Native forest spatiotemporal dynamics must be included into restoration programs to better estimate their expected benefits.
Abstract
Understanding the dynamics of native forest loss and gain is critical for biodiversity conservation and ecosystem services, especially in regions experiencing intense forest transformations. We quantified native forest cover dynamics on an annual basis from 1990 to 2017 in Brazil’s Atlantic Forest. Despite the relative stability of native forest cover during this period (~28 Mha), the ongoing loss of older native forests, mostly on flatter terrains, have been hidden by the increasing gain of younger native forest cover, mostly on marginal lands for mechanized agriculture. Changes in native forest cover and its spatial distribution increased forest isolation in 36.4% of the landscapes. The clearance of older forests associated with the recut of 27% of younger forests has resulted in a progressive rejuvenation of the native forest cover. We highlight the need to include native forest spatiotemporal dynamics into restoration programs to better estimate their expected benefits and unexpected problems.
INTRODUCTION
More than 100 Mha of tropical and subtropical forests have been degraded and deforested globally between 1980 and 2012 (1), mostly by the expansion of agricultural frontiers (2). To partially revert this trend and its negative environmental consequences, large-scale restoration and reforestation programs have been promoted globally (3), and many tropical regions are now experiencing a forest transition (shifts from net loss to net gain in tree cover) (4). However, the expansion of young secondary forests in marginal agricultural lands may hide the ongoing destruction of older forests in favorable areas for agro-pastoral production (5), so the quality of forest cover and its potential contribution to biodiversity conservation and ecosystem services provisioning may ultimately decline even if restoration or “zero deforestation” (6) targets are achieved (7, 8). Large-scale restoration should be monitored not only based on the quality and extension of the restored area but also considering the multiple consequences of forest cover transformations on targeted environmental benefits (9, 10).
Comprehensive forest restoration monitoring has to simultaneously map and track both forest loss and gain, distinguish native and exotic tree covers, and consider the age of native forests, as these factors are important determinants of biodiversity recovery and ecosystem services provisioning by restored tropical forests (11–13). Recent improvements in satellite imaging technology and cloud data processing have substantially increased our ability to map, quantify, and qualify tree cover changes at global scales (14, 15), incorporating an enormous amount of imagery information. Whereas past remote sensing studies have mostly focused on tropical deforestation—a discrete process that usually leads to an immediate loss of large forest patches—an emerging research challenge is to monitor native forest recovery—a long-term continuous and highly variable process, which occurs usually through the emergence of small patches of young forests in heterogeneous landscapes (7). Moreover, most of the past studies focused on changes in forest cover over continental scales have not distinguished between native and exotic tree covers (14–16), and when it was done, only large remnants of old-growth forests were considered (17–20).
The MapBiomas initiative, a collaborative consortium of multiple organizations initiated in Brazil, has produced annual land use and land cover (LULC) time series based on 30-m-resolution Landsat data using random forests machine learning algorithm, in which native and exotic tree covers are differentiated and the age of native forests is estimated (21). This novel methodological approach offers valuable opportunities for mapping native forests’ restoration and conservation dynamics (22, 23), which have a critical role in tracking the progress of ambitious restoration and tree planting commitments such as the Bonn Challenge and the 1t.org of the World Economic Forum, as well as the upcoming United Nations’ Decade on Ecosystem Restoration (2021–2030). It also allows for a long-term, in-depth analysis of yearly forest dynamics over large spatial scales, not only improving our understanding of forest spatial structure in tropical regions but also giving support for a better quantification of restoration benefits.
Here, we quantify the large-scale, long-term native forest dynamics in the Brazilian Atlantic Forest (24), a top priority hot spot for biodiversity conservation (25) and for tropical rainforest restoration (26). To do so, we rely on the MapBiomas annual LULC maps from 1985 to 2018 and cloud processing capabilities from the Google Earth Engine platform. We reveal that the apparent stability of native forest cover observed in the last decades has hidden the destruction of unreplaceable older forests. Our results indicate an alarming process of forest cover rejuvenation and uneven spatial distribution toward areas less attractive for mechanized agriculture, which can have deleterious effects on biodiversity conservation and ecosystem services. We highlight the critical need to develop policies that guarantee the conservation of older forests and differentiate younger and older native forest covers in the accountability of restoration and reforestation initiatives. Given Brazil’s pioneering policies on restoration and a specific law to protect Atlantic Forest remnants (27), this hidden destruction of older forests in the region is likely to happen at an even greater pace across the global tropics.
RESULTS
The challenges of a highly dynamic forest cover
Native forest cover within Brazil’s Atlantic Forest is nearly 28 to 30 Mha, an area that has remained relatively constant for the last 30 years (1989–2018; Fig. 1). Native forest cover (area with more than 0.5 ha in size, vegetation taller than 5 m in height, minimum canopy cover of 70%, and >4 years old, excluding monoculture tree plantations, mangroves, and savannas) appears “net stable,” but this relative stability hides a very dynamic process with detrimental effects for both biodiversity conservation (28) and carbon stocking in the region (29), as forest isolation increased in 36.4% of the landscapes (hexagonal polygons of 250 km2, 5231 polygons in total) between 1990 and 2017 (Fig. 2). When native forest cover gain and loss are considered, about 260,000 ha of forest loss and gain are detected each year since 2005 (Fig. 3). Older native forest cover (existing native forest cover in the 1985 map) loss ranged from 220,000 to 80,000 ha/year between 2000 and 2015, reaching its lowest level in 2015 (76,200 ha) (Fig. 3), whereas younger native forest cover (native forest cover first detected from 1988 forward) is attaining an annual rate of increase of ~156,000 ha in recent years (Fig. 3). Total native forest cover loss has declined in the period, and a net gain of native forest cover has been observed since 2005, which indicates that the ongoing loss of older native forest cover has been compensated in terms of area by the increase of younger native forest cover.
Fig. 1. Historical changes in the main land use/cover classes in the Brazilian Atlantic Forest.
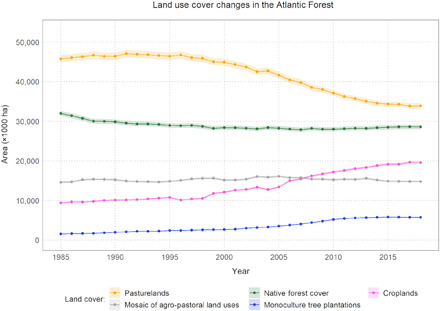
Shading represents 98% confidence interval.
