Abstract
Background
The prevalence of eating disorders, including binge eating disorder, is significantly higher in women. These findings are mirrored by preclinical studies, which indicate that female rats have a higher preference for palatable food and show greater binge-like eating compared with male rats.
Methods
Here, we describe a novel within-session behavioral-economic paradigm that allows for the simultaneous measurement of the intake at null cost (Q0) and normalized demand elasticity (α) of 3 types of palatable food (low fat, high fat, and chocolate sucrose pellets) via demand curve analysis. In light of evidence that the orexin (hypocretin) system is critically involved in reward and feeding behaviors, we also examined the role of orexin function in sex differences of economic demand for palatable foods.
Results
The novel within-session behavioral-economic approach revealed that female rats have higher intake (demand) than males for all palatable foods at low cost (normalized to body weight) but no difference in intake at higher prices, indicating sex-dependent differences in the hedonic, but not motivational, aspects of palatable food. Immediately following behavioral-economic testing, we observed more orexin-expressing neurons and Fos expression (measure of recent neural activation) in these neurons in female rats compared with male rats. Moreover, the orexin-1 receptor antagonist SB334867 reduced both low- and high-cost intake for palatable food in both male and female rats.
Conclusions
These findings provide evidence of higher demand at low prices for palatable food in females and indicate that these behavioral differences may be associated with sexual dimorphism in orexin system function.
Keywords: Hypocretin, high-fat food, sucrose, motivation, behavioral economics, SB334867
Significance Statement.
The prevalence of eating disorders, including binge eating disorder, is significantly higher in women. These findings are mirrored by preclinical studies, which indicate that female rats have a higher preference for palatable food and show greater binge-like eating compared with male rats. Here, we evaluated the demand for 3 different palatable rewards in male and female rats using a behavioral economics paradigm. We determined that female rats have a higher demand at null cost for all 3 of the palatable rewards. We did not observe sex differences in motivation to obtain the rewards. Furthermore, we indicate that these behavioral differences may be associated with differences in expression of the hypothalamic neuropeptide orexin (hypocretin) between male and female rats and show that orexin receptor-1 blockade generally reduces null cost and motivated responding for palatable food in both sexes.
Introduction
Eating disorders are the most sex-differentiated psychiatric disorders, with estimates of female-to-male ratios ranging from 4:1 (Hudson et al., 2007) to 10:1 (American Psychiatric Association, 2013). In clinical studies, females report a higher preference for high-fat, high-sugar foods and carbohydrates (Asarian and Geary, 2013) as well as enhanced neuronal responses to palatable foods in key brain reward regions (Cordeira et al., 2010; Legget et al., 2018). These findings are generally recapitulated in animal studies; female rats consume more palatable food and escalate their intake of palatable food at a faster rate compared with males (Babbs et al., 2011; Carlin et al., 2016; Hardaway et al., 2016). Thus, rodents offer a valuable model system to interrogate the biological factors that underpin sex differences in eating behavior.
Specialized assays are often used to measure the contribution of motivational vs hedonic processes to food intake in male vs female rats. For example, individual differences in the hedonic processing (“liking”) of food is typically inferred from measuring orofacial reactions to food (Grill and Norgren, 1978; Doyle et al., 1993; Ho and Berridge, 2013), whereas differences in motivation for a food (“wanting”) can be measured using tasks that require the animal to exert high amounts of physical effort (as in a progressive ratio [PR] task) or endure pain (typically foot shock) to earn the food reward (Oswald et al., 2011; Tapia et al., 2019). Although informative, these assays are limited by the fact that only 1 index (motivation or hedonia) can be measured at a time. Moreover, motivational measures such as PR do not account for individual differences in preferred levels of food intake. To this end, a behavioral-economic (BE) approach offers a unique means to measure several components of reinforcer value simultaneously and account for the effect of preferred intake on measures of motivation. By examining consumption of a reinforcer across multiple “prices” within a single session, it is possible to derive the preferred level of consumption under no-cost conditions (Q0) as well as demand elasticity, or the change in consumption with increasing price (α; an inverse index of motivation). Importantly, this approach offers a structured quantitative method to model behavior independently of differences in preferred intake at null cost, which can vary significantly between animals of different body weights and sex. Although such an approach has been successfully used to compare the reinforcing properties of drugs of abuse between sexes (Bentzley et al., 2013; Porter-Stransky et al., 2017; Kawa and Robinson, 2019), a within-session BE approach to study sex-specific differences relating to palatable food intake should be further explored.
Significant evidence indicates that signaling at the orexin receptor 1 (OxR1) is critically involved in the motivational properties of feeding. Notably, the selective OxR1 antagonist SB334867 (SB) reduces effortful responding for palatable and high-fat foods (Borgland et al., 2009; Cason and Aston-Jones, 2013a) and blocks consumption of a sweetened fat mixture in a binge-like eating paradigm (Piccoli et al., 2012). Orexin signaling also appears to be important for hedonic processing of food, as injections of exogenous orexin increase hedonic reactions to sucrose reward as measured by orofacial reactions (Ho and Berridge, 2013).
Here, we used a novel BE approach to directly compare males and females in terms of their low- and high-cost (i.e., low- and high-effort) consumption of both high-fat palatable and low-fat palatable (LFP) food pellets as well as for a highly palatable but non-fat alternative food (artificially) chocolate-flavored sucrose. We found that females have higher consumption of all foods tested at low cost (Q0) but do not differ from males with respect to their motivation (α) for these foods. These differences are associated with sexual dimorphism in orexin system function, as female rats exhibited greater activation and numbers of orexin-expressing neurons. Moreover, both low- and high-cost consumption of all palatable foods was reduced by the orexin-1 receptor antagonist SB comparably in both sexes.
MATERIALS AND METHODS
Animals
Male and female Sprague Dawley rats (approximately 225–275 g on arrival; Charles River Laboratories; n = 38) were single-housed and kept on a reverse 12-hour-light schedule (lights off at 6:00 am, on at 6:00 pm), with all behavioral testing occurring during the dark cycle. While rodents are social animals and benefit from group-housing, we single-housed the rats to control and monitor food intake for this dietary study, as done previously in other dietary studies (Babbs et al., 2011; Cason and Aston-Jones, 2013a, 2013b; Kay et al., 2014; Bello et al., 2019). Given that rats from this study were in the same room, they were still exposed to olfactory, visual, and auditory contact with conspecifics and therefore not totally isolated (Krohn et al., 2006). Furthermore, rats received frequent handling due to the nature of this study, which included daily training or testing. Rats were fed Harlan Teklad 8656 (males 25 g/d, females 18 g/d; this mild food restriction was proportional to mean differences in body weights between sexes) and were given ad libitum access to water. All protocols and procedures followed the NIH Guidelines for the Care and Use of Laboratory Animals and were approved by the Medical University of South Carolina Institutional Animal Care and Use Committee.
