Abstract
Introduction
Premature ventricular contractions (PVC) have been associated with mortality and heart failure (HF) regardless the presence of structural heart disease (SHD). The aim of this study was assessing the impact of burden and complexity of PVCs on prognosis, according to presence of SHD.
Methods
312 patients were retrospectively evaluated out of 1967 consecutive patients referred for 24‐hr Holter at a single hospital, with a PVC count >1% of total beats. Two groups with and without SHD. PVC burden (PVC%), presence of complex forms, incidence of all‐cause death, combined outcomes of all‐cause death and cardiovascular hospitalizations, HF death and HF hospitalizations and, sudden death (SD) or hospitalizations due to ventricular arrhythmias (VA)were assessed.
Results
Premature ventricular contraction burden was 2.7 (IQR: 1.6–6.7). SHD patients had more polymorphic PVCs, 77% versus 65%, p = .022, triplets and episodes of non‐sustained ventricular tachycardia (NSVT): 44% versus 27%, p = .002; 30% versus 12%, p < .0001. In idiopathic patients, a PVC% in the third quartile was independently associated with all‐cause mortality hazard ratio (HR) 2.288 (1.042–5.026) p = .039, but not in SHD. The complexity of the PVCs was not independently associated with outcomes in both groups. In SHD group, NSVT was associated with lower survival free from SD and VA hospitalizations, p = .028; after multivariable, there was a trend for a higher arrhythmic outcome with NSVT, HR 3.896 (0.903–16.81) p = .068.
Conclusion
Premature ventricular contractions in SHD showed more complex patterns. In idiopathic patients, a higher PVC count was associated with higher mortality but not is SHD patients. Complexity was not independently associated with worse prognosis.
Keywords: idiopathic, premature ventricular contractions, prognosis, PVC burden, PVC complexity, structural heart disease
1. INTRODUCTION
Premature ventricular contractions (PVCs) are a common finding during long‐term monitoring (Arnar et al., 2019). Although PVCs can occur in healthy persons, they are more frequent in the presence of structural heart disease (SHD), sleep apnea (Marinheiro et al., 2019), chronic obstructive pulmonary disease, and hyperthyroidism or stimulants (Einvik et al., 2017). They have been associated with a worse prognosis in patients with SHD, especially if frequent or presenting with complex forms (Kostis et al., 1987). Those findings were the basis for the treatment of PVCs with antiarrhythmics which proved harmful in patients with SHD (Echt et al., 1991). However, those studies are outdated and antiarrhythmics are no longer the only option. Catheter ablation is a successful treatment and has shown an improvement in left ventricular systolic function after successful ablation, but the impact on the prognosis is unproven (Baman et al., 2010; Bogun et al., 2007; Latchamsetty et al., 2015; Ling et al., 2014). Historically idiopathic PVCs have been considered benign (Gaita et al., 2001). Recently, a few population‐based studies have suggested that PVCs are associated with an adverse outcome, even in the absence of known SHD (Agarwal et al., 2012, 2017; Dukes et al., 2015; Lin et al., 2015, 2016).
The aim of this study was to evaluate the characteristics of the PVCs on the 24‐hr Holter recording in terms of frequency and complexity, and secondly assess their impact on prognosis in patients with and without SHD.
2. METHODS
2.1. Study population
We evaluated 1,967 consecutive patients older than 18 years who underwent 24‐hr Holter monitoring in our center, between June 2006 and December 2010. We selected patients with frequent PVC, defined as PVCs representing more than 1% of total number of heart beats (n = 530 patients). We select this value based on the work of Dukes et al (Dukes et al., 2015) that demonstrated that this cutoff value provides the best sensitivity/specificity relation for prediction of adverse outcomes. Patients with AF, atrial flutter, or any rhythm other than sinus during recording (n = 168) were excluded. Patients who were lost to follow‐up (n = 50) were also excluded. The final study population included 312 patients. For the purpose of assessing the combined endpoint of sudden death (SD) or hospitalizations due to VA, patients with unknown cause of death were excluded from the analysis (n = 35).
2.2. Study design
We retrospectively collected data from the medical records including demographic data, presence of SHD and its type, diabetes, hypertension, antiarrhythmic, and beta‐blocker use. Transthoracic echocardiographic data were retrospectively obtained. Echocardiographic evaluation included M‐mode measurements of the left atrium diameter (LAD), left ventricular end diastolic diameter (LVEDD), left ventricular end systolic diameter (LVESD), and left ventricular fractional shortening (LVFS) obtained in the left parasternal view. Holter recording was performed with the use of three‐channel tape recorders (GE SEER LIGHT®). Recordings had to exceed 20 hr and be of good quality to be analyzed and all of them were reviewed. SHD was ruled out based on echocardiogram, treadmill exercise test, coronariography, and cardiac magnetic resonance. Cardiac investigation was conducted at the discretion of the patient's physician.
On Holter evaluation, number of PVCs/24 hr, the percentage of PVCs (determined by dividing the total number of PVCs by the total number of beats recorded during Holter monitoring), the morphology of the PVCs, presence of couplets, triplets and runs of non‐sustained ventricular tachycardia (NSVT) defined as more than 3 PVCs in a run were evaluated. The presence of episodes of AF, defined as runs of irregular supraventricular rhythm lasting more than 30 s (Calkins et al., 2017) and runs of supraventricular ectopic beats, defined as more than 3 consecutive supraventricular beats with an accelerated cycle length lasting <30 s, was also assessed. The 24‐hr Holter reports were supervised by the same senior physician.
