Abstract
Background
The novel coronavirus (SARS-CoV-2) pandemic spread rapidly worldwide increasing exponentially in Italy. To date, there is lack of studies describing clinical characteristics of the people at high risk of infection. Hence, we aimed (i) to identify clinical predictors of SARS-CoV-2 infection risk, (ii) to develop and validate a score predicting SARS-CoV-2 infection risk, and (iii) to compare it with unspecific scores.
Methods
Retrospective case-control study using administrative health-related database was carried out in Southern Italy (Campania region) among beneficiaries of Regional Health Service aged over than 30 years. For each person with SARS-CoV-2 confirmed infection (case), up to five controls were randomly matched for gender, age and municipality of residence. Odds ratios and 90% confidence intervals for associations between candidate predictors and risk of infection were estimated by means of conditional logistic regression. SARS-CoV-2 Infection Score (SIS) was developed by generating a total aggregate score obtained from assignment of a weight at each selected covariate using coefficients estimated from the model. Finally, the score was categorized by assigning increasing values from 1 to 4. Discriminant power was used to compare SIS performance with that of other comorbidity scores.
Results
Subjects suffering from diabetes, anaemias, Parkinson’s disease, mental disorders, cardiovascular and inflammatory bowel and kidney diseases showed increased risk of SARS-CoV-2 infection. Similar estimates were recorded for men and women and younger and older than 65 years. Fifteen conditions significantly contributed to the SIS. As SIS value increases, risk progressively increases, being odds of SARS-CoV-2 infection among people with the highest SIS value (SIS = 4) 1.74 times higher than those unaffected by any SIS contributing conditions (SIS = 1).
Conclusion
Conditions and diseases making people more vulnerable to SARS-CoV-2 infection were identified by the current study. Our results support decision-makers in identifying high-risk people and adopting of preventive measures to minimize the spread of further epidemic waves.
Introduction
Since December 2019, the novel coronavirus (SARS-CoV-2) pandemic spread rapidly from the Hubei province in China to 185 countries causing over 3,000,000 cases [1]. The epidemic spread to and increased exponentially in Italy, earlier than in any other western Country, having generated at the current time (June 15) over 236,000 confirmed SARS-CoV-2 infections [2]. SARS-COV-2 causes a Coronavirus disease 2019 (Covid-19), for which minor symptoms are anosmia, ageusia, gastrointestinal symptoms, headache, and cutaneous manifestations and major symptoms are fever, cough, dyspnoea [3, 4]. Due to these major symptoms it may be considered necessary to hospitalize patients for respiratory complications [5].
Several hospital-based studies [6–10], including a systematic review of literature and meta-analysis [11], focused on the attempt for predicting the progression of the disease towards developing critical manifestations or death. These studies are important for the clinical practice point of view for identifying patients at whom early treatment must be guaranteed. However, as most infections are not life-threatening [7], for the public health point of view it becomes increasingly important stratifying population for identifying people at higher risk of infection. Despite this, at our best knowledge, no studies on this topic have been still published.
We therefore performed a large investigation based upon healthcare utilization database from the Italian Region of Campania aimed (1) to identify clinical predictors of the risk of SARS-CoV-2 infection, (2) to develop and validate a score overall predicting the risk of SARS-CoV-2 infection, and (3) to compare discriminant power of such a score with that from unspecific scores of clinical profile.
Methods
Target population and data source
Residents in Campania who were beneficiaries of the Regional Health Service (RHS) aged 30 years or older formed the target population (almost 3.9 million people, around 9% of the Italian population of that age group). Italian citizens have equal access to essential healthcare services provided by the National Health Service [12]. An automated system of healthcare utilization (HCU) databases allows managing NHS within each Italian region, including Campania. HCU data report a variety of information drawn from services provided fully or in part free of charge from NHS to beneficiaries of NHS (e.g. the ICD-CM-9 codes of inpatient diagnoses and services supplied from public or private hospitals, the ATC codes of outpatient drugs dispensed from pharmacies). This allowed to Campania Region of designing, building and routinely managing the so-called Campania Region Database (CaReDB) which formed the data source for the current study. Completeness and data validity of CaReDB being elsewhere reported [13–21]. Selected characteristics of CaReDB are described in S1 Table.
From the beginning of the Covid-19 epidemic, a surveillance system was implemented to detect all cases identified by reverse transcription-polymerase chain reaction (RT-PCR) testing for SARS-CoV-2. Diagnostic algorithm was based on the protocol released by the World Health Organization (WHO) [22], i.e., on nasopharyngeal swab specimens tested with at least two real-time RT PCT assays targeting different genes (E, RdRp and M) of SARS-CoV-2.
These various types of data (i.e., CaReDB and Covid-19 registry) can be interconnected, since a single individual identification code is used by all databases for each citizen enrolled. To preserve privacy, each identification code was automatically deidentified, the inverse process being allowed only to the Regional Health Authority on request from judicial authorities. Permission for using anonymized data for this study was granted to the researchers of the Centro di Ricerca in Farmacoeconomia e Farmacoutilizzazione (CIRFF) by the governance board of Unità del Farmaco della Regione Campania. According with Italian Data protection Authority, neither Ethical Committee approval, nor informed consent were required for carrying out observational studies based on HCU data as the our [23]. Our research protocol adhered to the tenets of the Declaration of Helsinki 1975 and its later amendments.
Cases and controls
The date of SARS-CoV-2 infection diagnosis was considered as the index date and patients were extracted from the Covid-19 registry until June 10, 2020. A total of 4,629 subjects positive to SARS-CoV-2 were identified. Among these, we excluded i) patients with missing demographic information (N = 469) and ii) patients younger than 30 years at the index date (N = 663). Finally, 3,497 patients were included into the study as cases. Among them, 453 patients died during the observational period.
For each case, up to five controls were randomly selected from the target population to be matched for gender, age at index date and municipality of residence. The density incidence approach was used for selecting controls since patients who had a confirmed diagnosis of SARS-CoV-2 infection were eligible as potential controls until they became cases, and all matches had to be at risk of SARS-CoV-2 infection.
Identifying clinical predictors of SARS-CoV-2 infection
A list of 47 diseases and conditions potentially predicting the risk of SARS-CoV-2 infection was developed starting from the lists included in several comorbidities scores, i.e., the Charlson [24], Elixhauser [25], Chronic Disease Scores [26] and RxRiskV Index [27, 28], and in some systematic reviews on Covid-19 risk factors [11, 29–31]. The algorithms for detecting patients who suffer from each of them from the above-mentioned databases were carefully chosen according to previously published papers on case-identification algorithms based on Italian healthcare data [32–36]. Individuals were classified as having one of the conditions listed if they received at least two consecutive dispensations of a drug for treatment of a specific class of disease and/or one hospital discharge with the diagnoses coded with the specific ICD-9-CM (S2 Table).
