Abstract
Objectives
To evaluate longitudinally the persistence of humoral immunity for up to 6 months in a cohort of hospital employees with mild coronavirus disease 2019 (COVID-19).
Methods
We measured anti-RBD (receptor binding domain of viral spike protein), anti-N (viral nucleoprotein) and neutralizing antibodies at 1, 3 and 6 months after mostly mild COVID-19 in 200 hospital workers using commercial ELISAs and a surrogate virus neutralization assay.
Results
Antibodies specific for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) persisted in all participants for up to 6 months. Anti-RBD geometric mean concentrations (GMCs) progressively increased between months 1 (74.2 U/mL, 95%CI: 62.7–87.8), 3 (103.2 U/mL, 95%CI: 87.9–121.2; p < 0.001), and 6 (123.3 U/mL, 95%CI: 103.4–147.0; p < 0.001) in the whole cohort. Anti-N antibodies were detectable in >97% at all times. Neutralizing antibodies were detectable in 99.5% of participants (195/196) at 6 months post infection. Their GMC progressively decreased between months 1 (20.1 AU/mL, 95%CI: 16.9–24.0), 3 (15.2 AU/mL, 95%CI: 13.2–17.6; p < 0.001) and 6 (9.4 AU/mL, 95%CI: 7.7–11.4; p < 0.001). RBD-ACE2-inhibiting antibody titres and anti-RBD antibody concentrations strongly correlated at each timepoint (all r > 0.86, p < 0.001). Disease severity was associated with higher initial anti-RBD and RBD-ACE2-inhibiting antibody titres, but not with their kinetics.
Conclusions
Neutralizing antibodies persisted at 6 months in almost all participants, indicating more durability than initially feared. Anti-RBD antibodies persisted better and even increased over time, possibly related to the preferential detection of progressively higher-affinity antibodies.
Keywords: Antibody persistence, COVID-19, Humoral immunity, Long-term immunity, Long-term protection, Persistence, SARS-CoV-2
Introduction
Most individuals infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) present self-limited disease (coronavirus disease 2019, COVID-19). Upon infection, SARS-CoV-2 elicits humoral responses, and within 3 weeks almost all infected patients develop antibodies against the receptor-binding domain (RBD) and the S1 and S2 domains of the spike (S) glycoprotein, as well as against the nucleocapsid protein (N) [[1], [2], [3], [4], [5], [6]]. Most also develop neutralizing antibodies [3,[5], [6], [7]]. Characterizing the breadth and the persistence of humoral responses over time in non-hospitalized SARS-CoV-2 individuals is of paramount importance in terms of public health, to assess the potential benefit of immunity and to design future preventive interventions. Lyer et al. showed that antibodies against the S-protein retained neutralizing capabilities and persisted for up to 75 days post infection in >95% of patients [6]. Recently, Gudbjartsson et al. showed that there was no sign of waning of antibody levels up to 4 months after infection [8]. On the other hand, some studies suggest a reduction of neutralizing capabilities during the early convalescent phase [9]. We have characterized humoral responses 6 months after the first pandemic wave in subjects with mostly mild COVID-19, as well as the host factors and disease patterns that have been implicated in impacting these responses [2,3,6,9,10].
Methods
Study design and participants
This prospective single-centre observational longitudinal study enrolled Geneva University Hospital (HUG) workers aged ≥18 years with SARS-CoV-2 infection detected by nasopharyngeal reverse-transcription polymerase chain reaction (RT-PCR). Exclusion criteria were the inability to provide informed consent (IC) and a period of >6 weeks between diagnosis and the first blood collection.
Study procedures
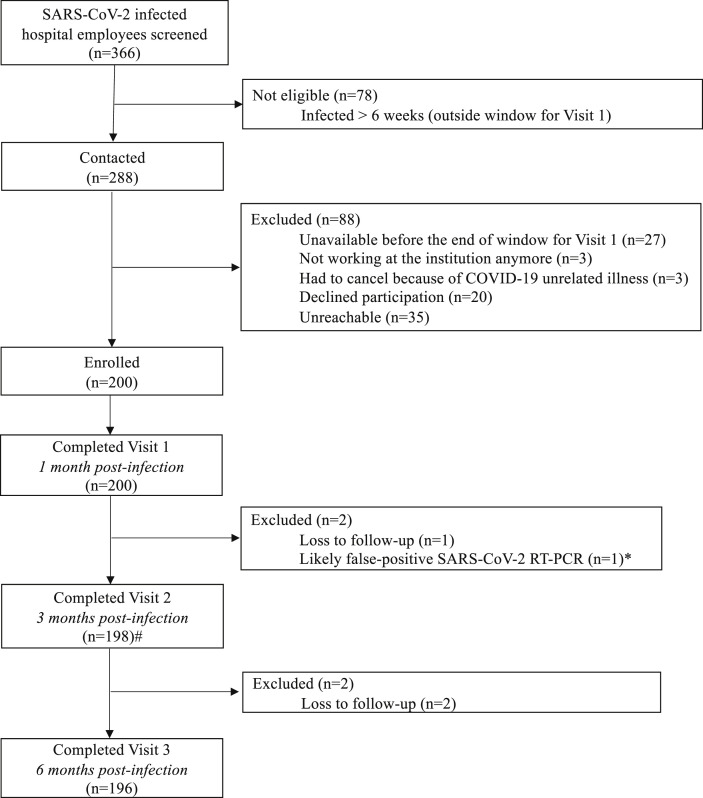
SARS-CoV-2-infected HUG workers were identified by Occupational Medicine through the hospital's surveillance network, which includes laboratory notification of SARS-CoV-2-positive specimens. Workers who agreed to participate and met the inclusion criteria signed the informed consent and then underwent blood draws 1, 3 and 6 months after diagnosis (Fig. 1 ). Participants' contact procedure and study visits are detailed in the Supplementary Material (Methods).
