Abstract
Background
Ceftriaxone (CTX) and penicillin G (PCN G) are considered reasonable treatment options for viridans group streptococci (VGS) bloodstream infections, but comparisons between these agents are limited. We evaluated clinical outcomes among patients treated with these agents for complicated VGS bacteremia.
Methods
This was a single-center retrospective study of adult patients with ≥1 positive VGS blood culture who were treated with either CTX or PCN G/ampicillin (both included in the PCN arm) between January 2013 and June 2019. The primary outcome was a composite of safety end points, including hospital readmission due to VGS bacteremia or adverse events (AEs) from therapy, Clostridioides difficile infections, treatment modification or discontinuation due to AEs from therapy, and development of extended-spectrum beta-lactamase resistance. Secondary outcomes included individual safety end points, VGS bacteremia recurrence, hospital readmission, and all-cause mortality.
Results
Of 328 patients screened, 94 met eligibility criteria (CTX n = 64, PCN n = 30). Streptococcus mitis was the most common isolate, and infective endocarditis was the predominant source of infection. CTX was not significantly associated with increased risk of the primary composite safety outcome (CTX 14% vs PCN 27%; P = .139). The driver of the composite outcome was hospital readmission due to VGS bacteremia or therapy complications. No secondary end points differed significantly between groups. On multivariate analysis, source removal was a protective factor of the primary composite safety outcome.
Conclusions
Despite potential safety concerns with the prolonged use of CTX in complicated VGS bacteremia, this study did not demonstrate higher rates of treatment failure, adverse events, or resistance.
Keywords: antimicrobial stewardship, bacteremia, ceftriaxone, penicillin, viridans group streptococci
Viridans group streptococci (VGS) are an infrequent cause of bloodstream infections, accounting for ~2% of positive blood cultures in immunocompetent adults in the United States [1, 2]. VGS commonly colonize the oral cavity, upper respiratory tract, female genital tract, and gastrointestinal tract and are often deemed to be contaminants in positive blood cultures [2]. However, the consequences of pathogenic VGS bacteremia can vary widely by patient population and infection source [2]. In immunocompetent patients with intact host defenses, VGS is considered to have low virulence. However, VGS can be a leading cause of bacteremia in febrile neutropenic patients, leading to shock, respiratory compromise, and mortality in up to 20% of patients [2–4]. Concomitant infections in VGS bacteremia such as endocarditis can occur, requiring prolonged antibiotic therapy, and are associated with mortality rates of 4% to 16% [5].
Optimal treatment strategies for VGS bacteremia have not been well defined. Both penicillin G (PCN G) and ceftriaxone (CTX) are frequently used for definitive treatment in clinical practice, and the American Heart Association (AHA) lists both agents as first-line treatment options for VGS infective endocarditis (IE). Ampicillin (AMP) is a reasonable alternative to PCN G if a drug shortage or patient intolerance exists [6]. Both PCN G and AMP require high total daily doses administered over several times per day. Specific dosing regimens and durations ultimately depend on VGS susceptibility results, site of infection, and patient factors.
Antimicrobial stewardship principles encourage the use of agents with both optimal effectiveness and safety. It is important to consider the risks for adverse effects and collateral damage in addition to in vitro activity, pharmacology properties, and convenience of administration during antibiotic treatment selection. While CTX offers an attractive once-daily dosing option, its use may be associated with increased risk of adverse events including Clostridioides difficile infection (CDI), laboratory abnormalities (increased serum transaminases, thrombocytopenia), and development of extended-spectrum beta-lactamase (ESBL) resistance [7, 8]. PCN G and AMP offer targeted, narrow-spectrum treatment options for VGS bacteremia with a relatively well-tolerated safety profile [9–11]. However, the frequent administration of these agents may be restrictive and potentially increase the risk of complications such as infusion line infections, particularly in patients requiring prolonged treatment in the outpatient setting [9, 10]. Alternatively, while not common practice at our institution, continuous infusions of PCN G and AMP can be considered for outpatient antibiotic therapy (OPAT) [11, 12]. The total sodium and potassium content in PCN G formulations can further exacerbate electrolyte abnormalities and pose additional risks for patients with comorbidities such as heart failure.
Previous observational studies have described the use of different treatment options for VGS bacteremia and IE, but comparative assessment of clinical outcomes between different agents is limited [13, 14]. We evaluated whether CTX is associated with more adverse effects than PCN G when used for complicated VGS bacteremia and whether these risks may outweigh the benefit of its once-daily dosing. The objective of this study was to compare clinical outcomes between CTX and PCN G for the prolonged treatment of complicated VGS bacteremia.
