Ppd-H1 integrates photoperiod and drought stress signals via FLOWERING LOCUS T1 and the downstream MADS-box genes BM3 and BM8 to modulate reproductive development, and shoot and spike morphology in barley.
Keywords: Barley, development, drought, flowering, FLOWERING LOCUS T, MADS-box genes, photoperiod, stress
Abstract
Drought impairs growth and spike development, and is therefore a major cause of yield losses in the temperate cereals barley and wheat. Here, we show that the photoperiod response gene PHOTOPERIOD-H1 (Ppd-H1) interacts with drought stress signals to modulate spike development. We tested the effects of a continuous mild and a transient severe drought stress on developmental timing and spike development in spring barley cultivars with a natural mutation in ppd-H1 and derived introgression lines carrying the wild-type Ppd-H1 allele from wild barley. Mild drought reduced the spikelet number and delayed floral development in spring cultivars but not in the introgression lines with a wild-type Ppd-H1 allele. Similarly, drought-triggered reductions in plant height, and tiller and spike number were more pronounced in the parental lines compared with the introgression lines. Transient severe stress halted growth and floral development; upon rewatering, introgression lines, but not the spring cultivars, accelerated development so that control and stressed plants flowered almost simultaneously. These genetic differences in development were correlated with a differential down-regulation of the flowering promotors FLOWERING LOCUS T1 and the BARLEY MADS-box genes BM3 and BM8. Our findings therefore demonstrate that Ppd-H1 affects developmental plasticity in response to drought in barley.
Introduction
Global warming increases the frequency and intensity of severe water scarcity events, which negatively affect the yield of rain-fed crops such as barley (Hordeum vulgare L.) and wheat (Triticum aestivum L.) (Xie et al., 2018; Kahiluoto et al., 2019). Drought during reproductive development impairs spike development and floret fertility, and is therefore a major cause of yield losses in these temperate cereals (Gol et al., 2017). At present, strategies to breed cereal varieties with improved yield under drought are limited due to a lack of knowledge of the genetic factors that control inflorescence and flower development under drought conditions. Understanding the plasticity and genetic control of stress-induced changes in reproductive development will be crucial to ensure future yield stability of temperate cereals.
The model plant Arabidopsis thaliana accelerates reproductive development under drought, a response that has been termed drought escape. In Arabidopsis, drought escape is triggered under inductive long-day (LD) conditions and is controlled by components of the circadian clock and the photoperiod response pathway (Riboni et al., 2013, 2016). Under drought conditions, the phytohormone abscisic acid (ABA) modulates the activity and signalling of the clock gene GIGANTEA (GI) and consequently its ability to activate FLOWERING LOCUS T (FT) under long photoperiods (Riboni et al., 2013, 2016). The FT protein acts as a florigenic signal, moving long distances from the leaf to the shoot apical meristem (SAM) to induce the floral transition (Abe et al., 2005; Wigge et al., 2005; Corbesier et al., 2007; Jaeger and Wigge, 2007; Mathieu et al., 2007; Tamaki et al., 2007; Jaeger et al., 2013). Under non-inductive short days (SDs), ABA delays flowering by repressing the flowering-promoting MADS-box gene SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1 (SOC1), encoding a transcription factor integrating floral cues in the shoot meristem (Riboni et al., 2016). In addition, it was shown that ABA-responsive element (ABRE)-binding factors (ABFs) interact with NUCLEAR FACTOR Y subunit C (NF-YC) 3/4/9 to promote flowering by inducing SOC1 transcription under drought conditions (Hwang et al., 2019). On the other hand, ABSCISIC ACID-INSENSITIVE 3/4/5 bZIP transcription factors involved in ABA signalling repress flowering by up-regulating the floral repressor and vernalization gene FLOWERING LOCUS C (FLC) (Wang et al., 2013; Shu et al., 2016). Consequently, drought cues depend on the photoperiod and interact with photoperiod response and vernalization genes to modulate flowering time in Arabidopsis. In contrast to Arabidopsis, rice (Oryza sativa L.) shows a delay in flowering in response to drought under inductive photoperiods, and this delay is accompanied by a down-regulation of the florigenic signals HEADING DATE 3a (Hd3a) and RICE FLOWERING LOCUS T 1 (RFT1) (Galbiati et al., 2016; Zhang et al., 2016). Consequently, the developmental response to drought varies within and between species, and is linked to the differential regulation of FT-like genes (Kazan and Lyons, 2016). However, the effects of drought on reproductive development and genetic components that modulate this response are not known in most crop species including the important temperate crop barley.
Barley germplasm is characterized by high genetic diversity and variation in response to abiotic stresses. While elite cultivars tend to be more stress susceptible, wild and landrace barley genotypes are well adapted to drought-prone environments and therefore represent a valuable resource for improving stress tolerance in elite barley (Baum et al., 2007; von Korff et al., 2008; Rollins et al., 2013b; Templer et al., 2017). It was demonstrated that yield stability in the field was associated with the major photoperiod response gene PHOTOPERIOD H1 (Ppd-H1) and the vernalization gene VERNALIZATION 1 (VRN1) (von Korff et al., 2008; Rollins et al., 2013a; Al-Ajlouni et al., 2016; Wiegmann et al., 2019). These findings suggested that the timing of reproductive development is crucial to maximize yield formation under harsh environmental conditions. However, it is not known if and how these floral regulators interact with stress cues to modulate development. Ppd-H1, a barley homologue of the PSEUDO RESPONSE REGULATOR (PRR) genes from the Arabidopsis circadian clock, induces the expression of FLOWERING LOCUS T1 (FT1), a homologue of Arabidopsis FT and rice Hd3a under LDs (Turner et al., 2005; Corbesier et al., 2007; Tamaki et al., 2007; Campoli et al., 2012a, b). In barley, the up-regulation of FT1 in the leaf is correlated with induction of the MADS-box genes VRN1 (BM5a), BARLEY MADS-box 3 (BM3) and BM8, barley homologues of Arabidopsis APETALA1/FRUITFUL (AP1/FUL), and the acceleration of inflorescence development (Schmitz et al., 2000; Trevaskis et al., 2007; Digel et al., 2015). Homologues of Ppd-H1/PRR37 function in the circadian clock in Arabidopsis and rice (Makino et al., 2001; Murakami et al., 2003; Turner et al., 2005). The circadian clock is an internal timekeeper that allows plants to anticipate predictable changes in the environment and controls a number of output traits including development and stress responses (Sanchez et al., 2011; Müller et al., 2014; Johansson and Staiger, 2015). In Arabidopsis, the central oscillator is composed of negative transcriptional feedback loops: the rise of CIRCADIAN CLOCK ASSOCIATED1 (CCA1) and LATE ELONGATED HYPOCOTYL (LHY) late at night inhibits the evening complex genes EARLY FLOWERING 3 (ELF3), EARLY FLOWERING 4 (ELF4), and LUX, which in turn repress the PRR genes at night. Barley homologues of these clock genes have been identified and their interactions are largely conserved in barley (Campoli et al., 2012b; Müller et al., 2020). Accordingly, elements of the evening complex genes repress Ppd-H1 at night and thereby control the photoperiod-dependent up-regulation of FT1 (Faure et al., 2012; Mizuno et al., 2012; Zakhrabekova et al., 2012; Campoli et al., 2013; Alvarez et al., 2016). In spring barley grown in northern latitudes, a recessive mutation in the CONSTANS, CONSTANS-like, and TOC1 (CCT) domain of ppd-H1 has been selected (Jones et al., 2008). This ppd-H1 allele delays flowering under LDs and thereby improves yield in temperate environments with long growing seasons (Cockram et al., 2007; Alqudah et al., 2014; Digel et al., 2015). In contrast, early flowering in response to LDs promoted by the wild-type Ppd-H1 allele was associated with improved yield under Mediterranean environments with terminal stress (Wiegmann et al., 2019). However, it is not known if the two Ppd-H1 variants also interact with stress cues to modulate reproductive development.
Here, we provide a detailed analysis of barley development under drought. We show that variation at Ppd-H1 interacts with drought to control flowering time, grain yield, as well as the expression of FT1 and the downstream MADS-box genes BM3 and BM8.
Materials and methods
Plant materials, growth conditions, and phenotyping
Drought responses were scored in the spring barley (H. vulgare L.) cultivars Scarlett, Golden Promise, and Bowman, and their derived introgression lines S42-IL107 (Scarlett), GP-fast (Golden Promise), and BW281 (Bowman). Scarlett, Golden Promise, and Bowman carry a natural mutation in the CCT domain of Ppd-H1, that causes a delay in flowering under LD conditions (Turner et al., 2005). The derived introgression lines S42-IL107 and BW281 carry a dominant Ppd-H1 allele introgressed from wild and winter barley, respectively (Druka et al., 2011; Schmalenbach et al., 2011). GP-fast was created via crossing of Golden Promise to the winter barley cultivar Igri, followed by two rounds of backcrossing to Golden Promise to reduce the size of the introgression.
The three spring barley cultivars and derived introgression lines were genotyped with the Barley 50k iSelect SNP Array at TraitGenetics GmbH (Gatersleben, Germany) (Bayer et al., 2017). Chromosomal positions for each marker were obtained from the POPSEQ_2017 genetic map (Cantalapiedra et al., 2015; Mascher et al., 2017). Sizes of the introgressions were calculated based on half the distance between the markers flanking donor introgressions and the first polymorphic markers within the introgressions (Supplementary Fig. S1; Supplementary Data S1 at JXB online).