Conditional logistic regression was used to estimate odds ratios (ORs), with 90% confidence intervals (CIs), for the association between candidate predictors and the odds of SARS-CoV-2 infection. Predictors entered as dichotomous covariates into the model, i.e., with value 0 or 1 according to whether the specific condition was not or was recorded at least once within two-years prior baseline (2018–2019). Unadjusted and mutually adjusted models were fitted by including one by one covariate, and all covariates together, respectively. Power considerations suggested of excluding covariates with prevalence ≤ 0.12% among controls, i.e., predictors for which our sample size was not enough for detecting OR of at least 3, with a 0.80 power, and by accepting a 0.10 two-sided first type error. In addition, some conditions were grouped together when strong uncertainty of algorithm did not allow for distinguishing them.
With the aim of testing the hypothesis that predictors may affect severity of clinical manifestations of SARS-CoV-2 infection, rather than infection per se, analyses were restricted to strata having fatal infection. Stratifications for sex and age categories (<65 years, ≥65 years) were performed as secondary analyses.
Developing and validating a score to predict SARS-CoV-2 infection
Seven out of ten of the 3,497 1:5 case-control sets were randomly selected to form the so-called training (derivation) set. The conditional logistic regression model was fitted to compute the ORs as above described. The least absolute shrinkage and selection operator (LASSO) method was applied for selecting the diseases / conditions able to independently predict the SARS-CoV-2 infection [37]. The coefficients estimated from the model were used for assigning a weight at each selected covariate. A weight was assigned to each coefficient by multiplying it by 10 and rounding it to the nearest whole number [38]. The weights thus obtained were then summed to generate a total aggregate score. To simplify the system, i.e., with the aim of accounting for excessive heterogeneity of the total aggregate score, the latter was categorized by assigning increasing values of 1, 2, 3 and 4 to the categories of the aggregate score of 0, 1–2, 3–4, ≥ 5, respectively. The so obtained index was denoted SARS-CoV-2 Infection Score (SIS).
Performance of SIS was explored by applying the corresponding weights to the so-called validation set consisting of the 1,048 1:5 case-control sets who did not enter the training set. To evaluate the clinical utility of SIS for predicting infection, we considered the receiver operating characteristic (ROC) curve analysis and used area under the ROC curve (AUC) as a global summary of the discriminatory capacity of the scores [39].
Comparing specific and unspecific predictors of SARS-CoV-2 infection
Some unspecific scores surrogating general clinical profile of each case and control included into the study were considered. In particular, the number of drugs with different 3rd level ATC dispensed to, and comorbidities with different ICD-9-CM experienced by each case and control within two-years prior baseline (2018–2019) were recorded. Categorization was made by assigning increasing values of 1, 2, 3 and 4 to 0, 1–4, 5–9 and ≥10 drugs (comedication score) and 1, 2, 3 and 4 to 0, 1–2 and ≥3 comorbidities (comorbidity score). In addition, cases and controls were categorized according to the Multisource Comorbidity Score (MCS), a new index of patients’ clinical status derived from inpatients diagnostic information and outpatient drug prescriptions provided by the regional Italian data and validated for outcome prediction [40, 41]. To simplify comparisons, the original five categories of worsening clinical profile (0, 1, 2, 3 and 4) as defined by MCS, were reduced to milder (MCS = 0), middle (1≤MCS≤3) and severe (MCS≥4) categories.
With the aim of comparing discriminatory ability of specific (SIS) and unspecific (comedications, comorbidities and MCS) predictors of SARS-CoV-2 infection, ROC curves and corresponding AUCs were again used.
All analyses were performed using SAS 9.4 (Cary, NC). A 2-sided p-value of 0.10 or less was considered significant.
Results
Clinical predictors of SARS-CoV-2 infection
Owing to their low prevalence, fourteen conditions were excluded from this analysis (tuberculosis, weight loss, disorders involving the immune mechanisms, disorders of fluid, electrolyte and acid-base balance, coagulation defects, bipolar disorders, alcohol abuse, drug addiction, multiple sclerosis, cystic fibrosis, chronic and acute pancreatitis, anchylosing spondylitis, systemic sclerosis, systemic sclerosis). Among the 33 remaining conditions, two were grouped, i.e., chronic pulmonary obstructive disease with asthma (chronic respiratory disease), and chronic renal disease with or without dialysis.
The characteristics of the cohort members are shown in Table 1. Among the 31 remaining conditions, 23 (74%) showed significant association with the risk of SARS-CoV-2 infection from univariate regression.
Table 1. Baseline characteristics of cohort members (Covid-19 cases and related controls), individual (one by one, univariate) Odds Ratio (OR), and 90% Confidence Intervals (CI), for the relationship between selected diseases/conditions and the risk of SARS-CoV-2 infection.
