Abstract
Rationale & Objective
During the coronavirus disease 2019 (COVID-19) pandemic, patients receiving maintenance dialysis are a highly vulnerable population due to their comorbidities and circumstances that limit physical distancing during treatment. This study sought to characterize the risk factors for and outcomes following COVID-19 in this population.
Study Design
Retrospective cohort study.
Setting & Participants
Maintenance dialysis patients in clinics of a midsize national dialysis provider that had at least 1 patient who tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from February to June 2020.
Predictors
Demographics, dialysis characteristics, residence in a congregated setting, comorbid conditions, measurements of frailty, and use of selected medications.
Outcomes
COVID-19, defined as having a positive SARS-CoV-2 test result, and all-cause mortality among those with COVID-19.
Analytical Approach
Logistic regression analyses conducted to identify clinical characteristics associated with COVID-19 and risk factors associated with mortality among patients following COVID-19.
Results
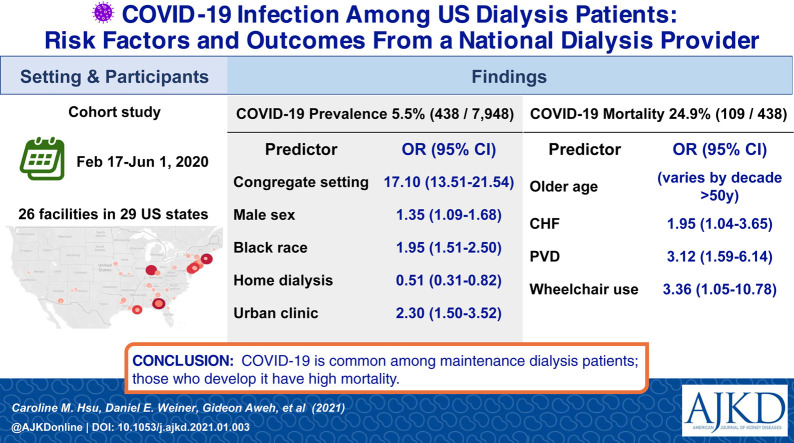
438 of 7948 (5.5%) maintenance dialysis patients developed COVID-19. Male sex, Black race, in-center dialysis (vs home dialysis), treatment at an urban clinic, residence in a congregate setting, and greater comorbidity were associated with contracting COVID-19. Odds of COVID-19 were 17-fold higher for those residing in a congregated setting (odds ratio [OR], 17.10 [95% CI, 13.51-21.54]). Of the 438 maintenance dialysis patients with COVID-19, 109 (24.9%) died. Older age, heart disease, and markers of frailty were associated with mortality.
Limitations
No distinction was detected between symptomatic and asymptomatic SARS-CoV-2 positivity, with asymptomatic screening limited by testing capacity during this initial COVID-19 surge period.
Conclusions
COVID-19 is common among patients receiving maintenance dialysis, particularly those residing in congregate settings. Among maintenance dialysis patients with COVID-19, mortality is high, exceeding 20%.
Index Words: coronavirus disease 2019 (COVID-19), death, dialysis clinic, dialysis, end-stage kidney disease (ESKD), end-stage renal disease (ESRD), epidemiology, mortality, nursing home, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)
Graphical abstract
Plain-Language Summary.
Maintenance dialysis patients are a vulnerable population during the COVID-19 pandemic due to their comorbid conditions and limited ability to physically distance themselves from others. This cohort study of risk factors for and outcomes from COVID-19 among patients receiving maintenance dialysis showed a 5.5% rate of infection in clinics with at least 1 case and that residence in a congregate setting was the strongest risk factor for disease. During this initial COVID-19 surge period, patients receiving maintenance dialysis who contracted COVID-19 had a mortality rate approaching 25%, far higher than that for the general population. These data suggest that the maintenance dialysis population requires substantial support during the pandemic, whether through expanded testing, early vaccination, or coordination of complex care.
Patients with kidney failure who receive maintenance dialysis are particularly vulnerable to coronavirus disease 2019 (COVID-19), the disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The average age of those receiving maintenance dialysis is 65 years old and these patients typically have multiple medical comorbid conditions, including diabetes, obesity, and uremia-induced impaired immunity, all of which increase their risk for poor outcomes should they develop COVID-19.1, 2, 3, 4, 5, 6 As a more socioeconomically disadvantaged group than the general population, including residence in more densely populated urban areas, this population is at higher risk of acquiring SARS-CoV-2.7 , 8 Additionally, most dialysis patients cannot practice effective physical distancing due to the need for in-center hemodialysis and associated transportation, as well as frequent reliance on social support and caregivers for assistance with daily activities or medication management. Confluence of these factors potentiates risk in the many who reside in long-term care facilities.9, 10, 11, 12
Studies in China, Italy, the United Kingdom, and the United States (New York and southern California) showed that maintenance dialysis patients with COVID-19 experience higher mortality and more severe disease than the general population.13, 14, 15, 16, 17, 18, 19 As of 2017, approximately 524,000 patients in the United States were receiving dialysis, constituting a large at-risk population.11 By June of 2020, the Centers for Medicare & Medicaid Services reported a COVID-19 prevalence of 3.8% in the dialysis population,20 but further characterization is needed. Given the lack of national, generalizable data on COVID-19 in the vulnerable maintenance dialysis population, we characterized risk factors for COVID-19 among dialysis patients treated by a medium-sized national dialysis organization. Additionally, among those with COVID-19, we characterized the risk of and risk factors for death.
Methods
Dialysis Clinic Inc. (DCI) is a national not-for-profit dialysis provider caring for more than 15,000 maintenance dialysis patients at 260 outpatient dialysis clinics in 29 states. All dialysis patients with the diagnosis of COVID-19 in DCI clinics between February 17, 2020, and June 1, 2020, were included in this retrospective cohort study, with outcomes ascertained through August 31, 2020. This initial 15-week period reflects the early surge of COVID-19 in the United States. We sought to describe our outpatient dialysis provider experience and determine risk factors for COVID-19 as well as for all-cause death within 90 days of a COVID-19 diagnosis. To mitigate bias from geographic imbalance of COVID-19 spread, in this analysis we only included patients from clinics with at least 1 case diagnosed with COVID-19. All patients in each of these clinics were included, but patients being treated with dialysis for acute kidney injury were excluded.