We conducted two different drought experiments. First, a continuous drought treatment was applied by a controlled dry down of the soil to a soil water content (SWC) of 15% of field capacity (FC), and this FC was maintained until plant maturity. In a second experiment, a transient drought treatment was applied by withholding water for eight consecutive days during floral development followed by rewatering to control levels. Both experiments were performed in a controlled-environment chamber under 60% relative humidity. Individual grains were sown in 7 cm×7 cm×8 cm black plastic pots; 40 pots (5×8 rows) per tray. Genotypes were distributed randomly on each tray and rearranged after each sampling to maintain the initial planting density. Additionally, trays were rotated and shuffled at least twice per week. Each pot was filled with exactly 150 g of soil mixture. A mixture of 93% (v/v) Einheitserde ED73 (Einheitserde Werkverband e.V., Sinntal_Altengronau, Germany), 6.6% (v/v) sand, and 0.4% (v/v) Osmocote exact standard 3–4M (Scotts Company LLC), was freshly prepared before sowing. This porous soil mixture with high organic matter content was selected to further aid the even distribution of moisture in the soil. Grains were stratified in well-watered soil at 4 °C in the dark for at least 4 d. Plants were then germinated under SD conditions (8 h, 22 °C day; 16 h, 18 °C night; photosynthetically active radiation ~250 µM m–2 s–1). For the continuous drought treatment, water was withheld after germination until the SWC reached 15% FC, while the control plants were watered to maintain 70% FC. The desired SWC of 15% FC was reached after 10 d when all plants were transferred from SDs to LDs and kept under LDs for the rest of the experiment (16 h, 22 °C day; 8 h, 18 °C night; photosynthetically active radiation ~250 µM m–2 s–1). For the application of severe transient drought, plants of Scarlett and S42-IL107 were germinated under SD conditions and shifted to LDs after 10 d. All plants were kept at 70% FC until they had reached the awn primordium stage [Waddington stage 3 (W3)]. Then watering was stopped for eight consecutive days. SWC in the pots reached a relative water content (RWC) of 8% FC on the eighth day. Control plants were kept at 70% FC during this time. Subsequently, all drought-treated pots were rewatered to control levels of 70% FC. FC was calculated from the difference in weight of fully hydrated and oven-dried soil. SWC was measured gravimetrically (Coleman, 1947). Pots were soaked with water and subsequently left to drain by gravity until their weight remained stable; this was set as 100% FC. Dry weight was measured after pots were dried in a drying cabinet at ~60°C until their weight remained stable. Measurements of FC were corrected for the biomass accumulation of growing plants as the experiments progressed by subtracting the weight of harvested plants from the measured soil weight. The weight of pots was checked daily and all plants were watered daily to maintain the same SWC throughout development. At least three replicate plants of all six genotypes were sown and germinated for each sampling time point.
The development of the main shoot apex (MSA) was scored in accordance with the stages described by Waddington et al. (1983) that is based on the progression of inflorescence initiation and then the most advanced floret primordium and pistil of the inflorescence. At W2 the first spikelets initiate and the MSA transitions to a reproductive inflorescence. The first floral organ primordia differentiate and stem elongation initiates at the stamen primordium stage (W3.5). New spikelet primordia are continuously initiated until about W5, which then mature into florets until anthesis and pollination at W10. MSA dissection was performed with microsurgical stab knives (SSC#72-1551; Sharpoint, Surgical Specialties Corporation). Images of developing apices were obtained using a Nikon stereo microscope (Nikon SMZ18), Nikon DS-U3 controller unit, and a Nikon DS-Fi2 digital camera. Nikon NIS-Elements software was used for image acquisition. Heading date was scored at Zadoks stage Z49 when first awns became visible, otherwise also referred to as tipping (Zadoks et al., 1974; Alqudah and Schnurbusch, 2017). Spike number, the number of grains per spike, the number of grains per plant, and thousand kernel weight (TKW) were scored at harvest.
Leaf RWC was determined from measurements of fresh, turgid, and dry weight of leaf sections from the middle part of the youngest fully expanded leaf. Turgid weight was measured after soaking the leaf sections in deionized water at 4 °C overnight in the dark. Dry weight of leaf sections was measured after drying at 70°C. The RWC was then calculated as (Smart and Bingham, 1974).
RNA extraction and gene expression analysis
Sections from the middle of the youngest fully emerged leaf were sampled for the developmental time courses at Zeitgeber time 8 (ZT8). Sampling was started on the first day after transfer to LDs in the continuous drought treatment and as soon as water was withheld in the severe drought experiment. Sampling was continued until flowering for both treatments. Samples for the diurnal expression analyses were harvested every 4 h starting at ZT0, with one additional sampling at ZT22. RNA extraction, reverse transcription, and quantitative real-time PCR (qRT-PCR) were performed as previously described (Campoli et al., 2012a, b; Digel et al., 2015). Several combinations of reference genes were tested for each experiment, and the genes with the most stable expression were chosen for normalization. The geometric mean of Actin and ADP-ribosylation factor 1-like protein (ADP) absolute expression was used for the calculation of relative gene expression levels for the developmental time courses. The geometric mean of ADP and Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) absolute expression was used for the calculation of relative gene expression levels for the diurnal time course. Normalization was performed by dividing target gene expression values by the obtained mean of the reference genes.
Statistical analysis
All statistical analyses were performed with R (R Core Team, 2020). Polynomial regressions (Loess smooth line) were calculated using second-degree polynomials and an alpha of 0.75, with a 95% confidence interval. Student’s t-test assuming two-tailed distribution and equal variance was used to compare group means for control and drought treatments at each time point of the time course analyses with a significance cut-off of P<0.05. Significant differences in trait expression between treatments and genotypes were compared by Kruskal–Wallis ANOVA followed by Conover–Iman test for multiple comparisons and Bonferroni correction with a significance cut-off of P<0.05.
Results
Drought interacts with Ppd-H1 to modulate flowering time
We aimed to characterize the effects of drought on the timing of reproductive development and on shoot and spike morphology. In addition, we tested if the major photoperiod response gene Ppd-H1 controlled reproductive development in response to drought. We quantified the effects of drought on developmental timing, growth, and inflorescence morphology in the spring barley genotypes Scarlett, Golden Promise, and Bowman with a natural mutation in the CCT domain of Ppd-H1 and in the derived introgression lines S42-IL107 (Scarlett), GP-fast (Golden Promise), and BW281 (Bowman) that carry wild-type Ppd-H1 alleles introgressed from wild barley (H. vulgare ssp. spontaneum) or winter barley (Supplementary Fig. S1) (Druka et al., 2011; Schmalenbach et al., 2011).
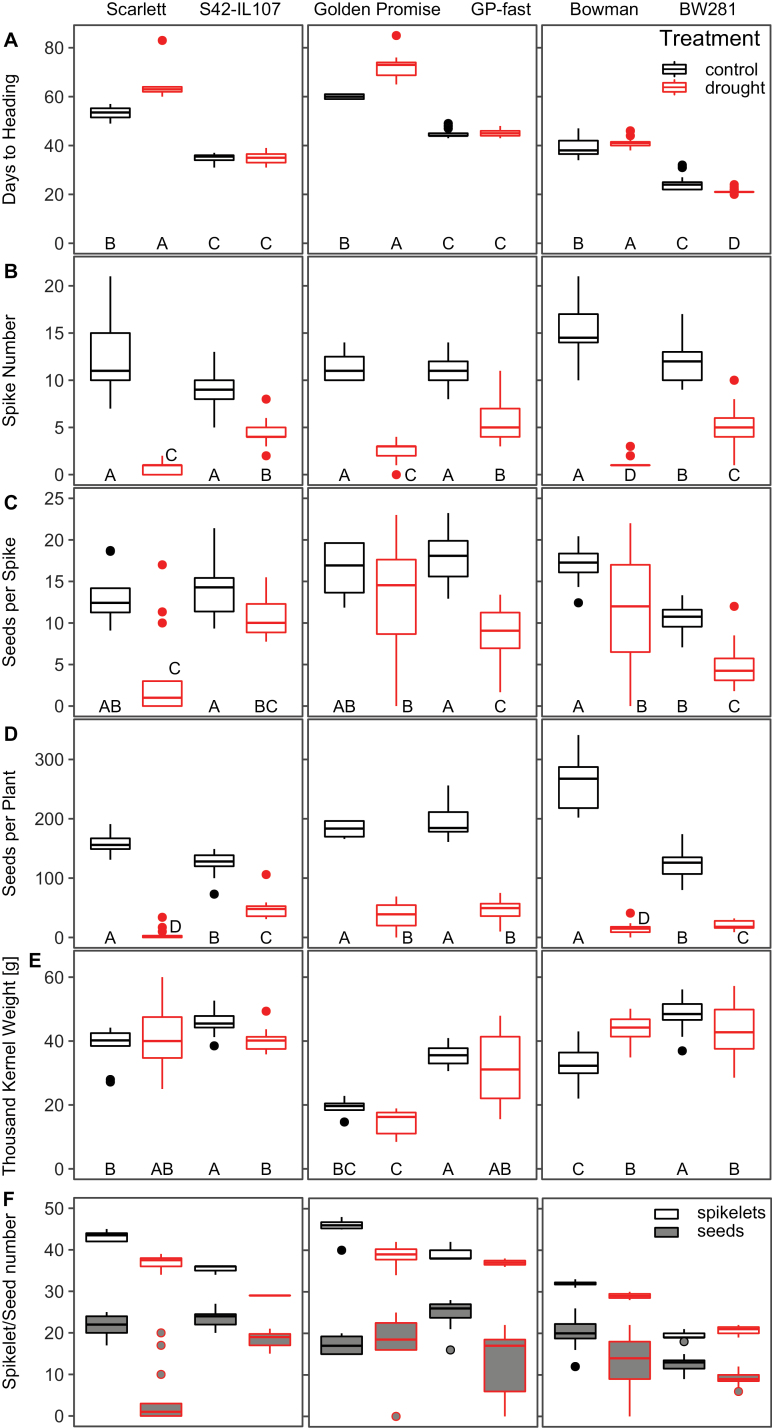
We developed an assay to apply drought starting from early vegetative growth and lasting until maturity. With this assay, drought effects on the transition of vegetative to reproductive development and on floral progression were examined. Heading date, scored as a proxy for flowering time, was significantly delayed in all parental spring barley genotypes (Supplementary Fig. S2). Heading date was delayed by 11 d in Scarlett, by 13 d in Golden Promise, and by 3 d in Bowman under drought compared with control conditions (Fig. 1A). In contrast, heading date was not significantly different under drought compared with control conditions in S42-IL107 and GP-fast, and was significantly accelerated in BW281. In the parental genotypes, the number of spikes per plant was strongly reduced under drought; all plants produced only a maximum of three spikes under drought compared with >10 spikes under control conditions (Fig. 1B). The introgression lines produced on average 5–6 spikes per plant under drought compared with twice as many under control conditions, and thus significantly more under drought compared with the parental genotypes. Drought also reduced the number of grains per spike in all genotypes (Fig. 1C). However, there were no consistent differences in the reduction of grain number between Ppd-H1 variants. The reductions in the number of spikes per plant and grains per spike resulted in a severely reduced number of grains per plant under drought (Fig. 1D). Total grain numbers under drought were significantly higher in the introgression lines S42-IL107 and BW281 than in the parental lines and not significantly different between Golden Promise and GP-fast. Drought did not strongly influence the TKW. Total yield per genotype was therefore primarily determined by the grain number (Fig. 1E).