| Cases (N = 3,497) | Controls (N = 17,358) | Individual OR (90% CI) | |
|---|---|---|---|
| Male gender | 1,945 (55.6%) | 9,640 (55.5%) | MV |
| Age (years) | |||
| 30–64 | 2,375 (67.9%) | 11,829 (68.1%) | MV |
| ≥65 | 1,122 (32.1%) | 5,538 (31.9%) | |
| Infectious and parasitic diseases | |||
| HIV infection | 68 (1.9%) | 301 (1.7%) | 1.12 (0.90 to 1.41) |
| Neoplasms | |||
| Malignant neoplasms | 155 (4.4%) | 661 (3.8%) | 1.18 (1.01 to 1.37) |
| Endocrine, nutritional and metabolic diseases, and immunity disorders | |||
| Thyroid disorders | 225 (6.4%) | 920 (5.3%) | 1.25 (1.10 to 1.42) |
| Diabetes | 411 (11.8%) | 1732 (10%) | 1.22 (1.10 to 1.35) |
| Hyperlipidaemia | 729 (20.8%) | 3708 (21.4%) | 0.97 (0.89 to 1.05) |
| Obesity | 48 (1.4%) | 153 (0.9%) | 1.58 (1.20 to 2.08) |
| Hyperuricemia/Gout | 180 (5.1%) | 711 (4.1%) | 1.28 (1.11 to 1.48) |
| Diseases of the blood and blood-forming organs | |||
| Anaemias | 265 (7.6%) | 927 (5.3%) | 1.48 (1.31 to 1.67) |
| Mental disorders | |||
| Dementia / Alzheimer | 48 (1.4%) | 89 (0.5%) | 2.79 (2.06 to 3.79) |
| Psychosis | 124 (3.5%) | 303 (1.7%) | 2.10 (1.75 to 2.52) |
| Depression | 233 (6.7%) | 1,003 (5.8%) | 1.17 (1.03 to 1.33) |
| Anxiety | 1,369 (39.1%) | 5,615 (32.3%) | 1.37 (1.29 to 1.47) |
| Diseases of the nervous system and sense organs | |||
| Parkinson’s disease | 67 (1.9%) | 188 (1.1%) | 1.78 (1.40 to 2.26) |
| Epilepsy | 176 (5%) | 660 (3.8%) | 1.35 (1.17 to 1.55) |
| Glaucoma | 119 (3.4%) | 482 (2.8%) | 1.25 (1.05 to 1.48) |
| Diseases of the circulatory system | |||
| Ischaemic Heart Disease/Angina | 213 (6.1%) | 841 (4.8%) | 1.29 (1.12 to 1.47) |
| Heart failure | 281 (8%) | 1,005 (5.8%) | 1.49 (1.31 to 1.69) |
| Arrhythmia | 196 (5.6%) | 738 (4.3%) | 1.36 (1.18 to 1.57) |
| Valvular diseases | 43 (1.2%) | 180 (1%) | 1.18 (0.88 to 1.57) |
| Vascular diseases | 52 (1.5%) | 186 (1.1%) | 1.41 (1.08 to 1.84) |
| Cerebrovascular diseases | 127 (3.6%) | 445 (2.6%) | 1.46 (1.22 to 1.74) |
| Hypertension | 826 (23.6%) | 3,731 (21.5%) | 1.15 (1.07 to 1.25) |
| Diseases of the respiratory system | |||
| Chronic respiratory diseases (COPD and asthma together) | 244 (7%) | 908 (5.2%) | 1.37 (1.21 to 1.56) |
| Diseases of the digestive system | |||
| Liver cirrhosis and other liver chronic diseases | 54 (1.5%) | 216 (1.2%) | 1.23 (0.96 to 1.59) |
| Inflammatory bowel diseases | 54 (1.5%) | 169 (1%) | 1.60 (1.23 to 2.07) |
| Diseases of the genitourinary system | |||
| Kidney disease with or without dialysis | 67 (1.9%) | 210 (1.2%) | 1.60 (1.26 to 2.03) |
| Diseases of the skin and subcutaneous tissues | |||
| Psoriasis | 23 (0.7%) | 113 (0.7%) | 1.02 (0.70 to 1.48) |
| Diseases of the musculoskeletal system and connective tissue | |||
| Rheumatologic conditions | 28 (0.8%) | 79 (0.5%) | 1.77 (1.23 to 2.56) |
| Other conditions | |||
| Transplantation | 13 (0.4%) | 59 (0.3%) | 1.10 (0.66 to 1.82) |
| Chronic pain | 89 (2.5%) | 378 (2.2%) | 1.19 (0.97 to 1.45) |
| Inflammation, not elsewhere specified | 410 (11.7%) | 2,244 (12.9%) | 0.89 (0.81 to 0.98) |
Abbreviation: MV, matching variable.
Table 2 reports multivariate association between the considered diseases/conditions and the risk of SARS-CoV-2 infection which results significant for 12 conditions (39%).
Table 2. Independent (all together, multivariate) Odds Ratio (OR), and 90% Confidence Intervals (CI), for the relationship between selected diseases/conditions and the risk of SARS-CoV-2 infection as a whole (3,497 cases and corresponding 17,358 controls), as well as the risk of fatal SARS-CoV-2 infection (435 cases and corresponding 2,154 controls).
| All Covid-19 cases | Fatal Covid-19 cases | |||
|---|---|---|---|---|
| Cases / Controls | OR (90% CI) | Cases / Controls | OR (90% CI) | |
| Infectious and parasitic diseases | ||||
| HIV infection | 68 / 301 | 1.07 (0.85 to 1.34) | 11 / 47 | 1.04 (0.58 to 1.86) |
| Neoplasms | ||||
| Malignant neoplasms | 155 / 661 | 0.99 (0.85 to 1.16) | 35 / 147 | 0.99 (0.70 to 1.42) |
| Endocrine, nutritional and metabolic diseases, and immunity disorders | ||||
| Thyroid disorders | 225 / 920 | 1.13 (0.99 to 1.29) | 31 / 133 | 0.93 (0.64 to 1.37) |
| Diabetes | 411 / 1,732 | 1.15 (1.03 to 1.28) | 88 / 327 | 1.30 (1.01 to 1.67) |
| Hyperlipidaemia | 729 / 3,708 | 0.86 (0.79 to 0.94) | 131 / 703 | 0.69 (0.55 to 0.86) |
| Obesity | 48 / 153 | 1.18 (0.89 to 1.57) | 6 / 18 | 1.08 (0.46 to 2.56) |
| Hyperuricemia/Gout | 180 / 711 | 1.08 (0.93 to 1.27) | 56 / 175 | 1.29 (0.95 to 1.76) |
| Diseases of the blood and blood-forming organs | ||||
| Anaemias | 265 / 927 | 1.24 (1.09 to 1.41) | 63 / 184 | 1.45 (1.07 to 1.95) |
| Mental disorders | ||||
| Dementia / Alzheimer | 48 / 89 | 2.14 (1.55 to 2.96) | 14 / 27 | 1.92 (1.02 to 3.63) |
| Psychosis | 124 / 303 | 1.71 (1.40 to 2.08) | 35 / 57 | 2.68 (1.76 to 4.08) |
| Depression | 233 / 1,003 | 0.98 (0.86 to 1.12) | 49 / 149 | 1.21 (0.88 to 1.67) |
| Anxiety | 1,369 / 5,615 | 1.26 (1.17 to 1.36) | 217 7,824 | 1.33 (1.07 to 1.65) |
| Diseases of the nervous system and sense organs | ||||
| Parkinson’s disease | 67 / 188 | 1.32 (1.02 to 1.70) | 18 / 47 | 1.32 (0.80 to 2.18) |
| Epilepsy | 176 / 660 | 1.10 (0.94 to 1.28) | 44 / 99 | 1.57 (1.11 to 2.22) |
| Glaucoma | 119 / 482 | 1.22 (1.03 to 1.46) | 27 / 95 | 1.32 (0.89 to 1.97) |
