Results of this study suggest that deep-sea sharks exhibit variable levels of physiological stress to longline capture. Shallower-dwelling and smaller-bodied sharks exhibited greater apparent physiological disruption. No increase in physiological disruption was detected with proximity of capture to the Deepwater Horizon oil spill site.
Keywords: Stress, elasmobranch, oil spill
ABSTRACT
Prior to the Deepwater Horizon (DWH) oil spill, little research effort was focused on studying deep-sea sharks in the Gulf of Mexico (GoM). While the biology of these fishes remains virtually unknown, they are routinely captured in commercial fisheries as bycatch. In the absence of basic biological data, and with the probability of post-release survival unknown for most species, effective management plans cannot be formulated, making populations highly susceptible to overfishing. Any potential detrimental effects of the DWH oil spill, which occurred at 1500 m deep, are also unknown. Following longline capture, we characterized the physiological blood biochemical parameters related to secondary stress and compared them among seven shark species occurring on the continental shelf edge and slope in the GoM at depths ranging from 200 to 2000 m. We also investigated the relationship between blood parameters and depth as well as proximity to the oil spill site. The deep-sea sharks examined here exhibited variability in blood chemistry associated with the secondary stress response, with values falling within published records for previously studied elasmobranchs. Results suggested that there is greater relative physiological stress in shallower-dwelling sharks as well as smaller-bodied sharks. Further, the rate of core temperature warming was fastest in smaller bodied sharks, which likely contributes to greater physiological stress. The core temperatures of the larger-bodied, deeper-dwelling species were not altered as drastically as the smaller-bodied sharks after being hauled to the surface. Any chronic physiological effects of the oil spill were not detectable as there were no relevant correlations between blood chemistry metrics and proximity to the DWH oil spill site.
Introduction
Elasmobranchs, the sharks and rays, are captured worldwide in many commercial and recreational fisheries, and ~18.8% of the group’s ~1250 extant species are designated as ‘threatened’ in the International Union for the Conservation of Nature (IUCN) Red List of Threatened Species (IUCN 2019). While a limited number of species are targeted, many are caught as bycatch and released because of low commercial value or fisheries regulations that limit their retention. Unfortunately, many individuals that are released as bycatch are either already dead, die post-release or suffer detrimental long-term sublethal effects from the capture event (e.g. Cooke et al. 2002; Morgan and Burgess 2007; Skomal 2007; Morgan et al. 2009; Skomal and Mandelman 2012; Brooks et al. 2015; Talwar et al. 2017). The life history of this group of fishes is generally characterized by slow growth, late maturity, a long lifespan and low fecundity, all characteristics that are even more extreme in deep-sea elasmobranchs (Simpfendorfer and Kyne 2009). These life history characteristics as well as increasing direct and indirect fishing pressures (Morato et al. 2006; Cotton and Grubbs 2015; Oliver et al. 2015) make elasmobranchs, particularly deep-sea elasmobranchs, susceptible to overexploitation worldwide (Bonfil 1994; Walker 1998; Dulvy et al. 2003).
Deep-sea sharks in the Gulf of Mexico (GoM), particularly Mustelus spp. and Squalus spp., are subject to bycatch and potential post-release mortality as they are captured and discarded in the deep reef-fish longline fisheries (Hale et al. 2011; Scott-Denton et al. 2011; Scott-Denton and Williams 2013) and shrimp trawl fisheries (Zhang et al. 2015). While Mustelus spp. are not currently directly harvested in the GoM, they are in the US Atlantic commercial shark fishery, with greater than 900 000 kg whole weight landed in 2012 (Cortés and Balchowsky 2015) and they make up the overwhelming majority of landed shark fins when examining both the small and large coastal shark complexes from 2016 to 2018 (NOAA Fisheries 2020). As traditional fisheries become overfished, the industry will begin to explore new and different habitats, like those in the deep ocean, which will also be fueled by the development of new technology (Cotton and Grubbs 2015). A better understanding of deep-sea taxa before they become targeted would allow for better management and conservation plans from the outset of these fisheries.
Understanding physiology is essential because physiology dictates the life history, behavior and fitness of an organism (Ricklefs and Wikelski 2002; Wikelski and Cooke 2006), and this information can be used in the development or adjustment of species-specific management and conservation plans (Ferguson and Tufts 1992; Wikelski and Cooke 2006; Young et al. 2006). For example, corticosterone levels in marine iguanas Amblyrhynchus cristatus in the Galapagos were studied during normal years and during El Niño years to predict overall population health and survival rates (Romero and Wikelski 2001). These data became particularly useful when an oil spill occurred in 2001, as researchers were able to predict oil spill-related mortality based on post-oil spill corticosterone levels (Wikelski et al. 2001). Previous work across a wide range of taxa including invertebrates and vertebrates has suggested that, in some cases, even closely related species can differ greatly in aspects of their physiology (e.g. Navas 1996; Tomanek and Somero 1999; Hoffman et al. 2000). This may give the species with the more robust physiological response to a stressor an advantage over its congener and may help define a species’ ecological niche and range. Congeneric differences have been observed in physiological markers of the stress response in several species of sharks (Manire et al. 2001; Mandelman and Skomal 2009; Gallagher et al. 2014; Butcher et al. 2015), though no comparable work has been done on deep-sea taxa. However, two studies have investigated mortality from longline capture among multiple deep-sea sharks (Brooks et al. 2015; Talwar et al. 2017).
Stress has been defined in many contexts, but a current working definition is a disruption of homeostasis of an organism by intrinsic or extrinsic stimuli, which can elicit a behavioral or physiological compensatory response (Wendelaar-Bonga 1997). Generally, there are metabolic costs associated with a stress response, and energy is reallocated from growth and reproduction toward respiration, locomotion and tissue repair (Wendelaar-Bonga 1997). Boonstra (2013) notes that the ecology of stress plays an essential role toward understanding a species’ distribution and abundance. In elasmobranchs, it has been suggested that responses are species specific rather than universal (Beerkircher et al. 2002; Morgan and Burgess 2007; Mandelman and Skomal 2009; Morgan and Carlson 2010; Hyatt et al. 2012; Marshall et al. 2012; Skomal and Mandelman 2012; Gallagher et al. 2014; Jerome et al. 2018), and they are likely influenced by many factors including capture method, capture duration, respiratory mode and metabolic scope (Skomal and Mandelman 2012; Dapp et al. 2016; Guida et al. 2016; Mohan et al. 2020). This response is often measured through physiological changes in blood chemistry (Skomal and Bernal 2010), such as glucose, lactate and acid–base status, which can determine the relative condition of the fish following a stressor such as capture (Cliff and Thurman 1984; Wells et al. 1986; Harrenstien et al. 2005; Skomal 2007).
The stress response in fishes can broadly be described by the primary (neuroendocrine), secondary (biochemical changes) and tertiary (fitness-related consequences) responses (Skomal and Mandelman 2012), of which the secondary response is the most widely studied in elasmobranchs. Briefly, this response involves numerous changes in blood chemistry that can be easily measured. The concentration of blood glucose is quantified as a proxy of the glucocorticoid hormone stress response, during which gluconeogenesis occurs and reserves of hepatic glycogen are converted to glucose and released to fuel muscle tissue (Barton and Iwama 1991; Hoffmayer and Parsons 2001). Elevated blood glucose, or hyperglycemia, has been observed in elasmobranchs in response to various stressors (Cliff and Thurman 1984; Hoffmayer and Parsons 2001; Skomal 2006; Frick et al. 2010). Generally, the partial pressure of carbon dioxide in the blood will also increase in fishes as a result of stressors that cause restricted ventilation, leading to respiratory acidosis, and can be inferred by a decline in blood pH. This can occur in a ram ventilating shark being captured on a line, for instance, or any elasmobranch being entangled in a gillnet (Manire et al. 2001; Mandelman and Farrington 2007). Elevated blood lactate leading to metabolic acidosis can occur when an animal switches from aerobic to anaerobic respiration in white muscle tissue as a result of increased energetic demands, such as when evading a predator. This results in a shift of lactate and H+ ions from the muscle to the blood (Black 1958; Schmidt-Nielsen 1997; Skomal 2007) and has been observed in elasmobranchs that are subjected to stress (Murdaugh Jr et al. 1965; Rasmussen and Rasmussen 1967; Piiper and Baumgarten 1969; Piiper et al. 1972; Martini 1974; Cliff and Thurman 1984). Hyperkalemia, an accumulation of potassium in the blood, has also been observed in elasmobranchs that have endured stress (Cliff and Thurman 1984; Wells et al. 1986; Manire et al. 2001) and can ensue from intracellular acidosis and the resulting shift of potassium from the muscle to the blood (Cliff and Thurman 1984; Moyes et al. 2006). This shift in potassium can modify muscle cell membrane excitability (Adams and Galvan 1986), which can result in myocardial dysfunction in spiny dogfish Squalus acanthias (Martini 1974) and muscle tetany in gummy sharks Mustelus antarcticus (Cliff and Thurman 1984; Frick et al. 2010). Shifts in serum ion concentrations may result in a compensatory mechanism called hemoconcentration (Piiper et al. 1972), where fluid shifts from the blood to the muscle to mitigate elevated lactate. Hemoconcentration can also be attributed to red blood cell swelling that increases blood oxygen carrying capacity (McDonald and Milligan 1992) or from catecholamine activation (Nikinmaa 1992). Hemoconcentration has been observed in spiny dogfishS. acanthias after trawl capture (Mandelman and Farrington 2007), in lemon sharks Negaprion brevirostris following exercise (Bushnell et al. 1982) and in capture-stressed shortfin mako sharks Isurus oxyrinchus (Wells and Davie 1985), sandbar sharks Carcharhinus plumbeus (Brill et al. 2008) and smalltooth sawfish Pristis pectinata (Prohaska et al. 2018). Some of the parameters described above can be indicators of immediate and post-release mortality or post-release behavior (Hoffmayer and Parsons 2001; Young et al. 2006; Arlinghaus et al. 2009; Talwar et al. 2017).
To accurately interpret the relative levels of physiological stress among individuals or taxa, baseline values of commonly assessed metrics are helpful (Skomal and Mandelman 2012). These reference points are uncommon because sharks are difficult to maintain and sample in captivity without causing some degree of stress through the capture and handling process. In the wild, sharks have been sampled after being placed into tonic immobility (Brooks et al. 2012) or sampled after limited capture durations in an attempt to provide ‘minimally stressed’ levels (Skomal and Mandelman 2012) as an alternative to absolute baselines. These ‘minimally stressed’ values have not been quantified in any deep-sea sharks, and measuring capture duration at depth is logistically difficult, making it difficult to characterize their physiological stress response to capture along that gradient. Conversely, blood chemistry parameters measured when animals become moribund are often reported in studies focused on stress and mortality in sharks (Moyes et al. 2006; Hight et al. 2007; Hutchinson et al. 2015; Wosnick et al. 2017). These values can provide useful end points to help researchers gauge how close an animal is to death as well as put ‘stressed’ values into context by providing an upper bound (Wosnick et al. 2017).
