Abstract
Objectives:
Laryngotracheal stenosis (LTS) is a fibrotic condition of the upper airway. Recent evidence suggests dysregulated host immunity plays a role in LTS development and progression. The programmed death-1 (PD-1)/programmed death-ligand 1 (PD-L1) axis, targeted by paradigm-shifting immunotherapies for cancer treatment, has also recently been implicated in the pathogenesis of fibrotic pulmonary disease. However, a role for the PD-1/PD-L1 axis in the proximal airway fibrosis seen in LTS patients has not been explored.
Study Design:
Controlled ex vivo study.
Methods:
Expression of PD-1, PD-L1, CD4, and CD8 were evaluated using immunohistochemical staining of cricotracheal resection specimens from postintubation iatrogenic laryngotracheal stenosis (iLTS), idiopathic subglottic stenosis (iSGS) patients, and normal controls derived from rapid autopsy (n = 8 per group). Fibroblasts derived from iLTS scar were also treated with transforming growth factor beta 1 (TGFβ1) and analyzed for PD-L1 expression by quantitative real-time polymerase chain reaction (n = 6).
Results:
iLTS specimens exhibited increased expression of PD-1, PD-L1, and CD4 (all P < .0167) compared to controls, whereas iSGS specimens exhibited increased expression of PD-1 and CD4 (P < .0167) compared to controls. PD-1, PD-L1, and CD4 showed periepithelial patterns of expression in both disease cohorts. TGFβ1 treatment of iLTS fibroblasts increased expression of PD-L1 (the cognate ligand for PD-1).
Conclusion:
Expression of both PD-1 and its ligand PD-L1 are significantly greater in patients with iLTS compared to controls, and PD-1 expression is also elevated in patients with iSGS. Given published evidence implicating the PD-1/PD-L1 axis in pulmonary fibrosis, this suggests a possible role for checkpoint inhibitors targeting the PD-1/PD-L1 axis for the treatment of LTS.
Level of Evidence:
N/A
Keywords: Laryngotracheal stenosis, PD-1, PD-L1, T lymphocytes
INTRODUCTION
Laryngotracheal stenosis (LTS) encompasses pathologic narrowing of the larynx, subglottis, and trachea due to fibrotic scar. LTS results from intubation injury (iatrogenic laryngotracheal stenosis [iLTS]), autoimmune disease, or radiation, or may be idiopathic (idiopathic subglottic stenosis [iSGS]). Regardless of the cause, LTS creates subjective dyspnea, dysphonia, and if left untreated, life-threatening airway compromise. iLTS is the most common form (representing 60%–75% of LTS cases) and follows prolonged intubation or tracheostomy.1 iSGS represents about 25% of cases and primarily affects healthy Caucasian women without a clearly understood underlying pathophysiology.2 Both iLTS and iSGS are primarily managed surgically, often requiring serial dilations or invasive open airway procedures to avoid tracheostomy.1,2 The lack of benefit derived from current medical therapies reflects our limited understanding of the pathologic mechanisms of disease.
Given the current lack of effective medical therapies for the treatment of LTS, we aimed to explore a potential role for immune-targeting therapies in the management of LTS. Our lab and others have previously described LTS as an immunologic disease, which could therefore benefit from therapeutics aimed at modifying the underlying dysregulated immune response.3-6
Both iLTS and iSGS have been linked to immune dysregulation leading to the pathologic deposition of collagen and extracellular matrix by fibroblasts. In particular, pathologic T-cell responses have been implicated in LTS.3-6 The inability of immunodeficient severe combined immunodeficient (SCID) mice to generate airway scar in response to injury highlights the role of adaptive immunity in LTS.7 Profibrotic responses from Th2 cells and M2 macrophages have been observed in animal models and patients with iLTS.3,4,8 Activation of the interleukin (IL)-17A/IL-23 axis, as well as alterations in the mucosal microbiome, have been observed in patients with iSGS.5,6,9,10 However, a specific and targetable modulator of these pathways has not yet been identified.
The immune system must balance the role of defense against diverse microbial viral and fungal pathogens while simultaneously avoiding self-reactivity. Whereas central tolerance mechanisms result in deletion of the majority of self-reactive T lymphocytes, some T-cells specific for self-antigens escape into the periphery.11 Over the last 15 years, periphery mechanisms to restrict adaptive immune activation have increasingly been defined. One of these mechanisms, immune checkpoints, regulates immune homeostasis and self-tolerance. The established checkpoint, programmed death-1 (PD-1) receptor (CD279); and its ligands, PD-L1 (B7-H1; CD274) and PD-L2 (B7-DC; CD273), deliver inhibitory signals that regulate the balance among T-cell activation, tolerance, and immune-mediated tissue damage.12 This pathway exerts an essential inhibitory function on T-cells in the setting of persistent antigenic stimulation such as during encounter of self-antigens, chronic viral infections, and tumors.13 Interestingly, a recent report indicated that PD-1 was upregulated in CD4+ T-cells and promoted bleomycin-induced pulmonary fibrosis.14 Complementary studies have shown the PD-1 ligand PD-L1 is upregulated on lung fibroblasts in idiopathic pulmonary fibrosis (IPF) and was required for their invasive phenotype.15
We hypothesized that, given the importance of T-cells in the pathogenesis of LTS and the potential for PD-1/PD-L1 interactions to regulate pathologic fibrosis, this axis may also be upregulated in patients with LTS. We utilized immunohistochemistry to evaluate PD-1 and PD-L1 expression in patients with LTS, then interrogated the response of its ligand in stromal fibroblasts derived from LTS airway scar. Identification of higher levels of PD-1 and PD-L1 in LTS patients supports investigation of Food and Drug Administration (FDA)-approved immunotherapies for the treatment of LTS.