| Diseases of the circulatory system | ||||
| Ischaemic Heart Disease/Angina | 213 / 841 | 0.99 (0.84 to 1.15) | 58 / 186 | 1.24 (0.88 to 1.76) |
| Heart failure | 281 / 1,005 | 1.24 (1.07 to 1.44) | 86 / 268 | 1.41 (1.04 to 1.90) |
| Arrhythmia | 196 / 738 | 1.14 (0.98 to 1.33) | 51 / 198 | 0.95 (0.68 to 1.31) |
| Valvular diseases | 43 / 180 | 0.80 (0.59 to 1.09) | 11 / 42 | 0.84 (0.44 to 1.60) |
| Vascular diseases | 52 / 186 | 1.00 (0.75 to 1.32) | 9 / 40 | 0.69 (0.36 to 1.33) |
| Cerebrovascular diseases | 127 / 445 | 1.00 (0.83 to 1.21) | 37 / 108 | 1.06 (0.72 to 1.56) |
| Hypertension | 826 / 3,731 | 1.12 (1.01 to 1.24) | 167 / 712 | 1.16 (0.94 to 1.43) |
| Diseases of the respiratory system | ||||
| Chronic respiratory diseases (COPD and asthma together) | 244 / 908 | 1.18 (1.03 to 1.35) | 50 / 178 | 1.14 (0.83 to 1.55) |
| Diseases of the digestive system | ||||
| Liver cirrhosis and other liver chronic diseases | 54 / 216 | 0.93 (0.71 to 1.21) | 14 / 35 | 1.42 (0.79 to 2.56) |
| Inflammatory bowel diseases | 54 / 169 | 1.47 (1.13 to 1.91) | 6 / 32 | 0.72 (0.33 to 1.56) |
| Diseases of the genitourinary system | ||||
| Kidney disease with or without dialysis | 67 / 210 | 1.10 (0.84 to 1.42) | 23 / 60 | 1.07 (0.60 to 1.90) |
| Diseases of the skin and subcutaneous tissues | ||||
| Psoriasis | 23 / 113 | 0.93 (0.63 to 1.36) | 2 / 19 | 0.39 (0.11 to 1.44) |
| Diseases of the musculoskeletal system and connective tissue | ||||
| Rheumatologic conditions | 28 / 79 | 1.54 (1.06 to 2.23) | 5 / 18 | 1.21 (0.50 to 2.91) |
| Other conditions | ||||
| Transplantation | 13 / 59 | 0.87 (0.52 to 1.46) | 3 / 8 | 1.45 (0.42 to 4.97) |
| Chronic pain | 89 / 378 | 1.06 (0.86 to 1.31) | 21 / 78 | 1.08 (0.69 to 1.70) |
| Inflammation, not elsewhere specified | 410 / 2,244 | 0.85 (0.77 to 0.93) | 74 / 316 | 1.12 (0.87 to 1.45) |
In particular, patients suffering from diabetes, anaemias, mental disorders (dementia / Alzheimer’s disease, psychosis and anxiety), Parkinson’s disease, glaucoma, diseases of the circulatory system (heart failure and hypertension), chronic respiratory, inflammatory bowel, and rheumatologic conditions showed statistical evidence of increased risk of infection with respect to patients who did not suffer from them. Likely because of low power, only 7 conditions resulted significantly associated with the risk of fatal Covid-19 disease, but there was no relevant difference in the estimates with respect to the risk of SARS-CoV-2 infection (Table 2).
Anaemias, dementia/Alzheimer, psychosis, anxiety, epilepsy, heart failure, kidney diseases and particularly cystic fibrosis increased the risk of SARS-CoV-2 infection among women, whereas higher risk of infection was observed among men suffering from diabetes, psychosis, anxiety, Parkinson, arrhythmia, chronic pulmonary disease, inflammatory bowel diseases and particularly dementia/Alzheimer and rheumatologic conditions (S3 Table).
Estimates were similar for Covid-19 patients younger and older than 65 years. Among the former group, a significant higher risk of infection was observed for diabetes, anxiety, Parkinson’s disease, arrhythmia, inflammatory bowel and chronic pulmonary diseases, particularly dementia/Alzheimer, whereas patients older than 65 years suffering from thyroid disorders, anaemias, dementia/Alzheimer, psychosis, anxiety, epilepsy and heart failure showed a significant higher risk infection (S4 Table).
SARS-CoV-2 Infection Score (SIS)
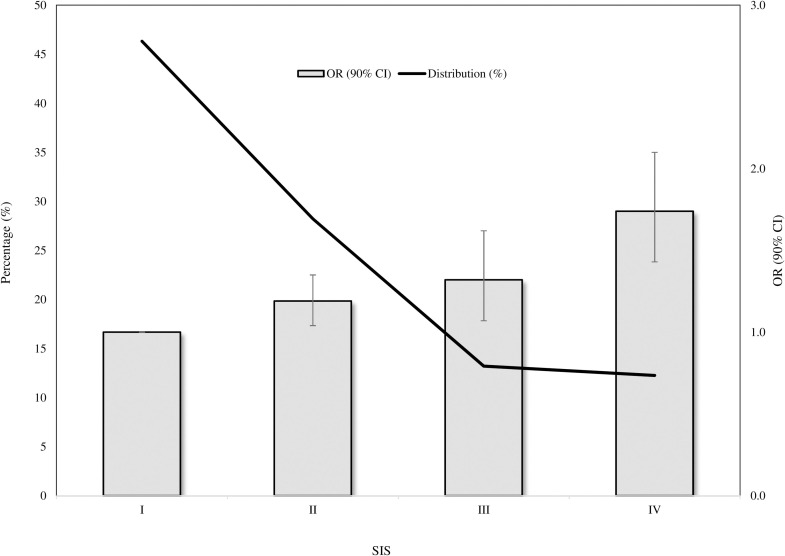
Fifteen conditions significantly contributed to the SIS, the corresponding weights being reported in Table 3. Factors which most contributed to the total aggregate score were dementia / Alzheimer’s disease, kidney disease, psychosis, inflammatory bowel disease and rheumatologic conditions, while diabetes, anaemias, anxiety, Parkinson’s disease, glaucoma, heart failure, hypertension, arrhythmia, thyroid disorders and chronic respiratory disease provided small, although significant, contributions. Fig 1 shows that, as the SIS value increases, the OR progressively increases, being the odds of SARS-CoV-2 infection among people with the highest SIS value (SIS = IV), 1.74 times higher than those unaffected by any SIS contributing conditions (SIS = I). The prevalence of controls stratified according to the SIS score gradually decreases from 50% (SIS = I) to 12% (SIS = IV).
Table 3. Weights, assigned to diseases that were significantly associated with the risk of Covid-19 disease, used to construct the SARS-CoV-2 Infection Score (SIS).
| Disease / Condition | Log (OR) | Weights |
|---|---|---|
| Thyroid disorders | 0.08 | 1 |
| Diabetes | 0.08 | 1 |
| Anaemias | 0.23 | 2 |
| Dementia / Alzheimer | 0.98 | 10 |
| Psychosis | 0.46 | 5 |
| Anxiety | 0.23 | 2 |
| Parkinson’s disease | 0.27 | 3 |
| Glaucoma | 0.15 | 2 |
| Heart failure | 0.27 | 3 |
| Arrhythmia | 0.12 | 1 |
| Hypertension | 0.12 | 1 |
| Chronic Pulmonary disease | 0.15 | 2 |
| Inflammatory bowel diseases | 0.40 | 4 |
| Kidney dialysis | 0.75 | 8 |
| Rheumatologic conditions | 0.55 | 6 |
Fig 1. SARS-CoV-2 Infection Score (SIS) distribution among controls, and corresponding trend in odds ratios (and 90% confidence intervals) along categories of SIS.
