Abstract
To investigate whether severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2)–induced myocarditis constitutes an important mechanism of cardiac injury, a review was conducted of the published data and the authors’ experience was added from autopsy examination of 16 patients dying of SARS-CoV-2 infection. Myocarditis is an uncommon pathologic diagnosis occurring in 4.5% of highly selected cases undergoing autopsy or endomyocardial biopsy. Although polymerase chain reaction–detectable virus could be found in the lungs of most coronavirus disease-2019 (COVID-19)–infected subjects in our own autopsy registry, in only 2 cases was the virus detected in the heart. It should be appreciated that myocardial inflammation alone by macrophages and T cells can be seen in noninfectious deaths and COVID-19 cases, but the extent of each is different, and in neither case do such findings represent clinically relevant myocarditis. Given its extremely low frequency and unclear therapeutic implications, the authors do not advocate use of endomyocardial biopsy to diagnose myocarditis in the setting of COVID-19.
Key Words: cardiovascular disease, COVID-19, heart, SARS-CoV-2
Abbreviations and Acronyms: CMR, cardiac magnetic resonance imaging; EMB, endomyocardial biopsy; SARS-CoV-2, severe acute respiratory syndrome-coronavirus-2; STEMI, ST-segment elevation myocardial infarction
Central Illustration
Infection with the severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) confers significant risk of morbidity and mortality. Previous epidemiological data suggest that approximately 20% of hospitalized patients have evidence of cardiac injury as indicated by elevated levels of high-sensitivity troponins (hs-cTnI) (1). In 1 study in which troponin I was measured within 24 h of admission to assess cardiac damage, 36% of patients had elevated troponin I concentrations. After adjusting for disease severity and relevant risk factor differences, even small amounts of myocardial injury were associated with increased mortality (2). However, much remains unknown about the nature of myocardial injury in patients with coronavirus disease-2019 (COVID-19). In the absence of obstructive epicardial coronary disease but evidence of myocardial injury (defined as positive troponin with or without wall motion abnormalities), physicians often default to the diagnosis of myocarditis as the underlying cause using data such as clinical and imaging markers of myocyte injury. Indeed, most published cases of presumed myocarditis induced by COVID-19 infection were based on blood troponin levels or cardiac magnetic resonance imaging (CMR) without tissue diagnosis (3, 4, 5). However, direct evidence for myocarditis in the setting of SARS-CoV-2 remains very limited (6,7). A complete understanding of the pathogenesis of cardiac injury in the setting of COVID-19 infection is critical for the development of appropriate treatments.
Pathophysiology of COVID-19–Induced Myocyte Infection/Injury
Acute myocarditis is a disease with variable clinical progression and presentation, making it one of the most challenging diagnoses in cardiology. Several mechanisms are hypothesized to be involved in the pathogenesis of COVID-19–induced myocarditis. To date, there is very little evidence supporting direct destruction of cardiomyocytes through the occurrence of virus-mediated lysis with damage to cardiac structures, resulting in myocyte injury and cardiac dysfunction. The quantification of viral load in 39 consecutive autopsy cases from Germany demonstrated that SARS-CoV-2 could be documented in 24 of 39 (61.5%) hearts with 16 of 39 (41%) having copy numbers higher than 1,000 copies per ug RNA (6). Virus replication of SARS-CoV-2 defined by detection of the (−) strand replicate of the RNA genome was documented in 5 of the patients with the highest viral load, but in situ hybridization confirmed the virus presence in interstitial cells within cardiac tissue but not in myocytes. Virus presence was not associated with increased infiltration of mononuclear cells into the myocardium, and no myocarditis was present in any of these cases according to the Dallas criteria.
Another potential mechanism of cardiac injury that has been proposed is direct entry of the virus into endothelial cells in the heart without necessarily entering myocytes. Direct endothelial infection has been documented in autopsy hearts as well as in glomerular endothelial cells using electronic microscopy with identification of virus particles, although in some cases their appearance and location within cells was not typical of coronavirus infected cells (7, 8, 9). We believe other techniques such as in situ hybridization should be used to confirm such findings. In our own experience using both techniques, we have not been able to document a single case of endothelial infection by SARS-CoV-2 in the heart. From these data, it seems difficult to conclude that endothelial tropism is a major mechanism of COVID-19–induced cardiac injury without more confirmation.
Another, and perhaps better supported, idea is that cardiac injury can be induced via hyperactivation of the immune system characterized by the release of multiple inflammatory mediators, including interleukins, tumor necrosis factors, and so on. Circulating levels of these factors that exceed normal thresholds can result in collateral damage. The term cytokine storm has been used to describe this condition, which has been reported in severely ill COVID-19 patients (10). Microvascular and macrovascular thrombi that result from the activation of platelets, neutrophils, and other proteins may contribute to vascular occlusion and cell death (11). We recently reported a case of myocardial infarction and cardiogenic shock caused by cardiac microvascular thrombosis in a young woman with COVID-19 (12). In this case, the virus was not detectable by polymerase chain reaction (PCR) in the heart. However, the exact pathogenesis of cardiac injury induced by COVID-19 infection remains to be elucidated, but we believe the most compelling evidence points toward cytokine-storm–related effects.
Clinical and Pathological Diagnosis of COVID-19-Induced Myocardial Injury
Despite having endured the COVID-19 pandemic for over 6 months, many questions still remain about the best approaches for determining causes of cardiac injury in both hospitalized and nonhospitalized patients. In most cases, cardiac injury appears to result within the context of the overall respiratory infection rather than the first manifestation of disease. Coagulation abnormalities that may result as a part of the immune response to the disease have been shown to predispose these patients toward thrombotic processes, both in the venous and arterial circulation (11). A single-center observational study on COVID-19 subjects with ST-segment elevation myocardial infarction (STEMI) documented higher troponin levels, multivessel thrombosis, and stent thrombosis when compared with COVID-19 patients without STEMI (13). However, a substantial number of cases in subjects with STEMI had no evidence of coronary occlusion. In 1 case series from New York involving 18 patients with a confirmed diagnosis of COVID-19 and ST-segment elevation, 44% received a diagnosis of acute coronary thrombosis causing myocardial infarction, and 56% had evidence of noncoronary myocardial injury (defined as nonobstructive disease on coronary angiography) (14). It is precisely in cases such as these, or in situations where troponin levels are elevated but clinical suspicion of acute coronary syndrome is low, where diagnostic dilemmas may occur. Because the clinical presentation of myocarditis may vary and may include vague or nonspecific symptoms such as fatigue, dyspnea, palpitations, and chest discomfort, the diagnosis of myocarditis in this setting is not straightforward.
The World Health Organization defines myocarditis as an inflammatory disease of the myocardium diagnosed by established histological, immunological, immunohistochemical, and molecular criteria with endomyocardial biopsy (EMB) used to gain certainty on the diagnosis and to potentially identify etiology (15). The current indication for EMB is “a strong reason to believe that the results will have a meaningful effect on subsequent therapeutic decisions” according to the American College of Cardiology guidelines (16). These guidelines state that EMB should be performed in the setting of new-onset heart failure with hemodynamic compromise or new ventricular arrhythmias (Class I recommendation), but the timing and exact criteria for EMB in cases of myocarditis, especially in the setting of COVID-19 infection, remain uncertain.