Given this inherent vulnerability of deep-sea sharks to fisheries (Dulvy et al. 2003; Simpfendorfer and Kyne 2009), as well as our limited knowledge of their biology, understanding the effects of extrinsic stressors on these animals is critical, particularly for those that can lead to animal or population-level consequences like major oil spills (Pulster et al. 2020). The Deepwater Horizon (DWH) oil spill of 2010 represents one such event that could have unknown effects on deep-sea elasmobranchs. Metabolites of polycyclic aromatic hydrocarbons (PAHs) are commonly studied to identify oil exposure in vertebrates (Gorbi and Regoli 2004; Ferreira et al. 2006), and, after the DWH oil spill, Leary (2015) found that exposure to PAHs in deep-sea sharks was highest close to the site of the spill and largely limited to within 100 km of the blowout (Fig. 1). Otherwise, the effects of the DWH oil spill on deep-sea sharks remain to be studied.
Figure 1.
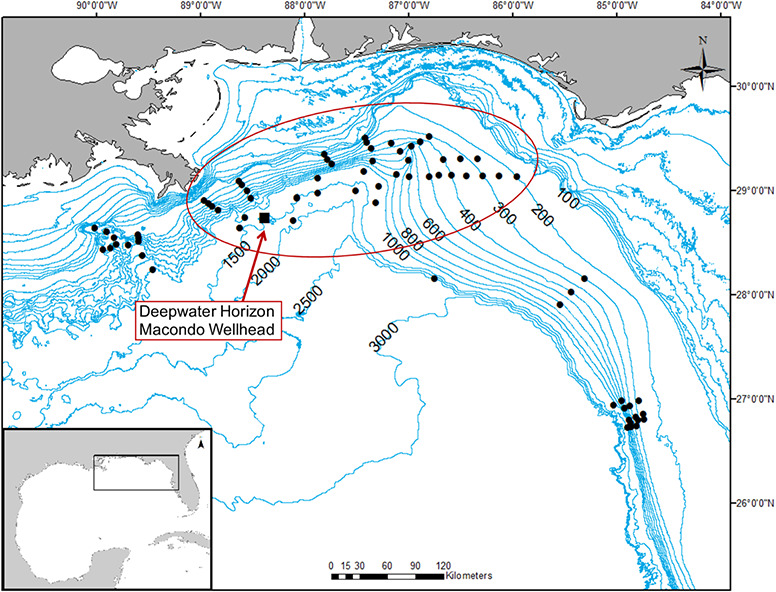
Map highlighting the locations of the fixed survey stations that were sampled during each research cruise, each black dot represents a station that was sampled during the larger survey, while the stations circled in red were locations where samples were collected for this study. The location of the DWH Macondo Wellhead is indicated by a black square with a red arrow.
The overarching objective of this study was to elucidate the species-specific capture-induced stress physiology of the seven most commonly captured sharks on a deep sea, fishery-independent survey in the GoM, which include the Gulf smooth-hound Mustelus sinusmexicanus, the dusky smooth-hound Mustelus canis, the Cuban dogfish Squalus cubensis, Genie’s dogfish Squalus clarkae, the little gulper shark Centrophorus uyato, the gulper shark Centrophorus granulosus and the bluntnose sixgill shark Hexanchus griseus. While we were not able to measure the specific capture duration of each individual sampled and baseline values for blood chemistry parameters are unavailable for these species, all animals were captured in the same manner, allowing us to investigate if observed differences between these species were mediated by habitat, body size, taxonomy (e.g. differences between species, genera or orders) or capture method, to inform fisheries conservation and management plans. Further, this study investigated differences in blood chemistry parameters relative to distance from the DWH oil spill to provide insights into the potential effects of oil spills on elasmobranch physiology.
Materials and methods
Deep-sea sharks were captured in the GoM and sampled during research cruises conducted by the Florida State University (FSU) aboard the R/V Apalachee. Each cruise consisted of 10–16 days as sea, and blood samples were collected during cruises in June and July 2014, October 2014, March and April 2015, July 2015, September 2015, April and May 2016 and April and May 2017.
Survey
Modified demersal longlines were used to capture fishes, and two or three longlines were deployed at a time in succession with targeted bottom soak times of 3–4 h. Each longline was ~550 m of 6.4 mm tarred, twisted, hard-laid nylon mainline set on the bottom and consisted of an anchor, followed by a baited chevron trap (60 cm × 60 cm × 38 cm with 2.5 cm mesh), then 50 circle hooks of five different sizes, ranging from 10/0 to 18/0 and spaced 10 m apart, followed by a baited hagfish trap (60 cm × 35 cm with 1.0 cm mesh), a 2-kg sash weight and a Lotek LAT2000 temperature-depth recorder (TDR), followed by scope line to a surface buoy and a strobed highflier. Bottom soak time was calculated as the time between the TDR reaching the bottom during a set and leaving the bottom upon retrieval. Based on average sinking rates of the gear, as measured by the TDR, the time between setting and retrieving the gear at each station was adjusted so that the time the gear soaked on the bottom was approximately the same across all depths and stations. Gear retrieval took ~15 to 45 min depending on the depth of the station. During each cruise, 30–56 demersal longlines were set and hauled at depths ranging from 200 to 2000 m. Sets were made at standard fixed stations located on the continental slope of the eastern GoM from the North Florida Slope across DeSoto Canyon to the Louisiana Slope, with varying distance (18–419 km) from the site of the DWH oil spill (Fig. 1).
Sampling
As soon as a live shark was landed, a core temperature was immediately assessed by inserting a 20 cm piercing thermometer (VWR, Radnor, PA) into the thickest portion of the dorsal musculature, which was typically 1 cm to either side of the first dorsal fin. The piercing thermometer was inserted 1.5 to 7 cm into the muscle, depending on the size of the shark, and the thermometer was allowed to stabilize (~15 s) before piercing slightly deeper into the muscle. If the temperature decreased, we let the thermometer stabilize again and repeated a deeper probe; however, if the temperature increased, we would pull back and read the coolest temperature of the muscle. Following a temperature reading, and typically 1 min or less after landing, a 5 ml blood sample was collected by caudal venipuncture using a 16–22 gauge needle attached to a heparinized syringe. Following blood collection, animals were euthanized and comprehensively sampled for ongoing and future studies. To assess lactate and pH, a small aliquot of blood was immediately loaded into a CG4+ cartridge and then inserted into a VetScan i-STAT 1 point of care device (Abaxis Inc., Union City, CA), which has been validated for use in elasmobranchs (Gallagher et al. 2010; Harter et al. 2015). The i-STAT is commonly used in elasmobranch stress studies to provide relative secondary stress data (e.g. Mandelman and Farrington 2007; Mandelman and Skomal 2009; Marshall et al. 2012; Guida et al. 2016; Talwar et al. 2017; Prohaska et al. 2018) and has been used on some of the same deep-sea sharks examined herein (Talwar et al. 2017). Glucose was measured using an Accu-Chek glucose meter (Roche Diagnostics, Basel, Switzerland), which has been validated for use on fishes (Cooke et al. 2008). Hematocrit was measured in duplicate by filling a capillary tube with the homogenized blood sample, capping one end with clay, and spinning the tube in a hematocrit centrifuge at 15 000 g for 5 min. Hematocrit was determined by calculating the red blood cell percentage of the whole blood volume. The remaining whole blood was then centrifuged at 1800 g for 5 min (Unico, Dayton, NJ). The separated plasma was stored at −20°C.
To quantify associations between morbidity and blood chemistry values in C. uyato, one of the most commonly captured species, we sampled blood from six individuals captured on the same longline immediately after they reached the deck. We then placed these individuals in a semi-circular tank with flowing, aerated sea surface water. Animals were monitored visually until they were moribund (determined when spiracle movement was negligible, animals were no longer responsive to touch, and there was no buccal reflex when removed from the water). They were then immediately sampled for blood as described previously. Temperature, dissolved oxygen, oxygen saturation and salinity were measured in the tank every 5–14 min using a handheld water quality meter (YSI Pro2030; Yellow Springs, OH, USA). Water conditions were stable during the trial.
In the laboratory, plasma potassium concentrations were measured using a Single-Channel Digital Flame Photometer (Model 02655-00, Cole-Parmer, Vernon Hills, IL, USA). Each sample was prepared using a 1:100 dilution of plasma to Cole-Parmer diluent. Potassium standards (K+: 0.5, 1, 2 and 5 ppm) were prepared with a 1000 ppm stock solution. Potassium ions were measured by running a standard curve (in triplicates) before the samples, which were then measured in triplicates and in groups of five. This process was repeated to ensure proper calibration. Measurement of each standard and sample dilution followed protocol developed by the manufacturer (Cole-Parmer), wherein the standard or sample was aspirated for 20 s prior to recording the concentration. Between each measurement, air was aspirated for 10 s, followed by Cole-Palmer diluent for 20 s and air again for 10 s.
Statistical analyses
Data for blood pH were temperature corrected to water temperature measurements at the depth of capture (Mandelman and Skomal 2009; Gallagher et al. 2010; Harter et al. 2015). Because hematocrit is a percentage, those data were arcsine transformed before analysis.
Prior to analyses, a non-metric multidimensional scaling (NMDS) was conducted of all blood chemistry metrics from all sharks sampled. The NMDS was followed by a series of permutational multivariate analyses of variance (PERMANOVA) of the NMDS scores as a function of the following independent variables: depth, species, core temperature change (ΔCT—the difference between capture temperature at depth and muscle temperature at vessel) and distance between the capture site and the DWH oil spill. These preliminary analyses were conducted to identify if one or more dependent variables were correlated. The NMDS plot and stress plot can be found in appendices 1 and 2. The results of the PERMANOVAs indicated that the dependent variables were not correlated and that independent statistical analyses were appropriate (Table 1).
Table 1.
Results of PERMANOVA on NMDS scores as a function of the species, the depth at which the shark was caught, the change in core temperature (core) and the distance from the DWH oil spill at which the animal was caught (distance)
| Parameter | F | DF | P-value |
|---|---|---|---|
| Species | 26.41 | 6192 | 0.001 |
| Depth | 93.57 | 1197 | 0.001 |
| Core temp | 4.67 | 1161 | 0.04 |
| Distance | 11.26 | 1197 | 0.001 |
One-way analyses of variance (ANOVA) were conducted between each species and their total lengths, capture temperature and capture depth to identify significant inter-specific differences. To investigate inter-specific differences in the physiological parameters glucose, lactate, pH, potassium and hematocrit, an ANOVA, a Welch’s ANOVA or a Kruskal–Wallis test was conducted depending on whether data were normally distributed and homoscedastic. When ANOVAs or their analogs were significant, a Tukey honestly significant difference or Dunn’s test was conducted to identify significant pairwise differences. Because these species inhabit discrete depth ranges, a one-way analysis of co-variance (ANCOVA) was conducted prior to the analyses to investigate inter-specific differences in the physiological parameters while controlling for the covariate ‘depth’; however, on further investigation across species, there were non-overlapping covariate ranges for these parameters and depth, making an ANCOVA an inappropriate analysis for these data.