SARS-CoV-2 Infection Score: I, II, III and IV to 0, 1–2, 3–4 and ≥5.
Comparing with unspecific predictors of SARS-CoV-2 infection
Generic/unspecific scores surrogating clinical profile showed to be associated with the risk of SARS-CoV-2 infection, showing patients with ≥ 10 drug treatments, those with ≥ 3 comorbidities, and those with MCS value ≥ 4, increased risk of 65%, 36% and 45% with respect to patients cotreatments, comorbidities and MCS value = I, respectively (Table 4).
Table 4. Relationship between selected score and the risk of SARS-CoV-2 infection.
| Scores | OR (90% CI) |
|---|---|
| SARS-CoV-2 Infection Score (SIS) | |
| I (0) | 1.00 (Ref.) |
| II (1–2) | 1.19 (1.03 to 1.36) |
| III (3–4) | 1.32 (1.10 to 1.58) |
| IV (≥5) | 1.74 (1.44 to 2.10) |
| Number of comedications | |
| I (0) | 1.00 (Ref.) |
| II (1–4) | 1.05 (0.91 to 1.21) |
| III (5–9) | 1.17 (0.97 to 1.41) |
| IV (≥10) | 1.65 (1.25 to 2.19) |
| Number of comorbidities | |
| I (0) | 1.00 (Ref.) |
| II (1–2) | 1.21 (1.05 to 1.38) |
| III (≥3) | 1.36 (1.15 to 1.60) |
| Multisource Comorbidity Score (MCS) | |
| I (0) | 1.00 (Ref.) |
| II (1–3) | 1.21 (1.03 to 1.41) |
| III (≥4) | 1.45 (1.23 to 1.70) |
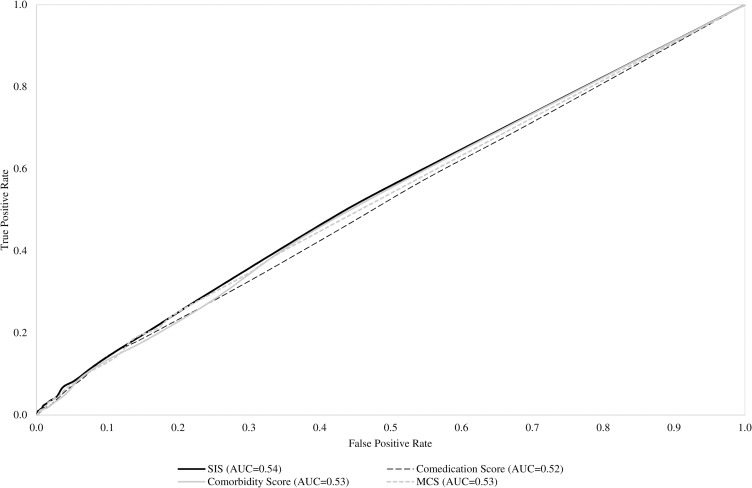
AUC (90% CI) of SIS, cotreatment and comorbidity scores and MCS respectively had values of 0.54 (0.52 to 0.56), 0.52 (0.50 to 0.54), 0.53 (0.51 to 0.55), and 0.53 (0.51 to 0.55) (Fig 2). There was no evidence that specific and unspecific scores had different discriminatory ability.
Fig 2. Receiver Operating Characteristics (ROC) curves comparing discriminant power of SARS-CoV-2 Infection Score (SIS), and selected unspecific score surrogating clinical profile (cotreatments, comorbidities and Multisource Comorbidity Score).
Discussion
Our study shows that several diseases and conditions are significantly and independently associated with the risk of SARS-CoV-2 infection. Beyond conditions making particularly vulnerable the respiratory system (e.g., chronic obstructive pulmonary disease and asthma), comorbidities positively associated with the infection practically included all diagnostic categories. Predictors belonging to nutritional and metabolic (diabetes), cardiovascular (heart failure and hypertension) and renal diseases were widely expected, since it has accepted that SARS-CoV-2 has major implications for the cardiovascular system. Indeed, patients with heart failure [42], diabetes [43–45], hypertension [12] and kidney disease [46–48] have been consistently identified as particularly vulnerable populations, and these findings were consistently found in our study. In addition, we confirmed that people with weakened immune systems from a medical condition or treatment are at a higher risk. Among these, those living with haemoglobin disorders [49], inflammatory bowel disease [50] and immune-rheumatological diseases [51] must be considered vulnerable groups for SARS-CoV-2 infection. Mental health and cognitive function might have independent utility in understanding the burden of respiratory disease, since they may influence the risk of contracting the infection, at least in part by impairing innate or adaptive immunity [52] and diminishing the precautions taken to minimize risk. Another explanation of our findings is that people with history of depression [53], psychosis [54] and stress disorders [55] could experience elevated rates of an array of respiratory infections because these conditions often require treatment in a psychiatric care facility, and the risk of infection can be particularly high in these structures. Finally, our study adds evidence regarding the impact of diseases and conditions on the risk of SARS-CoV-2 infection between men and women. As pointed out by a recent study [56], sex and age disaggregated data are essential for understanding the distributions of risk infection in the population and the extent to which they affect clinical outcomes.
Despite our results confirm that a wide range of diseases and conditions likely increase vulnerability to SARS-CoV-2 infection, and probably its more severe clinical manifestations, we have not been able to develop a score that accurately may predict the risk of infection. In addition, we found that predictive ability of the score obtained by weighting risk factors of SARS-CoV-2 infection did not overcome that of some generic scores of comorbidities and comedications. This expands upon previous findings of individual comorbidities as independent risk factors for SARS-CoV-2 infection [57, 58], and confirms our substantial inability to predict the risk of SARS-CoV-2 infection. This can be explained by several limitations of our approach, which generate estimates biased towards the null. First, exposure misclassification regards our inability to careful capturing conditions and diseases through algorithms based on healthcare utilization databases [59]. Second, it is well known that outcome misclassification can bias epidemiologic results. For Covid-19, suboptimal test sensitivity, despite excellent specificity, results in an overestimation of cases in the early stages of an outbreak, and substantial underestimation of cases as prevalence increases [60]. It should be noticed, however, that both, exposure and outcome misclassification likely drew estimates towards the null (i.e., underestimate the strength of the association between their presence and the outcome risk) so generating uncertainty for the weighting approach of score developing. Third, the lack of information on biologic markers potentially able to predict infection, and severity of its clinical manifestations, is another limitation of our study. For example, according to the current literature, some laboratory hallmarks have been shown to predict infection, particularly in more severe cases [61]. Finally, our choice of accepting a 0.10 first type error, and of consequently reporting 90% confidence intervals, is justified by the exploratory nature of our study, but at the same time likely generate false positive signals, so limiting discriminant power of the score.