Histological evidence from cardiac biopsies and autopsy hearts for myocarditis was initially diagnosed by the Dallas criteria (1985) and defined as histological evidence of inflammatory infiltrates within the myocardium associated with myocyte degeneration or necrosis (myocytolysis) of nonischemic origin (17). In the absence of necrosis and the presence of lymphocytic infiltrate, myocarditis was defined as borderline. In addition to a sampling error in EMBs containing PCR-detectable viral pathogens, the Dallas criteria were absent in 50% of virus positive cases (18). For these reasons, the use of Dallas criteria alone to diagnose myocarditis has been discouraged. More recently, immunohistochemical criteria have been added to address these shortcomings. By immunohistochemistry, myocarditis is defined according to the presence of an abnormal inflammatory infiltrate, defined as follows: ≥14 leukocytes/mm2 including up to 4 monocytes/mm2 with the presence of CD3-positive T-lymphocytes ≥7 cells/mm (19). Cell counts must be accompanied by evidence of myocyte degeneration and necrosis of nonischemic origin.
The appearance of inflammatory infiltrates alone in the absence of myocyte necrosis can be seen in normal myocardium as has been reported by us and others in individuals dying a noninfectious death (20,21). From our experience, it is the pattern of inflammatory infiltrate (concentrated collection of inflammatory cells [predominance of lymphocytes versus macrophages] around myocytes), extent, and presence of myocyte necrosis that defines myocarditis (16). Molecular biology diagnosis is defined as histological evidence for myocarditis associated with positive viral PCR to confirm the diagnosis of infectious myocarditis. Virus-negative myocarditis is defined as histological myocarditis with negative viral PCR.
Practically speaking, EMB is not routinely used to diagnose myocarditis and patients may be given the diagnosis of COVID-19 myocarditis based upon circumstantial evidence such as abnormal echocardiography or magnetic resonance imaging examinations. In 1 series where cardiac involvement was evaluated (using CMR) in 15 patients who reported cardiac symptoms but were considered recovered from COVID-19, 58% had abnormal CMR findings consisting mostly of myocardial edema, fibrosis, and impaired right ventricular function (3). In another series of 100 recovered patients, 78% had detectable cardiac involvement via CMR with 75% having detectable troponin levels (4). These studies raise the possibility that months after infection, ongoing myocardial inflammation with resultant left ventricular dysfunction may occur. In the following text, we will discuss data regarding the findings of EMB in cases of suspected COVID-19–induced myocarditis or unexplained heart failure and the low overall diagnostic yield of such investigations. Our opinion on the role of EMB in cases of suspected COVID-19 myocarditis is given later in this review.
What Is the Evidence for SARS-CoV-2 as a Cause of Myocarditis?
Coronaviruses are a family of enveloped, positive-sense, single-stranded, and highly diverse RNA viruses. To date, there have been 3 documented, highly pathogenic, and lethal human coronaviruses: SARS-CoV, Middle East Respiratory Syndrome–CoV, and SARS-CoV-2. SARS-CoV-2 shares approximately 79.5% genomic homology with SARS-CoV, but only about 50% with Middle East Respiratory Syndrome–CoV, indicating that SARS-CoV-2 is closer to SARS-CoV (22). SARS-CoV was highly lethal, but its presence was mitigated effectively by intense public health measures. Although many similarities exist between SARS-CoV and SARS-CoV-2, important differences also exist in transmissibility and disease pathogenesis. Nonetheless, lessons learned from the effects of SARS-CoV may have some degree of relevance to our current understanding of how SARS-CoV-2 might affect the heart. Autopsy data from 20 patients who died during the SARS outbreak in 2003 identified viral RNA in the hearts of 7 of these patients (23). Immunohistochemical staining of postmortem myocardial tissue using macrophage-specific marker (CD68) revealed a significant amount of macrophage infiltration, which was increased in patients who had evidence of SARS-CoV (by PCR) in their hearts with only minor elevation in those without SARS-CoV. By contrast, immunohistochemical staining for T-cell specific (CD3) cell surface markers showed that myocardial lymphocytic infiltration was minimal with no differences in lymphocyte counts between those with and without SARS-CoV in their hearts. Although virus entry occurs predominantly via the angiotensin-converting enzyme receptor 2 for both SARS-CoV and SARS-CoV-2, organ tropism may be different given the differing clinical presentations and infectiousness of the 2 pathogens.
To date, 9 cases have been reported where EMB was performed in COVID-19–positive subjects (24, 25, 26, 27). The diagnostic criteria for myocarditis were met in only 2 of these cases. In all studies, there was no direct evidence of SARS-CoV-2 within cardiomyocytes. For the most part, direct proof that SARS-CoV-2 infects myocytes leading to virus-induced necrosis and cell death with troponin release is lacking. In 1 of the cases that met the authors’ diagnostic criteria for myocarditis, a 43-year-old woman had an inverted Takotsubo pattern in the setting of COVID-19 infection (24). The final diagnosis was virus-negative immune-mediated myocarditis, because the PCRs of EMB samples were negative for the SARS-CoV-2 genome. In another case, a 48-year-old man with newly diagnosed heart failure in the setting of COVID-19 infection underwent EMB (26). Histological assessment met the Dallas criteria with necrosis of myocytes and pronounced myocardial inflammation with macrophages and lymphocytes, and PCR of the EMB sample was positive for the viral genome (albeit at a very low level). A summary of the results of prior studies published to date on EMB samples in the setting of COVID-19 infection is shown in Table 1 .
Table 1.