Fig. 1.
Continuous drought affects heading date, and shoot and spike morphology in barley. Days to heading (A), spike number per plant (B), grain number per spike (C), the number of grains per plant (D), thousand kernel weight (TKW) (E), and the maximum number of developed spikelets (unfilled boxes) and the number of grains (grey box) (F) were scored under control (black) and drought (red) conditions under LDs (16 h light/8 h night) in the spring barley cultivars Scarlett, Golden Promise, and Bowman, and the derived introgression lines S42-IL107, GP-fast, and BW281. Statistical groups were assigned using Kruskal–Wallis ANOVA and post-hoc Conover–Iman test and Bonferroni correction. Different letters indicate that groups differ (P<0.05).
We further investigated at which stage drought reduced final grain number and evaluated the effects of drought on spikelet versus grain number. Drought reduced the number of initiated spikelets in Scarlett, S42-IL107, Golden Promise, and Bowman by between 9% in Bowman to 18% in S42-IL107, while spikelet numbers were not significantly different between control and drought in BW281 and GP-fast (Fig. 1F). Furthermore, not all spikelets on the main spike developed grains. Under control conditions, the number of grains compared with initiated spikelets was reduced by 34–37% in the introgression lines and by 37% in Bowman, 50% in Scarlett, and 62% in Golden Promise. Consequently, in S42-IL107, GP-fast, and BW281, a higher percentage of spikelets developed grains compared with Scarlett, Golden Promise, and Bowman, respectively. Under drought conditions, the number of grains per spikelet was even more strongly reduced in all genotypes compared with control conditions, except for Golden Promise and S42-IL107. Under drought, relative grain numbers compared with spikelet numbers were reduced by 88% in Scarlett, by 64% in GP-fast, and by 56% and 57% in Bowman and BW281, respectively. Consequently, the reduction in grain number per spike under drought was primarily caused by an abortion of florets or floret sterility rather than a decrease in spikelet numbers.
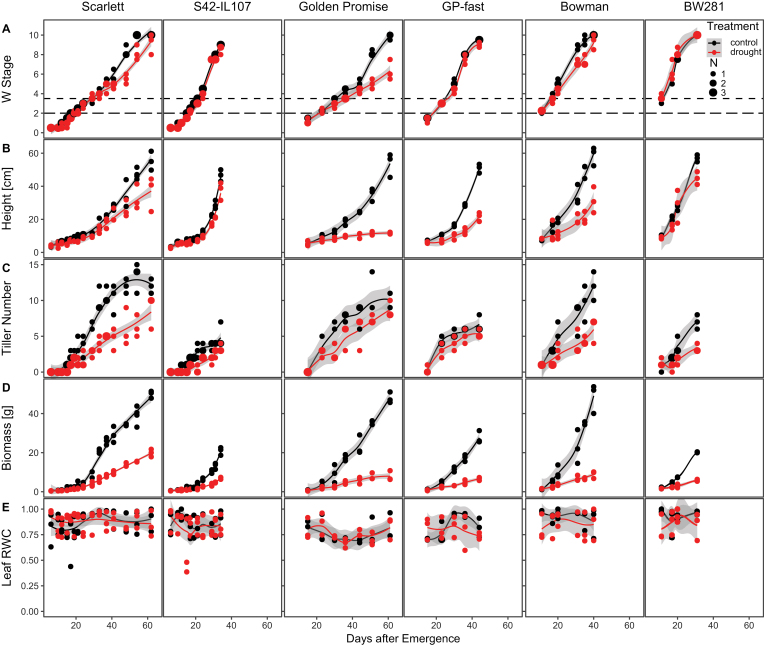
Development of the MSA was scored after microdissection according to the scale established by Waddington et al. (1983) (Supplementary Fig. S3). The timing of spikelet initiation was not significantly altered by drought in any of the genotypes (Fig. 2A). However, drought delayed floral progression in the parental genotypes, but not in the introgression lines. Similarly, stem elongation, measured as plant height, was strongly reduced under drought in the three parental genotypes, but was less affected in the introgression lines (Fig. 2B). Variation at Ppd-H1 and drought also had strong effects on the progression of tiller development (Fig. 2C). The introgression lines developed significantly fewer tillers than the parental lines under control and drought conditions. Drought delayed the development of tillers in Scarlett, Bowman, and BW281, but tiller development was not significantly different in S42-IL107, Golden Promise, and GP-fast. Consequently, drought had a much stronger effect on spike number than tiller number, demonstrating that the plants produced tillers during drought that did not develop a spike (Fig. 1B). The faster reproductive development in the introgression lines correlated with a reduced biomass accumulation compared with the parental lines under control and drought conditions. Drought reduced fresh weight biomass in all lines, and the relative reductions were similar between the parental genotypes and their respective introgression line. For example, 34 d after emergence, an ~70% reduction in biomass was observed in both Scarlett and S42-IL107 (Fig. 2D). We did not observe any effect of drought on the phyllochron and the number of leaves on the main culm, but leaf size was strongly reduced under drought (Supplementary Fig. S4). Leaf RWC was not altered under drought in any of the tested lines, indicating that all plants responded to the reduced water availability through a growth reduction and thus avoided tissue dehydration (Fig. 2E).
Fig. 2.
Continuous drought delays floral development in spring barley. Development of the main shoot apex (MSA) (A), plant height (B), the number of tillers (C), fresh weight biomass (D), and leaf relative water content (RWC) (E) were scored during development under control (black) and drought (red) conditions under LDs (16 h light/8 h night) in the spring barley cultivars Scarlett, Golden Promise, and Bowman, and their derived introgression lines S42-IL107, GP-fast, and BW281 according to the scale by Waddington et al. (1983). Dot sizes indicate the number of overlapping samples. Trend lines were calculated using a polynomial regression (Loess smooth line); grey areas show the 95% confidence interval.
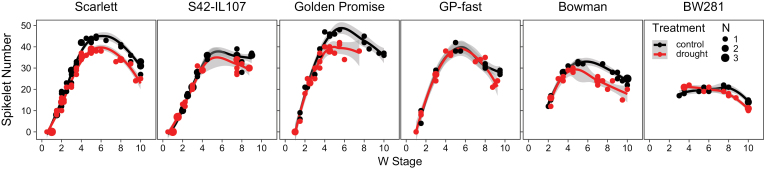
The induction of spikelets on the MSA terminated earlier in the introgression lines which therefore formed fewer spikelets compared with their respective parents. The introgression lines initiated spikelets until W4–5 while the parental lines formed new spikelets until W5–6 (Fig. 3). Under drought, the initiation of spikelets was slowed down in the parental lines, so that fewer spikelets were initiated under drought than under control conditions. However, in the introgression lines, there was no significant difference in the initiation of spikelet primordia between control and drought conditions. While the parental lines initiated more spikelets than the introgression lines, a higher proportion of spikelets did not develop florets in the parental genotypes, compared with the introgression lines. The introgression lines initiated fewer spikelets under control conditions, but drought did not reduce spikelet number further in these lines. The differences between spikelet number and grain number observed in the introgression lines (Fig. 1F) were therefore due to low floret fertility and not a failure in developing florets.
Fig. 3.
Continuous drought affects tillering and spikelet number in barley. The number of spikelets on the main shoot apex (MSA) (A) were scored during development under control (black) and drought (red) conditions under LDs (16 h light/8 h night) in the spring barley cultivars Scarlett, Golden Promise, and Bowman, and the derived introgression lines S42-IL107, GP-fast, and BW281. Dot sizes indicate the number of overlapping samples. Trend lines were calculated using a polynomial regression (Loess smooth line); grey areas show the 95% confidence interval.
Taken together, Ppd-H1 controlled the drought-induced changes in reproductive development, shoot and spike morphology, and plant height. Elite spring barley with a mutation in ppd-H1 displayed a strong delay in floral development and reductions in plant height and the number of spikelets initiated on the main inflorescence under drought, whereas these traits were scarcely affected under drought in the introgression lines with a wild-type Ppd-H1 allele. Finally, drought had a strong detrimental effect on floret fertility which resulted in a reduction of grains independent of the Ppd-H1 genotype.