Three other elements of weakness should be acknowledged. First, the lack of data regarding the clinical outcome experienced by SARS-CoV-2 positive patients in terms of home isolation, hospitalization and admission in intensive care. Second, because few people aged less than 30 years were diagnosed to be affected by SARS-CoV-2 infection during the investigated period, and few of them suffered from chronic conditions such as those considered in our study, patients with less than 30 years were excluded from the analysis. Although this reduced the uncertainty of the results, the generalisability of our findings requires extreme caution. Finally, because data on stays in long-term facilities are not recorded in our database, we cannot exclude that the higher risks associated with mental disorders observed in our study could be explained by confounding, i.e., patients who suffered from these conditions are often hospitalized in these structures where the risk of infection can be particularly high.
In conclusion, taking the limitations we discussed into account, we identified conditions and diseases that make people more vulnerable to SARS-CoV-2 infection. These findings contribute to inform public health, and clinical decisions regarding risk stratifying. However, further research is need for developing a score reliably predicting the risk, possibly by integrating healthcare utilization with clinical and biological data.
Our results can be an important tool supporting all clinical and political stakeholders allowing the identification of the population most at risk of contracting SARS-CoV-2 infection and facilitating the provision of appropriate preventive/therapeutic measures, especially with the hypothetic prediction of a new autumn outbreak. Adopting preventive measures can help to minimize the damage generated by a potential new relapse that the health systems will face.
Supporting information
ATC = Anatomical Therapeutic Chemical; ICD-9-CM = International Classification of Diseases, 9th Revision, Clinical Modification. aTime span covered was 2009–2018 for hospital-discharge records and 2014–2019 for outpatient pharmacy records.
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability
Permission use anonymized data to this study was granted to the researchers of the Centro di Ricerca in Farmacoeconomia e Farmacoutilizzazione (CIRFF) by the governance board of Unità del Farmaco della Regione Campania. The CIRFF has a regional decree that allow for conducting research by making secondary use of administrative data (DGRC n 276 23/05/2017). Furthermore, General Director of Health Care Services authorized the CIRFF to access the COVID-19 patients data in Campania Region (Protocol n. 0173642 of 26/03/2020). Requests for information on data access can be directed to Dr. Enrica Menditto, Director of CIRFF (contact via enrica.menditto@unina.it).
Funding Statement
The authors received no specific funding for this work.
References
- 1.Dong Ensheng, Du Hongru, Gardner Lauren. An interactive web-based dashboard to track COVID-19 in real time. The Lancet Infectious Diseases 2020;20:533–4. 10.1016/S1473-3099(20)30120-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Onder G, Rezza G, Brusaferro S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy [published online ahead of print, 2020 Mar 23]. JAMA. 2020; 10.1001/jama.2020.4683 [DOI] [PubMed] [Google Scholar]
- 3.Vaira LA, Hopkins C, Salzano G, Petrocelli M, Melis A, Cucurullo M, et al. Olfactory and gustatory function impairment in COVID-19 patients: Italian objective multicenter-study. Head Neck. 2020. July;42(7):1560–1569. Epub 2020 May 21. 10.1002/hed.26269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaira LA, Deiana G, Fois AG, Pirina P, Madeddu G, De Vito A, et al. Objective evaluation of anosmia and ageusia in COVID-19 patients: Single-center experience on 72 cases. Head Neck. 2020. June;42(6):1252–1258. Epub 2020 Apr 29. 10.1002/hed.26204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Vito A, Geremia N, Fiore V, Princic E, Babudieri S, Madeddu G. Clinical features, laboratory findings and predictors of death in hospitalized patients with COVID-19 in Sardinia, Italy. Eur Rev Med Pharmacol Sci 2020; 24 (14): 7861–7868. 10.26355/eurrev_202007_22291 [DOI] [PubMed] [Google Scholar]
- 6.Liang W, Liang H, Ou L, Chen B, Chen A, Li C, et al. Development and Validation of a Clinical Risk Score to Predict the Occurrence of Critical Illness in Hospitalized Patients With COVID-19. JAMA Intern Med. 2020;e202033 10.1001/jamainternmed.2020.2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie J, Hungerford D, Hui Chen H, Abrams ST, Li S, Wang G, et al. Development and external validation of a prognostic multivariable model on admission for hospitalized patients with COVID-19. medRxiv preprint 10.1101/2020.03.28.20045997 [DOI] [Google Scholar]
- 8.Li Q, Zhang J, Ling Y, Li W, Zhang X, Luet H, et al. A simple algorithm helps early identification of SARS-CoV-2 infection patients with severe progression. Infection. 2020; 1–8. 10.1007/s15010-019-01387-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun Y, Koh V, Marimuthu K, Tek Ng O, Young B, Vasoo S, et al. Epidemiological and Clinical Predictors of COVID-19. Clin Infect Dis. 2020;ciaa322 10.1093/cid/ciaa322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haimovich A, Ravindra NG, Stoytchev S, Young HP, Wilson FP, van Dijk D, et al. Development and validation of the COVID-19 severity index (CSI): a prognostic tool for early respiratory decompensation. medRxiv preprint. 10.1101/2020.05.07.20094573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng Z, Peng F, Xu B, Zhao J, Liu H, Peng J, et al. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J Infect. 2020; S0163-4453(20)30234-6. 10.1016/j.jinf.2020.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin-Angiotensin-Aldosterone System Blockers and the Risk of Covid-19. N Engl J Med. 2020;382(25):2431–2440. 10.1056/NEJMoa2006923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moreno Juste A, Menditto E, Orlando V, Monetti VM, Gimeno Miguel A, González Rubio F, et al. Treatment Patterns of Diabetes in Italy: A Population-Based Study. Front Pharmacol. 2019;10:870 Published 2019 Aug 6. 10.3389/fphar.2019.00870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guerriero F, Orlando V, Monetti VM, Russo V, Menditto E. Biological therapy utilization, switching, and cost among patients with psoriasis: retrospective analysis of administrative databases in Southern Italy. Clinicoecon Outcomes Res. 2017;9:741–748. Published 2017 Dec 1. 10.2147/CEOR.S147558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Russo V, Monetti VM, Guerriero F, Trama U, Guida A, Menditto E, et al. Prevalence of antibiotic prescription in southern Italian outpatients: real-world data analysis of socioeconomic and sociodemographic variables at a municipality level. Clinicoecon Outcomes Res. 2018;10:251–258. Published 2018 May 3. 10.2147/CEOR.S161299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iolascon G, Gimigliano F, Orlando V, Capaldo A, Di Somma C, Menditto E. Osteoporosis drugs in real-world clinical practice: an analysis of persistence. Aging Clin Exp Res. 2013;25 Suppl 1:S137–S141. 10.1007/s40520-013-0127-5 [DOI] [PubMed] [Google Scholar]
