Significance
Alcoholic hepatitis (AH) is a severe form of alcohol-associated liver disease (ALD) that is characterized by chronic liver injury and inflammation. The current treatment strategy for AH involves the use of corticosteroids, despite a lack of evidence supporting their efficacy, because other effective therapeutic agents are not available. Here, we demonstrate that activation of TLR7 signaling via the oral administration of 1Z1, which is a synthetic TLR7 ligand that does not elicit systemic side effects, protects against liver injury in an AH mouse model. The therapeutic effect of 1Z1 requires TLR7 expression and is mediated by intestinal IL-22 upregulation. Our study suggests that 1Z1-induced TLR7 activation and IL-22 induction may be a therapeutic approach for the treatment of AH.
Keywords: Toll-like receptor, alcoholic hepatitis, IL-22
Abstract
Effective therapies for alcohol-associated liver disease (ALD) are limited; therefore, the discovery of new therapeutic agents is greatly warranted. Toll-like receptor 7 (TLR7) is a pattern recognition receptor for single-stranded RNA, and its activation prevents liver fibrosis. We examined liver and intestinal damage in Tlr7−/− mice to determine the role of TLR7 in ALD pathogenesis. In an alcoholic hepatitis (AH) mouse model, hepatic steatosis, injury, and inflammation were induced by chronic binge ethanol feeding in mice, and Tlr7 deficiency exacerbated these effects. Because these results demonstrated that endogenous TLR7 signaling activation is protective in the AH mouse model, we hypothesized that TLR7 activation may be an effective therapeutic strategy for ALD. Therefore, we investigated the therapeutic effect of TLR7 agonistic agent, 1Z1, in the AH mouse model. Oral administration of 1Z1 was well tolerated and prevented intestinal barrier disruption and bacterial translocation, which thus suppressed ethanol-induced hepatic injury, steatosis, and inflammation. Furthermore, 1Z1 treatment up-regulated the expression of antimicrobial peptides, Reg3b and Reg3g, in the intestinal epithelium, which modulated the microbiome by decreasing and increasing the amount of Bacteroides and Lactobacillus, respectively. Additionally, 1Z1 up-regulated intestinal interleukin (IL)-22 expression. IL-22 deficiency abolished the protective effects of 1Z1 in ethanol-induced liver and intestinal damage, suggesting intestinal IL-22 as a crucial mediator for 1Z1-mediated protection in the AH mouse model. Collectively, our results indicate that TLR7 signaling exerts protective effects in the AH mouse model and that a TLR7 ligand, 1Z1, holds therapeutic potential for the treatment of AH.
Alcohol-associated liver disease (ALD) is caused by chronic and excessive consumption of alcohol. The disease ranges from alcohol-associated fatty liver to alcoholic hepatitis (AH), fibrosis, cirrhosis, and hepatocellular carcinoma (HCC) (1). Alcohol-associated fatty liver is considered reversible and nonprogressive. Nearly 35% of heavy alcohol drinkers develop AH, and up to 40% of severe AH patients die within 6 mo (2). AH patients who survive may progress to alcohol-associated cirrhosis. Treatment options for AH involve the use of corticosteroids and have remained largely unchanged since the early 1970s. Unfortunately, not all patients respond to corticosteroids, and the benefits are temporary in responders (1, 2). Early liver transplant has been shown to be superior to medical management for severe AH, but it still has limitations and can only be considered in a highly selective group of patients (1, 2). Thus, the identification of a better molecular therapeutic target for ALD is a significantly unmet medical need for the development of effective therapies for AH.
Previous studies have demonstrated the involvement of Toll-like receptors (TLRs), including TLR2, TLR4, and TLR9, in the development of ALD (3–8). In addition to the direct effect of alcohol and its metabolite, acetaldehyde, in hepatocytes, ethanol intake affects the function of the intestinal epithelial barrier. Chronic alcohol consumption disrupts intestinal tight junction integrity and increases gut permeability, resulting in elevated bacterial lipopolysaccharide (LPS) concentrations in the portal and systemic circulation (4). Translocated LPS activates resident hepatic macrophages, known as Kupffer cells, via TLR4, thereby promoting ALD (1, 2, 7, 8). Other TLRs, such as TLR2 and TLR9, recognize gram-positive bacterial components and bacterial CpG-DNA, respectively (3, 4). Furthermore, TLR2, TLR9, and MyD88 are required for the development of the preclinical AH murine model (5), whereas TLR4 and TLR9 exert protective effects against intestinal inflammation (9, 10).
TLR7 signaling has been shown to be protective against liver fibrosis in mice (11). Tlr7−/− mice exhibit augmented cholestasis and carbon tetrachloride (CCl4)-induced liver fibrosis (11). TLR7 signaling also induces IFN-α production in dendritic cells (DCs), followed by interleukin (IL)-1 receptor antagonist (IL-1Ra) induction in Kupffer cells. IL-1Ra suppresses IL-1-induced hepatic stellate cell (HSC) activation, resulting in inhibition of liver fibrosis (11). Among the TLRs, TLR3 and TLR7 activation has been reported to ameliorate some liver diseases (11, 12). However, a major disadvantage of the currently available synthetic ligands for TLR3 and TLR7, such as poly I:C, imiquimod, and R848, is the excessive induction of proinflammatory cytokines (3, 4). Thus, developing agents without undesirable adverse effects is of great clinical interest.
IL-22 is a hepatoprotective cytokine produced by T helper (Th) 17 cells, Th22 cells, γδ T cells, natural killer (NK) T cells, and innate lymphoid cells (ILCs) (13). Exogenous administration of IL-22 has a profound effect on tissue repair following liver injury via the promotion of proliferation and inhibition of apoptosis in hepatocytes of mouse models of AH (14), liver fibrosis, and drug- and LPS-induced liver injury. Also, IL-22 promotes tissue repair in the intestines and is protective against intestinal epithelial damage and inflammation (13). These findings suggest that IL-22 may suppress ALD via the maintenance of intestinal barrier function, thereby preventing increased intestinal permeability and bacterial translocation due to intestine-derived microbial products that promote ethanol-induced liver injury (15, 16).
Here, we have developed a synthetic TLR7 ligand, 1Z1, that possesses antiinflammatory effects via IL-22 induction and that is devoid of systemic toxicity after oral administration (17–19). Treatment with 1Z1 has already been reported to be effective for allergic encephalomyelitis, arthritis, dextran sodium sulfate (DSS)-induced colitis, and type I diabetes in mice (17–20). We hypothesize that targeting TLR7 activation may be an effective treatment strategy for ALD. Our experimental results demonstrate that 1Z1 oral administration inhibits ethanol-induced liver and intestinal damage and that these beneficial effects are due to intestinal IL-22 induction in an AH murine model.
Results
Tlr7−/− Mice Exhibited Exacerbated Hepatic Steatosis and Injury in an Alcoholic Hepatitis Murine Model.