Ppd-H1 affects the plasticity of reproductive development in response to a transient drought stress
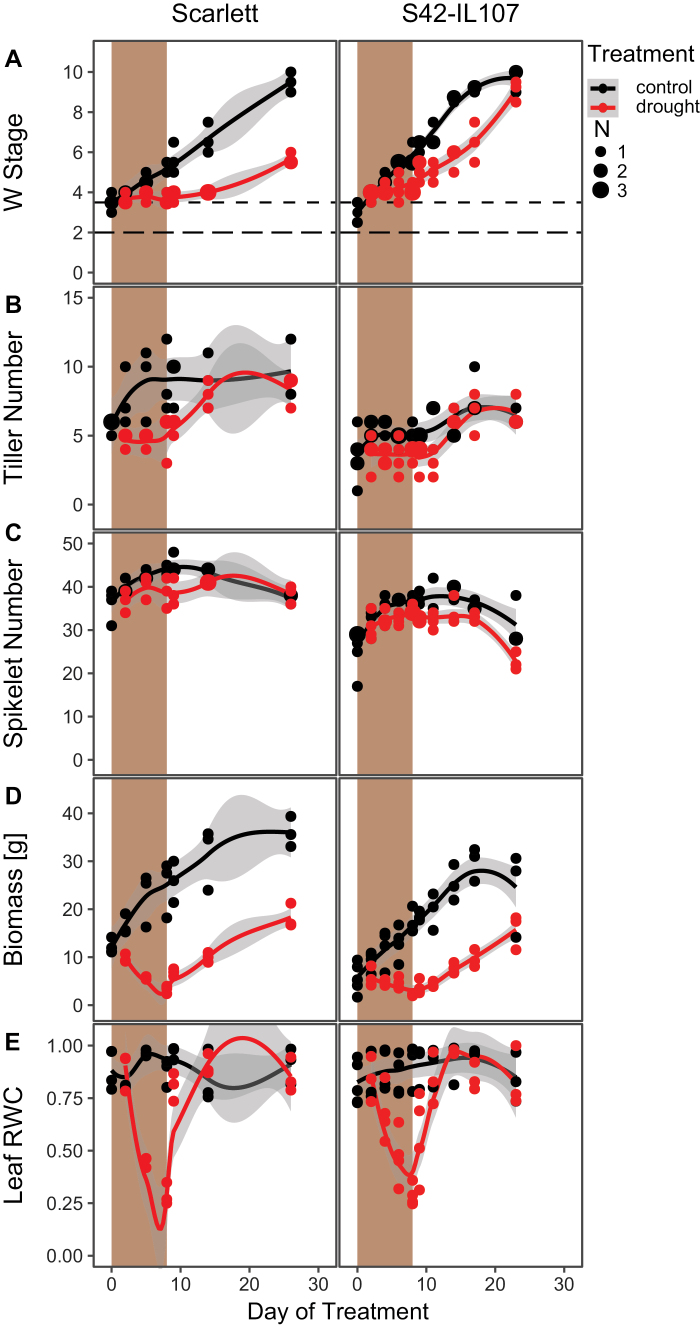
The severity, duration, and timing of drought events are highly variable in nature. We therefore tested if the observed effects of drought on reproductive development are dependent on the timing and severity of the stress. In addition, we investigated if Ppd-H1 also affected the plasticity of development in response to a transient drought stress followed by a recovery phase. Under severe drought, reproductive development stopped completely in Scarlett for the duration of the stress treatment and resumed after rewatering (Fig. 4A). However, the delay in development was maintained after the stress treatment, and stressed plants flowered significantly later than control plants. In S42-IL107, reproductive development only slowed down after the onset of drought stress and did not stop completely. After rewatering, reproductive development even accelerated so that control and stressed plants flowered almost at the same time (Fig. 4A; Supplementary Fig. S5). Tiller development was also halted in both genotypes upon the onset of stress, but both genotypes resumed tiller development after rewatering, and tiller numbers were not significantly different between control and stress conditions at flowering. Spikelet numbers were not strongly altered during development because at the onset of drought (W3) the majority of spikelets had already initiated. Drought, however, still caused a small reduction in spikelet initiation in both genotypes. The treatment completely stopped biomass accumulation in both genotypes already after 2 d of withholding water. On the eighth day, when the drought level was most severe, control plants of both Scarlett and S42-IL107 had accumulated almost nine times as much biomass compared with drought-stressed samples. The reductions in fresh biomass were also caused by a strong decline in the leaf RWC upon application of the severe drought stress (Fig. 4D, E). However, after rewatering, RWC levels rapidly increased again and were similar to RWC levels in control plants 6 d after rewatering in both genotypes. While RWC levels fully recovered after rewatering and stressed plants resumed growth, fresh weight biomass was significantly lower in stressed compared with control plants at flowering.
Fig. 4.
Severe drought delays MSA development in barley. MSA development (A), the number of tillers (B), the number of spikelets (C), fresh weight biomass (D), and leaf RWC (E) were scored under control (black) and severe drought (red) conditions and during recovery. Shaded areas indicate the period during which plants were not watered. Dot sizes indicate the number of overlapping samples. Trend lines were calculated using a polynomial regression (Loess smooth line), grey areas show the 95% confidence interval.
Taken together, transient severe stress applied during stem elongation also delayed floral development as observed under mild stress. Interestingly, the introgression line but not the parental line accelerated reproductive development after rewatering. Stressed and control S42-IL107 plants flowered nearly simultaneously, suggesting that Ppd-H1 affects the developmental plasticity in response to drought.
Drought alters the expression of clock and floral regulator genes in barley
Components of the circadian clock play important roles in the control of flowering time regulators in barley. Additionally, previous studies have found that abiotic stresses alter the diurnal gene expression of core clock genes and clock-regulated genes in barley (Habte et al., 2014; Ford et al., 2016; Ejaz and von Korff, 2017). We therefore examined whether reduced SWC affected reproductive development through alterations in the diurnal expression patterns of clock and flowering time genes. For this purpose, leaf samples of Scarlett and S42-IL107 plants grown under control and continuous mild drought conditions were harvested every 4 h over 24 h at the stamen primordium stage (≥W3.5).
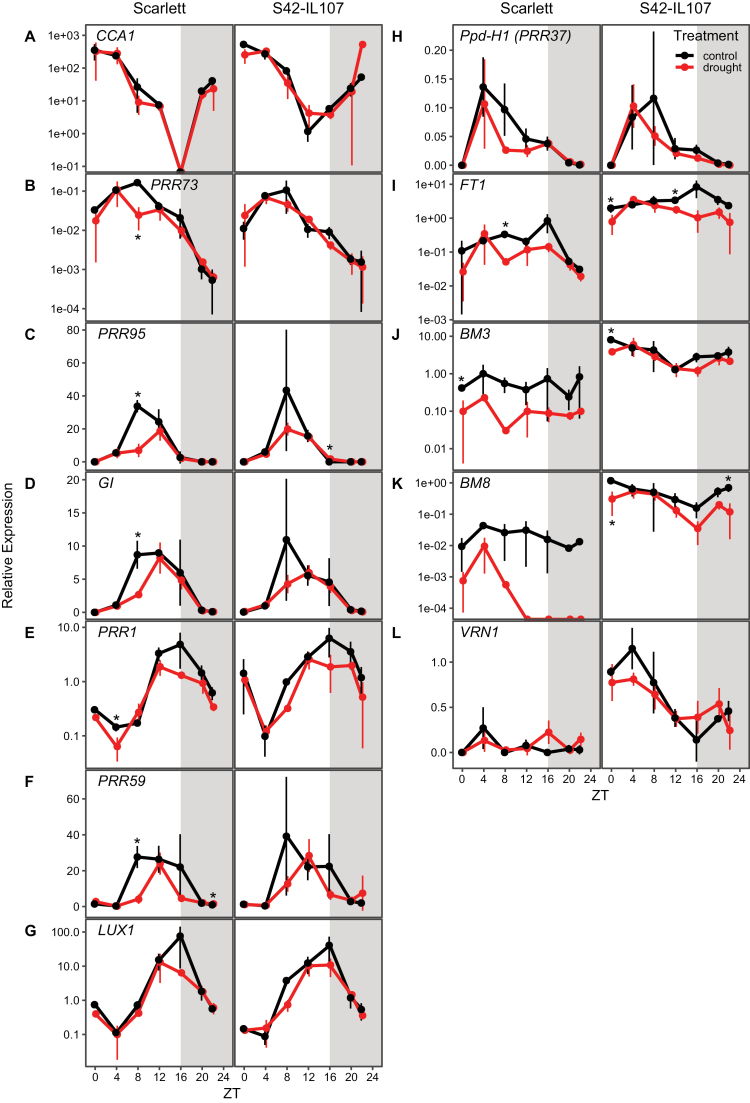
We investigated the expression of known barley core clock genes (Campoli et al., 2012b; Müller et al., 2020), with expression peaks at different times of the day (Fig. 5). The expression levels of the morning-expressed CCA1 and the evening-expressed LUX1 were not consistently altered between drought and control conditions (Fig. 5A, G). Expression levels of PRR59, PRR73, PRR95, and GIGANTEA (GI) were down-regulated at ZT8 under drought compared with control conditions in Scarlett (Fig. 5B–D, F). Drought also affected the peak time of expression of some clock transcripts. The expression peaks of PRR95 and GI were delayed by 4 h, while expression peaks of PRR1 and LUX1 were advanced by 4 h in both genotypes. There were no consistent differences in the expression levels and patterns of clock genes between Scarlett and S42-IL107 under both conditions. Similar to the clock genes, the floral regulator genes and putative downstream targets of Ppd-H1 were down-regulated under drought. Expression of Ppd-H1 itself was not strongly affected under drought in either genotype (Fig. 5H). However, the expression levels of floral regulator genes differed between the genotypes under control and drought conditions. Expression levels of FT1, and the barley MADS-box genes VRN1, BM3, and BM8, were overall higher in S42-IL107 than in Scarlett under both conditions (Fig. 5I–L). Drought reduced FT1 transcript levels in both genotypes, in particular at the evening peak time of expression. However, expression of FT1 under drought was at all time points higher in S42-IL107 than in Scarlett. BM3 and BM8 were down-regulated under drought specifically in Scarlett at the majority of time points (Fig. 5K). In S42-IL107, transcript levels of BM3 and BM8 were not strongly altered between control and drought conditions.
Fig. 5.
Continuous drought reduces the transcript levels of circadian clock and flowering time genes in barley. Diurnal gene expression of circadian clock genes was measured every 4 h for 24 h with an additional sampling at ZT22 under control (black) and drought (red) conditions under LDs (16 h light/8 h night) in the spring barley cultivars Scarlett and the derived introgression line S42-IL107. Grey areas indicate darkness. Error bars indicate ±SD of three biological replicates; an asterisk indicates a significant difference between control and drought at the respective time point (t-test, P<0.05).
In summary, drought decreased the expression levels of clock genes and floral regulator genes, and affected the peak time of expression of evening-expressed clock genes. Expression patterns of clock genes were similar between Scarlett and S42-IL107 under control and drought conditions; genetic variation at Ppd-H1 (PRR37) therefore did not affect the diel expression patterns of clock genes. However, expression of floral regulator genes was significantly different between Scarlett and S42-IL107 under control and drought conditions. In addition, expression levels of floral regulator genes were more strongly altered under drought in Scarlett than in S42-IL107, demonstrating that Ppd-H1 interacted with drought to control reproductive development and expression levels of major flowering time genes in barley.