MATERIALS AND METHODS
Human Tissue Sampling
Informed written consent was obtained from all participants in accordance with the institutional review boards at Johns Hopkins University (NA_00078310) (Baltimore, MD) and Vanderbilt University Medical Center (#140429) (Nashville, TN). Histology specimens were obtained during open tracheal or cricotracheal resections performed at Johns Hopkins and at Vanderbilt University. Control specimens with normal subglottic airways were obtained from rapid autopsies performed at Johns Hopkins. Specimens were fixed in formalin and embedded in paraffin.
Immunohistochemistry
Staining.
Formalin-fixed, paraffin-embedded specimens were stained with hematoxylin and eosin, CD4 (clone EP204, Sigma-Aldrich, St. Louis, MO), and CD8 (clone 4B11, Leica, Wetzlar, Germany) using standard automated protocols. Immunohistochemistry (IHC) for PD-1 (clone NAT105, Abcam, Cambridge, U.K.)16 and PD-L1 (clone SP142, Spring Bioscience, Pleasanton, CA)17 were performed as previously described, including the use of an isotype control for PD-L1 to control for potential false-positive staining.18 Eight patient samples from the control and iLTS cohorts were stained for CD4, CD8, PD-1, and PD-L1. Eight iSGS patient samples were stained for only CD4, PD-1, and PD-L1 due to limited sample supply.
Quantification.
Immunostained slides were imaged at high magnification (400×) from three areas of intense inflammation identified at low magnification (100×). Percentage of positive-stained cells was determined by diving the number of positive-staining cells by total cells in each high-power field (hpf).4
Multiplex Immunofluorescence
One iLTS tracheal resection specimen and one control specimen were stained by multiplex immunofluorescence (mIF) for PD-1, CD4, CD8, CD20, and cytokeratin, as previously described.19 iSGS was not included in the mIF analysis due to limited sample availability. Slides were scanned using Vectra Polaris Quantitative Pathology Imaging System (Perkin Elmer, Waltham, MA), and spectral unmixing was performed using InForm 2.4 Image Analysis software (Perkin Elmer).
Fibroblast Culture
Biopsy specimens of laryngotracheal scar were obtained during routine surgical dilation of subglottic and tracheal stenosis in six different patients with iLTS. This experiment was not conducted in iSGS due to limited patient specimens. Fibroblasts were isolated from the biopsy tissue and cultured as previously described in Dulbecco’s Modified Eagle Medium (DMEM) with 10% fetal bovine serum (FBS).20 2.5×105 cells were seeded in six well plates and allowed to adhere to the plate overnight. The cells were then serum-starved in 0.1% FBS/DMEM as previously described to obtain a baseline quiescent state.21 The following day, cells were treated with 10 ng/mL human recombinant transforming growth factor beta 1 (TGFβ1) (PeproTech, Rocky Hill, NJ) in complete media and harvested after 12 hours of treatment for subsequent analysis.
Gene Expression Analysis by Quantitative Real-Time Polymerase Chain Reaction
RNA was extracted and purified from cultured fibroblasts derived from the six iLTS patients using a RNeasy Mini Kit (Qiagen, Valencia, CA). RNA was quantified with a NanoDrop 2000 spectrophotometer (Thermo Scientific, Waltham, MA) and used for the creation of complementary DNA. Gene expression was quantified using quantitative real-time polymerase chain reaction (RT-PCR), as previously described.3 The cycle threshold value (CT) of PD-L1, collagen 1, and fibronectin 1 were normalized against β-actin (ΔCT) for all samples. Gene expression is displayed as the relative fold changes (2−ΔΔCT). All samples were investigated in triplicate. Primers sequences are shown in Supporting Information Table S1 (Integrated DNA Technologies, Coralville, IA).
Statistical Analysis
A nonparametric Mann–Whitney test was used to assess individual differences between control, iLTS, and iSGS patient groups. Results are displayed as percent positive cells per hpf, with the marker at the median value and P values as indicated with * denoting statistically significant differences. Categorical patient demographics were compared using a Fisher exact test to compare individual patient groups. Gene expression results are displayed as mean fold change, and error bars indicate standard deviation. A type I error rate (α) < 0.05 was considered statistically significant. A Bonferroni correction (α/m) was then applied to adjust for multiple hypotheses (m = 3) when comparing control, iLTS, and iSGS subgroups, setting the level of statistical significance at a P value < .0167. Data analysis was performed with Prism software (GraphPad Software Inc., La Jolla, CA).