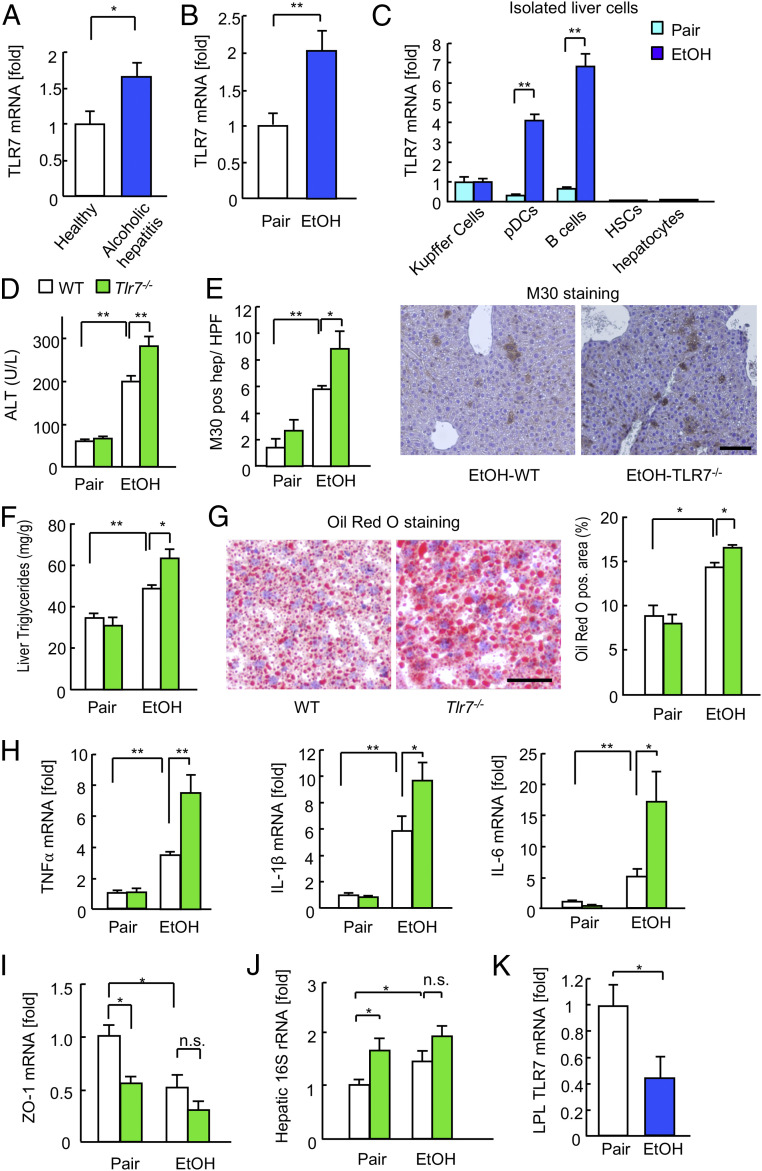
To validate the role of TLR7 signaling in ALD, we first investigated hepatic TLR7 expression during ALD in humans and mice. TLR7 mRNA expression was significantly increased in the liver tissues of AH patients (GSE28619) (21) as well as those of the AH murine model (Fig. 1 A and B). We next investigated the cell type responsible for the elevated expression of hepatic TLR7. TLR7 was predominantly expressed in Kupffer cells, plasmacytoid dendritic cells (pDCs), and B cells, but not hepatocytes or HSCs. Furthermore, it was dramatically up-regulated in the pDCs and B cells of the AH murine model (Fig. 1C).
Fig. 1.
Tlr7−/− mice were susceptible to alcohol-induced fatty liver and liver injury. (A) Hepatic TLR7 mRNA expression in AH patients (GSE28619; 15 AH patients, nine healthy controls). (B) Hepatic TLR7 mRNA expression in mice after chronic binge ethanol feeding (n = 6 to 10). (C) TLR7 mRNA expression in primary Kupffer cells, pDCs, B cells, HSCs, and hepatocytes isolated from pair- and ethanol-containing diet-fed mice (n = 4/cell type). (D–H) Wild-type and Tlr7−/− mice were subjected to chronic binge ethanol feeding (n = 6 to 14/group). (D) Serum ALT levels. (E) Quantification of M30-positive hepatocytes (Left). Representative images of M30 staining (Right). (Scale bar, 50 μm.) (F) Liver triglyceride levels. (G) Representative images of Oil Red O staining (Left). (Scale bar 50 μm.) Quantification of Oil Red O-positive area (Right). (H) qRT-PCR assays for TNFα, IL-1β, and IL-6 mRNA expression. (I) ZO-1 mRNA expression (n = 7, each). (J) Hepatic 16S rRNA levels (n = 7, each). (K) TLR7 expression in LPLs isolated from the small intestines of ethanol-fed mice (n = 9 to 11). Data are presented as the mean ± SEM *P < 0.05, **P < 0.01. Two-tailed Student’s t test (A–C and K); one-way ANOVA with Tukey’s post hoc analysis (D–J). n.s., not significant.
To elucidate the functional role of TLR7 in ALD, wild-type (WT) and Tlr7−/− mice were subjected to chronic binge ethanol feeding, as a murine model of AH. Chronic binge ethanol feeding induced hepatocyte damage and hepatic steatosis, which were demonstrated by increased levels of serum alanine aminotransferase (ALT), apoptotic hepatocytes, hepatic triglycerides, and lipid droplets (Fig. 1 D–G). The expression of hepatic proinflammatory cytokines, such as TNF-α, IL-1β, and IL-6, was also increased in ethanol-fed WT mice (Fig. 1H). Intriguingly, Tlr7−/− mice exhibited a higher degree of hepatocyte injury, fat accumulation, apoptosis, and inflammatory cytokine expression compared with WT mice (Fig. 1 D–H). These results suggest that endogenous TLR7 expression is protective against alcohol-induced inflammation, injury, and steatosis in the liver.
TLR7 Agonistic miRNAs Were Contained in Extracellular Vesicles and Induced Antiinflammatory Response.
Because intrinsic TLR7 signaling plays a protective role in ALD (Fig. 1), TLR7 could be activated by endogenous ligands. As previous studies reported that GU-rich microRNAs (miRNAs) could be endogenous ligands for TLR7 (22, 23), we examined the levels of miR-21a and miR-29a in the extracellular vesicle (EV) released from hepatocytes (24) and in the serum. The levels of miR-21a and miR-29a were elevated in the EVs from hepatocytes (24) and in the serum after ethanol treatment (SI Appendix, Fig. S1 A and B). These miRNAs up-regulated antiinflammatory IL-10, but not proinflammatory TNFα in WT Kupffer cells and pDCs (SI Appendix, Fig. S1 C and D), whereas these miRNAs did not up-regulate IL-10 in Tlr7−/− cells. These results suggest that the specific miRNAs activate TLR7 signaling, and induce antiinflammatory response, which could be associated with the protective effect of hepatic TLR7 signaling in ALD.