Follow‐up was performed in the second half of 2018, thus including up to 12 years of follow‐up in some patients. Data were retrieved from the national patient registry and from medical records or discharge letters and were validated by reviewing patients’ files. Patients who failed to have recent clinical records were contacted by phone. We assessed the occurrence of all‐cause death, combined rate of total death and cardiovascular (CV) hospitalizations, combined rate of heart failure (HF) death or hospitalizations due to HF, and combined rate of SD or hospitalizations due to sustained ventricular arrhythmias (VA). Death was ascertained by reviewing medical records or from national patient registry. HF death was defined as worsening HF, manifested as cardiogenic shock, pulmonary edema or increase in HF symptoms and drug therapy, or hospitalization due to decompensated HF prior to death. SD was defined as unexpected fatal event occurring within 1 hr of the onset of symptoms in an apparently healthy subject. If death is not witnessed, the definition applies when the victim was in good health 24 hr before the event (Priori et al., 2015). When the cause of death could not be established, the death was classified as of unknown cause. Hospitalization was defined as an overnight stay in a hospital ward. The cause of hospitalization was obtained from medical records, and CV hospitalization included the ones due to de novo or aggravated HF, ventricular arrhythmia, bradyarrhythmia, or acute coronary syndromes. Time‐to‐event for those without the event was censored either at death, or at the last follow‐up, whichever occurred earlier Patients were divided according to the presence or absence of SHD, and the characteristics of the PVCs as well as the outcomes during the follow‐up were compared in both groups. We assessed the association between PVC burden, PVC morphology, presence of triplets and NSVT and the occurrence of major cardiac events during follow‐up adjusting for the other covariables.
2.3. Statistical analysis
All analyses were performed using SPSS statistical software, version 25.0 (SPSS, Inc). Data are presented as median and lower and upper quartile (Q1–Q3) for continuous variables due to lack of normal distribution assessed with the Kolmogorov–Smirnov test. Categorical variables are presented as absolute numbers and percentages. Continuous variables were compared with the use of Mann–Whitney test. Categorical variables were compared with the use of two‐side Fischer's exact‐test or the chi square test as appropriate for independent samples. Patients were divided into quartiles according to the PVC percentage, and the PVC burden analyzed as percentage and as quartiles, taking the first quartile as reference.
Survival curves free from the analyzed outcomes were calculated by the Kaplan–Meier method and stratified by the burden and complexity of the PVCs, using the log‐rank test for comparison. The influence of Holter variables, on adverse events occurrence during follow‐up, was evaluated by univariate and multivariate Cox proportional‐hazards regression analysis. Unadjusted hazard ratios (HR) and their 95% confidence intervals (CI) for all‐cause mortality, all‐cause mortality or CV hospitalizations, HF death or HF hospitalizations, and SD or hospitalizations due to VA were calculated. The proportional hazard assumption was tested for each of the Cox models based on Schoenfeld residuals for continuous variables and on “log‐minus‐log” plot for categorical variables. The HRs were adjusted to potential confounders covariables that displayed p < .05 on univariable analysis. For all tests, a two‐tailed p‐value of < .05 was considered statistically significant.
3. RESULTS
3.1. Study population
The study population included 312 patients. The PVCs were idiopathic in 177, and SHD was present in 135 patients. The etiology of SHD was ischemic in 93 patients, non‐ischemic cardiomyopathy in 22, hypertrophic cardiomyopathy in 7, aortic stenosis in 9 and mitral stenosis in 3, left ventricular non‐compaction in 1 patient, and myocarditis in another. The baseline characteristics and comparison between patients with idiopathic PVCs and PVCs in the context of SHD are presented in Table 1. Patients with SHD were more frequently male, had a higher incidence of diabetes and hypertension, and were more frequently on beta‐blockers. The echocardiographic evaluation showed higher values of LAD, LVEDD, LVESD, and lower LVFS. (Table 1).
TABLE 1.
Baseline characteristics. Comparison between the two groups with idiopathic PVCs and with structural heart disease
| Overall sample (n = 312) | Idiopathic PVCs (n = 177) | Structural heart disease (n = 135) | p‐value | |
|---|---|---|---|---|
| Demographic data | ||||
| Age in years, median (Q1–Q3) | 69 (61–77) | 68 (60–77) | 70 (62–76) | .226 |
| Male gender, n (%) | 186 (60) | 85 (48) | 101 (75) | <.0001 |
| Risk factors | ||||
| Diabetes, n (%) | 64 (26) | 28 (20) | 36 (36) | .003 |
| Hypertension, n (%) | 212 (86) | 121 (82) | 91 (91) | .045 |
| Medications | ||||
| Beta‐blockers, n (%) | 104 (37) | 41 (25) | 63 (55) | <.0001 |
| Echocardiogram | ||||
| LAD in mm, median (Q1–Q3) | 40 (35–45) | 36 (34–42) | 42 (37–47) | <.0001 |
| LVEDD in mm, median (Q1–Q3) | 53 (49–59) | 50 (48–54) | 58 (53–64) | <.0001 |
| LVESD, in mm, median (Q1–Q3) | 35 (30–40) | 31 (29–35) | 42 (35–50) | <.0001 |
| LVFS (%), median (Q1–Q3) | 36 (28–40) | 39 (35–41) | 27 (20–36) | <.0001 |
| 24‐hr Holter recording | ||||
| Nº of PVCs/24 hr, median (Q1–Q3) | 2,894 (1,594–7,025) | 2,833 (1,619–7,192) | 3,050 (1,504–6,894) | .634 |
| PVC percentage, median (Q1–Q3) | 2.7 (1.6–6.7) | 2.7 (1.7–6.8) | 2.8 (1.6–6.6) | .997 |
| Polymorphic morphology, n (%) | 211 (70%) | 112 (65) | 99 (77) | .022 |
| Couplets, n (%) | 236 (75) | 118 (67) | 118 (87) | <.0001 |
| Triplets, n (%) | 106 (34) | 47 (27) | 59 (44) | .002 |
| NSVT, n (%) | 62 (20) | 22 (12) | 40 (30) | <.0001 |
| SV runs, n (%) | 108 (35) | 70 (40) | 38 (28) | .041 |
| Episodes of AF, n (%) | 3 (1) | 2 (1.1) | 1 (0.7) | 1.000 |
Abbreviations: AF, atrial fibrillation; LA, left atrium; LVEDD, left ventricular end diastolic diameter; LVESD, left ventricular end systolic diameter; LVFS, left ventricular fractional shortening; NSVT, non‐sustained ventricular tachycardia; PVCs, premature ventricular contractions; SV, supraventricular.