METHODS
Study Design and Population
A single-center retrospective study was performed between January 2013 and June 2019 at New York University Langone Health (NYULH), an 800-bed, urban, tertiary care academic medical center. Patients with 1 or more blood cultures positive for a VGS isolate who received at least 1 dose of either CTX, PCN G, or AMP were identified and screened for inclusion. Diagnosis of a true VGS bacteremia episode was determined based on at least 1 positive blood culture, documentation of VGS bacteremia by providers in the electronic health record (EHR), and initiation of antibiotic therapy for VGS bacteremia treatment. Complicated bacteremia was defined by the study investigators as cases requiring at least 4 weeks of therapy based on commonly prescribed durations for treatment of severe infections, such as IE [2, 6]. Patients receiving a study drug for at least 50% of the total treatment course were included in the study and assigned to the corresponding treatment arm, either CTX or PCN. Patients who received AMP for the minimum specified duration were assigned to the PCN group. The 50% threshold for study drug duration was chosen to increase the likelihood that outcomes would be associated with the study drug rather than other prescribed antibiotics.
Of note, for VGS bacteremia treatment at our institution, PCN G is typically dosed at 12–24 million units/d, while the total daily dose for AMP is 8–12 g/d, assuming normal renal function. Upon hospital discharge, continuous infusion PCN G and AMP are options for continued therapy in the OPAT setting, though not routinely done at NYULH. CTX can be administered once daily in most cases using a 1- or 2-g dose. Therapy selection is driven by provider preference in the absence of formal institutional guidelines for VGS bacteremia. This study was approved by the NYULH Institutional Review Board (IRB), and patient consent was not required.
Inclusion and Exclusion Criteria
Patients were included in the study if they were at least 18 years of age, received at least 4 weeks of overall treatment for a presumed complicated VGS bacteremia episode, and received study drug therapy (CTX, PCN G, or AMP) for at least 50% of the treatment course. Patients were excluded if they (i) had a polymicrobial blood culture with isolates other than VGS that were not considered contaminants, (ii) had a blood culture with VGS that was considered a contaminant, (iii) were transitioned to hospice care during their treatment course, (iv) died during treatment before receiving at least 2 weeks of therapy, (v) had their first VGS bacteremia episode before the study period, or (vi) received therapy at an outside hospital.
Data Collection
Data collection included patient demographics, comorbidities, hospital and intensive care unit admission details, VGS bacteremia presentation and management, medication therapy, and microbiological data by review of the EHR. Charlson comorbidity index (CCI) was calculated at time of hospital admission, and Pitt bacteremia score (PBS) was calculated using the most severe values from the 24-hour time period before and after the first positive blood culture was collected. Antibiotic therapy was categorized as empiric therapy (before availability of susceptibility results), definitive therapy (initial antibiotic prescribed after availability of susceptibility results), and treatment arm therapy (based on the study drug evaluated). Individual species of VGS and susceptibility results with minimum inhibitory concentration (MIC) values for each study drug were evaluated using the VITEK 2 system (bioMérieux).
Outcomes
The primary outcome was a composite of safety end points, including hospital readmission due to VGS bacteremia or an adverse event from antibiotic therapy, CDI, treatment modification or discontinuation due to an antibiotic-related adverse event, and development of ESBL resistance. All outcomes were assessed for the 6-month period following initial positive blood culture. Patients were censored after they experienced 1 event in the primary composite end point. Secondary outcomes included the individual safety end points, VGS bacteremia recurrence, hospital readmission, and all-cause mortality. Recurrent infection was defined as the development of a subsequent case of VGS bacteremia with the same species after completion of the initial treatment course. CDI was defined as a positive C. difficile test by polymerase chain reaction in symptomatic patients without alternative explanations for diarrhea. Development of ESBL resistance was defined as isolation of a gram-negative bacterium from any microbiological specimen with resistance to at least 1 third-generation cephalosporin. Source removal was defined as actions taken to control the presumed foci of infection and restore optimal function of the site, which included removal of prosthetic materials and debridement of infected foci (eg, valve replacement and repair).
Statistical Analysis
Data analysis was performed using IBM SPSS Statistics for Windows, version 25 (IBM Corp., Armonk, NY, USA). Variables were expressed as median values with interquartile ranges, as data were not normally distributed. Univariate comparisons between the CTX and PCN groups were conducted using the chi-square test or Fisher exact test for categorical variables and the Mann-Whitney U test for continuous variables. P values of ≤.05 denoted statistical significance. Variables with P values of ≤.2 on univariate analysis were included in the multivariate logistic regression model to identify risk factors associated with the primary composite safety outcome. The Hosmer-Lemeshow goodness-of-fit test was used to test the power of the model.