Ppd-H1 alters the effect of drought on flowering time gene expression during development
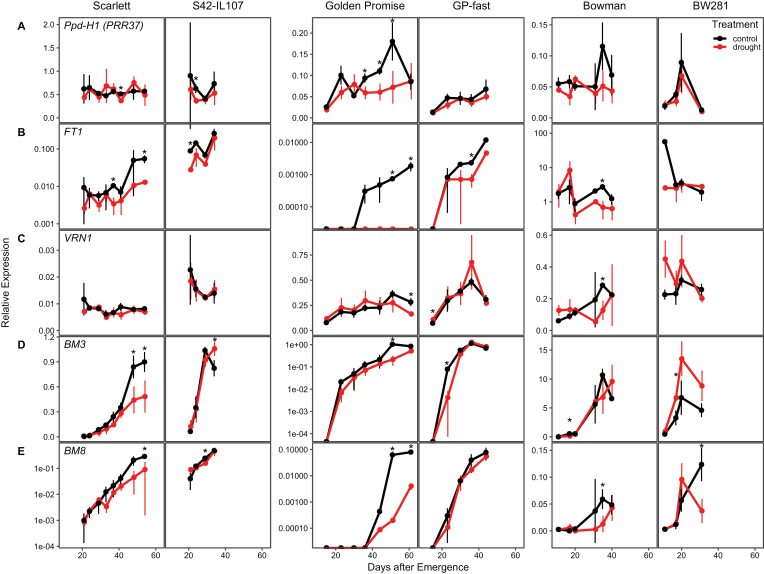
We further investigated how Ppd-H1 and drought affected expression of floral regulator genes during development in all six genotypes. Transcript levels of floral regulator genes were investigated in leaf samples from plants analysed for developmental traits as shown in Fig. 2. The youngest fully developed leaf was harvested at ZT8 in all genotypes starting from the first day after transfer to LDs until flowering. At transfer to LDs, all genotypes had formed a reproductive inflorescence at the double ridge stage (W2), with the exception of BW281, which was already at the awn primordium stage (W3).
The expression levels of Ppd-H1 were not strongly altered by the treatment or Ppd-H1 variant, with the exception of Golden Promise where Ppd-H1 transcript levels were significantly higher under control than drought conditions (W3.5–W5.5) (Fig. 6A). In contrast, FT1 expression levels were down-regulated under drought in all genotypes (Fig. 6B). FT1 transcript levels increased during development and this increase was slowed down under drought, in particular in the parental line Scarlett. In Golden Promise, no FT1 transcript was detected under drought at any time point. In the introgression lines, FT1 expression levels were only significantly different between conditions at single time points in S42-IL107 and GP-fast, and were not changed in BW281. These differences in FT1 transcript levels under drought correlated with the observed delay in floral progression in the parental genotypes as compared with the introgression lines under drought versus control conditions (Fig. 6A). Transcript levels of the vernalization gene VRN1 were higher in the introgression than parental lines, but not significantly different between control and drought conditions (Fig. 6C). Transcript levels of BM3 and BM8 increased during development in all genotypes, and this increase was delayed and reduced under drought in Scarlett, Golden Promise, and Bowman, but not significantly different in S42-IL107 and GP-fast under drought versus control treatments. In BW281, BM3 expression levels increased faster and to higher levels under drought compared with control conditions which correlated with the acceleration in floral development under drought in this line (Fig. 6D, E).
Fig. 6.
Continuous drought affects the expression of flowering time genes during development in barley. Transcript levels of flowering time genes was measured during development under control (black) and drought (red) conditions under LDs (16 h light/8 h night) in the spring barley cultivars Scarlett, Golden Promise, and Bowman, and the derived introgression lines S42-IL107, GP-fast, and BW281. Error bars indicate ±SD of three biological replicates; an asterisk indicates a significant difference between control and drought at the respective time point (t-test, P<0.05).
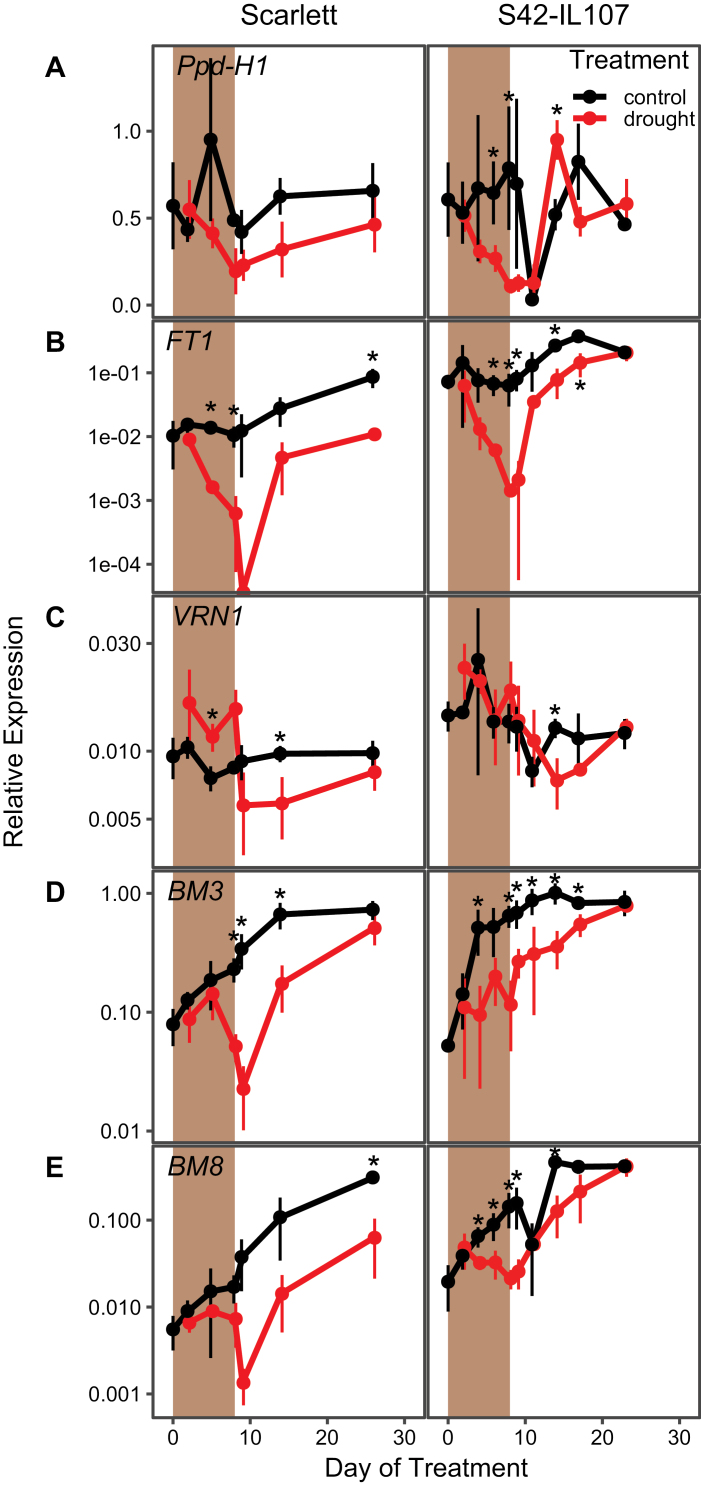
We also tested the effects of the transient severe drought stress on the expression of floral regulator genes in Scarlett and S42-IL107 (Fig. 7). During the transient drought treatment, transcript levels of Ppd-H1, FT1, BM3, and BM8 were strongly down-regulated compared with control conditions in both genotypes (Fig. 7A, B, D, E). In Scarlett, the down-regulation of these flowering inducers extended long into the recovery phase, even after leaf RWC had returned to control levels. In S42-IL107, transcript levels of floral inducers recovered rapidly after rewatering and eventually reached the same levels as observed under control conditions. Transcript levels of VRN1 were down-regulated after the transient drought stress in both genotypes, but matched VRN1 expression levels in control plants at flowering (Fig. 7C).
Fig. 7.
Severe drought interacts with Ppd-H1 to control transcript levels of flowering time genes during stress and recovery. Transcript levels of flowering time genes were measured during development under control (black) and drought (red) conditions under LDs (16 h light/8 h night) in the spring barley cultivar Scarlett and the derived introgression line S42-IL107. Shaded areas indicate the period during which plants were not watered; error bars indicate ±SD of three biological replicates; an asterisk indicates a significant difference between control and drought at the respective time point (t-test, P<0.05).
In summary, both mild continuous and severe transient drought reduced the transcript levels of flowering inducers. However, reductions in transcript levels were stronger in the parental than in the introgression lines with a wild-type Ppd-H1 allele. Ppd-H1 therefore modulated expression of floral inducers in response to drought in barley. In addition, transcript levels rapidly recovered after a transient drought stress to control levels in the introgression line but not the parental line, suggesting that Ppd-H1 affected transcriptional homeostasis in response to drought.
Discussion
Ppd-H1 was identified as a photoperiod response gene that controls adaptation to different environments by modulating flowering time in response to LDs (Turner et al., 2005; Cockram et al., 2007; Jones et al., 2008; Wiegmann et al., 2019). Here, we demonstrate that Ppd-H1 also integrates drought stress signals to modulate floral development in barley. Drought delayed floral development in the parental genotypes with a mutated ppd-H1 allele, while reproductive development was not affected by drought in genotypes with a wild-type Ppd-H1 allele (Figs 1, 2). This variation in developmental timing in response to drought was linked to variation in the number of initiated spikelet primordia on the main shoot. Spikelet initiation was reduced in the parental lines, but not in the introgression lines under drought (Fig. 3). Similarly, drought-triggered reductions in plant height, and tiller and spike number were more pronounced in the parental lines compared with the introgression lines. Under the severe transient stress, reproductive development slowed down in all genotypes; however, upon rewatering, the introgression line with a wild-type Ppd-H1 allele accelerated development so that control and stressed plants flowered simultaneously. In contrast, parental lines flowered significantly later after a transient stress than plants under control conditions (Fig. 4). Taken together, the results demonstrated that Ppd-H1 interacts with drought to control the development and morphology of the shoot and spike. Ppd-H1 has already been associated with a number of shoot- and spike-related traits in barley and acts as a key gene to coordinate the development of different plant organs with reproductive timing (Digel et al., 2015, 2016; Alqudah et al., 2016, 2018; Ejaz and von Korff, 2017; Pham et al., 2019; Shaaf et al., 2019). Furthermore, our results suggested that Ppd-H1 controlled the plasticity of reproductive development in response to drought. The parental lines with a mutation in ppd-H1 displayed a high trait variance between treatments and thus developmental plasticity. In contrast, the introgression lines exhibited a higher trait stability under drought, in particular for developmental timing and spikelet initiation, while biomass reductions under drought were comparable between genotypes. The identification of genes/alleles maintaining trait stability in response to environmental perturbations is interesting for breeding genotypes with high yield stability under global climatic changes and higher frequencies of extreme weather events.
