Abstract
Background
Haematopoietic stem-cell transplantation (HSCT) recipients are considered at high risk of poor outcomes after COVID-19 on the basis of their immunosuppressed status, but data from large studies in HSCT recipients are lacking. This study describes the characteristics and outcomes of HSCT recipients after developing COVID-19.
Methods
In response to the pandemic, the Center for International Blood and Marrow Transplant Research (CIBMTR) implemented a special form for COVID-19-related data capture on March 27, 2020. All patients—irrespective of age, diagnosis, donor type, graft source, or conditioning regimens—were included in the analysis with data cutoff of Aug 12, 2020. The main outcome was overall survival 30 days after a COVID-19 diagnosis. Overall survival probabilities were calculated using Kaplan-Meier estimator. Factors associated with mortality after COVID-19 diagnosis were examined using Cox proportional hazard models.
Findings
318 HSCT recipients diagnosed with COVID-19 were reported to the CIBMTR. The median time from HSCT to COVID-19 diagnosis was 17 months (IQR 8–46) for allogeneic HSCT recipients and 23 months (8–51) for autologous HSCT recipients. The median follow-up of survivors was 21 days (IQR 8–41) for allogeneic HSCT recipients and 25 days (12–35) for autologous HSCT recipients. 34 (18%) of 184 allogeneic HSCT recipients were receiving immunosuppression within 6 months of COVID-19 diagnosis. Disease severity was mild in 155 (49%) of 318 patients, while severe disease requiring mechanical ventilation occurred in 45 (14%) of 318 patients—ie, 28 (15%) of 184 allogeneic HSCT recipients and 17 (13%) of 134 autologous HSCT recipients. At 30 days after the diagnosis of COVID-19, overall survival was 68% (95% CI 58–77) for recipients of allogeneic HSCT and 67% (55–78) for recipients of autologous HSCT. Age 50 years or older (hazard ratio 2·53, 95% CI 1·16–5·52; p=0·020); male sex (3·53; 1·44–8·67; p=0·006), and development of COVID-19 within 12 months of transplantation (2·67, 1·33–5·36; p=0·005) were associated with a higher risk of mortality among allogeneic HSCT recipients, and a disease indication of lymphoma was associated with a higher risk of mortality compared with plasma cell disorder or myeloma (2·41, [1·08–5·38]; p=0·033) in autologous HSCT recipients.
Interpretation
Recipients of autologous and allogeneic HSCT who develop COVID-19 have poor overall survival. These data emphasise the need for stringent surveillance and aggressive treatment measures in HSCT recipients who develop COVID-19.
Funding
American Society of Hematology; Leukemia and Lymphoma Society; National Cancer Institute; National Heart, Lung and Blood Institute; National Institute of Allergy and Infectious Diseases; National Institutes of Health; National Cancer Institute; Health Resources and Services Administration; Office of Naval Research.
Introduction
COVID-19, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was declared a pandemic by WHO in March, 2020. By September, 2020, the disease had caused 1 million deaths worldwide. Patients with cancer have at least a two-times higher risk of COVID-19-associated intensive care unit admission, invasive ventilation, and death compared with the general population.1, 2, 3, 4, 5, 6, 7 Haematopoietic stem-cell transplantation (HSCT) recipients might be an especially vulnerable group due to nascent immune systems or organ impairment from treatment-related toxicities, specifically with respect to infection-related and respiratory complications. To date, data on outcomes of HSCT recipients with COVID-19 are limited to small case series and single-centre experiences.8, 9, 10, 11, 12 Better characterisation of HSCT patients infected with SARS-CoV-2 is needed. Here we describe the clinical characteristics, treatment patterns, and factors associated with outcomes of HSCT recipients who developed COVID-19 and were reported to the Center for International Blood and Marrow Transplant Research (CIBMTR).
Research in context.
Evidence before this study
For patients with cancer, the risk of COVID-19-associated intensive care unit admission, invasive ventilation, and death is twice that of the general population. However, data on outcomes of haematopoietic stem-cell transplantation (HSCT) recipients with COVID-19 are lacking. We searched PubMed for studies published between Dec 1, 2019, and Oct 19, 2020, using the terms “COVID”, “hematopoietic cell transplant”, and “bone marrow transplant”. No language restrictions were used. We identified four cohort studies reporting outcomes after COVID-19 infection in HSCT recipients. These studies reported high mortality in HSCT recipients after COVID-19. Older age, progressive disease, patients with active graft versus host disease, and patients receiving steroids were associated with higher mortality. However, all of these studies comprised modest numbers of patients, and did not have power to perform robust statistical analysis to assess factors associated with outcomes.
Added value of this study
To our knowledge, our study is the largest series to date summarising the clinical characteristics and outcomes of HSCT recipients with COVID-19. Using this relatively large cohort, we confirmed associations noted in the previous studies, and tested these associations for allogeneic and autologous HSCT recipients separately. We show that the estimated 30-day overall survival after COVID-19 diagnosis was poor, at 68% (95% CI 58–77) for allogeneic HSCT recipients. Although older age has previously been shown to be associated with worse outcomes, the age cutoff was even lower for HSCT recipients, probably representing the additional burden of toxicity among HSCT survivors. We also found an almost four-times increased risk of death among male recipients of allogeneic HSCT.
Implications of all the available evidence
Our results are consistent with, and extend, existing evidence suggesting higher mortality among HSCT recipients who developed COVID-19. Our data show a high risk of death even in patients who were more than a year from transplantation and patients not receiving immunosuppression. These data underscore the need for more stringent surveillance and pre-emptive measures for all HSCT recipients. Future research should focus on specific therapies that have been identified to improve outcomes in the general population, including the impact of vaccination in HSCT recipients.