Based on contemporary practices, SARS-CoV-2 infection status was assessed by nasopharyngeal or oropharyngeal swabs sent locally for reverse transcriptase–polymerase chain reaction (RT-PCR) testing that was approved by the US Food and Drug Agency under Emergency Use Authorization or, if unavailable locally, through DCI Labs contracted vendor beginning in April 2020 (using either the Hologic Panther Fusion or Cobas SARS-CoV-2 Assay [Roche Diagnostics]). Although facility and regional practices differed, positive test results were captured regardless of whether a patient was assessed in the dialysis clinic, or at a testing center, or at a hospital. In accordance with guidance from Centers for Disease Control and Prevention at the time,21 prior to each treatment, patients were identified for testing by the dialysis staff through screening for recent exposure to a known COVID-19 patient, travel history to an endemic area, and/or symptoms of cough, dyspnea, fever, sore throat, and unexplained oxygen saturation <92%. Of note, only 1 DCI clinic performed routine RT-PCR testing of dialysis patients regardless of screening results. All patients with a positive SARS-CoV-2 test result were defined as having COVID-19. Patients without a positive SARS-CoV-2 test result were considered to be without COVID-19. Procedures regarding isolation varied by center and by whether COVID-19 was suspected or confirmed, reflecting local resources and availability of isolation rooms. Depending on availability, many clinics cohorted patients with COVID-19 together, often on a dedicated COVID-19 shift, either at that facility or at a nearby clinic, while others treated patients with suspected or confirmed COVID-19 in isolation rooms with dedicated staff. Universal masking policies were adopted in all clinics by the end of March.
Demographic, comorbid, and clinical information including emergency department and/or hospitalization events and death reports (Centers for Medicare & Medicaid Services form 2746) were obtained from DCI’s proprietary outpatient electronic health record. Patient characteristics obtained from the electronic health record included age, sex, race, ethnicity, dialysis vintage, dialysis modality, treatment at an urban or rural clinic, use of a central venous catheter, living in a congregate setting at the time of diagnosis (eg, nursing home, skilled nursing facility, or rehabilitation facility), body mass index, number of comorbid conditions and specific comorbid conditions (eg, diabetes mellitus, hypertension, congestive heart failure, atherosclerotic heart disease, cerebrovascular disorders, other cardiac diseases, chronic obstructive pulmonary disease, peripheral vascular disease, cancer), history of limb amputation, use of a wheelchair, need for assistance to perform activities of daily living (ADL), alcohol abuse, drug abuse, tobacco use, vaccination for influenza during the 2019-2020 flu season; documented pneumococcal vaccination, serum albumin level most proximate to diagnosis, and use of selected medication groups (eg, angiotensin-converting enzyme inhibitors [ACEIs] or angiotensin receptor blockers [ARBs], inhaled respiratory agents [any anticholinergic, beta-agonist, and/or corticosteroid inhaler], or corticosteroids).
Primary outcomes of interest were diagnosis of COVID-19, and, among those with COVID-19, all-cause death within 90 days of COVID-19 diagnosis compared to those without a diagnosis of COVID-19. Among those patients without COVID-19, all-cause death was defined by death within 90 days of the first COVID-19 diagnosis at the source clinic. All available electronic records delineating potential cause(s) of deaths were reviewed by 2 investigators (E.C.L. and E.L., Jr.) to determine primary events leading to death as well as any delineation of the potential role of COVID-19. Furthermore, although hospitalization records were not routinely available, the time course of illness and relevant time to events were tracked through documented dates of COVID-19 diagnosis, hospital admission, hospital discharge, and death. Patients were followed until death or 90 days, whichever came sooner, through September 1, 2020.
Two sets of analyses were conducted. The first analysis explored risk factors for acquiring COVID-19 among dialysis patients, whereas the second analysis explored risk factors for death among the subset of dialysis patients with COVID-19. Factors associated with a COVID-19 diagnosis were assessed by using univariate logistic regression analyses, using the list in the previous paragraph. The comparator group included all patients without COVID-19 diagnoses treated in the origin clinics of the COVID-19 cases; clinics with no COVID-19 cases through June 1, 2020, were excluded from the comparator group. This approach was chosen to minimize the influence of geographic factors in models because COVID-19 prevalence varied greatly across the country during the study period. Quantitative variables were presented as means ± SD and medians. For simplicity, age was represented as decades between 50 and 80 years old. For albumin, concentrations were categorized as severe hypoalbuminemia (<3.5 g/dL), mild to moderate hypoalbuminemia (3.5-3.9 g/dL), and normal (≥ 4.0 g/dL). In addition to age, sex, and race, of the variables that were prespecified a priori, only candidate variables significant at P < 0.1 were included in multivariable analyses. Similarly, logistic regression models were constructed among patients with COVID-19 to assess patient characteristics related to mortality from COVID-19. Subgroup analyses were performed by stratifying on residence in a congregate setting. For consistency in comparisons, multivariable analyses of these subgroups incorporated the same variables used in the multivariable analysis of the aggregate. Statistical significance was defined as P ≤0.05.
This retrospective de-identified evaluation was performed under exemption from informed consent through the DCI “Chronic Kidney Disease Quality Improvement and Clinical Outcomes” evaluation process, through the Western Institutional Review Board (WIRB). All statistical analyses were performed using SAS version 9.4 software (SAS Software). We used t-tests, analysis of variance (ANOVA), and χ2 tests to compare data for most continuous, categorical, and dichotomous variables, respectively, by COVID-19 status. Exceptions were use of the Kruskal-Wallis test for dialysis vintage and body mass index and use of the Fisher exact test for assistance with ADLs.
