Significance
Low sunlight exposure and low vitamin D (vitD) levels are risk factors for the development of multiple sclerosis. However, there is still an ongoing debate, whether sunlight and vitD also modulate disease severity and worsening. Observational studies suggested vitD-dependent effects, but prospective supplementation studies have so far been inconclusive and reverse causality cannot be excluded as a source of bias. By using the sun-exposure measures vitD and latitude, we show correlations between vitD/latitude, vitD/disease severity, and latitude/disease severity in two multicentric cohorts. Although vitD cannot be proven as the causal factor, we provide evidence for clinically relevant effects of sunlight exposure. Furthermore, this study suggests sunlight-triggered pathways other than vitD could play additional and modulatory roles, as well.
Keywords: sunlight, multiple sclerosis, vitamin D, latitude, melanocortin 1 receptor
Abstract
Multiple sclerosis (MS) disease risk is associated with reduced sun-exposure. This study assessed the relationship between measures of sun exposure (vitamin D [vitD], latitude) and MS severity in the setting of two multicenter cohort studies (nNationMS = 946, nBIONAT = 990). Additionally, effect-modification by medication and photosensitivity-associated MC1R variants was assessed. High serum vitD was associated with a reduced MS severity score (MSSS), reduced risk for relapses, and lower disability accumulation over time. Low latitude was associated with higher vitD, lower MSSS, fewer gadolinium-enhancing lesions, and lower disability accumulation. The association of latitude with disability was lacking in IFN-β–treated patients. In carriers of MC1R:rs1805008(T), who reported increased sensitivity toward sunlight, lower latitude was associated with higher MRI activity, whereas for noncarriers there was less MRI activity at lower latitudes. In a further exploratory approach, the effect of ultraviolet (UV)-phototherapy on the transcriptome of immune cells of MS patients was assessed using samples from an earlier study. Phototherapy induced a vitD and type I IFN signature that was most apparent in monocytes but that could also be detected in B and T cells. In summary, our study suggests beneficial effects of sun exposure on established MS, as demonstrated by a correlative network between the three factors: Latitude, vitD, and disease severity. However, sun exposure might be detrimental for photosensitive patients. Furthermore, a direct induction of type I IFNs through sun exposure could be another mechanism of UV-mediated immune-modulation in MS.
Multiple sclerosis (MS), characterized by demyelinating lesions, is the most common neuroinflammatory disease of the central nervous system and presumably of autoimmune origin (1). In most cases, the disease is diagnosed at a young age, predominantly occurs in women, and follows a relapsing-remitting course, which can be superseded by a secondary, progressive stage (2). Etiologically, environmental factors have been shown to play an important role (3) and insufficient sunlight exposure has been suspected to be critical for the initial development of MS (4). The best characterized mediator of ultraviolet radiation (UVR)-dependent effects is vitamin D (vitD), which is generated from its precursor 7-dehydrocholesterole (7-DHC) in the skin, further metabolized in the liver and kidney, and that exerts its function in its active form 1-α,25-dihydroxyvitamin D3 [1α,25(OH)2D3], also known as calcitriol (5). Precursors of active vitD can also be found in food in the form of ergocalciferol (or vitamin D2), which is, however, of little relevance for total serum vitD levels (6). For MS, low vitD levels have been shown to be associated with disease risk (7, 8) and Mendelian randomization studies hint toward a causal role for vitD (9, 10). However, it is possible that alternative UVR-dependent pathways play a role as well (11). A study by Langer-Gould et al. (12) suggested that serum vitD is only a risk factor for MS in Whites, but not in people of color, although lifetime UVR-exposure was associated with reduced risk in both, arguing against vitD as the mediator of UVR-effects. Hedström et al. (13) report from a large population-based study that besides vitD-dependent effects, vitD-independent effects of UVR also play a role. Results from the PhoCIS (Phototherapy for Clinically Isolated Syndrome [CIS]) trial and its post hoc analysis suggest effects of UVR even in patients with a serum vitD status of ≥80 ng/mL (14, 15). Furthermore, it is still a topic of debate whether UVR/vitD only modulate disease risk, or if disease severity of established MS is affected, as well. In mouse models, UVR was shown to ameliorate disease, but this was independent of vitD and its receptor (16–19). Human observational studies suggested an influence of vitD on disease activity (20, 21) and prospective trials also suggested an effect on the formation of lesions, but the primary endpoints of supplementation studies have not been met so far, raising doubt about the role of vitD in ongoing disease (22–24). It has also been argued that reverse causation could influence the results from observational studies (i.e., low vitamin D could be caused by disease activity rather than vice versa) (4).
Reports on the effect of latitude (which can be regarded as an independent measure of sun-exposure) on disease severity are scarce and could either not identify any effects or showed contradictory effects (25, 26). Noteworthy, besides the assumed main effects of UVR and vitD on MS severity, there might be factors that could alter the effects of UVR/vitD. Medication—especially IFN-β therapy—has been suggested to modulate vitD-production [potentially by decreasing serum cholesterol (27)] and its treatment effect. Ambiguous results have been reported regarding the effect of vitD in IFN-β–treated patients: Two studies reported positive interactions between vitD and IFN-β (28, 29). Another study found the association between vitD and disease activity to disappear after IFN-β treatment onset, while in the BENEFIT and BEYOND trials the effect of vitD was apparent despite IFN-β treatment (20, 30, 31). Besides medication, genetic factors that determine pigmentation and photosensitivity could play an important role in modulating UVR effects. One genetic factor known to alter an individual’s response to UVR is the melanocortin 1 receptor (MC1R) genotype. The MC1R is responsible for melanin synthesis upon UVR exposure and carrying loss-of-function variants results in a red hair and fair skin phenotype with increased photosensitivity (32, 33). The MC1R is also known to confer immunosuppressive effects and functional signaling ameliorates disease course in mouse models of MS (34). Moreover, the MC1R agonist adrenocorticotropic hormone (ACTH) is used to treat disease exacerbations in MS and part of the effect of ACTH is assumed to be mediated through the MC1R (35). Therefore, the MC1R could be a mediator or modifier of UVR-effects in MS.