3.2. 24‐hr Holter recording
The comparison of the characteristics of the PVCs on the 24‐hr Holter recording in the two groups is displayed in Table 1. The PVC burden was not significantly different in patients with idiopathic PVCs and in those with SHD respectively, 2,833 (1,619–7,192) versus 3,050 (1,504–6,894), p = .634 and PVC percentage 2.7% (1.7%–6.8%) versus 2.8% (1.6%–6.6%), p = .997. Quartiles 1 through 4 represented PVC burdens of 1%–1.63%, 1.63%–2.74%, 2.74%–6.77%, and more than 6.67%, respectively. The median percentage in each quartile was as follows, quartile 1:1.25 (1.1–1.43); quartile 2:2.0 (1.8–2.4); quartile 3:4.3 (3.6–5.4) and quartile 4:11.4 (8.0–18.1). The complexity of the PVCs in terms of polymorphic morphologies and presence of couplets, triplets, and runs of NSVT was significantly higher in the group with SHD, respectively 77% versus 65%, p = .022; 87% versus 67%, p < .0001; 44% versus 27%, p = .002; 30% versus 12%, p < .0001. The presence of supraventricular runs was more frequent in idiopathic patients, 40% versus 28%, p = .041. Three patients had episodes of AF during Holter monitoring corresponding to 0.96%, two in the idiopathic group (1.1%) and one in the group with SHD (0.7%), p = 1.000.
3.3. Follow‐up
During a median follow‐up of 8.3 (5.1–9.9) years, 133 patients (43%) died, 157 (49%) had the combined outcome of all‐cause death or CV hospitalizations, 18 (5.8%) the combined outcome of HF death or HF hospitalizations, and 13 (4.2%) the combined outcome of SD or hospitalizations due to VA. The events per 1,000 person‐years observed in up to 12 years of follow‐up in the two studied groups are shown in Table 2. Patients with SHD had significantly higher rates of all‐cause mortality, combined outcome of all‐cause mortality, or CV hospitalizations and combined outcome of HF death or HF hospitalizations. The rate of SD or hospitalizations due to VA was also higher but did not reach statistical significance, 10.1 versus 3.7 per 1,000 person‐years, p = .089. The cause of death was non‐cardiac in 75 patients (24%), due to HF in 13 patients (4%), due to SD or documented VA in 8 patients (2.6%), and due to acute coronary syndrome in 2 patients (0.6%). In 35 patients (11%), the cause of death was unknown. The rate of different causes of death per 1,000 person‐years in patients with idiopathic PVCs and patients with SHD is shown in Table S1. Patients with SHD had significantly higher rates of death due to HF, 13.3 versus 0.7 per 1,000 person‐years, p < .0001. The comparison of baseline characteristics of patients that experienced the cardiac adverse outcomes versus patients that did not is presented in Table S2.
TABLE 2.
Events per 1,000 person‐years in patients with idiopathic PVCs and PVCs with SHD
| Overall sample (n = 312) | Idiopathic PVCs (n = 177) | PVCs in SHD (n = 135) | p value a | |
|---|---|---|---|---|
| All‐cause death | 57.2 | 42.8 | 80 | <.0001 |
| All‐cause death or CV hospitalizations | 71.4 | 51.2 | 106 | <.0001 |
| HF death or HF hospitalizations | 7.8 | 0.7 | 19.3 | <.0001 |
| SD or VA hospitalizations b | 6.1 | 3.7 | 10.1 | .089 |
Values are presented in number of events per 1,000 person‐years.
Abbreviations: CV, cardiovascular; HF, heart failure; PVCs, premature ventricular contractions; SD, sudden death; SHD, structural heart disease; VA, ventricular arrhythmia.
p values were calculated using the Log‐rank test.
For this outcome deaths of unknown cause were excluded (n = 35).
3.4. PVC burden and prognosis
The PVC burden as a continuous variable was not associated with a worse prognosis. In idiopathic patients, the analysis per quartile of the PVC% showed a lower overall survival in patients with a PVC% in the third quartile when compared with the lower quartile (Figure 1; Table 3), with an unadjusted HR (95% CI) of 2.331 (1.090–4.984), p = .029. After adjustment to significant covariables (Table S3), it was still associated with a higher all‐cause mortality, adjusted HR (95% CI) 2.288 (1.042–5.026), p = .039. The PVC burden was not significantly associated with the other adverse outcomes (Table 3; Figure 1; Figures S1 and S2).
FIGURE 1.
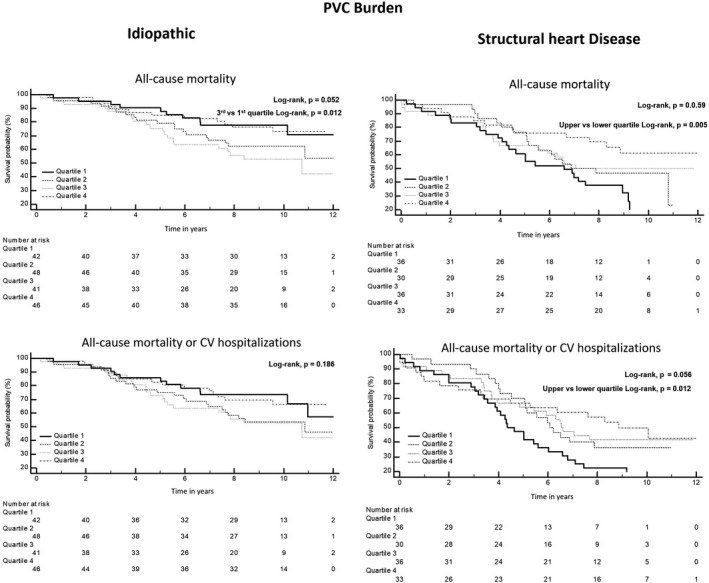
Kaplan–Meier survival estimate of overall survival and CV hospitalizations‐free survival stratified by the quartile of PVC percentage in both groups with and without structural heart disease. Quartiles 1 through 4 represented PVC burdens of 1%–1.63%, 1.63%–2.74%, 2.74%–6.77%, and more than 6.67%, respectively. CV, cardiovascular; PVC, premature ventricular contractions
TABLE 3.