Methods
Study design and participants
The CIBMTR is an outcome registry receiving data from more than 350 transplantation centres from 32 countries, which collect data on autologous HSCT, allogeneic HSCT, and other cellular therapies. Participating centres are required to report all transplantations consecutively, with long-term follow-up. The CIBMTR ensures data quality through computerised checks for discrepancies, physicians' review of submitted data, and onsite audits. CIBMTR staff members visit and audit sites to monitor reporting compliance. The CIBMTR complies with US federal regulations that protect research participants. All patients provide written informed consent for data use.
In response to the COVID-19 pandemic, a separate form to collect data on SARS-CoV-2 was implemented by the CIBMTR on March 27, 2020. This form was in addition to the robust data collection system wherein all the remaining patient-related, disease-related, and transplantation-related data were already being collected. All participating centres were requested to report data on any HSCT recipients with COVID-19. We included all patients who had HSCT and were reported to the CIBMTR as having COVID-19 up to Aug 12, 2020. All patient ages, diagnoses, donor types, graft sources, and conditioning regimens were included. See appendix pp 1–4 for the study protocol.
Outcomes
The primary outcome of this analysis was overall survival 30 days after a COVID-19 diagnosis. COVID-19 diagnosis was determined by the centre, and diagnostic method was requested. The severity of COVID-19 was defined as mild (no oxygen supplementation), moderate (supplemental oxygen needed), or severe (mechanical ventilation required). Duration of disease was defined as time from diagnosis to infection resolution or death, and infection status at last follow-up was as reported by the transplantation centre. Infection status was defined as ongoing if there were no signs of clinically significant improvement, improved if the patient remained on treatment but the signs and symptoms of infection were resolved, and resolved if all signs and symptoms were resolved and the patient had completed the planned course of treatment for the infection. We did not collect information if the patients were retested at the completion of treatment course. Surviving patients were censored at last follow-up. Time from HSCT to COVID-19 infection, time from acute and chronic graft-versus-host disease (GVHD) onset to COVID-19 infection, and cause of death were also predefined outcomes. GVHD outcomes will be reported elsewhere.
Statistical analysis
Descriptive statistics for the patient-related, disease-related, and transplantation-related variables were reported using frequency and percentages for categorical variables and median, range, and IQRs for continuous variables. Cox proportional hazard models were created to study the effect of various factors associated with mortality after COVID-19 diagnosis. Estimates are reported with 95% CIs. Separate models were created for recipients of allogeneic HSCT and autologous HSCT. Variables tested included: age at transplantation (dichotomised as <50 years and ≥50 years), sex (male vs female), race (White vs others [ie, African American, Asian, Pacific islander, Native American, more than one race]), ethnicity (Hispanic vs non-Hispanic vs non-US resident), time from HSCT to the diagnosis of COVID-19, immunosuppression within 6 months of COVID-19 diagnosis, conditioning intensity (myeloablative vs reduced intensity or non-myeloablative), and development of acute or chronic GVHD before COVID-19 diagnosis. For autologous HSCT, the effect of transplantation indication (myeloma vs lymphoma) was also examined. A stepwise model building approach was used, and variables that attained a p value of less than 0·05 were considered statistically significant and were held in the final multivariable model. All variables included in the final model met the assumptions for proportionality and we did not detect any first order interactions. The probabilities of overall survival were calculated using the Kaplan-Meier method. SAS, version 9.4, was used for all analyses.
Role of the funding source
The funding sources had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the Article for publication. All authors had full access to all data and the corresponding author had final responsibility for the decision to submit for publication.
Results
318 HSCT recipients diagnosed with COVID-19 were reported to the CIBMTR between March 27, 2020, and Aug 12, 2020. Baseline clinical characteristics are shown in table 1 . The majority (276 [87%] of 318) of the cohort was comprised of HSCT recipients from the USA with additional patients reported from five other countries. At the time of transplantation, 175 (55%) of 318 patients had a Lansky/Karnofsky performance score of 90% or more. The median time from diagnosis to allogeneic HSCT was 8 months (IQR 5–19). 140 (76%) of 184 grafts were derived from peripheral blood stem cells (PBSCs), 66 [36%] of 184 were obtained primarily from HLA-matched siblings, and 49 [27%] of 184 were from well matched unrelated donors. For GVHD prophylaxis, 90 (49%) of 184 patients received a calcineurin inhibitor with methotrexate, and 34 (18%) of 184 received post-transplantation cyclophosphamide. Plasma cell disorders (86 [64%] of 134) or lymphoma (41 [31%] of 134) were the most common indications for autologous HSCT and the median time from diagnosis to transplantation was 9 months (IQR 6–18).
Table 1.