Fig. 1.
Study flowchart. COVID-19, coronavirus disease 2019; RT-PCR, reverse-transcription polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2. ∗One patient was diagnosed with COVID-19 by RT-PCR during the peak of the outbreak. Viral load was very low (cyclethreshold [CT] value 37). Her antibody response and her memory B-cells were negative at 1 month using flow cytometry. Repeattesting of the original diagnostic nasopharyngeal swab was negative.#missing data for two participants
RT-PCR and viral load at diagnosis
SARS-CoV-2 RT-PCR was performed according to the manufacturers' instructions on various platforms. Viral load (E-gene) was expressed in log10 of RNA copies/mL as previously described [11]. Further details about RT-PCR and viral load are provided in the Supplementary Material (Methods).
SARS-CoV-2 antibody detection
SARS-CoV-2 antibodies were measured using the quantitative Elecsys anti-RBD and semi-quantitative Elecsys anti-N (both measuring total immunoglobulin levels) on the cobas e801 analyser (Roche Diagnostics Rotkreuz, Switzerland). Results for the quantitative Elecsys anti-RBD antibodies are reported as concentrations (U/mL), with a manufacturer's cuf-off >0.8 U/mL considered as positive. Results for the Elecsys anti-N antibodies are reported as a cut-off index (signal sample/cut-off or signal calibrator), with values > 1 considered as positive. Quality controls and coefficients of variation for both assays are provided in Supplementary Material Table S1.
Neutralization tests
Neutralization tests were performed using a commercially available surrogate virus neutralization assay (sVNT) measuring RBD-ACE2 (angiotensin-converting enzyme 2) inhibition as a surrogate for neutralization (GenScript cPass™ SARS-CoV-2 Neutralization Antibody Detection Kit, Genscript, The Netherlands) and a plaque reduction neutralization test (PRNT). More details about the sVNT and PRNT are provided in the Supplementary Material (Methods).
Variables
Collected clinical and host variables are detailed in the Supplementary Material (Methods). To assess disease severity, a symptom density score was calculated by adding the duration of each acute symptom in days (Supplementary Material (Methods).
Ethical considerations
The study was approved by the local ethics committee (CCER 2020-00516) and registered (NCT04329546) prior to initiation.
Statistics
The sample size was not calculated; logistical and funding considerations defined a target of 200 participants. Antigen-specific antibodies, viral loads at diagnosis, and symptom density scores were skewed and thus log-transformed for all analyses. For anti-N geometric mean concentrations (GMCs), no statistical comparisons were performed because the upper range of the results were occasionally outside the linear range of the assay.
First, data were compared using T-tests on log-transformed values. Evolution of anti-RBD and RBD-ACE2-inhibiting antibody titres over time was assessed using paired T-tests. Correlation between anti-RBD and RBD-ACE2-inhibiting antibody titres was performed using Pearson correlation. Reverse cumulative distribution curves were generated to show the evolution of antibody concentrations among the cohort.
Next, we used latent growth modelling (LGM) to estimate growth trajectories [12]. More details about the LGM are provided in the Supplementary Material (Methods). LGMs were done for the 198 patients who completed at least two study visits (Fig. 1). We also ran one sensitivity analysis, using the number of symptoms instead of symptom density score, with results similar to those reported in the Results section. All analyses were performed with SPSS software v23.0 (IBM Corp., Armonk, NY, USA), except LGM, which was done with R 4.0.3 (package lavaan version 0.6–7).
Results
Demographics
Three hundred and sixty-six hospital workers diagnosed with SARS-CoV-2 infection by RT-PCR between 17th March and 15th April were screened for the study. Two hundred were enrolled in the present study and 98% (196/200) completed the 6-month follow-up (Fig. 1). Only five patients (2.5%) were briefly hospitalized but without severe complications. Demographics and comorbidities are described in Table 1 .
Table 1.