Excessive Ethanol Consumption Suppressed TLR7 Expression in the Intestines.
Ethanol consumption damages the intestinal epithelial barrier and can lead to the translocation of bacteria or bacterial components to the liver, thus contributing to liver injury (3, 4). Therefore, we investigated whether the adverse effects of Tlr7 deficiency were associated with intestinal epithelial barrier dysfunction and bacterial translocation to the liver. We examined the effect of alcohol consumption on the expression of tight junction molecule zonulin-1 (ZO-1) in the intestinal epithelium and detection of bacterial 16S rRNA in the liver, as a marker of bacterial translocation due to increased intestinal permeability. Consistent with previous studies (25), intestinal ZO-1 expression was reduced and hepatic bacterial 16S rRNA levels were increased in ethanol-fed WT mice compared with controls (Fig. 1 I and J and SI Appendix, Fig. S1E). Both control and ethanol-fed Tlr7−/− mice exhibited significantly decreased intestinal ZO-1 expression and increased hepatic bacterial 16S rRNA levels compared with WT control mice (Fig. 1 I and J and SI Appendix, Fig. S1E). Notably, intestinal ZO-1 expression and hepatic bacterial 16S rRNA levels were not significantly changed between the ethanol-fed WT and Tlr7−/− mice (Fig. 1 I and J and SI Appendix, Fig. S1E). Intriguingly, TLR7 expression in lamina propria lymphocytes (LPLs) of the small intestine was dramatically decreased (Fig. 1K). These results suggest that decreased intestinal TLR7 expression due to excessive alcohol consumption may be sufficient to disrupt the intestinal epithelial barrier and result in bacterial translocation; therefore, further significant changes were not observed in response to TLR7 deficiency. The underlying mechanism of the intestinal damage due to reduced TLR7 expression was thus further investigated.
A TLR7 Agonist, 1Z1, Had a Low Proinflammatory Property and Counteracted TLR9 Signaling.
Because intrinsic TLR7 activation protected against alcohol-induced liver injury (Fig. 1), we postulated that exogenous TLR7 activation may be an effective therapeutic approach for ALD. Therefore, we evaluated the effects of a synthetic TLR7 ligand, 1Z1, which we developed via conjugation to a 6-polyethylene glycol (PEG) chain (SI Appendix, Fig. S2A), in Kupffer cells and pDCs that express TLR7. We compared the effects of 1Z1 to those of a commonly used TLR7/8 agonist, R848. Although standard concentrations of R848 induced high proinflammatory cytokine expression, a 10 times higher concentration of 1Z1 did not up-regulate proinflammatory cytokines (SI Appendix, Fig. S2B). Moreover, 1Z1 up-regulated IL-10, PD-L1, and IRAK-M antiinflammatory molecules in liver macrophages; however, this effect was not observed in Tlr7−/− cells, indicating that the 1Z1-induced up-regulation of antiinflammatory molecules was mediated through TLR7 (SI Appendix, Fig. S2C). In addition, 1Z1 inhibited the expression of proinflammatory cytokines induced by CpG-DNA (SI Appendix, Fig. S2D). These in vitro results suggest that 1Z1 may be a safer therapeutic agent than R848 and has an antiinflammatory property that inhibits TLR9 signaling, which is an essential pathway for the promotion of ALD (5).
Systemic Administration of 1Z1 Inhibited Alcohol-Induced Hepatic Steatosis and Injury.
Because 1Z1 exerted antiinflammatory effects on immune cells (SI Appendix, Fig. S2), we investigated its therapeutic potential in ALD development. Mice subjected to chronic binge ethanol feeding were treated with a subcutaneous (s.c.) injection of 1Z1 (SI Appendix, Fig. S3A). Vehicle-treated ethanol-diet-fed mice showed increased serum ALT levels, hepatocyte apoptosis, hepatic triglyceride levels, and accumulation of lipid droplets, whereas the s.c. injection of 1Z1 had reduced hepatic injury and fat accumulation induced by the ethanol-containing diet (SI Appendix, Fig. S3 B–E), indicating that the systemic administration of 1Z1 protected mice from ethanol-induced hepatic steatosis and injury.
Oral Ingestion of 1Z1 Protected Against Alcohol-Induced Fatty Liver and Liver Injury.
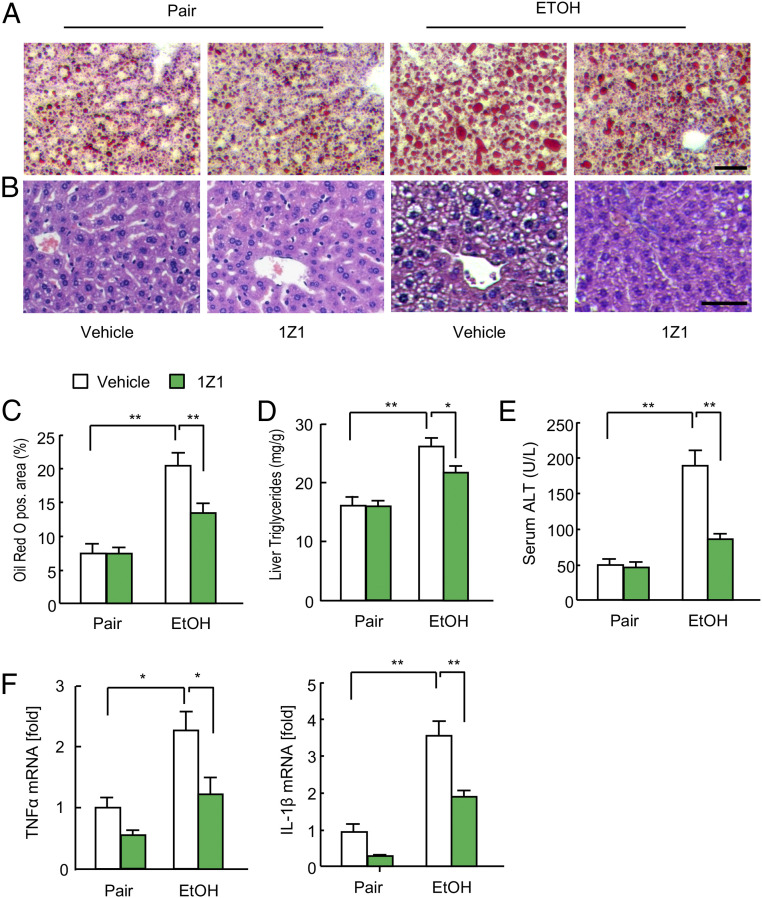
After the validation of the systemic effect of 1Z1, we examined the effect of 1Z1 via an oral route for consideration of future drug development. Mice were subjected to chronic binge ethanol feeding for 6 d to initiate ALD development. Mice were then divided into two groups and ethanol feeding was continued for progression to AH. One group was administered vehicle and the other was treated daily with 1Z1 (1 μmol) for an additional 8 d while ethanol feeding was continued (SI Appendix, Fig. S4A). In vehicle-treated mice, ethanol feeding resulted in increased lipid droplets within the cytoplasm of hepatocytes, elevated hepatic triglyceride and serum ALT levels, and increased expression of hepatic TNF-α and IL-1β mRNA, indicating hepatic fat deposition, hepatocyte injury, and liver inflammation (Fig. 2 A–F). In contrast, 1Z1-treated mice exhibited a lesser extent of ethanol-induced hepatic fat deposition, injury, and inflammation. These results suggest that orally administered 1Z1 has beneficial effects in this AH mouse model. Of note, pharmacokinetic analysis showed that orally administered 1Z1 (1 µmol) was not absorbed into systemic circulation when compared with i.v. injection (SI Appendix, Fig. S4B), suggesting that the effect of oral 1Z1 ingestion on ALD may be independent of its direct effect on the liver. We also confirmed that oral administration of 1Z1 did not cause body weight changes (SI Appendix, Fig. S4C), demonstrating that it is well tolerated.