Characteristics of hematopoietic stem cell transplantation recipients with COVID-19 diagnosis
| Allogeneic HSCT (n=184) | Autologous HSCT (n=134) | ||
|---|---|---|---|
| Number of centres | 76 | 64 | |
| Region | |||
| USA, northeast | 73 (40%) | 58 (43%) | |
| USA, midwest | 32 (17%) | 26 (19%) | |
| USA, south | 30 (16%) | 24 (18%) | |
| USA, west | 18 (10%) | 15 (11%) | |
| Canada | 4 (2%) | 3 (2%) | |
| Europe | 3 (2%) | 0 | |
| Central or South America | 20 (11%) | 7 (5%) | |
| Middle East or Africa | 4 (2%) | 1 (1%) | |
| Age, years | 47 (30–60) | 60 (49–65) | |
| ≤10 | 15 (8%) | 3 (2%) | |
| 11–20 | 11 (6%) | 0 | |
| 21–40 | 53 (29%) | 12 (9%) | |
| 41–60 | 65 (35%) | 61 (46%) | |
| ≥61 | 40 (22%) | 58 (43%) | |
| Sex | |||
| Female | 77 (42%) | 53 (40%) | |
| Male | 107 (58%) | 81 (60%) | |
| Race | |||
| White | 141 (77%) | 74 (55%) | |
| African-American | 13 (7%) | 33 (25%) | |
| Asian | 7 (4%) | 7 (5%) | |
| Pacific Islander | 0 | 1 (1%) | |
| Native American | 1 (1%) | 0 | |
| More than one race | 1 (1%) | 0 | |
| Missing | 21 (11%) | 19 (14%) | |
| Ethnicity | |||
| Hispanic or Latino | 44 (24%) | 30 (22%) | |
| Non-Hispanic or non-Latino | 108 (59%) | 90 (67%) | |
| Non-resident of the USA | 29 (16%) | 10 (7%) | |
| Missing | 3 (2%) | 4 (3%) | |
| HCT-CI score pre-transplantation | |||
| 0 | 49 (27%) | 30 (22%) | |
| 1–2 | 57 (31%) | 31 (23%) | |
| 3–4 | 51 (28%) | 41 (31%) | |
| ≥5 | 20 (11%) | 29 (22%) | |
| Not reported | 7 (4%) | 3 (2%) | |
| HSCT indication | |||
| Acute myeloid leukaemia | 66 (36%) | 1 (1%) | |
| Acute lymphocytic leukaemia | 45 (24%) | 0 | |
| Chronic myeloid leukaemia | 6 (3%) | 0 | |
| Myelodysplastic syndrome or myeloproliferative neoplasm | 29 (16%) | 0 | |
| Other acute leukaemia | 3 (2%) | 1 (1%) | |
| Non-Hodgkin lymphoma | 14 (8%) | 36 (27%) | |
| Hodgkin lymphoma | 4 (2%) | 5 (4%) | |
| Plasma cell disorder or multiple myeloma | 4 (2%) | 86 (64%) | |
| Solid tumours* | 0 | 4 (3%) | |
| Other malignancy | 2 (1%) | 0 | |
| Severe aplastic anaemia | 5 (3%) | 0 | |
| Sickle cell anaemia | 2 (1%) | 0 | |
| Immune disorders (SCID, other inherited immunodeficiency, autoimmune disease) | 4 (2%) | 1 (1%) | |
| Refined disease risk index | |||
| Low | 14 (8%) | N/A | |
| Intermediate | 88 (48%) | N/A | |
| High | 27 (15%) | N/A | |
| Very high | 7 (4%) | N/A | |
| NA (<18 years old or no disease risk index for subdisease) | 45 (24%) | N/A | |
| Missing | 3 (2%) | N/A | |
| Conditioning intensity (as reported by centre) | |||
| Myeloablative conditioning | 77 (42%) | N/A | |
| Reduced-intensity conditioning or non-myeloablative conditioning | 103 (56%) | N/A | |
| No conditioning given | 1 (1%) | N/A | |
| Not reported | 3 (2%) | N/A | |
| TBI-based conditioning, yes | 82 (45%) | N/A | |
| Graft versus host disease prophylaxis | |||
| Ex vivo T-cell depletion | 3 (2%) | N/A | |
| CD34 selection | 7 (4%) | N/A | |
| PTCy plus others | 32 (17%) | N/A | |
| PTCy alone | 2 (1%) | N/A | |
| Tacrolimus or cyclosporine A plus mycophenolate mofetil plus or minus others (except PTCy) | 25 (14%) | N/A | |
| Tacrolimus or cyclosporine A plus methotrexate plus or minus others (except mycophenolate mofetil, PTCy) | 90 (49%) | N/A | |
| Tacrolimus or cyclosporine A plus others (except mycophenolate mofetil, methotrexate, PTCy) | 5 (3%) | N/A | |
| Tacrolimus or cyclosporine A alone | 13 (7%) | N/A | |
| Other or none given | 4 (2%) | N/A | |
| Missing | 3 (2%) | N/A | |
| Anti-thymocyte globulin or alemtuzumab use at the time of HSCT | |||
| No | 94 (51%) | N/A | |
| Yes | 34 (18%) | N/A | |
| Not reported | 56 (30%) | N/A | |
| Donor type | |||
| HLA-identical sibling | 66 (36%) | N/A | |
| Haploidentical (≥2 MM) | 16 (9%) | N/A | |
| Other related (one antigen mismatched or fully matched but not identical) | 18 (10%) | N/A | |
| Well-matched unrelated (8 of 8) | 49 (27%) | N/A | |
| Partially matched unrelated (7 of 8) | 11 (6%) | N/A | |
| Mismatched unrelated (≤6 of 8) | 1 (1%) | N/A | |
| Unrelated (matching unknown) | 12 (7%) | N/A | |
| Cord blood | 11 (6%) | N/A | |
| Graft type | |||
| Bone marrow | 33 (18%) | 0 | |
| Peripheral blood | 140 (76%) | 134 (100%) | |
| Umbilical cord blood | 11 (6%) | 0 | |
| Year of transplant | |||
| 1999–2010 | 9 (5%) | 6 (5%) | |
| 2011–14 | 24 (13%) | 14 (10%) | |
| 2015–17 | 31 (17%) | 42 (31%) | |
| 2018–20 | 120 (65%) | 72 (54%) | |
Data are n (%), or median (IQR). HSCT=haematopoietic stem-cell transplantation. HCT-CI=haematopoietic cell transplantation-specific comorbidity index. HLA=human leucocyte antigen. PTCy=post-transplantation cyclophosphamide. NA=not applicable. MM=HLA mismatch. SCID=severe combined immunodeficiency syndrome. TBI=total body irradiation.
Includes CNS tumours, neuroblastoma, and germ-cell tumours.