RESULTS
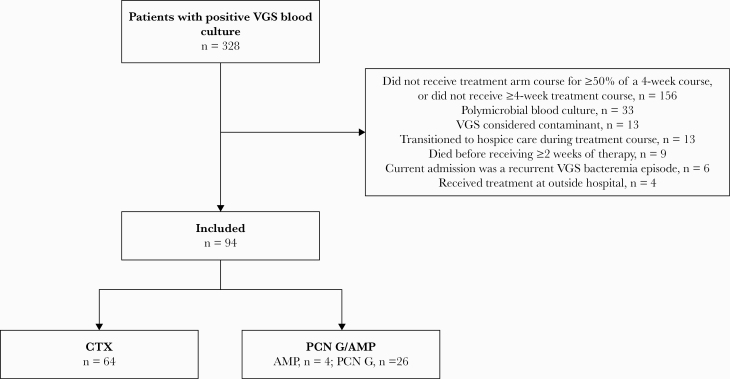
Three hundred twenty-eight patients were screened for potential study inclusion (Figure 1). After exclusion, there were 94 patients who met eligibility criteria, of whom 64 patients (68%) received CTX, 26 patients (28%) received PCN G, and 4 patients (4%) received AMP. Baseline characteristics are provided in Table 1. Patient demographics were similar between the 2 groups, and the majority of patients were elderly Caucasian men and had preexisting coronary artery disease and/or congestive heart failure. In general, study patients did not present with critical illness, as reflected by a median PBS score of 0 in the CTX group and 1 in the PCN group. More patients in the CTX group had a source of bacteremia identified (97% vs 84%; P = .032) and source removal (16% vs 12%; P = .045). S. mitis was the most common VGS species identified, and IE was the predominant source of infection in both treatment arms. The majority of patients were evaluated by the Infectious Diseases consult service, with a median evaluation time of 2 days from blood culture positivity. Treatment details are provided in Table 2. Vancomycin was chosen as empiric antibiotic therapy for most patients, and fewer patients in the CTX group received concomitant therapy with gentamicin (19% vs 43%; P = .012). Almost all patients who received CTX for definitive therapy (54/56, 96%) received 2 g every 24 hours. The majority of patients (97%) who received definitive therapy with PCN G or AMP received our recommended daily dose of 12–24 million units/d or 8–12 g/d, respectively. Almost all patients received continued outpatient antibiotic therapy via a peripherally inserted central catheter (PICC).
Figure 1.
Flowchart for patient inclusion and exclusion. Abbreviations: AMP, ampicillin; CTX, ceftriaxone; PCN G, penicillin G; VGS, viridans group streptococci.
Table 1.
Baseline Characteristics
| Variables | CTX (n = 64) | PCN G/AMP (n = 30) | P Value |
|---|---|---|---|
| Age, median (IQR), y | 69 (58–81) | 64 (52–80) | .422 |
| Male | 42 (65.6) | 22 (73) | .455 |
| Ethnicity | |||
| White | 50 (78.1) | 21 (70) | .393 |
| African American | 7 (10.9) | 2 (7) | .714 |
| Hispanic | 5 (7.8) | 2 (7) | 1.000 |
| Asian | 2 (3.1) | 4 (13) | .080 |
| Other/not specified | 0 (0) | 1 (3) | .319 |
| Medical history | |||
| Congestive heart failure | 17 (26.6) | 6 (20) | .490 |
| Coronary artery disease | 12 (18.8) | 6 (20) | .886 |
| Diabetes mellitus | 9 (14.1) | 7 (23) | .265 |
| End organ damage | 2/9 (22.2) | 2/7 (29) | .603 |
| Chronic kidney disease (moderate or severe) | 7 (10.9) | 2 (7) | .714 |
| Chronic obstructive pulmonary disease | 4 (6.3) | 2 (7) | 1.000 |
| Liver disease | 3 (4.7) | 0 (0) | .549 |
| Mild | 1/3 (33) | N/A | N/A |
| Moderate | 0/3 (0) | N/A | N/A |
| Severe | 2/3 (66) | N/A | N/A |
| Solid tumor | 2 (3.1) | 2 (7) | .590 |
| Metastatic | 1/2 (50) | 1/2 (50) | 1.000 |
| Leukemia | 2 (3.1) | 0 (0) | 1.000 |
| Lymphoma | 1 (1.6) | 1 (3) | .539 |
| Charlson comorbidity index, median (IQR) | 4 (2–5) | 3 (2–5) | .386 |
| Pitt bacteremia score, median (IQR) | 0 (0–2) | 1 (0–2) | .764 |
| Prior episode of C. difficile infection | 0 (0) | 0 (0) | n/a |
| Concomitant PPI or H2RA administration | 20 (32.3) | 10 (33) | .918 |
All data are expressed as No. (%) unless otherwise noted.
Abbreviations: AMP, ampicillin; CTX, ceftriaxone; H2RA, histamine type-2 receptor antagonist; IQR, interquartile range; PCN G, penicillin G; PPI, proton pump inhibitor.
Table 2.