The most plastic trait under drought in all genotypes was grain number. Drought caused a minor reduction in the number of spikelet primordia and in the number of spikelets/florets, but a major reduction in the final grain number (Fig. 1). This suggested that drought reduced grain number primarily by affecting floret fertility and tiller number. It has already been described that water deficit impairs pollen development. Altered tapetal degeneration and associated changes in nutrient provision and signalling have been identified as the primary causes for cellular defects in pollen maturation under drought stress (Saini et al., 1984; Lalonde et al., 1997; Saini, 1997; Saini and Westgate, 1999; Aloni et al., 2001; Pressman et al., 2002; Barnabás et al., 2008; De Storme and Geelen, 2014). Moreover, drought interferes with ovary survival or early grain development, potentially by restricting expansive growth, and thereby reduces the number of grains per spike (Guo et al., 2016; Oury et al., 2016a, b; Turc and Tardieu, 2018). In contrast to grain number, TKW was not very variable between drought and control conditions. We concluded that floral development is most susceptible to drought, while spikelet initiation as well as grain filling were less affected. These effects in controlled-environment chambers correspond to observations in field-grown wheat, where yield differences between environments were primarily controlled by variation in grain number while TKW was relatively stable across environments (Slafer et al., 2014, 2015). Our results therefore underline the importance of floral development and fertility for yield under drought, which supports recent studies that challenge the central importance of ‘terminal drought’ as the main cause for losses in cereal yield in drought-prone Mediterranean regions (Savin et al., 2015).
The circadian clock controls genes of the photoperiod response pathway, and Ppd-H1 itself is a barley homologue of an Arabidopsis clock gene (Faure et al., 2012; Campoli et al., 2013). Furthermore, the circadian clock controls stress adaptation and is itself regulated by stress cues (Liu et al., 2013; Tamaru et al., 2013; Habte et al., 2014; Grundy et al., 2015; Lee et al., 2016; Ejaz and von Korff, 2017; Guadagno et al., 2018). We consequently tested if drought interacted with variation at Ppd-H1 to affect the expression of barley clock genes. Indeed, drought marginally affected the amplitude and phase of clock gene expression. Clock gene transcripts were down-regulated under drought; however, variation at Ppd-H1 had no consistent effects on clock gene expression, under either control or drought conditions (Fig. 5). This supports earlier studies which demonstrated that the natural mutation in ppd-H1 did not affect the expression of other barley clock homologues either under control conditions or under osmotic and/or high temperature stress (Campoli et al., 2012b; Habte et al., 2014; Ejaz and von Korff, 2017). However, we cannot exclude that drought might have interacted with Ppd-H1 to affect clock proteins post-transcriptionally (Más et al., 2003; Kiba et al., 2007). Like the clock genes, the transcripts of the flowering time genes FT1, BM3, and BM8 were reduced under drought during floral development (Figs 5, 6). Similarly, in rice, the FT homologues Hd3a and RFT1 were down-regulated under drought stress and this correlated with a delay in floral transition under inductive SDs (Galbiati et al., 2016). In contrast, in Arabidopsis, drought induces early flowering through the ABA-dependent stimulation of GI or of ABFs that trigger SOC1 and FT transcriptional activation (Riboni et al., 2016; Hwang et al., 2019). On the other hand, it was also shown that ABSCISIC ACID-INSENSITIVE 4 (ABI4), a key component in the ABA signalling pathway, negatively regulated floral transition by directly promoting expression of the floral repressor FLC. Interestingly, the barley vernalization gene VRN1 was not consistently altered in expression under drought, suggesting that the vernalization response pathway is not involved in transmitting drought signals in barley. However, all genotypes carry spring alleles at VRN1; future research therefore needs to test the response of the winter vrn1 allele to drought and its effects on flowering.
Because negative and positive effects of drought and ABA on flowering time were observed, it was suggested that different levels of stress may elicit different developmental responses. A moderate level of drought and ABA levels may delay floral transition, allowing for flowering to occur after the stress, while a severe drought stress and high ABA levels promote flowering and drought escape to maximize reproductive success (Shu et al., 2018). However, we found that both mild and severe stress resulted in a delay in flowering time. Differential responses to drought were rather genetically controlled where Ppd-H1 controlled the drought-dependent down-regulation of FT1, BM3, and BM8, and correlated differences in reproductive development. Furthermore, after a transient drought stress, FT1, BM3, and BM8 transcript levels recovered fast after rewatering and eventually matched those under control conditions in the introgression but not in the parental line (Fig. 7). Consequently, Ppd-H1 also affected transcript homeostasis after a severe transient perturbation by stress. In contrast to reports from rice and Arabidopsis, drought did not strongly impact the timing of spikelet initiation but slowed down and impaired floral development and fertility. FT1, BM3, and BM8 have already been linked to inflorescence and floral development in barley, wheat, and rice (Digel et al., 2015; Wu et al., 2017; Callens et al., 2018; Shaw et al., 2019). In rice, simultaneous knockdown of OsMADS14 (VRN1, FUL1), OsMADS15 (BM3, FUL2), and OsMAD18 (BM8, FUL3) resulted in floral reversion and the formation of lateral vegetative tillers (Kobayashi et al., 2012). Similarly, triple wheat vrn1ful2ful3 mutants formed vegetative tillers instead of spikelets on lateral meristems and displayed a reduced stem elongation (Li et al., 2019). Reduced transcript levels of FT1, BM3, and BM8 might therefore have contributed to an impaired floral development and decreased stem elongation in the drought-stressed plants in our study. It has been shown in barley and rice, that FT homologues have positive effects on gibberellin (GA) biosynthesis or stem responsiveness to GA and thus stem elongation (Pearce et al., 2013; Gómez-Ariza et al., 2019). Reduced FT1 transcript levels might therefore have contributed to a reduction in stem elongation under drought; Golden Promise with the strongest FT1 down-regulation under drought was also characterized by the strongest reduction in plant height.
In summary, our results demonstrate that Ppd-H1 integrates photoperiod and drought stress signals to control reproductive timing and the plasticity of shoot and spike morphology in response to drought in barley. These differential responses to drought are linked to a differential down-regulation of FT1, BM3, and BM8 transcripts in the leaf. Future studies need to elucidate linked transcriptional changes in the inflorescences and further dissect the effects of drought on floral organ development. Furthermore, results obtained in this study under controlled conditions need to be verified under field conditions.
Supplementary data
Supplementary data are available at JXB online.
Table S1. Oligonucleotides used in this study.
Data S1. Genotyping of introgression lines used in this study.
Fig. S1. Genetic map of Ppd-H1 introgression in spring barley backgrounds, introgression size in centiMorgans (cM), and the polymorphic markers flanking the insertions.
Fig. S2. Flowering morphology in Golden Promise and Bowman background under continuous drought.
Fig. S3. MSA and pistil morphology in Golden Promise background.
Fig. S4. Continuous drought affects leaf size but not the phyllochron in barley.
Fig. S5. Severe drought delays flowering in barley.
Acknowledgements
We warmly thank Kerstin Luxa, Caren Dawidson, Andrea Lossow, and Thea Rütjes for excellent technical assistance. This work was funded by the Max Planck Society and the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany´s Excellence Strategy—EXC-2048/1—Project ID: 390686111 and the GRK 2064: Water use efficiency and drought stress responses: From Arabidopsis to Barley. Deutsche Forschungsgemeinschaft (DFG) - Projektnummer 252965955.
Glossary
Abbreviations
- ABA
abscisic acid
- FC
field capacity
- LD
long day
- MSA
main shoot apex
- RWC
relative water content
- SAM
shoot apical meristem
- SD
short day
- SWC
soil water content
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author contributions
LG and MvK conceived the project and planned the experiments. LG performed all experiments, except experiments with Golden Promise which were performed by LG and EBH. LG and MvK wrote the manuscript.