Fig. 2. Landscape structure dynamics in the Brazilian Atlantic Forest.
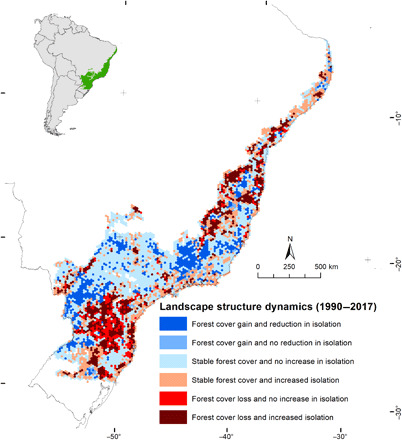
Hexagons in the maps represent 250-km2 landscapes.
Fig. 3. Historical annual native forest loss and gain in the Brazilian Atlantic Forest.
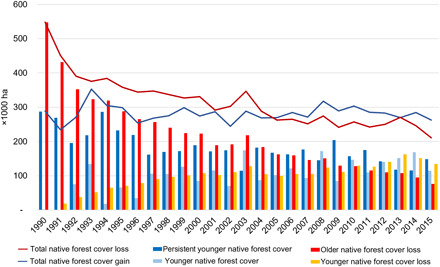
The steep decline in the rate of older native forest cover loss may reflect a propensity for the clearance of younger native forests (Fig. 3), as already observed in other human-dominated tropical landscapes (7). In regions with large continuous native forest cover, like the Amazon, clearing young forests for agricultural use may help protect old-growth forests from deforestation (30). However, this may not be the case in highly fragmented landscapes as those found in the Atlantic Forest. Despite the historical and continuous decline in the rate of older native forests loss, recent rates (~80,000 ha/year) are still markedly high for the Atlantic Forest, which is one of the most threatened and species-rich ecosystems worldwide (25, 31). It has very few old-growth remnants, 90% of the remaining native forest cover is privately owned (32), ~80% of the forest fragments are <50 ha (32), and most of its landscapes have less than 30% native forest cover, a critical threshold for long-term biodiversity conservation (28). Therefore, all native forest cover is needed, and such ongoing destruction of younger and especially older native forests makes species extinctions just a matter of time (33).
The spatiotemporal stability of native forest cover seems to be directly associated with the dynamics of agro-pastoral land uses in the region. While the area of croplands doubled and the area of monoculture tree plantations quadrupled in the past 30 years, the area of planted pasturelands declined by 20% (~13 Mha reduction) (Fig. 1). As a consequence of such historical transformation, the current area of anthropic land uses (monoculture tree plantations, croplands, pasturelands, urban infrastructure, and mining, excluding water reservoirs and nonforest native ecosystems) is ~75.8 Mha, which represent an increase of ~2.2 Mha since 1985. Native forest cover has, thus, remained almost stable in the last decade because croplands and monoculture tree plantations have mostly expanded over pasturelands (Fig. 1). However, the spatial distribution of native forest cover has been directly affected by the dynamics of agro-pastoral land uses’ expansion and retraction.
Native forest cover loss and gain are occurring in different contexts. The areas of native forest cover loss were recently occupied mostly by pasturelands (36%), mosaic of agro-pastoral land uses (26%), croplands (19%), and monoculture tree plantations (16%) (fig. S1 and table S1). Areas of native forests that were converted to croplands occurred on flatter terrains (average slope of 6.1°) when compared to the other anthropic land uses (pasturelands, mosaic of agro-pastoral land uses, and monoculture tree plantation), which are on steeper terrains (average slope of ~10°). Native forest gain was predominant in steeper areas (average slope of ~11.5°). It also occurred mostly in areas that were once occupied by native forests (39%, thus resulting in the rejuvenation of native forest cover in these areas), in the mosaic of agro-pastoral land uses (32%), and in pasturelands (29%). Native forest gain was much less common in flatter areas and regions dominated by croplands/monoculture tree plantations (table S1). The exception was on riparian buffers protected as areas of permanent vegetation by federal legislation, which accumulated 291,000 ha of younger native forest cover in the period (table S2). Consequently, the recent expansion of croplands and monoculture tree plantations in the Atlantic Forest has directly pushed deforestation on flatter areas. The predominant crops grown in the Atlantic Forest—sugarcane (~5.2 Mha), eucalyptus (~5.8 Mha), and soybean, maize, and coffee (~14.4 Mha all together)—are agricultural commodities produced in highly intensive and mechanized systems that rely mainly on flatter terrains, and their recent expansion over pasturelands may have displaced some cattle ranching activities to steeper areas, indirectly contributing to deforestation. On a broader scale, this pattern results in an uneven distribution of areas where persistent forest loss predominates compared to those where forest gain prevails (Fig. 2) and ultimately has promoted the concentration of native forests in marginal areas unsuited for intensive agriculture and forestry.
The rejuvenation of native forest cover and its consequences for conservation and restoration
The ongoing reduction of older native forest cover and the continuous increase of younger native forest cover are critical processes for biodiversity conservation (34) and have resulted in the reduction of the average age of the native forest cover in the Atlantic Forest. Presently, nearly 11% of the Atlantic Forest cover is <20 years old, and approximately one-third of the existing younger native forest cover is <10 years old (Fig. 3). This regional shift in the age structure of forests has intensified with time and at a relatively linear rate of 0.6% per year in the last two decades (Fig. 4). It represents a critical setback for biodiversity conservation and ecosystem services provisioning in the biome. Old-growth forests are irreplaceable for conserving tropical biodiversity, as many animal, plant, and microorganism species are unable to recolonize secondary forests and rely on older, less altered, more structurally developed, and biodiverse habitats to persist in human-modified landscapes (35, 36). Although tree species richness of young regenerating forests may reach nearly 80% of old-growth forest levels within 20 years (12, 13), the full recovery of tree species composition may take centuries or may never be reached (37). The same holds for ecosystem services, which may rely on well-developed, structurally complex forests to be maximized (8, 38). Limited expectation regarding the potential for restoration to recover predisturbance levels of ecosystem services and mitigate losses of old-growth forest biodiversity has been confirmed by global meta-analyses (36, 39, 40). We acknowledge, however, that our class “older native forest cover” is not totally composed of old-growth forests, as an important, yet unknown, portion of it can be potentially represented by native forest cover <35 years old, which regenerated few years before the first available Landsat image in 1985 used in the present analysis.
