 were detected in isolate 2012-019 and isolate 2013-005, respectively (Fig. 1). Previously, no endornavirus had been reported in M.phaseolina.
The putative viral sequence contig1085 and contig 371 both encoded a single large polyprotein that was similar to the polyprotein encoded by Hordeum vulgare endornavirus with 33% and 31% identity, respectively (Table 1). HvEV contained conserved motifs for a viral methyltransferase (pfam01660, 210 aa), Viral_helicase 1 (pfam01443, 239 aa), and RdRp_2 (pfam00978, 206 aa), but not UDP-glucosyltransferases (Candresse, Marais, and Sorrentino 2015). Likewise, the predicted polyprotein encoded by contig1085 and contig 371 also contained a viral methyltransferase and RdRp_2 domains (Fig. 3A). The pairwise alignment of amino acid sequences of polyprotein between contig1085 and contig 371 was 76.7% (Supplementary Additional file 1). These results suggested that contig 1085 and contig 371 were new endornaviruses in the family Endornaviridae, which were named M.phaseolina endornavirus 1 (MpEV1) and M.phaseolina endornavirus 2 (MpEV2), respectively. Phylogenic analysis was performed based on the conserved polyprotein among MpEV1, MpEV2, and other reported endornaviruses. The results further suggested that MpEV1 and MpEV2 were new virus species in the genus Betaendornavirus (Fig. 3B).
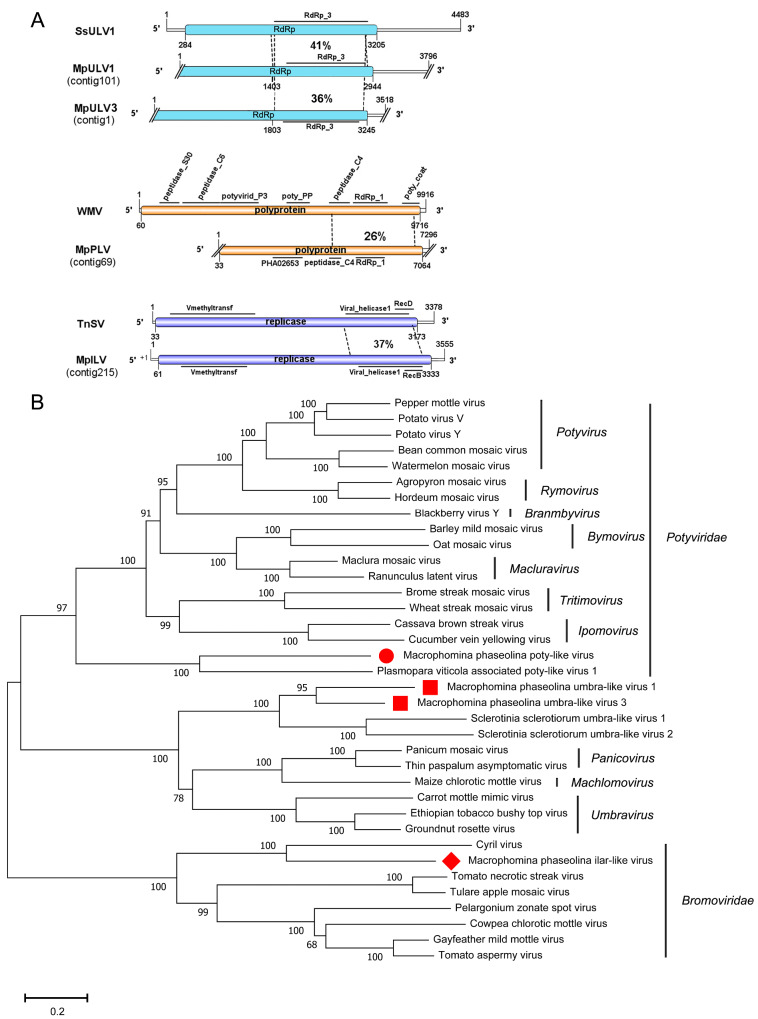
3.9 Characterization of one novel virus in the family Potyviridae
Potyvirus is the largest genus in the family Potyviridae, which contains a single molecule of linear, positive-sense ssRNA of approximately 9.7 kbp in size. The genome encodes a single major polyprotein that is self-cleaved into a set of functional proteins (Adams, 2012). One viral sequence showed similarity to members of the family Potyviridae. Contig 69 was 7,296 nt, containing one incomplete ORF predicted to encode a polyprotein of 2,343 aa. The predicted polyprotein showed similarity to the polyprotein of Watermelon mosaic virus (WMV) with 25% identity match. The polyprotein of WMV contained seven domains (Fig. 6A). However, the polyprotein of contig 69 only had RdRp_1 (pfam00680), Peptidase_C4 (pfam00863), and RNA helicase domains. It has been reported that Peptidase_C4 is present in the nuclear inclusion protein of potyvirus (Marchler-Bauer 2017). Contig 69 likely represented a novel potyvirus, and we named it M.phaseolina poty-like virus (MpPLV). Furthermore, a phylogenetic analysis of the conserved polyprotein domain among MpPLV and other related potyviruses was conducted, and a phylogenetic tree was constructed (Fig. 6B). The tree further suggested that contig 69 was a new virus species in the family Potyviridae and also the first mycovirus identified in the Potyviridae family. In addition, RT-PCR amplification revealed that MpPLV was present in isolates 2013-006 and 2013-037 (Fig. 1).
Figure 6.
Genome organizations and phylogenetic analysis of the putative viruses in family Potyviridae, Tombusviridae, and Bromoviridae detected from Macrophomina phaseolina. (A) Comparison of the organizations of putative viruses M.phaseolina umbra-like virus 1 (MpULV1) and M.phaseolina umbra-like virus 3 (MpULV3) to Sclerotinia sclerotiorum umbra-like virus 1 (SsULV1). Comparison of the organizations of putative viruses M.phaseolina poty-like virus (MpPLV) to Watermelon mosaic virus (WMV). Comparison of the organizations of putative viruses M.phaseolina ilar-like virus (MpILV) to Tomato necrotic streak virus (TNSV). (B) Predicted RdRp amino acid sequences of MpULV1, MpULV3, MpPLV, and MpILV were aligned and phylogenetic tree were constructed as described in Fig. 1. The viruses marked with red dots, red squares, and red diamonds were found in M.phaseolina.
3.10 Characterization of one novel virus in the family Bromoviridae
The family Bromoviridae contains six genera, Alfamovirus, Anulacirus, Bromovirus, Cucumovirus, Ilarvirus, and Oleavirus. The typical features of members of the Bromoviridae are a genome length of approximately 8 kb, which consists of three linear positive-sense ssRNAs, and segmented genomes packaged in separated virions that may also contain subgenomic RNAs (sgRNAs), defective RNAs or satellite RNAs. The 5′-terminus of Bromoviridae viruses has a cap structure, and the 3′-terminus is not polyadenylated but widely and highly conserved within a species or isolate, forming strong secondary structures (Bujarski, 2012). In the present study, one sequence, contig 215, was 3,555 nt and had one large ORF (61-3,333 nt) encoding a replicase of 1,090 aa. The 5′-UTR and 3′-UTR of contig 215 were 60 nt and 222 nt, respectively. Blastp analysis suggested that the predicted amino acid sequence of the replicase was similar to the replicase of Tomato necrotic streak virus with 37% identity. Like TNSV, the replicase of contig 215 not only included a Viral_helicase 1 and Vmethyltransf but also DEXXYc_viral_SF1-N and RecB domains (Fig. 6A). TNSV was a member of the genus Ilarvirus and the viral sequence of contig 215 represented a new ilar-like virus and was thus named M.phaseolina ilar-like virus (MpILV). RT-PCR amplification suggested that MpILV was harbored by isolate 2012-051 (Fig. 1). Phylogenetic analysis of the conserved protein among MpILV, TnSV, CV, and other viruses in the family Bromoviridae was conducted and a phylogenetic tree constructed (Fig. 6B). The tree revealed that MpILV clustered with CV to form a single branch, which also clustered with six ilarviruses (HMV, SLV, TnSV, TaMV, AMV, and FcLV) to form a larger branch. This branch included MpILV distinct from other genuses in the family Bromoviridae, and MpILV represented a new virus species in the family Bromoviridae; it is also the first mycovirus identified in this family.
