Abstract
Background:
Myocardial infarction is one of the leading causes of morbidity and mortality worldwide. Oxidative stress plays a vital role in the pathogenesis of atherosclerosis leading to myocardial infarction and Glutathione S-transferases (GSTs) act as detoxifying enzymes to reduce oxidative stress. The aim of the present study was to investigate the associations of the GST (T1 & M1) gene polymorphism with the susceptibility of myocardial infarction in the Bangladeshi population.
Methods:
A case-control study on 100 cardiac patients with MI and 150 control subjects was conducted. The genotyping of GST (T1 & M1) gene was done using conventional Polymerase Chain Reaction.
Results:
The percentage of GSTM1 genotypes was significantly (p< 0.01) lower in patients compared to control subjects while the GSTT1 genotypes were not significantly different between the study subjects. The individual with GSTM1 null allele was at 2.5-fold increased risk {odds ratio (OR)= 2.5; 95 % confidence interval (95 % CI)= 1.4 to 4.3; p< 0.01} of experiencing MI while individual with either GSTM1 or GSTT1 genotypes was at lower risk. In the case of GST M1 and GST T1 combined genotype, patients having both null genotypes for GST M1 and GST T1 gene showed significantly (p< 0.01) higher risk of experiencing MI when compared to control subjects (OR= 3.5; 95% CI= 1.7–7.2; p< 0.001).
Conclusion:
Thus our recent study suggested that GSTM1 alone and GSTM1 and T1 in combination augments the risk of MI in Bangladeshi population.
Key Words: Bangladesh, GST (T1 & M1), Myocardial infarction, PCR, Polymorphism
Introduction
Myocardial infarction (MI) is one of the major manifestations of coronary artery disease (CAD). CAD is considered to be one of the main causes of morbidity around the world (1). According to the World Health Organization (WHO), every year approximately 17.9 million people die of cardiovascular diseases worldwide which is 31% of all deaths and most of these occur in developing countries (2). Atherosclerotic plaque formation, hypercholesterolemia, hypertension, and diabetes are considered as major risk factors for CAD (3). The DNA oxidative stress plays a vital role in the pathogenesis of atherosclerosis and increased production of reactive oxygen species (ROS) through it has also been well noted (4). The higher levels of DNA adducts have been observed in vascular tissues compared to other tissues (5, 5). Higher levels of DNA adducts have been found in patients with severe CAD (7). In addition, high levels of DNA adducts were observed in heart tissues from patients with severe CAD, especially among patients who smoke cigarettes. Further, the antioxidant capacity of the body is largely diminished by DNA adducts and DNA adducts have been detected in smooth muscle cells of human who have atherosclerotic lesions in the abdominal aorta (5). However, CAD is a multifactorial disorder that is a consequence of an interaction between genetic background and environmental factors such as diet, smoking and physical activity (3, 8). The genes conferring susceptibility to CAD are largely unknown.
Glutathione S-transferases (GSTs) catalyse the detoxifying reaction of polycyclic aromatic hydrocarbons in detoxifying phase II. GSTs are members of a multigenic family that inhibit the formation of DNA adducts and provide protection against DNA damage by genotoxins (9). There are few polymorphisms in the genes of these enzymes that are considered to affect the activity of the enzyme. One notable polymorphism is the deletion of either GST theta 1 (T1) or GST mu 1 (M1) or both genes which result in loss of activity of the enzyme causing less detoxification of xenobiotic substances and a lower antioxidant activity which facilitates cellular damage by free radicals (10-13). However, GST enzymes play a pivotal role against the development of CAD by detoxifying the by-products of oxidative stress, such as the cytotoxic aldehydes generated from lipid peroxidation which is itself considered a risk factor for heart disease (14-16). The association of the polymorphic GSTT1 and GSTM1 genotypes with the risk of CAD and Myocardial Infarction (MI) (17) have been reported in different races and population worldwide (18-22). Investigations by Bhat et al. (23) in the North Indian population and Wang et al. (24) in the Chinese population also showed the association of GST (T1 & M1) polymorphisms with the occurrence of CAD and MI. In addition, a study by Nomani et al. reported GST (T1 & M1) polymorphisms as a susceptibility factor for CAD in Western Iran (25).