Results
A total of 438 maintenance dialysis patients had diagnoses of COVID-19 during the 15-week study period, representing approximately 3% of 15,200 patients treated in 260 DCI clinics. Not all states and DCI clinics were exposed to COVID-19 during this early surge; the true denominator included patients from 96 clinics from 22 states with at least 1 affected patient, making up 52% of all DCI patients (Fig S1). Therefore, these patients represented 438 of 7,948 (5.5%) of the dialysis population in impacted clinics. Compared to patients without COVID-19 in these clinics, dialysis patients with COVID-19 were older, more likely to be of Black race, more likely to be treated with in-center hemodialysis, more likely to be treated at an urban clinic, more likely to use respiratory inhalers, and more likely to reside in a congregate setting (eg, a nursing home). Additionally, those with COVID-19 had a higher burden of comorbidity, with higher prevalence of cardiovascular diseases, diabetes, and requirement for assistance with ADLs and/or use of a wheelchair (Table 1 ).
Table 1.
Characteristics and Mortality of Patients With and Without Diagnoses of COVID-19 From DCI Dialysis Facilities With at Least One Affected Patient From February-June 2020
| With COVID-19 Diagnosis | Without COVID-19 Diagnosis | P | |
|---|---|---|---|
| No. of patients | 438 (5.5%) | 7,510 (94.5%) | |
| Age, y | 65.2 ± 13.2 (67.0) | 62.0 ± 14.9 (63.4) | <0.001a |
| <50 y | 54 (12.3%) | 1,527 (20.3%) | |
| 51-59 y | 91 (20.8%) | 1,544 (20.6%) | |
| 60-69 y | 124 (28.3%) | 2,023 (26.9%) | |
| 70-79 y | 114 (26.0%) | 1,608 (21.4%) | |
| 80+ y | 55 (12.6%) | 808 (10.8%) | |
| Male sex | 264 (60.3%) | 4,281 (57.0%) | 0.2 |
| Race | <0.001a | ||
| White | 115 (26.3%) | 2,741 (36.5%) | |
| Black | 253 (57.8%) | 3,426 (45.6%) | |
| Other/not stated | 70 (16.0%) | 1,343 (17.9%) | |
| Ethnicity | 0.6 | ||
| Hispanic | 37 (8.5%) | 597 (8.0%) | |
| Non-Hispanic | 335 (76.5%) | 5,653 (75.3%) | |
| Not stated | 66 (15.1%) | 1,260 (16.8%) | |
| Dialysis vintage, mo | 56.0 ± 55.4 (39.4) | 54.9 ± 58.2 (36.9) | 0.2 |
| <3 mo | 29 (6.6%) | 536 (7.1%) | |
| 3-12 mo | 51 (11.6%) | 1,028 (13.7%) | |
| 12-36 mo | 120 (27.4%) | 2,137 (28.5%) | |
| >36 mo | 238 (54.3%) | 3,809 (50.7%) | |
| Home dialysis modality | 18 (4.1%) | 1,070 (14.3%) | <0.001a |
| Urban clinic | 412 (94.1%) | 6,613 (88.1%) | <0.001a |
| Central venous catheter | 104 (23.7%) | 1,621 (21.6%) | 0.3 |
| Congregate setting | 239 (54.6%) | 511 (6.8%) | <0.001a |
| Albumin, g/dL | 3.8 ± 0.4 (3.8) | 3.9 ± 0.4 (3.9) | <0.001a |
| <3.5 g/dL | 84 (19.2%) | 1,037 (13.8%) | |
| 3.5-3.9 g/dL | 184 (42.0%) | 2,853 (38.0%) | |
| ≥4 g/dL | 147 (35.8%) | 3,365 (44.8%) | |
| Body mass index, kg/m2 | 29.0 ± 7.5 (27.5) | 28.5 ± 7.5 (27.2) | 0.1 |
| Tobacco use | 72 (16.4%) | 1,230 (16.4%) | 0.9 |
| ACEI/ARB | 141 (32.2%) | 2,430 (32.4%) | 0.9 |
| Inhaled respiratory agent | 109 (24.9%) | 1,503 (20.0%) | 0.01a |
| Corticosteroid | 27 (6.2%) | 557 (7.4%) | 0.3 |
| Pneumococcal vaccination | 385 (87.9%) | 6,531 (87.0%) | 0.6 |
| 2019 Influenza vaccination | 362 (82.7%) | 6,072 (80.9%) | 0.4 |
| No. of comorbidities | 3.3 ± 1.9 (3.0) | 3.0 ± 1.8 (3.0) | 0.003a |
| Comorbidity | |||
| Diabetes mellitus | 306 (69.9%) | 4,367 (58.2%) | <0.001a |
| Hypertension | 346 (79.0%) | 6,208 (82.7%) | 0.05a |
| CHF | 104 (23.7 %) | 1,580 (21.0%) | 0.2 |
| Atherosclerotic heart disease | 118 (26.9%) | 1,650 (22.0%) | 0.02a |
| Stroke | 51 (11.6%) | 665 (8.9%) | 0.05a |
| Other cardiovascular | 162 (37.0%) | 2,550 (34.0%) | 0.2 |
| COPD | 66 (15.1%) | 1,053 (14.0%) | 0.5 |
| PVD | 68 (15.5%) | 1,115 (14.9%) | 0.7 |
| Amputation history | 14 (3.2%) | 182 (2.4%) | 0.3 |
| Assistance with ADL | 4 (0.9%) | 25 (0.3%) | 0.07 |
| Cancer history | 36 (8.2%) | 6161 (8.2%) | 0.9 |
| Alcohol abuse history | 56 (12.8%) | 907 (12.1%) | 0.7 |
| Drug abuse history | 29 (6.6%) | 427 (5.7%) | 0.4 |
| Wheelchair use | 15 (3.4%) | 147 (2.0%) | 0.03a |
| Death | 109 (24.9%) | 275 (3.7%) | <0.001a |
| Days to death | 16.7 ± 17.1 (11.0) | 43.4 ± 26.7 (45.0) | <0.001a |
Data are mean ± SD (median) or number (%). Race and ethnicity are independent of each other. We used t-test, ANOVA, and χ2 tests to compare data for most continuous, categorical, and dichotomous variables, respectively, by COVID-19 status. Exceptions are the use of the Kruskal-Wallis test for dialysis vintage and body mass index and use of the Fisher exact test for assistance with ADL.