3.11 Three novel umbra-like viruses
Members of the family Tombusviridae contain monopartite genomes including one or two 3.7–4.8 kbp linear ssRNA segments with up to six ORFs. Generally, the 5′-proximity ORF expresses the viral replicase, and the 3′-proximityORF is expressed by a subgenomic RNA and encodes the CP (Rochon, 2012). In our study, three contigs showed similarities to members of the family Tombusviridae. Contig 101 was 3,796 nt with a putative incomplete ORF encoding a protein most similar to the RdRp of S.sclerotiorum umbra-like virus 1with 41% identity (Table 1).The putative protein contained the RdRp_3 domain. We named this novel virus M.phaseolina umbra-like virus 1 (MpULV1). Contig 1 was 3,518 nt with an incomplete ORF encoding a putative protein most similar to the RdRp of S.sclerotiorum umbra-like virus 1 with 36% identity (Table 1), and the putative protein contained a RdRp_3 domain. It was designated as M.phaseolina umbra-like virus 3 (MpULV3). The viral sequence of contig 103 was shorter, but the predicted protein was similar to the RdRp of S.sclerotiorum umbra-like virus 1 with 45% identity (Table 1). It was named as M.phaseolina umbra-like virus 2 (MpULV2). The nucleotide sequences of MpULV2 showed strong similarly to MpULV3 and likely represented a different isolate of MpULV3 (Supplementary Additional file 2). RT-PCR amplification suggested that MpULV1 was detected in isolate 2012-019; MpULV2 was detected in isolates 2012-022, 2013-006, and 2012-036; and MpULV3 was detected in six isolates, including isolates 2012-022, 2012-161, 2013-037, 2013-006, 2012-036, and 2011-138 (Fig. 1). Moreover, a phylogenetic analysis based on the putative RdRp of MpULV1 and MpULV3 and other selected viruses was conducted. The result suggested that these two viruses likely represented new virus species in the genus Umbravirus (Fig. 6B).
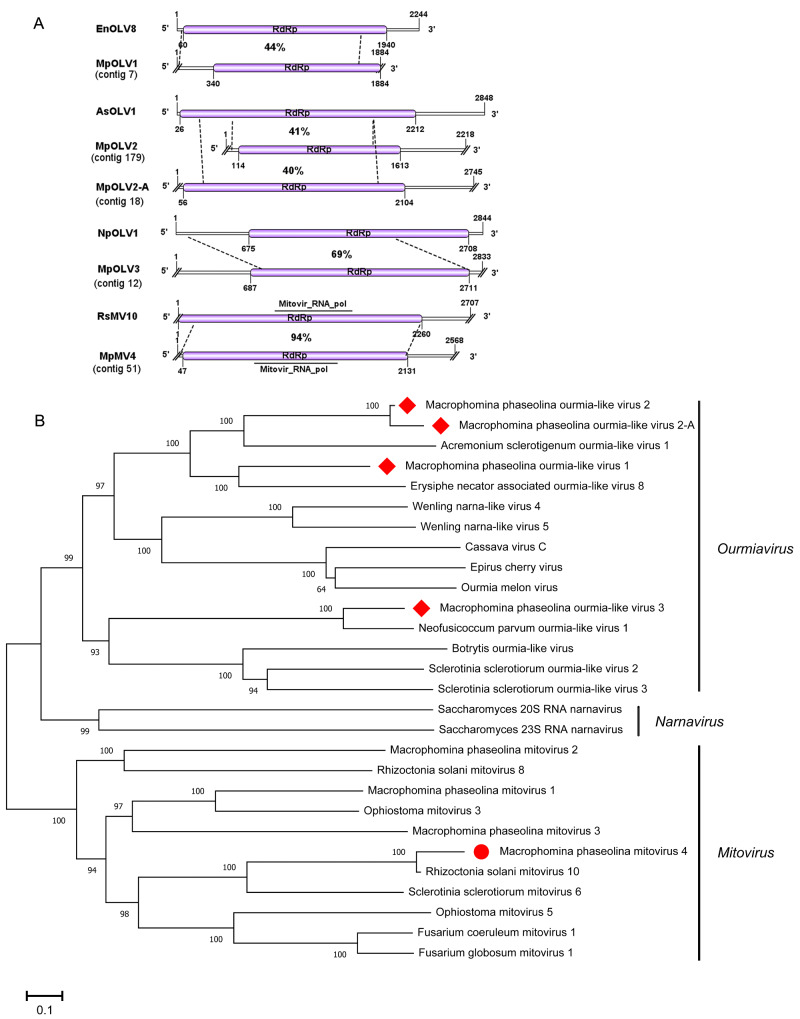
3.12 Characterization of one novel mitovirus
Viruses in the family Narnaviridae consist of a single molecule of positive-strand RNA of 2.3–2.9 kb, and a single ORF encoding only RdRp (Hillman 2012). Three mitoviruses have been reported to infect M.phaseolina, M.phaseolina mitovirus 1 (MpMV1), M.phaseolina mitovirus 2 (MpMV2), and M.phaseolina mitovirus 3 (MpMV3) (Marzano et al. 2016). In the present study, one viral sequence, contig 51, was 2,567 nt and contained a complete ORF encoding a putative protein of 694 aa. The protein contained a mitovir_RNA_Pol (pfam05919) domain. Blastp suggested that the predicted amino acid sequence of the putative protein was similar to the RdRp of R.solani mitovirus 10 with 94% identity (Table 1). Thus, contig 51 was likely to be a strain of RsMV10, and we named it M.phaseolina mitovirus 4 (MpMV4). Additionally, RT-PCR amplification revealed that MpMV4 was present in isolate 2015-003 (Fig. 1). A phylogenetic analysis based on the RdRp sequence of MpMV4, RsMV10, and other selected mitoviruses was conducted. The results suggested that MpMV4 was a new mitovirus in M.phaseolina (Fig. 7).
Figure 7.
Genome organizations and phylogenetic analysis of the putative viruses in genus Ourmiavirus and mitovirus detected from Macrophomina phaseolina. (A) Comparison of the organizations of putative viruses M.phaseolina ourmia-like virus 1 (MpOLV1), M.phaseolina ourmia-like virus 2 (MpOLV2), M.phaseolina ourmia-like virus 2-A (MpOLV2-A), and M.phaseolina ourmia-like virus 3 (MpOLV3) to Erysiphe necator associated ourmia-like virus 8 (EnOLV8), Acremonium sclerotigenum ourmia-like virus 1 (AsOLV1), and Neofusicoccum parvum ourmia-like virus 1 (NpOLV1), respectively. Comparison of the organizations of putative viruses M.phaseolina mitovirus 1 (MpMV1) to and Rhizoctonia solani mitovirus 10 (RsMV10). (B) Predicted RdRp amino acid sequences of MpOLV1, MpOLV2, MpOLV2-A, MpOLV3, and MpPLV, and MpILV were aligned and phylogenetic tree were constructed as described in Fig. 1. The viruses marked with red dots, red squares, and red diamonds were found in M.phaseolina.
3.13 Characterization of four novel ourmiaviruses
Genus Ourmiavirus belong to family Botourmiaviridae (María et al. 2020). The genome of ourmiavirus is composed of three positive-sense single-stranded RNAs of 2.8, 1.1, and 0.97 kb, respectively. RNA1 encodes RdRp, RNA2 encodes movement protein, and RNA3 encodes CP (Turina et al. 2017). Some ourmiaviruses have been reported to infect the phytopathogenic fungi R.solani, S.sclerotiorum (Marzano et al. 2016), Botrytis (Donaire et al. 2016), Phomopsis longicolla (Hrabáková, Koloniuk, and Petrzik 2017), and Magnaporthe oryzae (Li et al. 2019). In our study, four contigs showed high similarities to the reported fungus ourmiavirus.