. Demographics of patients infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)
| SARS-CoV-2 infection (n = 200) | |
|---|---|
| Demographics | |
| Age, y, median (IQR) | 40.6 (30.2–51.7) |
| Male sex, n (%) | 58 (29.0) |
| Ethnicity, n (%) | |
| Caucasian | 163 (81.5) |
| Hispanic | 13 (6.5) |
| Mixed | 8 (4.0) |
| African | 6 (3.0) |
| Asian | 4 (2.0) |
| Others | 2 (1.0) |
| Not provided | 4 (2.0) |
| Comorbidities, n (%) | |
| Obesity (BMI ≥30) | 23 (11.5) |
| Asthma | 17 (8.5) |
| Hypertension | 9 (4.5) |
| Cancera | 7 (3.5) |
| Autoimmune diseaseb | 6 (3.0) |
| Diabetes | 3 (1.5) |
| Chronic lung diseasec | 3 (1.5) |
| Eczema | 3 (1.5) |
| Inflammatory bowel disease | 2 (1.0) |
| Hepatic disease | 2 (1.0) |
| Primary immune deficiency | 1 (0.5) |
| Othersd | 4 (2.0) |
| No past medical history | 140 (70.0) |
| Habits, n (%) | |
| Smoking | 23 (11.5) |
| Vaping | 9 (4.5) |
| Neither smoking nor vaping | 173 (86.5) |
BMI, body mass index; IQR, interquartile range.
Solid tumour (n = 5); leukaemia (n = 1); melanoma (n = 1).
Psoriasis (n = 2), hyperthydoidism (n = 1); hypothyroidism (n = 1); lupus (n = 1), ankylosing spondylitis (n = 1).
Chronic obstructive pulmonary disease (n = 1); emphysema (n = 1); pulmonary sarcoidosis (n = 1).
Endometriosis (n = 2); obstructive apnoea (n = 1); pacemaker (n = 1).
Of note, 70.0% of participants (140/200) were without medical comorbidities nor known risk factors for severe COVID-19. Clinical characteristics and symptoms of COVID-19 are described in Table 2 . Median symptom density scores were higher among hospital workers with one or more comorbidities compared to those without (48, IQR 29–68 versus 33, IQR 18–51; p 0.005).
Table 2.
Coronavirus disease 2019 (COVID-19) disease characteristics
| SARS-CoV-2 infection (n = 200) | ||
|---|---|---|
| Patient management, n (%) | ||
| Ambulatory care | 195 (97.5) | |
| Hospital admission to ward | 5 (2.5) | |
| Hospital admission to ICU | 0 | |
| Perceived severity, n (%) | ||
| Asymptomatic | 1 (0.5) | |
| Mild | 73 (36.5) | |
| Moderate | 102 (51.0) | |
| Severe | 22 (11.0) | |
| Very severe | 2 (1.0) | |
| Impact on daily life, n (%)a | ||
| 1 (no or mild impact) | 28 (14.0) | |
| 2 | 19 (9.5) | |
| 3 (moderate impact) | 80 (40.0) | |
| 4 | 48 (24.0) | |
| 5 (very important impact) | 25 (12.5) | |
| Reported symptoms | ||
| Median number of symptoms (IQR) | 9 (6–11) | |
| Median symptom density score (IQR), symptom-daysb | 36 (19–58) | |
| Symptoms | Frequency, n (%) | Median durations, days (IQR) |
| Acute | ||
| Myalgia | 147 (73.5) | 5 (3–8) |
| Headache | 142 (71.0) | 6 (3–10) |
| Cough | 126 (63.0) | 10 (4–17) |
| Fever | 123 (61.5) | 3 (2–6) |
| Nasal discharge | 110 (55.0) | 7 (3–10) |
| Chills | 107 (53.5) | 3 (1–4) |
| Dyspnoea | 87 (43.5) | 7 (4–15) |
| Diarrhoea | 76 (38.0) | 2 (1–5) |
| Arthralgia | 75 (37.5) | 5 (3–10) |
| Thoracic pain | 56 (28.0) | 6 (3–10) |
| Nausea | 50 (25.0) | 4 (2–6) |
| Dysphagia | 45 (22.5) | 5 (2–8) |
| Abdominal pain | 40 (20.0) | 3 (2–6) |
| Rash | 22 (11.0) | 6 (2–14) |
| Vomiting | 12 (6.0) | 2 (1–4) |
| Subacute | ||
| Fatigue | 174 (87.0) | 15 (8–21) |
| Anosmia | 138 (69.0) | 19 (10–38) |
| Dysgeusia | 133 (66.5) | 14 (7–30) |
| Others | Frequency, n (%) | Kgs, median (IQR) |
| Weight loss | 81 (40.5) | 3 (2–4) |
| Mean viral load, SD (log10 copies/mL) | 6.8 ± 1.7 | |
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; ICU, intensive care unit; IQR, interquartile range.
Impact on daily life was assessed using scales with values in the range 1–5.
Symptom density score is the product of the total number of acute symptoms and the total duration (days) of each symptom.