Though the association between various types of GSTs genes and CAD has been established worldwide, to the best of our knowledge no such study has been conducted from Bangladesh. Therefore, we made an attempt to determine the frequency of GST (T1 & M1) genotypes and investigate the possible relationship between GST (T1 & M1) polymorphisms and MI in our population.
Materials and Methods
The study was a case-control study conducted on 250 subjects (Table 1). The case group comprises 100 cardiac patients who have experienced MI one or more times. There was no significant difference in baseline characteristics between control and cases (Table 1). The cardiac patients were recruited from the Coronary Care Unit (CCU) of different hospitals of Dhaka city without any medical history of other chronic diseases. A total of 150 healthy controls with no history of cardiac or chronic diseases were recruited from different hospitals of Dhaka city where they came for regular health check-ups (Table 1). All the participants completed a structured questionnaire covering information on age, gender, medical, family history of chronic diseases and smoking status. Smoking status was summarized as a smoker or non-smoker (Table 1).
Table 1.
Baseline characteristics of subjects.
| Healthy Control (n= 150) | CAD Patients (n= 100) | |
|---|---|---|
| Age | 53 ± 17 | 55 ± 12 |
| Gender (n, %) | ||
| Male | 113 (75) | 76 (76) |
| Female | 37 (25) | 24 (24) |
| Smoking Status | ||
| Non-Smoker | 60 (40) | 41 (41) |
| Smoker | 90 (60) | 59 (59) |
| Family history | ||
| Parents Affected | - | 28 (28) |
| Siblings Affected | - | 23 (23) |
| No comments | - | 22 (22) |
| No History | - | 27 (27) |
All participants were given an explanation about the nature of the study and written informed consent was obtained. The study was approved with Reference No. BMBDU-ERC/EC/16/007 by the ethical committee of the Department of Biochemistry and Molecular Biology, University of Dhaka. The study was conducted in accordance with the declaration of Helsinki and its subsequent revisions (26)
Sample collection
Approximately 5.0 mL of venous blood was drawn from each individual following all aseptic precautions with the help of a trained person, using a disposable syringe. The drawn blood was immediately transferred into a tube containing EDTA (1.20 mg/ml) and kept in an icebox for transportation to the laboratory. The blood samples of EDTA tubes were stored at −20 °C until genomic DNA extraction.
Genotyping of GST (T1 & M1) Genes
The GST (T1 & M1) genotypes were determined using the previously described PCR method (27). The genomic DNA was extracted from peripheral leukocytes according to our previous method (28). Then the genomic DNA was amplified by polymerase chain reaction (PCR). PCR conditions and primer sequences were used according to the method of Lutfa et al. (27). PCR was carried out using Go Taq polymerase (Go Taq® Flexi, Promega Corporation, USA). Genomic DNA (0.5 μg) was added to a PCR mix composed of 50 pmol of each primer, 200 μmol dNTPs, 2.5 units Taq polymerase, and PCR buffer composed of 10 mol/mL Tris-HCl (pH 8.3), 50 mol/mL KCl, and 2.5 mol/mL MgCl2 in a volume of 50 μL. The PCR products were separated by electrophoresis and visualized under UV light after ethidium bromide staining. The presence of either 466bp or 215bp band illustrates the presence of T1 or M1 genotype and both indicated the presence of both genotypes (T1 & M1). The 312bp fragment is an indicator of a positive polymerase chain reaction (Fig. 1).
Fig. 1.