References
- Abe M, Kobayashi Y, Yamamoto S, Daimon Y, Yamaguchi A, Ikeda Y, Ichinoki H, Notaguchi M, Goto K, Araki T. 2005. FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science 309, 1052–1056. [DOI] [PubMed] [Google Scholar]
- Al-Ajlouni Z, Al-Abdallat A, Al-Ghzawi A, Ayad J, Abu Elenein J, Al-Quraan N, Baenziger P. 2016. Impact of pre-anthesis water deficit on yield and yield components in barley (Hordeum vulgare L.) plants grown under controlled conditions. Agronomy 6, 33. [Google Scholar]
- Aloni B, Peet M, Pharr M, Karni L. 2001. The effect of high temperature and high atmospheric CO2 on carbohydrate changes in bell pepper (Capsicum annuum) pollen in relation to its germination. Physiologia Plantarum 112, 505–512. [DOI] [PubMed] [Google Scholar]
- Alqudah AM, Koppolu R, Wolde GM, Graner A, Schnurbusch T. 2016. The genetic architecture of barley plant stature. Frontiers in Genetics 7, 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alqudah AM, Schnurbusch T. 2017. Heading date is not flowering time in spring barley. Frontiers in Plant Science 8, 896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alqudah AM, Sharma R, Pasam RK, Graner A, Kilian B, Schnurbusch T. 2014. Genetic dissection of photoperiod response based on GWAS of pre-anthesis phase duration in spring barley. PLoS One 9, e113120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alqudah AM, Youssef HM, Graner A, Schnurbusch T. 2018. Natural variation and genetic make-up of leaf blade area in spring barley. Theoretical and Applied Genetics 131, 873–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez MA, Tranquilli G, Lewis S, Kippes N, Dubcovsky J. 2016. Genetic and physical mapping of the earliness per se locus Eps-A (m) 1 in Triticum monococcum identifies EARLY FLOWERING 3 (ELF3) as a candidate gene. Functional & Integrative Genomics 16, 365–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnabás B, Jäger K, Fehér A. 2008. The effect of drought and heat stress on reproductive processes in cereals. Plant, Cell & Environment 31, 11–38. [DOI] [PubMed] [Google Scholar]
- Baum M, Von Korff M, Guo P, et al. 2007. Molecular approaches and breeding strategies for drought tolerance in barley. In: Varshney R, Tuberosa R, eds. Genomic assisted crop improvement: Vol. 2: Genomics applications in crops. Dordrecht: Springer, 51–79. [Google Scholar]
- Bayer MM, Rapazote-Flores P, Ganal M, et al. 2017. Development and evaluation of a barley 50k iSelect SNP array. Frontiers in Plant Science 8, 1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callens C, Tucker MR, Zhang D, Wilson ZA. 2018. Dissecting the role of MADS-box genes in monocot floral development and diversity. Journal of Experimental Botany 69, 2435–2459. [DOI] [PubMed] [Google Scholar]
- Campoli C, Drosse B, Searle I, Coupland G, von Korff M. 2012a Functional characterisation of HvCO1, the barley (Hordeum vulgare) flowering time ortholog of CONSTANS. The Plant Journal 69, 868–880. [DOI] [PubMed] [Google Scholar]
- Campoli C, Pankin A, Drosse B, Casao CM, Davis SJ, von Korff M. 2013. HvLUX1 is a candidate gene underlying the early maturity 10 locus in barley: phylogeny, diversity, and interactions with the circadian clock and photoperiodic pathways. New Phytologist 199, 1045–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campoli C, Shtaya M, Davis SJ, von Korff M. 2012b Expression conservation within the circadian clock of a monocot: natural variation at barley Ppd-H1 affects circadian expression of flowering time genes, but not clock orthologs. BMC Plant Biology 12, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantalapiedra CP, Boudiar R, Casas AM, Igartua E, Contreras-Moreira B. 2015. BARLEYMAP: physical and genetic mapping of nucleotide sequences and annotation of surrounding loci in barley. Molecular Breeding 35, 13. [Google Scholar]
- Cockram J, Jones H, Leigh FJ, O’Sullivan D, Powell W, Laurie DA, Greenland AJ. 2007. Control of flowering time in temperate cereals: genes, domestication, and sustainable productivity. Journal of Experimental Botany 58, 1231–1244. [DOI] [PubMed] [Google Scholar]
- Coleman E 1947. A laboratory procedure for determining the field capacity of soils. Soil Science 63, 277–284. [Google Scholar]
- Corbesier L, Vincent C, Jang S, et al. 2007. FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science 316, 1030–1033. [DOI] [PubMed] [Google Scholar]
- De Storme N, Geelen D. 2014. The impact of environmental stress on male reproductive development in plants: biological processes and molecular mechanisms. Plant, Cell & Environment 37, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digel B, Pankin A, von Korff M. 2015. Global transcriptome profiling of developing leaf and shoot apices reveals distinct genetic and environmental control of floral transition and inflorescence development in barley. The Plant Cell 27, 2318–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digel B, Tavakol E, Verderio G, Tondelli A, Xu X, Cattivelli L, Rossini L, von Korff M. 2016. Photoperiod-H1 (Ppd-H1) controls leaf size. Plant Physiology 172, 405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druka A, Franckowiak J, Lundqvist U, et al. 2011. Genetic dissection of barley morphology and development. Plant Physiology 155, 617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejaz M, von Korff M. 2017. The genetic control of reproductive development under high ambient temperature. Plant Physiology 173, 294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure S, Turner AS, Gruszka D, Christodoulou V, Davis SJ, von Korff M, Laurie DA. 2012. Mutation at the circadian clock gene EARLY MATURITY 8 adapts domesticated barley (Hordeum vulgare) to short growing seasons. Proceedings of the National Academy of Sciences, USA 109, 8328–8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford B, Deng W, Clausen J, Oliver S, Boden S, Hemming M, Trevaskis B. 2016. Barley (Hordeum vulgare) circadian clock genes can respond rapidly to temperature in an EARLY FLOWERING 3-dependent manner. Journal of Experimental Botany 67, 5517–5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbiati F, Chiozzotto R, Locatelli F, Spada A, Genga A, Fornara F. 2016. Hd3a, RFT1 and Ehd1 integrate photoperiodic and drought stress signals to delay the floral transition in rice. Plant, Cell & Environment 39, 1982–1993. [DOI] [PubMed] [Google Scholar]
- Gol L, Tomé F, von Korff M. 2017. Floral transitions in wheat and barley: interactions between photoperiod, abiotic stresses, and nutrient status. Journal of Experimental Botany 68, 1399–1410. [DOI] [PubMed] [Google Scholar]
- Gómez-Ariza J, Brambilla V, Vicentini G, et al. 2019. A transcription factor coordinating internode elongation and photoperiodic signals in rice. Nature Plants 5, 358–362. [DOI] [PubMed] [Google Scholar]
- Grundy J, Stoker C, Carré IA. 2015. Circadian regulation of abiotic stress tolerance in plants. Frontiers in Plant Science 6, 648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadagno CR, Ewers BE, Weinig C. 2018. Circadian rhythms and redox state in plants: till stress do us part. Frontiers in Plant Science 9, 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Slafer GA, Schnurbusch T. 2016. Genotypic variation in spike fertility traits and ovary size as determinants of floret and grain survival rate in wheat. Journal of Experimental Botany 67, 4221–4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habte E, Müller LM, Shtaya M, Davis SJ, von Korff M. 2014. Osmotic stress at the barley root affects expression of circadian clock genes in the shoot. Plant, Cell & Environment 37, 1321–1327. [DOI] [PubMed] [Google Scholar]
- Hwang K, Susila H, Nasim Z, Jung JY, Ahn JH. 2019. Arabidopsis ABF3 and ABF4 transcription factors act with the NF-YC complex to regulate SOC1 expression and mediate drought-accelerated flowering. Molecular Plant 12, 489–505. [DOI] [PubMed] [Google Scholar]
- Jaeger KE, Pullen N, Lamzin S, Morris RJ, Wigge PA. 2013. Interlocking feedback loops govern the dynamic behavior of the floral transition in Arabidopsis. The Plant Cell 25, 820–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger KE, Wigge PA. 2007. FT protein acts as a long-range signal in Arabidopsis. Current Biology 17, 1050–1054. [DOI] [PubMed] [Google Scholar]
- Johansson M, Staiger D. 2015. Time to flower: interplay between photoperiod and the circadian clock. Journal of Experimental Botany 66, 719–730. [DOI] [PubMed] [Google Scholar]
- Jones H, Leigh FJ, Mackay I, Bower MA, Smith LM, Charles MP, Jones G, Jones MK, Brown TA, Powell W. 2008. Population-based resequencing reveals that the flowering time adaptation of cultivated barley originated east of the Fertile Crescent. Molecular Biology and Evolution 25, 2211–2219. [DOI] [PubMed] [Google Scholar]
- Kahiluoto H, Kaseva J, Balek J, et al. 2019. Decline in climate resilience of European wheat. Proceedings of the National Academy of Sciences, USA 116, 123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazan K, Lyons R. 2016. The link between flowering time and stress tolerance. Journal of Experimental Botany 67, 47–60. [DOI] [PubMed] [Google Scholar]
- Kiba T, Henriques R, Sakakibara H, Chua NH. 2007. Targeted degradation of PSEUDO-RESPONSE REGULATOR5 by an SCFZTL complex regulates clock function and photomorphogenesis in Arabidopsis thaliana. The Plant Cell 19, 2516–2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Yasuno N, Sato Y, Yoda M, Yamazaki R, Kimizu M, Yoshida H, Nagamura Y, Kyozuka J. 2012. Inflorescence meristem identity in rice is specified by overlapping functions of three AP1/FUL-like MADS box genes and PAP2, a SEPALLATA MADS box gene. The Plant Cell 24, 1848–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalonde S, Beebe DU, Saini HS. 1997. Early signs of disruption of wheat anther development associated with the induction of male sterility by meiotic-stage water deficit. Sexual Plant Reproduction 10, 40–48. [Google Scholar]
- Lee HG, Mas P, Seo PJ. 2016. MYB96 shapes the circadian gating of ABA signaling in Arabidopsis. Scientific Reports 6, 17754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Lin H, Chen A, Lau M, Jernstedt J, Dubcovsky J. 2019. Wheat VRN1, FUL2 and FUL3 play critical and redundant roles in spikelet development and spike determinacy. Development 146, dev175398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Carlsson J, Takeuchi T, Newton L, Farré EM. 2013. Direct regulation of abiotic responses by the Arabidopsis circadian clock component PRR7. The Plant Journal 76, 101–114. [DOI] [PubMed] [Google Scholar]
- Makino S, Matsushika A, Kojima M, Oda Y, Mizuno T. 2001. Light response of the circadian waves of the APRR1/TOC1 quintet: when does the quintet start singing rhythmically in Arabidopsis? Plant & Cell Physiology 42, 334–339. [DOI] [PubMed] [Google Scholar]