Anti-RBD antibody responses
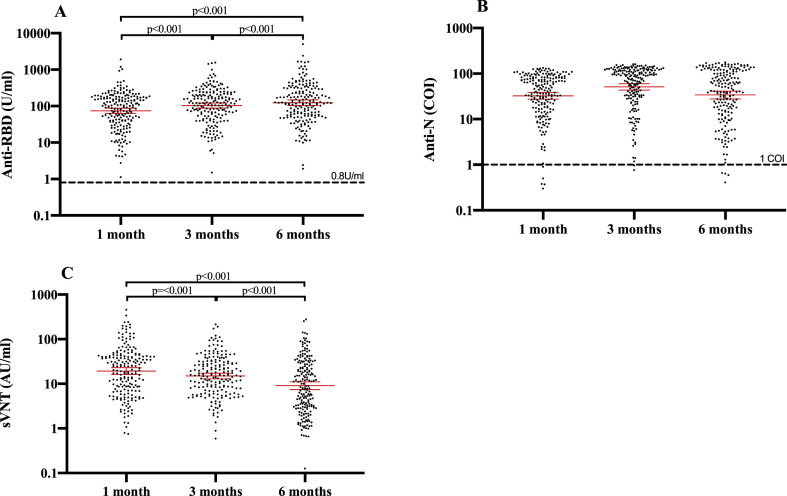
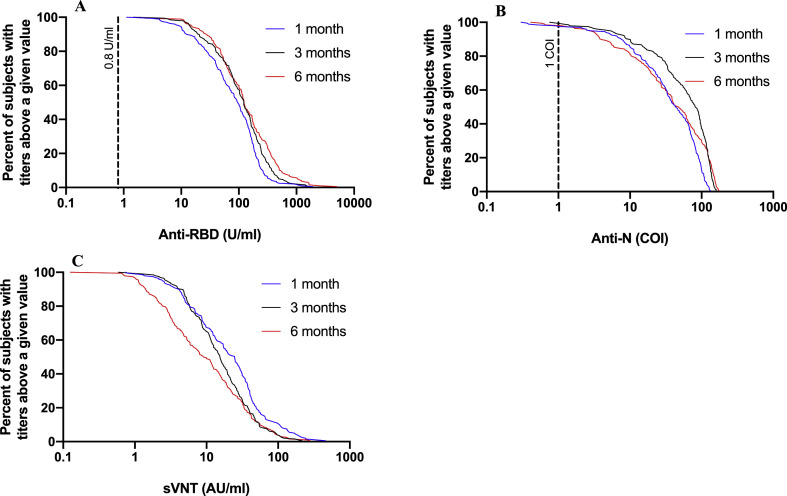
At 1, 3 and 6 months, all participants had detectable anti-RBD antibodies (Fig. 2 A). Anti-RBD GMCs increased progressively and significantly at each of the three visits (Fig. 2A): from 74.2 U/mL (95%CI: 62.7–87.8) at month 1 to 103.2 U/mL (95%CI: 87.9–121.2; p < 0.001) at month 3 and 123.3 U/mL (95%CI: 103.4–147.0; p < 0.001) at month 6. Anti-RBD antibodies increased over time among the whole cohort (Fig. 3 A). At 6 months, 36.7% of participants (72/196) showed anti-RBD values (U/mL) that were at least two-fold higher than at 1 month, while only 4.6% (9/196) had two-fold lower values, and anti-RBD antibodies remained stable (0.5–2-fold change) for 58.7% of participants (115/196) (Supplementary Material Fig. S1A).
Fig. 2.
Evolution of (A) anti-RBD (anti-receptor binding domain of viral spike protein), (B) anti-N (anti-viral nucleoprotein), and (C) surrogate virus neutralization assay (sVNT) between 1, 3 and 6 months following infection by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). COI, cut-off index; RT-PCR, reverse-transcription polymerase chain reaction. The bars represent the geometric mean concentration (GMC) with 95% confidence interval. The dashed lines represent the respective assays cut-offs.
Fig. 3.
Reverse cumulative distribution curves for (A) anti-RBD (anti-receptor binding domain of viral spike protein), (B) anti-N (anti-viral nucleoprotein), and (C) surrogate virus neutralization assay (sVNT) at 1, 3 and 6 months following infection by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). COI, cut-off index; RT-PCR, reverse-transcription polymerase chain reaction. The dashed lines represent the respective assays cut-offs.
In a latent growth model without covariates, anti-RBD concentrations increased with time (estimate = 0.040, 95%CI: 0.030; 0.050; p < 0.001), and their change over time differed among patients (estimate = 0.003, 95%CI: 0.001; 0.005; p 0.004) (Supplementary Material Fig. S1A). The level of anti-RBD antibodies at 1 month did not predict their kinetics in the following 5 months (estimate = –0.005, 95%CI: –0.011; 0.001; p 0.088).
Anti-N antibody responses
At 1, 3 and 6 months, 97.5% (194/199), 99.0% (194/196) and 98.0% (192/196) of participants had detectable anti-N antibodies, respectively (Fig. 2B). Anti-N GMCs progressively increased between months 1 (32.6, 95%CI: 27.5–38.6) and 3 (51.1, 95%CI: 43.5–60.1) and subsequently decreased between months 3 and 6 (34.0, 95%CI: 28.0–41.3) (Fig. 2B). Anti-N antibodies peaked at 3 months (Fig. 3B).