RESULTS
Demographics
Cricotracheal resection specimens were collected from a total of 16 patients with a diagnosis of iLTS (n = 8) or iSGS (n = 8). Control tracheal specimens were collected during rapid autopsies from patients without a history of large airway disease (n = 8). There were no significant differences in age, gender, race, rate of tracheostomy, diabetes, chronic obstructive pulmonary disease (COPD), or asthma among the cohorts (Table I). The Charlson comorbidity index was significantly greater in control patients compared to the iLTS (7 vs. 2, P = .0020) or iSGS groups (7 vs. 1, P = .0009), which is consistent with the use of rapid autopsy specimens in the control group, but was not significantly different between iLTS and iSGS groups. There was a trend toward more female patients in the iSGS group compared to the iLTS (100% vs. 50%, P = .0769) or control groups (100% vs. 50%, P = .0769), consistent with the known demographics of iSGS.1 There was also a trend toward more patients with a prior tracheostomy in the iLTS group compared to the control group (62.5% vs. 0%, P = .0256) and compared to the iSGS group (62.5% vs. 0%, P = .0256), which is also representative of the iLTS population at large.1
TABLE I.
Demographic Data of Study Patients.†
| Control (n = 8) |
Iatrogenic (n = 8) |
Idiopathic (n = 8) |
P Value | |
|---|---|---|---|---|
| Median age, y | 54 | 47 | 49.5 | NS for all comparisons |
| Sex | ||||
| Male | 4 (50) | 4 (50) | 0 (0) | NS for all comparisons |
| Female | 4 (50) | 4 (50) | 8 (100) | |
| Race | ||||
| Caucasian | 8 (100) | 5 (62.5) | 7 (87.5) | NS for all comparisons |
| Other | 0 (0) | 3 (37.5) | 1 (12.5) | |
| Tracheostomy | ||||
| Yes | 0 (0) | 5 (62.5) | 0 (0) | NS for all comparisons |
| No | 8 (100) | 3 (37.5) | 8 (100) | |
| Comorbidities | ||||
| Type 2 Diabetes | 1 (12.5) | 1 (12.5) | 2 (25) | NS for all comparisons |
| COPD | 0 (0) | 1 (12.5) | 0 (0) | NS for all comparisons |
| Asthma | 1 (12.5) | 0 (0) | 0 (0) | NS for all comparisons |
| Median | 7 | 2 | 1 | Control vs. iLTS |
| Charlson | *P = .0020 | |||
| Comorbidity | Control vs. iSGS | |||
| Index | *P = .0009 iLTS vs. iSGS NS | |||
Values are presented as n (%) unless otherwise specified.
COPD = chronic obstructive pulmonary disease; iLTS = iatrogenic laryngotracheal stenosis; iSGS = idiopathic subglottic stenosis; NS = nonsignificant.
PD-1 and PD-L1 Expression Is Increased in Patients With iLTS and iSGS
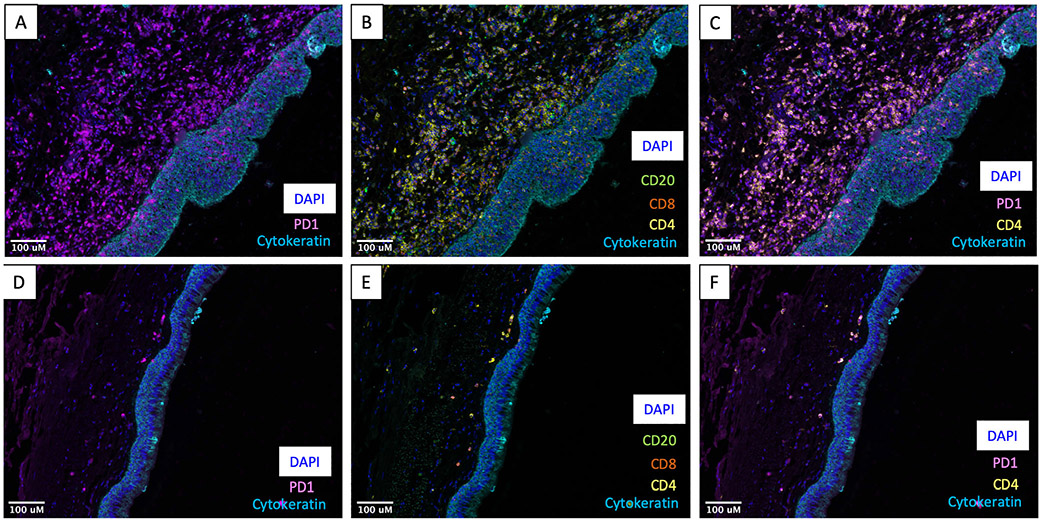
Multispectral staining of an iLTS resection specimen demonstrated intense infiltration of PD-1 positive cells (Fig. 1A) in the superficial lamina propria. Staining for additional immune cell markers demonstrated a high degree of CD4 expression in this area, with occasional CD8 and CD20 positivity (Fig. 1B). Costaining for PD-1 and CD4 shows a high degree of PD-1 expression on CD4 T-cells (Fig. 1C). In contrast, mIF staining of a control sample demonstrated minimal immune cell reactivity in the lamina propria (Fig. 1D-1F).