Published Cases of COVID-19 With Examination of the Heart at Autopsy or Biopsy for the Presence of Myocarditis
| PMID | First Author (Ref.), Journal | Total Number of Cases | Type of Sample | Age, yrs, Mean (Range) | Sex | H/O Underlying HD, Type | Impaired Cardiac Function | Virus Detected | Type and Cells | Case With Evidence of Myocarditis∗ According to Authors |
|---|---|---|---|---|---|---|---|---|---|---|
| 32267502 | Sala et al. (24), Eur Heart J | 1 | EMB | 43 | F | 0 | EF 43% | No | (T-cell)+ necrosis (limited) | 1 |
| 32275347 | Tavazzi et al. (25), Eur J Heart Fail | 1 | EMB | 69 | M | 0 | EF 34% | Yes | Low-grade inflammation, no necrosis | 0 |
| 32529795 | Escher et al. (26), ESC Heart Fail | 5 | EMB | 49 (36–62) | 4 M 1 F |
NA | 4/5 Impaired | 5 | Active lymphocytic myocarditis | 1 |
| 32562489 | Wenzel et al. (27), Cardiovasc Res | 2 | EMB | 39, 36 | 2 M | 1 HTN, 1 HF, 1 CAD | EF 60% EF 30% | 2 | Lymphocyte infiltration no necrosis | 0 |
| 32432787 | Pesaresi et al. (19), Eur Rev Med Pharmacol Sci | 1 | Aut | 84 | F | NA | NA | Yes (TEM) | Virus in myocytes by TEM, no inflammatory cells | 0 |
| 32085846 | Zhe et al. (35), Lancet Resp Med | 1 | Aut | 50 | M | 0 | NA | No | Interstitial inflammatory cells | 0 |
| 32275742 | Barton et al. (36), Am J Clin Pathol | 2 | Aut | 77, 42 | 2 M | 1 HTN | NA | NA | 0 | 0 |
| 32682491 | Beadley et al. (37), Lancet | 14 | Aut | 71 (42–84) | 6 M 8 F |
9 HTN, 3 CAD, 4 HF, 8 CKD, 5 DM, 5 obesity | NA | +† | Lymphocyte infiltration with necrosis | 1 |
| 32434133 | Buja et al. (38), Cardiovasc Path | 23 | Aut | NA (34–76) | 12 M 7 F 4 NA |
10 HTN, 5 DM 9 obesity | NA | NA | Lymphocytic myocarditis | 1 |
| 32374815 | Wichmann et al. (39), Ann Intern Med | 12 | Aut | 73 (52–87) | 9 M 3 F |
2 HTN, 6 CAD 2 CKD, 1 PAD 3 DM, 3 obesity | NA | NA‡ | Lymphocytic myocarditis | 1 |
| 32422076 | Lax et.al. (40), Ann Internal Med | 11 | Aut | 80 (66–91) | 8 M 3 F |
9 HTN, 5 DM, 3 CAD, 2 Malig | NA | NA | Focal lymphocytic infiltrate | 0 |
| 32291399 | Tian et al. (41), Modern Pathol | 4 | Aut | 73 (59–81) | 3 M 1 F |
1 DM, HTN | NA | NA | 0 | 0 |
| 32325026 | Varga et al. (8), Lancet | 3 | Aut | 66 (58–71) | 2 M 1 F |
2 HTN, 1 CAD 1 DM, 1 obesity | 1/3 EF low | NA | 0, Endothelialitis | 0 |
| NA | Bryce et al. (28), medRxiv | 25 | Aut | 69 (34–94) | NA | HTN 63%, CAD 31%, DM 40% | NA | NA | 2 cases of interstitial chronic inflammation | 0 |
| 32552178 | Beigmohammadi et al. (42), Int J Surg Pathol | 7 | Aut | 68 (46–84) | 5 M 2 F |
4 HTN, 1 IC 1 DM, 1 VD | NA | NA | Inflammation and necrosis but no myocarditis | 0 |
| 32766543 | Rapkiewicz et al. (43), EClinicalmedicine | 7 | Aut | 57 (44–65) | 3 M 4 F |
6 HTN, 5DM 5 obesity | NA | Non in 4 cases (EM) | 1 case of focal lymphocytic infiltration with myocardial necrosis | 1 |
| 32968776 | Basso et al. (29), Eur Heart J | 21 | Aut | 69 (44–86) | 15 M 6 F |
16 HTN, 7 DM, 3 CAD, 2 CKD | 2/21 died due to cardiogenic shock or cardiac arrest§ | NA | Multifocal lymphocyte infiltration with myocardial necrosis | 3 |
| 32689809; 32473124 | Fox et al. (7), Circulation; Fox et al. (44), Lancet Respir Med‖ | 22 | Aut | 69 (44–79) | NA | 18 HTN, 1 CAD, 11 DM, 4 CKD, 9 obesity | 2/22 HF | NA | Scattered single myocyte necrosis without significant lymphocyte infiltration | 0 |
| 32730555 | Lindner et al. (6), JAMA Cardiol | 39 | Aut | 85 (78–89) | 16 M 23 F |
17 HTN, 32 CAD, 7 DM | NA | 24¶ | NA | 0 |
| Total | 201 cases | EMB; 9 Aut; 192 | (34–94) | 89 M 61 F 51 NA |
Myocarditis Author Reported | |||||
| Myocarditis 9 (4.5%) | ||||||||||
Aut = autopsy; CAD = coronary artery disease; CHF = chronic heart failure; CKD = chronic kidney disease; COVID-19 = coronavirus disease-2019; DCM = dilated cardiomyopathy; DM = diabetes mellitus; EF = ejection fraction; EM = electron microscopy; EMB = endomyocardial biopsy; HCM = hypertrophic cardiomyopathy; HD = heart disease; HF = heart failure; H/O = history of; HTN = hypertension; IC = immunocompromised; Malig = malignancy; NA = no available information; PAD = peripheral artery disease; TEM = transmission electron microscopy; VD = valve disease.
“Borderline myocarditis” cases counted as not diagnostic of myocarditis.
Very low level of virus was detected by PCR (likely contamination by circulating virus rather than direct infection).
5 patients had virus detected in other tissues although not clearly stated which tissues virus was detected in.
12 patients showed new ECG abnormality including atrial fibrillation, premature ventricular beats, bundle branch block, and ST-segment abnormality. Only 5 cases were evaluated by cardiac ultrasound without any evidence of impaired cardiac function.
The same case series was reported in other papers (PMID 32689809, 32473124).
Virus load was lower than 1,000 copies in 8 patients and was above 1,000 copies in 16 patients.