Abbreviations: ADL, activities of daily living; ANOVA, analysis of variance; ARB, angiotensin receptor blocker; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; PVD, peripheral vascular disease.
Statistically significant.
In multivariable models, risk factors associated with COVID-19 infection in maintenance dialysis included male sex, Black race, treatment at an urban clinic, residence in a congregate setting, comorbid conditions, and carrying a diagnosis of atherosclerotic heart disease and diabetes. By contrast, individuals treated with home dialysis were less likely to have COVID-19 infection (Table 2 ).
Table 2.
Association Between Clinical Characteristics and COVID-19 Diagnoses Among Dialysis Patients
| OR (95% CI) | P | |
|---|---|---|
| Age | ||
| 60-69 y | 1.14 (0.80-1.64) | 0.5 |
| 70-79 y | 1.14 (0.78-1.65) | 0.5 |
| ≥80 y | 1.16 (0.75-1.78) | 0.5 |
| Male sex | 1.35 (1.09-1.68) | 0.01a |
| Black vs White race | 1.95 (1.51-2.50) | <0.001a |
| Other race vs White race | 1.27 (0.91-1.77) | 0.2 |
| Home dialysis | 0.51 (0.31-0.82) | 0.01a |
| Urban clinic | 2.30 (1.50-3.52) | 0.001a |
| Congregate setting | 17.10 (13.51-21.54) | <0.001a |
| Albumin <3.5 g/dL | 0.88 (0.66-1.17) | 0.3 |
| Inhaled respiratory agents | 1.15 (0.89-1.49) | 0.3 |
| No. of comorbiditiesb | 0.89 (0.81-0.97) | 0.01a |
| Diabetes mellitus | 1.36 (1.06-1.75) | 0.02a |
| Hypertension | 0.91 (0.67-1.22) | 0.5 |
| Atherosclerotic heart disease | 1.42 (1.04-1.94) | 0.03a |
| Stroke | 1.03 (0.72-1.47) | 0.9 |
| Assistance with ADL | 1.33 (0.39-4.48) | 0.7 |
| Wheelchair use | 0.89 (0.48-1.66) | 0.7 |
Entry in the logistic model requires P < 0.1 in univariate analysis, except for sex, which was forced in. N = 7,948. Abbreviation: ADL, activities of daily living.
Statistically significant.
Relative risk per additional comorbid illness category among comorbidities listed in Table 1.
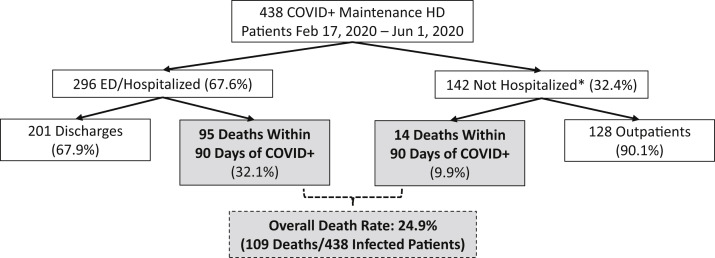
All 438 dialysis patients with diagnoses of COVID-19 were either followed until death or censored at 90 days. Death occurred in 109 of 438 COVID-19 patients (24.9%), compared to 275 of 7,510 (3.7%) of the non–COVID-19 cohort (Table 1, Fig 1 ). Among the 438 patients diagnosed with COVID-19, 296 patients (67.6%) received emergency department (ED) and/or inpatient hospital care either at the time of diagnosis or within 30 days following diagnosis (Fig 1). Of those 296 patients, 95 (32.1%) died. Of those 95 deaths, 86 deaths occurred during hospitalization, and 9 deaths occurred after initial hospital discharge. The remaining 201 patients (67.9%) with a hospital encounter were considered recovered by the end of follow-up. Among the 142 patients (32.4%) who were not initially hospitalized, 14 died within 30 days of diagnosis (8 in nursing homes, 2 in hospice, 2 with unknown site of death, and 2 at home), and another 9 patients were hospitalized 30 days or more after diagnosis. Three of those 9 patients were admitted due to COVID-19, whereas the other 6 received inpatient care for various medical conditions including vascular access issues. All 128 surviving outpatients were considered recovered from COVID-19 as of the end of follow-up.
Figure 1.
The patient flowchart tracks the clinical course of maintenance dialysis patients with SARS-CoV-2 infection (diagnosed COVID-19) treated in DCI clinics. ED, emergency department; DCI, Dialysis Clinic Inc; HD, hemodiaysis. ∗Hospitalization was defined as being in the ED or hospital on the date of COVID-19 diagnosis or within 30 days thereafter.
Among the 296 patients who went to the ED or hospital, including 25 patients who received diagnoses while hospitalized, more than half the patients were tested upon arrival to the ED or on the day of inpatient admission, with diagnosis based on results of that test. Thus, although the average time from COVID-19 diagnosis to hospitalization was 2.8 ± 5.9 days (range, 1-28 days), the median time corresponded to the first day. The overall length of stay after COVID-19 diagnosis for all hospitalized patients was 10.8 ± 10.7 days, with a median length of stay of 8 (interquartile range [IQR], 4-14) days. Among patients who survived until discharge, length of stay was 11.1 ± 11.7 days, with a median length of stay of 8 [IQR, 4-13] days.
Among the 109 patients who died, the mean time to death was 16.7 ± 17.1 days, with the median being 11 [IQR, 5.5-22] days. One patient died in the ED. Approximately half of the deaths had COVID-19 mentioned specifically as the primary or secondary cause of death, with additional notes in the electronic health records for all but 6 patients indicating that COVID-19 may have contributed to death. Of those 6 patients, one-half had a death date with no documented information surrounding the death. Immediate cause of death was most often attributed to pulmonary causes (51.9%), followed by cardiac (9.3%), and a combination of cardiac and pulmonary causes (7.4%). There were 8.3% of cases that specifically mentioned infection/sepsis and 2.8% due to hemorrhage. There were 20 deaths (18.5%) with the primary cause described only as cardiopulmonary arrest without further information or no information surrounding death circumstances recorded at all. Overall, 10 patients withdrew from dialysis (6 residing in a nursing home, 3 while in the hospital, and 1 at home), 9 of whom received hospice care prior to death.