Fig. 1.
Multispectral immunofluorescence of iLTS demonstrates expression of PD-1 on CD4 cells. (A) Photomicrograph from an iLTS patient demonstrating dense staining for PD-1 (purple) in the lamina propria beneath the cytokeratin-positive (blue) epithelium. (B) Photomicrograph of additional cell types in the multiplex panel including dense CD4 (yellow) expression with sparse CD8 (red) and CD20 (green) expression. (C) High degree of coexpression of PD-1 (purple) with CD4 (yellow). (D–F) Photomicrographs from a control specimen demonstrating limited immune cell staining. All specimens imaged at 200× magnification. iLTS = iatrogenic laryngotracheal stenosis; PD-1 = programmed death-1
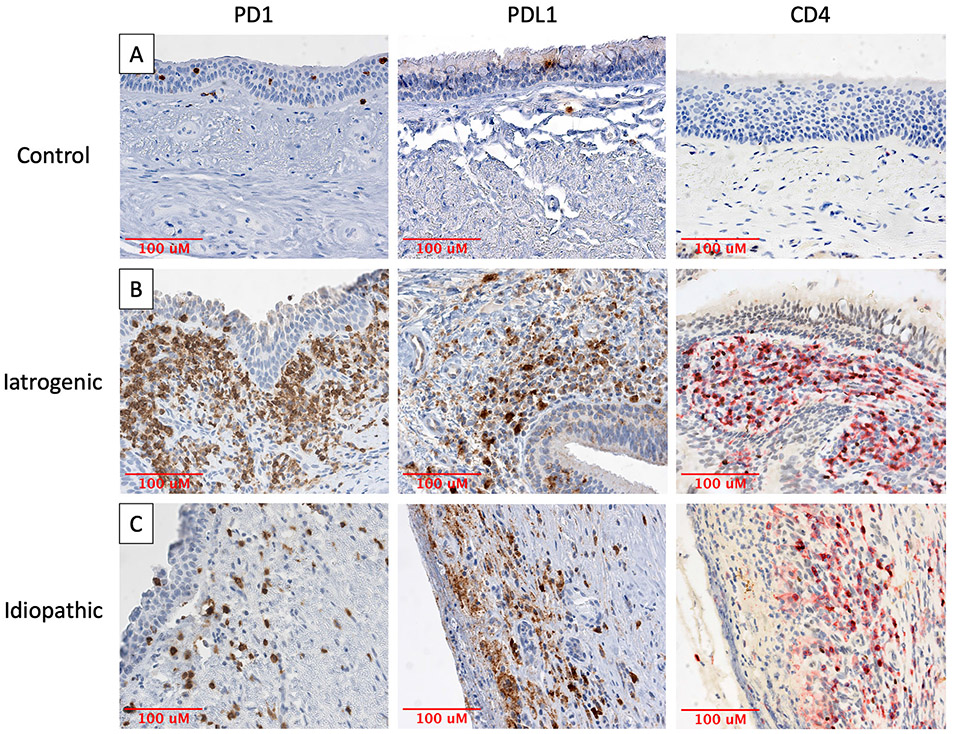
A quantitative comparison of PD-1 and PD-L1 expression in iLTS and iSGS patients was undertaken using traditional immunohistochemical staining (Figs. 2 and 3A,3B). PD-1 expression was significantly higher in both iLTS patients (median 21.65% positive cells per hpf vs. 7.29%, P = .0070) and iSGS patients (24.17% vs. 7.29%, P = .0006) compared to control samples (n = 8 per group). PD-1 expression was not significantly different between the iLTS and iSGS groups (21.65% vs. 24.17%, P = .3823).
Fig. 2.
PD-1, PD-L1, and CD4 expression in iLTS and iSGS specimens. Photomicrographs depicting (A) minimal PD-1, PD-L1, or CD4 staining in healthy tracheal samples and intense staining of PD-1, PD-L1, and CD4 in the subepithelial region of (B) iLTS and (C) iSGS patients. All specimens imaged at 400× magnification. iLTS = iatrogenic laryngotracheal stenosis; iSGS = idiopathic subglottic stenosis; PD-1 = programmed death-1.
Fig. 3.