The putative viral sequence of contig 7 was 1,884 nt and had one incomplete ORF encoding an RdRp of 514 aa. The sequence of contig 7 shared 44% amino acid sequence identity to Erysiphe necator associated ourmia-like virus 8 (Table 1), and we named it M.phaseolina ourmia-like virus 1 (MpOLV1). Contig 179 was 2,218 nt and had one incomplete ORF encoding an RdRp of 499 aa. The predicted amino acid sequence of contig 179 RdRp was 41% identical to that of Acremonium sclerotigenum ourmia-like virus 1 (Table 1). Furthermore, the predicted RdRp contained a Pneumo_att_G domain (pfam05539), and we named contig 179 M.phaseolina ourmia-like virus 2 (MpOLV2). Contig 18 was 2,745 nt and had one complete ORF encoding an RdRp of 682 aa. The predicted amino acid sequence of RdRp was similar to that of AsOLV1 with 40% identity. Moreover, the identity by pair wise alignments between contig 179 and contig 18 was 93% (Supplementary Additional file 3), and we named it M.phaseolina ourmia-like virus 2-A (MpOLV2-A). Contig 18 represented isolate of MpOLV2. Contig 12 was 2,833 nt and contained one complete ORF predicted to encode an RdRp of 674 aa. The predicted RdRp was similar to that of Neofusicoccum parvum ourmia-like virus 1with 69% identity (Table 1). Thus, we named it M.phaseolina ourmia-like virus 3 (MpOLV3).
RT-PCR amplification suggested that MpOLV1 and MpOLV2 were both detected in isolates 2012-019 and 2012-022 (Fig. 1), MpOLV2-A was detected in isolates 2012-019 and 2015-003 (Fig. 1), and MpOLV3 was detected in isolate 2012-019 (Fig. 1). These result revealed that M.phaseolina hosted various ourmiaviruses. Furthermore, the phylogenetic analysis indicated that MpOLV1, MpOLV2, MpOLV2-A, MpOLV3 and other fungus ourmia-like viruses clustered in one branch distinct from plant ourmiavirus (Fig. 7). Consequently, these four viruses represented novel species in the genus Ourmiavirus. In additon, Hrabáková, Koloniuk, and Petrzik (2017) propose to recognize ourmiaviruses from fungi as a separate genus with name ‘Ourmycovirus’.
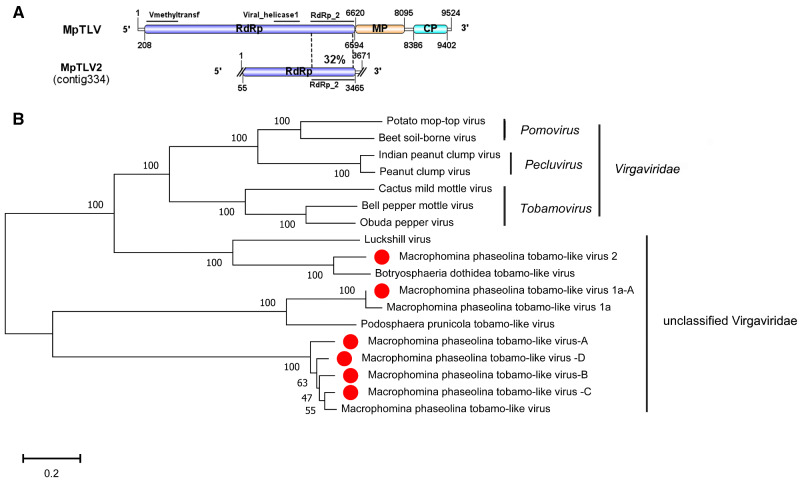
3.14 Six related viruses in the family Virgaviridae
Members of the family Virgaviridae have rod-shaped virions, and the genome of the 3’-terminal tRNA-like structure is a replication protein typical of alpha-like viruses (Adams, Antoniw, and Kreuze 2009). The viruses in this family almost always infect plants. Previously, M.phaseolina tobamo-like virus 1 (MpTLV1) and M.phaseolina tobamo-like virus 1a (MpTLV1a) have been reported in Virgaviridae, representing the first report of Tobamovirus-infected fungi (Marzano et al. 2016). The genomes of those two viruses all contain four ORFs encoding methyltransferase/helicase, RdRp, putative movement protein, and CP, respectively (Marzano et al. 2016). In our study, six contigs showed similarities to the members of Tobamovirus.
The viral sequence of contig 334 (3,671 nt) contained one incomplete ORF encoding an RdRp of 1,316 aa and a conserved RdRp_2 domain (pfam00978). The amino acid sequence of RdRp was 78% identical to that of Botryosphaeria dothideatobamo-like virus (BdTLV) followed by Luckshill virus (LV) with 41% identity (Table 1). Additionally, the putative RdRp was similar to Podosphaera prunicola tobamo-like virus (PpTLV) and MpTLV with 31% identity (Table 1). However, BdTLV and LV were not complete genomes, and LV was an unclassified ssRNA virus that infected pests (Medd et al. 2017). Thus, we named contig 334 M.phaseolina tobamo-like virus 2 (MpTLV2), which represented a new virus species in the family Virgaviridae. RT-PCR amplification suggested that MpTLV2 was detected in isolates 2012-051, 2013-037, 2015-003, 2014-017, 2012-036, and 2013-006 (Fig. 1).
The genome of contig 66 (2,073 nt), contig 76 (3,417 nt), contig 49 (3,155 nt), contig 15 (4,677 nt), and contig 47 (2,381 nt) all contained one incomplete ORF encoding an RdRp. The putative amino acid sequences of contig 66 contained RdRp_2 domain. The Blastp analysis suggested that the RdRp of contig 66 was similar to that of MpTLV1a with 98% identity (Table 1). Contig 66 represented an isolate of MpTLV1a, and we named it M.phaseolina tobamo-like virus 1a-A (MpTLV1a-A). RT-PCR amplification suggested that MpTLV1a-A was harbored in isolates 2012-019, 2015-003, 2013-037, 2011-138, and 2012-036 (Fig. 1). Furthermore, contig 76, contig 49, and contig 15 all contained a conserved domain, Viral_helicase 1, and showed strong relatedness to the RdRp of MpTLV with 88%-93% identity (Table 1). The putative amino acid sequence of Contig 47 RdRp contained no motif but was similar to the RdRp of MpTLV with 94% identity. Thus, contig76, contig 49, contig 15, and contig 47 represented different isolates of MpTLV, and we named them M.phaseolina tobamo-like virus-A (MpTLV-A), M.phaseolina tobamo-like virus-B (MpTLV-B), M.phaseolina tobamo-like virus-C (MpTLV-C), and M.phaseolina tobamo-like virus-D (MpTLV-D), respectively. The phylogenetic analysis showed that MpTLV2 was a new virus species in the family Virgaviridae. MpTLV1a-A, MpTLV-A, MpTLV-B, MpTLV-C, and MpTLV-D represented the strains of MpTLV1a and MpTLV in the family Virgaviridae (Fig. 8). These result confirmed that MpTLV1a and MpTLV were widely distributed in China.
Figure 8.
Genome organizations and phylogenetic analysis of the putative viruses in family Virgaviridae detected from Macrophomina phaseolina. (A) Comparison of the organizations of putative viruses M.phaseolina tobamo-like virus 2 (MpTLV2) to M.phaseolina tobamo-like virus (MpTLV). (B) Predicted RdRp amino acid sequences of MpTLV2 and other Tobamo-like viruses were aligned and phylogenetic tree were constructed as described in Fig. 1. The viruses marked with red dots were found in M.phaseolina.