Cox regression analysis of the association between PVC burden and complexity and outcomes in two groups
| Idiopathic | Structural heart disease | |||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | |||||
| HR (95% CI) | p‐value | HR (95% CI) | p‐value | HR (95% CI) | p‐value | HR (95% CI) | p‐value | |
| All‐cause death | ||||||||
| PVC% | 0.960 (0.912–1.011) | .119 | — | 0.944 (0.899–0.992) | .023 | 0.951 (0.905–1.000) | .048 | |
| PVC% per quartile | ||||||||
| Quartile 1 | Reference | Reference | Reference | Reference | ||||
| Quartile 2 | 1.733 (0.806–3.728) | .159 | — | 0.629 (80.335–1.181) | .149 | — | ||
| Quartile 3 | 2.331 (1.090–4.984) | .029 | 2.288 (1.042–5.026) | .039 | 0.645 (0.351–1.187) | .159 | — | |
| Quartile 4 | 1.247 (0.426–2.553) | .611 | — | 0.384 (0.191–0.772) | .007 | 0.417 (0.203–0.858) | .017 | |
| Polymorphic | 1.713 (0.952–3.085) | .073 | — | 1.275 (0.707–2.296) | .419 | — | ||
| Triplets | 1.982 (1.173–3.350) | .011 | 1.289 (0.740–2.245) | .370 | 1.117 (0.702–1.779) | .641 | — | |
| NSVT | 2.162 (1.149–4.070) | .017 | 1.898 (0.984–3.662) | .056 | 1.033 (0.621–1.717) | .902 | — | |
| All‐cause death or CV hospitalizations | ||||||||
| PVC% | 0.973 (0.931–1.016) | .210 | — | 0.960 (0.921–1.001) | .055 | — | ||
| PVC% per quartile | ||||||||
| Quartile 1 | Reference | Reference | Reference | |||||
| Quartile 2 | 1.659(0.840–3.276) | .145 | — | 0.594 (0.333–1.062) | .079 | — | ||
| Quartile 3 | 1.796 (0.891–3.618) | .101 | — | 0.572 (0.325–1.007) | .053 | — | ||
| Quartile 4 | 1.035 (0.493–2.176) | .927 | — | 0.462 (0.250–0.856) | .014 | 0.484 (0.257–0.911) | .025 | |
| Polymorphic | 1.785 (1.031–3.093) | .039 | 1.222 (0.679–2.201) | .504 | 1.075 (0.637–1.816) | .786 | — | |
| Triplets | 1.867 (1.145–3.045) | .012 | 1.004 (0.970–1.040) | .498 | 0.999 (0.651–1.5349 | .997 | — | |
| NSVT | 1.724 (0.926–3.211) | .086 | — | 1.006 (0.632–1.603) | .980 | — | ||
| HF death or HF hospitalizations a | ||||||||
| PVC% | — | 0.989 (0.913–1.072) | .796 | — | ||||
| PVC% per quartile | ||||||||
| Quartile 1 | Reference | Reference | ||||||
| Quartile 2 | — | 1.345 (0.301–6.014) | .698 | — | ||||
| Quartile 3 | — | 1.217 (0.272–5.449) | .798 | — | ||||
| Quartile 4 | — | 1.265 (0.282–5.666) | .759 | — | ||||
| Polymorphic | — | 4.086 (0.531–31.47) | .176 | — | ||||
| Triplets | — | 2.757 (0.942–8.069) | .064 | — | ||||
| NSVT | — | 1.620 (0.576–4.551) | 0.360 | — | ||||
| Sudden death or hospitalization due to VA | ||||||||
| PVC% | 0.817 (0.546–1.220) | .323 | — | 0.980 (0.872–1.102) | .736 | — | ||
| PVC% per quartile | ||||||||
| Quartile 1 | Reference | Reference | Reference | Reference | ||||
| Quartile 2 | 0.469 (0.043–5.174) | .536 | — | 0.246 (0.028–2.204) | .210 | — | ||
| Quartile 3 | 1.144 (80.161–8.137) | .893 | — | 0.223 (0.025–2.004) | .181 | — | ||
| Quartile 4 | 0.013 (000–1,209) | .459 | — | 0.430 (0.078–2.357) | .331 | — | ||
| Polymorphic | 44 (0.025–7,766) | .319 | — | 1.046 (0.211–5.187) | .956 | — | ||
| Triplets | 2.315 (0.387–13.86) | .358 | — | 0.188 (0.023–1.524) | .117 | — | ||
| NSVT | 2.498 (0.279–22.41) | .413 | — | 4.345 (1.037–18.20) | .044 | 3.896 (0.903–16.81) | .068 | |
Abbreviations: AF, atrial fibrillation; CV, cardiovascular; LAD, left atrium diameter; LVFS, left ventricular fractional shortening; NSVT, non‐sustained ventricular tachycardia; PVCs, premature ventricular contractions; SV, supraventricular; VA, ventricular arrhythmias.
The analysis of the combined outcome of HF death or HF hospitalizations the analysis was only done in the SHD group because there was only 1 event in the idiopathic group. In multivariable analysis, only covariables with a p < .05 in univariable analysis were included (Table S3), respectively: for all‐cause mortality, the covariables included were age, presence of supraventricular runs, presence of episodes of AF, LAD, and LVFS; for the combined outcome of all‐cause mortality and CV hospitalizations, the covariables included were age, gender, presence of episodes AF, LAD, and LVFS; for the combined outcome of sudden death or VA hospitalization, the only significant covariable included was LAD.