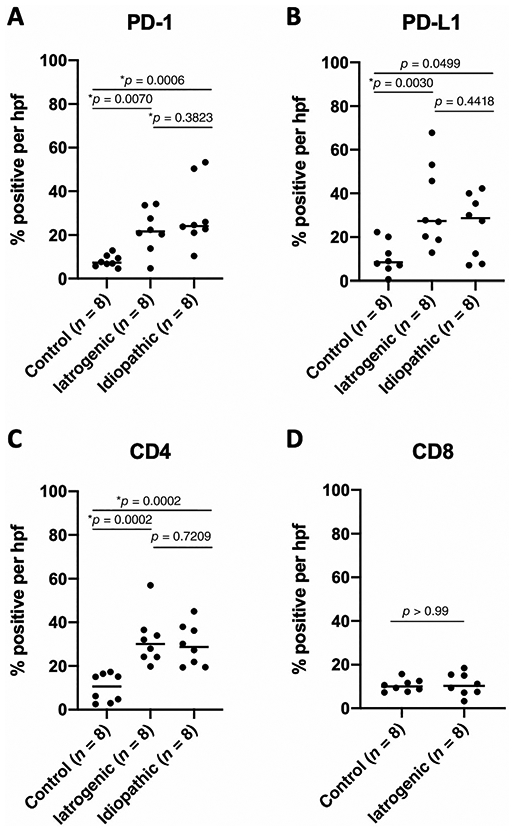
Significantly greater expression of PD-1, PD-L1, and CD4 are observed in iLTS and iSGS patients compared to controls. Quantification of IHC staining demonstrates (A) significantly greater expression of PD-1 in iLTS and iSGS patients compared to controls and (B) significantly greater expression of PD-L1 in iLTS and a trend toward greater expression of PD-L1 in iSGS patients compared to control. (C) CD4 expression is significantly elevated in iLTS and iSGS patients compared to controls. No significant differences were observed between iLTS and iSGS patient groups. (D) CD8 expression was not significantly different between iLTS patients and controls and was not evaluated in iSGS due to sample availability. iLTS = iatrogenic laryngotracheal stenosis; iSGS = idiopathic subglottic stenosis; PD-1 = programmed death-1; PD-L1 = programmed death-ligand 1.
PD-L1 expression was also significantly higher in the iLTS patients compared to controls (27.38% vs. 8.45%, P = .0030). The iSGS patients also exhibited a higher median expression of PD-L1 than controls; however, this did not reach statistical significance when using the Bonferroni correction (28.74% vs. 8.45%, P = .0499). Again, no significant difference in PD-L1 expression was observed between the iLTS and iSGS groups (P = .4418).
Correlation With CD4 and CD8 Expression
Given the high degree of PD-1 and CD4 coexpression seen in mIF staining of an iLTS patient sample (Fig. 1C), immunohistochemical staining for CD4 and CD8 was carried out (Figs. 2 and 3C,3D). CD4 expression was significantly higher in the iLTS patients (30.01% vs. 10.62%, P = .0002) and the iSGS patients (28.73% vs. 10.62%, P = .0002) compared to controls. No significant difference was observed in CD4 expression between iLTS and iSGS patients (P = .7209). CD8 expression was not significantly different between iLTS patients and controls (10.28% vs. 10.00%, P > .9999). CD4 expression was significantly higher than CD8 expression in iLTS patients (30.01% vs. 10.28%, P = .0078).
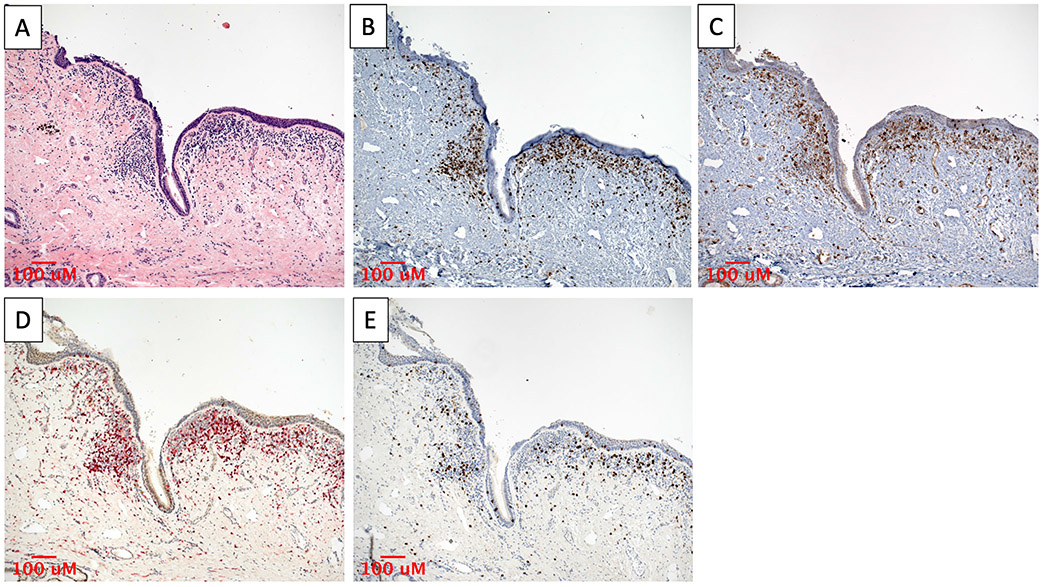
Qualitative assessment of the geographic colocalization of PD-1, PD-L1, CD4, and CD8 was carried out by imaging the same region of adjacent stained slides at low power (Fig. 4). Expression of all four markers was observed localizing to the same areas of intense subepithelial inflammation.