VGS Bacteremia Treatment Details
| VGS Bacteremia Treatment | CTX (n = 64) | PCN G (n = 30) | P Value |
|---|---|---|---|
| Hospital length of stay, median (IQR), d | 8 (5–11) | 10 (6–13) | .159 |
| Admission to ICU | 8 (12.5) | 7 (23) | .229 |
| Duration of ICU stay, median (IQR), d | 4.5 (1–9) | 6 (3–7) | .779 |
| Isolate group | |||
| S. mitis | 45 (70.3) | 18 (60) | .324 |
| S. anginosus | 8 (12.5) | 8 (27) | .095 |
| S. bovis | 2 (3.1) | 2 (7) | .592 |
| S. mutans | 5 (7.8) | 1 (3) | .661 |
| S. salivarius | 4 (6.3) | 1 (3) | 1.000 |
| Beta-lactam allergy | 14 (21.9) | 0 (0) | .004 |
| Penicillin | 13/14 (93) | N/A | N/A |
| Severe | 1/14 (7) | N/A | N/A |
| Cephalosporin | 1/14 (7) | N/A | N/A |
| Severe | 0/14 (0) | N/A | N/A |
| Source of bacteremia identified | 62 (96.9) | 25 (84) | .032 |
| Out of n = 62: | Out of n = 25: | ||
| Infective endocarditis | 41 (66) | 17 (68) | .847 |
| Prosthetic valve endocarditis | 20 (49) | 6 (35.3) | .347 |
| Skeletal | 8 (12.9) | 6 (24) | .213 |
| Skin and soft tissue | 7 (11.3) | 3 (12) | 1.000 |
| Intra-abdomina | 5 (8.1) | 0 (0) | .316 |
| Central line | 1 (1.6) | 0 (0) | 1.000 |
| Central nervous system | 2 (3.2) | 1 (4) | 1.000 |
| Pulmonary | 0 (0) | 1 (4) | .287 |
| Urinary tract | 0 (0) | 0 (0) | n/a |
| Multiple | 4 (4.8) | 3 (12) | .405 |
| Other | 2 (3.2) | 0 (0) | .045 |
| Source removal | 16/62 (25) | 12/25 (48) | .045 |
| Time to source removal, median (IQR), d | 1 (0–9) | 5 (2.25–8.50) | .277 |
| Susceptibility, susceptible + intermediate | |||
| Penicillin | 62 (96.9) | 30 (100) | 1.000 |
| Intermediate | 16/62 (25) | 4/30 (13.3) | .709 |
| Ampicillin | 61 (95.3) | 30 (100) | .549 |
| Intermediate | 9/61 (14.1) | 4/30 (13) | 1.000 |
| Ceftriaxone | 62 (96.9) | 24 (80) | .027 |
| Intermediate | 1/62 (1.6) | 1/24 (4.2) | .590 |
| Empiric therapy | |||
| Vancomycin | 57 (89.1) | 25 (83) | .512 |
| Ceftriaxone | 4 (6.3) | 0 (0) | .303 |
| Other | 3 (4.7) | 5 (17) | .105 |
| Definitive therapy | |||
| Ceftriaxone | 56 (87.5) | 0 (0) | - |
| Penicillin G | 6 (9.4) | 26 (87) | - |
| Ampicillin | 1 (1.6) | 4 (13) | - |
| Cefazolin | 1 (1.6) | 0 (0) | - |
| Infectious diseases consult | 63 (98.4) | 30 (100) | 1.000 |
| Time to consult, median (IQR), d | 2 (1–3) | 1.5 (1–2) | .826 |
| Combination therapy with gentamicin | 12 (18.8) | 13 (43) | .012 |
| Duration, median (IQR), d | 3 (1.5–13.5) | 14 (5.5–27.5) | .034 |
| Time to documented clearance of blood culture, median (IQR), d | 2 (1–3) | 1.5 (1–2) | .079 |
| Duration of total therapy, median (IQR), d | 41.5 (30–44) | 42.5 (31.75–47.25) | .334 |
| Duration of definitive therapy, median (IQR), d | 36.5 (25.25–42) | 39.5 (28–41.25) | .354 |
| Duration of treatment arm, median (IQR), d | 38 (27–43) | 39.5 (28–41.25) | .961 |
| Outpatient line accessa | 62 (97.9) | 30 (100) | 1.000 |
| PICC | 59 (95.2) | 30 (100) | .548 |
| Midline | 3/62 (4.8) | 0 (0) | .548 |
All data are expressed as No. (%) unless otherwise noted.
Abbreviations: CTX, ceftriaxone; ICU, intensive care unit; IQR, interquartile range; PCN G, penicillin G; PICC, peripherally inserted central catheter.
aOne patient completed therapy course inpatient; 1 patient completed therapy course with oral cefpodoxime.