Patient characteristics at the time of COVID-19 diagnosis are shown in table 2 . The median time from HSCT to the diagnosis of COVID-19 was 17 months (IQR 8–46; range 1–243) for allogeneic HSCT recipients and 23 months (IQR 8–51; range 1–169) after autologous HSCT. 60 (33%) of 184 allogeneic HSCT recipients had grade 2–4 acute GVHD before COVID-19 diagnosis. 66 (36%) of 184 allogeneic HSCT recipients had evidence of chronic GVHD before COVID-19. However, only 34 (18%) of 184 patients had received immunosuppression within 6 months of COVID-19 diagnosis.
Table 2.
Patient characteristics at diagnosis and clinical features of COVID-19
| Allogeneic HSCT (n=184) | Autologous HSCT (n=134) | |
|---|---|---|
| Time from HSCT to COVID-19 | ||
| Date of COVID-19 diagnosis not reported | 19 (10%) | 18 (13%) |
| Time, months | 17 (1–243; 8–46) | 23 (1–169; 8–51) |
| Acute GVHD grade 2–4 before COVID-19 | ||
| No | 100 (54%) | N/A |
| Yes | 60 (33%) | N/A |
| Not reported | 24 (13%) | N/A |
| Chronic GVHD before COVID-19 | ||
| No | 118 (64%) | N/A |
| Yes | 66 (36%) | N/A |
| On immunosuppression in 6 months before COVID-19 diagnosis | ||
| No | 131 (71%) | N/A |
| Yes | 34 (18%) | N/A |
| Not reported | 19 (10%) | N/A |
| White blood cell count at COVID-19 diagnosis | ||
| Not reported | 52 (28%) | 48 (36%) |
| Reported | 132 (72%) | 86 (64%) |
| Median, 1×109 cells per L | 6 (3–9) | 4 (3–6) |
| Absolute lymphocyte count at COVID-19 diagnosis | ||
| Not reported | 56 (30%) | 53 (40%) |
| Reported | 128 (70%) | 81 (60%) |
| Median, 1×109 cells per L | 1 (0–2) | 1 (0–1) |
Data are n (%), median (range; IQR), or median (IQR). HSCT=haematopoietic stem cell transplantation. GVHD=graft-versus-host disease.
Of the 318 patients included in this cohort, a nasal swab was the predominant method of diagnosis (n=261 [82%]). Six (2%) patients were reportedly diagnosed by symptoms alone, and 49 (15%) patients had no diagnostic method reported. The median duration of COVID-19 infection was 14 days (IQR 7–31) for recipients of allogeneic HSCT and 19 days (11–31) for recipients of autologous HSCT. Disease severity was mild in 155 (49%) of 318 patients. Moderate disease occurred in 49 (27%) of 184 allogeneic HSCT recipients and 27 (20%) of 134 autologous HSCT recipients. Severe disease occurred in 28 (15%) of 184 allogeneic HSCT recipients and 17 (13%) of 134 autologous HSCT recipients. There was no information on oxygen requirements reported for 42 (13%) of 318 patients. Regarding treatment, of the 318 patients reported, 123 (39%) did not receive any therapy, and no information about therapy was provided for another 44 (14%) patients. Agents used for treatment for the remaining 151 (47%) patients based on disease severity are shown in table 3 .
Table 3.
Treatment administered (n=151) for COVID-19 based on disease severity
|
Mild disease (no supplemental oxygen required) |
Moderate or severe disease (supplemental oxygen or mechanical ventilation required, or both) |
Disease severity unknown |
||||
|---|---|---|---|---|---|---|
| Allogeneic HSCT (n=86) | Autologous HSCT (n=69) | Allogeneic HSCT (n=77) | Autologous HSCT (n=44) | Allogeneic HSCT (n=21) | Autologous HSCT (n=21) | |
| No medications reported | 2 (2%) | 0 | 2 (3%) | 0 | 20 (95%) | 20 (95%) |
| No treatment given | 57 (66%) | 44 (64%) | 13 (17%) | 8 (18%) | 0 | 1 (5%) |
| COVID-19 convalescent plasma | 2 (2%) | 1 (1%) | 9 (12%) | 6 (14%) | 1 (5%) | 0 |
| Remdesivir | 2 (2%) | 4 (6%) | 22 (29%) | 9 (20%) | 1 (5%) | 0 |
| Tocilizumab | 0 | 0 | 4 (5%) | 3 (7%) | 0 | 0 |
| Hydroxychloroquine | 4 (5%) | 7 (10%) | 18 (23%) | 11 (25%) | 0 | 0 |
| Azithromycin | 7 (8%) | 3 (4%) | 5 (6%) | 1 (2%) | 0 | 0 |
| Lopinovir or ritonovir (Kaletra) | 0 | 1 (1%) | 0 | 0 | 0 | 0 |
| Methylprednisolone | 0 | 0 | 1 (1%) | 1 (2%) | 0 | 0 |
| Oseltamivir | 1 (1%) | 0 | 3 (4%) | 2 (5%) | 0 | 0 |
| Ribavirin | 0 | 1 (1%) | 0 | 0 | 0 | 0 |
| DAS181 | 0 | 0 | 1 (1%) | 0 | 0 | 0 |
| Acyclovir or valacyclovir | 7 (8%) | 5 (7%) | 7 (9%) | 4 (9%) | 0 | 0 |
| Famciclovir | 0 | 0 | 0 | 1 (2%) | 0 | 0 |
| Antibacterial agent | 2 (2%) | 7 (10%) | 6 (8%) | 9 (20%) | 0 | 0 |
| Other drug* | 3 (3%) | 1 (1%) | 1 (1%) | 0 | 0 | 0 |
Data are n (%). Treatments are not mutually exclusive and are as reported to the Center for International Blood and Marrow Transplant Research (CIBMTR). HSCT=haematopoietic stem cell transplantation.