Reward Pellets
Three different rewards were utilized during this study. The LFP reward was a 45-mg grain-based pellet (Bio-Serv, Frenchtown, NJ). This pellet delivers 0.85 kcal/g from protein, 0.34 kcal/g from fat, and 2.16 kcal/g from carbohydrate. The main fat sources are soybean oil and porcine animal fat. This pellet also contains banana flakes, beet pulp, sucrose, fructose, and dextrose, contributing to the palatability. The pellet contains a standard mineral and vitamin mix. The high-fat palatable reward (HFP) was a 45 mg Pellet (Bio-Serv). This pellet delivers 0.72 kcal/g from protein, 2.11 kcal/g from fat, and 1.77 kcal/g from carbohydrate. The fat sources are hydrogenated cottonseed oil and soybean oil. This pellet also contains dextrose and sucrose contributing to the palatability. The pellet also contains a standard mineral and vitamin mix. Lastly, the chocolate-flavored sucrose pellet (CSP) is a 45-mg Chocolate-Flavored Sucrose Pellet (Bio-Serv) reward. This pellet delivers 3.5 kcal/g from carbohydrate. This is an artificially flavored chocolate pellet and only contains sucrose and dextrose; there is no fat. There are no vitamins or minerals.
Behavioral Economics Paradigm
Rats were trained to lever press for food rewards in an operant chamber (Med Associates) housed inside a sound attenuating cubicle, which contained a red house light, 2 retractable levers with white cue lights above them, a food hopper, and a tone generator. Rats underwent fixed-ratio 1 (FR1) training followed by FR3, FR10, FR32 and FR100, each for a minimum of 2 days during which they were required to meet criteria of at least 60 lever presses/d (there was no minimum lever press criteria for FR100). Animals that did not progress through each FR value were removed from the study (n = 2). After 2 days of FR100 training, rats started BE training and testing. During the 105 minutes BE session, a 5 minute “active period” was signaled by the illumination of the house light and extension of the levers. During the active periods, responses on the active lever resulted in delivery of a reward pellet on an FR schedule. The first active period was the highest schedule of reinforcement (FR100) followed by FR32, FR10, FR3, and FR1. There was no maximum for pellets earned during training or testing. Instead, between each of these active periods, there was a 20 minutes time-out period signaled by darkness in the chamber and retraction of the levers; the time-out and the reverse-order of FR schedules were employed to limit satiation. Responses on an inactive lever were not reinforced. This design produced full demand curves (see Figure 1e). Rats were trained on a minimum of 6 BE sessions and until α (demand elasticity) varied by less than 25% across the last 3 days.
Figure 1.
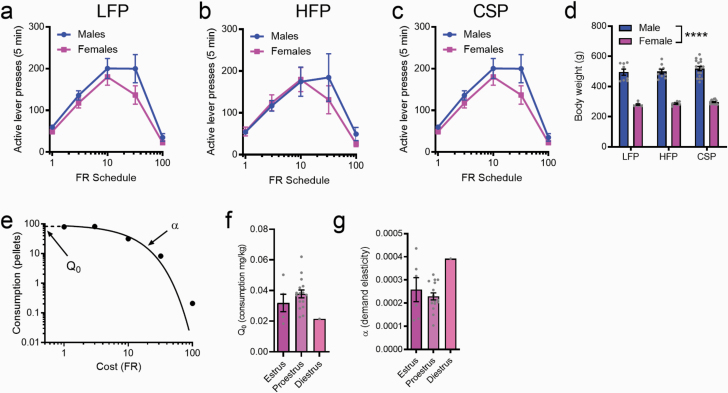
Establishment and validation of a behavioral-economic (BE) procedure for palatable food. Active lever presses at each price during the within-session BE protocol were similar across sexes (a, LFP [low-fat palatable reward]; b, HFP [high-fat palatable reward], c, CSP [chocolate sucrose palatable reward]). However, body weights were significantly higher in males compared with females (d). An example demand curve from the within-session protocol (e) and strong demand curve fits (R2 = 0.974 ± 0.023) across all animals supports feasibility of the BE procedure for palatable food. Estrus cycle was evaluated in female rats; Q0 and α values did not differ between estrus and proestrus (f and g); all rats were either in estrus or proestrus (except for 1 rat in diestrus) at the time of testing. ****P < .0001.
Peripheral Administration of the Selective Orexin-1 Receptor Antagonist (SB334867)
Once the rats reached a stable baseline α, they were treated with SB (0, 30 mg/kg; i.p.) in a counterbalanced fashion 30 minutes prior to BE testing. This dose of SB was selected based on previous studies indicating that this dose reliably affects motivated reward seeking, including for food (Fragale et al., 2019; James et al., 2019a, 2019b; Barson, 2020; Mohammadkhani et al., 2020; Wiskerke et al., 2020). The drug and vehicle were prepared as described previously (Smith et al., 2009).
Locomotor Testing
Locomotor analysis was evaluated at the end of the study, as described previously (Smith et al., 2009). Animals were given SB or vehicle (counterbalanced between groups) 30 minutes prior to a 120-minute test session.
Tissue Preparation
A subgroup was re-stabilized on BE for CSP; the next session animals were killed 90 minutes after Pmax (point of highest responding, determined on that test day). Animals were anesthetized with an overdose of ketamine/xylazine, perfused transcardially with 0.9% saline and 4% paraformaldehyde in 0.1 M phosphate buffered saline, and brains were collected. This time point was chosen because Fos expression is maximal at 60–90 minutes after a behavioral manipulation. Brains were kept in 4% paraformaldehyde for 24 hours and then submerged in a 20% sucrose-azide solution. Coronal brain sections were cut at the level of the lateral hypothalamus on a cryostat at 40 µm thick and stored in phosphate buffered saline-azide.
Immunohistochemistry
Sections from the hypothalamus were processed for Fos immunohistochemistry as done previously (Mahler and Aston-Jones, 2012). Briefly, every 6th section through the hypothalamus was selected to visualize orexin neurons throughout the longitudinal axis of the lateral hypothalamus. Sections were incubated in a rabbit anti-Fos primary antibody overnight (1:1000; Millipore ABE457), followed by 2 hours in a donkey anti-rabbit secondary (1:500, Jackson ImmunoResearch Laboratories, West Grove, PA). The same sections from the hypothalamus were then processed for Orx-A immunohistochemistry in a goat anti-Orx A primary antibody overnight (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA) and then incubated for 2 hours in a donkey anti-goat secondary antibody (1:500, Jackson ImmunoResearch Laboratories, West Grove, PA).