This study aims to provide a better understanding of the effects of UVR and vitD on MS severity, and to deduce the role of modulatory factors like medication and photosensitivity-associated genotypes. In the setting of two large, independent multicenter studies, the effects of the sun-exposure measures vitD and latitude on disease severity were investigated. To assess the role of further mechanistic pathways in mediating or modifying UVR and vitD effects, interactions between sun-exposure measures and either medication or the MC1R genotype were assessed. Finally, in an unbiased exploratory approach the UVB-induced transcriptomic changes in immune cells of MS patients from our 2014 pilot study were assessed (18), illuminating the pathways triggered by UVR in peripheral immune cells of MS patients.
Results
Cohort Characteristics.
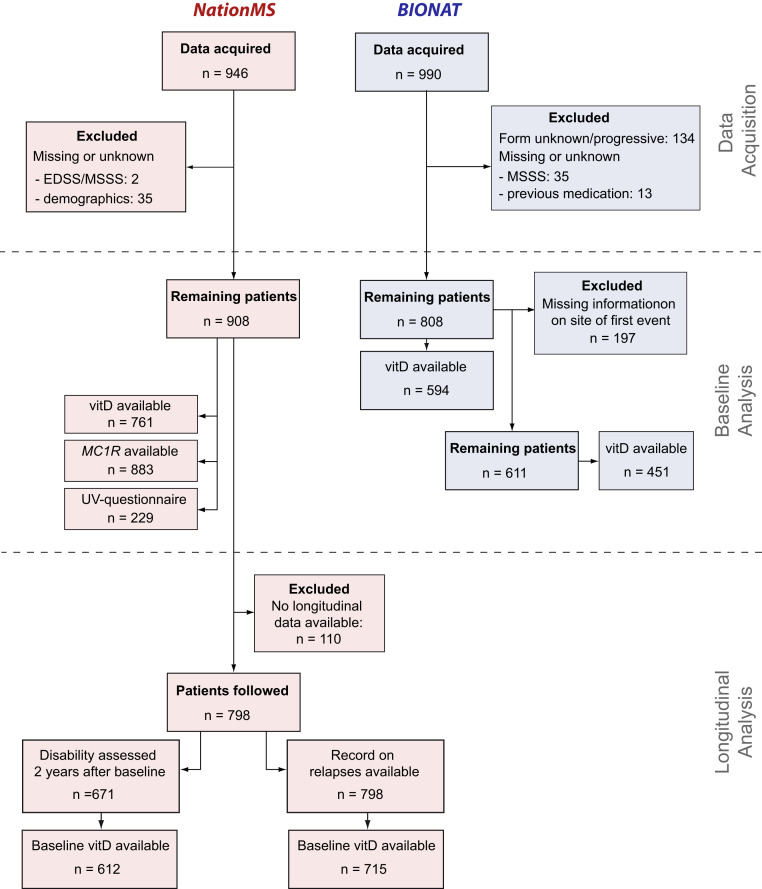
For the NationMS cohort, data of 946 treatment-naïve patients at baseline with CIS or relapsing-remitting multiple sclerosis (RRMS) were acquired (disease duration <2 y). The study procedure is shown in Fig. 1. Baseline characteristics are described in SI Appendix, Table S1. Genotyping for MC1R was successful and passed quality control (QC) for 883 (97.25%) of the 908 patients that had full demographic and clinical information available. The most common MC1R variant allele was rs1805008:T (n = 148 of 883, 16.76% with ≥1 risk allele, minor allele frequency [MAF] = 8.60%), followed by rs2228479:A (n = 140 of 883, 15.86% with ≥1 risk allele, MAF = 8.10%), and rs885479:A (n = 76 of 883, 8.61% with ≥1 risk allele, MAF = 4.42%). Patients were subjected to regular follow-ups. In total, for 798 patients, longitudinal data were available (Fig. 1). Of these, 148 (18.55%) patients remained untreated after baseline assessment, while 650 (81.46%) received treatment. The most prescribed medication was IFN-β (n = 364, 45.61% of all patients, including untreated) (SI Appendix, Table S2). A change in medication was recorded for 233 patients (29.20% ever changed). Moreover, 355 patients (44.49%) reported a relapse in the time after baseline assessment with a median time to relapse of 245 d (interquartile range = 102.0–470.5). For another 671 patients, disability was assessed 2 y after baseline, and the median change in expanded disability status scale (EDSS) was 0 (interquartile range = −0.5–0.5), with 233 patients (34.72%) who presented with an increase after those 2 y, 220 (32.79%) patients with a decrease, and 218 (32.49%) whose EDSS remained unchanged.
Fig. 1.