- Más P, Kim WY, Somers DE, Kay SA. 2003. Targeted degradation of TOC1 by ZTL modulates circadian function in Arabidopsis thaliana. Nature 426, 567–570. [DOI] [PubMed] [Google Scholar]
- Mascher M, Gundlach H, Himmelbach A, et al. 2017. A chromosome conformation capture ordered sequence of the barley genome. Nature 544, 427–433. [DOI] [PubMed] [Google Scholar]
- Mathieu J, Warthmann N, Küttner F, Schmid M. 2007. Export of FT protein from phloem companion cells is sufficient for floral induction in Arabidopsis. Current Biology 17, 1055–1060. [DOI] [PubMed] [Google Scholar]
- Mizuno N, Nitta M, Sato K, Nasuda S. 2012. A wheat homologue of PHYTOCLOCK 1 is a candidate gene conferring the early heading phenotype to einkorn wheat. Genes & Genetic Systems 87, 357–367. [DOI] [PubMed] [Google Scholar]
- Müller L, Mombaerts L, Pankin A, Davis SJ, Webb AA, Goncalves J, von Korff M. 2020. Differential effects of day–night cues and the circadian clock on the barley transcriptome. Plant Physiology (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller LM, von Korff M, Davis SJ. 2014. Connections between circadian clocks and carbon metabolism reveal species-specific effects on growth control. Journal of Experimental Botany 65, 2915–2923. [DOI] [PubMed] [Google Scholar]
- Murakami M, Ashikari M, Miura K, Yamashino T, Mizuno T. 2003. The evolutionarily conserved OsPRR quintet: rice pseudo-response regulators implicated in circadian rhythm. Plant & Cell Physiology 44, 1229–1236. [DOI] [PubMed] [Google Scholar]
- Oury V, Caldeira CF, Prodhomme D, Pichon JP, Gibon Y, Tardieu F, Turc O. 2016. Is change in ovary carbon status a cause or a consequence of maize ovary abortion in water deficit during flowering? Plant Physiology 171, 997–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oury V, Tardieu F, Turc O. 2016. Ovary apical abortion under water deficit is caused by changes in sequential development of ovaries and in silk growth rate in maize. Plant Physiology 171, 986–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce S, Vanzetti LS, Dubcovsky J. 2013. Exogenous gibberellins induce wheat spike development under short days only in the presence of VERNALIZATION1. Plant Physiology 163, 1433–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham AT, Maurer A, Pillen K, Brien C, Dowling K, Berger B, Eglinton JK, March TJ. 2019. Genome-wide association of barley plant growth under drought stress using a nested association mapping population. BMC Plant Biology 19, 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressman E, Peet MM, Pharr DM. 2002. The effect of heat stress on tomato pollen characteristics is associated with changes in carbohydrate concentration in the developing anthers. Annals of Botany 90, 631–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. 2020. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Riboni M, Galbiati M, Tonelli C, Conti L. 2013. GIGANTEA enables drought escape response via abscisic acid-dependent activation of the florigens and SUPPRESSOR OF OVEREXPRESSION OF CONSTANS. Plant Physiology 162, 1706–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riboni M, Robustelli Test A, Galbiati M, Tonelli C, Conti L. 2016. ABA-dependent control of GIGANTEA signalling enables drought escape via up-regulation of FLOWERING LOCUS T in Arabidopsis thaliana. Journal of Experimental Botany 67, 6309–6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins JA, Drosse B, Mulki MA, Grando S, Baum M, Singh M, Ceccarelli S, von Korff M. 2013a Variation at the vernalisation genes Vrn-H1 and Vrn-H2 determines growth and yield stability in barley (Hordeum vulgare) grown under dryland conditions in Syria. Theoretical and Applied Genetics 126, 2803–2824. [DOI] [PubMed] [Google Scholar]
- Rollins JA, Habte E, Templer SE, Colby T, Schmidt J, von Korff M. 2013b Leaf proteome alterations in the context of physiological and morphological responses to drought and heat stress in barley (Hordeum vulgare L.). Journal of Experimental Botany 64, 3201–3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini HS 1997. Effects of water stress on male gametophyte development in plants. Sexual Plant Reproduction 10, 67–73. [Google Scholar]
- Saini H, Sedgley M, Aspinall D. 1984. Development anatomy in wheat of male sterility induced by heat stress, water deficit or abscisic acid. Functional Plant Biology 11, 243. [Google Scholar]
- Saini HS, Westgate ME. 1999. Reproductive development in grain crops during drought. Advances in Agronomy 68, 59–96. [Google Scholar]
- Sanchez A, Shin J, Davis SJ. 2011. Abiotic stress and the plant circadian clock. Plant Signaling & Behavior 6, 223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savin R, Slafer GA, Cossani CM, Abeledo LG. 2015. Cereal yield in Mediterranean-type environments: challenging the paradigms on terminal drought, the adaptability of barley vs wheat and the role of nitrogen fertilization. In: Sadras VO, Calderini DF, eds. Crop physiology. Applications for genetic improvement and agronomy, 2nd edn Academic Press, 141–158. [Google Scholar]
- Schmalenbach I, March TJ, Bringezu T, Waugh R, Pillen K. 2011. High-resolution genotyping of wild barley introgression lines and fine-mapping of the threshability locus thresh-1 using the illumina goldengate assay. G3 (Bethesda, Md.) 1, 187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz J, Franzen R, Ngyuen TH, Garcia-Maroto F, Pozzi C, Salamini F, Rohde W. 2000. Cloning, mapping and expression analysis of barley MADS-box genes. Plant Molecular Biology 42, 899–913. [DOI] [PubMed] [Google Scholar]
- Shaaf S, Bretani G, Biswas A, Fontana IM, Rossini L. 2019. Genetics of barley tiller and leaf development. Journal of Integrative Plant Biology 61, 226–256. [DOI] [PubMed] [Google Scholar]
- Shaw LM, Lyu B, Turner R, Li C, Chen F, Han X, Fu D, Dubcovsky J. 2019. FLOWERING LOCUS T2 regulates spike development and fertility in temperate cereals. Journal of Experimental Botany 70, 193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu K, Chen Q, Wu Y, Liu R, Zhang H, Wang S, Tang S, Yang W, Xie Q. 2016. ABSCISIC ACID-INSENSITIVE 4 negatively regulates flowering through directly promoting Arabidopsis FLOWERING LOCUS C transcription. Journal of Experimental Botany 67, 195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu K, Luo X, Meng Y, Yang W. 2018. Toward a molecular understanding of abscisic acid actions in floral transition. Plant & Cell Physiology 59, 215–221. [DOI] [PubMed] [Google Scholar]
- Slafer GA, Kantolic AG, Appendino ML, Tranquilli G, Miralles DJ, Savin R. 2015. Genetic and environmental effects on crop development determining adaptation and yield. In: Sadras VO, Calderini DF, eds. Crop physiology. Applications for genetic improvement and agronomy, 2nd edn Academic Press, 285–319. [Google Scholar]
- Slafer GA, Savin R, Sadras VO. 2014. Coarse and fine regulation of wheat yield components in response to genotype and environment. Field Crops Research 157, 71–83. [Google Scholar]
- Smart RE, Bingham GE. 1974. Rapid estimates of relative water content. Plant Physiology 53, 258–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki S, Matsuo S, Wong HL, Yokoi S, Shimamoto K. 2007. Hd3a protein is a mobile flowering signal in rice. Science 316, 1033–1036. [DOI] [PubMed] [Google Scholar]
- Tamaru T, Hattori M, Ninomiya Y, et al. 2013. ROS stress resets circadian clocks to coordinate pro-survival signals. PLoS One 8, e82006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templer SE, Ammon A, Pscheidt D, et al. 2017. Metabolite profiling of barley flag leaves under drought and combined heat and drought stress reveals metabolic QTLs for metabolites associated with antioxidant defense. Journal of Experimental Botany 68, 1697–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevaskis B, Tadege M, Hemming MN, Peacock WJ, Dennis ES, Sheldon C. 2007. Short vegetative phase-like MADS-box genes inhibit floral meristem identity in barley. Plant Physiology 143, 225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turc O, Tardieu F. 2018. Drought affects abortion of reproductive organs by exacerbating developmentally driven processes via expansive growth and hydraulics. Journal of Experimental Botany 69, 3245–3254. [DOI] [PubMed] [Google Scholar]
- Turner A, Beales J, Faure S, Dunford RP, Laurie DA. 2005. The pseudo-response regulator Ppd-H1 provides adaptation to photoperiod in barley. Science 310, 1031–1034. [DOI] [PubMed] [Google Scholar]
- von Korff M, Grando S, Del Greco A, This D, Baum M, Ceccarelli S. 2008. Quantitative trait loci associated with adaptation to Mediterranean dryland conditions in barley. Theoretical and Applied Genetics 117, 653–669. [DOI] [PubMed] [Google Scholar]
- Waddington SR, Cartwright PM, Wall PC. 1983. A quantitative scale of spike initial and pistil development in barley and wheat. Annals of Botany 51, 119–130. [Google Scholar]
- Wang Y, Li L, Ye T, Lu Y, Chen X, Wu Y. 2013. The inhibitory effect of ABA on floral transition is mediated by ABI5 in Arabidopsis. Journal of Experimental Botany 64, 675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegmann M, Maurer A, Pham A, et al. 2019. Barley yield formation under abiotic stress depends on the interplay between flowering time genes and environmental cues. Scientific Reports 9, 6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigge PA, Kim MC, Jaeger KE, Busch W, Schmid M, Lohmann JU, Weigel D. 2005. Integration of spatial and temporal information during floral induction in Arabidopsis. Science 309, 1056–1059. [DOI] [PubMed] [Google Scholar]
- Wu F, Shi X, Lin X, Liu Y, Chong K, Theißen G, Meng Z. 2017. The ABCs of flower development: mutational analysis of AP1/FUL-like genes in rice provides evidence for a homeotic (A)-function in grasses. The Plant Journal 89, 310–324. [DOI] [PubMed] [Google Scholar]
- Xie W, Xiong W, Pan J, Ali T, Cui Q, Guan D, Meng J, Mueller ND, Lin E, Davis SJ. 2018. Decreases in global beer supply due to extreme drought and heat. Nature Plants 4, 964–973. [DOI] [PubMed] [Google Scholar]
- Zadoks JC, Chang TT, Konzak CF. 1974. A decimal code for the growth stages of cereals. Weed Research 14, 415–421. [Google Scholar]
- Zakhrabekova S, Gough SP, Braumann I, et al. 2012. Induced mutations in circadian clock regulator Mat-a facilitated short-season adaptation and range extension in cultivated barley. Proceedings of the National Academy of Sciences, USA 109, 4326–4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Liu J, Zhao T, et al. 2016. A drought-inducible transcription factor delays reproductive timing in rice. Plant Physiology 171, 334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.