Fig. 4.
Colocalization of PD-1 and PD-L1 expression with T-cell infiltrates in iLTS. Immunostaining of a representative iLTS patient sample showing (A) subepithelial lymphocytic infiltrate on H&E, which is associated with (B) PD-1, (C) PD-L1, (D) CD4, and (E) CD8 staining. All specimens imaged at 100× magnification. H&E = hematoxylin and eosin; iLTS = iatrogenic laryngotracheal stenosis; PD-1 = programmed death-1; PD-L1 = programmed death-ligand 1.
TGFβ1 Induces PD-L1, Collagen 1, and Fibronectin 1 Expression in iLTS Fibroblasts
Fibroblasts isolated from six individual iLTS patients demonstrated significantly greater PD-L1 expression (mean 3.8-fold, P = .0022) (Fig. 5A) after 12 hours of treatment with TGFβ1 compared to untreated scar fibroblasts. TGFβ1 also increased fibroblast expression of collagen-1 (Col1; 1.8-fold, P = .0476) and fibronectin-1 (FN1; 2.3-fold higher, P = .0022) (Fig. 5B,5C) after 12 hours of treatment.
Fig. 5.
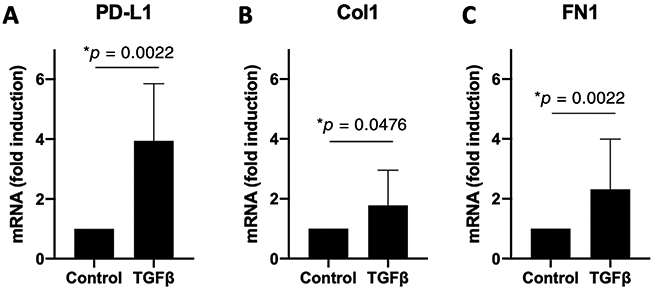
TGFβ1 increases fibroblast expression of PD-L1, Col1, and FN1. Scar fibroblasts derived from six patients with iLTS were treated with TGFβ1 for 12 hours. RT-PCR demonstrated (A) mean 3.8-fold increase in PD-L1 expression, (B) 1.8-fold increase in Col-1, and (C) 2.3-fold increase in FN1 expression. Error bars indicate standard deviation. iLTS = iatrogenic laryngotracheal stenosis; PD-L1 = programmed death-ligand 1; RT-PCR = real-time polymerase chain reaction; TGFβ1 = transforming growth factor beta 1; Col1 = Collagen 1; FN1 = Fibronectin 1.
DISCUSSION
This is the first study to evaluate the expression of the immune checkpoint PD-1 and its primary ligand, PD-L1, in patients with LTS. Immunohistochemical staining of laryngotracheal resection specimens demonstrated higher expression of both PD-1 and PD-L1 in patients with iLTS, and higher PD-1 in patients with iSGS compared to controls (Fig. 3A,3B). Expression of PD-1, PD-L1, CD4, and CD8 colocalized to the same periepithelial inflammatory hotspots. This suggests that coexpression of these markers on the same cells and/or on adjacent cells interact to promote fibrosis (Fig. 4). Preliminary mechanistic in vitro studies demonstrated iLTS fibroblasts cultured ex vivo responded to the profibrotic cytokine TGFβ1 by upregulating PD-L1 expression in addition to the fibrotic mediators Col1 and FN1 (Fig. 5).
Increased expression of both PD-1 and its ligand PD-L1 in LTS patients suggests that rather than PD-1 being upregulated purely as a reaction to underlying inflammatory processes, PD-1/PD-L1 interactions could play a role in the development of airway scar. The PD-1/PDL-1 axis has recently been implicated in IPF, a fibrotic condition of the lower airways that shares many similarities with LTS. PD-1 upregulation on Th17 cells in IPF patients has been shown to promote fibrosis through increased secretion of IL-17A and TGFβ1.14 PD-1 blockade in cancer patients has also been shown to inhibit the Th2 T-cell response in favor of the Th1 response,22 which attenuates fibrotic activity in LTS.20 In IPF, PD-L1 has also been identified as a marker of invasive fibroblasts, and inhibition of PD-L1 in a murine model of IPF resulted in attenuation of fibrosis.15
The upregulation of PD-L1 on iLTS patient fibroblasts in response to TGFβ1 provides a potential source for the observed increase in PD-L1 in iLTS patients. Our observation that TGFβ1 induces Col1 and FN1 in iLTS fibroblasts is consistent with the known function of TGFβ1, which has been implicated in fibrosis in a variety of organ systems and is elevated in human airway stenosis.23 Our finding of PD-L1 upregulation by TGFβ1 also supports prior findings of PD-L1 induction by TGFβ1 in pulmonary fibroblasts.21 Future investigations into the functional role of the PD-1/PD-L1 axis and the impact of its inhibition on fibrosis in LTS could pave the way for the novel application of FDA-approved checkpoint inhibitors to this disease.