Fig. 4. Forest cover dynamics and forest age pattern in the Brazilian Atlantic Forest.
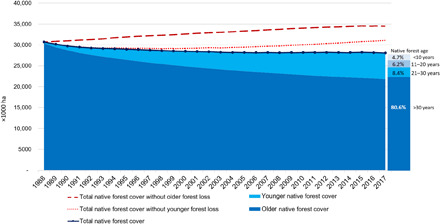
Dashed lines represent estimates in which older and younger native forest cover loss are summed up to total native forest cover.
Implications for large-scale restoration
There are many forest restoration commitments pledged for the Atlantic Forest in the coming years, including the one made by the Atlantic Forest Restoration Pact (15 Mha by 2050) and others at the national level that include part of this biome, like Brazil’s National Determined Contribution to the Paris Climate Agreement and the National Native Vegetation Recovery Plan (12 Mha by 2030). If the mean rate of native forest cover gain in the last decade remains constant and all recut of younger native forests ceases immediately, 4.2 Mha of native forests would be recovered by 2030 in the Atlantic Forest alone, which represent 35% of Brazil’s overarching commitment in a region that covers only 13% of its national territory. However, if the clearance of younger native forests continues at current rates, only 2.3 Mha of native forests would be recovered by 2030 (20% of the national commitment). If older forest cover loss is simultaneously considered at its current rate with younger forest loss, then only 0.49 Mha of additional native forest cover would be expected by 2030 (4.1% of the aforementioned national commitment). If the impacts of this ongoing destruction of both younger and older forests are considered on the quality, age, and spatial structure of native forest cover, then a net increase of a few million hectares in forest cover may result in negligible restoration benefits or even in a net decrease in species conservation and ecosystem services.
DISCUSSION
The consequences of the observed forest dynamics for biodiversity and ecosystem services can be drastic, including the increase of habitat isolation (Fig. 2), the destruction of habitats, and the loss of endemic species that occur exclusively in areas that are more suitable for agriculture (35), as well as the reduction of agricultural yields by losses in ecosystem services (41–43). Although agriculture intensification has spared lands for restoration, it also promotes the direct and indirect destruction of older forests with potentially high conservation value, thus having an ultimate net negative impact for biodiversity.
Just as reductions in infant mortality have boosted average human life expectancy, avoiding the clearance of younger native forests may increase the average age of the Atlantic Forest cover (Fig. 4). Younger native forests are recut for many reasons, such as to support a shifting cultivation system, to demonstrate land ownership, and to prevent regenerating forests to become developed enough to be legally protected. Because of the low persistence of the younger native forest cover in the Atlantic Forest (27% of them were cleared before 2019), as already observed in the Amazon (30) and Costa Rica (7), there is a huge potential to increase native forest cover by avoiding clearance of young forests through legal enforcement and economic incentives, like supply chain interventions and certification (44). Recent studies have highlighted the role of assisted natural regeneration to upscale forest restoration (45, 46), and some others have developed predictive models to identify areas with potential for natural forest regrowth (47, 48). Here, we also show that protecting younger native forests, as well as older native forests, is a critical step to ensure large-scale, long-lasting forest restoration. In this vein, we highlight the importance of clarifying which biophysical, ecological, socio-economic, and political factors influence the persistence of younger native forests, as well as which command-and-control mechanisms and economic incentives would best prevent native forest clearance.
Nearly 200 Mha of forest restoration commitments were pledged by more than 60 national and subnational programs as part of the Bonn Challenge, most of them located in tropical developing countries (3). The hidden loss of older native forest cover during the implementation of restoration programs across the global tropics can be even worse than what we observed in the Atlantic Forest, as Brazil is (or at least, was) globally recognized by its successful policies and tools to reduce deforestation (49). Brazil has a considerably strict legislation to protect forests and mandate forest recovery on private lands (27). In addition, the Atlantic Forest has specific legislations to protect all forest patches of intermediate and late-successional stages (>10 years old) from deforestation. Such legal protections, alongside the economic development of several regions within this biome, have promoted forest transitions regionally [e.g., (50–52)], but these favorable conditions are hardly found in other tropical developing countries.
The most favorable areas for restoring tropical rainforests in the world coincide with those with higher conservation priorities (26), which reinforce the need to favor forest restoration with native species (11) and the implementation of forest protection interventions as part of restoration programs (53). We here demonstrate how native and exotic tree covers can be differentiated, native forest cover can be classified according to age, and the impacts of native forest cover dynamics on habitat isolation can be assessed, which are crucial elements for the accountability of the several ambitious forest restoration programs planned for the next decade. Our findings highlight the opportunity to promote environmental policies to reconcile the conservation of older native forests with the protection of younger ones (i.e., limiting recut rates). Such measures would ultimately contribute to mitigate the ongoing perverse rejuvenation and spatial displacement of native forests in agricultural landscapes, which can be particularly harmful for some of the most biodiverse and carbon-rich ecosystems on Earth.
MATERIALS AND METHODS
Study region
The Brazilian Atlantic Forest region is one of the most emblematic of the global hot spots for conservation priorities (25) and restoration opportunities (26). The region has a long history of land use changes and widespread deforestation, as the original forest cover has been drastically reduced (54). Recent urbanization and agricultural development have resulted in highly fragmented landscapes such that more than 80% of the remnant forest are composed of patches <50 ha (32, 52). Previous studies estimated that 12 to 16% of the original 129 Mha of native forest cover remains in the biome (31, 55). These estimates, however, only included forest patches >3 ha. A more recent analysis based on 5-m-resolution imagery showed that native vegetation in 2013 was 28% (56). Global (15), regional (57), and local studies (51, 58, 59) have shown a consistent increase in forest cover across the region in recent decades, yet at the regional scale, the dynamics of native forest loss and gain are still unknown. The Brazilian Atlantic Forest represents a valuable case for exploring the interactions between forest loss and gain and their consequences in a region with ambitious restoration commitments, urgent needs to prevent species extinctions, and high demand of ecosystem services (26).