Quantification of Immunoreactivity
Photomicrographs were collected using Openlab image processing software (Improvision) and a Leica microscope (10× magnification). The number of orexin-positive neurons (brown reaction product) and neurons double-labeled for Fos + orexin were quantified using a point-counter tool (ImageJ) bilaterally through the main rostrocaudal extent of the hypothalamic orexin neuron region (2–3 sections/rat) by an experimenter blind to experimental groups. For all cell counting, the number of immunoreactive cells was averaged across all sections for each rat.
Estrous Cycle Monitoring
Throughout FR training and BE testing, estrous cycle was evaluated via vaginal lavage as described previously (Cox et al., 2013; Bello et al., 2019).
Experimental Design
Male and female rats were randomly divided into groups to receive either the LFP or HFP. All animals were given FR training and then BE testing. Animals were stabilized on BE over a minimum of 6 days and then tested with vehicle or SB. A subgroup of animals received SB and vehicle testing 1 day apart (n = 13); however, the majority of animals were given a minimum of an additional 3 sessions between testing days. Once completing SB and vehicle testing, all animals were switched to the CSP reward. Rats were tested for a minimum of 6 days on BE for CSP until demand stabilized and were then retested under vehicle/SB. Because demand for CSP and the effects of SB did not differ between those rats with a history of LFP vs HFP, groups were collapsed to make 2 groups (male CSP, female CSP). Next, animals (n = 28) underwent locomotor testing. Four sessions were administered: habituation, baseline testing, vehicle testing, and SB testing (counterbalanced with vehicle and SB). Finally, a subgroup of animals was retested on BE for CSP until stable behavior was again achieved. In the following session, demand was measured in real-time, and animals were perfused 90 minutes following Pmax (as described above; n = 14).
Data Analyses
An exponential demand equation was applied to BE data to generate demand curves, α, and Q0 values (Hursh and Silberberg, 2008). To normalize Q0 values, the raw Q0 value was multiplied by calories provided by a particular pellet and divided by the animal’s body weight in grams. Differences in baseline BE values between males and females were examined using a 2-way ANOVA (sex × diet). Effects of vehicle and SB were analyzed by calculating the percent change relative to baseline followed by repeated measures 2-way ANOVAs (sex × treatment) for each food type. Overall locomotor activity was analyzed using a 2-way repeated-measures ANOVA (time × treatment). To determine the relationship between SB on locomotor activity and BE parameters, we correlated the percent change in locomotor activity following SB (vs vehicle) to the percent change in behavior following SB (vs vehicle) using Pearson’s correlations. For immunohistochemistry, the number of orexin-expressing neurons, and the percentage of Fos+ orexin neurons were analyzed using 2-way ANOVA (sex × region).
RESULTS
Establishment of a Within-Session Behavioral Economics Procedure for Palatable Food Pellets
We first sought to establish a within-session BE procedure for palatable food pellets (HFP, LFP, and CSP). Animals initially underwent 2-hour training sessions for each of the FR schedules; the mean number of active lever presses and pellets earned over the last 2 days of FR responding at each price, for both male and female rats, is presented in Table 1. Rats were then trained on the within-session threshold procedure for a mean of 8.87 ± 2.59 days before reaching a stable baseline. The average number of active lever presses at each price during the threshold procedure for each food type is shown in Figure 1 (a, HFP; b, LFP, c, CSP). For all food types, there was no main effect of sex (P > .05) nor a sex × price interaction (P > .05), indicating that responding was similar across sexes at all prices. This is despite there being a significant difference in body weights between male and female rats when tested on all foods (Figure 1d; P < .0001). We used the exponential demand equation from Hursh and Silberberg (Hursh and Silberberg, 2008) to generate demand curves for each animal across all food pellet types (example demand curve is shown in Figure 1e). This approach was associated with strong demand curve fits (R2 = 0.974 ± 0.023) and stable α and Q0 values across consecutive days. In a subpopulation of female rats, we examined estrus cycle stage prior to BE testing. Only 1 rat was found to be in diestrus at the time of testing; all others were either in estrus or proestrus. For α and Q0, there was no main effect of food type, and thus values were collapsed across LFP, HFP, and CSP. Comparisons revealed no effect of estrus stage for either parameter (P > .05; Figure 1f+g).
Table 1.
Average Number of Active/Inactive Lever Responses and Pellets Earned During 2-Hour FR Training Sessions Prior to Testing
| Food pellet | FR | Sex | Active Lever | Inactive Lever | Pellets |
|---|---|---|---|---|---|
| HFP | 1 | M | 275 (58) | 15 (6) | 215 (42) |
| F | 195 (40) | 29 (10) | 121 (22) | ||
| 3 | M | 429 (76) | 18 (4) | 134 (24) | |
| F | 442 (104) | 15 (6) | 121 (28) | ||
| 10 | M | 627 (122) | 15 (3) | 61 (12) | |
| F | 731 (141) | 19 (8) | 70 (13) | ||
| 32 | M | 567 (155) | 16 (3) | 17 (5) | |
| F | 785 (236) | 12 (5) | 32 (10) | ||
| 100 | M | 395 (136) | 17 (3) | 3 (1) | |
| F | 508 (286) | 8 (4) | 5 (3) | ||
| LFP | 1 | M | 213 (40) | 14 (3) | 159 (28) |
| F | 101 (10) | 26 (4) | 81 (6) | ||
| 3 | M | 362 (53) | 16 (7) | 113 (17) | |
| F | 262 (28) | 27 (8) | 78 (8) | ||
| 10 | M | 1056 (137) | 23 (9) | 104 (13) | |
| F | 488 (76) | 14 (3) | 47 (7) | ||
| 32 | M | 985 (199) | 34 (16) | 30 (6) | |
| F | 571 (115) | 15 (3) | 17 (4) | ||
| 100 | M | 676 (165) | 24 (8) | 6 (1) | |
| F | 276 (103) | 16 (4) | 2 (1) |
Abbreviations: FR, fixed ratio; HFP, high-fat palatable reward; LFP, low-fat palatable reward.
Values are n (SEM).