To investigate how core temperature warming may affect physiological parameters in each species, linear regressions were conducted between each parameter and ΔCT. Linear regressions were also conducted for each species, investigating the relationship between each individual physiological parameter and the distance from the DWH oil spill at which they were captured. When data were not normally distributed, a Kendall’s rank correlation was conducted. A small number of S. cubensis (n = 9) and C. uyato (n = 8) were captured in chevron traps, and, to identify significant differences in the physiological disturbance induced by longline versus trap capture methods, t-tests or Wilcoxon rank sum tests were conducted depending on data normality and homoscedasticity. To account for multiple comparisons of physiological parameters in our analyses, we calculated a Bonferroni-corrected  of 0.007 for lactate, pH and hematocrit and 0.008 for potassium and glucose when conducting linear regressions, t-tests and their non-parametric analogs for consideration when interpreting our results.
of 0.007 for lactate, pH and hematocrit and 0.008 for potassium and glucose when conducting linear regressions, t-tests and their non-parametric analogs for consideration when interpreting our results.
Results
During the seven research cruises, 265 longline sets were made. The mean soak time was 3.93 h (SD 0.66 h), and soak time did not differ as a function of depth (adjusted r2 = 0.003, P = 0.703). Blood samples were collected for blood chemistry analyses from a total of 450 sharks representing seven species. The catch consisted of 19 M. sinusmexicanus (102 ± 2.95 cm; ‘average total length (TL) ± SE’), 17 M. canis (111 ± 3.79 cm), 94 S. cubensis (47 ± 0.65 cm), 79 S. clarkae (61 ± 0.77 cm), 209 C. uyato (83 ± 0.94 cm), 21 C. granulosus (132 ± 5.09 cm) and 11 H. griseus (426 ± 27.09 cm) (Fig. 2A).
Figure 2.
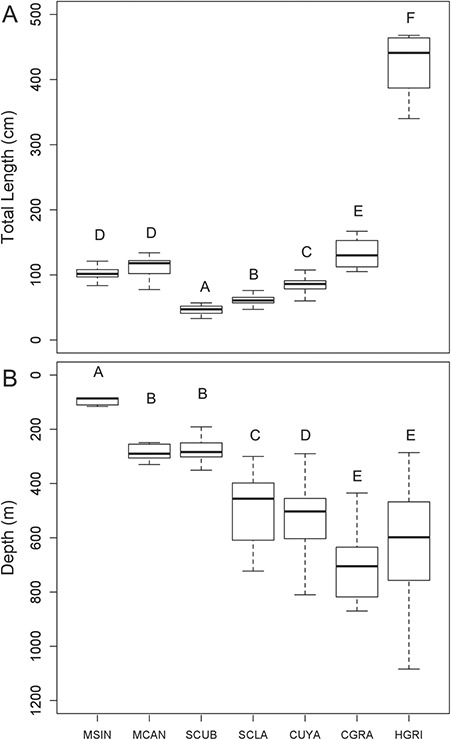
Box plots of (A) total length (cm) and (B) depth (m) of capture of M. sinusmexicanus (MSIN), M. canis (MCAN), S. cubensis (SCUB), S. clarkae (SCLA), C. uyato (CUYA), C. granulosus (CGRA) and H. griseus (HGRI), letters above bars indicate significant pairwise differences within each plot.
These seven shark species varied significantly in both length and capture depth (length: Welch’s ANOVA: F6,56.2 = 280.72, P < 2e-16; depth: Welch’s ANOVA: F6,59.4 = 327.21,P < 2e-16). M. sinusmexicanus were caught at the shallowest depths of all species (100 ± 6.98 m; ‘average depth ± SE’). M. canis and S. cubensis were found at significantly deeper depths than M. sinusmesicanus; however, not significantly different from one another at 279 ± 17.95 m and 270 ± 4.79 m, respectively. S. clarkae were captured significantly deeper at an average depth of 472 ± 12.77 m. C. uyato were found significantly deeper at an average of 535 ± 7.95 m. C. granulosus and H. griseus were captured significantly deeper yet at average depths of 711 ± 39.45 m and 679 ± 92.73 m, respectively (Fig. 2B). Temperature was negatively correlated with depth; therefore, capture temperature was also significantly different between species (Kruskal–Wallis: Χ2 = 275.53, df = 6, P = 2.2e-16). The warmest average capture temperatures were found where the shallowest species, M. sinusmexicanus, was captured (18.24 ± 0.30°C; ‘average temperature ± SE’). Mean capture temperature was slightly cooler at the depth that M. canis and S. cubensis were caught, with temperatures averaging 13.97 ± 0.49°C and 13.56 ± 0.17°C, respectively. The mean capture temperature for the next deepest dwelling species, S. clarkae, was significantly cooler at 9.39 ± 0.28°C. There were no significant differences in mean capture temperatures for C. uyato (8.08 ± 0.11°C), C. granulosus (7.20 ± 0.50°C), and H. griseus (7.72 ± 0.78°C).
Inter-specific differences in blood chemistry metrics
There were significant differences in median blood glucose concentrations between the six species of sharks for which glucose was analyzed (Kruskal–Wallis: Χ2 = 40.56, df = 5, P = 1.1e-7). M. canis had a significantly higher median blood glucose concentration than S. cubensis, S. clarkae, C. uyato and C. granulosus, but not H. griseus. S. cubensis had the next highest median blood glucose concentration, higher than that of S. clarkae and C. uyato; however, it was not higher than that of C. granulosus or H. griseus. Lastly, S. clarkae and C. uyato displayed the lowest median blood glucose concentrations; however, they were not significantly different from that of C. granulosus or H. griseus (Table 2; Fig. 3A). While M. sinusmexicanus was not included in the statistical comparison because of low sample size, this species’ mean blood glucose concentration was high compared to that of other species, and similar to that of M. canis (Fig. 3A). These results should be interpreted with caution, as the intraspecific variability in at-vessel blood glucose concentrations was high (Fig. 3A).
Table 2.
Mean (± SE) at-vessel blood chemistry parameters of longline captured M. sinusmexicanus (MSIN), M. canis (MCAN), S. cubensis (SCUB),S. clarkae (SCLA), C. uyato (CUYA), C. granulosus (CGRA) and H. griseus (HGRI)
| Glucose (mmol l−1) | Lactate (mmol l−1) | pH | Potassium (mmol l−1) | Hematocrit (%) | |
|---|---|---|---|---|---|
| MSIN | 5.11 ± 0 | 6.25 ± 0.86 (11) | 7.31 ± 0.03 (11) | 3.06 ± 0 (1) | 23.32 ± 1.22 (19) |
| MCAN | 4.83 ± 0.41 | 11.40 ± 1.47 (14) | 7.32 ± 0.06 (16) | 3.51 ± 0.24 (15) | 25.07 ± 1.18 (17) |
| SCUB | 4.45 ± 0.38 | 10.35 ± 0.38 (62) | 7.16 ± 0.01 (63) | 4.41 ± 0.13 (81) | 22.88 ± 0.40 (92) |
| SCLA | 3.43 ± 0.22 | 6.22 ± 0.31 (62) | 7.23 ± 0.01 (63) | 3.77 ± 0.13 (73) | 26.15 ± 0.38 (79) |
| CUYA | 3.69 ± 0.21 | 4.97 ± 0.23 (85) | 7.31 ± 0.01 (85) | 3.29 ± 0.10 (98) | 20.52 ± 0.20 (209) |
| CUYA* | 2.90 ± 0.24 | 11.16 ± 0.35 (6) | 7.17 ± 0.04 (6) | 23.17 ± 1.19 (6) | |
| CGRA | 5.04 ± 0.88 | 5.30 ± 0.76 (15) | 7.26 ± 0.03 (14) | 3.16 ± 0.36 (14) | 19.35 ± 0.57 (21) |
| HGRI | 4.28 ± 0.85 | 4.35 ± 0.29 (8) | 7.27 ± 0.05 (9) | 6.44 ± 0.93 (10) | 23.33 ± 0.95 (10) |
CUYA* are parameters for at-moribund individuals that were kept in an on-board observation tank. Sample sizes are in parentheses.
Figure 3.
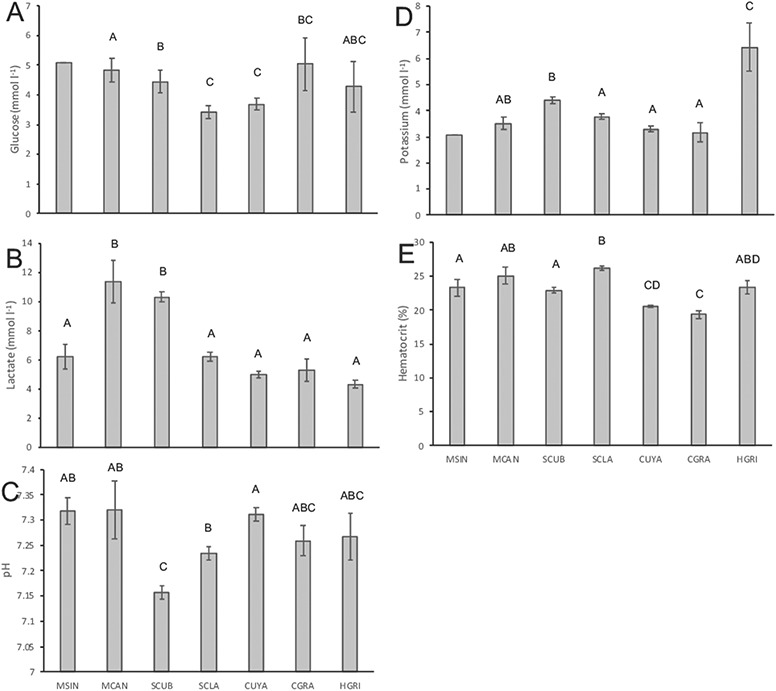
Mean (± SE) of the at-vessel blood chemistry parameters (A) glucose (mmol l−1), (B) lactate (mmol l−1), (C) pH, (D) potassium (mmol l−1) and (E) hematocrit (%) of longline-captured M. sinusmexicanus (MSIN), M. canis (MCAN), S. cubensis (SCUB), S. clarkae (SCLA), C. uyato (CUYA), C. granulosus (CGRA) and H. griseus (HGRI), letters indicate significant pairwise differences within each plot.
There were significant inter-specific differences in mean blood lactate concentrations (Welch’s ANOVA: F6,45.8 = 31.35, P = 1.2e-14). Mean blood lactate was significantly higher in M. canis and S. cubensis than in the other five sharks analyzed, including M. sinusmexicanus, S. clarkae, C. uyato, C. granulosus and H. griseus (Table 2; Fig. 3B).