In patients with SHD, the PVC burden was associated with a better overall survival and survival free from CV hospitalizations (Figure 1), the increase in PVC percentage was associated with a reduction of all‐cause mortality and combined all‐cause mortality or CV hospitalizations (Table 3). The per quartile analysis has shown that having a PVC percentage above the upper quartile versus below the lower quartile was associated with a better overall survival and survival free from CV hospitalizations (Figure 1). The unadjusted and adjusted HR (95% CI) for a PVC percentage above the fourth quartile was respectively 0.384 (0.191–0.772), p = .007 and 0.417 (0.203–0.858), p = .017 for all‐cause mortality, and 0.462 (0.250–0.856), p = .014 and 0.484 (0.257–0.911), p = .025, for all‐cause mortality or CV hospitalizations. In the SHD group, the PVC burden was not associated with the other combined outcomes either (Table 3; Figures S1 and S2).
3.5. Prognostic implications of PVC complexity on survival
In the idiopathic group, patients with triplets had lower overall survival or survival free from CV hospitalizations (Figure 2). Patients with NSVT or polymorphic pattern had a lower survival free from CV hospitalization (Figures 3 and 4). In univariable analysis, the unadjusted HR (95% CI) was respectively, 1.982 (1.173–3.350), p = .011 and 1.867 (1.145–3.045), p = .012 for triplets, and 2.162 (1.149–4.070), p = .017 and 1.785 (1.031–3.093), p = .039, respectively for the NSVT and polymorphic morphology (Table 3). After adjustment for the other confounding covariables, the presence of triplets, NSVT, or polymorphic morphology was no longer associated with the outcomes (Table 3).
FIGURE 2.
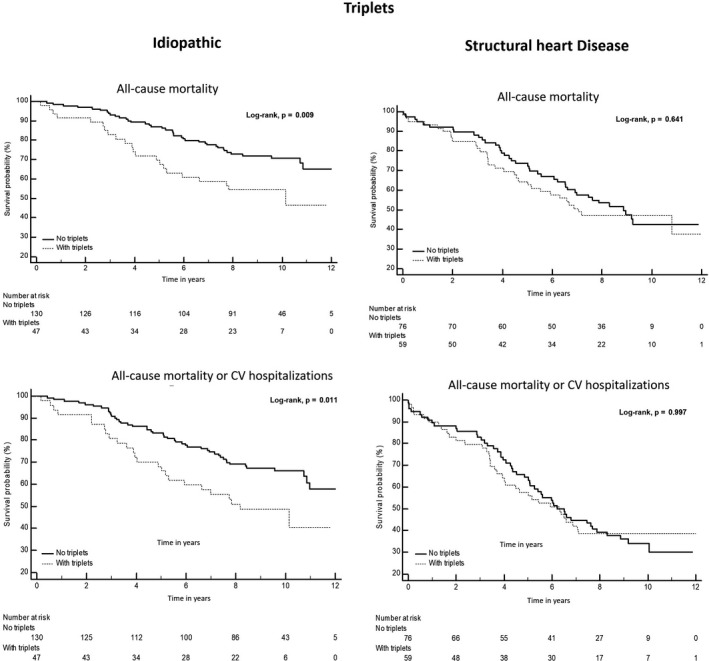
Kaplan–Meier survival estimate of overall survival and CV hospitalizations‐free survival stratified by the presence of triplets in both groups with and without structural heart disease. CV, cardiovascular
FIGURE 3.
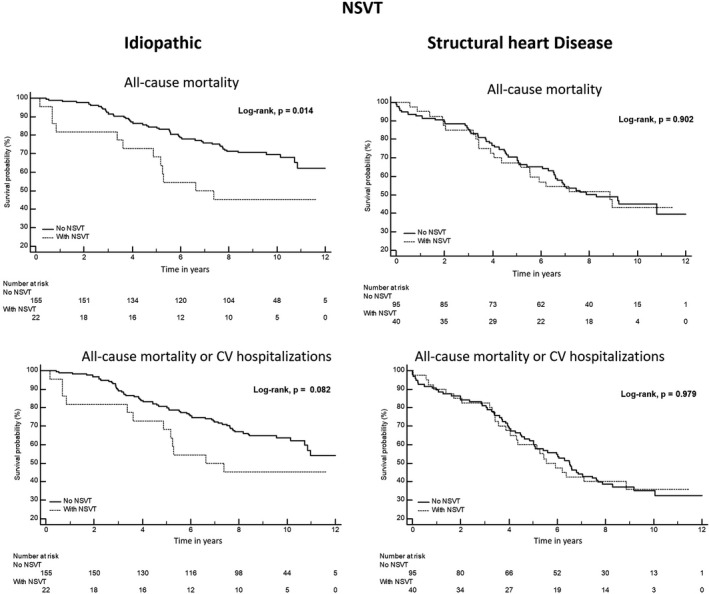
Kaplan–Meier survival estimate of overall survival and CV hospitalizations‐free survival stratified by the presence of NSVT in both groups with and without structural heart disease. CV, cardiovascular; NSVT, non‐sustained ventricular tachycardia
FIGURE 4.