Females Have Higher Demand at Null Cost for All Food Types
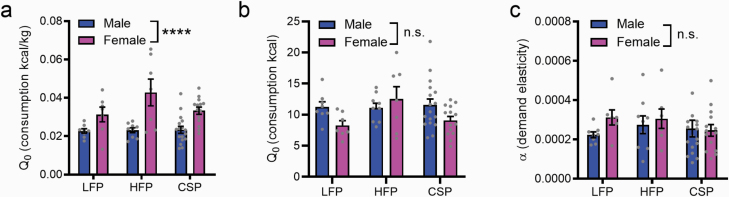
We next used the BE paradigm to determine sex differences in preferred intake at null cost for each of the 3 palatable food pellets. Q0, or estimated consumption at null cost, was used as an index of animals’ preferred level of intake of each food under free access conditions. Because kilocalories per pellet differed significantly across food types (LFP: 3.35 kcal/g; HFP: 4.60 kcal/g; CSP: 3.50 kcal/g) and body weight differed significantly between male and female rats (Figure 1d), consumption values were normalized by multiplying Q0 by the kilocalories per 45-mg pellet and divided by the rat’s body weight during testing. Across all food types, females had higher demand at null cost compared with males (F1,56 = 29.00, P < .0001; Figure 2a); there was no effect of food type (P = .1396) nor a sex × food type interaction (P = .1433). Without accounting for body size, there were no significant differences in Q0 (kcals consumed; Figure 2b) for sex (P = .1317) or food type (P = .1960) nor a sex × food type interaction (P = .1097), indicating males and females consumed similar calories from the palatable rewards despite significant differences in body weight (Figure 1d). In contrast to Q0, males and females did not differ with respect to demand elasticity (α; Figure 2b) for any food type (all main effects and interactions P > .05).
Figure 2.
Females have higher demand at null cost for all palatable foods compared with males. Within-session behavioral-economic (BE) analyses revealed that female rats had higher demand at null cost (Q0; panel a) for all palatable foods (low-fat palatable reward [LFP], high-fat palatable reward [HFP], and CSP [chocolate sucrose palatable reward]) compared with male rats when consumption was adjusted for kcal/kg. In contrast, female and male rats did not differ significantly on demand at null cost (Q0; calories consumed, without adjustment for body weight; panel b) or demand elasticity (α; panel c). Data depict mean values (±SEM) from 3 BE sessions. ****P < .0001. n.s., not significant.
Orexin-1 Receptor Antagonist SB Reduces Demand and Motivation in Both Male and Female Rats
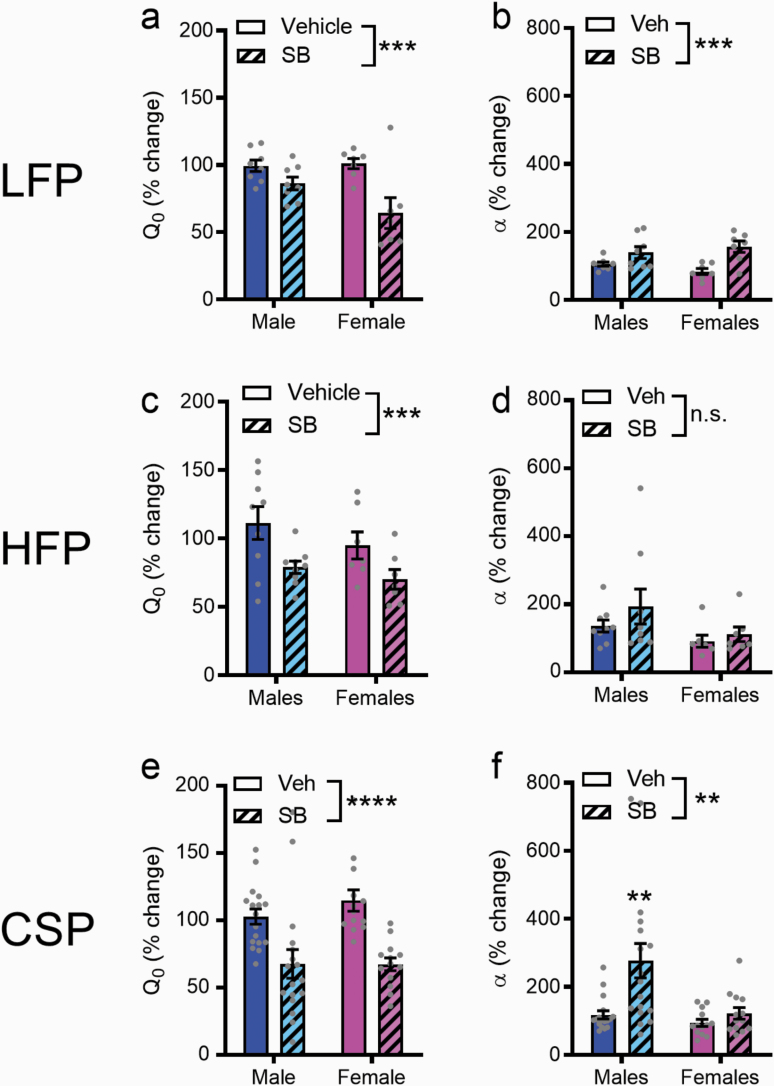
We next sought to determine the effect of systemic treatment with the OxR1 antagonist SB on demand and motivational measures for palatable food in male vs female rats. For LFP, there was a significant main effect of “treatment” on Q0 (F1,13 = 20.28, P = .0006; Figure 3a) and α (F1,13 = 21.71, P = .0004; Figure 3b) values, indicating that SB reduced both low- and high-cost responding for this palatable food type. Despite a trend towards stronger effects of SB on Q0 in female rats (interaction: P = .0528), effects were overall similar between sexes.
Figure 3.
Effects of an orexin-1 receptor antagonist (SB) on demand and motivation for palatable foods in both sexes. Across both male and female rats, SB reduced low (Q0, panel a) and high cost (α, panel b) responding for low-fat palatable reward (LFP). In rats tested on behavioral-economic (BE) for high-fat palatable reward (HFP), SB reduced low- (Q0, panel c), but not high-cost (α, panel d) responding in both male and female rats. When tested on chocolate sucrose palatable reward (CSP), SB reduced low- (Q0, panel e) and high-cost (α, panel f) responding in both males and females; posthoc analyses revealed that α values were significantly higher (motivation was lower) following SB in males compared with females (P = .0017). **P < .01, ***P < .001, ****P < .0001.
For HFP, there was a significant main effect of treatment on Q0 (F1,14 = 14.17, P = .0021; Figure 3c), indicating that SB reduced null cost intake in both male and female rats. There was no effect of treatment on α (P = .2717; Figure 3d). There was no sex × treatment interaction for Q0 or α (P > .05).
For CSP, there was a significant main effect of “treatment” on Q0 (F1,28 = 42.71, P < .0001; Figure 3e) and α (F1,13 = 21.71, P = .0004; Figure 3f) values, indicating that SB reduced both low- and high-cost responding. There was a significant sex × treatment interaction for α (F1,28 = 4.462, P = .0437), with Sidak post-hoc analyses revealing that α values were significantly higher following SB in males compared with females (P = .0017). No such interaction was observed for Q0 (P > .05).