Characteristics of patients who died compared to those who survived are shown in Table 3 . Older patients had higher mortality, particularly those over 80 years old. Comorbidity related to chronic heart failure, hypertension, and other cardiovascular diseases as well as peripheral vascular disease, low albumin level, and use of a wheelchair were more prevalent in patients who died. In multivariable models, older age became strongly associated with death, with increased risk in each decade after age 50, along with peripheral vascular disease, chronic heart failure, hypertension, and wheelchair use remaining significantly associated with mortality risk (Table 4 ).
Table 3.
Characteristics of Patients With Documented COVID-19 by Vital Status
| Died | Survived | |
|---|---|---|
| No. of patients | 109 (24.9%) | 329 (75.1%) |
| Age, y | 70.8 ± 10.3 (70.5) | 63.3 ± 13.6 (64.2) |
| <50 y | 2 (1.8%) | 52 (15.8%) |
| 51-59 y | 17 (15.6%) | 74 (22.5%) |
| 60-69 y | 34 (31.2%) | 90 (27.4%) |
| 70-79 y | 33 (30.3%) | 81 (24.6%) |
| 80+ y | 23 (21.1%) | 32 (9.7%) |
| Male sex | 70 (64.2%) | 194 (59.0%) |
| Race | ||
| White | 36 (33.0%) | 79 (24.0%) |
| Black | 56 (51.4%) | 197 (60.0%) |
| Other/not stated | 17(15.6%) | 53(16.1%) |
| Ethnicity | ||
| Hispanic | 10 (9.2%) | 27 (8.2%) |
| Non-Hispanic | 87 (79.8%) | 248 (75.4%) |
| Not stated | 12 (11.0%) | 54 (16.4%) |
| Dialysis vintage, mo | 63.4 ± 61.6 (43.6) | 53.6 ± 53.0 (39.3) |
| <3 mo | 5 (4.6%) | 24 (7.3%) |
| 3-12 mo | 10 (9.2%) | 41 (12.5%) |
| 12-36 mo | 28(25.7%) | 92(28.0%) |
| >36 mo | 66(60.6%) | 172(52.3%) |
| Home dialysis modality | 3(2.8%) | 15(4.6%) |
| Central venous catheter | 30(27.5%) | 74(22.5%) |
| Congregate setting | 65(59.6%) | 174(52.9%) |
| Urban clinic | 103(94.5%) | 309(93.9%) |
| Albumin, g/dL | 3.7 ± 0.5 (3.7) | 3.8 ± 0.4 (3.9) |
| <3.5 g/dL | 28 (25.7%) | 56 (17.0%) |
| 3.5-3.9 g/dL | 45 (41.3%) | 139 (42.3%) |
| ≥4 g/dL | 36 (33.0%) | 121 (36.8%) |
| Body mass index, kg/m2 | 28.9 ± 6.9 (26.9) | 29.1 ± 7.7 (27.5) |
| Tobacco use | 19 (17.4%) | 53 (16.1%) |
| ACEI/ARBs | 28 (25.7%) | 113 (34.4%) |
| Inhaled respiratory agent | 35 (32.4%) | 74 (22.5%) |
| Corticosteroid | 12 (11.0%) | 15 (4.6%) |
| Pneumococcal vaccination | 94 (86.2%) | 291 (88.5%) |
| 2019 Influenza vaccination | 88 (80.7%) | 274 (83.3%) |
| No. of comorbidities | 3.7 ± 2.2 (3.0) | 3.2 ± 1.8 (3.0) |
| Comorbidity | ||
| Diabetes mellitus | 75 (68.8%) | 231 (70.2%) |
| Hypertension | 79 (72.5%) | 267 (81.2%) |
| CHF | 37 (33.9%) | 67 (20.4%) |
| Atherosclerotic heart disease | 33 (30.3%) | 84 (25.8%) |
| Stroke | 13 (11.9%) | 38 (11.6%) |
| Other Cardiovascular | 53 (48.6%) | 109 (33.1) |
| COPD | 19 (17.4%) | 47 (14.3%) |
| PVD | 29 (26.6%) | 39 (11.9%) |
| Amputation history | 5 (4.6%) | 9 (2.7%) |
| Assistance with ADL | 2 (1.8%) | 2 (0.6%) |
| Cancer history | 11 (10.1%) | 25 (7.6%) |
| Alcohol abuse history | 13 (11.9%) | 43 (13.1%) |
| Drug abuse history | 5 (4.6%) | 24 (7.3%) |
| Wheelchair use | 8 (7.3%) | 7 (2.1%) |
Data are mean ± SD (median) or number (%). Age is reported in years and dialysis duration (vintage) in months. Race and ethnicity are independent of each other.
Abbreviations: ADL, activities of daily living; ARB, angiotensin receptor blocker; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; PVD, peripheral vasculardisease.
Table 4.