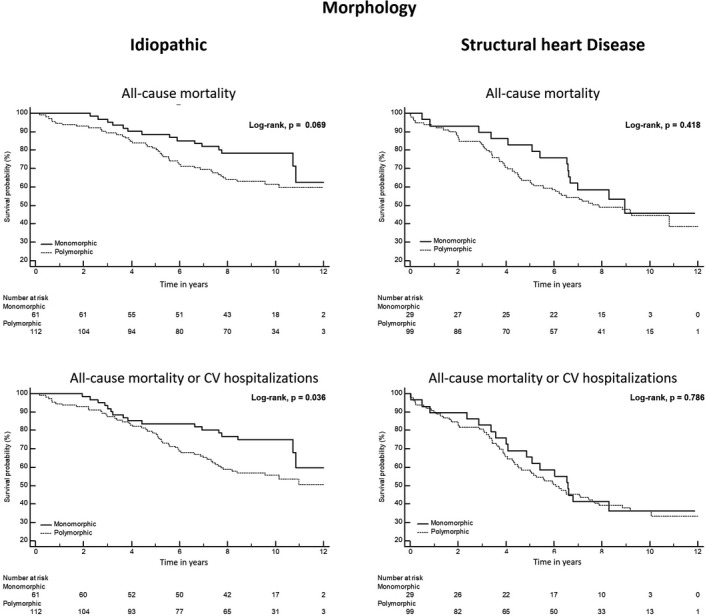
Kaplan–Meier survival estimate of overall survival and CV hospitalizations‐free survival stratified by the morphology of the PVCs in both groups with and without structural heart disease. CV, cardiovascular; PVC, premature ventricular contractions
In patients with SHD, the complexity of the PVCs was not associated with all‐cause mortality or the combined outcomes, the only exception was the presence of NSVT. Patients with SHD and runs of NSVT had a lower survival free from the arrhythmic combined outcome of SD or hospitalizations due to VA (Figures 2, 3, 4; Figures S1 and S2). The presence of NSVT was associated with that outcome in univariable analysis, HR (95% CI) 4.345 (1.037–18.20), p = .044, but after adjustment, the significance was lost, although there was a trend toward a worse outcome, HR (95% CI) 3.896 (0.903–16.81), p = .068 (Table 3).
4. DISCUSSION
In our population of almost 2,000 consecutive patients that underwent 24‐hr Holter monitoring, more than one fourth had a percentage of PVCs above 1% of total beats with a median PVC% of 2.7 (1.6–6.7) %. This value is much higher than the observed in previous studies evaluating the impact of the burden or the complexity of the PVC on prognosis. Dukes et al. (2015) in a population‐based study evaluated 1,139 Cardiovascular Health Study participants randomly assigned to 24‐hr Holter monitoring that had a normal left ventricular ejection fraction and no history of CHF. The median PVC percentage was 0.011% (0.002%–0.123%). Lin et al. (2016) in a hospital population of 3,767 patients referred for 24‐hr Holter monitoring at Taipei Veterans General Hospital reported a mean PVC% of 176 ± 423 beats per day and none of the patients had a PVC burden of more than 5% of the total beats.
In our group of patients, the PVC burden was not significantly different between idiopathic patients and patients with SHD. However, in the former the PVCs had a less complex pattern. Idiopathic patients with a higher PVC burden had a lower overall survival and survival free from CV hospitalizations. The lower survival however was not associated with the upper quartile but rather with the third. These results may be related with the fluctuations in the PVC burden from day to day. Mullis et al, studied the variation in 24‐hr PVC burden over up to 14 days in 59 patients (Mullis et al., 2019). The authors found that the median of the absolute 24‐hr PVC burden change was 9.9% (IQR: 5.4%–14.5%) and 72.9% patients fell into at least two categories of PVC burden depending on the 24‐hr period considered.
The survival free from adverse outcomes was also significantly lower in idiopathic patients with complex PVCs. The prognostic value of PVC morphology (monomorphic vs. polymorphic) or presence of triplets or NSVT has been investigated previously. Lin et al followed 3,351 patients with apparently normal hearts for 10 ± 1 years and found that patients with polymorphic PVCs had an increased risk of mortality, CV hospitalization, ischemic stroke, and new‐onset HF (Lin et al., 2015). Later, the same authors reported that NSVT was also independently associated with a higher incidence of the same outcomes (Lin et al., 2016).
In patients with SHD, neither the burden nor the complexity of the PVCs was associated with a worse prognosis. Remarkably, in this group the higher PVC burden acted as a protective factor, in fact a PVC% in the upper quartile was independently associated with a better prognosis. The reason for these unexpected results is unknown. However, we may speculate that the presence of SHD may dilute the effect of the PVC burden. Furthermore, the studies assessing the impact of PVC burden on adverse events have shown that the existence of PVCs per se is responsible for the adverse outcomes, whereas the further increase in the burden or complexity does not bring about significant additional risk. In a population‐based study, Agarwal et al. have shown that the risk associated with having just one PVC on a 2 min ECG recording was the same as having a higher burden or complexity (Agarwal et al., 2012). Likewise, in the study by Dukes, the authors showed that patients in the upper quartile of PVC% had a 48% increased risk of incident HF. The chart displaying the PVC% plotted against incident HF shows a plateau in the incidence of HF for additional increases in PVC% above 1.5% (Dukes et al., 2015). Noteworthy, in our study the lower quartile corresponded to a median PVC% of 1.25% which corresponds to the fourth quartile of the work by Dukes. This fact may explain why a PVC burden in the upper quartile is not more deleterious than one in the lower in patients with a high competing risk of death or adverse outcome. Besides, the absence of prognostic impact of the PVC burden and complexity in patients with SHD it is not totally unexpected and has been observed previously. Agarwal et al in a retrospective population‐based study concluded that the impact of the PVC burden on the incidence of HF was higher in young idiopathic patients without competing risks for adverse outcomes than in patients with SHD (Agarwal et al., 2017).
Another important finding was the absence of an association between PVC burden and complexity and prognosis after multivariable analysis. This probably results from the fact that patients who experienced the adverse outcomes were also older, had more frequently comorbidities, had a lower LVFS and a larger LAD. These last two covariables reflect the severity of both systolic and diastolic left ventricular disfunction. The dimension of the LA reflects the diastolic function (Yoshida et al., 2009), meaning that the diastolic function might be impaired in patients with LA dilation. Furthermore, LAD has been independently associated with a worse prognosis if the patients are in sinus rhythm (Tsang et al., 2006).