Prior studies have highlighted distinct lymphocytic mechanisms in iLTS and iSGS, with upregulation of the CD4 axis seen in iLTS3,4 and the IL-17A/IL-23 axis in iSGS.5,6 This study is the first to directly compare CD4 expression between iLTS and iSGS patients, showing that CD4 T-cell infiltration, as well as overall expression of PD-1 and PD-L1, are very similar. Whereas many iLTS and iSGS patients have disparate demographics1 and distinct outcomes to treatment,1,24 overlapping immune responses are seen in both clinical entities. One limitation of our study is the inability to obtain CD8 staining in the iSGS cohort due to limited availability of patient samples. Future studies comparing CD8 expression between iLTS and iSGS are warranted.
We have previously demonstrated a predominantly CD4+ lymphocytic infiltrate in the subepithelium of iLTS patients, as well as in our bleomycin-induced murine model of iLTS.3,4 This study corroborates these prior findings by once again demonstrating significantly greater CD4 than CD8 expression in this iLTS cohort. We also observed spatial association of PD-1, PD-L1, and CD4 expression within inflammatory “hotspots,” suggesting that PD-1 may be expressed on CD4 T-cells and could be interacting with PD-L1 within the vicinity to mediate changes in the profibrotic immune response. However, further investigations into the specific cell types expressing PD-1 and PD-L1 are warranted.
This study also demonstrates for the first time that novel mIF technology developed to study immune cell infiltrates in the tumor microenvironment19 can be effectively applied to healthy and iLTS tracheal specimens. This technology has previously been utilized to quantitatively map the proximity between PD-1 and PD-L1 expression in patients with Merkel cell carcinoma, which was found to be associated with response to anti-PD-1 therapy.19 Future studies using this evolving technology will be able to quantify the immune cell infiltrates and the associated expression of PD-1 and PD-L1 in LTS. Mapping algorithms quantifying the spatial relationships among these cells will allow us to better understand the functional implications of their interactions. Mapping the proximity between PD-1 and PD-L1 in situ can capture direct binding between PD-1 and PD-L1 to better understand their interactions at the immune synapse.
CONCLUSION
Expression of the immune checkpoint PD-1 and its ligand PD-L1 are significantly elevated in iLTS patients compared to controls. iSGS patients also demonstrate a significant elevation in PD-1 expression. These results suggest a potential role for the PD-1/PD-L1 axis in the profibrotic immune responses that characterize iLTS and iSGS. The upregulation of PD-L1 expression by iLTS fibroblasts in conjunction with profibrotic signals in response to TGFβ1 provides a potential mechanism of PD-L1 upregulation in LTS. These novel findings provide the foundation for future functional and quantitative studies into the application of FDA-approved immunotherapies for the treatment of LTS.
Supplementary Material
ACKNOWLEDGMENT
Research reported in this publication was supported by an American Academy of Otolaryngology–Head and Neck Surgery Foundation Resident Research Grant (r.j.d.) and the National Institute on Deafness and Other Communication Disorders of the National Institutes of Health under award numbers 1R01DC018567, 1K23DC014082, and R21DC017225. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This study was also financially supported by the Triological Society and American College of Surgeons (a.t.h.).
Footnotes
Presented as a virtual oral presentation at the virtual American Bronchoesophagological Association (ABEA) Meeting at the Combined Otolaryngology Spring Meetings (COSM), April 23, 2020. Originally accepted for podium presentation at the cancelled ABEA meeting at COSM, Atlanta, Georgia, U.S.A., April 23, 2020.
Additional supporting information may be found in the online version of this article.
Contributor Information
Ruth J. Davis, Department of Otolaryngology—Head and Neck Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, U.S.A..
Ioan Lina, Department of Otolaryngology—Head and Neck Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, U.S.A..
Dacheng Ding, Department of Otolaryngology—Head and Neck Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, U.S.A..
Elizabeth L. Engle, Department of Otolaryngology, Bloomberg-Kimmel Institute for Cancer Immunotherapy, Baltimore, MD, U.S.A.; Department of Dermatology, Johns Hopkins University School of Medicine, Baltimore, MD, U.S.A..
Janis Taube, Department of Otolaryngology, Bloomberg-Kimmel Institute for Cancer Immunotherapy, Baltimore, MD, U.S.A.; Department of Dermatology, Johns Hopkins University School of Medicine, Baltimore, MD, U.S.A.; Department of Oncology, Johns Hopkins University School of Medicine, Baltimore, MD, U.S.A.; Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, MD, U.S.A..
Alexander Gelbard, Department of Otolaryngology, Vanderbilt University School of Medicine, Nashville, TN, U.S.A..
Alexander T. Hillel, Department of Otolaryngology—Head and Neck Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, U.S.A..