Other drugs include albuterol, ascorbic acid, fluconazole, and ruxolitinib.
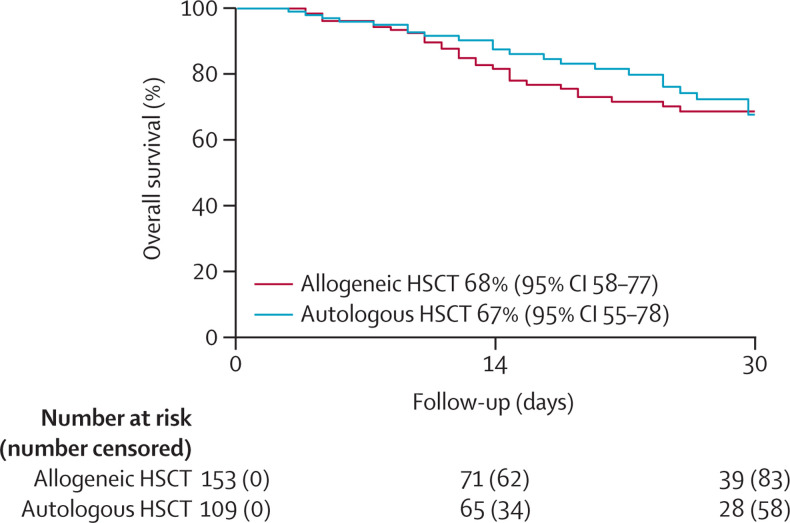
From the diagnosis of COVID-19, the median follow-up of survivors was 21 days (IQR 8–41, range 1–93) for allogeneic HSCT recipients (n=184), and 25 days (IQR 12–35, range 1–109) for autologous HSCT recipients (n=134). At the time of last follow-up, the infection was considered resolved in 126 (40%) of 318 patients, 30 (9%) patients were improving, and infection was ongoing for 49 (15%) patients. 66 (21%) of 318 HSCT recipients reported to the registry had died and infection resolution status was unknown or not reported for another 48 (15%) patients. Among the allogeneic HSCT recipients, 40 (22%) of 184 died, of whom the majority (37 [93%] of 40) died due to complications of COVID-19, and the remaining due to their primary or underlying disease. Of the 26 (19%) of 134 autologous HSCT recipients who died, COVID-19 was the primary cause of death in 19 (73%) of 26, with relapse (n=4), organ failure (n=1), and new malignancy (n=2) as other causes. Overall probability of survival at 30 days after diagnosis of COVID-19 was 68% (95% CI 58–77) for recipients of allogeneic HSCT and 67% (55–78) for recipients of autologous HSCT (figure ). We compared the overall survival of patients diagnosed with COVID-19 without a diagnostic method reported (n=55) and patients with a diagnostic method reported (n=263). We compared the overall survival at 14 days after COVID-19 diagnosis, and it was similar between the two groups (79% [95% CI 65–90] vs 85% [80–90]; p=0·31). The overall proportion of deaths in the two groups were also similar (12 [22%] of 55 vs 54 [21%] of 263; p=0·58).
Figure.
Overall survival after COVID-19 diagnosis
The results of the multivariable analysis for factors associated with mortality after COVID-19 diagnosis in allogeneic HSCT and autologous HSCT recipients are reported in table 4 , showing that age 50 years or older, male sex, and development of COVID-19 within 12 months of transplantation were associated with a higher risk of mortality among allogeneic HSCT recipients, and that a disease indication of lymphoma was associated with a higher risk of mortality compared with plasma cell disorder or myeloma in autologous HSCT recipients. Notably, race, ethnicity, haematopoietic cell transplantation-comorbidity index at the time of HSCT, and immunosuppression in the 6 months before COVID-19 were not associated with increased mortality. Absolute lymphocyte count of 0·3 × 109 cells per L or less at COVID-19 diagnosis was associated with worse survival (56% [95% CI 34–76] vs 85% [78–90]; p=0·003). Given that the duration of follow-up was short and some patients did not have PCR-confirmed, virologically documented disease, a subgroup analysis of PCR-confirmed patients who had a minimum of 10 days follow-up was done and the results are included in the appendix (pp 5–12). The results of the multivariable analysis of this subgroup were similar to the whole cohort (appendix pp 13–14).
Table 4.