- 17.Orlando V, Guerriero F, Putignano D, Monetti VM, Tari DU, Farina G, et al. Prescription Patterns of Antidiabetic Treatment in the Elderly. Results from Southern Italy. Curr Diabetes Rev. 2015;12(2):100–106. 10.2174/1573399811666150701120408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menditto E, Cahir C, Aza-Pascual-Salcedo M, Bruzzese D, Poblador-Plou B, Malo S, et al. Adherence to chronic medication in older populations: application of a common protocol among three European cohorts. Patient Prefer Adherence. 2018; 12:1975–1987. Published 2018 Oct 5. 10.2147/PPA.S164819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casula M, Catapano AL, Piccinelli R, Menditto E, Manzoli L, De Fendi L, et al. Assessment and potential determinants of compliance and persistence to antiosteoporosis therapy in Italy. Am J Manag Care. 2014;20(5):e138–e145. [PubMed] [Google Scholar]
- 20.Orlando V, Monetti VM, Moreno Juste A, Russo V, Mucherino S, Trama U, et al. Drug Utilization Pattern of Antibiotics: The Role of Age, Sex and Municipalities in Determining Variation. Risk Manag Healthc Policy. 2020; 13:63–71. Published 2020 Jan 29. 10.2147/RMHP.S223042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orlando V, Coscioni E, Guarino I, Mucherino S, Perrella A, Trama U, et al. Drug-utilisation Profiles and COVID-19: Retrospective Cohort Study in Italy, 29 May 2020, PREPRINT (Version 1) available at Research Square. 10.21203/rs.3.rs-31829/v [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DKW, et al. Detection of 2019 novel coronavirus (2019-nCoV) by 9 real-time RT-PCR. Euro Surveill. 2020. January;25(3):2000045 doi: 10.2807/1560-10 7917.ES.2020.25.3.2000045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Italian Data Protection Authority. General authorisation to process personal data for scientific research purposes– 1 March 2012 [1884019]. 10.1094/PDIS-11-11-0999-PDN.
- 24.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 25.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998. January;36(1):8–27. 10.1097/00005650-199801000-00004 [DOI] [PubMed] [Google Scholar]
- 26.Von Korff M, Wagner EH, Saunders K. A chronic disease score from automated pharmacy data. J Clin Epidemiol. 1992. February;45(2):197–203. 10.1016/0895-4356(92)90016-g [DOI] [PubMed] [Google Scholar]
- 27.O’Shea M, Teeling M, Bennett K. The prevalence and ingredient cost of chronic comorbidity in the Irish elderly population with medication treated type 2 diabetes: A retrospective cross-sectional study using a national pharmacy claims database. BMC Health Serv Res 2013;13(1):1 10.1186/1472-6963-13-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pratt NL, Kerr M, Barratt JD, Kemp-Casey A, Kalisch Ellett LM, Ramsay E, et al. The validity of the Rx-Risk Comorbidity Index using medicines mapped to the Anatomical Therapeutic Chemical (ATC) Classification System. BMJ Open 2018;8(4):1–8. 10.1136/bmjopen-2017-021122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wynants L, Van Calster B, Collins GS, Riley RD, Heinze G, Schuit E, et al. Prediction models for diagnosis and prognosis of covid-19 infection: systematic review and critical appraisal. BMJ. 2020. April 7;369:m1328 Erratum in: BMJ. 2020 Jun 3;369:m2204. 10.1136/bmj.m1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aghagoli G, Gallo Marin B, Soliman LB, Sellke FW. Cardiac involvement in COVID-19 patients: Risk factors, predictors, and complications: A review. J Card Surg. 2020. June;35(6):1302–1305. 10.1111/jocs.14538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang B, Li R, Lu Z, Huang Y. Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging (Albany NY). 2020. April 8;12(7):6049–6057. 10.18632/aging.103000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maio V, Yuen E, Rabinowitz C, Louis D, Jimbo M, Donatini A, et al. Using pharmacy data to identify those with chronic conditions in Emilia Romagna, Italy. Journal of health services research & policy, 2005, 104: 232–238. 10.1258/135581905774414259 [DOI] [PubMed] [Google Scholar]
- 33.Canova C, Danieli S, Barbiellini CA, Simonato L, Di RD, Cappai G, et al. A Systematic Review of Case-Identification Algorithms Based on Italian Healthcare Administrative Databases for Three Relevant Diseases of the Nervous System: Parkinson's Disease, Multiple Sclerosis, and Epilepsy. Epidemiol Prev. 2019;43(4 Suppl 2):62–74. 10.19191/EP19.4.S2.P062.093 [DOI] [PubMed] [Google Scholar]
- 34.Bezzini D, Policardo L, Meucci G, Ulivelli M, Bartalini S, Profili F, et al. Prevalence of Multiple Sclerosis in Tuscany (Central Italy): A Study Based on Validated Administrative Data. Neuroepidemiology. 2016;46(1):37–42. 10.1159/000441567 [DOI] [PubMed] [Google Scholar]
- 35.Bargagli AM, Colais P, Agabiti N, Mayer F, Buttari F, Centonze D, et al. Prevalence of multiple sclerosis in the Lazio region, Italy: use of an algorithm based on health information systems. J Neurol. 2016;263(4):751–759. 10.1007/s00415-016-8049-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chini F, Pezzotti P, Orzella L, Borgia P, Guasticchi G. Can we use the pharmacy data to estimate the prevalence of chronic conditions? a comparison of multiple data sources. BMC Public Health. 2011;11:688 Published 2011 Sep 5. 10.1186/1471-2458-11-688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tibshirani R. The lasso method for variable selection in the Cox model. Stat Med. 1997;16(4):385–395. [DOI] [PubMed] [Google Scholar]