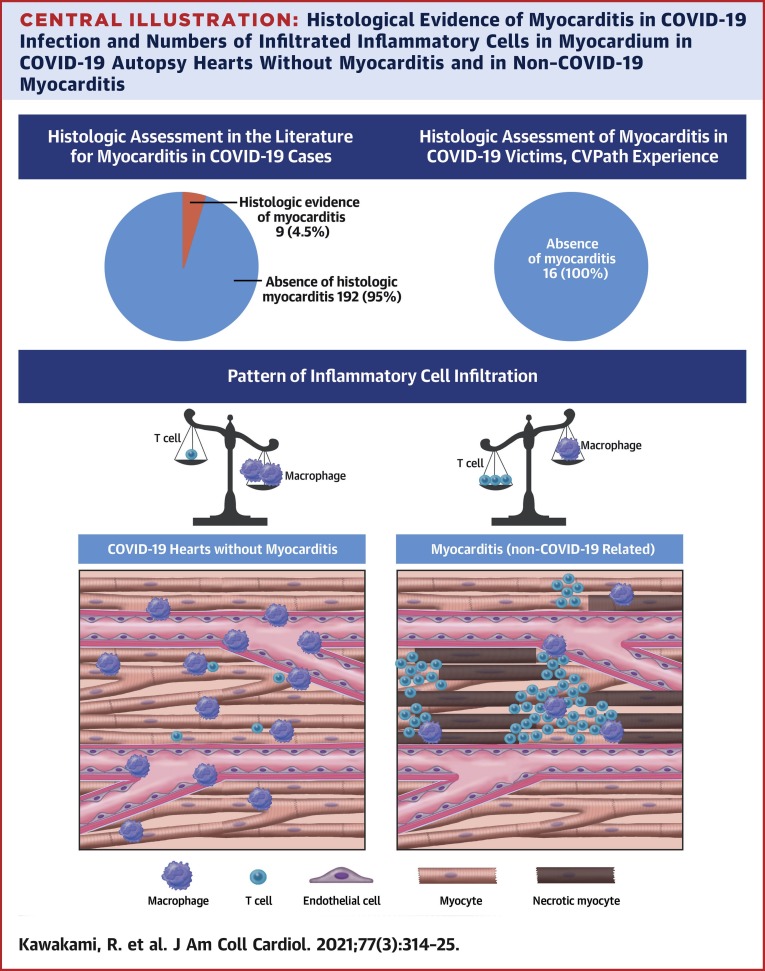
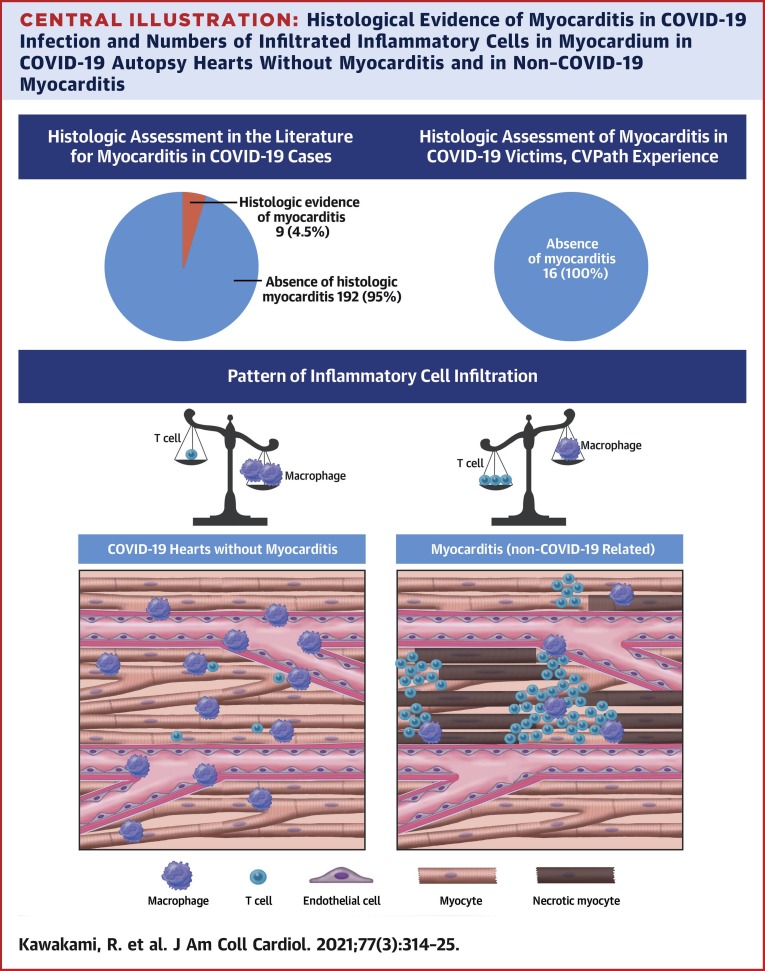
Another method to demonstrate the effect of COVID-19 on the heart is through autopsy studies. Although many studies have focused on the pulmonary findings of COVID-19, few studies have been conducted specifically examining the effects of COVID-19 on the heart. In the largest series of autopsies conducted in a New York hospital in subjects dying of COVID-19 infection, hearts from 25 cases were evaluated (28). Most hearts showed evidence of pre-existing atherosclerotic or hypertensive heart disease, with 60% of cases showing nonspecific patchy, mild interstitial chronic inflammation within the myocardium without associated myocyte necrosis. More recently, and as mentioned in the previous text, Lindner et al. (6) quantified viral load in 39 consecutive autopsy cases from Germany and demonstrated that SARS-CoV-2 could be documented in 24 of 39 (61.5%); yet, none of these cases were found to meet the criteria for myocarditis. Recently Basso et al. (29) reported a multicenter autopsy case series examining 21 hearts from COVID-19 cases. They found myocarditis in 3 cases (3 of 21; 14.2%), defined as the presence of an inflammatory infiltrate associated with myocyte injury not due to other causes and observed in multiple foci (29). All 3 cases had CD3-positive lymphocytes (2 had CD4, 1 had CD8 predominance) as well as macrophages. The high rate of myocarditis reported in this paper may in part be due to selection bias, as cases were referred from 4 separate institutions. A recently published literature review of 277 autopsied hearts across 22 publications found that myocarditis was present in 20 hearts (7.2%), with closer examination by the authors revealing that most cases were likely not functionally significant, and in their opinion, the true prevalence was much lower (<2%) (30). A list of the findings of published papers that included pathological analysis of hearts from COVID-19–positive subjects using specific and well-adopted criteria for the diagnosis of myocarditis are listed in Table 1. Of the collective 201 hearts (or EMB samples) examined in published series, in 9 cases (4.5%), investigators found some evidence of myocarditis of unclear extent and nature. In most of these cases, analysis for the virus in the heart was not performed and evidence for direct infection of cardiac myocytes has not been shown.
An import caveat to the analysis of these studies (as well as to the clinical diagnosis of SARS-CoV-2) is the possibility of false-negative results, which in the case of tissue samples, might be caused by either sampling errors or by degradation of viral RNA during the process of fixation prior to analysis (31). Indeed, a recent case report documented the occurrence of 2 subjects with clinically suspected myocarditis whose nasopharyngeal swab tests for COVID-19 were negative but the virus was recovered by PCR from EMB specimens (27). It is important to recognize the reliability of SARS-CoV-2 RNA tests when examining diagnostic pathology reports.
In addition to the possibility of false-negative PCR results during tissue sampling, it is also likely that given the low overall expression of ACE2 receptor in myocardial cells, tropism of SARS CoV-2 for the heart is not likely (32). Takotsubo syndrome is another potential cause of myocardial injury in the setting of electrocardiographic abnormalities and troponin-positive results with normal coronaries and should also be considered. In such cases, patients are known to have regional wall motion abnormalities, injury to myocytes, and infiltration of lymphocytes and macrophages in autopsy specimens. However, to be sure of the diagnosis, this requires exclusion of myocarditis. Troponin levels and CMR signs of edema can be found in both entities and each can mimic the other.
CVPath Experience
In a series of 384 autopsy cases (239 cardiac, 51 noncardiac, and 94 non-natural deaths), inflammatory infiltrates were found in the heart in 18% of all cases, with multifocal infiltrates in 9%. Incidental infiltrates were most frequent in natural noncardiac deaths (31%), but were also seen in drug-related deaths (20%) and cardiac deaths (16%), and were least frequent in traumatic deaths (16%) (21). We previously reported autopsy findings in the hearts of patients dying during viral infection (in this case during the AIDS epidemic) and showed that focal mild inflammatory infiltrates not meeting the definition of myocarditis in the heart were not uncommon (31%) (20). We also found focal mild mononuclear inflammatory infiltrates in 10 of 24 (42%) hearts in sudden traumatic deaths. Because these foci were not associated with myocyte necrosis, they were not diagnosed as myocarditis.