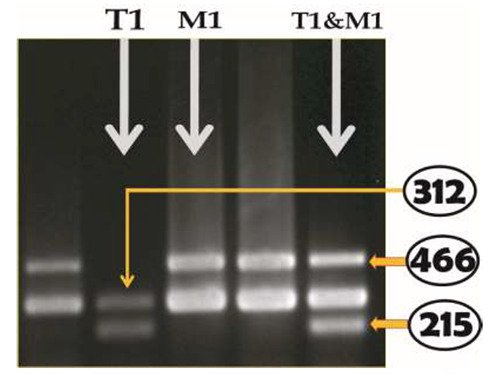
Banding pattern of GSTT1 and GSTM1 genes in 2% agarose gel. Presence of either 466 bp or 215 bp-band illustrated the presence of T1 or M1 genotype respectively and both bands indicated the presence of both genotypes (T1 & M1). The 312 bp-fragment is an indicator of positive polymerase chain reaction or internal control.
Statistical Analysis
Statistical analyses were performed using Statistical Package for Social Science (SPSS), windows version 17.0. The relative association between cases and controls was assessed by calculating the odds ratio (OR). ORs, as a measure of relative risk, at 95% confidence intervals (95% CI) were estimated using logistic regression models. P-value< 0.05 were considered as statistically significant.
Results
In this study, we examined the association of GST (T1 & M1) genes polymorphisms with the susceptibility of MI in the Bangladeshi population. The genotypic distribution (p< 0.01) of GST M1 genotypes among the study subjects were significantly different (Table 2). In contrast, the genotypic proportions of GST T1 genotypes in both groups were not significantly different. The percentages of GST M1 genotypes and GST M1 null genotypes were 78% and 22% respectively in control subjects and 59% and 41% respectively in MI patients. On the other hand, the percentages of different variants of GST T1 genotypes were almost similar among the study subjects (Table 2). The genotypic frequencies of GST T1 and GST M1 combined genotypes are presented in Table 3. Among the patients, 39% have got both GST (M1 & T1) genotypes, 34% got at least one genotype and 27% were null for both genotypes. On the other hand, 58% of control subjects have gained both genotypes, 30.7% got either M1 or T1 genotypes and 11.3% got no genotypes.
Table 2.
Glutathione S-transferase theta 1 (GSTT1) and glutathione S-transferase mu 1 (GSTM1) genotype frequency in the study subjects.
| Genotypes | Controls subjects (n= 150, %) | CAD patients (n= 100, %) | p value |
|---|---|---|---|
| GST T1 variants | |||
| Present | 122 (81.3) | 73 (73) | ns |
| Null | 28 (18.7) | 27 (27) | |
| GST M1 variants | |||
| Present | 117 (78) | 59 (59) | < 0.01 |
| Null | 33 (22) | 41 (41) | |
Results are expressed as number (percentage). Chi-square test was performed to analyse significance between two groups. p< 0.05 was taken as level of significance. ns, not significant.
Table 3.
Significance and risk assessment of glutathione S-transferase theta 1 (GSTT1) and glutathione S-transferase mu 1 (GSTM1) genotypes in study subjects.
| z | Controls (n= 150) | CAD Patients (n= 100) | p value | Odd ratio (95% CI) |
|---|---|---|---|---|
| GST T1 genotypes | ||||
| Present | 122 | 73 | - | 1 (Ref.) |
| Null | 28 | 27 | ns | 1.6 (0.9 to 3.0) |
| GST M1 genotypes | ||||
| Present | 117 | 59 | - | 1 (Ref.) |
| Null | 33 (22) | 41 (41) | < 0.01 | 2.5 (1.4 to 4.3) |
| GST T1 & M1 combined genotypes | ||||
| Both Present | 87 (58) | 39 (39) | - | 1 (Ref.) |
| At least one Present | 46 (30.7) | 34 (34) | ns | 1.6 (0.9 to 3.0) |
| Both Null | 17 (11.3) | 27 (27) | < 0.001 | 3.5 (1.7 to 7.2) |
Odds ratios (OR) and 95% confidence interval (95% CI), OR adjusted for ages and gender. Fisher’s exact test was performed. p< 0.05 was taken as level of significance.
Genotypic analysis of GST (T1 & M1) Genes
The risk for MI was also estimated in relation to the GSTT1 and GSTM1 (present and null) genotypes presented in Table 3. In the case of the GSTT1 gene, patients having a null genotype are at higher risk of developing coronary artery diseases when compared to control subjects (OR= 1.6; 95% CI= 0.9–3.0; p> 0.05) although not statistically significant. For the GST M1 gene, the GST M1 present and null genotypes were significantly (p< 0.01) different among study subjects and the risk of developing the coronary artery diseases was also higher in the case of Null genotypes when compared to control subjects (OR= 2.5; 95% CI= 1.4–4.3; p< 0.01).