Data used
We used the LULC data from the fifth collection of MapBiomas, a Brazilian Annual Land Use and Land Cover Mapping Project (MapBiomas Collection 5). This dataset reconstructs annual LULC information at 30-m spatial resolution from 1985 to 2019 for every Brazilian biome, based on random forest algorithm (60) applied to Landsat archive using Google Earth Engine (21). In the highly dynamic Atlantic Forest, the MapBiomas initiative arises as an unprecedented tool for understanding forest dynamics using medium-resolution remote sensing data with detailed land use classification. Consistent monitoring of forest dynamics in the region was not possible until the creation of the MapBiomas project. The MapBiomas analyses of accuracy were performed using the method described by Pontius and Millones (61), which indicated a global accuracy of 85.5% for the Atlantic Forest in the most detailed legend, with an allocation disagreement of 7.8% and an area disagreement of 6.5% (table S3) with consistent accuracy for the entire time series (fig. S2). The population bias from more than 12,000 reference points was used to estimate the unbiased land cover area for each class according to good practice guidance (table S3) (62, 63).
Data preparation
The legend of annual LULC maps was simplified as binary “native forests” (natural forest formation) and “anthropic” (corresponding to monoculture tree plantations, croplands, pasturelands, urban infrastructure and mining). Other classes were not considered in the present analysis [savanna formation, mangrove, nonforest natural formation (including all subclasses), beach and dune, and water (including all subclasses)]. A postclassification temporal filter based on a moving window (fig. S4) was applied in simplified maps to reduce uncertainty and year-to-year fluctuations in native forest loss and gain (4). Native forest loss and gain with less than 11 connected pixels (approximately 1 ha) in the accumulated forest gain and forest loss across the entire time series were considered scattered and excluded from the present analysis.
Additional analysis with native forest loss and gain
To evaluate the percentage of native forest gain that was formerly attributed to pasturelands or croplands, we used the LULC map from 1990. To evaluate the percentage of native forest loss and recent LULC, we used the map from 2017. We also calculated the mean value of the slope for each LULC in younger forest and forest loss areas using Shuttle Radar Topography Mission Digital Elevation Data 30 m (64) as reference. Brazil’s environmental legislation protects a minimum of 30 m of riparian areas along both sides of rivers and streams, the so-called permanent protected areas (PPAs) (27). The distance is based on the river width and may reach a maximum of 500 m. The map of riparian PPAs for the entire Atlantic Forest based on high-resolution RapidEye imagery (56) was used as reference in our analysis to quantify the LULC in these areas from the MapBiomas Collection 5 in 1990 and 2015.
Accuracy assessment
MapBiomas LULC accuracy assessment was produced using more than 12,000 random points distributed throughout the Atlantic Forest (62). The points were inspected by an independent team in dry/wet period using a set of Landsat images together with Google Earth imagery with the Temporal Visual Inspection tool (tvi.lapig.iesa.ufg.br). Three different interpreters inspected each point, and we applied the same legend simplification used in annual maps (63). The LULC maps for Atlantic Forest produced by the MapBiomas have a global accuracy that varies according to the level of detail of the legend (table S3) (65, 66). MapBiomas LULC maps from 1985 to 2019 (35 maps) were reclassified into binary maps. We assigned the value “1” for all pixels in the forest formation class of the MapBiomas product (legend ID: 3) and “0” for the “anthropic” LULC classes including monoculture tree plantations, croplands, pasturelands, urban infrastructure, and mining (legend ID: 9, 14, 24, and 30). Transition with other classes like savanna and water were excluded from our analyses (legend ID: 4, 5, 10, 23, and 25). This binary map has a consistent time-series global accuracy with a mean value for all years of 93.8% with a minimum value of 91.2% in 1985 (fig. S5). The separation of tree cover into “native forest” and “monoculture tree plantations” is present in MapBiomas original maps (21) and is consistent throughout the time series with a global accuracy mean value for all years of 96.0% and a minimum value of 94.3% in 2012 (fig. S6). Forest gain and loss have consistent results with the Global Forest Change products (15) when filtering canopy closure greater than 70% and removing monoculture tree plantations (fig. S7). Mapping forest cover based on Landsat imagery and monitoring forest loss and gain using supervised classification are a consolidated and well-accepted methodology for regional scale studies. Our results reported for the whole Brazilian Atlantic Forest are consistent with previous studies that identified similar forest dynamics for specific regions of the biome (50, 58). The reported accuracy for detection of changes in forest cover based on Landsat guided by specialists varies between 75 and 91% (67, 68). We created 350 random points in native forest loss and gain and used visual inspection on annual Landsat images from 1985 to 2018 to verify whether they are correctly mapped. Of 350 random points, 289 (83%) are correctly mapped in native forest loss and 257 (73%) are correctly mapped in native forest gain.
Regional analysis of forest and landscape dynamics
Regional maps of forest and landscape dynamics were produced by calculating the difference in native forest cover and landscape metrics from 1990 and 2017 within hexagons of 250 km2 in Fragstats 4.2.1 (69). The analysis allowed quantifying the forest loss and gain and forest isolation (mean distance to the nearest neighbor) for each landscape across the whole Atlantic Forest (4). We considered regions that presented a native forest cover variation <3% of landscape area as stable regions. To evaluate changes in forest isolation, we considered only hexagons with forest cover >0.01% in 1995 and in 2017. Hexagon isolation change <1% was considered as stable.
Future dynamics
Future forest dynamics were calculated by using the average value for the younger native forest cover gain (155,000 ha/year), younger native forest cover loss (129,000 ha/year), and older native forest cover loss (122,000 ha/year) between 2006 and 2015. These values were multiplied by 15 years to predict forest dynamics for 2030 and compare with Brazil’s 12-Mha restoration goals established in the National Plan for Native Vegetation Recovery (70).
Limitations
The threshold of 1 ha was applied to sum native forest gain and loss in the period. This threshold could limit the ability to identify the gain and loss of small patches of native forests, but it is necessary considering the use of Landsat images with 30-m spatial resolution. Considering that our time-series data start in 1985, it is not possible either to identify the forests that regenerated in the Atlantic Forest right before this date. Therefore, part of the forests lost in the beginning of our time series and classified as “older native forest cover loss” could be composed of young native forests. We analyzed the reduction of older native forest cover after 2000 to reduce this uncertainty.