We have examined the hearts of 15 subjects dying with COVID-19 infection from the Lombardy region outbreak (Papa Giovanni Hospital, Bergamo, Italy) and 1 case from the Medical Examiner in Baltimore, Maryland. The hearts of 15 unselected patients dying from COVID-19 at Ospedale Papa Giovanni XXIII (Bergamo, Italy) and undergoing autopsy were collected and sent to CVPath Institute (Gaithersburg, Maryland) for detailed pathological analysis. The study protocol was reviewed and approved by the ethical committee at Ospedale Papa Giovanni XXIII Bergamo (2020-0056) and by CVPath Institute IRB (RP0112), and was registered at ClinicalTrials.gov (NCT04367792). The specimens were anonymized before shipping, and the examining pathologist (R.V., M.R., R.K.) was blinded to the clinical details. All tissue specimens were fixed in 10% buffered formalin for at least 72 to 96 h prior to shipment. Whole hearts and lungs (either paraffin blocks or tissues), were sent to CVPath Institute for pathological examination.
As shown in Table 2 , the average age of the subjects was 70 years, and 69% were men. In total, 4 subjects had a history of prior cardiovascular intervention and 4 had other relevant past medical histories. The average length of hospitalization until death was 6 days. A complete autopsy with detailed cardiac examination (histological sections examined from all 4 walls, at 2 levels of the heart) was conducted. Overall, none of the cases met the criteria for myocarditis, although 3 had evidence of either microvascular or epicardial thrombosis in the setting of acute myocardial infarction, and these cases all had neutrophilic infiltration. Inflammatory cell infiltration was found in the epicardium in 10 of 16 cases (63%), consisting mostly of lymphocytes, and myocardial mononuclear inflammatory cell infiltration was found in 5 of 16 (31%) cases (Table 3 ).
Table 2.
Relevant Clinical and Laboratory Information of the 16 COVID-19 Autopsy Cases Examined by CVPath
| Case | Age (yrs) | Sex | Relevant Past Medical History | History of CV Disease | Duration of Hospitalization (Days) | Max Ventilatory Support | ICU Stay | ECG Suggestive of Ischemia∗ | Troponin I Level (ng/l) | D-Dimer (ng/ml) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 80 | M | None | None | 7 | CPAP | No | No | N/A | N/A |
| 2† | 28 | M | None | None | 0 | None | No | N/A | N/A | N/A |
| 3 | 80 | F | GIM | None | 3 | CPAP | No | No | N/A | 1,790 |
| 4 | 65 | F | HM | None | 11 | Mechanical | Yes | No | 79 | 26,421 |
| 5 | 80 | F | None | None | 3 | None | No | No | N/A | N/A |
| 6 | 43 | F | None | None | 3 | Mechanical | Yes | Yes | 45,844 | 662 |
| 7 | 86 | M | None | CAD (post-stent implantation), PM implantation | 14 | CPAP | No | No | N/A | N/A |
| 8 | 57 | M | None | None | 9 | CPAP | No | N/A | N/A | 4,918 |
| 9 | 81 | M | None | OMI (post-stent implantation), A Fib, Valve surgery | 1 | Mechanical | Yes | Yes | 125,000 | N/A |
| 10 | 66 | M | None | OMI (post-stent implantation) | 7 | Mechanical | Yes | No | 381 | 35,000 |
| 11 | 73 | M | HM | None | 3 | CPAP | No | No | N/A | 2,672 |
| 12 | 63 | M | None | CAD (post-stent implantation) | 17 | Mechanical | Yes | No | 1,000 | 5,000 |
| 13 | 86 | M | None | None | 29 | CPAP | No | No | N/A | N/A |
| 14 | 57 | M | None | None | 8 | Mechanical | Yes | No | N/A | N/A |
| 15 | 56 | M | None | None | 2 | Mechanical | Yes | No | 25 | 19,118 |
| 16 | 82 | F | Liver cirrhosis, breast cancer | None | 5 | CPAP | No | No | N/A | N/A |
| Total | 70 (57–80) | Male 11 (69%) | — | Coronary stent implantation 4 (25%) | 6 (3.0–9.5) | Mechanical 7 (44%) | 7 (44%) | 2 (13%) |
AFib = atrial fibrillation; CAD = coronary artery disease; CPAP = continuous positive airway pressure therapy; CV = cardiovascular; ECG = electrocardiogram; GIM = gastrointestinal malignancy; HM = hematology malignancy; Mechanical = mechanical ventilation; N/A = not available; OMI = old myocardial infarction; PCI = percutaneous coronary intervention; PM = pacemaker.
Ischemic ECG changes defined as ST-segment elevation/depression >0.1 mV, new left bundle branch block, inverted T-wave.
Died suddenly at home (not hospitalized).
Table 3.
Pathological Findings of 16 COVID-19 Autopsy Cases Examined by CVPath
| Case | Age (yrs) | Sex | Autopsy Determined Cause of Death | Heart Weight (g) | Pericardial Effusion at Autopsy | Quantification of Effusion (ml) | Epicardial Inflammation |
AMI | Myocardial Inflammation |
Nonischemic Myocardial Necrosis | Virus RT-PCR |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes/No (Degree)∗ | Cell Type | Yes/No | Cell Type | Heart | Lung | |||||||||
| 1 | 80 | M | DAD | 385 | Yes | 50–100 | No | No | No† | No | +(LA) | + | ||
| 2 | 28 | M | AH | 444 | No | <20 | Yes (Mild) | Lym | No | No | No | ND | ND | |
| 3 | 80 | F | DAD | 415 | Yes | 50–100 | No | No | No† | No | ND | + | ||
| 4 | 65 | F | DAD | 350 | Yes | 50–100 | No | No | No | No | ND | + | ||
| 5 | 80 | F | DAD, AH, PT | 304 | Yes | 50–100 | Yes (Mild) | Lym | No | No† | No | ND | + | |
| 6 | 43 | F | AMI, DAD, PT | 278 | Yes | 50–100 | Yes (Mild) | Neut, Lym | Yes | Yes | Neut‡ | No | ND | + |
| 7 | 86 | M | DAD, AH | 477 | Yes | 50–100 | Yes (Mild) | Lym | No | No† | No | ND | + | |
| 8 | 57 | M | DAD, BP | 393 | Yes | 50–100 | Yes (Mild) | Lym | No | Yes† | Lym | No | ND | + |
| 9 | 81 | M | AMI | 900 | Yes | 50–100 | No | Yes | Yes | Neut‡ | No | ND | ND | |
| 10 | 66 | M | DAD, AH | 450 | Yes | 50–100 | Yes (Moderate) | Lym | No | Yes | Lym | No | ND | + |
| 11 | 73 | M | DAD, AH, PT | 468 | Yes | 50–100 | Yes (Moderate) | Lym | No | No | No | +(RA) | + | |
| 12 | 63 | M | DAD, AH, PT | 641 | Yes | 50–100 | No | No | No | No | ND | + | ||
| 13 | 86 | M | DAD, AH, PT | 384 | Yes | 50–100 | Yes (Mild) | Neut, Lym, Plasma | No | No | No | ND | + | |
| 14 | 57 | M | DAD, PT | 373 | Yes | 50–100 | No | No | No | No | ND | + | ||
| 15 | 56 | M | DAD, PT | 353 | Yes | 50–100 | Yes (Mild) | Lym | No | No | No | ND | ND | |
| 16 | 82 | F | DAD, AMI | 475 | Yes | 50–100 | Yes (Mild) | Lym | Yes | Yes | Neut‡ | No | ND | ND |
| Total | 70 (57–80) | Male 11 (69%) | AMI 3 (19%), DAD 13 (81%), PT 8 (50%) | 404 (368–470) | 15 (94%) | 10 (63%) | — | 3 (19%) | 5 (31%) | — | 0 (0%) | 2 (13%) | 12 (75%) | |
Continuous values are expressed by a median (interquartile range).