There were significant inter-specific differences in mean blood pH (ANOVA: F6,254 = 11.53, P = 2.08e-11). S. cubensis had significantly lower mean blood pH than M. sinusmexicanus, M. canis, S. clarkae and C. uyato. S. clarkae had the next lowest blood pH, but it was only significantly lower than that of C. uyato. Mean blood pH in C. granulosus and H. griseus was not significantly different than that of any other species (Table 2; Fig. 3C).
There were significant inter-specific differences in mean blood potassium concentrations (ANOVA: F5,285 = 18.78, P = 3.93e-16). H. griseus had the highest mean concentration of the six species examined. S. cubensis had the next highest potassium concentration; however, it was not significantly higher than that of M. canis. The species with the lowest mean blood potassium concentration were S. clarkae, C. uyato and C. granulosus; however, those values were not significantly lower than that of M. canis. While the mean blood potassium concentration of M. sinusmexicanus was not included in this analysis because of low sample size, it was similar to that of the four shark species with low blood potassium—M. canis, S. clarkae, C. uyato and C. granulosus (Table 2; Fig. 3D).
There were significant inter-specific differences in mean hematocrit (ANOVA: F6,440 = 31.7, P < 2e-16). S. clarkae had the highest mean hematocrit of any species examined, which was significantly higher than that of M. sinusmexicanus, S. cubensis, C. uyato and C. granulosus, but not significantly different than that of M. canis or H. griseus. M. sinusmexicanus and S. cubensis had the next highest hematocrit, although it was not significantly different than that of M. canis or H. griseus, while C. uyato and C. granulosus had significantly lower hematocrit levels relative to other species (Table 2; Fig. 3E).
Change in core temperature
Average (± SE) changes between at-vessel core temperature and bottom temperature (ΔCT) were 2.05 ± 0.71°C for M. sinusmexicanus, 3.49 ± 0.39°C for M. canis, 6.70 ± 0.29°C for S. cubensis, 10.30 ± 0.47°C for S. clarkae, 6.29 ± 0.19°C for C. uyato, 2.31 ± 0.21°C for C. granulosus and 3.75 ± 0.92°C for H. griseus.
No significant relationships were found between the seven blood chemistry parameters and ΔCT for M. sinusmexicanus, M. canis or C. granulosus (Table 3). In S. cubensis, pH significantly decreased (R2 = 0.14, P = 0.01) and hematocrit significantly increased (R2 = 0.09, P = 0.01) with increasing ΔCT. When considering the Bonferroni correction, neither aforementioned parameter significantly changed with ΔCT. Glucose, lactate and potassium did not change with ΔCT in S. cubensis (Table 3).
Table 3.
Results of linear regressions investigating how at-vessel blood chemistry parameters of longline captured M. sinusmexicanus (MSIN), M. canis (MCAN), S. cubensis (SCUB), S. clarkae (SCLA), C. uyato (CUYA), C. granulosus (CGRA) and H. griseus (HGRI) change with core temperature
| Species | Parameter | P or NP | Test statistics (F or Z) | DF | R 2 or tau | P-value |
|---|---|---|---|---|---|---|
| MSIN | ||||||
| Lactate | P | 0.46 | 1, 1 | 0.31 | 0.62 | |
| pH | P | 0.91 | 1, 1 | 0.48 | 0.51 | |
| Hct | P | 24.06 | 1, 1 | 0.96 | 0.13 | |
| MCAN | ||||||
| Glucose | P | 1.21 | 1, 8 | 0.13 | 0.30 | |
| Lactate | P | 1.40 | 1, 7 | 0.17 | 0.28 | |
| pH | P | 0.32 | 1, 7 | 0.04 | 0.59 | |
| K | P | 0.01 | 1, 8 | 1.9e-3 | 0.91 | |
| Hct | P | 0.001 | 1, 8 | 8.4e-5 | 0.98 | |
| SCUB | ||||||
| Glucose | NP | 0.48 | 1, 67 | 0.04 | 0.63 | |
| Lactate | P | 0.01 | 1, 43 | 1.2e-4 | 0.94 | |
| pH | P | 6.94 | 1, 43 | 0.14 | 0.01 * | |
| K | P | 0.24 | 1, 68 | 3.5e-3 | 0.63 | |
| Hct | P | 6.96 | 1, 72 | 0.09 | 0.01 * | |
| SCLA | ||||||
| Glucose | NP | 2.96 | 1, 53 | 0.28 | 3.1e-3 ** | |
| Lactate | P | 2.92 | 1, 38 | 0.07 | 0.10 | |
| pH | P | 5.75 | 1, 39 | 0.13 | 0.02 * | |
| K | P | 1.42 | 1, 54 | 0.03 | 0.24 | |
| Hct | P | 3.35 | 1, 54 | 0.09 | 0.07 | |
| CUYA | ||||||
| Glucose | NP | 6.57 | 1, 159 | 0.35 | 5.1e-11 ** | |
| Lactate | P | 0.003 | 1, 69 | 5.0e-5 | 0.95 | |
| pH | P | 1.08 | 1, 69 | 0.02 | 0.30 | |
| K | P | 0.28 | 1, 92 | 3.0e-3 | 0.60 | |
| Hct | P | 0.44 | 1, 179 | 2.5e-3 | 0.51 | |
| CGRA | ||||||
| Glucose | P | 3.26 | 1, 10 | 0.25 | 0.10 | |
| Lactate | P | 0.002 | 1, 7 | 3.2e-4 | 0.96 | |
| pH | P | 0.67 | 1, 8 | 0.08 | 0.44 | |
| K | P | 0.51 | 1, 10 | 0.05 | 0.49 | |
| HCT | P | 0.78 | 1, 10 | 0.07 | 0.40 | |
| HGRI | ||||||
| Glucose | P | 5.45 | 1, 4 | 0.58 | 0.08 | |
| Lactate | P | 0.27 | 1, 4 | 0.06 | 0.63 | |
| pH | P | 1.38 | 1, 4 | 0.26 | 0.31 | |
| K | P | 13.99 | 1, 3 | 0.82 | 0.03 * | |
| Hct | P | 13.05 | 1, 3 | 0.81 | 0.04 * |
P denotes a parametric test was conducted, while NP denotes a non-parametric test was conducted. A * following a P-value denotes significance at an  level of 0.05, while ** denotes significance at the Bonferroni-corrected
level of 0.05, while ** denotes significance at the Bonferroni-corrected  level.
level.
In S. clarkae, there was a significant increase in glucose (tau = 0.28, P = 3.1e-3) and a significant decrease in pH (R2 = 0.13, P = 0.02) with increasing ΔCT; however, the change in pH was not significant with the Bonferroni correction. No significant relationships were found between lactate, potassium or hematocrit and ΔCT in S. clarkae (Table 3).
In C. uyato, a significant increase in glucose (tau = 0.35, P = 5.1e-11) was found with increasing ΔCT. No significant changes were observed in the six other physiological parameters relative to ΔCT (Table 3).
In H. griseus, hematocrit increased significantly (R2 = 0.81, P = 0.04) while potassium decreased significantly (R2 = 0.82, P = 0.03) with increasing ΔCT. The remaining parameters (glucose, lactate and pH) had no relationship with ΔCT (Table 3). It is important to note that none of these parameters significantly changed with ΔCT when considering the Bonferroni correction.
Moribund blood chemistry metrics
Six mature gulper sharks C. uyato were captured on one longline set for 371 minutes at a maximum depth of 711 m and a minimum temperature of 6.41°C. Because of sea conditions, this set had among the longest soak times of any in the survey. All individuals were hooked in the jaw on 10/0, 12/0 or 14/0 hooks. Once placed in an on-deck tank, each shark floated upside down and buccal pumped and/or respired through its spiracles for 14 to 47 min before becoming moribund. No sharks showed signs of recovery. Blood chemistry metrics for at-moribund sharks appear in Table 2.
Capture method
Two species, S. cubensis and C. uyato, were also captured in chevron traps in large enough numbers (albeit fewer than on longlines) to allow for a comparison of physiological parameters between longline- and trap-captured sharks (Table 4). S. cubensis had significantly higher lactate after longline capture than after trap capture (Table 5). C. uyato had significantly higher blood potassium after trap capture than after longline capture (Table 6). No other blood chemistry parameters were significantly different between capture methods in either species.
Table 4.
Mean (± SE) at-vessel blood chemistry parameters of trap and longline (LL) captured S. cubensis (SCUB) and C. uyato (CUYA)
| Glucose (mmol l−1) | Lactate (mmol l−1) | pH | Potassium (mmol l−1) | Hematocrit (%) | ||
|---|---|---|---|---|---|---|
| SCUB | Trap | 4.70 ± 1.72 (9) | 5.83 ± 0.39 (9) | 6.48 ± 0.03 (9) | 4.43 ± 0.55 (4) | 25.06 ± 1.44 (8) |
| LL | 4.79 ± 0.40 (89) | 10.42 ± 0.39 (63) | 7.16 ± 0.01 (63) | 4.41 ± 0.14 (77) | 22.38 ± 0.41 (100) | |
| CUYA | Trap | 2.78 ± 0.37 (8) | 3.60 ± 0.45 (8) | 6.48 ± 0.03 (8) | 4.60 ± 0.41 (5) | 22.13 ± 1.19 (8) |
| LL | 3.83 ± 0.23 (175) | 5.03 ± 0.24 (78) | 7.31 ± 0.01 (78) | 3.20 ± 0.10 (94) | 20.47 ± 0.19 (219) |
Sample sizes in parentheses.
Table 5.
Statistical results of t-tests or Wilcoxon rank sum tests comparing at-vessel blood chemistry parameters of S. cubensis caught by trap or longline (LL)
| Sample size | Test statistics | |||||
|---|---|---|---|---|---|---|
| Trap | LL | P or NP | (T or W) | DF | P-value | |
| Glucose | 9 | 89 | NP | 509 | 96 | 0.19 |
| Lactate | 9 | 63 | P | 3.78 | 70 | 3.2e-3 |
| pH | 9 | 63 | P | −1.12 | 70 | 0.27 |
| K | 4 | 77 | P | 0.03 | 79 | 0.98 |
| Hct | 8 | 100 | P | −1.72 | 106 | 0.09 |
P denotes a parametric test was conducted, while NP denotes a non-parametric test was conducted.
Table 6.
Statistical results of t-tests or Wilcoxon rank sum tests comparing at-vessel blood chemistry parameters of C. uyato caught by trap or longline (LL)
| Sample size | Test statistics | |||||
|---|---|---|---|---|---|---|
| Trap | LL | P or NP | (T or W) | DF | P-value | |
| Glucose | 8 | 178 | NP | 805 | 181 | 0.48 |
| Lactate | 8 | 78 | NP | 391 | 84 | 0.25 |
| pH | 8 | 78 | P | 0.44 | 84 | 0.6 |
| K | 5 | 94 | P | −3.24 | 97 | 0.0016 |
| Hct | 8 | 219 | P | −1.59 | 225 | 0.11 |
P denotes a parametric test was conducted, while NP denotes a non-parametric test was conducted.