Fig. 2.
Oral administration of 1Z1 mitigated alcohol-induced fatty liver and liver injury. Mice undergoing chronic binge ethanol feeding were treated with 1Z1 (1 µmol/mouse) for the last 8 d (n = 7/group). (A and B) Representative images of Oil Red O staining (A) and hematoxylin and eosin staining (B). (Scale bar, 50 μm.) (C) Quantification of Oil Red O-positive area. (D) Liver triglyceride levels. (E) Serum ALT levels. (F) qRT-PCR assays for TNFα and IL-1β mRNA expression. Data are presented as the mean ± SEM *P < 0.05, **P < 0.01. One-way ANOVA with Tukey’s post hoc analysis (C–F).
Oral Ingestion of 1Z1 Prevented Alcohol-Mediated Disruption of Gut Tight Junctions, Bacterial Translocation, and Bacterial Overgrowth.
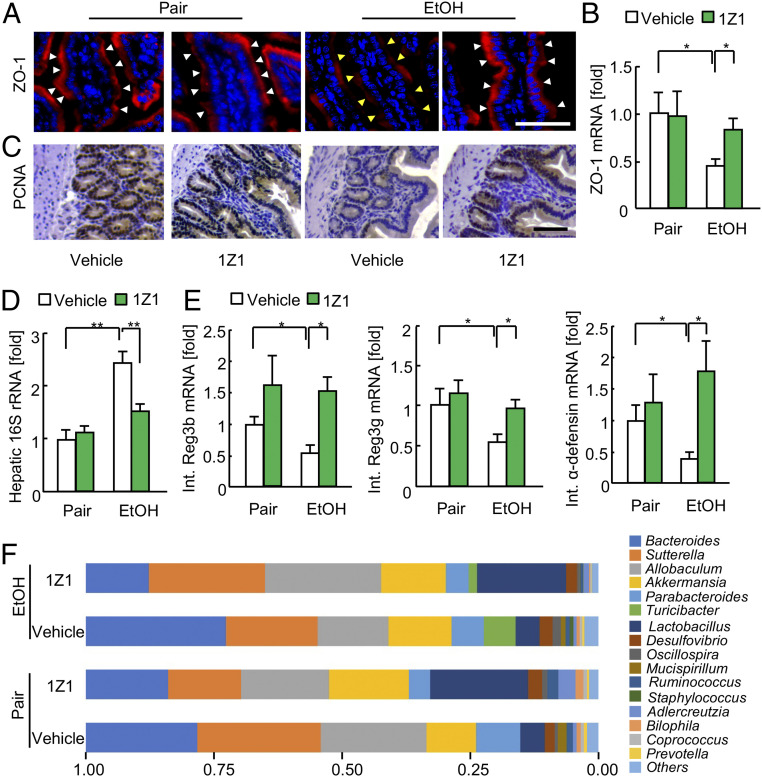
Because little absorption of 1Z1 was observed via the oral route (SI Appendix, Fig. S4B), we postulated that the protective mechanism of oral 1Z1 treatment against alcohol-induced liver injury may be due its local effects in the intestines, including the inhibition of gut epithelial damage, tight junction disruption, and bacterial translocation. Indeed, oral 1Z1 treatment inhibited ethanol-mediated ZO-1 reduction (Fig. 3 A and B). Furthermore, ethanol suppressed the physiological proliferation of intestinal epithelial cells, which was shown by proliferating cell nuclear antigen (PCNA) expression. However, 1Z1 treatment preserved the intestinal epithelial cell proliferation (Fig. 3C). Moreover, 1Z1 treatment reduced the ethanol-induced levels of bacterial 16S rRNA in the liver (Fig. 3D). Also, ethanol suppressed the expression of antimicrobial peptides, Reg3b, Reg3g, and α-defensin (Fig. 3E), and this effect was again mitigated by 1Z1 treatment (Fig. 3E). Consistently, 1Z1 also affected the gut bacterial composition by increasing the relative abundance of beneficial Lactobacillus and reducing that of Bacteroides (Fig. 3F). These findings suggest that intestinal TLR7 signaling activation via oral administration of 1Z1 ameliorates ethanol-induced intestinal epithelial damage and facilitates epithelial proliferation, which maintains the function of the intestinal epithelial barrier and subsequently prevents the translocation of gut bacterial products to the liver. Moreover, 1Z1 treatment increases the amount of beneficial Lactobacillus in the intestines, which is likely mediated through the induction of antimicrobial peptides (26).
Fig. 3.
Oral administration of 1Z1 ameliorated alcohol-induced intestinal barrier dysfunction and bacterial translocation. Mice undergoing chronic binge ethanol feeding were treated with 1Z1 (1 µmol/mouse) for the last 8 d (n = 4 to 9/group). (A) Representative immunofluorescence staining images for ZO-1 in the small intestines. White arrowhead, maintained ZO-1 expression; yellow arrowhead, decreased ZO-1 expression. (Scale bar, 50 μm.) (B) ZO-1 mRNA expression. (C) Representative images of staining for PCNA in the small intestines. (Scale bar, 50 μm.) (D) Hepatic 16S rRNA levels. (E) qRT-PCR assays for intestinal Reg3b, Reg3g, and α-defensin mRNA expression. (F) Fecal samples were collected and 16S rRNA sequences were analyzed. The graph demonstrates the relative abundance of each genus. Data are presented as the mean ± SEM *P < 0.05, **P < 0.01. One-way ANOVA with Tukey’s post hoc analysis (B and D–E).
Oral Ingestion of 1Z1 Contributed to the Induction of IL-22 and Antimicrobial Peptides in the Intestines.