In the previous abovementioned studies, the PVC burden and complexity remained significantly associated with a worse prognosis after multivariable analysis. However, in those studies the authors did not include in their analysis the LA dimension.
Regarding the incidence of the combined outcome of HF death or HF hospitalizations, no association was detected between the PVC burden or complexity and the outcomes. During up to 12 years of follow‐up, this outcome occurred in 18 patients (5.8%), much more uncommon than previously reported, respectively 27% by Dukes (Dukes et al., 2015) and 19.4% by Argawal (Agarwal et al., 2012). These results are conflicting since not only our PVC burden was much higher than in those studies, but also because HF hospitalizations only occurred in patients with SHD. Frequent PVCs are usually defined as the presence of at least one PVC on a 12‐lead ECG or >30 PVCs per hr (Al‐Khatib et al., 2018). But although this is a widely accepted guideline, in most of the studies assessing the impact of the PVC burden on the left ventricular dysfunction, the reported PVC burden is above 10% and usually higher than 20% of total beats (Arnar et al., 2019).Thus, higher than the PVC burden of our population and much higher than the PVC burden reported by Dukes and Argawal. It is possible that some subjects in those population‐based cohorts simply present PVCs as the first evidence of a subclinical cardiomyopathy that would have been the cause of the incident HF during the follow‐up.
Finally, regarding the incidence of the combined outcome of SD or VA hospitalizations, it would be expected that at least in the presence of SHD, the presence of frequent PVCs or complex forms would have an impact on prognosis. In fact, the presence of NSVT was associated with a lower survival free from SD or hospitalizations for VA. However, once again after adjustment to covariables the association was lost although a trend was maintained.
While previous studies seem to demonstrate increased mortality from ventricular ectopy even with very low PVC counts, there is little evidence to support prophylactic intervention to suppress PVCs in patients without underlying SHD with such low PVC burden. Higher PVC burdens should be treated in idiopathic patients and the presence of PVCs should lead to prompt closer evaluation for possible undiagnosed cardiac disease or reversible underlying etiology.
5. CONCLUSIONS
In this group of patients, the PVCs had a more complex pattern if SHD was present. The complexity of PVCs was not independently associated with worse prognosis; however, in patients with SHD, the presence of NSVT showed a trend toward a higher incidence of the combined outcome of SD or hospitalizations due to VA. In idiopathic patients, a higher PVC count was independently associated with higher mortality, but in patients with SHD, it was not.
5.1. Limitations
This was a retrospective study so we cannot rule out selection bias due to the fact that patients were referred for a 24‐hr Holter recording because of symptoms, for risk stratification or due to the presence of SHD, so these results cannot be extrapolated to other populations. The sample size was small and the population with SHD was very heterogeneous. The cause of death was unknown in 26% of cases, and we cannot exclude the possibility that some of those deaths might have been sudden and due to CV causes.
CONFLICT OF INTEREST
None declared.
ETHICAL APPROVAL
The Ethical Committee of the Centro Hospitalar de Setubal approved the study. The study is in compliance with the Helsinki Declaration. The informed consent was waived via the Ethical Committee.
Supporting information
Supplementary Material
Parreira L, Marinheiro R, Amador P, et al. Frequent premature ventricular contractions. Association of burden and complexity with prognosis according to the presence of structural heart disease. Ann Noninvasive Electrocardiol. 2021;26:e12800 10.1111/anec.12800
DATA AVAILABILITY STATEMENT
Data available on request due to privacy/ethical restrictions.
REFERENCES
- Agarwal, S. , Simpson, R. , Rautaharju, P. , Alonso, A. , Shahar, E. , Massing, M. , … Heiss, G. (2012). Relationship of ventricular premature complexes to heart failure (from the Atherosclerosis Risk in Communities [ARIC] study). American Journal of Cardiology, 109, 105–109. 10.1016/j.amjcard.2011.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal, V. , Vittinghoff, E. , Whitman, R. , Dewland, T. , Dukes, J. , & Marcus, G. (2017). Relation between ventricular premature complexes and incident heart failure. American Journal of Cardiology, 119, 1238–1242. 10.1016/j.amjcard.2016.12.029 [DOI] [PubMed] [Google Scholar]
- Al‐Khatib, S. , Stevenson, W. , Ackerman, M. , Bryant, W. , Callan, D. , Curtis, A. , … Page, R..(2018). 2017 AHA/ACC/HRS guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Circulation, 138, e272–e391. 10.1161/CIR.0000000000000549 [DOI] [PubMed] [Google Scholar]
- Arnar, D. O. , Mairesse, G. H. , Boriani, G. , Calkins, H. , Chin, A. , Coats, A. , … Heinzel, F. R. (2019). Management of asymptomatic arrhythmias: A European Heart Rhythm Association (EHRA)consensus document, endorsed by the Heart Failure Association (HFA), Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), Cardiac Arrhythmia Society of Southern Africa (CASSA), and Latin America Heart Rhythm Society (LAHRS). Europace, 1–32. 10.1093/europace/euz046 [DOI] [PubMed] [Google Scholar]