Distance from DWH oil spill
No significant relationships were found between the seven blood chemistry parameters and the distance between the DWH oil spill site and the capture site for M. sinusmexicanus, M. canis, S. clarkae, C. granulosus or H. grisius (Table 7). A significant decrease in hematocrit was found in S. cubensis with increasing distance from the DWH oil spill site (Table 7). In C. uyato, glucose and potassium significantly increased, while lactate significantly decreased with increasing distance from the DWH oil spill site (Table 7). When considering the Bonferroni correction, the only significant change was the increase in glucose in C. uyato.
Table 7.
Results of linear regressions investigating how at-vessel blood chemistry parameters of longline captured M. sinusmexicanus (MSIN), M. canis (MCAN), S. cubensis (SCUB), S. clarkae (SCLA), C. uyato (CUYA), C. granulosus (CGRA) and H. griseus (HGRI) change with distance between the site of the DWH oil spill and the animal capture site
| Species | Parameter | P or NP | Test statistics (F or Z) | DF | R 2 or tau | P-value |
|---|---|---|---|---|---|---|
| MSIN | ||||||
| Lactate | P | 2.77 | 1, 9 | 0.24 | 0.13 | |
| pH | P | 5.1e-4 | 1, 9 | 5.7e-5 | 0.98 | |
| Hct | P | 1.16 | 1, 17 | 0.06 | 0.30 | |
| MCAN | ||||||
| Glucose | P | 0.85 | 1, 13 | 0.06 | 0.37 | |
| Lactate | P | 1.91 | 1, 12 | 0.14 | 0.19 | |
| pH | P | 1.41 | 1, 14 | 0.09 | 0.26 | |
| K | P | 0.15 | 1, 13 | 0.01 | 0.70 | |
| Hct | P | 0.35 | 1, 15 | 0.02 | 0.56 | |
| SCUB | ||||||
| Glucose | NP | 0.81 | 1, 83 | 0.07 | 0.42 | |
| Lactate | P | 0.52 | 1, 60 | 8.6e-3 | 0.47 | |
| pH | P | 2.11 | 1, 61 | 0.03 | 0.15 | |
| K | P | 0.52 | 1, 79 | 6.6e-3 | 0.47 | |
| Hct | P | 6.72 | 1, 90 | 0.07 | 0.01 * | |
| SCLA | ||||||
| Glucose | NP | 0.74 | 1, 69 | 0.07 | 0.46 | |
| Lactate | P | 0.02 | 1, 60 | 2.7e-4 | 0.90 | |
| pH | P | 2.26 | 1, 60 | 0.04 | 0.14 | |
| K | P | 0.09 | 1, 71 | 13.e-3 | 0.76 | |
| Hct | P | 3.37 | 1, 77 | 0.04 | 0.07 | |
| CUYA | ||||||
| Glucose | NP | 4.59 | 1, 179 | 0.25 | 4.3e-6 ** | |
| Lactate | P | 6.77 | 1, 83 | 0.07 | 0.01 * | |
| pH | P | 3.9e-3 | 1, 83 | 4.7e-5 | 0.95 | |
| K | P | 5.87 | 1, 96 | 0.06 | 0.02 * | |
| Hct | P | 3.77 | 1, 207 | 0.02 | 0.05 | |
| CGRA | ||||||
| Glucose | P | 3.49 | 1, 12 | 0.23 | 0.09 | |
| Lactate | P | 3.07 | 1, 13 | 0.19 | 0.10 | |
| pH | P | 0.19 | 1, 12 | 0.02 | 0.67 | |
| K | P | 0.68 | 1, 12 | 0.05 | 0.43 | |
| HCT | P | 1.06 | 1, 19 | 0.05 | 0.32 | |
| HGRI | ||||||
| Glucose | P | 1.05 | 1, 8 | 0.12 | 0.34 | |
| Lactate | P | 1.13 | 1, 6 | 0.16 | 0.33 | |
| pH | P | 4.56 | 1, 7 | 0.39 | 0.07 | |
| K | P | 0.02 | 1, 8 | 2.8e-3 | 0.88 | |
| Hct | P | 4.12 | 1, 8 | 0.34 | 0.08 |
P denotes a parametric test was conducted, while NP denotes a non-parametric test was conducted. A * following a P-value denotes significance at an  level of 0.05, while ** denotes significance at the Bonferroni-corrected
level of 0.05, while ** denotes significance at the Bonferroni-corrected  level.
level.
Discussion
This is the third study following Barkley et al. (2017) and Talwar et al. (2017) to examine blood chemistry after capture in deep-sea sharks. The seven species of sharks examined herein vary in size from the small S. cubensis to the large H. griseus and also vary in the depths at which they reside, with M. sinusmexicanus, M. canis and S. cubensis inhabiting depths shallower than 300 m and S. clarkae, C. uyato, C. granulosus and H. griseus inhabiting depths of about 500 m and greater (Fig. 2B).
We documented variability in blood chemistry values after capture for these seven species of deep-sea shark. In general, values suggested more disturbed blood chemistry than reported for shallower-dwelling demersal elasmobranch species such as the southern stingray Hypanus americana (Cain et al. 2004), Port Jackson shark Heterodontus portusjacksoni (Frick et al. 2010) and smalltooth sawfish P. pectinata (Prohaska et al. 2018). Values were similar to those of gillnet- and longline-captured gummy sharksM. antarcticus (Frick et al. 2010) and trawl-captured spiny dogfish S. acanthias (Mandelman and Farrington 2007). While multiple blood chemistry parameters were similar between the species examined here and some coastal and pelagic sharks studied to date (Marshall et al. 2012; Gallagher et al. 2014), they generally suggested less physiological disturbance for those individuals examined here than reported for species such as the great hammerhead Sphyrna mokarran (Gallagher et al. 2014), blacktip Carcharhinus limbatus, porbeagle Lamna nasus and pelagic thresher Alopias pelagicus (Marshall et al. 2012), which are considered particularly susceptible to the stress of capture.
Post-release mortality may be high for some of these species. In Exuma Sound, The Bahamas, Centrophorus sp. exhibited an 83% post-release mortality rate in the first 24 h following longline capture after a mean soak time of 3.5 h (Talwar et al. 2017). Blood chemistry data from this study support that finding; the mean moribund blood lactate (11.16 mmol/L) and pH (7.17) values for C. uyato in this study were similar to the at-vessel blood lactate (9.29 mmol/L) and pH values (7.14) of those animals. Satellite telemetry data also suggest high post-release mortality for longline-caught C. uyato in the northern GoM (~29°N latitude; Grubbs, unpublished data) and for Centrophorus spp. in The Bahamas (Brooks et al. 2015). Using a predictive equation for post-release mortality based on lactate, glucose, and total length of longline-caught S. cubensis (Talwar et al. 2017), we suggest a higher post-release mortality rate for individuals caught in this study (73.4%) than in The Bahamas (49.7%). This difference is probably because of our catch being composed of smaller animals (mean TL: 47 cm here, 58 cm there) caught on longlines with slightly longer mean soak times (3.93 h here, 3.5 h there), which could influence higher blood lactate values (10.35 mmol/l here, 9.8 mmol/l there).
Inter-specific differences
Blood glucose, lactate and pH values were similar in the two Mustelus species and S. cubensis and indicated a more pronounced secondary physiological stress response in those species than in S. clarkae, the two Centrophorus species and H. griseus. When investigating the three pairs of congeners independently, there were no significant differences in the majority of the physiological parameters assessed between Mustelus congeners and Centrophorus congeners; however, there was always a significant difference between Squalus congeners. The similarities between the Centrophorus congeners, S. clarkae and H. griseus could be attributed to their taxonomic relatedness as the Centrophorus congeners andS. clarkae are members of the same order, Squaliformes, and they are all members of the same superorder as H. griseus, Squalomorphii (Naylor et al. 2012). The two Mustelus congeners are members of the order Carcharhiniformes, which are more distantly related to the Centrophorus and Squalus congeners and H. griseus, and are members of a different superorder, Galeomorphii (Naylor et al. 2012). Interestingly, the blood chemistry profiles of the two Squalus congeners were different, with greater relative physiological disturbance in S. cubensis than S. clarkae, and similar blood chemistry values were observed between S. cubensis and M. canis despite being distantly related evolutionarily. While all congener pairs inhabit statistically different depths, the difference in depth between the two Squalus species is the greatest and may demarcate a difference between the shallower- and deeper-dwelling sharks in this study—those shallower than 300 m that inhabit the continental shelf edge and upper slope and those deeper than ~ 500 m that inhabit the mid-continental slope. With this difference in depth comes a difference in average bottom temperature, with significantly warmer and fluctuating temperatures in the shallower habitats that receive mixed epipelagic water and experience seasonality, and the relatively aseasonal, permanently cold temperatures in the deeper habitats. Furthermore, Larsen et al. (2020) found deeper-dwelling sharks had smaller relative heart size resulting in lower metabolic capacity than shallower-dwelling species. These results suggest that differences observed in at-vessel blood chemistry parameters between the Mustelus congeners and S. cubensis versus the remaining Squalomorphii may be driven by habitat use, rather than evolutionary history.
While individual capture duration was not measured in this study, we do know that the deeper-dwelling species experienced longer total time on hook than shallow-dwelling species after consistent soak times on bottom as it took longer to retrieve gear from deeper depths. While we do not suggest the time between hooking and gear retrieval is non-stressful, the process of retrieval from deep depths, sometimes greater than 1000 m, likely induces significant stress relative to the hooking event itself. Furthermore, only one species investigated herein, H. griseus, is an obligate ram ventilator, meaning that the other species are capable of lying motionless and breathing normally once hooked. Benthic-associated buccal/spiracular pumping species like those herein exhibit more subdued responses to longline capture than more active ram ventilators (Talwar et al. 2020), and resting on the bottom while hooked can mitigate the effects of capture on physiological disturbance (Guida et al. 2016; Bouyoucos et al. 2018).
However, even when considering the stress of retrieval, we see a greater magnitude of acidosis in the shallower-dwelling S. cubensis than the deeper-dwelling S. clarkae, for instance. One possible explanation for this could be the difference in capture temperature because of depth, with warmer capture temperatures at shallower depths driving a more pronounced response to capture. Previous studies examining capture stress in elasmobranchs have found that capture temperature may have an effect on blood chemistry. For example, blood glucose and plasma lactate were positively related to sea surface temperature at the time of hook-and-line capture in Atlantic sharpnose sharks Rhizoprionodon terraenovae (Hoffmayeret al. 2012). Similarly, in longline-captured nurse sharks, both blood glucose and plasma potassium concentrations were positively related to sea surface temperature (Bouyoucoset al. 2018). In trawl-captured little skates, elevations in plasma lactate and ions (Na+, Cl−, Ca2+, Mg2+, K+) were more extreme after aerial exposure during the summer than during the winter (Cicia et al. 2012). While it appears that at-vessel blood chemistry in the seven species of sharks examined herein is species specific, there are similarities between closely related species and, simultaneously, the depth and temperatures at which they inhabit may be strong drivers of their physiological response.