CTX was not significantly associated with increased events in the primary composite safety outcome (Table 3). The driver of the primary composite safety outcome was hospital readmission due to VGS bacteremia or therapy complications. Specifically, 7/64 (11%) in the CTX group and 6/30 patients (20%) in the PCN group met this end point. Reasons for these readmissions included valve repair surgery for new development or worsening of preexisting valve insufficiency (n = 5), infection complications with septic emboli (n = 3), spinal osteomyelitis progression (n = 3), and concern for or confirmed PICC-associated bacteremia (n = 2). The clinical characteristics of the patients readmitted due to VGS or therapy complications are provided in Supplementary Table 1. None of the secondary end points differed significantly between the groups. Microbiological evidence of ESBL resistance and development of CDI were infrequent. Neither therapy modification nor discontinuation due to an adverse drug event or infection recurrence occurred in any study patients. CTX was not associated with increased risk of all-cause mortality or 6-month readmission rates, although the time to first readmission was numerically shorter in the CTX group (31 vs 53 day; P = .135). Of note, similar results were demonstrated in the subgroup of patients with IE (n = 58). CTX was not significantly associated with increased risk of safety outcomes when compared with the PCN group (12.5% vs 23.5%), even in the patients who received CTX and gentamicin for any duration. Among the 25 patients who received concomitant gentamicin for any duration (range, 1–38 days), the majority of patients (n = 21) had IE.
Table 3.
Outcomes
| CTX (n = 64) | PCN G (n = 30) | P Value | |
|---|---|---|---|
| Primary end point | |||
| Composite safety end point | 9 (14.1) | 8 (26.7) | .139 |
| Secondary end points | |||
| Composite safety end point components | |||
| At least 1 readmission due to VGS or therapy | 7 (10.9) | 6 (20) | .336 |
| Microbiological evidence of ESBL | 1 (1.6) | 1 (3) | .539 |
| C. difficile infection | 1 (1.6) | 1 (3) | .539 |
| Therapy modification or discontinuation due to adverse drug event | 0 (0) | 0 (0) | .549 |
| Infection recurrence | 0 (0) | 0 (0) | N/A |
| Hospital readmission | |||
| At least 1 readmission in 6 mo | 24 (37.5) | 16 (53) | .148 |
| No. of readmissions, median (IQR), d | 0 (0–1) | 1 (0–1.25) | .341 |
| Time to first readmission, median (IQR), d | 31 (15–60) | 53 (29–91) | .135 |
| All-cause mortality | 0 (0) | 2 (7) | .100 |
All data expressed as No. (%) unless otherwise noted.
Abbreviations: CTX, ceftriaxone; ESBL, extended-spectrum beta-lactamase; IQR, interquartile range; PCN G, penicillin G; VGS, viridans group streptococci.
Univariate and multivariate analyses were conducted to identify patient demographics and treatment details that predicted events in the primary composite safety outcome (Table 4). There were no variables significantly associated with the primary composite safety outcome on univariate analysis. In the multivariate logistic regression model after including variables with P ≤ .2 and adjusting for covariates, source removal was found to be a protective factor (odds ratio [OR], 0.116; 95% CI, 0.020–0.6771; P = .017). Patients who received at least 2 weeks of gentamicin combination therapy were selected for the univariate and multivariate analyses based on the minimum duration recommended by AHA guidelines for IE therapy when indicated. Concurrent therapy with gentamicin for ≥2 weeks (OR, 6.553; 95% CI, 0.893–48.106; P = .065) trended toward significance as an independent predictor of the primary composite safety outcome.
Table 4.
Univariate and Multivariate Analysis
| Primary Composite Safety Outcome Met (n = 17) | Primary Composite Safety Outcome Not Met (n = 77) | Univariate OR (CI) | P Value | Multivariate OR (CI) | P Value | |
|---|---|---|---|---|---|---|
| CTX | 9 (52.9) | 55 (71.4) | 0.45 (0.154–1.316) | .139 | 0.53 (0.144–1.957) | .342 |
| Duration of treatment arm ≥4 wk | 14 (82.4) | 52 (67.5) | 2.24 (0.590–8.526) | .227 | 1.84 (0.433–7.776) | .410 |
| Source removal | 2 (11.8) | 26 (33.8) | 0.26 (0.056–1.231) | .073 | 0.13 (0.021–0.822) | .030 |
| Coronary artery disease | 1 (5.9) | 18 (24) | 0.2 (0.025–1.598) | .181 | 0.10 (0.010–1.126) | .063 |
| Gentamicin ≥2 wk duration | 4 (23.5) | 6 (7.8) | 3.64 (0.901–14.713) | .078 | 6.55 (0.893–48.106) | .065 |
| Congestive heart failure | 3 (17.6) | 20 (26) | 0.59 (0.153–2.268) | .547 | - | - |
| Endocarditis | 10 (52) | 48 (62) | 0.86 (0.296–2.517) | .787 | - | - |
| CCI, median (IQR) | 4 (3–4.5) | 4 (2–5) | - | .784 | - | - |
| PBS, median (IQR) | 1 (0–2) | 0 (0–2) | - | .781 | - | - |
| Age ≥65 y | 8 (47.1) | 46 (59.7) | 0.60 (0.208–1.722) | .338 | - | - |
| S. mitis | 6 (37.5) | 23 (29.9) | 1.41 (0.458–4.333) | .563 | - | - |
| Infection source identified | 15 (88.2) | 72 (93.5) | 0.54 (0.095–3.028) | .606 | - | - |
| Moderate or severe CKD | 2 (11.8) | 7 (9.1) | 1.33 (0.252–7.065) | .664 | - | - |
All data expressed as No. (%) unless otherwise noted.