BIBLIOGRAPHY
- 1.Gelbard A, Francis DO, Sandulache VC, Simmons JC, Donovan DT, Ongkasuwan J Causes and consequences of adult laryngotracheal stenosis. Laryngoscope 2015;125:1137–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gelbard A, Donovan DT, Ongkasuwan J, et al. Disease homogeneity and treatment heterogeneity in idiopathic subglottic stenosis. Laryngoscope 2016;126:1390–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Motz KM, Yin LX, Samad I, et al. Quantification of inflammatory markers in laryngotracheal stenosis. Otolaryngol Head Neck Surg 2017;157:466–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hillel AT, Ding D, Samad I, Murphy MK, Motz K T-helper 2 lymphocyte Immunophenotype is associated with iatrogenic laryngotracheal stenosis. Laryngoscope 2018;129:177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gelbard A, Katsantonis N-G, Mizuta M, et al. Idiopathic subglottic stenosis is associated with activation of the inflammatory IL-17A/IL-23 axis. Laryngoscope 2016;126:E356–E361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morrison RJ, Katsantonis N-G, Motz KM, et al. Pathologic fibroblasts in idiopathic subglottic stenosis amplify local inflammatory signals. Otolaryngol Neck Surg 2019;160(1):107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghosh A, Malaisrie N, Leahy KP, et al. Cellular adaptive inflammation mediates airway granulation in a murine model of subglottic stenosis. Otolaryngol Neck Surg 2011;144:927–933. [DOI] [PubMed] [Google Scholar]
- 8.Hillel AT, Samad I, Ma G, et al. Dysregulated macrophages are present in bleomycin-induced murine laryngotracheal stenosis. Otolaryngol Head Neck Surg 2015;153:244–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gelbard A, Katsantonis N-G, Mizuta M, et al. Molecular analysis of idiopathic subglottic stenosis for mycobacterium species. Laryngoscope 2017;127:179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hillel AT, Tang SS, Carlos C, et al. Laryngotracheal microbiota in adult laryngotracheal stenosis. mSphere 2019;4:e00211–e00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lohmann T, Leslie RDG, Londei M T cell clones to epitopes of glutamic acid decarboxylase 65 raised from Normal subjects and patients with insulin-dependent diabetes. J Autoimmun 1996;9:385–389. [DOI] [PubMed] [Google Scholar]
- 12.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol 2007;8:239–245. [DOI] [PubMed] [Google Scholar]
- 13.Keir ME, Butte MJ, Freeman GJ, Sharpe AH PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008;26:677–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Celada LJ, Kropski JA, Herazo-Maya JD, et al. PD-1 up-regulation on CD4+ T cells promotes pulmonary fibrosis through STAT3-mediated IL-17A and TGF-β1 production. Sci Transl Med 2018;10:eaar8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geng Y, Liu X, Liang J, et al. PD-L1 on invasive fibroblasts drives fibrosis in a humanized model of idiopathic pulmonary fibrosis. JCI Insight 2019;4:e125326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lipson EJ, Lilo MT, Ogurtsova A, et al. Basal cell carcinoma: PD-L1/PD-1 checkpoint expression and tumor regression after PD-1 blockade. J Immunother Cancer 2017;5:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sunshine JC, Nguyen PL, Kaunitz GJ, et al. PD-L1 expression in melanoma: a quantitative Immunohistochemical antibody comparison. Clin Cancer Res 2017;23:4938–4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res 2014;20:5064–5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giraldo NA, Nguyen P, Engle EL, et al. Multidimensional, quantitative assessment of PD-1/PD-L1 expression in patients with Merkel cell carcinoma and association with response to pembrolizumab. J Immunother Cancer 2018;6:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Motz K, Samad I, Yin LX, et al. Interferon-γ treatment of human Laryngotracheal stenosis-derived fibroblasts. JAMA Otolaryngol Head Neck Surg 2017;143:1134–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang J, Jung M, Choudhury M, Leof EB Transforming growth factor beta induces fibroblasts to express and release the immunomodulatory protein PD-L1 into extracellular vesicles. FASEB J 2019;34:2213–2226. [DOI] [PubMed] [Google Scholar]
- 22.Dulos J, Carven GJ, van Boxtel SJ, et al. PD-1 blockade augments Th1 and Th17 and suppresses Th2 responses in peripheral blood from patients with prostate and advanced melanoma cancer. J Immunother 2012;35:169–178. [DOI] [PubMed] [Google Scholar]
- 23.Karagiannidis C, Velehorschi V, Obertrifter B, Macha H-N, Linder A, Freitag L High-level expression of matrix-associated transforming growth factor-beta1 in benign airway stenosis. Chest 2006;129:1298–1304. [DOI] [PubMed] [Google Scholar]
- 24.Gadkaree SK, Pandian V, Best S, et al. Laryngotracheal stenosis: risk factors for tracheostomy dependence and dilation interval. Otolaryngol Head Neck Surg 2017;156:321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.