Study flow chart. Data of 946 therapy-naïve patients were acquired for the NationMS cohort and data of 990 BIONAT patients with a history of previous medication for MS have been acquired. Datasets were filtered for missing information with 908 NationMS and 808 BIONAT patients remaining at baseline. For the analysis of disease severity, BIONAT patients were further excluded when information on site of first event was unknown/unavailable (remaining total n = 611, n for remaining patients with available information on serum vitD = 451). To assess the influence of sun-exposure measures on serum vitD levels, this information was not necessary and n = 594 patients were assessed. For NationMS, longitudinal information on relapses was available for n = 798 and information on disability 2 y after baseline was available for 671 patients.
For the BIONAT cohort, data of 990 MS patients at their baseline assessment were acquired (Fig. 1). Full information on demographics, clinical subtype, medication, and the multiple sclerosis severity score (MSSS) were available for 808 patients (81.62%). Most patients had already received therapy before the baseline assessment. The most prescribed medication was IFN-β (59.77%) (SI Appendix, Table S2).
The characteristics of the five patients treated with UVB-phototherapy were previously published (18). Two of the patients were treated with IFN-β, one with Glatirameracetate (GA), one with Natalizumab, and one had not received any treatment. All patients with available biomaterial were included for this study and there were no specific selection criteria besides those already published in Breuer et al. (18).
Latitude and Prior Medication Determine Serum vitD Levels.
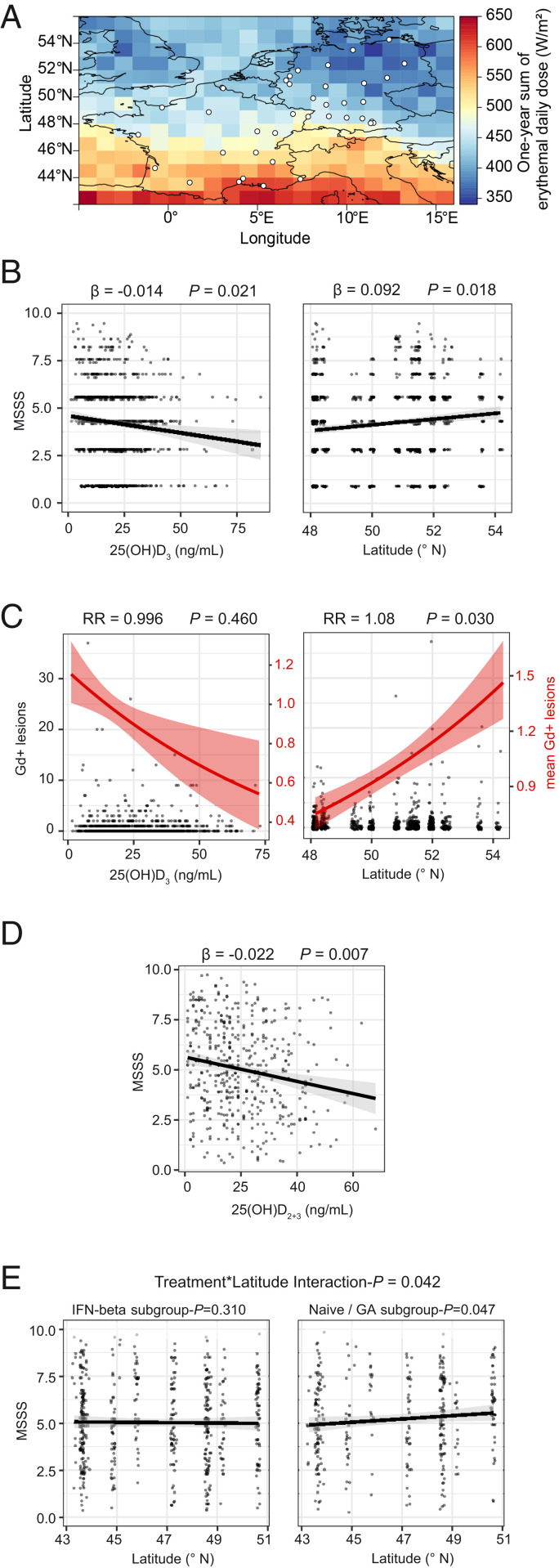
With decreasing latitude, the amount of UVR reaching the earth’s surface increases; thus, latitude can be regarded as a measure of sun exposure. Indeed, a north–south gradient of UVR can be observed along the latitude axis in both Germany and France, with an increase in average yearly UVR of about twofold from the north of Germany to the south of France, as measured by NASA satellite data (Fig. 2A). To demonstrate that lower latitude also correlates with higher actual UVR exposure on a population level, the correlation between latitude and serum vitD levels was tested in both cohorts. As expected, lower latitude was associated with higher vitD levels (β = −0.56, 95% confidence interval [CI] = [−1.030 to −0.090], P = 0.020) in the NationMS cohort. Consistent with the results from the NationMS cohort, in the BIONAT cohort lower latitude was also associated with higher vitD levels (β = −0.44, 95% CI = [−0.87 to −0.018], P = 0.041) (SI Appendix, Fig. S1 A and B). However, not all patients from the BIONAT cohort were untreated and IFN-β treatment has been shown to modulate vitD levels, potentially by decreasing serum cholesterol, which in turn modulates the amount of the vitD-precursor 7-DHC (27, 29). In a subgroup analysis of patients who had received IFN-β therapy, there seemed to be no effect of latitude on vitD levels, while untreated and GA-treated patients showed the expected increase of vitD as latitude decreases (SI Appendix, Fig. S1C). In line with previous reports, IFN-β–treated patients also had higher vitD levels than therapy-naïve patients (β = 4.020, 95% CI = [0.450–7.560], P = 0.031) (SI Appendix, Fig. S1D) (29).