Abbreviations: CCI, Charlson comorbidity index; CKD, chronic kidney disease; CTX, ceftriaxone; PBS, Pitt bacteremia score.
DISCUSSION
There are very few clinical studies evaluating commonly used treatment options for VGS bacteremia, and none focus on the comparison of individual beta-lactams and their associated adverse events. The prolonged course of treatment required for complicated VGS bacteremia raises theoretical safety concerns, particularly for the collateral damage historically associated with third-generation cephalosporins. CTX was selected for definitive treatment in more than double the number of cases as PCN G and AMP combined within our 6-year study period. This decision may have been driven not only by its once-daily dosing option, but also by the number of VGS isolates with intermediate susceptibilities to PCN G and/or AMP and by allergies to PCN. Contrary to our study hypothesis, CTX was not associated with greater incidence of hospital readmission due to VGS or an adverse event from antibiotic therapy, CDI, treatment modification or discontinuation due to an antibiotic-related adverse event, or development of ESBL resistance when compared with PCN G and AMP for the treatment of complicated VGS bacteremia. Our findings suggest that source removal is protective against poor safety outcomes, while gentamicin therapy for at least 2 weeks may be a potential predictor for increased risk of poor safety outcomes.
Despite the paucity of safety comparisons between agents in this setting, there is prior literature evaluating the efficacy of beta-lactams for VGS IE. Knoll and colleagues conducted a single-site observational study focusing on treatment efficacy in 29 adult patients with IE due to penicillin-resistant VGS [13]. Of the patients considered cured, treatment varied with respect to antibiotic regimen chosen: 9 were treated with PCN and an aminoglycoside, 8 received a bimodal combination regimen with PCN or CTX plus an aminoglycoside or CTX monotherapy, and 9 were treated with either a combination regimen or monotherapy with vancomycin or CTX. The only adverse event reported was the development of seizures in 1 patient receiving high-dose (40 million units/d) PCN G. Another study demonstrated that a 2-week course of once-daily CTX plus netilmicin was comparable to a historical control of PCN G plus gentamicin in 52 patients with IE; VGS was the causative organism in 31 cases. Other causative organisms were Streptococcus bovis (18 patients), Gemella morbillorum (2), and Group C Streptococcus (1) [14]. In both of the studies mentioned, the antibiotic regimens used may not reflect preferred treatment in current real-world practice, and safety parameters were not specifically measured.
Third-generation cephalosporins are thought to be associated with more collateral damage than narrower-spectrum beta-lactam agents. Internationally, increased rates of ESBL and vancomycin-resistant Enterococcus (VRE) have been linked to extensive use of these antibiotics [15–18]. Development of antibiotic resistance is promoted by both prolonged antibiotic exposure and the spectrum of activity for agent(s) utilized [16]. Additionally, cephalosporin use and its association with CDI has been established in many case–control studies [19, 20]. The Centers for Disease Control and Prevention (CDC) attribute improved appropriate antibiotic use to the overall reduction in deaths from antibiotic resistance both in the general population and hospitalized population over the years [19]. A 2-year pre–post study at a 500-bed hospital reported that extensive restriction of cephalosporins led to an 80% decrease in use and a 44% reduction in the incidence of ESBL-producing Klebsiella infections [21]. Additionally, restriction of injectable third-generation cephalosporins at another site led to a 92% reduction in use and a 50% reduction of CDI cases [22]. CDI and ESBL isolation were infrequent in our study, with only 1 occurrence for each end point per treatment arm. In comparison, reported incidences of CDI and ESBL in the aforementioned studies, following interventions targeting cephalosporins, were significant at 22 cases per 1000 admissions and 0.48 cases per 1000 patient-days, respectively [21, 22]. Antimicrobial stewardship principles are still important in regulating the use of these agents to minimize adverse effects on both an individual level and a population level [18].
In the ambulatory setting, prolonged OPAT can result in many potential safety concerns. Complications include line-related infections, line occlusions, and drug intolerances, all of which can ultimately lead to hospital readmissions [23, 24]. Duncan and colleagues evaluated safety outcomes in >1300 cases with CTX via OPAT from 2001 to 2010. VGS accounted for ~10% of isolates, and IE accounted for 25% of indications [25]. Interestingly, CTX was found to have an excellent safety profile overall in the OPAT setting without a strong link to CDI, suggesting other patient and environmental factors contributing to CDI risk [25]. In our study, the majority of patients completed most of their treatment course with OPAT. The low incidence of CDI in our study’s CTX group (only 1 patient per group) is consistent with literature describing CTX in the OPAT setting, although our study was limited by a small sample size. Administration of PCN G and AMP in the outpatient setting may be accomplished via a multidose intermittent infusion or continuous infusion strategy. While continuous infusion AMP is not offered upon discharge from our institution due to prior data reporting the short stability of AMP, published experience supports the feasibility of both continuous infusion AMP and PCN G as options for more targeted therapy with relatively well-tolerated safety profiles [11, 12].