Association Between Clinical Characteristics and All-Cause Death Among Dialysis Patients With COVID-19
| OR (95% CI) | P | |
|---|---|---|
| Age category | ||
| <50 y | 1.00 (reference) | |
| 50-59 y | 5.57 (1.18-26.39) | 0.03a |
| 60-69 y | 8.52 (1.87-38.80) | 0.01a |
| 70-79 y | 9.48 (2.07-43.37) | 0.004a |
| 80+ y | 15.55 (3.25-74.34) | 0.001a |
| Male sex | 1.25 (0.75-2.08) | 0.4 |
| Black vs White race | 0.69 (0.40-1.20) | 0.2 |
| Other race vs White race | 0.68 (0.32-1.43) | 0.3 |
| Congregate setting | 0.91 (0.55-1.52) | 0.7 |
| Albumin <3.5 g/dL | 1.37 (0.77-2.46) | 0.3 |
| ACEI/ARB | 0.78 (0.45-1.33) | 0.4 |
| Inhaled respiratory agent | 1.33 (0.75-2.36) | 0.3 |
| Corticosteroid | 2.19 (0.89-5.37) | 0.09 |
| No. of comorbidities | 0.86 (0.68-1.09) | 0.2 |
| Hypertension | 0.50 (0.26-0.96) | 0.04a |
| Congestive heart failure | 1.95 (1.04-3.65) | 0.04a |
| Other cardiovascular | 1.74 (0.88-3.43) | 0.1 |
| Peripheral vascular disease | 3.12 (1.59-6.14) | 0.001a |
| Wheelchair use | 3.36 (1.05-10.78) | 0.04a |
Entry in logistic model requires P < 0.1 in univariate analysis, except for sex and race, which were forced in. N = 438.
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Statistically significant.
In subgroup analysis with stratification by residence in a congregate setting, 199 of 7,198 patients (2.8%) in noncongregate settings had COVID-19, compared to 239 of 750 patients (31.9%) among residents of congregate settings. Of those with COVID-19, noncongregate mortality was 22.1% and congregate mortality was 27.2% (Tables S1-S2). Multivariable analysis of the subgroups showed risk factors for COVID-19 to be similar to risk factors identified in aggregate analysis (Tables S3-S4).
Discussion
Maintenance dialysis patients were found to be at risk of developing COVID-19, and among those with COVID-19, 90-day mortality approached 25%. Of note, the current study extended outcome follow-up to 90 days, compared to most initial reports that covered only early and short-term outcomes. Among dialysis patients, key risk factors for COVID-19 included residence in a long-term care facility, Black race, male sex, diabetes, receipt of in-center compared to home dialysis, and treatment at an urban dialysis center. In dialysis patients who developed COVID-19, older age, hypertension, congestive heart failure, peripheral vascular disease, and wheelchair use were associated with higher risk of death.
Studies have shown a close interrelationship between socioeconomic status and health outcomes, further exacerbated by systemic racial biases.22 The ability to maintain physical distancing through low household density, working from home, or accepting a loss of income, is a matter of privilege and more often the territory of more affluent communities. In contrast, urban areas of high density, which tend to have a greater non-White population, reflect communities in which a pandemic is more likely to take hold,8 highlighted by a higher prevalence of medical comorbid conditions. That our study shows worse outcomes with non-White race, more comorbid conditions, and treatment at urban dialysis centers reflects this tangle of many factors. Of note, unlike in the general population, Hispanic ethnicity was not associated with either risk of acquiring COVID-19 or risk of mortality in patients with COVID-19. It is possible that the locations of DCI clinics with COVID-19 patients generally had a lower proportion of people who identify as Hispanic or that missing ethnicity data impacted results.
Among dialysis patients, those residing in a congregate setting had more than a 17-fold higher rate of COVID-19 than those residing independently, a pattern that, although commented on, has not been well quantified in national studies.23 , 24 In congregate residences such as group homes and nursing facilities, the capacity to isolate COVID-positive patients is often limited, staffing may be stretched as employees fall ill, and staff members carry infectious risk to the community when they leave the facility. There may be a role for universal screening in such congregate settings, particularly when at least 1 case is identified.25 Moreover, patients living in congregate settings are often older and more likely to be frail; the association between mortality and markers of frailty such as age, peripheral vascular disease, and decreased mobility shows that COVID-19 in the congregate setting is all the more damaging.
Reflecting those factors, communities with dense urban populations, Black and Hispanic neighborhoods, and high numbers of nursing home residents have the greatest need for support during this pandemic. Measures such as expanded testing, community education, promoting capacity for telemedicine, and financial support to facilitate physical distancing would reduce transmission. Because a large proportion of dialysis patients derive from these communities, nephrologists have a responsibility to advocate better disease containment and greater relief for these communities. Furthermore, compared to other disadvantaged groups, dialysis patients almost constantly interface with the health care system; accordingly, public health departments can and should coordinate with dialysis providers to provide interventions such as expanded testing and possibly vaccination prioritization.
The mandatory congregate settings and the associated commutes can create additional exposure risk for the majority of dialysis patients, as demonstrated by the increased risk associated with in-center dialysis relative to home dialysis. Patients performing home dialysis tend to be younger and healthier than patients treated by in-center hemodialysis.11 For in-center hemodialysis patients, enhanced and universal sanitation protocols are a necessity, including for the transportation services involved, and dialysis facilities have taken steps to protect patients, including emptying waiting rooms, screening patients for symptoms, and isolating those who screen positive, increasing spacing within dialysis facilities, and implementing universal masking for patients and staff.9 , 26 Indeed, while transmission of COVID-19 has been demonstrated to occur in congregate settings such as in nursing homes, it has seldom been reported to occur within the outpatient dialysis clinic where infection control precautions are closer to those in the hospital setting than those of nursing homes. Our experience underscores the very high risk of infection in dialysis patients residing in congregate facilities, emphasizing the need to view these patients as being at high risk and potential candidates for enhanced screening, including asymptomatic testing, and highlighting the critical need for improved communication between long-term care facilities and dialysis facilities. Critically, the COVID-19 pandemic has emphasized the need to increase the use of home dialysis and kidney transplantation for treatment of kidney failure.