Supplementary Material
Acknowledgments
We thank the comments of S. Sloan in an earlier version of this manuscript and the MapBiomas project for the production and availability of annual LULC maps for the Brazilian Atlantic Forest. Funding: P.H.S.B. acknowledges the São Paulo Research Foundation (FAPESP, grant #2018/18416-2) for financial support. Author contributions: Conceptualization, M.R.R., P.H.S.B., R.C., L.R.T., and J.P.M. Methodology, M.R.R., P.H.S.B., R.C., L.R.T., F.E.B.L., and J.P.M. Software, M.R.R. and F.E.B.L. Validation, M.R.R. Formal analysis, M.R.R., P.H.S.B., R.C., L.R.T., P.R.P., and J.P.M. Investigation, M.R.R., P.H.S.B., R.C., L.R.T., P.R.P., F.E.B.L., M.H., E.S., and J.P.M. Writing—original draft preparation, M.R.R., P.H.S.B., R.C., L.R.T., and P.R.P. Writing—review and editing, M.R.R., P.H.S.B., R.C., L.R.T., P.R.P., F.E.B.L., M.H., E.S., and J.P.M. Supervision, M.R.R., P.H.S.B., and J.P.M. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/4/eabc4547/DC1
REFERENCES AND NOTES
- 1.Lewis S. L., Edwards D. P., Galbraith D., Increasing human dominance of tropical forests. Science 349, 827–832 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Curtis P. G., Slay C. M., Harris N. L., Tyukavina A., Hansen M. C., Classifying drivers of global forest loss. Science 361, 1108–1111 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Chazdon R., Brancalion P., Restoring forests as a means to many ends. Science 364, 24–25 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Nanni A. S., Sloan S., Aide T. M., Graesser J., Edwards D., Grau H. R., The neotropical reforestation hotspots: A biophysical and socioeconomic typology of contemporary forest expansion. Glob. Environ. Chang. 54, 148–159 (2019). [Google Scholar]
- 5.Brancalion P. H. S., Chazdon R. L., Beyond hectares: Four principles to guide reforestation in the context of tropical forest and landscape restoration. Restor. Ecol. 25, 491–496 (2017). [Google Scholar]
- 6.Brown S., Zarin D., What does zero deforestation mean? Science 342, 805–807 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Reid J. L., Fagan M. E., Lucas J., Slaughter J., Zahawi R. A., The ephemerality of secondary forests in southern Costa Rica. Conserv. Lett. 12, e12607 (2019). [Google Scholar]
- 8.Wilson S. J., Schelhas J., Grau R., Nanni A. S., Sloan S., Forest ecosystem-service transitions: The ecological dimensions of the forest transition. Ecol. Soc. 22, 38 (2017). [Google Scholar]
- 9.Sloan S., Meyfroidt P., Rudel T. K., Bongers F., Chazdon R., The forest transformation: Planted tree cover and regional dynamics of tree gains and losses. Glob. Environ. Chang. 59, 101988 (2019). [Google Scholar]
- 10.Holl K. D., Brancalion P. H. S., Tree planting is not a simple solution. Science 368, 580–581 (2020). [DOI] [PubMed] [Google Scholar]
- 11.Lewis S. L., Wheeler C. E., Mitchard E. T. A., Koch A., Restoring natural forests is the best way to remove atmospheric carbon. Nature 568, 25–28 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Poorter L., Bongers F., Aide T. M., Almeyda Zambrano A. M., Balvanera P., Becknell J. M., Boukili V., Brancalion P. H. S., Broadbent E. N., Chazdon R. L., Craven D., De Almeida-Cortez J. S., Cabral G. A. L., De Jong B. H. J., Denslow J. S., Dent D. H., DeWalt S. J., Dupuy J. M., Durán S. M., Espírito-Santo M. M., Fandino M. C., César R. G., Hall J. S., Hernandez-Stefanoni J. L., Jakovac C. C., Junqueira A. B., Kennard D., Letcher S. G., Licona J. C., Lohbeck M., Marín-Spiotta E., Martínez-Ramos M., Massoca P., Meave J. A., Mesquita R., Mora F., Munõz R., Muscarella R., Nunes Y. R. F., Ochoa-Gaona S., De Oliveira A. A., Orihuela-Belmonte E., Penã-Claros M., Pérez-Garciá E. A., Piotto D., Powers J. S., Rodríguez-Velázquez J., Romero-Pérez I. E., Ruíz J., Saldarriaga J. G., Sanchez-Azofeifa A., Schwartz N. B., Steininger M. K., Swenson N. G., Toledo M., Uriarte M., Van Breugel M., Van Der Wal H., Veloso M. D. M., Vester H. F. M., Vicentini A., Vieira I. C. G., Bentos T. V., Williamson G. B., Rozendaal D. M. A., Biomass resilience of Neotropical secondary forests. Nature 530, 211–214 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Rozendaal D. M. A., Bongers F., Aide T. M., Alvarez-Dávila E., Ascarrunz N., Balvanera P., Becknell J. M., Bentos T. V., Brancalion P. H. S., Cabral G. A. L., Calvo-Rodriguez S., Chave J., César R. G., Chazdon R. L., Condit R., Dallinga J. S., De Almeida-Cortez J. S., De Jong B., De Oliveira A., Denslow J. S., Dent D. H., DeWalt S. J., Dupuy J. M., Durán S. M., Dutrieux L. P., Espírito-Santo M. M., Fandino M. C., Fernandes G. W., Finegan B., García H., Gonzalez N., Moser V. G., Hall J. S., Hernández-Stefanoni J. L., Hubbell S., Jakovac C. C., Hernández A. J., Junqueira A. B., Kennard D., Larpin D., Letcher S. G., Licona J. C., Lebrija-Trejos E., Marín-Spiotta E., Martínez-Ramos M., Massoca P. E. S., Meave J. A., Mesquita R. C. G., Mora F., Müller S. C., Muñoz R., De Oliveira Neto S. N., Norden N., Nunes Y. R. F., Ochoa-Gaona S., Ortiz-Malavassi E., Ostertag R., Peña-Claros M., Pérez-García E. A., Piotto D., Powers J. S., Aguilar-Cano J., Rodriguez-Buritica S., Rodríguez-Velázquez J., Romero-Romero M. A., Ruíz J., Sanchez-Azofeifa A., De Almeida A. S., Silver W. L., Schwartz N. B., Thomas