Neutralizing capacity
At 1, 3 and 6 months, the level of neutralizing antibodies was assessed in all patients by sVNT that measures inhibition of RBD-ACE2 binding. To validate whether sVNT is a good surrogate for neutralizing antibody titres, we measured the neutralizing activity of a selected set of 45 serum samples ranging from 0.13 to 279.3 AU/mL using a plaque reduction neutralization test (PRNT) with a cut-off at 90% or 50% reduction (PRNT90 or PRNT50, respectively). Overall, we found a very high correlation between sVNT and PRNT (r 0.936; p < 0.0001), but also noticed that for low RBD-ACE2-inhibiting antibody titres (AU/mL < 5) the sVNT did not predict the presence of neutralizing antibodies accurately (Supplementary Material Fig. S2). GMCs measured by sVNT progressively decreased between months 1 (19.2 AU/mL, 95%CI: 16.1–22.9) and 3 (15.0 AU/mL, 95%CI: 12.9–17.3; p < 0.001) and between months 3 and 6 (9.1 AU/mL, 95%CI: 7.4–11.1; p < 0.001) (Fig. 2C). RBD-ACE2-inhibiting antibody titres decreased over time among the whole cohort (Fig. 3C). At 6 months, 7.7% of participants (15/196) showed RBD-ACE2-inhibiting antibody titres that were at least two-fold higher than at 1 month, while 58.2% (114/196) had two-fold lower titres and RBD-ACE2-inhibiting antibodies remained stable (0.5–2-fold change) for 34.2% of participants (67/196) (Supplementary Material Fig. S1B). To reliably determine the proportion of patients still showing neutralizing activity at 6 months post infection, we tested all samples with a titre of <10 AU/mL using the PRNT. Only one participant did not have a PRNT50 titre of ≥10, and 14 subjects did not have a PRNT90 titre of ≥10. Based on our correlation analysis, we assumed that all subjects with an RBD-ACE2-inhibiting antibody titre of ≥10 AU/mL also showed neutralizing activity in the PRNT. Therefore, 92.9% (182/196, PRNT90) and 99.5% of participants (195/196, PRNT50) retained functional neutralizing antibodies at 6 months post infection.
The latent growth model without covariates indicated that RBD-ACE2-inhibiting antibody titres decreased with time (estimate = –0.066, 95%CI: –0.078; –0.053, p < 0.001), but their change over time did not significantly differ among participants (estimate = –0.002, 95%CI: –0.006; 0.001, p 0.207) (Supplementary Material Fig. S1B). The decrease in RBD-ACE2-inhibiting antibody titres was faster for participants with higher initial concentrations (estimate = 0.009, 95%CI: 0.000; 0.018, p 0.048).
RBD-ACE2-inhibiting and anti-RBD antibody titres strongly correlated at 1 (r 0.877; p < 0.001), 3 (r 0.865; p < 0.001) and 6 months (r 0.893; p < 0.001) (Supplementary Material Fig. S3A-C).
Host and clinical factors associated with persistence of humoral responses
The symptom density score was significantly and positively associated with the concentration of anti-RBD (p 0.001) and RBD-ACE2-inhibiting antibody titres (p 0.004) 1 month after diagnosis, indicating higher antibodies in those with more symptoms (Table 3 ). However, the symptom density score did not impact the kinetics of the anti-RBD and RBD-ACE2-inhibiting antibody responses (Table 3). The inclusion of persistent symptoms (anosmia, dysgeusia and fatigue) generated similar results (data not shown). Similarly, analyses of specific symptoms individually (cough, fever) did not impact the kinetics of the anti-RBD and RBD-ACE2-inhibiting antibody responses (data not shown). We observed faster anti-RBD increase in women than in men (p 0.010) (Table 3).
Table 3.