- 38.Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64(7):749–759. 10.1016/j.jclinepi.2010.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu H, Qian J, Paynter NP, Zhang X, Whitcomb BW, Tworoger SS, et al. Estimating the receiver operating characteristic curve in matched case control studies. Stat Med. 2019;38(3):437–451. 10.1002/sim.7986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Corrao G, Rea F, Di Martino M, De Palma R, Scondotto S, Fusco D, et al. Developing and validating a novel multisource comorbidity score from administrative data: a large population-based cohort study from Italy. BMJ Open. 2017;7(12):e019503 Published 2017 Dec 26. 10.1136/bmjopen-2017-019503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Corrao G, Rea F, Carle F, Di Martino M, De Palma R, Francesconi P, et al. Measuring multimorbidity inequality across Italy through the multisource comorbidity score: a nationwide study [published online ahead of print, 2020 May 20]. Eur J Public Health. 2020;ckaa063 10.1093/eurpub/ckaa063 [DOI] [PubMed] [Google Scholar]
- 42.Wang BX. Susceptibility and prognosis of COVID-19 patients with cardiovascular disease. Open Heart. 2020;7(1):e001310 10.1136/openhrt-2020-001310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carey IM, Critchley JA, DeWilde S, Harris T, Hosking FJ, Cook DG. Risk of Infection in Type 1 and Type 2 Diabetes Compared With the General Population: A Matched Cohort Study. Diabetes Care. 2018;41(3):513–521. 10.2337/dc17-2131 [DOI] [PubMed] [Google Scholar]
- 44.Geerlings SE, Hoepelman AI. Immune dysfunction in patients with diabetes mellitus (DM). FEMS Immunol Med Microbiol. 1999;26(3–4):259–265. 10.1111/j.1574-695X.1999.tb01397.x [DOI] [PubMed] [Google Scholar]
- 45.Peleg AY, Weerarathna T, McCarthy JS, Davis TM. Common infections in diabetes: pathogenesis, management and relationship to glycaemic control. Diabetes Metab Res Rev. 2007;23(1):3–13. 10.1002/dmrr.682 [DOI] [PubMed] [Google Scholar]
- 46.Perico L, Benigni A, Remuzzi G. Should COVID-19 Concern Nephrologists? Why and to What Extent? The Emerging Impasse of Angiotensin Blockade. Nephron. 2020;144(5):213–221. 10.1159/000507305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–838. 10.1016/j.kint.2020.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Diao B, Feng Z, Wang C, Feng Z, Tan Y, Wang H, et al. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection medRxiv. In press; 10.1101/2020.03.04.20031120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chowdhury SF, Anwar S. Management of Hemoglobin Disorders During the COVID-19 Pandemic. Front Med (Lausanne). 2020;7:306 Published 2020 Jun 9. 10.3389/fmed.2020.00306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de León-Rendón JL, Hurtado-Salazar C, Yamamoto-Furusho JK. Aspects of inflammatory bowel disease during the COVID-19 pandemic and general considerations. Aspectos y consideraciones generales en la enfermedad inflamatoria intestinal durante la pandemia por COVID-19. Rev Gastroenterol Mex. 2020;S0375-0906(20)30054-9. 10.1016/j.rgmx.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Favalli EG, Ingegnoli F, De Lucia O, Cincinelli G, Cimaz R, Caporali R. COVID-19 infection and rheumatoid arthritis: Faraway, so close!. Autoimmun Rev. 2020;19(5):102523 10.1016/j.autrev.2020.102523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cohen S, Tyrrell DA, Smith AP. Psychological stress and susceptibility to the common cold. N Engl J Med. 1991;325(9):606–612. 10.1056/NEJM199108293250903 [DOI] [PubMed] [Google Scholar]
- 53.Andersson NW, Goodwin RD, Okkels N, Gustafsson LN, Taha F, Cole SW et al. Depression and the risk of severe infections: prospective analyses on a nationwide representative sample. Int J Epidemiol. 2016;45(1):131–139. 10.1093/ije/dyv333 [DOI] [PubMed] [Google Scholar]
- 54.Seminog OO, Goldacre MJ. Risk of pneumonia and pneumococcal disease in people with severe mental illness: English record linkage studies. Thorax. 2013;68(2):171–176. 10.1136/thoraxjnl-2012-202480 [DOI] [PubMed] [Google Scholar]
- 55.Jiang T, Farkas DK, Ahern TP, Lash TL, Sørensen HT, Gradus JL. Posttraumatic Stress Disorder and Incident Infections: A Nationwide Cohort Study. Epidemiology. 2019;30(6):911–917. 10.1097/EDE.0000000000001071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sharma G, Volgman AS, Michos ED. Sex Differences in Mortality from COVID-19 Pandemic: Are Men Vulnerable and Women Protected? JACC Case Rep. 2020;10.1016/j.jaccas.2020.04.027. 10.1016/j.jaccas.2020.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guan WJ, Liang WH, Zhao Y, Liang H, Chen Z, Li Y et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55(5):2000547 Published 2020 May 14. 10.1183/13993003.00547-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Christensen DM, Strange JE, Gislason G, Torp-Pedersen C, Gerds T, Fosbøl E et al. Charlson Comorbidity Index Score and Risk of Severe Outcome and Death in Danish COVID-19 Patients. J Gen Intern Med. 2020;1–3. 10.1007/s11606-020-05991-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Burstyn I, Yang Y, Schnatter AR. Effects of non-differential exposure misclassification on false conclusions in hypothesis-generating studies. Int J Environ Res Public Health. 2014;11(10):10951–10966. Published 2014 Oct 21. 10.3390/ijerph111010951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Burstyn I, Goldstein ND, Gustafson P. Towards reduction in bias in epidemic curves due to outcome misclassification through Bayesian analysis of time-series of laboratory test results: Case study of COVID-19 in Alberta, Canada and Philadelphia, USA. Preprint. medRxiv. 2020;2020.04.08.20057661. Published 2020 Apr 11. 10.1101/2020.04.08.20057661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vultaggio A, Vivarelli E, Virgili G, Lucenteforte E, Bartoloni A, Nozzoli C et al. Prompt predicting of early clinical deterioration of moderate-to-severe COVID-19 patients: usefulness of a combined score using IL-6 in a preliminary study. J Allergy Clin Immunol Pract 2020:S2213-2198(20)30611-5. 10.1016/j.jaip.2020.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ATC = Anatomical Therapeutic Chemical; ICD-9-CM = International Classification of Diseases, 9th Revision, Clinical Modification. aTime span covered was 2009–2018 for hospital-discharge records and 2014–2019 for outpatient pharmacy records.
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
Permission use anonymized data to this study was granted to the researchers of the Centro di Ricerca in Farmacoeconomia e Farmacoutilizzazione (CIRFF) by the governance board of Unità del Farmaco della Regione Campania. The CIRFF has a regional decree that allow for conducting research by making secondary use of administrative data (DGRC n 276 23/05/2017). Furthermore, General Director of Health Care Services authorized the CIRFF to access the COVID-19 patients data in Campania Region (Protocol n. 0173642 of 26/03/2020). Requests for information on data access can be directed to Dr. Enrica Menditto, Director of CIRFF (contact via enrica.menditto@unina.it).