W. W., Toledo M., Uriarte M., De Sá Sampaio E. V., Van Breugel M., Van Der Wal H., Martins S. V., Veloso M. D. M., Vester H. F. M., Vicentini A., Vieira I. C. G., Villa P., Williamson G. B., Zanini K. J., Zimmerman J., Poorter L., Biodiversity recovery of Neotropical secondary forests. Sci. Adv. 5, eaau3114 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song X. P., Hansen M. C., Stehman S. V., Potapov P. V., Tyukavina A., Vermote E. F., Townshend J. R., Global land change from 1982 to 2016. Nature 560, 639–643 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansen M. C., Potapov P. V., Moore R., Hancher M., Turubanova S. A., Tyukavina A., Thau D., Stehman S. V., Goetz S. J., Loveland T. R., Kommareddy A., Egorov A., Chini L., Justice C. O., Townshend J. R. G., High-resolution global maps of 21st-century forest cover change. Science 850, 850–854 (2013). [DOI] [PubMed] [Google Scholar]
- 16.Kim D. H., Sexton J. O., Noojipady P., Huang C., Anand A., Channan S., Feng M., Townshend J. R., Global, Landsat-based forest-cover change from 1990 to 2000. Remote Sens. Environ. 155, 178–193 (2014). [Google Scholar]
- 17.Tyukavina A., Baccini A., Hansen M. C., Potapov P. V., Stehman S. V., Houghton R. A., Krylov A. M., Turubanova S., Goetz S. J., Aboveground carbon loss in natural and managed tropical forests from 2000 to 2012. Environ. Res. Lett. 10, 074002 (2015). [Google Scholar]
- 18.Turubanova S., Potapov P. V., Tyukavina A., Hansen M. C., Ongoing primary forest loss in Brazil, Democratic Republic of the Congo, and Indonesia. Environ. Res. Lett. 13, 074028 (2018). [Google Scholar]
- 19.Potapov P., Hansen M. C., Laestadius L., Turubanova S., Yaroshenko A., Thies C., Smith W., Zhuravleva I., Komarova A., Minnemeyer S., Esipova E., The last frontiers of wilderness: Tracking loss of intact forest landscapes from 2000 to 2013. Sci. Adv. 3, e1600821 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansen M. C., Wang L., Song X. P., Tyukavina A., Turubanova S., Potapov P. V., Stehman S. V., The fate of tropical forest fragments. Sci. Adv. 6, eaax8574 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Souza C. M. Jr., Shimbo J. Z., Rosa M. R., Parente L. L., Alencar A. A., Rudorff B. F. T., Hasenack H., Matsumoto M., Ferreira L. G., Souza-Filho P. W. M., de Oliveira S. W., Rocha W. F., Fonseca A. V., Marques C. B., Diniz C. G., Costa D., Monteiro D., Rosa E. R., Vélez-Martin E., Weber E. J., Lenti F. E. B., Paternost F. F., Pareyn F. G. C., Siqueira J. V., Viera J. L., Neto L. C. F., Saraiva M. M., Sales M. H., Salgado M. P. G., Vasconcelos R., Galano S., Mesquita V. V., Azevedo T., Reconstructing three decades of land use and land cover changes in Brazilian biomes with Landsat Archive and Earth Engine. Remote Sens. (Basel) 12, 2735 (2020). [Google Scholar]
- 22.Silva Junior C. H. L., Heinrich V. H. A., Freire A. T. G., Broggio I. S., Rosan T. M., Doblas J., Anderson L. O., Rousseau G. X., Shimabukuro Y. E., Silva C. A., House J. I., Aragão L. E. O. C., Benchmark maps of 33 years of secondary forest age for Brazil. Zenodo 7, 269 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crouzeilles R., Santiami E., Rosa M., Pugliese L., Brancalion P. H. S., Rodrigues R. R., Metzger J. P., Calmon M., Scaramuzza C. A. d. M., Matsumoto M. H., Padovezi A., de M. Benini R., Chaves R. B., Metzker T., Fernandes R. B., Scarano F. R., Schmitt J., Lui G., Christ P., Vieira R. M., Senta M. M. D., Malaguti G. A., Strassburg B. B. N., Pinto S., There is hope for achieving ambitious Atlantic Forest restoration commitments. Perspect. Ecol. Conserv. 17, 80–83 (2019). [Google Scholar]
- 24.Joly C. A., Joly C. A., Metzger J. P., Tabarelli M., Experiences from the Brazilian Atlantic Forest: Ecological findings and conservation initiatives. New Phytol. 204, 459–473 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Laurence W., Laurance, conserving the hottest of the hotspots. Biol. Conserv. 142, 1137 (2009). [Google Scholar]
- 26.Brancalion P. H. S., Niamir A., Broadbent E., Crouzeilles R., Barros F. S. M., Almeyda Zambrano A. M., Baccini A., Aronson J., Goetz S., Leighton Reid J., Strassburg B. B. N., Wilson S., Chazdon R. L., Global restoration opportunities in tropical rainforest landscapes. Sci. Adv. 5, eaav3223 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brancalion P. H. S., Garcia L. C., Loyola R., Rodrigues R. R., Pillar V. D., Lewinsohn T. M., A critical analysis of the Native Vegetation Protection Law of Brazil (2012): Updates and ongoing initiatives. Nat. e Conserv. 14, 1–15 (2016). [Google Scholar]
- 28.Banks-Leite C., Pardini R., Tambosi L. R., Pearse W. D., Bueno A. A., Bruscagin R. T., Condez T. H., Dixo M., Igari A. T., Martensen A. C., Metzger J. P., Using ecological thresholds to evaluate the costs and benefits of set-asides in a biodiversity hotspot. Science 345, 1041–1045 (2014). [DOI] [PubMed] [Google Scholar]
- 29.Pütz S., Groeneveld J., Henle K., Knogge C., Martensen A. C., Metz M., Metzger J. P., Ribeiro M. C., De Paula M. D., Huth A., Long-term carbon loss in fragmented Neotropical forests. Nat. Commun. 5, 5037 (2014). [DOI] [PubMed] [Google Scholar]