In the case of GST M1 and GST T1 combined genotype, patients having both null genotypes for GST M1 and GST T1 gene showed significantly (p< 0.01) higher risk of experiencing MI when compared to control subjects (OR= 3.5; 95% CI= 1.7–7.2; p< 0.001) (Table 3). On the other hand, patients having at least one present genotype for either GST T1 or GST M1 gene showed lower risk (OR= 1.6; 95% CI= 0.9–3.0; p> = 0.05) of experiencing MI.
Discussion
Myocardial infarction (MI) is a devastating disorder that evolved from the interaction of several genetic and environmental factors. These factors affect various ethnic groups differently. Though numerous investigations have been performed to find the relation of environmental and genetic factors with MI, the underpinning mechanism of these disorders is still elusive. There are several candidate genes have been documented to be associated with MI worldwide (29-31). Sequence variants of the components of the GSTs isoforms are suggested to have significant influences on the lower antioxidant activity facilitating atherosclerotic plaque formation. The polymorphisms of GST (M1 & T1) gene have been investigated and reported to be involved with coronary artery disease and myocardial infarction in several studies (21, 23, 32, 33). In our present study, we have investigated the relationship between the most common polymorphisms of GST (M1 & T1) gene and MI in Bangladesh.
In this study, the GST M1 genotypes and allele frequencies were significantly different between the two groups and the M1 allele was more frequent in patients with MI than normal subjects. The risk of occurring MI was 2.5-fold (OR= 2.5; 95% CI= 1.4–4.3; p< 0.01) in patients with M1 null genotypes. Moreover, we found strong association of GST gene with MI, where patients with both null genotypes were 3.5-fold (OR= 3.5; 95% CI= 1.7–7.2; pp< 0.001) likely to develop cardiovascular diseases. On the other hand, individuals with at least one GSTT1 or GSTM1 gene showed a lower risk of experiencing MI.
In our present study, the GSTM1 null genotype provided a 2.5-fold increased risk of developing CAD which was in agreement with the previous studies of Bhat et al. (23) who reported that the GSTM1 null genotype conferred a two-fold increased risk of developing CAD in a North Indian Punjabi population. Another investigation by Abu-Amero et al. (32) in the Saudi Arabian population showed that individual with GSTM1 null genotype was nine fold prone to experience MI. Wang et al. investigation also described a significant association between the GSTM1 null genotype and CHD risk in the Chinese population (24). In contrast, our results are not in concordance with the results of Girisha et al. (33) and Singh et al. (21) who observed no significant association of GSTM1 null genotype with CAD and acute myocardial infarction in a north Indian population, respectively. Syed et al. also found no significant association with GSTM1 null genotype and CAD in Hyderabad (34).
Though we have found no association between GSTT1 genotype and CAD, several previous studies have shown contradictory results. Abu-Amero et al. observed that the GSTT1 null genotype showed an eightfold increased risk of CAD in a Saudi Arabian population (33). The study by Manfredi et al. in an Italian population, also documented that patients with a GSTT1 null genotype had a significant 2.5-fold increased risk of developing CAD (35). Nomani et al. described the association of the GSTT1 null genotype with a 2.2-fold increased risk of developing CAD (25). Cora et al. found that the GSTT1 null genotype was associated with the susceptibility of developing myocardial infarction in a Turkish population (36). However, Yeh et al. observed no significant association between the GSTT1and CAD in a Taiwanese population (22). These conflicting results might be attributed to ethnic differences and variations of environmental factors.