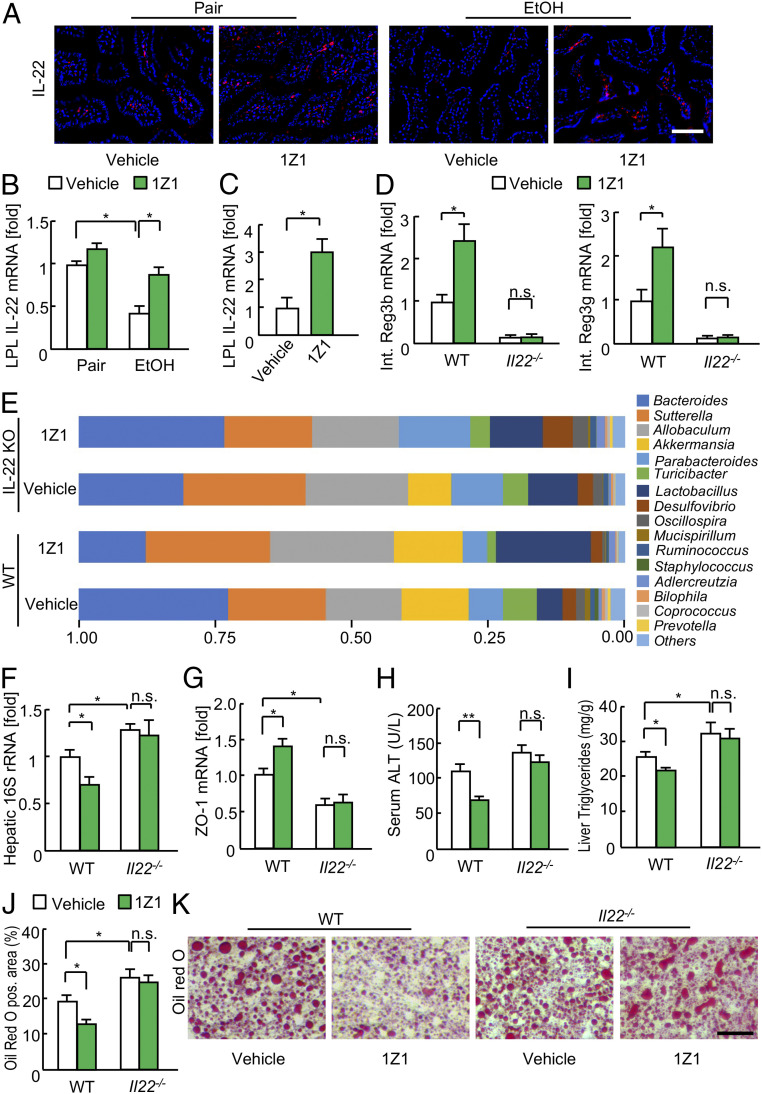
Next, we explored the underlying mechanism of the TLR7-mediated protection against alcohol-induced alterations in the intestines. IL-22 has proliferative, antiapoptotic, and antimicrobial effects in various experimental models, and it is also induced by TLR7 signaling (27–29). We hypothesized that TLR7-mediated IL-22 expression may have protective effects on the function of the intestinal epithelial barrier. IL-22 was expressed in intestinal immune cells, including LPLs, and ethanol ingestion dramatically reduced its expression (Fig. 4 A and B). However, 1Z1 treatment increased the IL-22 expression in LPLs both in vivo and in vitro (Fig. 4 A–C). The flow cytometry (FACS) analysis revealed that the dominant IL-22-producing cells in the naïve intestine are the RORγt+CD3− lymphocyte subset, which includes innate lymphoid cell type 3 (ILC3), whereas after oral 1Z1 treatment the cells expressing RORγt, CD3, and/or CD4, such as Th17/22 cells and γδ T cells, also produce IL-22 (SI Appendix, Fig. S5). To test whether IL-22 was responsible for the gut phenotype of 1Z1-treated mice (30), ethanol-fed WT and Il22−/− mice were treated with vehicle or 1Z1. The beneficial effects of 1Z1 were not observed in the Il22−/− mice, as no changes were detected in the expression of antimicrobial peptides, gut bacterial composition, bacterial translocation, or tight junction molecule (Fig. 4 D–G and SI Appendix, Fig. S6). These results suggest that intestinal IL-22 up-regulation is responsible for the beneficial effects of 1Z1 on the gut bacterial composition and against bacterial translocation.
Fig. 4.
The beneficial effects of 1Z1 were abolished in Il-22−/− mice. (A) Il-22 promoter-driven tdTomato reporter mice undergoing chronic binge ethanol feeding were treated with 1Z1 (1 µmol/mouse) for the last 8 d. Representative fluorescence images. (Scale bar, 50 μm.) (B) IL-22 mRNA expression in LPLs isolated from the small intestines of ethanol-fed mice (n = 3 to 4, each). (C) IL-22 mRNA expression in LPLs treated with 1Z1 (5 μM) for 16 h (n = 3 to 4, each). (D–J) Wild-type and Il-22−/− mice undergoing chronic binge ethanol feeding were treated with 1Z1 (1 µmol/mouse) for the last 8 d (n = 6 to 9/group). (D) qRT-PCR assays for intestinal Reg3b and Reg3g mRNA expression. (E) Fecal samples were collected and 16S rRNA sequences were analyzed. The graph demonstrates the relative abundance of each genus. (F) Hepatic 16S rDNA levels. (G) ZO-1 mRNA expression. (H) Serum ALT levels. (I) Liver triglyceride levels. (J) Quantification of the Oil Red O-positive area. (K) Representative images of Oil Red O staining. (Scale bar, 50 μm.) *P < 0.05, **P < 0.01. One-way ANOVA with Tukey’s post hoc analysis (B–D, F, and J). n.s., not significant.
IL-22 Was Crucial for the Inhibition of Alcohol-Mediated Steatohepatitis by 1Z1.
Lastly, we wanted to know whether IL-22 was indeed crucial for the 1Z1-mediated protection against alcohol-induced hepatic steatosis and injury. Compared with WT mice, Il22−/− mice displayed slightly higher degrees of hepatic steatosis without a significant increase in serum ALT levels (Fig. 4 H–K). Although our previous results showed that 1Z1 treatment suppressed hepatic steatosis and injury in WT mice, 1Z1 treatment did not have any therapeutic effects in the Il22−/− mice (Fig. 4 H–K). Collectively, these results suggest that the therapeutic effects of 1Z1 for alcohol-induced steatohepatitis are mediated through IL-22. Furthermore, the 1Z1-mediated effects on the liver may be secondary to the protective effects of intestinal IL-22 against gut damage and bacterial translocation.