3.15 The distribution of detected viruses in a single isolate of M.phaseolina
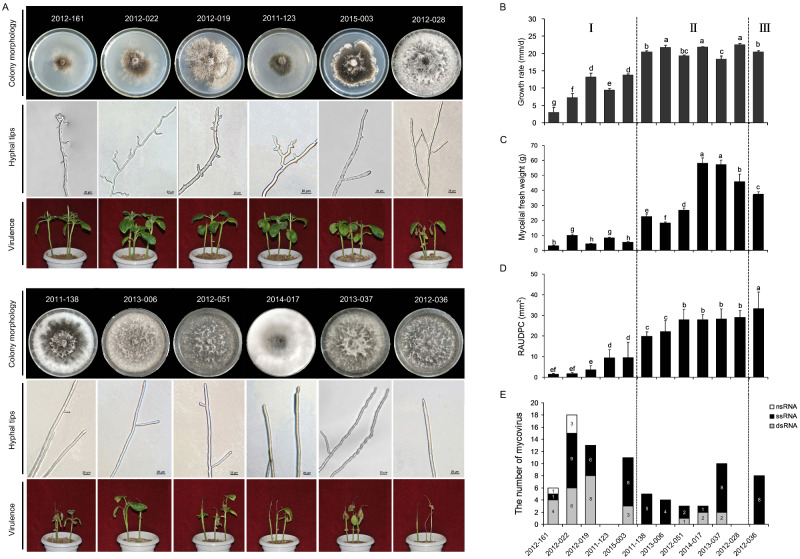
The viruses harbored by each isolate were detected by RT-PCR amplification with virus-specific primers (Fig. 1). Ten of these twelve isolates were virus-positive by RT-PCR detection. Moreover, 10 isolates showed coinfection with more than one virus (Fig. 9E). Among these viruses, 43% of the viral genomes were predicted to represent dsRNA virus, 47% ssRNA+ virus, and 10% ssRNA− virus (Fig. 9E). The virus-infected isolates 2012-161 and 2012-019 both showed three nucleic acid types genomes (dsRNA, ssRNA+, and ssRNA−) (Fig. 9E). Notably, isolate 2012-019 harbored 18 different viruses belonging to seven distinct lineages: Bunyavirales, Totiviridae, Endornaviridae, Tombusviridae, Virgaviridae, ‘Fusagraviridae’, and Ourmiavirus (Fig. 1). Isolates 2012-022 harbored thirteen different viruses belonging to seven distinct lineages: Totiviridae, Chrysoviridae, Hypoviridae, Tombusviridae, Virgaviridae, ‘Fusagraviridae’, and Ourmiavirus. Moreover, isolates of 2015-003, 2012-051, 2014-017, and 2013-037 were infected by viruses containing dsRNA and ssRNA+ genomes (Fig. 9E), whereas the viral genome of the viruses infecting isolates 2012-036, 2013-006 and 2011-138 had only ssRNA+ genomes (Fig. 9E). In addition, isolates 2011-123 and 2012-028 did not harbor any viruses (Fig. 1). This result revealed that M.phaseolina isolated from sesame in China harbored huge resources of virus; however, a small number of isolates did not harbor the virus.
Figure 9.
The comparison of different biological characteristic and quantity of mycoviruses among 12 isolates of Macrophomina phaseolina. (A) Compare the colony morphology, hyphal tips, and virulence of 12 isolates. All fungal strains were grown on PDA for 4 days at 30°C and photographed. (B) Growth rate of 12 isolates were measured on PDA for 48 h at 30°C. (C) Biomass of 12 isolates was measured in PDB for 8 days at 30°C. (D) The data of pathogenicity was calculated base on RAUDPC. (E) The number of ssRNA−, ssRNA+, and dsRNA viruses contained by 12 isolates respectively. Means followed by the different letters on the top of each column are significantly different at the P < 0.05 level of confidence according to Duncan’s multiple range test.
3.16 Effect of viruses on the biological characteristics of M.phaseolina
The 12 M.phaseolina isolates were cultured at 30°C on PDA to observe their morphology. Compared with the virus-negative isolates 2012-028, four isolates, 2012-161, 2012-019, 2012-022 and 2015-003, harbored multiple viruses and showed a significantly abnormal colony morphology with sectoring and restricted growth at the colony margin on PDA plates (Fig. 9A), which is similar to the hypovirulence traits of S.sclerotiorum isolates containing RNA virus (Liu et al. 2014). The colony color of the other seven isolates was light or dark gray, and the growth rates and biomass were significantly reduced compared with the virus-free isolate of 2012-028 (P < 0.05) (Fig. 9B and C). The hyphal tips of each isolate were checked under a microscope, revealing the similarity of six isolates (2011-138, 2013-006, 2012-051, 2014-017, 2013-037, 20120-036) to the virus-free isolate 2012-028, although the six isolates harbored viruses. The isolate 2015-003 showed an abnormal colony phenotype, but it had normal hyphal tips (Fig. 9A). However, the hyphal tips of isolates 2012-161, 2012-019, 2011-123, 2012-022 were shortened and multibranched; additionally, some tips of small branches were capreolary, and the morphology was abnormal (Fig. 9A).
The pathogenicity of each isolate was tested on sesame. The results showed that isolates 2012-022, 2012-019, 2012-161, 2011-123, and 2015-003 caused slight necrosis at the tip of sesame stem at 8 days postinoculation (dpi) and hardly extended to the unifoliate node with a significantly lower RAUDPC than the virus-free isolate (Fig. 9A). In contrast, the virus-negative isolate 2012-028 caused stem necrosis reaching down to the unifoliate node after 2 dpi and extending to the second unifoliate node after 6 dpi, but no significant drying up at 8 dpi (Fig. 9A). Interestingly, isolate 2012-036 harboring MpULV1, MpULV2, and MpTLV2 showed a significantly higher RAUDPC of 44.3 mm2 than the other eleven isolates, with stem necrosis reaching down to the unifoliate node after 2 dpi and extending below the second unifoliate node after 6 dpi. Most stems then driedup and became covered with pycnidia and microsclerotia at 8 dpi (Fig. 9A). Therefore, both the colony phenotype and pathogenicity analysis suggested that isolates 2012-022, 2012-161, 2012-019, 2011-123, and 2015-003 were hypovirulent strains.
4. Discussion
To the best of our knowledge, there have been few reports to date on viruses in M.phaseolina (Arora, Dilbaghi, and Chaudhury 2012; Marzano et al. 2016). However, only Marzano et al. (2016) analyzed the viral sequences, while they did not mention the phenotypes and viral types of each isolates. Hence, the relationship between viruses and the phenotype of M.phaseolina is still unknown. This is the first report of the virus diversity of M.phaseolina isolates collected from sesame in China, and the morphology of each isolate was described. We identified forty putative viral sequences, most of which were nearly the full-length genome. Four viruses provided a complete genome sequence by RACE. In addition, these viral genomes showed similarity to twelve distinct lineages: Hypoviridae, Totiviridae, Chrysoviridae, Endornaviridae, Partitiviridae, Bunyavirales, Virgaviridae, Potyviridae, Bromoviridae, Narnaviridae, Tombusviridae, ‘Fusagraviridae’, and Ourmiavirus. Furthermore, 14 viral sequences grouped into 6 different lineages (Chrysoviridae, Tombusviridae, Virgaviridae, Narnaviridae, Hypoviridae, and Bunyavirales), and two unclassified RNA viruses were identified from the forty-eight M.phaseolina isolates collected from the soybean in North America (Marzano et al. 2016) (Supplementary Table S4). The viruses in families Hypoviridae, Bunyavirales, Chrysoviridae, Virgaviridae, and Narnaviridae were detected in both Chinese and American isolates of M.phaseolina. However, sixteen viral sequences showed similarity to six families in this research, Totiviridae, Partitiviridae, Endornaviridae, Potyviridae, Bromoviridae, and Ourmiavirus, which previously contained no viruses identified from M.phaseolina. Jo et al. (2020) identified two viruses (Uromyces potyvirus A and Uromyces anulavirus A) from public fungal transcriptomes datasets, which showed similarities to the members of family Potyviridae and Bromoviridae, they suggest the existence of putative fungal bromovirus and potyvirus. This study greatly expands the number of M.phaseolina viruses and further shows the abundant diversity of viruses in M.phaseolina, revealing many new viruses for exploration. These findings also revealed that virus are commonly present in M.phaseolina. Moreover, the viruses of M.phaseolina collected worldwide are mostly different, consistent with the observation in other fungi such as S.sclerotiorum (Mu et al. 2018). Moreover, the variety of viruses in each isolate was confirmed by RT-PCR, and the results suggested that ten of twelve isolates were infected by various viruses. The most interesting one was isolate 2012-019 harboring eighteen different viruses belonging to seven different lineages. The isolate (2014-017, 2011-138, 2012-051, and 2012-036) for which dsRNA segment detection failed but that provided results by deep mRNA sequencing and RT-PCR suggested that these viruses have low accumulation levels in the host, and metatranscriptomics is sufficiently sensitive to discover such new virus types. Analysis of the genome type of the viruses suggested that most of them were dsRNA and ssRNA+; only 10% were ssRNA−, and no DNA virus existed in these twelve isolates. Similar results have been reported previously showing that DNA and ssRNA− viruses are less common than dsRNA and ssRNA+ viruses in fungi (Yu et al. 2010; Liu et al. 2014).