Fig. 2.
Influence of sun-exposure measures on clinical severity. (A) Map of 1-y averaged and erythemically weighted radiation reaching the earth’s surface for Germany and France for the year 2015. White dots represent the locations of the medical centers that contributed participants to either the NationMS (only Germany) or the BIONAT (only France) cohort. (B) Dotplots for MSSS in relation to 25(OH)D3 levels and latitude (of the patients’ medical center) with least-squares linear regression lines ± SE for NationMS (nvitD = 761, nlat = 908). (C) Dotplot for Gd-enhancing lesions in relation to 25(OH)D3 levels (n = 761) and latitude (n = 908) for the NationMS cohort. Left y axis corresponds to the dotplot displaying observed counts; right y axis corresponds to the red line displaying mean number of lesions ± SE. (D) Dotplot for MSSS in relation to 25(OH)D2+3 with least-squares linear regression line ± SE for BIONAT. (E) Dotplots for MSSS in relation to latitude stratified by previous treatment (nIFN-β=363, nNaive/GA = 248) with least-squares regression line ± SE. Analyses for NationMS are adjusted for age, sex, BMI, smoking, alcohol consumption, clinical subtype, neurological site of first manifestation, month of assessment, and center. Analyses for BIONAT are adjusted for age, sex, neurological site of first manifestation, month of assessment, and center. Adjustment for center was omitted when analyzing the effect of latitude.
Low vitD Levels and High Latitude Are Associated with Clinical Disease Severity.
Based on the available evidence before the start of the study, the association between latitude, vitD, and clinical severity was assessed (Fig. 2). In the NationMS cohort vitD levels were associated with lower disability, as assessed by the MSSS (β = −0.014, 95% CI = [−0.026 to −0.002], P = 0.021). Higher latitude (corresponding to lower sun exposure) was associated with worse MSSS scores in the NationMS cohort (β = 0.092, 95% CI = [−0.016–0.168], P = 0.018) (Fig. 2B). A similar result was obtained when substituting latitude by satellite-derived UVR estimates (SI Appendix, Table S3). The risk for gadolinium (Gd)-enhancing lesions increased by 8.31% for every 1° increase in latitude (risk ratio [RR] = 1.08, 95% CI = [1.01–1.16], P = 0.030) (Fig. 2C).
The Association of Latitude with Disability Is Absent in Patients Treated with IFN-β.
The hypotheses were then also tested in the BIONAT cohort. Here, higher vitD levels showed an association with lower disability as well (β = −0.022, 95% CI = [−0.037 to −0.007], P = 0.007) (Fig. 2D). However, in contrast to the results from the NationMS cohort, there was no clear trend for an effect of latitude on the MSSS (β = −0.004, 95% CI = [−0.076–0.068], P = 0.913). As not all BIONAT patients were therapy-naïve at baseline and IFN-β therapy has been shown to modulate vitD synthesis, but also to eradicate the association between vitD and severity measures, it was hypothesized that the missing effect of latitude could also be due to confounding by IFN-β therapy (28–30). Interaction analyses indeed showed that the effect of latitude on disability was significantly different depending on IFN-β treatment status (β = 0.145, 95% CI[0.005–0.283], interaction-P = 0.042). Subsequent subgroup analyses confirmed that higher latitude was associated with a worse MSSS in patients, whose last medication was not IFN-β (β = 0.115, 95% CI = [0.001–0.227], P = 0.047), while patients treated with IFN-β did not show this effect (β = −0.048, 95% CI = [−0.141–0.045], P = 0.310) (Fig. 2E).
High vitD and Low Latitude Are Associated with Reduced Risk for Relapses and Disability Accumulation.
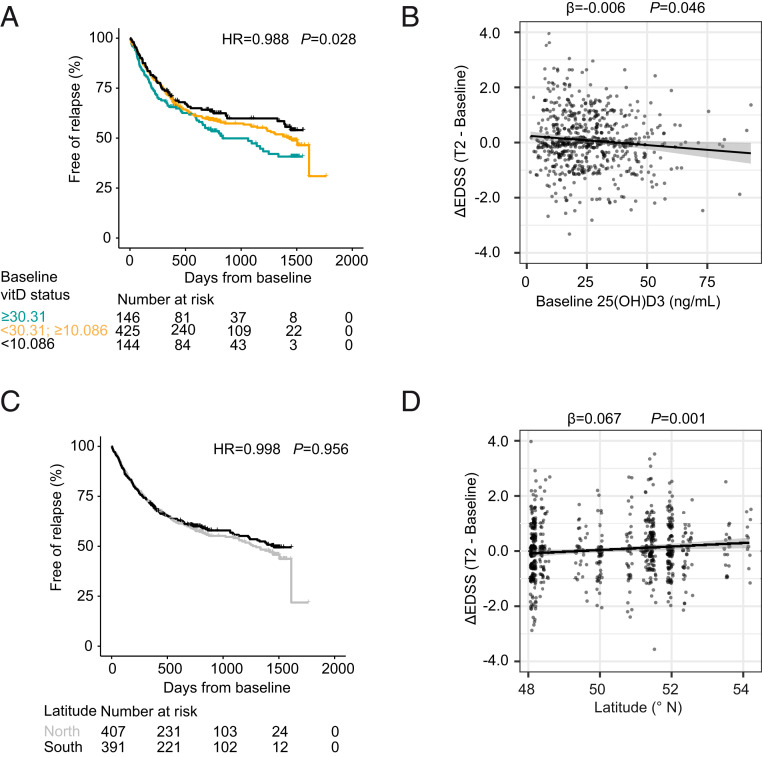
To further test the predictive capacity of vitD and latitude with regard to accumulation of disease burden, data on confirmed relapses after baseline in the NationMS cohort were assessed (Fig. 3). In a mixed-effects Cox regression vitD (for statistical inference treated as continuous variable) reduced the risk for a relapse by 1% for every 1 ng/mL of serum vitD at baseline (hazard ratio [HR] = 0.988, 95% CI = [0.978–0.999], P = 0.028). For the purpose of visualization, vitD was grouped into three categories: 1) The 20% of patients with the highest vitD levels (≥30.31 ng/mL); 2) the 20% of patients with the lowest vitD levels (<10.86 ng/mL); and 3) the patients in between (Fig. 3A). This categorization is also close to common definitions of optimal (≥30 ng/mL) and deficient (<10 ng/mL) vitD levels (36, 37). As categorization exclusively served the purpose of visualization, results from statistical models were not influenced by this and relied on continuous scale serum vitD. Furthermore, baseline vitD was associated with a lower grade of disability accumulation, as measured by the ΔEDSS (β = −0.006, 95% CI = [−0.012 to −0.0003], P = 0.046) (Fig. 3B). Latitude showed no significant effect on the risk for relapses (Fig. 3C). However, in line with the results from the baseline analysis, lower latitude was associated with significantly lower ΔEDSS (β = 0.67, 95% CI = [0.026–0.106], P = 0.001) (Fig. 3D).