In conclusion, we found that GSTM1 alone, and GSTM1 & T1 in combination augments the risk of MI in the Bangladeshi population. The M1 allele was significantly lower in MI patients compared to control subjects. Thus, GSTM1 null genotype would be considered as a risk factor and the GSTT1 genotype would be a molecular marker of reducing CAD. Therefore, genotyping of GST (M1 & T1) gene would be a biomarker of early diagnosis of CAD and also be helpful to intervene in personalized medicine as a novel treatment of CAD. However, our study was conducted on a small number of samples which was one of the main limitations that necessitate careful interpretation of results. Studies on a larger population would certainly be more conclusive.
Acknowledgements
We would like to acknowledge the support and cooperation of the participants of this study. The study was conducted at the Department of Biochemistry and Molecular Biology, University of Dhaka. All the authors reported that there is no conflict of interest.
References
- 1.Gouvinhas C, Severo M, Azevedo A, Lunet N. Worldwide patterns of ischemic heart disease mortality from 1980 to 2010. International Journal of Cardiology. 2014;170(3):309–314. doi: 10.1016/j.ijcard.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization (WHO). 2019. Jul 21, Available from: http://www.who.int/cardiovascular_diseases.
- 3.Smith F, Lee A, Fowkes F, Price J, Rumley A, Lowe GD. Hemostatic factors as predictors of ischemic heart disease and stroke in the Edinburgh Artery Study. . Arterioscler Thromb Vasc Biol. 1997;17(11):3321–3325. doi: 10.1161/01.atv.17.11.3321. [DOI] [PubMed] [Google Scholar]
- 4.Harrison D, Griendling KK, Landmesser U, Hornig B, Drexler H. Role of oxidative stress in atherosclerosis. Am J Cardiol. 2003;91(3A):7A–11A. doi: 10.1016/s0002-9149(02)03144-2. [DOI] [PubMed] [Google Scholar]
- 5.De flora S, Izzotti A, Walsh D, Degan P, Petrilli GL, Lewtas J. Molecular epidemiology of atherosclerosis. FASEB J. . 1997;11(12):1021–31. [PubMed] [Google Scholar]
- 6.Penn A, Snyder C. Arteriosclerotic plaque development is promoted by polynuclear aromatic hydrocarbons. Carcinogenesis. 1998;9(12):2185–2189. doi: 10.1093/carcin/9.12.2185. [DOI] [PubMed] [Google Scholar]
- 7.Van Schooten FJ, Hirvonen A, Maas LM, De Mol BA, Kleinjans JC, Bell DA, et al. Putative susceptibility markers of coronary artery disease: association between VDR genotype, smoking and aromatic DNA adducts levels in human right atrial tissue. . FASEB J. 1998;12(13):1409–17. doi: 10.1096/fasebj.12.13.1409. [DOI] [PubMed] [Google Scholar]
- 8.Prins BP, Lagou V, Asselbergs FW, Snieder H, Fu J. Genetics of coronary artery disease: genome-wide association studies and beyond. Atherosclerosis. 2012;225(1):1–10. doi: 10.1016/j.atherosclerosis.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 9.Ryberg D, Skaug V, Hewer A, Philips DH, Harries LW, Wolf CR. Genotypes of glutathione transferase M1 and P1 and their significance for lung DNA adduct levels and cancer risk. . Carcinogenesis. 1997;18(7):1285–9. doi: 10.1093/carcin/18.7.1285. [DOI] [PubMed] [Google Scholar]
- 10.Yalin S, Hatungil R, Tamer L, Ates NA, Dogruer N, Yildirim H, et al. Glutathione S-transferase gene polymorphisms in Turkish patients with diabetes mellitus. . Cell Biochem Funct. . 2007;25(5):509–13. doi: 10.1002/cbf.1339. [DOI] [PubMed] [Google Scholar]