- 30.Wang Y., Ziv G., Adami M., de Almeida C. A., Antunes J. F. G., Coutinho A. C., Esquerdo J. C. D. M., Gomes A. R., Galbraith D., Upturn in secondary forest clearing buffers primary forest loss in the Brazilian Amazon. Nat. Sustain. 3, 290–295 (2020). [Google Scholar]
- 31.Sloan S., Jenkins C. N., Joppa L. N., Gaveau D. L. A., Laurance W. F., Remaining natural vegetation in the global biodiversity hotspots. Biol. Conserv. 177, 12–24 (2014). [Google Scholar]
- 32.Ribeiro M. C., Metzger J. P., Martensen A. C., Ponzoni F. J., Hirota M. M., The Brazilian Atlantic Forest: How much is left, and how is the remaining forest distributed? Implications for conservation. Biol. Conserv. 142, 1141–1153 (2009). [Google Scholar]
- 33.Lira P. K., de Souza Leite M., Metzger J. P., Temporal lag in ecological responses to landscape change: Where are we now? Curr. Landsc. Ecol. Reports. 4, 70–82 (2019). [Google Scholar]
- 34.Zhai D., Xu J., Dai Z., Schmidt-Vogt D., Lost in transition: Forest transition and natural forest loss in tropical China. Plant Divers. 39, 149–153 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gibson L., Lee T. M., Koh L. P., Brook B. W., Gardner T. A., Barlow J., Peres C. A., Bradshaw C. J. A., Laurance W. F., Lovejoy T. E., Sodhi N. S., Primary forests are irreplaceable for sustaining tropical biodiversity. Nature 478, 378–381 (2011). [DOI] [PubMed] [Google Scholar]
- 36.Barlow J., Gardner T. A., Araujo I. S., Ávila-Pires T. C., Bonaldo A. B., Costa J. E., Esposito M. C., Ferreira L. V., Hawes J., Hernandez M. I. M., Hoogmoed M. S., Leite R. N., Lo-Man-Hung N. F., Malcolm J. R., Martins M. B., Mestre L. A. M., Miranda-Santos R., Nunes-Gutjahr A. L., Overal W. L., Parry L., Peters S. L., Ribeiro M. A., Da Silva M. N. F., Da Silva Motta C., Peres C. A., Quantifying the biodiversity value of tropical primary, secondary, and plantation forests. Proc. Natl. Acad. Sci. U.S.A. 104, 18555–18560 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin P. A., Newton A. C., Bullock J. M., Carbon pools recover more quickly than plant biodiversity in tropical secondary forests. Proc. R. Soc. B Biol. Sci. 280, 20132236 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chazdon R. L., Broadbent E. N., Rozendaal D. M. A., Bongers F., Zambrano A. M. A., Aide T. M., Balvanera P., Becknell J. M., Boukili V., Brancalion P. H. S., Craven D., Almeida-Cortez J. S., Cabral G. A. L., De Jong B., Denslow J. S., Dent D. H., DeWalt S. J., Dupuy J. M., Durán S. M., Espírito-Santo M. M., Fandino M. C., César R. G., Hall J. S., Hernández-Stefanoni J. L., Jakovac C. C., Junqueira A. B., Kennard D., Letcher S. G., Lohbeck M., Martínez-Ramos M., Massoca P., Meave J. A., Mesquita R., Mora F., Muñoz R., Muscarella R., Nunes Y. R. F., Ochoa-Gaona S., Orihuela-Belmonte E., Peña-Claros M., Pérez-García E. A., Piotto D., Powers J. S., Rodríguez-Velazquez J., Romero-Pérez I. E., Ruíz J., Saldarriaga J. G., Sanchez-Azofeifa A., Schwartz N. B., Steininger M. K., Swenson N. G., Uriarte M., Van Breugel M., Van Der Wal H., Veloso M. D. M., Vester H., Vieira I. C. G., Bentos T. V., Williamson G. B., Poorter L., Carbon sequestration potential of second-growth forest regeneration in the Latin American tropics. Sci. Adv. 2, e1501639 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meli P., Holl K. D., Benayas J. M. R., Jones H. P., Jones P. C., Montoya D., Mateos D. M., A global review of past land use, climate, and active vs. passive restoration effects on forest recovery. PLoS One. 12, e0171368 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moreno-Mateos D., Barbier E. B., Jones P. C., Jones H. P., Aronson J., López-López J. A., McCrackin M. L., Meli P., Montoya D., Rey Benayas J. M., Anthropogenic ecosystem disturbance and the recovery debt. Nat. Commun. 8, 14163 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saturni F. T., Jaffé R., Metzger J. P., Landscape structure influences bee community and coffee pollination at different spatial scales. Agric. Ecosyst. Environ. 235, 1–12 (2016). [Google Scholar]
- 42.Aristizábal N., Metzger J. P., Landscape structure regulates pest control provided by ants in sun coffee farms. J. Appl. Ecol. 56, 21–30 (2018). [Google Scholar]
- 43.González-Chaves A., Jaffé R., Metzger J. P., Kleinert A. d. M. P., Forest proximity rather than local forest cover affects bee diversity and coffee pollination services. Landsc. Ecol. 35, 1841–1855 (2020). [Google Scholar]
- 44.Chazdon R. L., Lindenmayer D., Guariguata M. R., Crouzeilles R., Fostering natural forest regeneration on former agricultural land through economic and policy interventions. Environ. Res. Lett. 15, 043002 (2020). [Google Scholar]
- 45.Molin P. G., Chazdon R., Frosini de Barros Ferraz S., Brancalion P. H. S., A landscape approach for cost-effective large-scale forest restoration. J. Appl. Ecol. 55, 2767–2778 (2018). [Google Scholar]
- 46.Chazdon R. L., Guariguata M. R., Natural regeneration as a tool for large-scale forest restoration in the tropics: prospects and challenges. Biotropica. 48, 716–730 (2016). [Google Scholar]
- 47.Crouzeilles R., Beyer H. L., Monteiro L. M., Feltran-Barbieri R., Pessôa A. C. M., Barros F. S. M., Lindenmayer D. B., Lino E. D. S. M., Grelle C. E. V., Chazdon R. L., Matsumoto M., Rosa M., Latawiec A. E., Strassburg B. B. N., Achieving cost-effective landscape-scale forest restoration through targeted natural regeneration. Conserv. Lett. 13, e12709 (2020). [Google Scholar]