In 2016, the first virus identified in the family Virgaviridae was reported by analysis of tobamo-like virus in M.phaseolina (Marzano et al. 2016). In our study, nine M.phaseolina isolates harbored tobamo-like virus. The findings suggested that tobamo-like virus was presented worldwide and prevalent in the plant-pathogenic fungus M.phaseolina. Moreover, the tobamo-like virus 1a (MpTLV1a; KP900897) was identified in three hypovirulent isolates, suggesting that MpTLV1a might be associated with hypovirulence. However, the other tobamo-like virus was not only detected in hypovirulent but also in virulent isolates. Furthermore, seven isolates tested in this research contained umbra-like virus, while only one umbra-like virus was detected in S.sclerotiorum collected from North America (Marzano et al. 2016). This result suggests that the virus of M.phaseolina in China was significantly different from the North American isolate.
A previous report revealed that viruses in Mononegavirales typically have negative single-stranded genomes of 8.9–19 kb and contains eight families (Easton 2012). Some new members of the Mymonaviridae have been identified in fungi, such as S.sclerotiorum negative-stranded RNA virus 1 (SsNSRV1; YP_009094317; 10,002 nt) and Fusarium graminearum negative-stranded RNA virus 1 (FgNSRV1; MF 276904; 9,072 nt) (Amarasinghe et al. 2017; Wang et al. 2018). A recently reported B.cinerea negative-stranded RNA virus 1 (BcNSRV1; NC_028466; 8,543 nt), R.solani negative-stranded RNA virus 4 (RsNSRV4; KP900923; 7,224 nt), and M.phaseolina negative-stranded RNA virus 1 (MpNSRV1; KP900899; 6,081 nt) are new members of the order Bunyavirales (Marzano et al. 2016; Donaire, Pagán, and Ayllón 2016). In this study, MpMBV3 was identified as a strain of MpNSRV1. However, MpMBV3 was longer than MpNSRV1. Hence, further research is needed to address this difference.
Compared with co-infections, single virus infection is rare (Jiang, Fu, and Ghabrial 2013). In this research, 10 isolates showed coinfection by various viruses. Interestingly, the hypovirulent isolate 2012-019 contained up to 18 viruses belonging to seven distinct lineages. Similarly, the avirulent R.solani isolate DC-17 harbored 17 different mycovirus species belonging to at least eight different families (Bartholomäus, Wibberg, and Winkler 2016). In 2016, Zhang and Nuss (2016) engineered a super donor strain that were able to transmit hypoviruses more efficiently and enhance the biological control potential of CHV1. Similar to isolate 2012-019 and DC-17, they displayed the ability to harbor many different viruses. Hence, these strains have propensity to harbor different viruses which may help with horizontal transmission. In addition, isolate 2012-019 could be a valuable experimental material to study the mechanism of M.phaseolina anti-viral immunity.
Prediction of virus localization revealed that the cytoplasm is a more common area of localization than mitochondria in M.phaseolina, of which only one isolate, 2015-003, harbored a mitovirus that was predicted to replicate within mitochondria. Consistent with previous reports, mitoviruses are frequently identified in the viromes of S.sclerotiorum and R.solani, but not in M.phaseolina (Marzano et al. 2016). As mentioned above, it is possible that the mitochondria of M.phaseolina have a higher immunity to virus than S.sclerotiorum and R.solani. The isolates 2015-003 containing mitovirus 4 showed hypovirulent traits, implying that this mitovirus might be hypovirulent. However, Ran et al. (2016) described a limited effect of S.sclerotiorum mitovirus 4 on S.sclerotiorum.
Viruses that attenuate fungal virulence may be welcome additions for the mitigation of plant diseases. Similar to previous reports showing that most viruses are latent infections and do not cause obviously symptoms (Pearson et al. 2009; Ghabrial and Suzuki 2009), we found thirteen viruses (MpV3, MpPV1, MpChrV4, MpULV2, MpULV3, MpILV, MpPLV, MpTLV2, MpTLV1a-A, and MpTLVA-D) infecting the isolates that showed no significant change in phenotype. Comprehensive analysis of the kind of virus and phenotype of each isolate suggested that the M.phaseolina virus of Hypovirus 2, Fusagravirus2, 3,4, and 5, Victorivirus 2, Chrysovirus 2, 3, and 5, Endornavirus 1 and 2, Partitivirus 2 and 3, Mycobunyavirus1, 2, 3, and 4, Umbra-like virus 1, Ourmia-like virus 1, 2, and 3, and Mitovirus 4 were only present in hypovirulent isolates, indicating that these viruses might be associated with hypovirulence traits of M.phaseolina (Fig. 1). Thus, we speculated that those viruses might be associates with hypovirulence. More interestingly, the reported virus MpTLV was detected in three isolates exhibiting attenuated virulence.
The M.phaseolina virus of Fusagravirus 3, 4, and 5 was detected in three different hypovirulent isolates, suggesting that this virus likely contributes to the hypovirulence traits of M.phaseolina, and might be transmitted much more easily in M.phaseolina than other hypovirulence-associated viruses. Using isolates only harboring MpFV3 to test the features of this virus will help test this hypothesis.
Alternaria alternata infecting Japanese pear can produces a host-specific AK-toxin. The mycovirus Alternaria alternate chrysovirus 1 (AaCV1) was identified in a strain that showed an impaired growth phenotype. The effect of AaCV1 on the A.alternate exhibited two contrasting features, impaired growth of the host fungus while rendering the host hypervirulent to the plant (Okada, 2018). Interestingly, isolate 2012-036 in this study harbored MpULV2, MpULV3, MpTLV2, MpTLV1a, and MpTLVA-D, showing normal growth phenotypes while enhancing virulence to sesame. These results implied that these viruses might have a positive effect on the fungus. Thus, this performance might be induced by multiple virus infection or just the biological trait of this isolate. Thus, further analysis of the influence of each virus on isolate 2012-36 is needed. Additionally, umbra-like viruses were detected in seven M.phaseolina isolates, suggesting that this virus widespread in the host of M.phaseolina.