Fig. 3.
Influence of vitD and latitude on risk for relapses and disability accumulation. (A) Time-to-event curves displaying the proportion of relapse-free patients over time for the NationMS cohort grouped by serum 25(OH)D3 levels (color code: jade = the 20% of patients with the lowest 25(OH)D3 levels, cutpoint: <10.086 ng/mL; black = the 20% of patients with the highest 25(OH)D3 levels, cutpoint: ≥30.31 ng/mL; orange = patients in between) and complemented with a table displaying the number at risk over time (n = 715). (B) Dotplot for the change in EDSS (ΔEDSS) in relation to baseline 25(OH)D3 levels with least-squares linear regression line ± SE (n = 612). (C) Time-to-event curves displaying the proportion of relapse-free patients over time for the NationMS cohort grouped by latitude (north defined as greater than or equal to median latitude within the cohort = 50.85 °N) and complemented with a table displaying the number at risk over time (n = 798). (D) Dotplot for the ΔEDSS in relation to latitude with least-squares regression line ± SE (n = 671). Analyses are adjusted for age, sex, BMI, smoking, alcohol consumption, clinical subtype, neurological site of first manifestation, month of assessment, medication after baseline, whether medication was changed during the study, and center variability. Differences in baseline severity were adjusted by using the baseline MSSS and the number of Gd-enhancing lesions at baseline as covariates. Adjustment for center was omitted when analyzing the effect of latitude.
Photosensitivity-Associated MC1R Missense Variants Might Modify UVR-Mediated Effects.
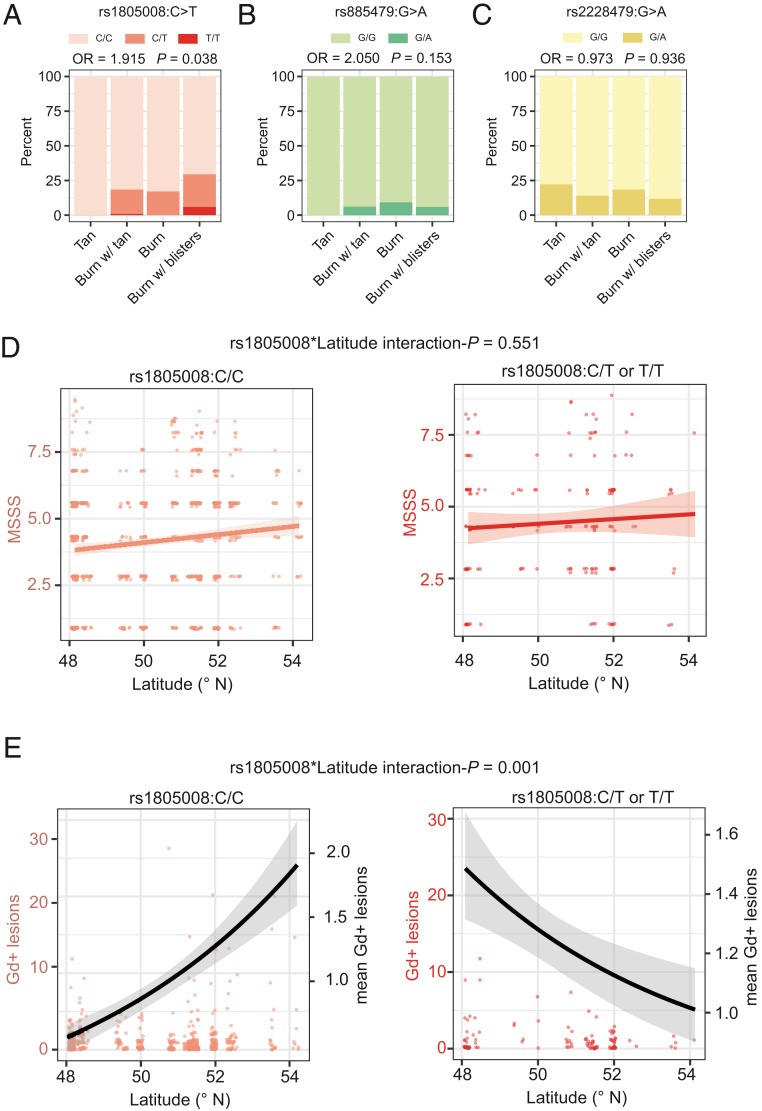
MC1R missense variants strongly increase an individual’s sensitivity to UVR and could, therefore, modify the beneficial effect of UVR on MS disease severity. First, the previously described influence of MC1R genotype on photosensitivity was confirmed using ordinal regression (Fig. 4 A–C). Whereas there was no statistically significant effect for the low-penetrance variants rs885479 (odds ratio [OR] = 2.05, 95% CI = [0.764–5.541], P = 0.153) and rs2228479 (OR = 0.97, 95% CI = [0.492–1.911], P = 0.936), the T allele of the high-penetrance variant rs1805008 increased the odds for a severe reaction to sunlight by 91.5% (OR = 1.92, 95% CI = [1.040–3.560], P = 0.038) (Fig. 4A). Biological sex did not modify the effect of rs1805008 on photosensitivity (interaction-P = 0.308). Next, as rs1805008:T carriers reported severe reactions to sunlight, the effect of an interaction term between measures of sun exposure (latitude, vitD) and rs1805008 on clinical severity was assessed (at baseline). No significant interactions were found regarding the MSSS (Fig. 4D). The interaction term of rs1805008:T and latitude was nominally significant in the analysis of Gd-enhancing lesions (interaction-P = 0.001). In subgroup analyses for carriers of rs1805008:T, the risk for Gd-enhancing lesions increased by 20.5% for every 1° decrease in latitude (RR = 1.21, 95% CI = [1.010–1.437], P = 0.034), while it was reduced by 11.6% in noncarriers for every 1° decrease in latitude (RR = 0.88, 95% CI = [0.808–0.947], P = 9.2 × 10−4) (Fig. 4E). Both subgroup analyses were nominally significant. Furthermore, the statistical significance of the interaction between rs1805008 and latitude held in subgroup analyses stratified by biological sex (Pmale = 0.036, Pfemale = 0.018). Inconsistencies between read-outs (MSSS and Gd-enhancing lesions) might be due to the known weak correlation between Gd-enhancing lesions and the MSSS (ρ = 0.03, P = 0.33). No interaction between rs1805008:T and vitD levels regarding Gd-enhancing lesions was found (RR = 1.00, 95% CI = [0.968–1.137], P = 0.825).