IE was the predominant source of infection in our study. Treatment of VGS IE may consist of beta-lactam monotherapy or in combination with aminoglycosides [5]. The AHA guidelines suggest gentamicin combination therapy to shorten the total duration of treatment, depending on PCN G MIC [6]. Sexton and colleagues yielded a cure rate of 96% for both a 2-week course of CTX plus once-daily gentamicin and a regimen of CTX alone for 4 weeks [26]. Approximately 50% of patients with IE in our study received gentamicin, with a wide duration range of 1 to 38 days. Although we did not evaluate cure rates, there were no cases of infection recurrence identified in our study, and IE was not associated with increased risk of the primary composite end point. Additionally, aminoglycosides also carry significant safety concerns that can be exacerbated in patients with preexisting renal impairment or concomitant exposure to other potentially nephrotoxic agents [14]. In our study, combination therapy for at least 2 weeks with gentamicin demonstrated a trend toward increased risk of poor safety outcomes, supporting the concerns of potential negative safety consequences with aminoglycosides. Similar to observational studies for streptococcal IE, we demonstrated that source removal was a protective factor in the overall cohort of patients with VGS bacteremia [27, 28].
There are several limitations to this study. First, it is a single-center retrospective study with a small sample size. In the absence of prior literature in this setting, we were unable to conduct a power calculation to determine the number of patients needed to detect a difference between groups. Furthermore, the study period chosen was dictated by the timeframe of available data in the EHR. Our study captured a very small number of patients with malignancies. Therefore, it does not sufficiently describe safety outcomes in patients with febrile neutropenia, in whom higher rates of clinically significant VGS infections have been reported, with associated mortality rates, compared with the general population. Event rates for both CDI and development of ESBL resistance were low, limiting the ability to compare treatment groups with respect to these outcomes. Finally, although patients received agents other than their assigned treatment arm therapy during the bacteremia courses, efforts were made to evaluate the antibiotic most likely attributing to end points evaluated based on duration of each agent relative to the total course. The selection of antibiotic regimens for empiric vs definitive treatment as well as inpatient vs outpatient management reflects management of VGS bacteremia in real-world practice.
To our knowledge, this study is the first to directly compare safety outcomes of CTX vs PCN G or AMP in complicated VGS bacteremia treatment. We did not identify any overt differences in safety between CTX, PCN G, and AMP in their propensity to cause readmission due to VGS or an adverse event from antibiotic therapy, CDI, treatment modification or discontinuation due to an antibiotic-related adverse event, and development of ESBL resistance. The overall rate of patients who experienced an event in the primary composite safety outcome highlights the importance of identifying associated risk factors. Source control was identified as a protective factor, while use of combination therapy with gentamicin may be associated with poor safety outcomes. We did not find clinical outcomes to justify limiting the liberal use of CTX for definitive therapy in this setting; however, we cannot exclude the risk of collateral damage and increased risk of other safety concerns over PCN G and AMP given the limitations of a single-center retrospective study. Our findings warrant further exploration in a larger prospective trial.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Financial support. None.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Patient consent. This study did not include factors necessitating patient consent. The NYULH IRB approved this study, which conforms to current standards.