In addition to identifying new potential biological control agents, these results expand our overall view of the diversity of viruses. Phylogenetic analyses of viruses infecting lower eukaryotes often show clusters of viral genomes that are more closely related to viruses that infect the same host species than to viruses that infect other species (Arjona-Lopez et al. 2018). In contrast, in this research, only a few virus sequences matched the virus associated with M.phaseolina. Most of the novel viruses from M.phaseolina in China in this research showed the best match with viruses described from animal, plant, and other soil-inhabitant fungi such as S.sclerotiorum and R.solani. Similarly, Arjona-Lopez et al. reported that many viruses detected in R.necatrix show a closer relationship to soil-borne fungi other than R.nectarix, and they hypothesized that horizontal viral transfer occurs between soil-inhabitant fungi (Arjona-Lopez et al. 2018). In addition, increasing evidence supports the horizontal transfer of viruses between fungi and plants. For example, Cryphonectria hypovirus 4 reveals the phylogenetic relatedness between the hypovirus and plant RNA virus potyvirus (Linder-Basso, Dynek, and Hillman 2005). In our study, two putative viral sequences, contig 69 and contig 215, showed similarity to the plant virus family Potyviridae and Bromoviridae, and the discovery of MpEV1-2 provides further proof for the hypothesis that unencapsidated dsRNA-like genomes appear to have a common ancestry with plant (+) strand RNA viruses (Roossinck et al. 2011). In addition, Song et al. (2013) found three glycome-related viral genes in endornavirus that were acquired from marine bacteria by horizontal gene transfer. Mycovirus of P.longicolla RNA virus 1 (PlRV1) and Botrytis ourmia-like virus (BOLV) were recently discovered, which are related to plant ourmiaviruses and form one clade, which might represent the closest link between fungal Narnavirus and plant Ourmiavirus (Donaire et al. 2016; Marzano et al. 2016; Hrabáková, Koloniuk, and Petrzik 2017). These findings suggest the origin of BOLV and support the so-called plant virus hypothesis (Ghabrial 1998). Furthermore, horizontal transmission from animals to plants by invertebrate parasites of both hosts has been reported for negative-stranded RNA viruses (Dolja and Koonin 2011). The B.cinerea negative-stranded RNA virus 1 (BcNSRV-1) is hypothesized to be derived from an invertebrate and vertebrate-infecting virus (Donaire, Pagán, and Ayllón 2016). Similarly, MpTLV2 showed similarity to Luckshill virus identified in invertebrate animal, indicating that MpTLV2 could be derived from an invertebrate-infecting virus.
The novel viruses described in our study will further supplement the virus sequence in the database and supply strong evidence explaining the horizontal virus transfer among animals, plants, and fungi. The results also facilitate understanding of the origins of all viruses to solve difficulties in viral identification and classification, and provide new insights into the coevolutionary biology of fungal viruses and their hosts.
5. Conclusions
In this study, we used a high throughput sequencing-based metatranscriptomic approach to detect viral sequences of M.phaseolina isolates collected from sesame in China. The forty viruses were contained by eleven Chinese isolates and grouped into twelve distinct lineages, which is significantly more abundant than previously reported for fourteen new viruses detected from forty-eight M.phaseolina isolates collected from soybean in North America (Marzano et al. 2016). These results support a great diversity of viruses in Chinese isolates. Interestingly, one virus, M.phaseolina tobamo-like virus 1a, was also detected in Chinese isolates, indicating that MpTLV1a is widespread in China and North America. Importantly, many detected viruses showed the closest relationship to viruses reported from soil-inhabitant fungi (Sclerotina spp., Rhizoctonia spp., Verticillium spp.) that are sympatric to M.phaseolina. This result is consistent with previous reports on viruses in R.necatrix (Arjona-Lopez et al. 2018). Most importantly, our results also showed that coinfection was very common in M.phaseolina. Four hypovirulent isolates contained four to eighteen different viruses, including three types of nucleotide genomes (dsRNA, ssRNA+, and ssRNA−). In summary, this study explored many viruses that could be potential biocontrol agents, providing many new insights into the taxonomy and evolutionary biology of viruses.
Supplementary Material
Acknowledgements
We are extremely grateful to the Integrated Experiment Station of Characteristic Oil Crop Research System for supplying the diseased sesame samples. We also deeply appreciate Dr. Jiatao Xie, professor in the Department of Plant Pathology at the University of Huazhong Agriculture, who provided constructive comments and language editions. We sincerely thank the anonymous reviewers for their detailed comments that greatly improved the manuscript.
Funding
This research work is supported by the China Agriculture Research System (CARS-14-1-19). The funder had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Data availability
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. The GenBank accession numbers for the nucleotide sequence of 38 mycoviruses are listed in the Table 1. Additional data related to this paper may be requested from the authors.
Supplementary data
Supplementary data are available at Virus Evolution online.
Conflict of interest: None declared.
References
- Adams M. J., Antoniw J. F., Kreuze J. (2009) ‘ Virgaviridae: A New Family of Rod-Shaped Plant Viruses’, Archives of Virology, 154: 1967–72. [DOI] [PubMed] [Google Scholar]
- Adams Z. (2012) ‘Family Potyviridae’, in King A.M.Q., Adams M.J., Carstens E.B., Lefkowitz E.J. (eds.) Virus Taxonomy: Classification and Nomenclature of Viruses, Ninth Report of the International Committee on Taxonomy of Viruses, pp.1069–89. Waltham, MA, USA. [Google Scholar]
- Aiewsakun P., Katzourakis A. (2015) ‘ Endogenous Viruses: Connecting Recent and Ancient Viral Evolution’, Virology, 479–480: 26–37. [DOI] [PubMed] [Google Scholar]
- Amarasinghe G. K. et al. (2017) ‘ Taxonomy of the Order Mononegavirales: Update 2017’, Archives of Virology, 162: 2493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammon V., Wyllie T. D., Brown M. F. (1974) ‘ An Ultrastructural Investigation of Pathological Alterations Induced by Macrophomina Phaseolina (Tassi) Goid in Seedlings of Soybean, Glycine max (L.) Merrill’, Physiological Plant Pathology, 4: 1–4. [Google Scholar]
- Arjona-Lopez J. M. et al. (2018) ‘ Novel, Diverse RNA Viruses from Mediterranean Isolates of the Phytopathogenic Fungus, Rosellinia Necatrix: Insights into Evolutionary Biology of Fungal Viruses’, Environmental Microbiology, 20: 1464–83. [DOI] [PubMed] [Google Scholar]
- Arora P., Dilbaghi N., Chaudhury A. (2012) ‘ Detection of Double Stranded RNA in Phytopathogenic Macrophomina Phaseolina Causing Charcoal Rot in Cyamopsis Tetragonoloba’, Molecular Biology Reports, 39: 3047–54. [DOI] [PubMed] [Google Scholar]
- Badillo-Vargas I. E, et al. (2016) Genomic and Biological Characterization of Tomato Necrotic Streak Virus, a Novel Subgroup 2 Ilarvirus Infecting Tomato in Florida, Plant Disease, 100: 1046–53. [DOI] [PubMed] [Google Scholar]
- Baird R. E., Watson C. E., Scruggs M. (2003) ‘ Relative Longevity of Macrophomina Phaseolina and Associated Mycobiota on Residual Soybean Roots in Soil’, Plant Disease, 87: 563–6. [DOI] [PubMed] [Google Scholar]