Importantly, although tests of general locomotor activity revealed modest SB-induced reductions in general activity in both male and female rats, individual changes in locomotor activity did not correlate with changes in BE parameters (supplementary Figure 1), indicating that any soporific effects of SB were not significantly related to its effects on Q0 or α. We conducted locomotor testing in locomotor analysis chambers, as done previously (Smith et al., 2009; Bentzley and Aston-Jones, 2015; Porter-Stransky et al., 2017). However, a limitation to these findings is that locomotor analyses were conducted in a separate chamber from the operant chamber.
Increased Orexin Cell Number and cFos+ Orexin Cells in Female Rats During the BE Task
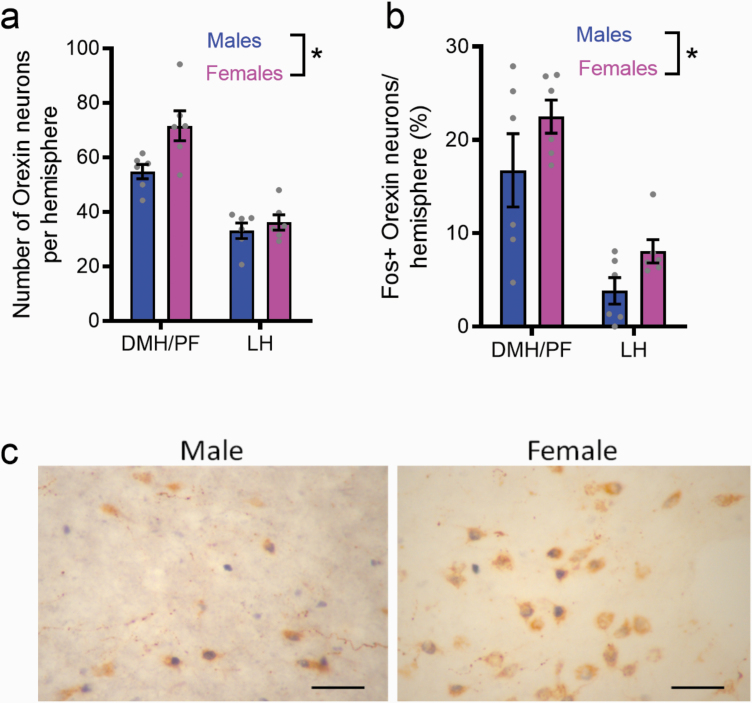
We euthanized a subgroup of rats 90 minutes after Pmax in the BE task for CSP; this allowed us to examine any sex differences in orexin neuron cell number and activity (as gauged by cFos expression) relevant to responding during the BE task. Separate counts were made for medial (DMH/PF) and lateral (LH) orexin cell populations. With respect to the number of orexin-expressing neurons, we observed a significant main effect of sex (F1,20 = 7.448, P = .0129; Figure 4a), indicating a higher overall number of orexin neurons in female rats compared with males. There was a trend towards a sex × region interaction; however, this failed to reach significance (P = .0728), indicating that the increased number of orexin neurons in females was not specific to the medial or lateral subpopulation. Across both sexes, there was no relationship between the number of orexin-expressing neurons in DMH/PF or LH and any behavioral variables from the final CSP BE session (P > .05, data not shown). We observed similar results with respect to the percentage of cFos+ orexin neurons; a significant main effect of sex (F1,20 = 4.532, P = .0459; Figure 4b), but no sex × region interaction (P = .7488), indicated that females also exhibited greater activity in all orexin neurons during the BE task relative to males. Interestingly, across both male and female rats, alpha values from the final CSP BE test positively correlated with the percentage of cFos+ orexin neurons in DMH/PF (R2 = 0.546, P = .006); a similar nonsignificant trend was observed in LH (R2 = 0.309, P = .061). No such relationship was observed for Q0.
Figure 4.
Higher number and activity of orexin-expressing cells in female rats. Immunohistochemical studies revealed that female rats had a higher number of orexin-expressing neurons across the dorsomedial hypothalamus/perifornical (DMH/PF) and lateral hypothalamus (LH) of the hypothalamus compared with males (a). Females also had an increased percentage of orexin cells that expressed cFos in the hypothalamus compared with males (b). Representative micrographs (40×) for males and females are presented (c). Orexin label is brown, cFos is black. Scale bar = 50 μm. *P < .05.
Discussion
We used a BE approach to reveal sex differences in demand for highly palatable food rewards. Females had higher demand at null cost (Q0) for an LFP reward, HFP reward, and a CSP reward compared with males, but there was no significant difference between sexes on an index of food motivation (demand elasticity [α]). Using the OxR1 antagonist SB, we determined a role for the orexin system in demand at null cost and motivated responding for palatable food in both males and females. Finally, we observed significantly higher numbers of orexin-expressing neurons as well as greater activation of orexin cells during food seeking in female rats. Together, these findings provide evidence of increased demand for palatable food in females and indicate that these behavioral differences may be associated with sexual dimorphism in orexin system function.
We describe a novel within-session BE paradigm that allows for demand curve analysis of palatable food pellets. In these BE sessions, consumption was measured at 5 FR schedules, which were presented in reverse order of “price” (FR100-FR1) with inter-trial intervals between each FR value to limit satiety; this resulted in robust responding at all prices. Using the exponential demand equation (Hursh and Silberberg, 2008), we generated demand curves with strong fits that were highly reliable over repeated sessions, as we have previously reported for various drugs of abuse (Bentzley et al., 2014; Porter-Stransky et al., 2017; James et al., 2019a, 2019b). We report that female rats had higher Q0 values (adjusted for body weight) compared with males across all palatable foods. We and others have previously suggested that Q0 might reflect the hedonic value (i.e., liking) of a reinforcer, indicating that the pleasurable properties of palatable food might be higher in female rats. This interpretation is consistent with evidence that female rats show higher intake of palatable foods in binge-eating paradigms (Babbs et al., 2011; Klump et al., 2013) as well as clinical evidence that obese women report a stronger preference for high-fat and high-carbohydrate foods, and particularly those high in sugar (Drewnowski and Almiron-Roig, 2010). The palatable rewards administered in this study had different levels of fat and carbohydrate, which also contributed to the differences in caloric values. We have described the rewards as LFP, HFP, and CSP. An alternative description could include “high carbohydrate palatable” (for LFP), “low carbohydrate palatable” (for HFP), and “very high carbohydrate palatable” (for CSP). Given that we did not determine significant differences in Q0 between rewards, we hypothesize that both fat and carbohydrate content contributed to the reward palatability. However, while not statistically significant, females revealed the greatest Q0 values for the HFP reward compared with LFP and CSP; males did not reveal any differences in Q0 between rewards. Further investigation will be necessary, but it is possible that high fat is a stronger driver for reward in females compared with other types of palatable rewards.