Change in core temperature
When an ectothermic fish is hauled to the surface after capture, their body temperature begins to adjust to the ambient temperature, with temporary insulation from this change corresponding to the amount of muscle tissue on their body (Stevens and Sutterlin 1976). Rapid alterations in body temperature can trigger a stress response (Crawshaw 1979). In this study, the difference between the bottom temperature and sea surface temperature in the GoM was as much as 15°C at depths of 200–300 m and 25°C at depths greater than 1000 m. The subsequent change in core temperature appeared to affect at-vessel blood chemistry most prominently in the two smallest species examined, S. cubensis and S. clarkae, as well as the largest species examined, H. griseus. The average ΔCT was greatest in the two Squalus species, likely because their body sizes were the smallest. Significant changes in at-vessel blood pH were observed in both Squalus species as a function of ΔCT, but glucose only significantly increased with increasing ΔCT in S. clarkae. Changes in an ectotherm’s core body temperature can affect its metabolic rate, and, in smaller bodied ectotherms that are not able to insulate as well against this change (Stevens and Sutterlin 1976), this may result in a quicker change in metabolic rate and subsequently a greater observed stress response (Hopkins and Cech 1994; Morgan and Burgess 2007; Guida et al. 2016). Talwar et al. (2017) did not find a significant difference in many of the blood chemistry parameters examined between S. cubensis and Centrophorus sp.; however, their sharks were hauled to the surface at a rate of 0.3 m s−1 from an average depth of 760 m, while our sharks were hauled at an approximate rate of 1 m s−1 from a mean depth of 711 m or 535 m (depending on species). Because their sharks were hauled at a slower rate and deeper depth (but similar temperature) there was likely more time for Centrophorus sp. core temperature to warm, potentially resulting in an increased metabolic rate and subsequently a greater physiological disturbance, unlike in our study where sharks were hauled much more rapidly, not allowing the larger-bodied, deeper-dwelling sharks to warm as much as the shallower species. This also is corroborated by the greater metabolic stress observed in the Centrophorus sp. examined in Talwar et al. (2017) versus this study.
The significant changes observed in H. griseus potassium and hematocrit with increasing ΔCT are interesting and contradictory to the interpretation of the results in the two Squalus species; however, it should be noted that the temperature probe may not have been long enough to assess a true core temperature, and the sample size for the change in core analyses in H. griseus was only five sharks. Future work using a longer temperature probe and larger sample size is warranted. Furthermore, when using the Bonferroni correction  , no significant changes were detected in stress physiology parameters for H. griseus.
, no significant changes were detected in stress physiology parameters for H. griseus.
Capture method
In S. cubensis, longline capture induced higher at-vessel blood lactate than trap capture; in C. uyato, trap capture induced higher potassium than longline capture. The remaining blood chemistry parameters were not significantly different between capture methods. The chevron traps that were used measured 60 cm×60 cm×38 cm, and the average total length of the sharks captured in the traps was 48 and 68 cm for S. cubensis and C. uyato, respectively. Given the relatively small size of S. cubensis, this species was likely still able to move easily once in the trap. S. cubensis have been observed swimming in similar deep enclosures while ventilating normally and resting on the bottom (Talwar et al. 2017). As C. uyato is larger in size, this species may have bumped the edges of the trap more frequently than S. cubensis, potentially eliciting the more elevated levels of blood potassium. In past research, Centrophorus sp. has exhibited abnormal swimming behavior inside deep enclosures and may thus respond negatively to trap capture in a similar manner (Talwar et al. 2017). Regardless, no significant differences in pH, which can be a proxy for respiratory stress, were noted between the two capture methods for this species, most likely because they are able to respire without moving, and there would be no respiratory impediment in either scenario. S. cubensis exhibited higher at-vessel blood lactate after longline capture, probably because hooking induced a greater, and earlier, fight-or-flight response than trap capture because of the physical injury from the hook going through the fish’s jaw. Trap capture, in comparison, may not elicit stress until gear retrieval. Furthermore, the traps were typically 10% full or less by volume at retrieval, which may have reduced the incidence of physical trauma, which has been attributed to stress in trap-captured fish (Davis 2002). While we could not identify any studies that compared the stress response in fish between longline and trap capture, Hopkins and Cech (1992) did find a more pronounced stress response in gillnet-captured striped bass, Morone saxatilis, than those captured in traps. In elasmobranchs, gillnet capture is more stressful than longline capture (Manire et al. 2001; Hyatt et al. 2012; Guida et al. 2016; Prohaska et al. 2018). Here, it appears that in the smaller-bodied S. cubensis, longline capture was more stressful than trap capture, probably because of the hooking injury and the resulting behavioral and physiological response, whereas trap capture was more stressful in the larger-bodied C. uyato, potentially because of more limited mobility inside the trap. The interpretation of these results should be considered with caution because of the low number of S. cubensis and C. uyato that were captured in traps for comparison with longline-captured individuals.
Distance from DWH oil spill
Given that all sharks in this study were captured in the same manner, from the same fixed stations (Fig. 1), we investigated at-vessel blood chemistry as a function of distance from the DWH oil spill site. We know that oil exposure likely occurred at this study site between 2011 and 2014 given the analysis of PAH metabolites for sharks collected in this same survey by Leary (2015). Furthermore, while catch rates at individual sampling stations varied predictably among stations and regions, they did not vary seasonally, suggesting these taxa are non-migratory. To our knowledge, no previous studies have investigated the effects of oil exposure on the physiological metrics examined herein over this length of time as they are not well suited for this examination; however, given this unique data set and the wider context of the expeditions, we were interested in this question. Of all 61 analyses for seven blood chemistry parameters, only four relationships were found to be significant, three of which had coefficients of determination less than 0.08, indicating minor relationships, and only one of which was significant at the Bonferroni-corrected  . That significant relationship was between glucose in C. uyato and distance from the DWH oil spill site; results indicated that at-vessel blood glucose was higher further away. However, this range of values was similar to that measured in Centrophorus sp. after longline capture in areas unaffected by oil exposure (Talwar et al. 2017). Overall, our results indicate that there are no biologically significant relationships between these blood chemistry metrics and distance from the DWH oil spill and the relationships that we did observe are likely because of factors other than the oil spill. These results are not surprising as sharks are highly mobile organisms that, in the absence of significant barriers, can move away from locally unsuitable habitats or environmental conditions (Schlaff et al. 2014). Sessile organisms, on the other hand, are unable to move long distances once settled on the substrate. In addition, the oil spill occurred in 2010, and our sampling began in 2014, so we assume a low likelihood of seeing an effect of the oil spill so long after the event occurred in the physiological markers that we investigated, which are best suited to characterize acute physiological stress. Thus, the long-term effects of the DWH oil spill are less likely to be observed in sharks’ secondary stress responses, but rather in toxicological examinations which may indicate contaminants from the oil spill entering the food web through sessile organisms, then bioaccumulating and biomagnifying in these larger mobile vertebrates (Gelsleichter et al. 2005).
. That significant relationship was between glucose in C. uyato and distance from the DWH oil spill site; results indicated that at-vessel blood glucose was higher further away. However, this range of values was similar to that measured in Centrophorus sp. after longline capture in areas unaffected by oil exposure (Talwar et al. 2017). Overall, our results indicate that there are no biologically significant relationships between these blood chemistry metrics and distance from the DWH oil spill and the relationships that we did observe are likely because of factors other than the oil spill. These results are not surprising as sharks are highly mobile organisms that, in the absence of significant barriers, can move away from locally unsuitable habitats or environmental conditions (Schlaff et al. 2014). Sessile organisms, on the other hand, are unable to move long distances once settled on the substrate. In addition, the oil spill occurred in 2010, and our sampling began in 2014, so we assume a low likelihood of seeing an effect of the oil spill so long after the event occurred in the physiological markers that we investigated, which are best suited to characterize acute physiological stress. Thus, the long-term effects of the DWH oil spill are less likely to be observed in sharks’ secondary stress responses, but rather in toxicological examinations which may indicate contaminants from the oil spill entering the food web through sessile organisms, then bioaccumulating and biomagnifying in these larger mobile vertebrates (Gelsleichter et al. 2005).
Conclusions
Deep-sea sharks inhabiting depths shallower than 500 m generally exhibited larger relative secondary physiological stress to capture compared to those inhabiting depths greater than 500 m. We hypothesize that this pattern could be species specific but also related to their ecology. Further, small sharks like S. cubensis have lower thermal inertia than large sharks, and thus temperature-related stress can be greatest for small demersal sharks captured and hauled to the surface.
Funding
This work was supported by the Gulf of Mexico Research Initiative as part of the Deep Sea to Coast Connectivity in the Eastern Gulf of Mexico (Deep-C) Consortium and through the Florida Resources and Ecosystem Sustainability, Tourist Opportunities and Revived Economies of the Gulf Coast Act of 2012 (RESTORE Act) Center of Excellence Program (4710-1126-00-F). The corresponding author, Bianca K. Prohaska, was supported in part by the Philanthropic Educational Organization’s Scholar Award, Florida State University Biology Department’s Jack Winn Gramling Endowed Scholarship, the Florida State University’s Coastal and Marine Lab Graduate Student Scholarship and the Florida Sea Grant Guy Harvey Scholarship.
Acknowledgements
Thank you to all of the employees and volunteers who helped with the survey and sample collection. Thank you to John Mandelman, Ryan Knotek, the New England Aquarium and University of Massachusetts Boston for assistance in quantifying plasma potassium. Thank you to all of the FSU Grubbs Lab and University of North Florida Gelsleichter Lab members who participated in these surveys, in particular Johanna Imhoff for collecting and processing blood samples in the corresponding author’s absence. All samples were collected under FSU Institutional Animal Care and Use Committee Protocols 1411 and 1708.
Appendix 1
Stress plot of the NMDS, which included all of the dependent variables regardless of the species. The dependent variables were at-vessel blood glucose, lactate, pH, potassium and hematocrit.
Appendix 2
NMDS plot color coded by species; however, not analyzed by species. This included all of the dependent variables regardless of the species, which were glucose, lactate, pH, potassium and hematocrit.