- Bartholomäus A., Wibberg D., Winkler A. (2016) ‘ Deep Sequencing Analysis Reveals the Mycoviral Diversity of the Virome of an Avirulent Isolate of Rhizoctonia solani AG-2-2 IV’, PLoS One, 11: e0165965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujarski F. (2012) ‘Family Bromoviridae’, in King A.M.Q., Adams M.J., Carstens E.B., Lefkowitz E.J. (eds.) Virus Taxonomy: Classification and Nomenclature of Viruses, Ninth Report of the International Committee on Taxonomy of Viruses, pp.965–76. Waltham, MA, USA. [Google Scholar]
- Candresse T., Marais A., Sorrentino R. (2015) ‘ Complete Genomic Sequence of Barley (Hordeum Vulgare) Endornavirus (HvEV) Determined by Next-Generation Sequencing’, Archives of Virology, 161: 741–3. [DOI] [PubMed] [Google Scholar]
- Cañizares M. C. (2014) The Complete Nucleotide Sequence of a Novel Partitivirus Isolated from the Plant Pathogenic Fungus Verticillium Albo-Atrum, Archives of Virology, doi: 10.1007/s00705-014-2156-6. [DOI] [PubMed] [Google Scholar]
- Chiba S. et al. (2009) ‘ A Novel Bipartite Double-Stranded RAN Mycovirus from the White Root Rot Fungus Rosellinia Necatrix: Molecular and Biological Characterization, Taxonomic Considerations, and Potential for Biological Control’, Journal of Virology, 83: 12801–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolja V. V., Koonin E. V. (2011) ‘ Common Origins and Host-Dependent Diversity of Plant and Animal Viromes’, Current Opinion in Virology, 1: 322–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaire L. et al. (2016) ‘ Molecular Characterization of Botrytis ourmia-like Virus, a Mycovirus Close to the Plant Pathogenic Genus Ourmiavirus’, Virology, 489: 158–64. [DOI] [PubMed] [Google Scholar]
- Donaire L., Pagán I., Ayllón M. A. (2016) ‘ Characterization of Botrytis Cinerea, Negative-Stranded RNA Virus 1, a New Mycovirus Related to Plant Viruses, and a Reconstruction of Host Pattern Evolution in Negative-Sense ssRNA Viruses’, Virology, 499: 212–8. [DOI] [PubMed] [Google Scholar]
- Easton P. (2012) ‘Order Mononegavirales’, in King A.M.Q., Adams M.J., Carstens E.B., Lefkowitz E.J. (eds.) Virus Taxonomy: Classification and Nomenclature of Viruses, Ninth Report of the International Committee on Taxonomy of Viruses, pp.654–57. Waltham, MA, USA. [Google Scholar]
- Ejmal M. A. (2018) A Novel Chrysovirus from a Clinical Isolate of Aspergillus Thermomutatus Affects Sporulation, PLoS One, doi:10.1371/journal.pone.0209443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Pedrajas M. D. et al. (2019) ‘ Mycoviruses in Biological Control: From Basic Research to Field Implementation’, Phytopathology, 109: 1828–39. [DOI] [PubMed] [Google Scholar]
- Ghabrial S. A., Suzuki N. (2009) Viruses of Plant Pathogenic Fungi, Annual Review of Phytopathology, 47: 353–84. [DOI] [PubMed] [Google Scholar]
- Ghabrial S. A. (1998) ‘ Origin, Adaptation and Evolutionary Pathways of Fungal Viruses’, Virus Genes, 16: 119–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghabrial S. A. et al. (2015) ‘ 50-plus Years of Fungal Viruses’, Virology, 479–480: 356–68. [DOI] [PubMed] [Google Scholar]
- Hao F. M. et al. (2018) ‘ Two Novel Hypovirulence-Associated Mycoviruses in the Phytopathogenic Fungus Botrytis Cinerea: Molecular Characterization and Suppression of Infection Cushion Formation’, Viruses, 10: 254–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman E. (2012) ‘Family Nanaviridae’, in King A.M.Q., Adams M.J., Carstens E.B., Lefkowitz E.J.(eds.) Virus Taxonomy: Classification and Nomenclature of Viruses, Ninth Report of the International Committee on Taxonomy of Viruses, pp.1055–60. Waltham, MA, USA. [Google Scholar]
- Hrabáková L., Koloniuk I., Petrzik K. (2017) ‘ Phomopsis longicolla RNA Virus 1-Novel Virus at the Edge of Myco-and Plant Viruses’, Virology, 506: 14–22. [DOI] [PubMed] [Google Scholar]
- Jiang D. H., Fu Y. P., Ghabrial S. A. (2013) ‘ Chapter eight-Viruses of the Plant Pathogenic Fungus Sclerotinia sclerotiorum’, Advances in Virus Research, Mycoviruses, 86: 215–48. [DOI] [PubMed] [Google Scholar]
- Jo Y. et al. (2020) ‘ Identification of Viruses from Fungal Transcriptomes’, bioRxiv, preprint, doi:10.1101/2020.02.26.966903. [Google Scholar]
- Kaur S. et al. (2012) ‘ Emerging Phytopathogen Macrophomina Phaseolina: Biology, Economic Importance and Current Diagnostic Trends’, Critical Reviews in Microbiology, 38: 136–51. [DOI] [PubMed] [Google Scholar]
- Komatsu K., et al. (2016) Genome Sequence of a Novel Victorivirus Identified in the Phytopathogenic Fungus Alternaria Arborescens, Archives of Virology, 161: 1701–4. [DOI] [PubMed] [Google Scholar]
- Kondo H., Kanematsu S., Suzuki N. (2013) ‘ Viruses of the White Root Rot Fungus, Rosellinia Necatrix’, Advances in Virus Research, 86: 177–214. [DOI] [PubMed] [Google Scholar]
- Li C. X. et al. (2019) ‘ Characterization of a Novel Ourmia-Like Mycovirus Infecting Magnaporthe oryzae and Implications for Viral Diversity and Evolution’, Viruses, 11: 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L. L. (1989) ‘ The Disease Type, Research Overview and Prospects of Sesame in China’, Chin Oil, 1: 11–5. [Google Scholar]
- Li P. F. et al. (2020) ‘ A Tripartite ssDNA Mycovirus from a Plant Pathogenic Fungus is Infectious as Cloned DNA and Purified Virions’, Science Advances, 6: eaay9634–9643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder-Basso D., Dynek J. N., Hillman B. I. (2005) ‘ Genome Analysis of Cryphonectria Hypovirus 4, the Most Common Hypovirus Species in North America’, Virology, 337: 192–203. [DOI] [PubMed] [Google Scholar]
- Liu H. Q. et al. (2009) ‘ A Novel Mycovirus That is Related to the Human Pathogen Hepatitis E Virus and Rubi-like Viruses’, Journal of Virology, 83: 1981–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L. J. et al. (2014) ‘ Fungal Negative-Stranded RNA Virus That is Related to Bornaviruses and Nyaviruses’, Proceedings of the National Academy of Sciences, 111: 12205–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. et al. (2016) ‘ Fungal DNA Virus Infects a Mycophagous Insect and Utilizes it as a Transmission Vector’, Proceedings of the National Academy of Sciences, 113: 12803–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchler-Bauer A. et al. (2017) ‘ CDD/SPARCLE: Functional Classification of Proteins via Subfamily Domain Architectures’, Nucleic Acids Research, 45: D200–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- María A. et al. (2020) ‘ ICTV Virus Taxonomy Profile: Botourmiaviridae’, Journal of General Virology, 101: 454–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvelli R. A. et al. (2014) ‘ Identification of Novel Double-Stranded RNA Mycoviruses of Fusarium Virguliforme and Evidence of Their Effects on Virulence’, Archives of Virology, 159: 349–52. [DOI] [PubMed] [Google Scholar]
- Marzano S.-Y. L. et al. (2016) ‘ Identification of Diverse Mycoviruses through Metatranscriptomics Characterization of the Viromes of Five Major Fungal Plant Pathogens’, Journal of Virology, 90: 6846–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medd N. C. et al. (2017) ‘ The Virome of Drosophila Suzukii, an Invasive Pest of Soft Fruit’, Virus Evolution, 4: vey009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milgroom M. G., Cortesi P. (2004) ‘ Biological Control of Chestnut Blight with Hypovirulence: A Critical Analysis’, Annual Review of Phytopathology, 42: 311–38. [DOI] [PubMed] [Google Scholar]
- Mokili J. L., Rohwer F., Dutilh B. E. (2012) ‘ Metagenomics and Future Perspectives in Virus Discovery’, Current Opinion in Virology, 2: 63–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu F. et al. (2018) ‘ Virome Characterizatiodn of a Collection of S. sclerotium from Australia’, Microbiology, 8: 2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedveckyte I., Pečiulyte D., Buda V. (2014) ‘ Entomopathogenic Fungi: Diversity and Peculiarities of Interactions with Insects’, Ekologija, 60: 39–53. [Google Scholar]
- Nerva L.(2019) Isolation, Molecular Characterization and Virome Analysis of Culturable Wood Fungal Endophytes in Esca Symptomatic and Asymptomatic Grapevine Plants, Environmental Microbiology, doi: 10.1111/1462-2920.14651. [DOI] [PubMed] [Google Scholar]