Fig. 4.
Interaction of MC1R genotype and sun exposure. (A–C) Barplots displaying the fraction of total patients (ntotal = 229) who reported their reactions to sun exposure at noon in summer for the MC1R genotypes (A) rs1805008(C>T), (B) rs885479(G>A), and (C) rs2228479(G>A). This analysis was adjusted for age, sex, BMI, smoking, alcohol consumption, and population stratification. (D) Dotplots for MSSS in relation to latitude with least-squares linear regression line stratified by rs1805008 genotype. (E) Dotplots for Gd-enhancing lesions in relation to latitude (left y axis) complemented with the mean number of lesions (black line, right y axis). Analyses for D and E were adjusted for age, sex, BMI, smoking, alcohol consumption, clinical subtype, neurological site of first manifestation, month of assessment, and center. Adjustment for center was omitted when analyzing the effect of latitude.
The vitD and the Type I IFN Pathway Are Up-Regulated upon UVB Phototherapy in MS Patients.
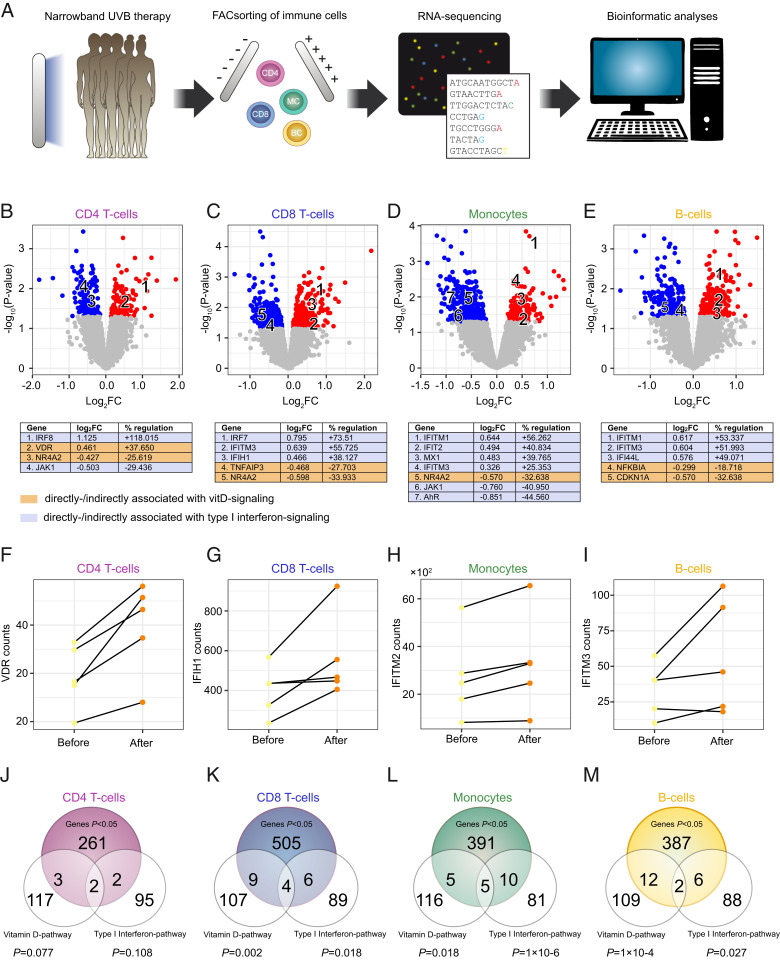
As it is unclear which molecular pathways mediate the beneficial effects of UVR in MS, peripheral blood mononuclear cells (PBMC) samples of five UVB-treated MS patients from our 2014 pilot study (18) were FACsorted for CD4 T cells, CD8 T cells, monocytes, B cells, and subjected to RNA-sequencing (RNA-seq) analysis (Fig. 5A). For CD4 T cells there were 268 genes that were regulated with P < 0.05, 524 genes for CD8 T cells, 411 genes for monocytes, and 407 genes for B cells (Datasets S1–S4). Among these were previously described markers of vitD signaling: For example, VDR (CD4 T cells), NR4A2, NR4A3 (CD4, CD8 T cells, and monocytes), and CD14 (monocytes) (Fig. 5 B–E). Furthermore, a first visual inspection suggested an enrichment of genes belonging to the type I IFN-family—including IFITM1, IFITM2, IFITM3, MX1, IRF8, IRF7, IFI44L, IFIT2, and IFIT3—and this was most apparent in monocytes (Fig. 5 B–E). Regulation of exemplary genes for both pathways are plotted in Fig. 5 F–I. A down-regulation of the Aryl-hydrocarbon receptor-associated genes AHR and TIPARP was observed as well. Next, to check whether the vitD and the type I IFN pathway were significantly enriched, permutation tests using reference gene sets from wikipathways were performed. A significant enrichment of vitD-associated genes was found in CD8 T cells (gene ratio [GR] = 0.108, P = 0.002), monocytes (GR = 0.079, P = 0.018), and B cells (GR = 0.114, P = 1 × 10−4) (Dataset S5). Furthermore, type I IFN pathway-associated genes were significantly enriched in CD8 T cells (GR = 0.101, P = 0.018), monocytes (GR = 0.156, P = 1 × 10−6), and B cells (GR = 0.083, P = 0.027) (Fig. 5 J–M and Dataset S6).
Fig. 5.
Transcriptional effects of UVB-phototherapy in immune cells. (A) MS patients had been treated with UVB-phototherapy during the course of our 2014 pilot study (18). PBMC samples of five patients before and 6 wk after phototherapy (10 samples in total) have been used to isolate CD4+ T cells, CD8+ T cells, B cells, and monocytes, that were then subjected to RNA-isolation and RNA-seq with subsequent bioinformatics analyses, including differential expression analysis and gene set enrichment analysis (overrepresentation