Parameter estimates of latent growth models for anti-RBD and sVNT
| Covariates | Bivariate models |
Multivariate model |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial antibody levels |
Evolution of antibody levels |
Initial antibody levels |
Evolution of antibody levels |
|||||||||
| Estimate | 95%CI | p | Estimate | 95%CI | p | Estimate | 95%CI | p | Estimate | 95%CI | p | |
| Anti-RBD | ||||||||||||
| Sex (ref. female) | 0.114 | –0.044; 0.272 | 0.157 | –0.028 | –0.049; –0.007 | 0.008 | 0.116 | –0.041; 0.273 | 0.147 | –0.028 | –0.049; –0.007 | 0.010 |
| Age | <0.001 | –0.006; 0.007 | 0.984 | <0.001 | –0.001; 0.001 | 0.955 | –0.003 | –0.010; 0.004 | 0.381 | <0.001 | –0.001; 0.001 | 0.668 |
| Any comorbidity | 0.106 | –0.049; 0.262 | 0.181 | 0.008 | –0.016; 0.026 | 0.631 | 0.012 | –0.148; 0.172 | 0.882 | 0.016 | –0.005; 0.038 | 0.134 |
| Viral load | 0.020 | –0.026; 0.065 | 0.395 | 0.008 | –0.001; 0.011 | 0.139 | 0.006 | –0.039; 0.050 | 0.804 | 0.005 | –0.001; 0.011 | 0.105 |
| Symptom density score |
0.284 |
0.113; 0.454 |
0.001 |
<0.001 |
–0.023; 0.024 |
0.979 |
0.310 |
0.129; 0.490 |
0.001 |
–0.013 |
–0.037; 0.011 |
0.292 |
| sVNT | ||||||||||||
| Sex (ref. female) | 0.096 | –0.042; 0.235 | 0.173 | 0.104 | –0.036; 0.244 | 0.144 | ||||||
| Age | 0.005 | –0.001; 0.011 | 0.083 | 0.002 | –0.004; 0.008 | 0.443 | ||||||
| Any comorbidity | 0.170 | 0.035; 0.306 | 0.014 | 0.110 | –0.032; 0.252 | 0.130 | ||||||
| Viral load | 0.021 | –0.020; 0.062 | 0.320 | 0.007 | –0.033; 0.047 | 0.734 | ||||||
| Symptom density score | 0.252 | 0.102; 0.402 | 0.001 | 0.239 | 0.078; 0.399 | 0.004 | ||||||
RBD, receptor binding domain of spike protein; sVNT, surrogate virus neutralization assay; CI, confidence interval.
Discussion
Antibodies against SARS-CoV-2 persisted in all of 200 hospital workers at least 6 months after documented mild COVID-19. Initial studies demonstrated a rapid decrease in IgG concentrations against both S- and N-proteins in the first 2 months following infection [9,13], while recent large-scale studies have shown antibody persistence for up to 4 months after diagnosis [6,8]. That antibody persistence can now be extended to 6 months, with stable or slowly decreasing antibody concentrations, is thus promising given the traditional importance of antibodies for protection.
An initial peak and early decline of antibodies is often observed following infection or immunization, as most short-lived antibody-secreting plasmablasts responsible for the early antibody peak have died by month 3 [14]. At month 6 and thereafter, antibody production essentially results from long-lived plasma cells responsible for the longer-term persistence of antigen-specific antibodies. Unexpectedly, anti-RBD titres increased over time, which contrasts with other recent publications that found a slight decrease in anti-RBD antibodies [6,15]. This could have several explanations. First, the assay used in this study measures total Ig instead of only IgG. Second, the anti-RBD assay intrinsically favours detection of higher-affinity antibodies [16]. Therefore, the increase in signal in the RBD assay could reflect antibody affinity maturation over time, while the total amount of anti-RBD antibodies actually remains stable or even decreases slightly. Similar results were observed after yellow fever vaccination, where affinity maturation increased over 6–9 months despite a slight decrease in serum neutralization titres [17]. Finally, a boosting effect secondary to re-exposure to SARS-CoV-2 is very unlikely to explain the increase in anti-RBD concentrations seen in most participants, given the relatively low circulation of the virus between month 1 and month 6.
Thus, we interpret the strong persistence/increase of anti-RBD antibodies as reflecting the detection of progressively higher-affinity antibodies over time.
Interestingly, the kinetics of anti-N antibodies differed from those of anti-RBD, following the traditional pattern of an early peak followed by a decline, thus suggesting differences in long-lived plasma-cell activation by surface (RBD) or internal (N) antigens.
As neutralizing antibodies can block viral entry into cells, their presence reflects an important (although not exhaustive) part of antibody functionality. Published data show that antibodies against SARS-CoV-2 retain their neutralizing capabilities until 75 days [6] and in a smaller sample until 7 months [18] post-infection. In our study, although RBD-ACE2-inhibiting antibody titres declined between 1 and 6 months, 92.9% or 99.5% of participants (PRNT90 or PRNT50, respectively) retained neutralizing capabilities at 6 months post-infection. However, since no correlate of protection has been established for SARS-CoV-2 infection, it remains unknown which neutralizing titre protects patients from reinfection or disease. We observed a significant covariance between the initial concentrations and the subsequent decrease in RBD-ACE2-inhibiting antibodies—i.e. a faster decrease in participants with higher concentrations at 1 month—as expected given the early but transient contribution of short-lived plasmablasts. Thus, anti-SARS-CoV-2 RBD-ACE2-inhibiting antibodies peak earlier (at 1 month) [9] than anti-SARS-CoV-1 neutralizing antibodies, which peak at 4 months post infection [19]. At the individual level, anti-RBD concentrations were strongly correlated with RBD-ACE2-inhibiting antibody concentrations. The strong correlation between RBD-ACE2-inhibiting antibody titres and anti-RBD concentrations most likely reflects the fact that the former are essentially directed against the RBD [20]. Importantly, however, our findings indicate that the Roche assay cannot be used directly as a surrogate for neutralizing antibodies in individuals as it shows an increase in concentrations despite a decrease in neutralization capacity.