We also examined demand elasticity (α), or sensitivity of demand to changes in price, which reflects animals’ willingness to “work” to maintain their preferred level of food intake (Bentzley et al., 2014). Unlike PR breakpoints, α values self-normalize to differences in baseline consumption (Q0), thus allowing for the comparison of elasticity across reinforcers and body weights independent of consumption. Interestingly, we observed no sex differences in α across all food types, indicating that the motivational properties of palatable foods under high-cost conditions are similar for male and female rats. Collectively, our data indicate that female rats consume higher amounts of palatable foods under low-cost conditions compared with male rats but show no difference in intake at higher prices. The ability to simultaneously measure motivational vs putative hedonic properties of palatable food within an individual subject and session using the BE paradigm thus offers significant advantages over alternative approaches (home cage intake or PR) to interrogating biological systems contributing to differences in feeding behaviors.
A major advantage of the demand analysis approach is the ability to investigate how within-session measures of food demand are affected by pharmacological intervention. Because the orexin system is important for motivated seeking of rewards, including food, here we tested the effect of SB on demand measures for each of the palatable foods. We administered a 30-mg/kg dosage of SB given previous work indicating this dosage is effective at reducing motivated seeking of rewards with little to no effect on general motor activity (Moorman and Aston-Jones, 2009; Smith et al., 2009; LeSage et al., 2010; Porter-Stransky et al., 2017; James et al., 2019b; Mohammadkhani et al., 2020). We show that for both sexes, SB reduced Q0 for all types of palatable food. Further investigation will be necessary to determine whether lower doses of SB would result in sex-dependent reduction of Q0. SB also reduced animals’ willingness to work for both LFP and CSP (and tended to do the same for HFP), as indicated by higher demand elasticity (higher α values). These findings are consistent with evidence linking orexin signaling with both low- and high-effort intake of palatable food (Barson, 2020). SB is also effective at reducing food-seeking behaviors in paradigms where effortful responding is required to earn food reward. For example, SB reduces PR breakpoints in rats trained to respond for sucrose or sweet-fat pellets (Borgland et al., 2009; Choi et al., 2010; Kay et al., 2014); similar effects are observed following viral-mediated knockdown of orexin neurons in the hypothalamus (Schmeichel et al., 2018). The orexin system appears to be especially important for regulating reward seeking under conditions of augmented motivation (Mahler et al., 2014). SB reduces intake of palatable foods at doses that do not affect regular chow intake (Alcaraz-Iborra et al., 2014; Vickers et al., 2015), and greater effects of SB are observed when animals are food restricted (Cason and Aston-Jones, 2013b; Kay et al., 2014). Moreover, in the case of cocaine demand, SB is only effective when drug infusions are paired with conditioned stimuli (Bentzley and Aston-Jones, 2015), which are known to imbue additional motivational valence (Robinson and Berridge, 1993; Mahler and Berridge, 2009; Anselme et al., 2013). Thus, the fact that animals in the current study were mildly food restricted and pellet deliveries in the BE program were paired with light- + tone-conditioned stimuli likely contributed to the effects of SB reported here. Together, these data indicate that OxR1 antagonists effectively reduce both low- and high-effort responding for palatable food in both sexes and thus might represent an effective strategy for reducing palatable food intake in clinical populations. Of note, here we tested the effects of SB at a single dose (30 mg/kg) only; future studies should investigate the effect of lower doses (e.g., 10 mg/kg) in male vs female rats, especially because emerging evidence from the addiction field indicates that lower doses are effective at reducing motivated behavior specifically in high demand rats (Fragale et al., 2019; James et al., 2019a, 2020; Mohammadkhani et al., 2020). Indeed, it is possible female rats might be more sensitive to the Q0-reducing effects of SB at lower doses given their higher baseline consumption at null cost. The lack of a statistically significant effect of SB vs vehicle on α for HFP was particularly evident in females. Given that demand for HFP was highest (while not statistically significant) for females, these data may indicate sex differences in response to SB specifically for the HFP reward.
Because of recent evidence linking numbers of orexin-immunoreactive cells with motivation for cocaine and opioids (Thannickal et al., 2018; James et al., 2019a; Fragale et al., 2020; Pantazis et al., 2020), we examined orexin expression in male vs female rats. Consistent with previous studies (Jöhren et al., 2003), we observed a significantly higher number of orexin-expressing neurons in female rats compared with males, indicating that the relationship between orexin cell numbers and reward seeking extends to demand for palatable food. We also observed in female rats that orexin neurons exhibited greater activation (as gauged by cFos expression) at the point of maximal responding during the chocolate sucrose BE task. We specifically evaluated cFos expression 90 minutes after Pmax to capture orexin activity relevant to this BE task. Together, these data point to an overall enhanced orexin system functioning in females that is associated with higher demand at null cost for palatable food.
Given our reported sex differences in Q0, orexin cell numbers, and cFos + orexin cell numbers, we hypothesize a role of sex hormones in these differences. For example, female rats exhibit increased expression of OxR1 and OxR2 during proestrus compared with rats in other estrus stages (Silveyra et al., 2010), revealing cycle-dependent differences in orexin. The neuroendocrine role of orexin is complex with site-specific effects on release of hormones (Jöhren et al., 2003). Orexin A has dual effects on luteinizing hormone release in vivo; in ovariectomized rats, i.c.v. administration of orexin-A inhibits luteinizing hormone, whereas luteinizing hormone is stimulated in the presence of estradiol and progesterone supplementation (Pu et al., 1998). In the present study, estrus cycle was monitored throughout FR training and BE testing. No significant differences were observed in α or Q0 values whether females were in proestrus or estrus. However, only 1 rat was in diestrus for their stable BE values. Therefore, diestrus may affect the ability to reach a stable α in this study. Further studies will need to be performed to better understand the role of diestrus in palatable food responding and orexin cell activation.
In conclusion, we describe a novel within-session BE approach for examining demand and motivation for palatable food pellets. These analyses revealed that female rats have significantly higher demand at null cost for 3 types of palatable foods compared with males. These differences in demand were associated with higher numbers and activity of orexin neurons in female rats, indicating that sexual dimorphism in the orexin system function might underlie greater binge-like behavior in women. We also show that the selective OxR1 antagonist SB is highly effective at reducing demand and motivation for palatable foods in both male and female rats, indicating that orexin-based therapeutics might be effective for treating disordered eating in both sexes.
Supplementary data
Supplementary data are available at International Journal of Neuropsychopharmacology (IJNPPY) online.