AH = alveolar hemorrhage; AMI = acute myocardial infarction; BP = bronchial pneumonia; DAD = diffuse alveolar damage due to coronavirus; LA = left atrium; Lym = lymphocytes; ND = not detected; Neut = neutrophils; Plasma = plasma cells; PT = pulmonary thrombus; RA = right atrium; y/n = yes or no; other abbreviations as in Table 2.
Degree of inflammation in epicardium was classified into 3: mild (≥50 and <100 per mm2), moderate (≥100 and <500 per mm2), and severe (≥500 per mm2).
Additional examination by immunohistochemistry was done (see Figure 1).
Neutrophil infiltration in the area of acute myocardial infarction.
Total RNA was extracted from myocardial tissue (1 sample from each chamber of the heart) from each of the 16 cases. Concentration and quality of RNA samples were measured. Quantitative reverse transcriptase PCR was performed using specifically designed primers for SARS-CoV-2 (N1 and N2 from CDC EUA essay). Virus copy number was quantified based on the reverse transcriptase PCR results with standard control containing the complete nucleocapsid gene from SARS-CoV-2. RNase P was used as a control. The detection of SARS-CoV-2 RNA was determined by the amplification at Ct ≤40 (33). In only 2 cases was the virus detected in the heart by PCR and both were in the atria (1 left atrium and 1 right atrium) and not in the ventricles, and neither was accompanied by inflammation, although in most of the 16 cases the virus was detected in the lung (Table 3).
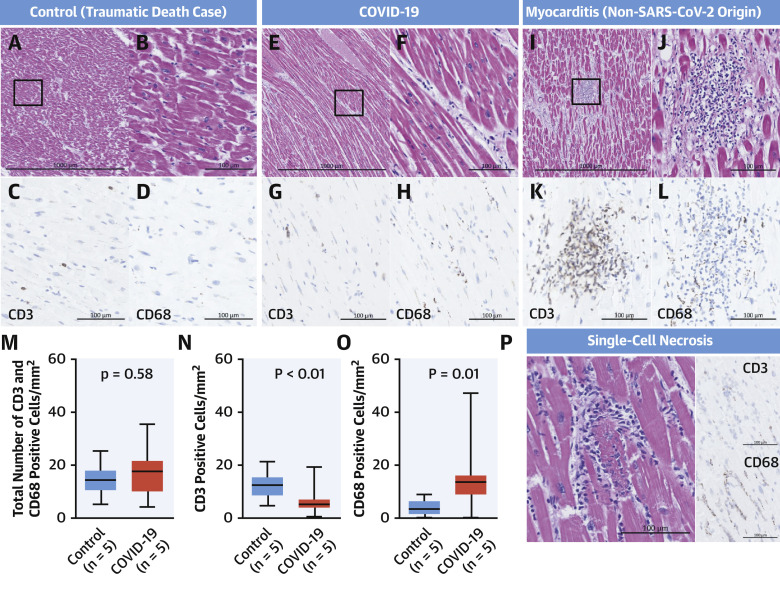
We also evaluated the nature of the inflammatory cells found in the myocardium in cases of (noninfectious) traumatic (control) versus COVID-19 deaths. We examined histological sections from the left ventricles from 5 randomly selected cases of control death from our CVPath registry and from 5 randomly selected cases of subjects dying of COVID-19 (see Table 2 for list of cases selected). We stained left ventricular myocardial sections using antibodies against CD3 (T-cell marker) and CD68 (macrophage). Overall, there was no difference in the total number of T cells and macrophages in the 2 groups (Figure 1 ). However, the cell counts in COVID-19 hearts were higher than what others have used to diagnose myocarditis. We believe because these cells are scattered and not accompanied by myocyte necrosis, these cases do not fulfill the criteria of myocarditis. Moreover, CD3 cells were significantly more frequent in control cases than in COVID-19 cases, whereas macrophages were more frequent in COVID-19 than in control cases, but we did not determine their exact location (i.e., interstitium vs. intravascular). Figure 1 contrasts these results with a typical case of mild myocarditis from the CVPath biobank, which showed greater CD3 abundance than macrophages, but both are typically more frequently observed in myocarditis than in control deaths and COVID-19 cases.
Figure 1.
Myocardial Inflammatory Cell Infiltrates in Cases of Traumatic Death, COVID-19, and Myocarditis
Representative low and high power of hematoxylin and eosin images, and immunostains for CD3 and CD68 from cases of traumatic death (control) (A to D and P), COVID-19 (E to H), and myocarditis (I to L). Bar graphs represent total inflammatory (i.e., CD3 and CD68) cell counts in control subjects and COVID-19 cases (n = 5 each) (M, N, and O). A photomicrograph of single-cell necrosis with CD3 and CD68 staining is shown in P. CD3 = cluster of differentiation 3; CD68 = cluster of differentiation 68; COVID-19 = coronavirus disease-2019; SARS-CoV-2 = severe acute respiratory syndrome-coronavirus-2.
The Authors’ Position on Usefulness of EMB in Cases of Suspected COVID-19-induced Myocarditis
The EMB procedure itself comes with inherent risks, including cardiac perforation, pericardial tamponade, bleeding, and so on, although these have declined with the use of flexible bioptomes with smaller jaws. The incidence of complications was 1.2%, with perforation occurring in 0.42% and death in 0.03% (16). The reason for EMB should be based upon the anticipated yield of the disorder (presumed probability and risk of sampling error) and the presence of an effective therapy. Certainly, the procedure should only be recommended in cases where benefits of the information obtained outweighs the potential risks of the procedure. The preponderance of autopsy and EMB evidence to date in cases of suspected myocarditis after COVID-19 infection suggests that myocarditis is an uncommon diagnosis. Moreover, the limited sampling of the heart (confined usually to the right ventricular septum) combined with the rarity of COVID-19 myocarditis makes diagnostic yield very unlikely, as has been shown in published cases where EMB has been conducted in cases of suspected myocarditis in the setting of COVID-19 infection. Further complicating the issue of histological diagnosis is that the typical criteria for myocarditis based upon the presence of certain types of inflammation may be different in cases of COVID-19 where very ill patients tend to have lymphopenia (34). It remains unclear to what extent lymphocytic infiltration should be expected in these cases (as it is in typical myocarditis). Moreover, the therapeutic implications are even less clear given there is no data supporting the effectiveness of particular therapies for myocarditis in the setting of COVID-19. Thus, at present, the authors believe EMB should not be routinely performed in such cases and should perhaps be reserved for worst case scenarios, such as patients with new-onset fulminant heart failure with hemodynamic compromise in the setting of documented COVID-19 infection, and the absence of coronary artery disease.