In our dataset there was a positive association between disease severity, expressed as a symptom density score, and initial anti-RBD and RBD-ACE2-inhibiting antibody titres. However, there was no association between symptom density score and the evolution of anti-RBD and RBD-ACE2-inhibiting antibody titres over time, suggesting that disease severity impacts the initial magnitude but not the persistence of antibodies. These data, which are in line with data showing weaker humoral responses among pauci- or asymptomatic patients [2,6,9,10], are best explained by the strong activation of short-lived plasmablasts by inflammation/prolonged disease.
This study has limitations. First, most participants had mild disease as per the World Health Organization's criteria [21]. Therefore, antibody kinetics may differ in patients with severe COVID-19. However, as mild infections represent the vast majority of cases [21], their contribution to protection in the community is the most important. Second, hospital workers were first seen 1 month after diagnosis, so that one cannot exclude a recall bias in terms of symptom reporting. However, our design allowed the capture of protracted symptoms that occurred after RT-PCR diagnosis, and the symptoms density scores correlated well with antibody responses. As the duration of symptoms before diagnosis was not registered, associations between viral load and humoral responses might have been missed given the rapid decline in viral load in the first few days [22]. However, the vast majority of our hospital workers were likely tested within the first 24–48 hours following symptom onset. Third, because of the modest sample size, we could not add more variables to the multivariate models. Further studies with larger numbers of participants are needed to achieve a better understanding of the role of host and clinical factors on antibody levels and their change over time. In addition, we did not investigate the presence of memory B and T cells, which can also contribute to the persistence of immunity. Last, but not least, this study used a surrogate assay for the detection of neutralizing antibodies which reliably measures RBD-directed neutralizing antibodies but not neutralizing antibodies directed against the S2 or N-terminal domain of the S protein [23,24]. However, our own validation dataset as well as those of other studies have shown a high correlation between cell-based neutralization assays and the surrogate assay used in this study to enable high-throughput analyses [25,26].
This study shows the persistence of several SARS-CoV-2 antigen-specific antibodies 6 months following COVID-19. It shows that almost all patients with mild COVID-19 retain neutralizing antibodies at 6 months after infection. Although this is reassuring, antibody concentrations required to protect against reinfection are not established. Thus, large follow-up studies are needed to establish correlates of protection against reinfection and/or disease and the duration of antibody-mediated protection.
Author contributions
AGL’H, CSE, AH, KMP-B, NV, SY, CAS and LK designed the study. AGL’H, BM, DOA, IA-V, SB, IE, CG-S, ISR, JAP, NV and SY acquired the data. AGL’H, BM, DOA, IA-V, SB, AD, CSE, IE, AH, ISR, NV, SY, CAS and LK interpreted the data. AGL’H, BM, DOA, SB, AH, SY, CAS and LK drafted the manuscript. All authors critically revised the manuscript for its intellectual content and approved the submitted version. All authors had full access to all the data in the study and agree to be accountable for all aspects of the work. AGL’H and SB verified the underlying data.
Transparency declaration
The authors have no conflicts of interest to declare. The study was funded by the Fondation de Bienfaisance du Groupe Pictet, the Fondation Ancrage, the Fondation Privée des HUG, the Ernst and Schmidheiny foundation, the Geneva University Hospitals PRD grant, the Center for Vaccinology and the Centre for Emerging Viral Diseases.
Data sharing statement
Study protocols, statistical analysis plan and individual participant data after de-indentification that underlie the results reported in this article can be made available after de-identification to any reasearcher who makes a methodologically sound proposal. Proposals should be directed to arnaud.lhuillier@hcuge.ch. To gain access, data requestors will need to sign a data access agreement.
Acknowledgements
We would like to thank Olivier Golaz and Laure Vieux for their technical support and Erik Boehm for revising the manuscript. We would also like to thank the Platerforme de Recherche Clinique en Pediatrie for its logistical support throughout the study.