Discussion
The development and validation of novel, effective therapeutic agents for the treatment of severe AH are critically needed because the current treatments are limited to corticosteroids and liver transplantation. Corticosteroids are potent antiinflammatory agents with unfavorable side effects, including increased risk of infection. Liver transplantation is only considered in a highly selective group of patients, and the limited availability of donor livers, high cost, and need for life-long immunosuppressants remain as significant concerns. Here, we have demonstrated that endogenous TLR7 signaling intrinsically inhibits the development of ALD and that exogenous TLR7 activation by oral administration of a synthetic TLR7 ligand, 1Z1, inhibits the development of AH in mice. Mechanistically, we found that intestinal IL-22 expression is required for the 1Z1-mediated protective effects against AH. Because IL-22 has been proposed as a therapeutic agent for AH and is currently being tested in clinical trials (31, 32), 1Z1 may also be an attractive agent that induces IL-22 for the treatment of AH.
TLR7 is mainly expressed in immune cells, such as macrophages, DCs, and B cells. TLR7 has been implicated in the promotion of some autoimmune diseases, including plaque psoriasis, systemic lupus erythematosus, and autoimmune cholangitis (33–35). However, TLR7 plays beneficial roles in other diseases. For instance, in cancer immunotherapy, TLR7 activation inhibits cancer growth by enhancing myeloid cell presentation of cancer antigens in the tumor microenvironment and tumor-specific T cell-dependent cancer cytotoxicity (36). Additionally, in viral infections, TLR7 activation induces type I IFN production, which enhances Th1 and cytotoxic T cell responses (37). TLR7 signaling also inhibits the progression of liver fibrosis and nonalcoholic fatty liver disease in mice (11, 38). The 1Z1 molecule was designed to maximize the tissue protective effects of TLR7 signaling, while avoiding systemic toxicity due to inflammatory cytokine release. Consistent with this pharmacological profile, our previous studies showed that 1Z1 treatment prevented the development of allergic encephalomyelitis, arthritis, DSS-induced colitis, and diabetes in mice (17, 18, 20).
In the present study, we found that hepatic TLR7 expression is up-regulated in patients with AH and in an AH murine model, indicating that excessive alcohol consumption enhances TLR7 expression in the liver. However, because Tlr7−/− mice display exacerbated alcohol-induced hepatic steatosis, injury, and inflammation compared with WT mice, it is conceivable that hepatic TLR7 signaling serves as a compensatory response and suppresses alcohol-induced liver inflammation. Activation of TLR7 signaling in Kupffer cells and pDCs induces the production of antiinflammatory IL-10 and IFN-α (11). IL-10 is a well-known antiinflammatory cytokine, and genetic deficiency of the type I IFN receptor causes augmented alcohol-induced liver injury in mice (6). Therefore, the decreased expression of IFN-α may be an underlying mechanism of the exacerbated hepatic inflammation and injury observed in the Tlr7−/− mice (11). In ALD, TLR7 signaling could be activated endogenously. MicroRNAs are single-stranded RNAs that can be natural ligands for TLR7. Among microRNAs, GU-rich miRNAs, such as miR-21a and miR-29a, exhibit TLR7 agonistic effects (22, 23). These miRNAs induce IL-10 expression in Kupffer cells and pDCs. Moreover, primary hepatocytes that are exposed to ethanol release EVs in which these miRNAs are contained. EVs act as a vehicle to transfer their cargo to target cells. EVs transfer hepatocyte-derived miRNAs to immune cells to regulate immune functions. Through phagocytosis or endocytosis machinery, EVs can deliver miRNAs intercellularly and engage to intracellular TLR7 to induce antiinflammatory response, negatively controlling the development of ALD. Our findings also indicate another potential mechanism of alcohol-induced liver injury associated with lowered TLR7 expression in the intestines, which may be due to an increased translocation of bacterial products, such as LPS and bacterial CpG-DNA, from the intestines to the liver.
Our study demonstrates the effective treatment of mice via systemic as well as oral administrations of 1Z1 for AH. Of note, orally administered 1Z1 does not enter the systemic circulation. Although some of the drug may have passed through the alcohol-damaged epithelial barrier of the intestines, the data are consistent with the interpretation that the intestines are the site of the orally administered 1Z1-mediated protection against AH. Oral 1Z1 treatment also maintains the expression of ZO-1 and PCNA after ethanol feeding, suggesting that 1Z1 is capable of stimulating the repair and regeneration of the damaged intestinal barrier, which in turn may prevent the translocation of intestinal bacterial products to the liver (SI Appendix, Fig. S7). Moreover, oral 1Z1 treatment increases the expression of antimicrobial peptides, which likely affect the gut bacterial composition, thus increasing the amount of beneficial Lactobacillus and reducing that of Bacteroides (SI Appendix, Fig. S7) (26). Mechanistically, we determined that intestinal IL-22 is responsible for the 1Z1-mediated tissue protection in the liver and intestines (SI Appendix, Fig. S7). IL-22 is an IL-10 family member cytokine that has protective and regenerative functions in tissues as well as antiapoptotic and antiinflammatory properties. IL-22 is produced by Th17 cells, Th22 cells, γδ T cells, NKT cells, and ILC3s (13). Our FACS analysis suggested that ILC3, Th17 cells, Th22 cells, and γδ T cells are the major sources of IL-22. Upon 1Z1 treatment, the CD3-expressing T cells produced more IL-22 (SI Appendix, Fig. S5), suggesting that Th17 cells, Th22 cells, and γδ T cells could be the major IL-22-producing cells in response to 1Z1. Previous studies have demonstrated that hepatic IL-22 is not endogenously up-regulated in AH and liver fibrosis mouse models (31, 39, 40). In contrast, exogenous administration of IL-22 has a profound effect on tissue repair following liver injury via the promotion of proliferation and inhibition of apoptosis in the hepatocytes of AH, nonalcoholic steatohepatitis, liver fibrosis, and acute-on-chronic liver failure mouse models (31, 39–42).
In the intestines, IL-22 contributes to epithelial cell regeneration and antimicrobial peptide production (30, 43). Indeed, the 1Z1-induced tissue protection in the liver and intestines is lost in Il22−/− mice along with the induction of intestinal antimicrobial peptides and the beneficial changes to the gut bacterial composition. In particular, intestinal Reg3g expression is associated with an increased amount of Lactobacilli (26), but this is not observed in the Il22−/− mice. The protective role of IL-22 has been previously described in preclinical studies using various experimental hepatitis animal models (31). IL-22 has recently been studied in an open-label clinical trial for AH treatment and has shown promising results (32). Additionally, IL-22-mediated signaling has been reported to prevent Clostridioides difficile infection, which is a life-threatening condition in hospitalized patients (44, 45). Thus, because IL-22 increases the amount of Lactobacilli that is associated with Reg3g expression and because it prevents C. difficile infection (46), it is conceivable that 1Z1-induced IL-22 may also be beneficial for the treatment of recurrent C. difficile infections and other intestinal diseases in which IL-22 induction is beneficial. Notably, 1Z1 may be a more cost-effective therapy than recombinant IL-22 because 1Z1 is a small water-soluble molecule that can be synthesized and formulated at a much lower cost than recombinant proteins.