References
- Adams PR, Galvan M (1986) Voltage-dependent currents of vertebrate neurons and their role in membrane excitability. Adv Neurol 44: 137–170. [PubMed] [Google Scholar]
- Arlinghaus R, Klefoth T, Cooke SJ, Gingerich AJ, Suski CD (2009) A combined laboratory and field study to understand physiological and behavioral disturbance and recovery from catch-and-release recreational angling in northern pike (Esox lucius). Fish Res 97: 223–233. [Google Scholar]
- Barkley AN, Cooke SJ, Fisk AT, Hedges K, Hussey NE (2017) Capture-induced stress in deep-water Arctic fish species. Polar Biol 40: 213–220. [Google Scholar]
- Barton BA, Iwama G (1991) Physiological changes in fish from stress in aquaculture with emphasis on the response and effects of corticosteroids. Annu Rev Fish Dis 1: 3–26. [Google Scholar]
- Beerkircher LR, Cortes E, Shivji M (2002) Characteristics of shark bycatch on pelagic longlines off the southeastern United States, 1992–2000. Mar Fish Rev 64: 40–49. [Google Scholar]
- Black EC. (1958) Hyperactivity as a lethal factor in fish. J Fish Res Board Can 15: 573–586. [Google Scholar]
- Bonfil R. (1994) Overview of world elasmobranch fisheries. FAO Fisheries Technical Paper 341, Rome.
- Boonstra R. (2013) The ecology of stress: a marriage of disciplines. Funct Ecol 27: 7–10. [Google Scholar]
- Bouyoucos IA, Talwar BS, Brooks EJ, Brownscombe JW, Cooke SJ, Suski CD, Mandelman JW (2018) Exercise intensity while hooked is associated with physiological status of longline-captured sharks. Conserv Physiol 6: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill RW, Bushnell PG, Schroff S, Seifert R, Galvin M (2008) Effects of anaerobic exercise accompanying catch-and-release fishing on blood-oxygen affinity of the sandbar shark (Carcharhinus plumbeus, Nardo). J Exp Mar Biol Ecol 354: 132–143. [Google Scholar]
- Brooks EJ, Mandelman JW, Sloman KA, Liss S, Danylchuk AJ, Cooke SJ, Skomal GB, Philipp DP, Sims DW, Suski CD (2012) The physiological response of the Caribbean reef shark (Carcharhinus perezi) to longline capture. Comp Biochem Physiol A 162: 94–100. [DOI] [PubMed] [Google Scholar]
- Brooks EJ, Brooks AM, Williams S, Jordan LKB, Abercrombie D, Chapman DD, Howey-Jordan LA, Grubbs RD (2015) First description of deep-water elasmobranch assemblages in the Exuma Sound, The Bahamas. Deep Sea Res II 115: 81–91. [Google Scholar]
- Bushnell PG, Lutz PL, Steffensen JF, Oikari A, Gruber SH (1982) Increases in arterial blood oxygen during exercise in the lemon shark (Negaprion brevirostris). J Comp Physiol B 147: 41–47. [Google Scholar]
- Butcher PA, Peddemors VM, Mandelman JW, McGrath SP, Cullis BR (2015) At-vessel mortality and blood biochemical status of elasmobranchs caught in an Australian commercial longline fishery. Glob Ecol Conserv 3: 878–889. [Google Scholar]
- Cain DK, Harms CA, Segars A (2004) Plasma biochemistry reference values of wild-caught southern stingrays (Dasyatis americana). J Zoo Wildl Med 35: 471–476. [DOI] [PubMed] [Google Scholar]
- Cicia AM, Schlenker LS, Sulikowski JA, Mandelman JM (2012) Seasonal variations in the physiological stress response to discrete bouts of aerial exposure in the little skate, Leucoraja erinacea. Comp Biochem Physiol A 162: 130–138. [DOI] [PubMed] [Google Scholar]
- Cliff G, Thurman G (1984) Pathological and physiological effects of stress during capture and transport in the juvenile dusky shark, Carcharhinus obscurus. Comp Biochem Physiol A 78: 167–173. [Google Scholar]
- Cooke SJ, Schreer JF, Dunmall KM, Philipp DP (2002) Strategies for quantifying sublethal effects of marine catch-and-release angling: insights from novel freshwater applications. In Lucy JA, Studholme AL, eds, Catch and Release in Marine Recreational Fisheries: American Fisheries Society Symposium 30, American Fisheries Society, Bethesda, MD, pp. 121–134. [Google Scholar]
- Cooke SJ, Suski CD, Danylchuk SE, Danylchuk AJ, Donaldson MR, Pullen C, Bulte G, O’Toole A, Murchie KJ, Koppelman JB et al. (2008) Effects of different capture techniques on the physiological condition of bonefish Albula vulpes evaluated using field diagnostic tools. J Fish Biol 73: 1351–1375. [Google Scholar]
- Cortés E, Balchowsky H (2015) Preliminary catches of smoothhound sharks. SEDAR39-DW-03. SEDAR, North Charleston, SC.
- Cotton CF, Grubbs RD (2015) Biology of deep-water chondrichthyans: Introduction. Deep Sea Res Part II 115: 1–10. [Google Scholar]
- Crawshaw LI. (1979) Responses to rapid temperature change in vertebrate ectotherms. Am Zool 19: 225–237. [Google Scholar]
- Dapp DR, Walker TI, Huveneers C, Reina RD (2016) Respiratory mode and gear type are important determinants of elasmobranch immediate and post-release mortality. Fish Fish 17: 507–524. [Google Scholar]
- Davis MW. (2002) Key principles for understanding fish bycatch discard mortality. Can J Fish Aquat Sci 59: 1834–1843. [Google Scholar]
- Dulvy NK, Sadovy Y, Reynolds JD (2003) Extinction vulnerability in marine populations. Fish Fish 4: 25–64. [Google Scholar]
- Ferguson RA, Tufts BL (1992) Physiological effects of brief air exposure in exhaustively exercised rainbow trout (Oncorhynchus mykiss): implications for ‘catch and release’ fisheries. Can J Fish Aquat Sci 49: 1157–1162. [Google Scholar]
- Ferreira M, Moradas-Ferreira P, Reis-Henriques MA (2006) The effect of long-term depuration on phase I and phase II biotransformation in mullets (Mugil cephalus) chronically exposed to pollutants in River Douro Estuary, Portugal. Mar Environ Res 61: 326–338. [DOI] [PubMed] [Google Scholar]
- Frick LH, Reina RD, Walker TI (2010) Stress related physiological changes and post-release survival of Port Jackson sharks (Heterodontus portusjacksoni) and gummy sharks (Mustelus antarcticus) following gill-net and longline capture in captivity. J Exp Mar Biol Ecol 386: 29–37. [Google Scholar]
- Gallagher AJ, Frick LH, Bushnell PG, Brill RW, Mandelman JW (2010) Blood gas, oxygen saturation, pH, and lactate values in elasmobranch blood measured with a commercially available portable clinical analyzer and standard laboratory instruments. J Aquat Anim Health 22: 229–234. [DOI] [PubMed] [Google Scholar]
- Gallagher AJ, Serafy JE, Cooke SJ, Hammerschlag N (2014) Physiological stress response, reflex impairment, and survival of five sympatric shark species following experimental capture and release. Mar Ecol Prog Ser 496: 207–218. [Google Scholar]
- Gelsleichter J, Manire CA, Szabo NJ, Cortéz E, Carlson J, Lombardi-Carlson L (2005) Organochlorine concentrations in bonnethead sharks (Sphyra tiburo) from four Florida estuaries. Arch Environ Con Tox 48: 474–483. [DOI] [PubMed] [Google Scholar]
- Gorbi S, Regoli F (2004) Induction of cytochrome P4501A and biliary PAH metabolites in European eel Anguilla anguilla: seasonal, dose- and time-responsive variability in field and laboratory conditions. Mar Environ Res 58: 511–515. [DOI] [PubMed] [Google Scholar]
- Guida L, Walker TI, Reina RD (2016) Temperature insensitivity and behavioural reduction of the physiological stress response to longline capture by the gummy shark, Mustelus antarcticus. PLoS One 11: e0148829. doi: 10.1371/journal.pone.0148829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale LF, Gulak SJB, Napier AM, Carlson JK (2011) Characterization of the shark bottom longline fishery: 2010. NOAA Tech Memo NMFS-SEFSC-611.
- Harrenstien LA, Tornquist ST, Miller-Morgan TJ, Fodness BG, Clifford KE (2005) Evaluation of a point-of-care blood analyzer and determination of reference ranges for blood parameters in rockfish. J Am Vet Med Assoc 226: 255–265. [DOI] [PubMed] [Google Scholar]
- Harter TS, Morrison PR, Mandelman JW, Rummer JL, Farrell AP, Brill RW, Brauner CJ (2015) Validation of the i-STAT system for the analysis of blood gases and acid-base status in uvenile sandbar shark (Carcharhinus plumbeus). Conserv Physiol 3. doi: 10.1093/conphys/cov002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hight BV, Holts D, Graham JB, Kennedy BP, Taylor V, Sepulveda CA, Bernal D, Ramon D, Rasmussen R, Lai NC (2007) Plasma catecholamine levels as indicators of the post-release survivorship of juvenile pelagic sharks caught on experimental drift longlines in the Southern California Bight. Mar Fresh Res 58: 145–151. [Google Scholar]
- Hoffman GE, Buckley BA, Airaksinen S, Keen J, Somero GN (2000) The Antarctic fish Trematomus bernacchii lacks heat-inducible heat shock protein synthesis. J Exp Biol 203: 2331–2339. [DOI] [PubMed] [Google Scholar]
- Hoffmayer ER, Parsons GR (2001) The physiological response to capture and handling stress in the Atlantic sharpnose shark, Rhizoprionodon terraenovae. Fish Physiol Biochem 25: 277–285. [Google Scholar]
- Hoffmayer ER, Hendon JM, Parsons GR (2012) Seasonal modulation in the secondary stress response of a carcharhinid shark Rhizoprionodo terraenovae. Comp Biochem Physiol A 162: 81–87. [DOI] [PubMed] [Google Scholar]
- Hopkins TE, Cech JJ (1992) Physiological effects of capturing striped bass in gill nets and fyke traps. T Am Fish Soc 121: 819–822. [Google Scholar]
- Hopkins TE, Cech JJ (1994) Effect of temperature on oxygen consumption of the bat ray, Myliobatis californica (Chondrichthyes, Myliobatididae). Copeia 1994: 529–532. [Google Scholar]
- Hutchinson MR, Itano DG, Muir JA, Holland KN (2015) Post-release survival of juvenile silky sharks captured in a tropical tuna purse seine fishery. Mar Ecol Prog Ser 521: 143–154. [Google Scholar]
- Hyatt MW, Anderson PA, O’Donnell PM, Berzins IK (2012) Assessment of acid–base derangements among bonnethead (Sphyrna tiburo), bull (Carcharhinus leucas), and lemon (Negaprion brevirostris) sharks from gillnets and longline capture and handling methods. Comp Biochem Physiol A 162: 113–120. [DOI] [PubMed] [Google Scholar]
- IUCN 2019(IUCN) Red List Assessment. https://www.iucnssg.org/uploads/5/4/1/2/54120303/rl_assessment_results_190124.pdf (Accessed 1 August 2020).