Editor: F. Allerberger
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2021.01.005.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Grzelak L., Temmam S., Planchais C., Demeret C., Tondeur L., Huon C. A comparison of four serological assays for detecting anti-SARS-CoV-2 antibodies in human serum samples from different populations. Sci Transl Med. 2020;12 doi: 10.1126/scitranslmed.abc3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao J., Yuan Q., Wang H., Liu W., Liao X., Su Y. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.To K.K., Tsang O.T., Leung W.S., Tam A.R., Wu T.C., Lung D.C. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vabret N., Britton G.J., Gruber C., Hegde S., Kim J., Kuksin M. Immunology of COVID-19: current state of the science. Immunity. 2020;52:910–941. doi: 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fafi-Kremer S., Bruel T., Madec Y., Grant R., Tondeur L., Grzelak L. Serologic responses to SARS-CoV-2 infection among hospital staff with mild disease in eastern France. EBioMedicine. 2020;59:102915. doi: 10.1016/j.ebiom.2020.102915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iyer A.S., Jones F.K., Nodoushani A., Kelly M., Becker M., Slater D. Persistence and decay of human antibody responses to the receptor binding domain of SARS-CoV-2 spike protein in COVID-19 patients. Sci Immunol. 2020;5 doi: 10.1126/sciimmunol.abe0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ju B., Zhang Q., Ge J., Wang R., Sun J., Ge X. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature. 2020;584:115–119. doi: 10.1038/s41586-020-2380-z. [DOI] [PubMed] [Google Scholar]
- 8.Gudbjartsson D.F., Norddahl G.L., Melsted P., Gunnarsdottir K., Holm H., Eythorsson E. Humoral immune response to SARS-CoV-2 in Iceland. N Engl J Med. 2020 doi: 10.1056/NEJMoa2026116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long Q.X., Tang X.J., Shi Q.L., Li Q., Deng H.J., Yuan J. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26:1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 10.Jiang C., Wang Y., Hu M., Wen L., Wen C., Wang Y. Antibody seroconversion in asymptomatic and symptomatic patients infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Transl Immunol. 2020;9 doi: 10.1002/cti2.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baggio S., L’Huillier A.G., Yerly S., Bellon M., Wagner N., Rohr M. SARS-CoV-2 viral load in the upper respiratory tract of children and adults with early acute COVID-19. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Preacher K.J., Wichman A.L., MacCallum R.C., Briggs N.E. 1st ed. SAGE Publications; 2008. Latent growth curve modeling. [Google Scholar]
- 13.Adams E.R., Ainsworth M., Anand R., Andersson M.I., Auckland K., Baillie J.K. Antibody testing for COVID-19: a report from the national COVID Scientific Advisory Panel. medRxiv. 2020 doi: 10.1101/2020.04.15.20066407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith K.G., Hewitson T.D., Nossal G.J., Tarlinton D.M. The phenotype and fate of the antibody-forming cells of the splenic foci. Eur J Immunol. 1996;26:444–448. doi: 10.1002/eji.1830260226. [DOI] [PubMed] [Google Scholar]
- 15.Ibarrondo F.J., Fulcher J.A., Goodman-Meza D., Elliott J., Hofmann C., Hausner M.A. Rapid decay of anti-SARS-CoV-2 antibodies in persons with mild covid-19. N Engl J Med. 2020;383:1085–1087. doi: 10.1056/NEJMc2025179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elecsys® Roche. Anti-SARS-CoV-2 S. https://diagnostics.roche.com/global/en/products/params/elecsys-anti-sars-cov-2-s.html Available from:
- 17.Wec A.Z., Haslwanter D., Abdiche Y.N., Shehata L., Pedreno-Lopez N., Moyer C.L. Longitudinal dynamics of the human B cell response to the yellow fever 17D vaccine. Proc Natl Acad Sci USA. 2020;117:6675–6685. doi: 10.1073/pnas.1921388117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ripperger T.J., Uhrlaub J.L., Watanabe M., Wong R., Castaneda Y., Pizzato H.A. Orthogonal SARS-CoV-2 serological assays enable surveillance of low prevalence communities and reveal durable humoral immunity. Immunity. 2020 doi: 10.1016/j.immuni.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin Q., Zhu L., Ni Z., Meng H., You L. Duration of serum neutralizing antibodies for SARS-CoV-2: lessons from SARS-CoV infection. J Microbiol Immunol Infect. 2020 doi: 10.1016/j.jmii.2020.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barnes C.O., West A.P., Jr., Huey-Tubman K.E., Hoffmann M.A.G., Sharaf N.G., Hoffman P.R. Structures of human antibodies bound to SARS-CoV-2 spike reveal common epitopes and recurrent features of antibodies. Cell. 2020;182:828–842. doi: 10.1016/j.cell.2020.06.025. e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization Clinical management of COVID-19—interim guidance 27 May 2020. https://www.who.int/publications/i/item/clinical-management-of-covid-19 Available from:
- 22.Wolfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Muller M.A. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 23.Chi X., Yan R., Zhang J., Zhang G., Zhang Y., Hao M. A neutralizing human antibody binds to the N-terminal domain of the spike protein of SARS-CoV-2. Science. 2020;369:650–655. doi: 10.1126/science.abc6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duan J., Yan X., Guo X., Cao W., Han W., Qi C. A human SARS-CoV neutralizing antibody against epitope on S2 protein. Biochem Biophys Res Commun. 2005;333:186–193. doi: 10.1016/j.bbrc.2005.05.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyer B., Reimerink J., Torriani G., Brouwer F., Godeke G.J., Yerly S. Validation and clinical evaluation of a SARS-CoV-2 surrogate virus neutralisation test (sVNT) Emerg Microbe. Infect. 2020:1–18. doi: 10.1080/22221751.2020.1835448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan C.W., Chia W.N., Qin X., Liu P., Chen M.I., Tiu C. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein–protein interaction. Nat Biotechnol. 2020;38:1073–1078. doi: 10.1038/s41587-020-0631-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.