Multivariable analysis for risk factors associated with death from COVID-19
| Number events/evaluable | Hazard ratio (95% CI) | p value | ||
|---|---|---|---|---|
| Allogeneic HSCT | ||||
| Age, years | ||||
| <50 | 10/84 | 1·00 | NA | |
| ≥50 | 26/66 | 2·53 (1·16–5·52) | 0·020 | |
| Sex | ||||
| Female | 7/64 | 1·00 | NA | |
| Male | 29/86 | 3·53 (1·44–8·67) | 0·006 | |
| Time from HSCT to COVID-19, months | ||||
| >12 | 15/96 | 1·00 | NA | |
| ≤12 | 21/54 | 2·67 (1·33–5·36) | 0·005 | |
| Race | ||||
| White | 31/121 | 1·00 | 0·74 | |
| African American, Asian, Pacific islander, Native American, or more than one race | 3/16 | 1·52 (0·46–5·06) | 0·49 | |
| Not reported | 2/16 | 1·95 (0·31–12·04) | 0·48 | |
| Ethnicity | ||||
| Not Hispanic or Latino | 24/92 | 1·00 | 0·62 | |
| Hispanic or Latino | 5/38 | 0·67 (0·23–1·99) | 0·47 | |
| Non-US resident or ethnicity not reported | 7/23 | 1·24 (0·50–3·08) | 0·64 | |
| HCT-CI score pre-transplantation | ||||
| 0–2 | 19/86 | 1·00 | 0·26 | |
| ≥3 | 16/60 | 1·81 (0·89–3·69) | 0·11 | |
| Not reported | 1/7 | 1·70 (0·20–14·13) | 0·63 | |
| Immunosuppression within 6 months before COVID-19 | ||||
| No | 21/107 | 1·00 | 0·45 | |
| Yes | 13/29 | 1·55 (0·74–3·26) | 0·24 | |
| Not reported | 2/17 | 0·77 (0·18–3·38) | 0·73 | |
| Autologous HSCT | ||||
| Age, years | ||||
| <50 | 2/25 | 1·00 | NA | |
| ≥50 | 22/78 | 3·31 (0·77–14·19) | 0·11 | |
| Sex | ||||
| Female | 8/41 | 1·00 | NA | |
| Male | 16/62 | 1·90 (0·80–4·51) | 0·14 | |
| Time from HSCT to COVID-19, months | ||||
| >12 | 15/69 | 1·00 | NA | |
| ≤12 | 9/34 | 0·94 (0·39–2·22) | 0·88 | |
| Race | ||||
| White | 17/58 | 1·00 | 0·47 | |
| African American, Asian, Pacific islander, Native American, or more than one race | 5/32 | 0·60 (0·21–1·67) | 0·33 | |
| Not reported | 2/13 | 0·51 (0·12–2·24) | 0·38 | |
| Ethnicity | ||||
| Not Hispanic or Latino | 13/69 | 1·00 | 0·53 | |
| Hispanic or Latino | 7/23 | 1·28 (0·48–3·42) | 0·62 | |
| Non-US resident or ethnicity not reported | 4/11 | 1·93 (0·61–6·14) | 0·26 | |
| HCT-CI score pre-transplantation | ||||
| 0–2 | 9/45 | 1·00 | 0·34 | |
| ≥3 | 13/56 | 1·07 (0·45–2·54) | 0·88 | |
| Not reported | 2/2 | 3·25 (0·66–15·91) | 0·15 | |
| Disease for HSCT | ||||
| Plasma cell disorder or myeloma | 12/69 | 1·00 | NA | |
| Lymphoma | 12/34 | 2·41 (1·08–5·38) | 0·033 | |
HSCT=haematopoietic stem cell transplantation. HCT-CI=haematopoietic cell transplantation-specific comorbidity index. NA=not applicable.
Discussion
Here we report, to our knowledge, the largest cohort of HSCT recipients diagnosed with COVID-19 using the international CIBMTR database. The goal of this initial analysis from CIBMTR was to describe outcome trends of COVID-19 among HSCT recipients and investigate potential risk factors for mortality. In this cohort, the median time from HSCT to COVID-19 diagnosis was 17 months for allogeneic HSCT recipients and 23 months for autologous HSCT recipients, yet both groups had a 30 day survival from COVID-19 of only approximately 70%.
Patients with cancer and patients with haematological malignancies have a high risk of developing severe disease and death from COVID-19 (13–39%).2, 3, 4, 6, 7, 8, 12 After HSCT, patients might be at additional risk of severe COVID-19 due to GVHD prophylaxis or treatment and transplantation-related morbidities. Not surprisingly, in a study from Italy, of 82 patients who developed COVID-19 after receiving an HSCT, death occurred in 11 (35%) of 31 patients who had received an allogeneic HSCT and in 17 (33%) of 51 recipients of an autologous HSCT.7 Increasing age, progressive disease status, diagnosis of acute myeloid leukaemia, non-Hodgkin lymphoma, or plasma cell neoplasms were associated with worse survival.7 A report from the Spanish Hematopoietic Stem Cell Transplantation and Cell Therapy Group reported a 45-day overall mortality rate of 17% for 65 allogeneic HSCT recipients and 18% for 58 autologous HSCT recipients.11 In this cohort, age older than 70 years and the presence of hypertension were associated with a doubling of the odds of mortality.11 There are two North American cohorts (Chicago and New York City) comprising 111 HSCT recipients (allogeneic HSCT n=55, autologous HSCT n=51, chimeric antigen receptor T-cell therapy n=5), of which 15% (n=17) required intubation and mechanical ventilation.10, 12 In the two cohorts, 19 (17%) patients died—the majority of these patients had received an allogeneic HSCT and most were within 1 year from transplantation.10, 12 Patients with active GVHD and patients receiving steroids were more likely to have poor outcomes.10 In our cohort, the estimated 30-day overall survival after COVID-19 diagnosis was comparable to the Italian and North American cohorts. As previously reported, we found being within a year from HSCT to be associated with worse survival in our allogeneic HSCT cohort.10 It has been hypothesised that because patients with cancer have an impaired immune response or are immunosuppressed, a reduction in the intensity of lung inflammation might be associated with a reduction in mortality. Indeed, at least one study showed reduced mortality in HSCT recipients, including autologous HSCT recipients, compared with patients with haematological malignancies who did not receive HSCT (17–18% vs 31%, respectively; p=0·02).11 However, immunosuppression within 6 months of developing COVID-19 was not associated with survival in our cohort.