analysis). (B–E) Labeled, volcano plots for CD4+ T cells, CD8+ T cells, monocytes, and B cells. (F–I) Before and after plots for exemplary genes associated with either the vitD or the type I IFN pathway, regulated by phototherapy in respective cell types. (J–M) Venn diagrams for the overlap between significantly regulated genes in the respective cell types and the vitD and type I IFN gene sets (extracted from wikipathways). P values were calculated from distribution-free permutation tests. The numbers for the reference gene sets refer to the number of genes belonging to the reference gene set and that have detectable expression in the respective cell type.
Discussion
High latitude has been identified as a major risk factor for the development of MS (4). This association is suspected to be mediated by low exposure to UVR and subsequent low vitD levels. Although this is a worldwide phenomenon, there are exceptions to the rule. An unexpectedly high MS prevalence has been described for the island of Sardinia, despite high exposure to beneficial environmental factors like UVR, but also a seafood-rich diet. This exemplifies the importance of the genetic background, especially in historically rather isolated populations. Even though environmental factors are assumed to contribute more to overall MS risk than genetics, certain genetic compositions seem to be able to invert this balance (3, 38). Notably, populations like the Maori, Amerindian, and Inuit have been described to have a low MS risk, despite living in high-risk areas, which is of relevance for this study as in these populations hyporesponsive MC1R variants are rare (39).
Besides the risk to develop MS, it is still a topic of debate whether UVR, vitD, or other UVR-dependent pathways, have an influence on disease severity and worsening over time, as well. Most work has focused on vitD and only few studies investigated alternative UVR-mediated pathways, although several candidate molecules have been proposed to mediate UVR-effects (e.g., nitric oxide, cis-urocanic acid, and tryptophan metabolites) (11, 40, 41). In the present study, data of two large, independent multicenter cohorts was used to test the association between latitude and vitD with MS disease severity. In this regard, modification of UVR-dependent effects by medication and photosensitivity-associated MC1R missense alleles was investigated as well. To explore by which mechanisms UVR could exert immunomodulation in MS, next-generation sequencing was performed to assess the UVB phototherapy-induced changes in immune cells in a small group of patients.
In line with previous studies, we observed lower disease severity and a lower tendency toward disease worsening in patients with higher vitD levels in the NationMS cohort (4). Cutoffs for immunomodulation by vitD have been discussed previously in the post hoc analyses of the BENEFIT trial, suggesting ≥50 nmol/mL (equal to 20 ng/mL) to sensibly discriminate patients with low or high risk for disease activity and progression (20). Furthermore, results from BEYOND indicate that for optimal immunomodulation an even higher serum vitD status (≥100 nmol/mL) might be necessary (31). In line with these observations, our study indicates a linear relationship with no natural cutoff. Furthermore, confounding of our results by genetic predisposition is unlikely, as two large studies showed no interaction between the effects of UVR-/vitD and HLA-DRB1*15:01, a major genetic risk factor for MS (31, 42).