Conclusions
SARS-CoV-2 infection carries significant morbidity and mortality, especially in the setting of concomitant cardiac injury. However, the mechanism by which the virus causes cardiac damage remains uncertain. Because of the known relationship between viral infection and myocarditis, COVID-19–induced myocarditis has been suggested to be a major mechanism. A thorough review of the literature describing both EMB and autopsy sample analysis suggests that myocarditis is an uncommon diagnosis occurring in 4.5% of highly selected cases undergoing autopsy or EMB (Central Illustration ). Given the referral bias of autopsy studies, it is likely the number of COVID-19 cases complicated by myocarditis is even lower, because the majority of cases do not have evidence of myocardial injury and do not result in death. However, it should also be appreciated that myocardial inflammation involving infiltration by macrophages and T cells can also be seen in noninfectious deaths (control) and COVID-19 cases, but the extent of each is different, and in neither case do such findings represent clinically relevant myocarditis. Indeed, in the hearts of SARS-CoV-2 infected subjects, nonspecific inflammatory infiltrate characterized by macrophages was more abundant whereas T cells were lower compared with hearts from cases of control deaths (Central Illustration). This finding may be consistent with the known lymphope5nic effects of the virus, but deserves further investigation. Nonetheless, our data suggest evidence that COVID-19–induced myocarditis is an uncommon phenomenon. Given this fact and the unclear therapeutic implications of identifying viral myocarditis in subjects with COVID-19, the authors believe EMB should not be routinely used and perhaps should be reserved for worst case scenarios such as patients with new-onset fulminant heart failure with hemodynamic compromise in the setting of documented COVID-19 infection.
Central Illustration.
Histological Evidence of Myocarditis in COVID-19 Infection and Numbers of Infiltrated Inflammatory Cells in Myocardium in COVID-19 Autopsy Hearts Without Myocarditis and in Non–COVID-19 Myocarditis
According to published data on pathological evidence for myocarditis in subjects with coronavirus disease-2019 (COVID-19), the rate of myocarditis is 4.5%. In our experience with 16 COVID-19 autopsy cases, no case met diagnostic criteria for myocarditis. In a comparison of inflammatory cells in the myocardium of subjects dying from traumatic versus COVID-19 deaths (but without a diagnosis of myocarditis), there were less cluster of differentiation (CD) 3–positive cells in COVID-19 cases and more CD68-positive cells.
Author Disclosures
This study was supported by the CVPath Institute, a 501(c)(3) foundation. CVPath Institute has received institutional research support from R01 HL141425 Leducq Foundation Grant, 480 Biomedical, 4C Medical, 4Tech, Abbott, Accumedical, Amgen, Biosensors, Boston Scientific, Cardiac Implants, Celonova, Claret Medical, Concept Medical, Cook, CSI, DuNing, Inc, Edwards LifeSciences, Emboline, Endotronix, Envision Scientific, Lutonix/Bard, Gateway, Lifetech, Limflo, MedAlliance, Medtronic, Mercator, Merill, Microport Medical, Microvention, Mitralign, MitrAssist, NAMSA, Nanova, Neovasc, NIPRO, Novogate, Occulotech, OrbusNeich Medical, Phenox, Profusa, Protembis, Qool, Recor, Senseonics, Shockwave, Sinomed, Spectranetics, Surmodics, Symic, Vesper, W.L. Gore, and Xeltis. Dr. Cornelissen has received research grants from University Hospital RWTH Aachen. Dr. Guagliumi has received institutional research grants from Abbott Vascular, Boston Scientific, and Infraredx; and has served as a consultant for Abbott Vascular and Boston Scientific. Dr. Virmani has received honoraria from Abbott Vascular, Biosensors, Boston Scientific, Celonova, Cook Medical, Cordis, CSI, Lutonix Bard, Medtronic, OrbusNeich Medical, CeloNova, SINO Medical Technology, Recor Medical, Terumo Corporation, W. L. Gore, and Spectranetics; and has served as a consultant for Abbott Vascular, Boston Scientific, Celonova, Cook Medical, Cordis, CSI, Edwards Lifesciences, Lutonix Bard, Medtronic, OrbusNeich Medical, ReCore, Sinomedical Technology, Spectranetics, Surmodics, Terumo Corporation, W. L. Gore, and Xeltis. Dr. Finn has received honoraria from Abbott Vascular, Biosensors, Boston Scientific, Celonova, Cook Medical, CSI, Lutonix Bard, Sinomed, and Terumo Corporation; and has served as a consultant to Amgen, Abbott Vascular, Boston Scientific, Celonova, Cook Medical, Lutonix Bard, and Sinomed. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Shi S., Qin M., Shen B. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lala A., Johnson K.W., Januzzi J.L. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol. 2020;76:533–546. doi: 10.1016/j.jacc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang L., Zhao P., Tang D. Cardiac involvement in patients recovered from COVID-2019 identified using magnetic resonance imaging. J Am Coll Cardiol Img. 2020;13:2330–2339. doi: 10.1016/j.jcmg.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Puntmann V.O., Carerj M.L., Wieters I. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajpal S., Tong M.S., Borchers J. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19 infection. JAMA Cardiol. 2020 Sep 11 doi: 10.1001/jamacardio.2020.4916. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindner D., Fitzek A., Brauninger H. Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol. 2020;5:1281–1285. doi: 10.1001/jamacardio.2020.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fox S.E., Li G., Akmatbekov A. Unexpected features of cardiac pathology in COVID-19 infection. Circulation. 2020;142:1123–1125. doi: 10.1161/CIRCULATIONAHA.120.049465. [DOI] [PubMed] [Google Scholar]
- 8.Varga Z., Flammer A.J., Steiger P. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldsmith C.S., Miller S.E., Martines R.B., Bullock H.A., Zaki S.R. Electron microscopy of SARS-CoV-2: a challenging task. Lancet. 2020;395:e99. doi: 10.1016/S0140-6736(20)31188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang Y., Liu J., Zhang D., Xu Z., Ji J., Wen C. Cytokine storm in COVID-19: the current evidence and treatment strategies. Front Immunol. 2020;11:1708. doi: 10.3389/fimmu.2020.01708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bikdeli B., Madhavan M.V., Jimenez D. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guagliumi G., Sonzogni A., Pescetelli I., Pellegrini D., Finn A.V. Microthrombi and ST-segment-elevation myocardial infarction in COVID-19. Circulation. 2020;142:804–809. doi: 10.1161/CIRCULATIONAHA.120.049294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choudry F.A., Hamshere S.M., Rathod K.S. High thrombus burden in patients With COVID-19 presenting with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2020;76:1168–1176. doi: 10.1016/j.jacc.2020.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bangalore S., Sharma A., Slotwiner A. ST-segment elevation in patients with Covid-19 - a case series. N Engl J Med. 2020;382:2478–2480. doi: 10.1056/NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richardson P., McKenna W., Bristow M. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of Cardiomyopathies. Circulation. 1996;93:841–842. doi: 10.1161/01.cir.93.5.841. [DOI] [PubMed] [Google Scholar]