Figure S1. SB-induced changes to locomotor did not correlate with changes in BE parameters. Locomotor behavior analyses following SB injection revealed a modest but significant decrease in the total distance traveled compared with vehicle injection in both male (main effect of treatment, F1,12 = 6.18, P = .029, a) and female (F1,11 = 9.701, P = .0098, b) rats. To determine whether these soporific effects may have been linked to behavioral effects on the BE paradigm, we correlated the effect of SB on locomotor activity (% of vehicle) with the effect of SB on Q0 and alpha (% of vehicle) across all reinforcers, collapsed across male and female rats. For LFP (c), the magnitude of the effect of SB on locomotor activity was not correlated with change in Q0 (R2 = 0.066, P = .803) or alpha (R2 = 0.080, P = .374). For HFP (d), the magnitude of the effect of SB on locomotor activity was not correlated with change in Q0 (R2 = 0.196, P = .130) or alpha (R2 = 0.002, P = .880). For CSP (e), the magnitude of the effect of SB on locomotor activity was not correlated with change in Q0 (R2 = 0.046, P = .314) or alpha (R2 = 0.006, P = .711). *P < .05. **P < .01.
Acknowledgments
This work was supported by United States Public Health Service (PHS) Awards T32DA7288-22 to L.R.F. and F30DA035065 to B.S.B., a National Health and Medical Research Council of Australia C.J. Martin fellowship (1072706) and National Institute on Drug Abuse (K99DA045765) grant to M.H.J., and by a PHS Award from NIDA (R01-DA006214) to G.A.J.
Interest Statement
G.A.J. has consulted for Merck Pharmaceuticals, but that relationship does not present a conflict of interest for this publication. The authors declare that the research was conducted without any commercial or financial relationships that could be considered a potential conflict of interest.
References
- Alcaraz-Iborra M, Carvajal F, Lerma-Cabrera JM, Valor LM, Cubero I (2014) Binge-like consumption of caloric and non-caloric palatable substances in ad libitum-fed C57BL/6J mice: pharmacological and molecular evidence of orexin involvement. Behav Brain Res 272:93–99. [DOI] [PubMed] [Google Scholar]
- Anselme P, Robinson MJ, Berridge KC (2013) Reward uncertainty enhances incentive salience attribution as sign-tracking. Behav Brain Res 238:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asarian L, Geary N (2013) Sex differences in the physiology of eating. Am J Physiol Regul Integr Comp Physiol 305:R1215–R1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Press. [Google Scholar]
- Babbs RK, Wojnicki FH, Corwin RL (2011) Effect of 2-hydroxyestradiol on binge intake in rats. Physiol Behav 103:508–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barson JR. (2020) Orexin/hypocretin and dysregulated eating: promotion of foraging behavior. Brain Res 1731:145915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello NT, Yeh CY, James MH (2019) Reduced sensory-evoked locus coeruleus-norepinephrine neural activity in female rats with a history of dietary-induced binge eating. Front Psychol 10:1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzley BS, Aston-Jones G (2015) Orexin-1 receptor signaling increases motivation for cocaine-associated cues. Eur J Neurosci 41:1149–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzley BS, Fender KM, Aston-Jones G (2013) The behavioral economics of drug self-administration: a review and new analytical approach for within-session procedures. Psychopharmacology (Berl) 226:113–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzley BS, Jhou TC, Aston-Jones G (2014) Economic demand predicts addiction-like behavior and therapeutic efficacy of oxytocin in the rat. Proc Natl Acad Sci U S A 111:11822–11827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Chang SJ, Bowers MS, Thompson JL, Vittoz N, Floresco SB, Chou J, Chen BT, Bonci A (2009) Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J Neurosci 29:11215–11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin JL, McKee SE, Hill-Smith T, Grissom NM, George R, Lucki I, Reyes TM (2016) Removal of high-fat diet after chronic exposure drives binge behavior and dopaminergic dysregulation in female mice. Neuroscience 326:170–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cason AM, Aston-Jones G (2013a) Attenuation of saccharin-seeking in rats by orexin/hypocretin receptor 1 antagonist. Psychopharmacology (Berl) 228:499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cason AM, Aston-Jones G(2013b) Role of orexin/hypocretin in conditioned sucrose-seeking in rats. Psychopharmacology (Berl) 226:155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DL, Davis JF, Fitzgerald ME, Benoit SC (2010) The role of orexin-A in food motivation, reward-based feeding behavior and food-induced neuronal activation in rats. Neuroscience 167:11–20. [DOI] [PubMed] [Google Scholar]
- Cordeira JW, Frank L, Sena-Esteves M, Pothos EN, Rios M (2010) Brain-derived neurotrophic factor regulates hedonic feeding by acting on the mesolimbic dopamine system. J Neurosci 30:2533–2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox BM, Young AB, See RE, Reichel CM (2013) Sex differences in methamphetamine seeking in rats: impact of oxytocin. Psychoneuroendocrinology 38:2343–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle TG, Berridge KC, Gosnell BA (1993) Morphine enhances hedonic taste palatability in rats. Pharmacol Biochem Behav 46:745–749. [DOI] [PubMed] [Google Scholar]
- Drewnowski A, Almiron-Roig E (2010) Human perceptions and preferences for fat-rich foods. In: Fat Detection: Taste, Texture, and Post Ingestive Effects (Montmayeur JP, le Coutre J eds). Boca Raton, FL: CRC Press/Taylor & Francis. [PubMed] [Google Scholar]
- Fragale JE, Pantazis CB, James MH, Aston-Jones G (2019) The role of orexin-1 receptor signaling in demand for the opioid fentanyl. Neuropsychopharmacology 44:1690–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragale JE, James MH, Aston-Jones G (2020) Intermittent self-administration of fentanyl induces a multifaceted addiction state associated with persistent changes in the orexin system. bioRxiv. doi: 10.1101/2020.04.23.055848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill HJ, Norgren R (1978) The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res 143:263–279. [DOI] [PubMed] [Google Scholar]
- Hardaway JA, Jensen J, Kim M, Mazzone CM, Sugam JA, Diberto JF, Lowery-Gionta EG, Hwa LS, Pleil KE, Bulik CM, Kash TL (2016) Nociceptin receptor antagonist SB 612111 decreases high fat diet binge eating. Behav Brain Res 307:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho CY, Berridge KC (2013) An orexin hotspot in ventral pallidum amplifies hedonic ‘liking’ for sweetness. Neuropsychopharmacology 38:1655–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson JI, Hiripi E, Pope HG Jr, Kessler RC (2007) The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry 61:348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR, Silberberg A (2008) Economic demand and essential value. Psychol Rev 115:186–198. [DOI] [PubMed] [Google Scholar]