- Nogawa M., et al. (1996) Cloning and Characterization of Mycovirus Double-Stranded RNA FROM THE Plant Pathogenic Fungus, Fusarium Solani f. sp. robiniae, Bioscience, Biotechnology and Biochemistry, 60: 784–8. [DOI] [PubMed] [Google Scholar]
- Nuss D. L. (2005) ‘ Hypovirulence: Mycoviruses at the Fungal-Plant Interface’, Nature Reviews Microbiology, 3: 632–42. [DOI] [PubMed] [Google Scholar]
- Okada R., et al. (2018) Molecular Characterization of a Novel Mycovirus in Alternaria Alternata Manifesting Two-Sided Effects: Down-Regulation of Host Growth and up-Regulation of Host Plant Pathogenicity, Virology, 519: 23–32. [DOI] [PubMed] [Google Scholar]
- Pearson M. N. et al. (2009) ‘ Mycoviruses of Filamentous Fungi and Their Relevance to Plant Pathology’, Molecular Plant Pathology, 10: 115–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picarelli M. A. S. C. et al. (2019) ‘ Extreme Diversity of Mycoviruses Present in Isolates of Rhizoctonia solani AG2-2 LP from Zoysia Japonica from Brazil’, Frontiers in Cellular and Infection Microbiology, 9: 244–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preisig O. (1998) Coinfection of a Fungal Pathogen by Two Distinct Double-Stranded RNA Viruses, Virology, 252: 399–406. [DOI] [PubMed] [Google Scholar]
- Pun K. B., Sabitha D., Valluvaparidasan V. (1998) ‘ Studies on Seed-Borne Nature of Macrophomina Phaseolina in Okra’, Plant Disease Research, 13: 249–90. [Google Scholar]
- Ran H. C. et al. (2016) ‘ Co-Infection of a Hypovirulent Isolate of Sclerotinia sclerotiorum with a New Botybirnavirus and a Strain of a Mitovirus’, Virology Journal, 13: 92–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochon L. (2012) ‘Family Tombusviridae’, in King A.M.Q., Adams M.J., Carstens E.B., Lefkowitz E.J. (eds.) Virus Taxonomy: Classification and Nomenclature of Viruses, Ninth Report of the International Committee on Taxonomy of Viruses, pp.1111–38. Waltham, MA, USA. [Google Scholar]
- Rodrigo A. et al. (2019) ‘ ICTV Virus Taxonomy Profile: Endornaviridae’, Journal of General Virology, 100: 1204–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roossinck M. J. (2014) ‘ Metagenomics of Plant and Fungal Viruses Reveals an Abundance of Persistent Lifestyles’, Frontiers in Microbiology, 5: 767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roossinck M. J. et al. (2011) ‘ The Remarkable Evolutionary History of Endornaviruses’, Journal of General Virology, 92: 2674–8. [DOI] [PubMed] [Google Scholar]
- Rosseto P. et al. (2016) ‘ Investigation of Mycoviruses in Endophytic and Phytopathogenic Strains of Colletotrichum from Different Hosts’, Genetics and Molecular Research, 15: 15017651. [DOI] [PubMed] [Google Scholar]
- Shi M. et al. (2016) ‘ Redefining the Invertebrate RNA Virosphere’, Nature, 540: 539–43. [DOI] [PubMed] [Google Scholar]
- Short G. E., Wyllie T. D., Bristow P. R. (1980) ‘ Survival of M. phaseolina in Soil and Residue of Soybeans’, Phytopathology, 70: 13–7. [Google Scholar]
- Song D. et al. (2013) ‘ Evolution of and Horizontal Gene Transfer in the Endornavirus Genus’, PLoS One, 8: e64270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan A. et al. (2009) ‘ Cutaneous Infection Caused by Macrophomina Phaseolina in a Child with Acute Myeloid Leukemia’, Journal of Clinical Microbiology, 47: 1969–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N. et al. ; ICTV Report Consortium (2018) ‘ ICTV Virus Taxonomy Profile: Hypoviridae’, Journal of General Virology, 99: 615–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turina M. et al. ; ICTV Report Consortium (2017) ‘ ICTV Virus Taxonomy Profile: Ourmiavirus’, Journal of General Virology, 98: 129–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twizeyimana M. et al. (2012) ‘ A Cut-Stem Inoculation Technique to Evaluate Soybean for Resistance to Macrophomina Phaseolina’, Plant Disease, 96: 1210–5. [DOI] [PubMed] [Google Scholar]
- Vainio E. J. et al. ; ICTV Report Consortium (2018) ‘ ICTV Virus Taxonomy Profile: Partitiviridae’, Journal of General Virology, 99: 17–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varsani A., Krupovic M. (2017) ‘ Sequence-Based Taxonomic Framework for the Classification of Uncultured Single-Stranded DNA Viruses of the Family Genomoviridae’, Virus Evolution, 3: 37–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. et al. (2019) ‘ A Novel Double-Stranded RNA Mycovirus That Infects Macrophomina Phaseolina’, Archives of Virology, 164: 2411–6. [DOI] [PubMed] [Google Scholar]
- Wang J. et al. (2020) ‘ Complete Genome Sequence of a Novel Victorivirus Isolated from the Sesame Charcoal Rot Fungus Macrophomina Phaseolina’, Archives of Virology, 165: 509–14. [DOI] [PubMed] [Google Scholar]
- Wang L. et al. (2016) ‘ Two Novel Relative Double-Stranded RNA Mycoviruses Infecting Fusarium Poae Strain SX63’, International Journal of Molecular Sciences, 17: 641–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. et al. (2018) ‘ Evidence for a Novel Negative-Stranded RNA Mycovirus Isolated from the Plant Pathogenic Fungus fusarium graminearum’, Virology, 518: 232–40. [DOI] [PubMed] [Google Scholar]
- Wickner G. (2012) ‘Family Totiviridae’ in King A.M.Q., Adams M.J., Carstens E.B., Lefkowitz E.J. (eds.) Virus Taxonomy: Classification and Nomenclature of Viruses, Ninth Report of the International Committee on Taxonomy of Viruses, pp.639–50. Waltham, MA, USA. [Google Scholar]
- Wrather J. A., Koenning S. R. (2006) ‘ Estimates of Disease Effects on Soybean Yields in the United States 2003 to 2005’, Journal of Nematology, 38: 173–80. [PMC free article] [PubMed] [Google Scholar]
- Wu M. D. et al. (2007) ‘ Hypovirulence and Double-Stranded RNA in Botrytis Cinerea’, Phytopathology, 97: 1590–9. [DOI] [PubMed] [Google Scholar]
- Wyllie T. D. (1988) ‘Charcoal Rot of Soybean-Current Status’, inWyllie T.D., Scott D.H. (eds.) Soybean Diseases of the North Central Region, pp. 106–13. Saint Paul, MN. [Google Scholar]
- Xie J. T., Ghabrial S. A. (2012) ‘ Molecular Characterizations of Two Mitoviruses Coinfecting a Hypovirulent Isolate of the Plant Pathogenic Fungus Sclerotinia sclerotiorum’, Virology, 428: 77–85. [DOI] [PubMed] [Google Scholar]
- Xie J. T., Jiang D. H. (2014) ‘ New Insights into Mycoviruses and Exploration for the Biological Control of Crop Fungal Diseases’, Annual Review of Phytopathology, 52: 45–68. [DOI] [PubMed] [Google Scholar]
- Yu L. et al. (2015) ‘ Novel Hypovirulence-Associated RNA Mycovirus in the Plant-Pathogenic Fungus Botrytis Cinerea: Molecular and Biological Characterization’, Applied and Environmental Microbiology, 81: 2299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X. et al. (2010) ‘ A Geminivirus-Related DNA Mycovirus That Confers Hypovirulence to a Plant Pathogenic Fungus’, Proceedings of the National Academy of Sciences, 107: 8387–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X. et al. (2013) ‘ Extracellular Transmission of a DNA Mycovirus and its Use as a Natural Fungicide’, Proceedings of the National Academy of Sciences, 110: 1452–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D. X., Nuss D. L. (2016) ‘ Engineering Super Mycovirus Donor Strains of Chestnut Blight Fungus by Systematic Disruption of Multilocus Vic Genes’, Proceedings of the National Academy of Sciences, 113: 2062–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J., et al. (2014) Complete Genome Sequence and Organization of a Novel Virus from the Rice False Smut Fungus Ustilaginoidea Virens, Virus Genes, 48: 329–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. The GenBank accession numbers for the nucleotide sequence of 38 mycoviruses are listed in the Table 1. Additional data related to this paper may be requested from the authors.