Older age and having comorbid medical conditions (eg, diabetes, hypertension, or cardiac disease) are related to poor outcomes in the general population1 and in patients with cancer.2, 3, 4, 6, 7, 10, 12 Most studies have identified cancer survivors older than 60 years to be at the greatest risk of mortality or adverse outcomes after COVID-19.2, 3, 4, 6, 7, 10, 11 In our study, the age cutoff was slightly lower, at age 50 years, which is similar to another analysis of HSCT patients, where age older than 40 years was a risk factor.10 Patients with comorbidities are more likely to have severe or critical COVID-19 disease,7 but even after adjusting for comorbid conditions, patients with cancer have double the odds of death or severe symptoms after COVID-19 when compared with the general population.3 The younger age at which HSCT recipients have adverse outcomes might represent the additional burden of toxicity that HSCT survivors endure, and possibly higher frailty and burden of comorbidities among the HSCT survivors. However, unlike other studies, haematopoietic cell transplantation-comorbidity index at the time of HSCT, which was used as a surrogate for pre-existing comorbid conditions was not associated with survival in this analysis.
Several earlier studies1, 4, 5, 9, 13, 14 have indicated a male bias in SARS-CoV-2 infections, suggesting a biological basis in skewed infectivity and increased mortality. The mortality difference between male and female patients with COVID-19 tends to increase with increasing age, indicating a compound synergistic effect of cumulative comorbidities.5 In our study, we found an almost four-times higher risk of death among male recipients of allogeneic HSCT.
In our cohort, patients with moderate or severe COVID-19 infection were more likely to receive presumed COVID-19-directed therapies. However, due to the rapidly changing landscape and unknown efficacy of treatments, a plethora of agents were reported (table 3). Although our study provides an overview of the COVID-19 treatment patterns in HSCT recipients, due to limited number of patients treated with each of these drugs and patients receiving multiple agents concurrently or sequentially, we cannot comment on the efficacy of these drugs individually. Subsequent analyses from the CIBMTR might inform evidence-based treatment guidelines as this dataset matures.
We recognise several limitations inherent to our observational cohort study. A significant proportion of data was missing, and patient follow-up was relatively short. The management of patients with COVID-19 within and across centres might not be directly comparable, because there has been considerable centre-to-centre heterogeneity in treatment approaches. This heterogeneity was both due to rapidly evolving data related to investigational and repurposed drugs, as well as access to medications early in the course of pandemic. However, these data reflect real-world use and outcomes in HSCT recipients with COVID-19. The modest number of patients and observational method of this study might have resulted in overestimation of certain associations; hence, we emphasise the exploratory nature of this analysis, which was not driven by formal a priori hypotheses. We could not assess and account for various comorbidities or in patients receiving active therapy, because full details about comorbidities for the patients included in the analysis were not available. Additionally, these data were voluntarily reported to the CIBMTR, and there is a potential for selection bias given the SARS-CoV-2 PCR testing during the initial phase of the pandemic was limited to symptomatic or hospitalised individuals. Finally, some patients included in the current analysis might have been previously reported in the other two published North American cohorts.10, 12 Nevertheless, the rapidity with which the CIBMTR garnered a data-collection mechanism linked to the already extensive CIBMTR transplantation data will provide a deeper understanding of this pandemic for HSCT recipients.
This Article summarises the clinical characteristics and outcomes of HSCT recipients with COVID-19 from six countries in, to our knowledge, the largest reported series to date. Our data suggest considerably higher mortality among HSCT recipients who developed COVID-19 compared with the general population. Most of these patients were more than a year from transplantation and were not receiving immunosuppression. The current data underscore the need for more stringent surveillance and pre-emptive measures for all HSCT recipients. As more data become available, future directions will include assessments of treatment impacts on outcome, associated coinfections, and better determination of risk factors for mortality in HSCT recipients with COVID-19.
This online publication has been corrected. The corrected version first appeared at thelancet.com/haematology on May 25, 2021
Data sharing
Center for International Blood and Marrow Transplant Research (CIBMTR) supports accessibility of research in accordance with the National Institutes of Health (NIH) Data Sharing Policy and the NCI Cancer Moonshot Public Access and Data Sharing Policy. The CIBMTR only releases de-identified datasets (including data dictionary) that comply with all relevant global regulations regarding privacy and confidentiality. These data will be made available on the CIBMTR website—https://www.cibmtr.org/ReferenceCenter/PubList/PubDsDownload/Pages/default.aspx) with the publication.
Acknowledgments
Acknowledgments
The authors sincerely thank the data operations and IT groups in CIBMTR (on both the MCW and NMDP campuses) for their assistance in implementation of these data collection mechanisms. Without their dedication, the current analysis would not be possible. The CIBMTR is supported primarily by Public Health Service grant number U24CA076518 from the NCI, the National Heart, Lung and Blood Institute (NHLBI), and the National Institute of Allergy and Infectious Diseases; grant number U24HL138660 from NHLBI and NCI; grant number U01HL128568 from the NHLBI; grant numbers HHSH250201700006C and HHSH250201700007C from the Health Resources and Services Administration (HRSA); and grant number N00014–18–1-2850, N00014–18–1-2888, and N00014–20–1-2705 from the Office of Naval Research. Additional federal support was provided by the NIH (grant numbers P01CA111412, R01CA152108, R01CA215134, R01CA218285, R01CA231141, R01AI128775, R01HL126589, R01HL129472, R01HL130388, R01HL131731, U01AI069197, U01AI126612, and BARDA). Support is also provided by Be the Match Foundation, Boston Children's Hospital, Dana Farber, St Baldrick's Foundation, Stanford University, the Medical College of Wisconsin the National Marrow Donor Program, and from the following commercial entities: AbbVie, Actinium Pharmaceuticals, Adaptive Biotechnologies, Adienne SA, Allovir, Amgen, Angiocrine Bioscience, Astellas Pharma US, AstraZeneca, Atara Biotherapeutics, Bluebird bio, Bristol Myers Squibb, Celgene, CSL Behring, CytoSen Therapeutics, Daiichi Sankyo, Gamida-Cell, Genentech, HistoGenetics, Incyte Corporation, Janssen Biotech, Jazz Pharmaceuticals, Johnson & Johnson, Kiadis Pharma, Kite (a Gilead Company), Kyowa Kirin, Legend Biotech, Magenta Therapeutics, Mallinckrodt, Merck & Company, Merck Sharp & Dohme, Millennium, the Takeda Oncology, Miltenyi Biotec, Novartis, Omeros, Oncoimmune, Orca Biosystems, Pfizer, Pharmacyclics, Sanofi Genzyme, Shire, Sobi, Stemcyte, Takeda, Terumo, Viracor Eurofins, Vor Bio Pharma, and Xenikos. The views expressed in this Article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, HRSA or any other agency of the US Government.