- 11.Azarpira N, Nikeghbalian S, Geramizadeh B, Darai M. Influence of glutathione S-transferase M1 and T1 polymorphisms with acute rejection in Iranian liver transplant recipients. Mol Bio Rep. . 2010;37(1):21–25. doi: 10.1007/s11033-009-9487-5. [DOI] [PubMed] [Google Scholar]
- 12.Rafiee L, Saadat I, Saadat M. Glutathione S-transferase genetic polymorphisms (GSTM1, GSTT1 and GSTO2) in three Iranian populations. Mol Biol Rep. 2010;37(1):155–8. doi: 10.1007/s11033-009-9565-8. [DOI] [PubMed] [Google Scholar]
- 13.Saadat I, Saadat M. Influence of genetic polymorphisms of glutathione S-transferase T1 (GSTT1) and M1 (GSTM1) on hematological parameters. . Mol Biol Rep. . 2010;37(1):249–53. doi: 10.1007/s11033-009-9662-8. [DOI] [PubMed] [Google Scholar]
- 14.Katoh T, Yamano Y, Tsuji M, Watanabe M. Genetic polymorphisms of human cytosol glutathione S-transferases and prostate cancer. . Pharmacogenomics. . 2008;9(1):93–104. doi: 10.2217/14622416.9.1.93. [DOI] [PubMed] [Google Scholar]
- 15.Miller EA, Pankow JS, Millikan RC, Bray MS, Ballantyne CM, Bell DA, et al. Glutathione-S-transferase genotypes, smoking, and their association with markers of inflammation, hemostasis, and endothelial function: the atherosclerosis risk in communities (ARIC) study. . Atherosclerosis. 2003;171(2):265–272. doi: 10.1016/j.atherosclerosis.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Khan MA, Baseer A. Increased malondialdehyde levels in coronary heart disease. J Pak Med Assoc. . 2000;50(8):261–64. [PubMed] [Google Scholar]
- 17.Wilson M, Grant P, Hardie L, Wild CP. Glutathione-Stransferase M1-null genotype is associated with a decreased risk of myocardial infarction. FASEB J. . 2000;14(5):791–6. doi: 10.1096/fasebj.14.5.791. [DOI] [PubMed] [Google Scholar]
- 18.Masetti S, Botto N, Manfredi S, Colombo MG, Rizza A, Vassalle C, et al. Interactive effect of the glutathione S-transferase genes and cigarette smoking on occurrence and severity of coronary artery risk. . J Mol Med (Berl). . 2003;81(8):488–94. doi: 10.1007/s00109-003-0448-5. [DOI] [PubMed] [Google Scholar]
- 19.Tamer L, Ercan B, Camsari A, Yildirim H, Ciçek D, Sucu N, et al. Glutathione S-transferase gene polymorphism as a susceptibility factor in smoking-related coronary artery disease. . Basic Res Cardiol. . 2004;99(3):223–9. doi: 10.1007/s00395-004-0465-8. [DOI] [PubMed] [Google Scholar]
- 20.Kim SJ, Kim MG, Kim KS, Song JS, Yim SV, Chung JH. Impact of glutathione S-transferase M1 and T1 gene polymorphisms on the smoking-related coronary artery disease. . J Korean Med Sci. 2008;23(3):365–372. doi: 10.3346/jkms.2008.23.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh N, Sinha N, Kumar S, Pandey CM, Agrawal S. Glutathione S-transferase gene polymorphism as a susceptibility factor for acute myocardial infarction and smoking in the North Indian population. Cardiology. 2011;118(1):16–21. doi: 10.1159/000324066. [DOI] [PubMed] [Google Scholar]
- 22.Yeh HL, Kuo LT, Sung FC, Chiang CW, Yeh CC. GSTM1, GSTT1, GSTP1 and GSTA1 genetic variants are not associated with coronary artery disease in Taiwan. . Gene. . 2013;523(1):64–9. doi: 10.1016/j.gene.2013.02.052. [DOI] [PubMed] [Google Scholar]
- 23.Bhat MA, Gandhi G. Association of GSTT1 and GSTM1 gene polymorphisms with coronary artery disease in North Indian Punjabi population: a case-control study. Postgrad Med J. 2016;92(1094):701–706. doi: 10.1136/postgradmedj-2015-133836. [DOI] [PubMed] [Google Scholar]