Improving outcomes in patients with COVID-19 requires particular attention in the dialysis population, focusing on prevention of SARS-CoV-2 infection as well as evaluation of vaccines and novel treatments in the vulnerable dialysis population. Although COVID-19 care in dialysis patients remains largely supportive, practices implemented at the systems level can have a meaningful impact. Similar to other studies,17 , 19 we observed that approximately 60% of COVID-positive dialysis patients were hospitalized, translating to a need for adequate resource planning for in-hospital dialysis equipment, consumables, and staffing to minimize strain, particularly given the associated system strain with the high incidence of acute kidney injury requiring dialysis among critically ill COVID-19 patients. With admissions averaging 11 days’ duration in an already frail population, a number of patients may be expected to need rehabilitation; therefore, coordination among hospitals, dialysis centers, rehabilitation facilities, and long-term care facilities is critical to avoid medical errors and readmissions. In early studies, COVID-19 survivors presented a spectrum of persistent pulmonary dysfunction, and they are expected to have a high prevalence of post–intensive care syndrome, which encompasses reduced physical, cognitive, and mental health after critical illness.27 Therefore, maintenance dialysis patients who survive COVID-19 may benefit from interdisciplinary posthospitalization care. Also, this study and others demonstrate that mortality exceeds 20% among maintenance dialysis patients.17, 18, 19 , 28 , 29 Therefore, discussions around advance care planning should be considered to avoid aggressive medical therapy when it is incongruent with the patient’s life goals and values; this is particularly true of those with advanced age and with other indicators of frailty, which were associated with mortality in our study. The appropriate planning for and implementation of palliative care and hospice is critical in this population, particularly with the devastating impact of acute illnesses like COVID-19.
Our study of the COVID-19 pandemic in the outpatient dialysis setting uses a large population derived from a national dialysis provider with longitudinal patient follow-up for outcomes. We nevertheless acknowledge the study’s limitations. It focuses on observational data acquired from the dialysis provider, rendering specific details surrounding in-hospital events less available. Second, very limited testing availability, particularly in March through June 2020, may have resulted in underestimation of COVID-19 cases and misclassification of deaths. Third, given the extensive comorbid condition burden in dialysis patients with COVID-19, determining the cause of death in this population remains somewhat subjective, and we have restricted analyses to all-cause death. Fourth, we excluded dialysis clinics with no COVID-19 cases to facilitate comparison between COVID-19 and non–COVID-19 patients. This may result in other biases being introduced into models, and this restriction prevents comparisons with geographic regions where the incidence of COVID-19 was very low during this early pandemic surge (ie, where COVID-19 cases were unlikely in the dialysis clinic). Fifth, because our study defined all patients with a positive SARS-CoV-2 test result as having COVID-19, we did not distinguish asymptomatic carriers from those with symptomatic disease; currently, the impact of SARS-CoV-2 vaccines on asymptomatic carriage and transmission is unknown, so this distinction will need greater investigation and scrutiny as vaccinations occur. Sixth, we found very few home dialysis patients diagnosed during this period, which may be due in part to more frequent opportunities for screening and testing for the in-center hemodialysis population. However, we note that there are far fewer home dialysis patients living in congregate settings and overall testing capacity was very limited during this time frame, so symptomatic patients were likely to be brought to the ED and/or be hospitalized, potentially mitigating this issue.
In conclusion, dialysis patients are at high risk of COVID-19, with 5.5% of patients with a diagnosis of COVID-19 in facilities with at least 1 COVID-19 case during the first 3 months of the pandemic in the United States. In-center dialysis, residence in a long-term care facility, and Black race are all major risk factors associated with COVID-19, suggesting that the ability to maintain physical distance is critical to controlling COVID-19. Mortality among dialysis patients with COVID-19 exceeds 20%. To address COVID-19 while awaiting availability of safe and effective vaccines and therapeutics, dialysis facilities should recognize risk factors, maximize utility of telemedicine, promote home dialysis, encourage transplantation where appropriate, and optimize socioculturally adapted education regarding physical distancing and universal precautions including masking, not limited to the dialysis clinic but relevant to all other at-risk settings.
Article Information
Authors’ Full Names and Academic Degrees
Caroline M. Hsu, MD, Daniel E. Weiner, MD, MS, Gideon Aweh, MS, Dana C. Miskulin, MD, MS, Harold J. Manley, PharmD, Carol Stewart, RN, Vlad Ladik, MS, John Hosford, MBA, Edward C. Lacson, Douglas S. Johnson, MD, JD, and Eduardo Lacson Jr, MD, MPH.
Authors’ Contributions
Study design: CMH, DEW, EL; collected and verified data: ECL, GA, CS, JH, VL, EL; analyzed the data: GA, EL; interpreted the data: CMH, DEW, DCM, HJM, DSJ, EL. Each author contributed important intellectual content during manuscript drafting or revision and agrees to be personally accountable for the individual’s own contributions and to ensure that questions pertaining to the accuracy or integrity of any portion of the work, even one in which the author was not directly involved, are appropriately investigated and resolved, including with documentation in the literature if appropriate.
Support
This report was supported by DCI. Dr Hsu receives support from grant T32DK00777 from National Institute of Diabetes and Digestive and Kidney Diseases, which had no role in study design, data collection, reporting, or decision to submit.
Financial Disclosure
Mr Aweh, Dr Manley, Ms Stewart, Mr Ladik, Mr Hosford, Dr Johnson, and Dr Lacson Jr are all employees of DCI, where Dr Johnson is vice chair of the board. Drs Weiner and Miskulin receive salary support to their institution from DCI. The other authors declare that they have no relevant financial interests.
Peer Review
Received October 20, 2020. Evaluated by 2 external peer reviewers, with direct editorial input from a Statistics/Methods Editor, an Associate Editor, and the Editor-in-Chief. Accepted in revised form January 11, 2021.
Footnotes
Complete author and article information provided before references.
Figure S1. Map illustrating locations of the clinics treating dialysis patients with COVID-19.
Table S1. Characteristics and mortality of patients with and without diagnosed COVID-19, subset of patients not residing in a congregate setting.
Table S2. Characteristics and mortality of patients with and without diagnosed COVID-19, subset of patients residing in a congregate setting.
Table S3. Association between clinical characteristics and COVID-19 diagnosis among dialysis patients, subset of patients not residing in a congregate setting.
Table S4. Association between clinical characteristics and COVID-19 diagnosis among dialysis patients, subset of patients residing in a congregate setting.
Supplementary Material
Figure S1; Tables S1-S4.