The limitation of this study is that TLR7 activation may promote some liver diseases, including primary sclerosing cholangitis (47). However, 1Z1 has antiinflammatory properties and does not cause the production of proinflammatory cytokines, even when given systemically. These properties may help avoid the unfavorable side effects that are seen with other synthetic TLR7 ligands, and 1Z1 may also effectively treat primary sclerosing cholangitis, similar to ALD. However, further investigations are warranted to address this possibility.
Our collective data provide evidence that targeting TLR7 and IL-22 with an oral agent may be an effective therapeutic approach for AH. Because the mortality of severe AH is very high and no effective therapies exist beyond alcohol abstinence and liver transplantation, exogenous TLR7 activation by 1Z1 may be a safe and effective therapeutic option for AH as well as other liver and enteric diseases; however, further investigation is warranted.
Materials and Methods
Mice.
C57BL/6 WT and Il22−/− mice (no. 027524) were purchased from The Jackson Laboratory. The Tlr7−/− mice were originally provided by S. Akira, Osaka University, Osaka, Japan. Mice were backcrossed at least 10 generations with the C57BL/6 background. For AH induction in the mice, we followed a previously published protocol for the chronic-binge ethanol-feeding model with slight modifications (48). Briefly, female mice were fed a control liquid diet ad libitum for the first 5 d as an acclimatization step. The mice were subsequently fed a Lieber-DeCarli diet (Bio-Serv) containing 5 to 6% ethanol (vol/vol) for 10 or 14 d. In the morning on the 11th or 15th day, mice received a single dose of ethanol (5 g/kg of bodyweight) and were killed 9 h later. Each experiment included six to eight mice/group. For some experiments, a synthetic TLR7 ligand 1Z1 was orally administered at the dose of 1 µmol/mouse once daily from the 7th to the 14th day of ethanol feeding, or s.c. injected at the dose of 0.4 µmol/mouse once daily throughout the ethanol-feeding period. The mice received humane care according to the National Institutes of Health recommendations outlined in the Guide for the Care and Use of Laboratory Animals (49). All animal experiments were approved by the Institutional Animal Care and Use Committees of Cedars-Sinai Medical Center and the University of California, San Diego.
Human Data Analysis.
For the analysis of TLR7 mRNA expression in human livers, the GSE28619 dataset (15 AH patients, nine healthy controls) was analyzed using R software (21).
Histological Analysis.
Formalin-fixed, paraffin-embedded liver tissues were used. For immunohistochemical analysis, tissues were deparaffinized and rehydrated, and then endogenous peroxidase was blocked and antigen retrieval was performed. After blocking, sections were incubated with primary antibodies against cleaved cytokeratin 18 (clone M30; #12140322001, Sigma) or PCNA (#307902, BioLegend). Secondary antibodies were used. The number of M30-positive cells was counted from 8 to 10 randomly selected fields per slide at a magnification of 100× using ImageJ software (https://imagej.nih.gov/ij). Neutral lipids were analyzed by Oil Red O staining. The Oil Red O-positive area was evaluated from 10 randomly selected fields per slide at a magnification of 200×.
Immunofluorescence Staining.
For ZO-1 staining, small intestine sections were incubated with ZO-1 antibody (1:100, #216880, Abcam), followed by Alexa Fluor 594-conjugated goat anti-rabbit IgG (1:200, Thermo Fisher). Imaging analyses were performed using a Zeiss LSM800 inverted microscope and Axio Imager Z2m confocal system or a Keyence BZ-X710 fluorescence microscope.
Bacterial DNA Isolation and qPCR for 16S rRNA.
Genomic DNA was isolated from livers using the QIAamp DNA Mini Kit (51306, Qiagen). The qPCR value of the 16S rRNA was normalized to that of the host 18S rRNA.
Statistical Analysis.
Statistical significance was assessed using GraphPad Prism 8.01 software (GraphPad Software). Differences between two groups were determined using a two-tailed, unpaired Student’s t test. Differences among multiple groups were determined using one-way ANOVA, followed by Tukey’s post hoc analysis. P values <0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
We thank Ms. F. Miao for technical support for the preparation of the histological sections. This work was supported by the NIH (R01AA027036 to E.S. and T.H., R21AA025841 to E.S. and S.C.L., R01AA026759 to S.C.L., R01DK085252 to E.S., and P01CA233452 to E.S. and S.C.L.), a Winnick Research Award from Cedars-Sinai Medical Center (to E.S.), the Center for Integrated Research in Cancer and Lifestyle Award by the Samuel Oschin Comprehensive Cancer Institute at Cedars-Sinai Medical Center (to E.S.), and a scholarship from the Suntory Global Innovation Center (to E.S.).
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2020868118/-/DCSupplemental.
Data Availability.
The 16S sequence data have been deposited in NCBI Bioproject (PRJNA667517).