- 24.Wang LS, Tang JJ, Tang NP, Wang MW, Yan JJ, Wang QM, et al. Association of GSTM1 and GSTT1 gene polymorphisms with coronary artery disease in relation to tobacco smoking. Clin Chem Lab Med. 2008;46(12):1720–5. doi: 10.1515/CCLM.2008.353. [DOI] [PubMed] [Google Scholar]
- 25.Nomani H, Mozafari H, Ghobadloo SM, Rahimi Z, Raygani AV, Rahimi MA, et al. The association between GSTT1, M1 and P1 polymorphisms with coronary artery disease in Western Iran. . Mol Cell Biochem. . 2011;354(1-2):181–7. doi: 10.1007/s11010-011-0817-2. [DOI] [PubMed] [Google Scholar]
- 26.World Medical Association. World medical association declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–4. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 27.Akther L, Rahman MM, Bhuiyan MES, Hosen MB, Nesa A, Kabir Y. Association of Glutathione S-transferase theta 1 (GSTT1) and Glutathione S-transferase mu 1 (GSTM1) gene polymorphism with the risk of preeclampsia during pregnancy in Bangladesh. . J Obstet Gynaecol Res. . 2019;45(1):113–118. doi: 10.1111/jog.13791. [DOI] [PubMed] [Google Scholar]
- 28.Hosen MB, Salam MA, Islam MF, Howlader MZH, Hossain A, Kabir Y. Association of TP53 gene polymorphisms with susceptibility of bladder cancer in Bangladeshi population. Tumour Biol. . 2015;36(8):6369–74. doi: 10.1007/s13277-015-3324-3. [DOI] [PubMed] [Google Scholar]
- 29.Cicoira M, Zanolla L, Rossi A, Golia G, Franceschini L, Cabrini G. Failure of aldosterone suppression despite angiotensin-converting enzyme (ACE) inhibitor administration in chronic heart failure is associated with ACE DD genotype. . J Am Coll Cardiol. . 2001;37(7):1808–12. doi: 10.1016/s0735-1097(01)01237-2. [DOI] [PubMed] [Google Scholar]
- 30.Cine N, Hatemi AC, Erginel-Unaltuna N. Association of a polymorphism of the ecNOS gene with myocardial infarction in a subgroup of Turkish MI patients. . Clin genet. 2002;61(1):66–70. doi: 10.1034/j.1399-0004.2002.610113.x. [DOI] [PubMed] [Google Scholar]
- 31.Oniki K, Hori M, Takata K, Yokoyama T, Mihara S, Marubayashi T, et al. Association between glutathione Stransferase A1, M1 and T1 polymorphisms and hypertension. . Pharmacogenet Genomic. 2008;18(3):275, 7. doi: 10.1097/FPC.0b013e3282f56176. [DOI] [PubMed] [Google Scholar]
- 32.Abu-Amero KK, Al-Boudari OM, Mohamed GH, Dzimiri N. T null and M null genotypes of the glutathione S-transferase gene are risk factor for CAD independent of smoking. . BMC Med Genet. . 2006;7:38. doi: 10.1186/1471-2350-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Girisha KM, Gilmour A, Mastana S, Singh VP, Sinha N, Tewari S, et al. T1 and M1 polymorphism in glutathione S-transferase gene and coronary artery disease in North Indian population. . Indian J Med Sci. 2004;58:520–6. [PubMed] [Google Scholar]
- 34.Syed R, Deeba F, Jamil K. Role of GSTM1 gene polymorphism and its association with coronary artery disease. . Journal of Clinical Medicine Research. 2010;2:22–25. [Google Scholar]
- 35.Manfredi S, Federici C, Picano E, Botto N, Rizza A, Andreassi MG. GSTM1, GSTT1 and CYP1A1 detoxification gene polymorphisms and susceptibility to smoking-related coronary artery disease: a case-only study. Mut Res. 2007;621(1-2):106–12. doi: 10.1016/j.mrfmmm.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 36.Cora T, Tokae M, Acar H, Soylu A, Inan Z. Glutathione S-transferase M1 and T1 genotypes and myocardial infarction. . Mol Biol Rep. 2013;40(4):3263–7. doi: 10.1007/s11033-012-2401-6. [DOI] [PubMed] [Google Scholar]