References
- 1.Gupta S., Hayek S.S., Wang W. Factors Associated With Death in Critically Ill Patients With Coronavirus Disease 2019 in the US. JAMA Intern Med. 2020;180(11):1–12. doi: 10.1001/jamainternmed.2020.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richardson S., Hirsch J.S., Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323(20):2052. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petrilli C.M., Jones S.A., Yang J. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garg S., Kim L., Whitaker M. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 - COVID-NET, 14 states, March 1-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fung M., Babik J.M. COVID-19 in immunocompromised hosts: what we know so far. Clin Infect Dis. 2021;72(2):340–350. doi: 10.1093/cid/ciaa863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Syed-Ahmed M., Narayanan M. Immune dysfunction and risk of infection in chronic kidney disease. Adv Chronic Kidney Dis. 2019;26(1):8–15. doi: 10.1053/j.ackd.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Webb Hooper M., Nápoles A.M., Pérez-Stable E.J. COVID-19 and racial/ethnic disparities. JAMA. 2020;323(24):2466. doi: 10.1001/jama.2020.8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhayani S., Sengupta R., Markossian T. Dialysis, COVID-19, poverty, and race in greater chicago: an ecological analysis. Kidney Med. 2020;2(5):552–558. doi: 10.1016/j.xkme.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiner D.E., Watnick S.G. Hemodialysis and COVID-19: An Achilles’ heel in the pandemic health care response in the United States. Kidney Med. 2020;2(3):227–230. doi: 10.1016/j.xkme.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McMichael T.M., Clark S., Pogosjans S. COVID-19 in a long-term care facility---King County, Washington, February 27-March 9, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):339–342. doi: 10.15585/mmwr.mm6912e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saran R., Robinson B., Abbott K.C. US renal data system 2018 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2019;73(3):A7–A8. doi: 10.1053/j.ajkd.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall R.K., O’Hare A.M., Anderson R.A., Colón-Emeric C.S. End-stage renal disease in nursing homes: a systematic review. J Am Med Dir Assoc. 2013;14(4):242–247. doi: 10.1016/j.jamda.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiong F., Tang H., Liu L. Clinical characteristics of and medical interventions for COVID-19 in hemodialysis patients in Wuhan, China. J Am Soc Nephrol. 2020;31(7):1387–1397. doi: 10.1681/ASN.2020030354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alberici F., Delbarba E., Manenti C. A report from the Brescia Renal COVID Task Force on the clinical characteristics and short-term outcome of hemodialysis patients with SARS-CoV-2 infection. Kidney Int. 2020;98(1):20–26. doi: 10.1016/j.kint.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang H., Tian J.B., Dong J.W. Serologic detection of SARS-CoV-2 infections in hemodialysis centers: a multicenter retrospective study in Wuhan, China. Am J Kidney Dis. 2020;76(4):490–499. doi: 10.1053/j.ajkd.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corbett R.W., Blakey S., Nitsch D. Epidemiology of COVID-19 in an urban dialysis center. J Am Soc Nephrol. 2020;31(8):1815–1823. doi: 10.1681/ASN.2020040534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weiss S., Bhat P., Del Pilar Fernandez M., Bhat J.G., Coritsidis G.N. COVID-19 infection in ESKD: findings from a prospective disease surveillance program at dialysis facilities in New York City and Long Island. J Am Soc Nephrol. 2020;31(11):2517–2521. doi: 10.1681/ASN.2020070932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valeri A.M., Robbins-Juarez S.Y., Stevens J.S. Presentation and outcomes of patients with ESKD and COVID-19. J Am Soc Nephrol. 2020;31(7):1409–1415. doi: 10.1681/ASN.2020040470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sim J.J., Huang C.W., Selevan D.C., Chung J., Rutkowski M.P., Zhou H. COVID-19 and survival in maintenance dialysis. Kidney Med. 2021;3(1):132–135. doi: 10.1016/j.xkme.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Medicare & Medicaid Services. Preliminary Medicare COVID-19 data snapshot. 2020. Accessed March 11, 2021. https://www.cms.gov/research-statistics-data-systems/preliminary-medicare-covid-19-data-snapshot
- 21.Centers for Disease Control and Prevention. Updated guidance on evaluating and testing persons for coronavirus disease 2019 (COVID-19). 2020. Accessed September 23, 2020. https://emergency.cdc.gov/han/2020/HAN00429.asp
- 22.Yancy C.W. COVID-19 and African Americans. JAMA. 2020;323(19):1891–1892. doi: 10.1001/jama.2020.6548. [DOI] [PubMed] [Google Scholar]
- 23.Landes S.D., Turk M.A., Formica M.K., McDonald K.E., Stevens J.D. COVID-19 outcomes among people with intellectual and developmental disability living in residential group homes in New York State. Disabil Health J. 2020;13(4):100969. doi: 10.1016/j.dhjo.2020.100969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu C.K., Ghai S., Waikar S.S., Weiner D.E. COVID-19 infection risk among hemodialysis patients in long-term care facilities. Kidney Med. 2020;2(6):810–811. doi: 10.1016/j.xkme.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hatfield K.M., Reddy S.C., Forsberg K. Facility-wide testing for SARS-CoV-2 in nursing homes---seven U.S. Jurisdictions, March-June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(32):1095–1099. doi: 10.15585/mmwr.mm6932e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kliger A.S., Silberzweig J. Mitigating risk of COVID-19 in dialysis facilities. Clin J Am Soc Nephrol. 2020;15(5):707–709. doi: 10.2215/CJN.03340320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lutchmansingh D.D., Knauert M.P., Antin-Ozerkis D.E. A clinic blueprint for post-COVID-19 RECOVERY: learning from the past, looking to the future. Chest. 2021;159(3):949–958. doi: 10.1016/j.chest.2020.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Couchoud C., Bayer F., Ayav C. on behalf of the French REIN registry. Low incidence of SARS-CoV-2, risk factors of mortality and the course of illness in the French national cohort of dialysis patients. Kidney Int. 2020 Dec;98(6):1519–1529. doi: 10.1016/j.kint.2020.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jager K.J., Kramer A., Chesnaye N.C. Results from the ERA-EDTA registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int. 2020;98(6):1540–1548. doi: 10.1016/j.kint.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1; Tables S1-S4.