References
- 1.Gao B., Bataller R., Alcoholic liver disease: Pathogenesis and new therapeutic targets. Gastroenterology 141, 1572–1585 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lucey M. R., Mathurin P., Morgan T. R., Alcoholic hepatitis. N. Engl. J. Med. 360, 2758–2769 (2009). [DOI] [PubMed] [Google Scholar]
- 3.Yang L., Seki E., Toll-like receptors in liver fibrosis: Cellular crosstalk and mechanisms. Front. Physiol. 3, 138 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seki E., Schnabl B., Role of innate immunity and the microbiota in liver fibrosis: Crosstalk between the liver and gut. J. Physiol. 590, 447–458 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roh Y. S., Zhang B., Loomba R., Seki E., TLR2 and TLR9 contribute to alcohol-mediated liver injury through induction of CXCL1 and neutrophil infiltration. Am. J. Physiol. Gastrointest. Liver Physiol. 309, G30–G41 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petrasek J., et al. , Interferon regulatory factor 3 and type I interferons are protective in alcoholic liver injury in mice by way of crosstalk of parenchymal and myeloid cells. Hepatology 53, 649–660 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hritz I., et al. , The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology 48, 1224–1231 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inokuchi S., et al. , Toll-like receptor 4 mediates alcohol-induced steatohepatitis through bone marrow-derived and endogenous liver cells in mice. Alcohol. Clin. Exp. Res. 35, 1509–1518 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rachmilewitz D., et al. , Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology 126, 520–528 (2004). [DOI] [PubMed] [Google Scholar]
- 10.Lee J., et al. , Maintenance of colonic homeostasis by distinctive apical TLR9 signalling in intestinal epithelial cells. Nat. Cell Biol. 8, 1327–1336 (2006). [DOI] [PubMed] [Google Scholar]
- 11.Roh Y. S., et al. , Toll-like receptor 7-mediated type I interferon signaling prevents cholestasis- and hepatotoxin-induced liver fibrosis. Hepatology 60, 237–249 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yin S., Gao B., Toll-like receptor 3 in liver diseases. Gastroenterol. Res. Pract. 2010, 750904 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dudakov J. A., Hanash A. M., van den Brink M. R., Interleukin-22: Immunobiology and pathology. Annu. Rev. Immunol. 33, 747–785 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiang X., Hwang S., Feng D., Shah V. H., Gao B., Interleukin-22 in alcoholic hepatitis and beyond. Hepatol. Int. 14, 667–676 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang L., et al. , Intestinal REG3 lectins protect against alcoholic steatohepatitis by reducing mucosa-associated microbiota and preventing bacterial translocation. Cell Host Microbe 19, 227–239 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartmann P., et al. , Deficiency of intestinal mucin-2 protects mice from diet-induced fatty liver disease and obesity. Am. J. Physiol. Gastrointest. Liver Physiol. 310, G310–G322 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayashi T., et al. , Prevention of autoimmune disease by induction of tolerance to Toll-like receptor 7. Proc. Natl. Acad. Sci. U.S.A. 106, 2764–2769 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayashi T., et al. , Induction of tolerogenic dendritic cells by a PEGylated TLR7 ligand for treatment of type 1 diabetes. PLoS One 10, e0129867 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan M., et al. , Synthesis and characterization of PEGylated toll like receptor 7 ligands. Bioconjug. Chem. 22, 445–454 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayashi T., et al. , Mast cell-mediated inhibition of abdominal neutrophil inflammation by a PEGylated TLR7 ligand. Mediators Inflamm. 2012, 262394 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Affò S., et al. , Transcriptome analysis identifies TNF superfamily receptors as potential therapeutic targets in alcoholic hepatitis. Gut 62, 452–460 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fabbri M., et al. , MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc. Natl. Acad. Sci. U.S.A. 109, E2110–E2116 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen X., Liang H., Zhang J., Zen K., Zhang C. Y., microRNAs are ligands of Toll-like receptors. RNA 19, 737–739 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Momen-Heravi F., Bala S., Kodys K., Szabo G., Exosomes derived from alcohol-treated hepatocytes horizontally transfer liver specific miRNA-122 and sensitize monocytes to LPS. Sci. Rep. 5, 9991 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stärkel P., Schnabl B., Bidirectional communication between liver and gut during alcoholic liver disease. Semin. Liver Dis. 36, 331–339 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Huang Y., et al. , Gut REG3γ-associated Lactobacillus induces anti-inflammatory macrophages to maintain adipose tissue homeostasis. Front. Immunol. 8, 1063 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abt M. C., et al. , TLR-7 activation enhances IL-22-mediated colonization resistance against vancomycin-resistant enterococcus. Sci. Transl. Med. 8, 327ra25 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wohn C., et al. , Langerin(neg) conventional dendritic cells produce IL-23 to drive psoriatic plaque formation in mice. Proc. Natl. Acad. Sci. U.S.A. 110, 10723–10728 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Belle A. B., et al. , IL-22 is required for imiquimod-induced psoriasiform skin inflammation in mice. J. Immunol. 188, 462–469 (2012). [DOI] [PubMed] [Google Scholar]
- 30.Hendrikx T., et al. , Bacteria engineered to produce IL-22 in intestine induce expression of REG3G to reduce ethanol-induced liver disease in mice. Gut 68, 1504–1515 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ki S. H., et al. , Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: Role of signal transducer and activator of transcription 3. Hepatology 52, 1291–1300 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arab J. P., et al. , An open-label, dose-escalation study to assess the safety and efficacy of IL-22 agonist F-652 in patients with alcohol-associated hepatitis. Hepatology 72, 441–453 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lai C. Y., Su Y. W., Lin K. I., Hsu L. C., Chuang T. H., Natural modulators of endosomal toll-like receptor-mediated psoriatic skin inflammation. J. Immunol. Res. 2017, 7807313 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Souyris M., Mejía J. E., Chaumeil J., Guéry J. C., Female predisposition to TLR7-driven autoimmunity: Gene dosage and the escape from X chromosome inactivation. Semin. Immunopathol. 41, 153–164 (2019). [DOI] [PubMed] [Google Scholar]
- 35.Bae H. R., et al. , The interplay of type I and type II interferons in murine autoimmune cholangitis as a basis for sex-biased autoimmunity. Hepatology 67, 1408–1419 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kobold S., Wiedemann G., Rothenfußer S., Endres S., Modes of action of TLR7 agonists in cancer therapy. Immunotherapy 6, 1085–1095 (2014). [DOI] [PubMed] [Google Scholar]
- 37.Zhang S. Y., et al. , Human Toll-like receptor-dependent induction of interferons in protective immunity to viruses. Immunol. Rev. 220, 225–236 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim S., Park S., Kim B., Kwon J., Toll-like receptor 7 affects the pathogenesis of non-alcoholic fatty liver disease. Sci. Rep. 6, 27849 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Radaeva S., Sun R., Pan H. N., Hong F., Gao B., Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology 39, 1332–1342 (2004). [DOI] [PubMed] [Google Scholar]
- 40.Kong X., et al. , Interleukin-22 induces hepatic stellate cell senescence and restricts liver fibrosis in mice. Hepatology 56, 1150–1159 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hwang S., et al. , Interleukin-22 ameliorates neutrophil-driven nonalcoholic steatohepatitis through multiple targets. Hepatology 72, 412–429 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiang X., et al. , Interleukin-22 ameliorates acute-on-chronic liver failure by reprogramming impaired regeneration pathways in mice. J. Hepatol. 72, 736–745 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hammer A. M., et al. , Interleukin-22 prevents microbial dysbiosis and promotes intestinal barrier regeneration following acute injury. Shock 48, 657–665 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagao-Kitamoto H., et al. , Interleukin-22-mediated host glycosylation prevents Clostridioides difficile infection by modulating the metabolic activity of the gut microbiota. Nat. Med. 26, 608–617 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hasegawa M., et al. , Interleukin-22 regulates the complement system to promote resistance against pathobionts after pathogen-induced intestinal damage. Immunity 41, 620–632 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Capurso L., Thirty years of lactobacillus rhamnosus GG: A review. J. Clin. Gastroenterol. 53, S1–S41 (2019). [DOI] [PubMed] [Google Scholar]
- 47.Katt J., et al. , Increased T helper type 17 response to pathogen stimulation in patients with primary sclerosing cholangitis. Hepatology 58, 1084–1093 (2013). [DOI] [PubMed] [Google Scholar]
- 48.Bertola A., Mathews S., Ki S. H., Wang H., Gao B., Mouse model of chronic and binge ethanol feeding (the NIAAA model). Nat. Protoc. 8, 627–637 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.National Research Council , Guide for the Care and Use of Laboratory Animals (National Academies Press, Washington, DC, 2011), ed. 8. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The 16S sequence data have been deposited in NCBI Bioproject (PRJNA667517).