Contributors
AS, NB, MBA, LG, GLS, and MLR designed the study. ASM, JB, EET, BES, and MLR acquired the data and verified it. AS, NB, ASM, MBA, LG, GLS, and MLR analysed the data. AS, NB, GLS, and MLR wrote the Article, and ASM, MBA, RFC, CD, JG, LG, SS, AMZ, and MAP critically reviewed the Article. AS and NB contributed equally to this work. AS is listed first because he initiated the project. All authors agree with, and take full responsibility for, the content of this Article. AS confirms that all authors had full access to the data reported in the study.
Declaration of interests
AS receives support for the conduct of industry-sponsored trials from Vertex Pharmaceuticals, CRISPR Therapeutics, and Novartis, and consulting fee from Spotlight Therapeutics. AMZ received research funding (institutional) from Celgene/BMS, AbbVie, Astex, Pfizer, Medimmune/AstraZeneca, Boehringer Ingelheim, Trovagene/Cardiff Oncology, Incyte, Takeda, Novartis, Aprea, and ADC Therapeutics. AMZ participated in advisory boards, or had a consultancy with, or both, and received honoraria from AbbVie, Otsuka, Pfizer, Celgene/BMS, Jazz, Incyte, Agios, Boehringer Ingelheim, Novartis, Acceleron, Astellas, Daiichi Sankyo, Cardinal Health, Taiho, Seattle Genetics, BeyondSpring, Trovagene/Cardiff Oncology, Takeda, Ionis, Amgen, Janssen, Epizyme, Syndax, and Tyme. AMZ served on clinical trial committees for Novartis, AbbVie, Geron, and Celgene/BMS. AMZ received travel support for meetings from Pfizer, Novartis, and Trovagene/Cardiff Oncology. MLR participated in advisory board for BioIntelect. GLS has received research funding from Janssen and Amgen. None of these relationships were related to the development of this Article. AS is supported in part by a Scholar Award by the American Society of Hematology. AMZ is a Leukemia and Lymphoma Society Scholar in Clinical Research and is also supported by a National Cancer Institute (NCI) Cancer Clinical Investigator Team Leadership Award. This research was supported in part by National Institutes of Health (NIH) award number P01 CA23766 and NIH/NCI Cancer Center Support grant P30 CA008748 (MAP and GLS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. NB, ASM, MBA, JB, RFC, CD, JG, LG, SS, BES, and EET declare no competing interests.
Supplementary Material
References
- 1.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dai M, Liu D, Liu M, et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10:783–791. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuderer NM, Choueiri TK, Shah DP, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta V, Goel S, Kabarriti R, et al. Case fatality rate of cancer patients with COVID-19 in a New York Hospital system. Cancer Discov. 2020;10:935–941. doi: 10.1158/2159-8290.CD-20-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Passamonti F, Cattaneo C, Arcaini L, et al. Clinical characteristics and risk factors associated with COVID-19 severity in patients with haematological malignancies in Italy: a retrospective, multicentre, cohort study. Lancet Haematol. 2020;7:e737–e745. doi: 10.1016/S2352-3026(20)30251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malard F, Genthon A, Brissot E, et al. COVID-19 outcomes in patients with hematologic disease. Bone Marrow Transplant. 2020;55:2180–2184. doi: 10.1038/s41409-020-0931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vicent MG, Martinez AP, Trabazo Del Castillo M, et al. COVID-19 in pediatric hematopoietic stem cell transplantation: the experience of Spanish Group of Transplant (GETMON/GETH) Pediatr Blood Cancer. 2020;67 doi: 10.1002/pbc.28514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varma A, Kosuri S, Ustun C, et al. COVID-19 infection in hematopoietic cell transplantation: age, time from transplant and steroids matter. Leukemia. 2020;34:2809–2812. doi: 10.1038/s41375-020-01019-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piñana JL, Martino R, García-García I, et al. Risk factors and outcome of COVID-19 in patients with hematological malignancies. Exp Hematol Oncol. 2020;9:21. doi: 10.1186/s40164-020-00177-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah GL, DeWolf S, Lee YJ, et al. Favorable outcomes of COVID-19 in recipients of hematopoietic cell transplantation. J Clin Invest. 2020;130:6656–6667. doi: 10.1172/JCI141777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boulad F, Kamboj M, Bouvier N, Mauguen A, Kung AL. COVID-19 in children with cancer in New York City. JAMA Oncol. 2020;6:1459–1460. doi: 10.1001/jamaoncol.2020.2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu X, Zhang L, Du H, et al. SARS-CoV-2 infection in children. N Engl J Med. 2020;382:1663–1665. doi: 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Center for International Blood and Marrow Transplant Research (CIBMTR) supports accessibility of research in accordance with the National Institutes of Health (NIH) Data Sharing Policy and the NCI Cancer Moonshot Public Access and Data Sharing Policy. The CIBMTR only releases de-identified datasets (including data dictionary) that comply with all relevant global regulations regarding privacy and confidentiality. These data will be made available on the CIBMTR website—https://www.cibmtr.org/ReferenceCenter/PubList/PubDsDownload/Pages/default.aspx) with the publication.