- 48.Crouzeilles R., Barros F. S. M., Molin P. G., Ferreira M. S., Junqueira A. B., Chazdon R. L., Lindenmayer D. B., Tymus J. R. C., Strassburg B. B. N., Brancalion P. H. S., A new approach to map landscape variation in forest restoration success in tropical and temperate forest biomes. J. Appl. Ecol. 56, 2675–2686 (2019). [Google Scholar]
- 49.Nepstad D., Mcgrath D., Stickler C., Alencar A., Azevedo A., Swette B., Bezerra T., Digiano M., Shimada J., Seroa R., Armijo E., Castello L., Brando P., Hansen M. C., Mcgrath-horn M., Carvalho O., Hess L., Slowing Amazon deforestation through public policy and interventions in beef and soy supply chains. Science 344, 1118–1123 (2014). [DOI] [PubMed] [Google Scholar]
- 50.Baptista S. R., Rudel T. K., A re-emerging Atlantic forest? Urbanization, industrialization and the forest transition in Santa Catarina, southern Brazil. Environ. Conserv. 33, 195–202 (2006). [Google Scholar]
- 51.Rezende C. L., Uezu A., Scarano F. R., Araujo D. S. D., Atlantic Forest spontaneous regeneration at landscape scale. Biodivers. Conserv. 24, 2255–2272 (2015). [Google Scholar]
- 52.Lira P. K., Tambosi L. R., Ewers R. M., Metzger J. P., Land-use and land-cover change in Atlantic Forest landscapes. For. Ecol. Manage. 278, 80–89 (2012). [Google Scholar]
- 53.Brancalion P. H. S., Holl K. D., Guidance for successful tree planting initiatives. J. Appl. Ecol. 57, 2349–2361 (2020). [Google Scholar]
- 54.Pinto S. R., Melo F., Tabarelli M., Padovesi A., Mesquita C. A., de Mattos Scaramuzza C. A., Castro P., Carrascosa H., Calmon M., Rodrigues R., César R. G., Brancalion P. H. S., Governing and delivering a biome-wide restoration initiative: The case of Atlantic Forest Restoration Pact in Brazil. Forests 5, 2212–2229 (2014). [Google Scholar]
- 55.M. Hirota, Atlas dos remanescentes florestais da mata atlântica mapeamento dos sistemas costeiros. Fundação SOS Mata Atlântica Inst. Nac. Pesqui. Espac. (2018), p. 43.
- 56.Rezende C. L., Scarano F. R., Assad E. D., Joly C. A., Metzger J. P., Strassburg B. B. N., Tabarelli M., Fonseca G. A., Mittermeier R. A., From hotspot to hopespot: An opportunity for the Brazilian Atlantic Forest. Perspect. Ecol. Conserv. 16, 208–214 (2018). [Google Scholar]
- 57.Aide T. M., Clark M. L., Grau H. R., López-Carr D., Levy M. A., Redo D., Bonilla-Moheno M., Riner G., Andrade-Núñez M. J., Muñiz M., Deforestation and reforestation of Latin America and the Caribbean (2001-2010). Biotropica. 45, 262–271 (2013). [Google Scholar]
- 58.Bicudo da Silva R. F., Batistella M., Moran E. F., Lu D., Land changes fostering Atlantic Forest transition in Brazil: Evidence from the Paraíba Valley. Prof. Geogr. 69, 80–93 (2016). [Google Scholar]
- 59.Costa R. L., Prevedello J. A., de Souza B. G., Cabral D. C., Forest transitions in tropical landscapes: A test in the Atlantic Forest biodiversity hotspot. Appl. Geogr. 82, 93–100 (2017). [Google Scholar]
- 60.Breiman L., Random forests. Mach. Learn. 45, 5–32 (2001). [Google Scholar]
- 61.Pontius R. G., Millones M., Death to Kappa: Birth of quantity disagreement and allocation disagreement for accuracy assessment. Int. J. Remote Sens. 32, 4407–4429 (2011). [Google Scholar]
- 62.Stehman S. V., Estimating area and map accuracy for stratified random sampling when the strata are different from the map classes. Int. J. Remote Sens. 35, 4923–4939 (2014). [Google Scholar]
- 63.Olofsson P., Foody G. M., Herold M., Stehman S. V., Woodcock C. E., Wulder M. A., Good practices for estimating area and assessing accuracy of land change. Remote Sens. Environ. 148, 42–57 (2014). [Google Scholar]
- 64.Farr T. G., Rosen P. A., Caro E., Crippen R., Duren R., Hensley S., Kobrick M., Paller M., Rodriguez E., Roth L., Seal D., Shaffer S., Shimada J., Umland J., Werner M., Oskin M., Burbank D., Alsdorf D., The shuttle radar topography mission. Rev. Geophys. 45, 33 (2007). [Google Scholar]
- 65.Olofsson P., Foody G. M., Stehman S. V., Woodcock C. E., Making better use of accuracy data in land change studies: Estimating accuracy and area and quantifying uncertainty using stratified estimation. Remote Sens. Environ. 129, 122–131 (2013). [Google Scholar]
- 66.Stehman S. V., Foody G. M., Key issues in rigorous accuracy assessment of land cover products. Remote Sens. Environ. 231, 111199 (2019). [Google Scholar]
- 67.Coppin P. R., Bauer M. E., Processing of multitemporal Landsat TM imagery to optimize extraction of forest cover change features. IEEE Trans. Geosci. Remote Sens. 32, 918–927 (1994). [Google Scholar]
- 68.Saksa T., Uuttera J., Kolström T., Lehikoinen M., Pekkarinen A., Sarvi V., Clear-cut detection in boreal Forest aided by remote sensing. Scand. J. For. Res. 18, 537–546 (2003). [Google Scholar]
- 69.K. McGarigal, C. SA, E. E, Spatial pattern analysis program for categorical and continuous maps. Computer software program produced by the authors at the University of Massachusetts, Amherst. FRAGSTATS v4 (2020).
- 70.Ministério do Meio Ambiente, Plano Nacional de Recuperação da Vegetação Nativa (Brasília, DF, 2017), vol. 53; https://mma.gov.br/images/arquivos/florestas/planaveg_plano_nacional_recuperacao_vegetacao_nativa.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/4/eabc4547/DC1