- James MH, Mahler SV, Moorman DE, Aston-Jones G (2017) A decade of orexin/hypocretin and addiction: where are we now? Curr Top Behav Neurosci 33:247–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, Stopper CM, Zimmer BA, Koll NE, Bowrey HE, Aston-Jones G (2019a) Increased number and activity of a lateral subpopulation of hypothalamic orexin/hypocretin neurons underlies the expression of an addicted state in rats. Biol Psychiatry 85:925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, Bowrey HE, Stopper CM, Aston-Jones G (2019b) Demand elasticity predicts addiction endophenotypes and the therapeutic efficacy of an orexin/hypocretin-1 receptor antagonist in rats. Eur J Neurosci 50:2602–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, Fragale JE, Aurora RN, Cooperman NA, Langleben DD, Aston-Jones G (2020) Repurposing the dual orexin receptor antagonist suvorexant for the treatment of opioid use disorder: why sleep on this any longer? Neuropsychopharmacology 45:717–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jöhren O, Brüggemann N, Dendorfer A, Dominiak P (2003) Gonadal steroids differentially regulate the messenger ribonucleic acid expression of pituitary orexin type 1 receptors and adrenal orexin type 2 receptors. Endocrinology 144:1219–1225. [DOI] [PubMed] [Google Scholar]
- Kawa AB, Robinson TE (2019) Sex differences in incentive-sensitization produced by intermittent access cocaine self-administration. Psychopharmacology (Berl) 236:625–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay K, Parise EM, Lilly N, Williams DL (2014) Hindbrain orexin 1 receptors influence palatable food intake, operant responding for food, and food-conditioned place preference in rats. Psychopharmacology (Berl) 231:419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Racine S, Hildebrandt B, Sisk CL (2013) Sex differences in binge eating patterns in male and female adult rats. Int J Eat Disord 46:729–736. [DOI] [PubMed] [Google Scholar]
- Krohn TC, Sorensen D, Ottesen J, Kornerup Hansen A (2006) The effects of individual housing on mice and rats: a review. Animal Welfare 15:343–352. [Google Scholar]
- Legget KT, Cornier MA, Bessesen DH, Mohl B, Thomas EA, Tregellas JR (2018) Greater reward-related neuronal response to hedonic foods in women compared with men. Obesity (Silver Spring) 26:362–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeSage MG, Perry JL, Kotz CM, Shelley D, Corrigall WA (2010) Nicotine self-administration in the rat: effects of hypocretin antagonists and changes in hypocretin mRNA. Psychopharmacology (Berl) 209:203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Aston-Jones GS (2012) Fos activation of selective afferents to ventral tegmental area during cue-induced reinstatement of cocaine seeking in rats. J Neurosci 32:13309–13326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Berridge KC (2009) Which cue to “want?” Central amygdala opioid activation enhances and focuses incentive salience on a prepotent reward cue. J Neurosci 29:6500–6513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Moorman DE, Smith RJ, James MH, Aston-Jones G (2014) Motivational activation: a unifying hypothesis of orexin/hypocretin function. Nat Neurosci 17:1298–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadkhani A, James MH, Pantazis CB, Aston-Jones G (2020) Persistent effects of the orexin-1 receptor antagonist SB-334867 on motivation for the fast acting opioid remifentanil. Brain Res 1731:146461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman DE, Aston-Jones G (2009) Orexin-1 receptor antagonism decreases ethanol consumption and preference selectively in high-ethanol–preferring Sprague–Dawley rats. Alcohol 43:379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald KD, Murdaugh DL, King VL, Boggiano MM (2011) Motivation for palatable food despite consequences in an animal model of binge eating. Int J Eat Disord 44:203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantazis CB, James MH, Bentzley BS, Aston-Jones G (2020) The number of lateral hypothalamus orexin/hypocretin neurons contributes to individual differences in cocaine demand. Addict Biol 25:e12795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccoli L, Micioni Di Bonaventura MV, Cifani C, Costantini VJ, Massagrande M, Montanari D, Martinelli P, Antolini M, Ciccocioppo R, Massi M, Merlo-Pich E, Di Fabio R, Corsi M (2012) Role of orexin-1 receptor mechanisms on compulsive food consumption in a model of binge eating in female rats. Neuropsychopharmacology 37:1999–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter-Stransky KA, Bentzley BS, Aston-Jones G (2017) Individual differences in orexin-I receptor modulation of motivation for the opioid remifentanil. Addict Biol 22:303–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu S, Jain MR, Kalra PS, Kalra SP (1998) Orexins, a novel family of hypothalamic neuropeptides, modulate pituitary luteinizing hormone secretion in an ovarian steroid-dependent manner. Regul Pept 78:133–136. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC (1993) The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev 18:247–291. [DOI] [PubMed] [Google Scholar]
- Schmeichel BE, Matzeu A, Koebel P, Vendruscolo LF, Sidhu H, Shahryari R, Kieffer BL, Koob GF, Martin-Fardon R, Contet C (2018) Knockdown of hypocretin attenuates extended access of cocaine self-administration in rats. Neuropsychopharmacology 43:2373–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveyra P, Cataldi NI, Lux-Lantos VA, Libertun C (2010) Role of orexins in the hypothalamic-pituitary-ovarian relationships. Acta Physiol (Oxf) 198:355–360. [DOI] [PubMed] [Google Scholar]
- Smith RJ, See RE, Aston-Jones G (2009) Orexin/hypocretin signaling at the orexin 1 receptor regulates cue-elicited cocaine-seeking. Eur J Neurosci 30:493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapia MA, Lee JR, Weise VN, Tamasi AM, Will MJ (2019) Sex differences in hedonic and homeostatic aspects of palatable food motivation. Behav Brain Res 359:396–400. [DOI] [PubMed] [Google Scholar]
- Thannickal TC, John J, Shan L, Swaab DF, Wu MF , Ramanathan L, McGregor R , Chew KT, Cornford M, Yamanaka A, Inutsuka A, Fronczek R, Lammers GJ, Worley PF, Siegel JM (2018) Opiates increase the number of hypocretin-producing cells in human and mouse brain and reverse cataplexy in a mouse model of narcolepsy. Sci Transl Med 10:eaao4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers SP, Hackett D, Murray F, Hutson PH, Heal DJ (2015) Effects of lisdexamfetamine in a rat model of binge-eating. J Psychopharmacol 29:1290–1307. [DOI] [PubMed] [Google Scholar]
- Wiskerke J, James MH, Aston-Jones G (2020) The orexin-1 receptor antagonist SB-334867 reduces motivation, but not inhibitory control, in a rat stop signal task. Brain Res 1731:146222. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.