- 16.Cooper L.T., Baughman K.L., Feldman A.M. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Endorsed by the Heart Failure Society of America and the Heart Failure Association of the European Society of Cardiology. J Am Coll Cardiol. 2007;50:1914–1931. doi: 10.1016/j.jacc.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 17.Aretz H.T. Myocarditis: the Dallas criteria. Hum Pathol. 1987;18:619–624. doi: 10.1016/s0046-8177(87)80363-5. [DOI] [PubMed] [Google Scholar]
- 18.Dennert R., Crijns H.J., Heymans S. Acute viral myocarditis. Eur Heart J. 2008;29:2073–2082. doi: 10.1093/eurheartj/ehn296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caforio A.L., Pankuweit S., Arbustini E. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–2648. doi: 10.1093/eurheartj/eht210. 2648a–d. [DOI] [PubMed] [Google Scholar]
- 20.Anderson D.W., Virmani R., Reilly J.M. Prevalent myocarditis at necropsy in the acquired immunodeficiency syndrome. J Am Coll Cardiol. 1988;11:792–799. doi: 10.1016/0735-1097(88)90213-6. [DOI] [PubMed] [Google Scholar]
- 21.Zhang M., Tavora F., Zhang Y. The role of focal myocardial inflammation in sudden unexpected cardiac and noncardiac deaths--a clinicopathological study. Int J Legal Med. 2013;127:131–138. doi: 10.1007/s00414-011-0634-x. [DOI] [PubMed] [Google Scholar]
- 22.Zhu Z., Lian X., Su X., Wu W., Marraro G.A., Zeng Y. From SARS and MERS to COVID-19: a brief summary and comparison of severe acute respiratory infections caused by three highly pathogenic human coronaviruses. Respir Res. 2020;21:224. doi: 10.1186/s12931-020-01479-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oudit G.Y., Kassiri Z., Jiang C. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest. 2009;39:618–625. doi: 10.1111/j.1365-2362.2009.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sala S., Peretto G., Gramegna M. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur Heart J. 2020;41:1861–1862. doi: 10.1093/eurheartj/ehaa286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tavazzi G., Pellegrini C., Maurelli M. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020;22:911–915. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Escher F., Pietsch H., Aleshcheva G. Detection of viral SARS-CoV-2 genomes and histopathological changes in endomyocardial biopsies. ESC Heart Fail. 2020;7:2440–2447. doi: 10.1002/ehf2.12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wenzel P., Kopp S., Gobel S. Evidence of SARS-CoV-2 mRNA in endomyocardial biopsies of patients with clinically suspected myocarditis tested negative for COVID-19 in nasopharyngeal swab. Cardiovasc Res. 2020;116:1661–1663. doi: 10.1093/cvr/cvaa160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bryce C., Grimes Z., Pujadas E. Pathophysiology of SARS-CoV-2: targeting of endothelial cells renders a complex disease with thrombotic microangiopathy and aberrant immune response. The Mount Sinai COVID-19 autopsy experience. medRxiv. 2020 May 22 [E-pub ahead of print] [Google Scholar]
- 29.Basso C., Leone O., Rizzo S. Pathological features of COVID-19-associated myocardial injury: a multicentre cardiovascular pathology study. Eur Heart J. 2020;41:3827–3835. doi: 10.1093/eurheartj/ehaa664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halushka M.K., Vander Heide R.S. Myocarditis is rare in COVID-19 autopsies: cardiovascular findings across 277 postmortem examinations. Cardiovasc Pathol. 2020;50:107300. doi: 10.1016/j.carpath.2020.107300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woloshin S., Patel N., Kesselheim A.S. False negative tests for SARS-CoV-2 infection - challenges and implications. N Engl J Med. 2020;383:e38. doi: 10.1056/NEJMp2015897. [DOI] [PubMed] [Google Scholar]
- 32.Chen L., Li X., Chen M., Feng Y., Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res. 2020;116:1097–1100. doi: 10.1093/cvr/cvaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramlall V., Thangaraj P.M., Meydan C. Immune complement and coagulation dysfunction in adverse outcomes of SARS-CoV-2 infection. Nat Med. 2020;26:1609–1615. doi: 10.1038/s41591-020-1021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan L., Wang Q., Zhang D. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5:33. doi: 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu Z., Shi L., Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. The Lancet Respiratory Medicine. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barton L.M., Duval E.J., Stroberg E., Ghosh S., Mukhopadhyay S. COVID-19 Autopsies, Oklahoma, USA. Am J Clin Pathol. 2020;153:725–733. doi: 10.1093/ajcp/aqaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bradley B.T., Maioli H., Johnston R. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. The Lancet. 2020;396:320–332. doi: 10.1016/S0140-6736(20)31305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buja L.M., Wolf D.A., Zhao B. The emerging spectrum of cardiopulmonary pathology of the coronavirus disease 2019 (COVID-19): Report of 3 autopsies from Houston, Texas, and review of autopsy findings from other United States cities. Cardiovasc Pathol. 2020;48:107233. doi: 10.1016/j.carpath.2020.107233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wichmann D., Sperhake J.P., Lutgehetmann M. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med. 2020;173:268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lax S.F., Skok K., Zechner P. Pulmonary arterial thrombosis in COVID-19 with fatal outcome: results from a prospective, single-enter, Clinicopathologic Case Series. Ann Intern Med. 2020;173:350–361. doi: 10.7326/M20-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tian S., Xiong Y., Liu H. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol. 2020;33:1007–1014. doi: 10.1038/s41379-020-0536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beigmohammadi MT, Jahanbin B, Safaei M, et al. Pathological findings of postmortem biopsies from lung, heart, and liver of 7 deceased COVID-19 patients. International Journal of Surgical Pathology 0:1066896920935195. [DOI] [PMC free article] [PubMed]
- 43.Rapkiewicz A.V., Mai X., Carsons S.E. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: A case series. EClinicalMedicine. 2020;24:100434. doi: 10.1016/j.eclinm.2020.100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fox S.E., Akmatbekov A., Harbert J.L., Li G., Quincy Brown J., Vander Heide R.S. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med. 2020;8:681–686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]