References
- 1. Swenson FJ, Rubin SJ. Clinical significance of viridans streptococci isolated from blood cultures. J Clin Microbiol 1982; 15:725–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sinner SW, Tunkel AR. Viridans streptococci, nutritionally variant streptococci, groups C and G streptococci, and other related organisms. In: Bennett J, Dolin R, Blaser MJ, et al. eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 8th ed Philadelphia, PA: Saunders; 2015:2349–61. [Google Scholar]
- 3. Han SB, Bae EY, Lee JW, et al. Clinical characteristics and antimicrobial susceptibilities of viridans streptococcal bacteremia during febrile neutropenia in patients with hematologic malignancies: a comparison between adults and children. BMC Infect Dis 2013; 13:273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dulanto Chiang A, Sinaii N, Palmore TN. Risk factors for viridans group streptococcal bacteremia in neutropenic and non-neutropenic patients: a single center case-case-control study. Open Forum Infect Dis 2018; 5:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goldstein EJ, Le T, Bayer AS. Combination antibiotic therapy for infective endocarditis. Clin Infect Dis 2003; 365:615–21. [DOI] [PubMed] [Google Scholar]
- 6. Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications. Circulation 2015; 132:1435–86. [DOI] [PubMed] [Google Scholar]
- 7. Ceftriaxone [prescribing information]. Lake Forest, IL: Hospira; 2017. [Google Scholar]
- 8. Park SH. Third-generation cephalosporin resistance in gram-negative bacteria in the community: a growing public health concern. Korean J Intern Med 2014; 29:27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Penicillin G [prescribing information]. Deerfield, IL: Baxter Healthcare Corporation; 2016. [Google Scholar]
- 10. Ampicillin [prescribing information]. New York: Pfizer Labs; 2010. [Google Scholar]
- 11. Walton AL, Howden BP, Grayson LM, Korman TM. Continuous-infusion penicillin home-based therapy for serious infections due to penicillin-susceptible pathogens. Int J Antimicrob Agents 2007; 29:544–8. [DOI] [PubMed] [Google Scholar]
- 12. Lewis PO, Jones A, Amodei RJ, Youssef D. Continuous infusion ampicillin for the outpatient management of enterococcal endocarditis: a case report and literature review. J Pharm Pract 2020; 33:392–4. [DOI] [PubMed] [Google Scholar]
- 13. Knoll B, Tleyjeh IM, Steckelberg JM, et al. Infective endocarditis due to penicillin-resistant viridans group streptococci. Clin Infect Dis 2007; 44:1585–92. [DOI] [PubMed] [Google Scholar]
- 14. Francioli P, Ruch W, Stamboulian D. Treatment of streptococcal endocarditis with a single daily dose of ceftriaxone and netilmicin for 14 days: a prospective multicenter study. Clin Infect Dis 1995; 21:1406–10. [DOI] [PubMed] [Google Scholar]
- 15. Pinto Pereira LM, Phillips M, Ramlal H, et al. Third generation cephalosporin use in a tertiary hospital in Port of Spain, Trinidad: need for an antibiotic policy. BMC Infect Dis 2004; 4:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Livermore DM. Minimising antibiotic resistance. Lancet Infect Dis 2005; 5:450–9. [DOI] [PubMed] [Google Scholar]
- 17. Cižman M, Plankar Srovin T. Antibiotic consumption and resistance of gram-negative pathogens (collateral damage). GMS Infect Dis 2018; 6:Doc05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee SH, Lee ES, Park SY, et al. Reduced use of third-generation cephalosporins decreases the acquisition of extended spectrum beta-lactamase–producing Klebsiella pneumonia. Infect Control Hosp Epidemiol 2004; 10:832–7. [DOI] [PubMed] [Google Scholar]
- 19. CDC. Antibiotic Resistance Threats in the United States, 2019. Atlanta, GA: U.S. Department of Health and Human Services, CDC; 2019. [Google Scholar]
- 20. Paterson DL. “Collateral damage” from cephalosporin or quinolone antibiotic therapy. Clin Infect Dis 2004; 38(Suppl 4):S341–5. [DOI] [PubMed] [Google Scholar]
- 21. Rahal JJ, Urban C, Horn D, et al. Class restriction of cephalosporin use to control total cephalosporin resistance in nosocomial Klebsiella. JAMA 1998; 280:1233–7. [DOI] [PubMed] [Google Scholar]
- 22. Ludlam H, Brown N, Sule O, et al. An antibiotic policy associated with reduced risk of Clostridium difficile–associated diarrhoea. Age Ageing 1999; 28:578–80. [DOI] [PubMed] [Google Scholar]
- 23. Cervera C, del Río A, García L, et al. ; Hospital Clinic Endocarditis Study Group Efficacy and safety of outpatient parenteral antibiotic therapy for infective endocarditis: a ten-year prospective study. Enferm Infecc Microbiol Clin 2011; 29:587–92. [DOI] [PubMed] [Google Scholar]
- 24. Amodeo MR, Clulow T, Lainchbury J, et al. Outpatient intravenous treatment for infective endocarditis: safety, effectiveness and one-year outcomes. J Infect 2009; 59:387–93. [DOI] [PubMed] [Google Scholar]
- 25. Duncan CJ, Barr DA, Seaton RA. Outpatient parenteral antimicrobial therapy with ceftriaxone, a review. Int J Clin Pharm 2012; 34:410–7. [DOI] [PubMed] [Google Scholar]
- 26. Sexton DJ, Tenenbaum MJ, Wilson WR, et al. Ceftriaxone once daily for four weeks compared with ceftriaxone plus gentamicin once daily for two weeks for treatment of endocarditis due to penicillin-susceptible streptococci. Endocarditis Treatment Consortium Group. Clin Infect Dis 1998; 27:1470–4. [DOI] [PubMed] [Google Scholar]
- 27. Lebeaux D, Fernández-Hidalgo N, Pilmis B, et al. Aminoglycosides for infective endocarditis: time to say goodbye? Clin Microbiol Infect 2020; 26:723–8. [DOI] [PubMed] [Google Scholar]
- 28. Pilmis B, Lourtet-Hascoët J, Barraud O, et al. ; GMC Study Group Be careful about MICs to amoxicillin for patients with streptococci-related infective endocarditis. Int J Antimicrob Agents 2019; 53:850–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.