- Baman, T. S. , Lange, D. C. , Ilg, K. J. , Gupta, S. K. , Liu, T.‐Y. , Alguire, C. , … Bogun, F. (2010). Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm: the Official Journal of the Heart Rhythm Society, 7, 865–869. 10.1016/j.hrthm.2010.03.036 [DOI] [PubMed] [Google Scholar]
- Bogun, F. , Crawford, T. , Reich, S. , Koelling, T. M. , Armstrong, W. , Good, E. , … Morady, F. (2007). Radiofrequency ablation of frequent, idiopathic premature ventricular complexes: Comparison with a control group without intervention. Heart Rhythm: the Official Journal of the Heart Rhythm Society, 4, 863–867. 10.1016/j.hrthm.2007.03.003 [DOI] [PubMed] [Google Scholar]
- Calkins, H. , Hindricks, G. , Cappato, R. , Kim, Y.‐H. , Saad, E. B. , Aguinaga, L. , … Yamane, T. (2017). HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm: the Official Journal of the Heart Rhythm Society, 14, e275–e444. 10.1016/j.hrthm.2017.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukes, J. , Dewland, T. , Vittinghoff, E. , Mandyam, M. , Heckbert, S. , Siscovick, D. , … Marcus, G. (2015). Ventricular ectopy as a predictor of heart failure and death. Journal of the American College of Cardiology, 66, 101–109. 10.1016/j.jacc.2015.04.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echt, D. S. , Liebson, P. R. , Mitchell, L. B. , Peters, R. W. , Obias‐Manno, D. , Barker, A. H. , … Richardson, D. W. (1991). Mortality and morbidity in patients receiving encainide, flecainide, or placebo—The cardiac arrhythmia suppression trial. New England Journal of Medicine, 324, 781–788. 10.1056/NEJM199103213241201 [DOI] [PubMed] [Google Scholar]
- Einvik, G. , Bhatnagar, R. , Holmedahl, N. , Neukamm, A. , Omland, T. , & Søyseth, V. (2017). Premature ventricular complex is more prevalent during acute exacerbated than stable states of chronic obstructive pulmonary disease, and Is Related to Cardiac Troponin T. COPD: Journal of Chronic Obstructive Pulmonary Disease, 14, 318–323. 10.1080/15412555.2017.1298085 [DOI] [PubMed] [Google Scholar]
- Gaita, F. , Giustetto, C. , Di Donna, P. , Richiardi, E. , Libero, L. , Brusin, M. C. R. , … Trevi, G. (2001). Long‐term follow‐up of right ventricular monomorphic extrasystoles. Journal of the American College of Cardiology, 38, 364–370. 10.1016/S0735-1097(01)01403-6 [DOI] [PubMed] [Google Scholar]
- Kostis, J. , Byington, R. , Friedman, L. , Goldstein, S. , & Furberg, C. (1987). Prognostic significance of ventricular ectopic activity in survivors of acute myocardial infarction. Journal of the American College of Cardiology, 10, 231–242. 10.1016/s0735-1097(87)80001-3 [DOI] [PubMed] [Google Scholar]
- Latchamsetty, R. , Yokokawa, M. , Morady, F. , Kim, H. , Mathew, S. , Tilz, R. , … Bogun, F. (2015). Multicenter outcomes for catheter ablation of idiopathic premature ventricular complexes. JACC: Clinical Electrophysiolog, 1, 116–123. 10.1016/j.jacep.2015.04.005 [DOI] [PubMed] [Google Scholar]
- Lin, C.‐Y. , Chang, S.‐L. , Chung, F.‐P. , Chen, Y.‐Y. , Lin, Y.‐J. , Lo, L.‐W. , … Chen, S.‐A. (2016). Long‐term outcome of non‐sustained ventricular tachycardia in structurally normal hearts. PLoS One, 11, e0160181 10.1371/journal.pone.0160181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, C.‐Y. , Chang, S.‐L. , Lin, Y.‐J. , Lo, L.‐W. , Chung, F.‐P. , Chen, Y.‐Y. , … Chen, S.‐A. (2015). Long‐term outcome of multiform premature ventricular complexes in structurally normal heart. International Journal of Cardiology, 180, 80–85. 10.1016/j.ijcard.2014.11.110 [DOI] [PubMed] [Google Scholar]
- Ling, Z. , Liu, Z. , Su, L. I. , Zipunnikov, V. , Wu, J. , Du, H. , … Zrenner, B. (2014). Radiofrequency ablation versus antiarrhythmic medication for treatment of ventricular premature beats from the right ventricular outflow tract prospective randomized study. Circulation: Arrhythmia and Electrophysiology, 7, 237–243. 10.1161/CIRCEP.113.000805 [DOI] [PubMed] [Google Scholar]
- Marinheiro, R. , Parreira, L. , Amador, P. , Mesquita, D. , Farinha, J. , Fonseca, M. , … Caria, R. (2019). Ventricular arrhythmias in patients with obstructive sleep apnea. Current Cardiology Reviews, 15, 64–74. 10.2174/1573403X14666181012153252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullis, A. H. , Ayoub, K. , Shah, J. , Butt, M. , Suffredini, J. , Czarapata, M. , … Elayi, C. S. (2019). Fluctuations in premature ventricular contraction burden can affect medical assessment and management. Heart Rhythm: the Official Journal of the Heart Rhythm Society, 16, 1570–1574. 10.1016/j.hrthm.2019.04.033 [DOI] [PubMed] [Google Scholar]
- Priori, S. G. , Blomström‐Lundqvist, C. , Mazzanti, A. , Blom, N. , Borggrefe, M. , Camm, J. , … Van Veldhuisen, D. J. (2015). ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. European Heart Journal, 36, 2793–2867. 10.1093/eurheartj/ehv316 [DOI] [PubMed] [Google Scholar]
- Tsang, T. S. M. , Abhayaratna, W. P. , Barnes, M. E. , Miyasaka, Y. , Gersh, B. J. , Bailey, K. R. , … Seward, J. B. (2006). Prediction of cardiovascular outcomes with left atrial size is volume superior to area or diameter? Journal of the American College of Cardiology, 47, 1018–1023. 10.1016/j.jacc.2005.08.077 [DOI] [PubMed] [Google Scholar]
- Yoshida, C. , Nakao, S. , Goda, A. , Naito, Y. , Matsumoto, M. , Otsuka, M. , … Masuyama, T. (2009). Value of assessment of left atrial volume and diameter in patients with heart failure but with normal left ventricular ejection fraction and mitral flow velocity pattern. European Journal of Echocardiography, 10, 278–281. 10.1093/ejechocard/jen234 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
Data available on request due to privacy/ethical restrictions.