- Jerome JM, Gallagher AJ, Cooke SJ, Hammerschlag N (2018) Integrating reflexes with physiological measures to evaluate coastal shark stress response to capture. ICES J Mar Sci 75: 796–804. [Google Scholar]
- Larsen ME, Abel DC, Crane DP, Grubbs RD (2020) Differences in relative heart mass among deep-sea and coastal sharks with increasing depth. Mar Biol 167: 1–8. [Google Scholar]
- Leary AE. (2015) Effects of the Deepwater Horizon oil spill on deep sea fishes. Master’s thesis. University of North Florida, Jacksonville, FL.
- Mandelman JW, Farrington MA (2007) The physiological status and mortality associated with otter trawl capture, transport, and captivity of an exploited elasmobranch Squalus acanthias. ICES J Mar Sci 64: 122–130. [Google Scholar]
- Mandelman JW, Skomal GB (2009) Differential sensitivity to capture stress assessed by blood acid-base status in five carcharhinid sharks. J Comp Physiol B 179: 267–277. [DOI] [PubMed] [Google Scholar]
- Manire C, Hueter R, Hull E, Spieler R (2001) Serological changes associated with gill-net capture and restraint in three species of sharks. T Am Fish Soc 130: 1038–1048. [Google Scholar]
- Marshall H, Field L, Afiadata A, Sepulveda C, Skomal G, Bernal D (2012) Hematological indicators of stress in longline-captured sharks. Comp Biochem Physiol A 162: 121–129. [DOI] [PubMed] [Google Scholar]
- Martini FH. (1974) Effects of capture and fasting confinement on an elasmobranch, Squalus acanthias. PhD dissertation. Cornell University, Ithaca, NY.
- McDonald DG, Milligan CL (1992) Chemical properties of the blood. Fish Physiol. 12B: 56–135. [Google Scholar]
- Mohan JA, Jones ER, Hendon JM, Falterman B, Boswell KM, Hoffmayer ER, Wells RJD (2020) Capture stress and post-release mortality of blacktip sharks in recreational charter fisheries of the Gulf of Mexico. Conserv Physiol 8. doi: 10.1093/conphys/coaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morato T, Watson R, Pitcher TJ, Pauly D (2006) Fishing down the deep. Fish Fish 7: 24–34. [Google Scholar]
- Morgan A, Burgess GH (2007) At-vessel fishing mortality for six species of sharks caught in the northwest Atlantic and Gulf of Mexico. Gulf Caribb Res 19: 123–130. [Google Scholar]
- Morgan A, Cooper PW, Curtis T, Burgess GH (2009) Overview of the U.S. east coast bottom longline shark fishery, 1994-2003. Mar Fish Rev 71: 23–38. [Google Scholar]
- Morgan A, Carlson J (2010) Capture time, size and hooking mortality of bottom longline-caught sharks. Fish Res 101: 32–37. [Google Scholar]
- Moyes CD, Fragoso N, Musyl M, Brill RW (2006) Predicting post-release survival in large pelagic fish. Trans Am Fish Soc 135: 1389–1397. [Google Scholar]
- Murdaugh HV Jr, Robin ED, Theodore J, Drewry W (1965) Studies of lactate metabolism in Squalus acanthias. Bull Mt Des Is Biol Lab 5: 30–31. [Google Scholar]
- Navas CA. (1996) Metabolic physiology, locomotor performance, and thermal niche breadth in neotropical anurans. Physiol Zool 69: 1481–1501. [Google Scholar]
- Naylor GJP, Caira JN, Jensen K, Rosana KAM, Straube N, Lakner C (2012) Elasmobranch phylogeny: a mitochondrial estimate based on 595 Species. In Carrier JC, Musick JA, Heithaus MR, eds, Biology of Sharks and Their Relatives, Ed 2 CRC Press, Boca Raton, pp. 31–56. [Google Scholar]
- Nikinmaa M. (1992) Membrane transport and the control of haemoglobin oxygen affinity in nucleated erythrocytes. Physiol Rev 72: 301–321. [DOI] [PubMed] [Google Scholar]
- NOAA Fisheries (2020) 2019 Stock assessment and fishery evaluation report for Atlantic highly migratory species. Atlantic Highly Migratory Species Management Division, Silver Spring, MD.
- Oliver S, Braccini M, Newman SJ, Harvey ES (2015) Global patterns in the bycatch of sharks and rays. Mar Policy 54: 86–97. [Google Scholar]
- Piiper J, Baumgarten D (1969) Blood lactate and acid-base balance in the elasmobranch Scyliorhinus stellaris after exhausting activity. Publ Staz Zool Napoli 37: 84–94. [Google Scholar]
- Piiper J, Meyer M, Drees F (1972) Hydrogen ion balance in the elasmobranch, Scyliorhinus stellaris, after exhausting activity. Resp Physiol 16: 290–303. [DOI] [PubMed] [Google Scholar]
- Prohaska BK, Bethea DM, Poulakis GR, Scharer RM, Knotek R, Carlson JK, Grubbs RD (2018) Physiological stress in the smalltooth sawfish: effects of ontogeny, capture method, and habitat quality. Endanger Species Res 36: 121–135. [Google Scholar]
- Pulster EL, Gracia A, Snyder SM, Deak K, Fogelson S, Murawski SA (2020) Chronic sub-lethal effects observed in wild-caught fishes following two major oil spills in the Gulf of Mexico: Deepwater Horizon and Ixtoc 1. In Murawski SA, Ainsworth CH, Gilbert S, Hollander DJ, Paris CB, Schlüter M, Wetzel DL, eds, Deep Oil Spills: Facts, Fate, and Effects. Springer, Cham, pp. 388–413. [Google Scholar]
- Rasmussen RA, Rasmussen LE (1967) Some observations on the protein and enzyme levels and fractions in normal and stressed elasmobranchs. Trans NY Acad Sci 29: 397–413. [DOI] [PubMed] [Google Scholar]
- Ricklefs RE, Wikelski M (2002) The physiology/life-history nexus. Trends Ecol Evol 17: 462–468. [Google Scholar]
- Romero LM, Wikelski M (2001) Corticosterone levels predict survival probabilities of Galapagos marine iguanas during El Niño events. Proc Natl Acad Sci USA 98: 7366–7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaff AM, Heupel MR, Simpfedorfer CA (2014) Influence of environmental factors on shark and ray movement, behaviour and habitat use: a review. Rev Fish Biol Fisher 24: 1089–1103. [Google Scholar]
- Schmidt-Nielsen K. (1997) Animal Physiology: Adaptation and the Environment. Cambridge University Press, Cambridge, MA [Google Scholar]
- Scott-Denton E, Cryer PF, Gocke JP, Harrelson MR, Kinsella DL, Pulver JR, Smith RC, Williams JA (2011) Descriptions of the U.S. Gulf of Mexico reef fish bottom longline and vertical line fisheries based on observer data. Mar Fish Rev 72: 1–26. [Google Scholar]
- Scott-Denton E, Williams JA (2013) Observer coverage of the 2010–2011 Gulf of Mexico reef fish fishery. NOAA Tech Memo NMFS-SEFSC-646.
- Simpfendorfer CA, Kyne PM (2009) Limited potential to recover from overfishing raises concerns for deep-sea sharks, rays and chimaeras. Environ Conserv 36: 97–103. [Google Scholar]
- Skomal GB. (2006) The physiological effects of capture stress on post-release survivorship of sharks, tunas, and marlin. PhD dissertation. Boston University, Boston, MA.
- Skomal GB. (2007) Evaluating the physiological and physical consequences of capture on post-release survivorship in large pelagic fishes. Fisheries Manag Ecol 14: 81–89. [Google Scholar]
- Skomal GB, Bernal D (2010) Physiological responses to stress in sharks. In Carrier JC, Musick JA, Heithaus MR, eds, Biology of Sharks and Their Relatives, Ed 2 CRC Press, Boca Raton, pp. 459–490. [Google Scholar]
- Skomal GB, Mandelman JW (2012) The physiological response to anthropogenic stressors in marine elasmobranch fishes: a review with a focus on the secondary response. Comp Biochem Phys A 162: 146–155. [DOI] [PubMed] [Google Scholar]
- Stevens ED, Sutterlin AM (1976) Heat transfer between fish and ambient water. J Exp Biol 65: 131–145. [DOI] [PubMed] [Google Scholar]
- Talwar B, Brooks EJ, Mandelman JW, Grubbs RD (2017) Stress, post-release mortality, and recovery of commonly discarded deep-sea sharks caught on longlines. Mar Ecol Prog Ser 582: 147–161. [Google Scholar]
- Talwar BS, Bouyoucos IA, Brooks EJ, Brownscombe JW, Suski CD, Cooke SJ, Grubbs RD, Mandelman JW (2020) Variation in behavioural responses of sub-tropical marine fishes to experimental longline capture. ICES J Mar Sci . 10.1093/icesjms/fsaa146. [DOI] [Google Scholar]
- Tomanek L, Somero GN (1999) Evolutionary and acclimation-induced variation in the heat shock responses of congeneric marine snails (genus Tegula) from different thermal habitats: implications for limits of thermotolerance and biogeography. J Exp Biol 202: 2925–2936. [DOI] [PubMed] [Google Scholar]
- Walker FI. (1998) Can shark resources be harvested sustainably? A question revisited with a review of shark fisheries. Mar Fresh Res 49: 533–572. [Google Scholar]
- Wells RMG, Davie PS (1985) Oxygen binding by the blood and hematological effects of capture stress in two big gamefish: mako shark and striped marlin. Comp Biochem Physiol A 81: 643–646. [DOI] [PubMed] [Google Scholar]
- Wells RMG, McIntyre RH, Morgan AK, Davie PS (1986) Physiological stress responses in big gamefish after capture: observations on plasma chemistry and blood factors. Comp Biochem Physiol A 84:565–571. [DOI] [PubMed] [Google Scholar]
- Wendelaar-Bonga SE. (1997) The stress response in fish. Physiol Rev 77: 591–625. [DOI] [PubMed] [Google Scholar]
- Wikelski M, Romero LM, Snell HL (2001) Marine iguanas oiled in Galapagos. Science 292: 437–438. [DOI] [PubMed] [Google Scholar]
- Wikelski M, Cooke SJ (2006) Conservation physiology. Trends Ecol Evol 21: 38–46. [DOI] [PubMed] [Google Scholar]
- Wosnick N, Bornatowski H, Ferraz C, Afonso A, Rangel BS, Hazin FHV, Freire CA (2017) Talking to the dead: using post-mortem data in the assessment of stress in tiger sharks (Galeocerdo cuvier) (Péron and Lesueur, 1822). Fish Physiol Biochem 43: 165–178. [DOI] [PubMed] [Google Scholar]
- Young JL, Bornik Z, Marcotte M, Charlie K, Wagner GN, Hinch SG, Cooke SJ (2006) Integrating physiology and life history to improve fisheries management and conservation. Fish Fish 7: 262–283. [Google Scholar]
- Zhang X, Cortés E, Courtney D, Scott-Denton E (2015) Shrimp fishery bycatch estimates for smoothhound sharks in the Gulf of Mexico, 1972–2012. SEDAR39-DW-05. SEDAR, North Charleston, SC.