Expectedly, vitD levels were also strongly associated with latitude. Therefore, latitude itself is a sound proxy for sun exposure on a population level, especially if adjusted for the sampling month as well. Consistently, latitude also showed a significant association with disease severity and worsening in the NationMS cohort. Confirming this, substituting a center’s latitude by satellite-derived UVR-estimates and rerunning the analyses revealed similar associations. Although this cannot directly prove a causal effect of vitD, the intercorrelative network between latitude, vitD (two measures of sun exposure on conceptually different levels), and disability is strongly suggestive of positive effects of UVR in established MS. In the BIONAT cohort vitD was also associated with reduced disability. Interestingly, the effect of latitude was only observed in patients who were not treated with IFN-β before. Since interactions between IFN-β treatment and vitD have been shown before and it is known that IFN-β modulates vitD synthesis (28–30), it is possible that the normal association between latitude and vitD is altered by IFN-β treatment, which is also in line with the observation that patients with IFN-β therapy had significantly higher vitD levels compared to treatment-naïve patients, and vitD levels seemed not to be different across northern and southern latitudes for IFN-β–treated patients. However, previous studies analyzing the interaction between UVR/vitD and IFN-β have provided ambiguous results; for example, Løken-Amsrud et al. (30) report that after initiation of IFN-β the association between disease activity and vitD was lost, whereas data from Ascherio et al. (20) suggest that even in IFN-β–treated patients the effect of vitD persists. Although the reasons for this discrepancy cannot be fully explained by our data, it supports the previous notion that there is a strong relationship between vitD and IFN-β signaling.
To uncover the UVR-induced transcriptomic changes in immune cells in MS patients, the effect of UVB phototherapy was assessed using next-generation sequencing. Due to the exploratory nature of this approach, the limited sample size and the high false-discovery rate burden, genes with P < 0.05 were regarded as regulated. The regulation of genes such as NR4A2, NR4A3, CD14, and the gene for the vitD receptor (VDR), which have been reported multiple times to indicate a vitD response (43, 44), underpins the integrity of the approach and is in line with the profound vitD increase upon phototherapy that was previously demonstrated in these patients (18). The up-regulation of VDR can be interpreted as an intrinsic sensitivity analysis and demonstrates that despite the limited number of samples, biologically meaningful results could be obtained. As vitD counteracts parathyroid hormone (PTH) expression (45), it is likely that the down-regulation of the NR4A family is due to a reduction of PTH, as PTH has been shown to increase NR4A expression (46). This is also in line with the observed general enrichment of vitD-associated genes in permutation tests.
Moreover, we identified regulation of the type I IFN pathway by phototherapy. As an up-regulation of type I IFN-associated genes was observed in all donors of whom only two received IFN-β therapy, this relationship cannot be explained by IFN-β therapy, but instead is likely to be a direct result of phototherapy. Type I IFN induction was most apparent in monocytes, the natural producers of type I IFNs in the context of pathogen control (47). A UVR-dependent induction of type I IFNs could provide an alternative explanation why the effect of latitude on disability was absent in IFN-β–treated patients. If part of the effect of UVR is mediated directly through type I IFNs, it is possible that IFN-β treatment could mask the effects of UVR/latitude. Of note, there is no known association between latitude and prevalence of neuromyelitis optica, an MS-related disease that worsens upon IFN-β therapy (48). In the present analysis, the type I IFN genes found to be regulated have previously been identified to be regulated upon IFN-β therapy in RRMS patients who were classified as IFN-β therapy responders (49, 50). The down-regulation of AHR and TIPARP, known counter-actors of type I IFN-signaling, further supports a direct up-regulation of type I IFNs through phototherapy (51). Mechanistically, it is possible that the type I IFN expression is modulated by vitD, as interactions between vitD and IFN-α/β have been described previously (28, 29, 52). This is also in line with reports showing that vitD and IFN-β–triggered gene expression partly overlaps (53).
Moreover, studies have proposed that UVR could also induce type I IFNs via nucleic acid-damage and induction of damage-associated molecular patterns that can be sensed by Toll-like receptors and the stimulator of IFN genes (54, 55). Interestingly, type I IFNs were also shown to be induced in the skin of healthy human individuals upon UVR exposure, which is in lines with the results from this study (56). If the type I IFN pathway was indeed regulated by UVR, this could provide a link between MS and lupus. In direct contrast to MS, in lupus, an autoimmune disease that can manifest locally (e.g., cutaneous lupus erythematosus) or systemically (systemic lupus erythematosus), exposure to UVR is known to be disease-worsening (57). Furthermore, lupus is known to be partly driven by type I IFNs and patients often show an increased type I IFN blood signature (58). Blockade of the type I IFN pathway has been demonstrated to reduce disease severity in systemic lupus erythematosus (59). In contrast, MS is associated with a reduced type I IFN signature and patients respond positively to IFN-β therapy (28).
Furthermore, although sun exposure seems to be beneficial for MS patients, it is also important to consider individual patient characteristics, such as skin type and photosensitivity, and we therefore also investigated modulatory effects of photosensitivity-associated MC1R variants. In line with the concept of weak and strong MC1R variant alleles (32), only the high-penetrance variant rs1805008(C>T) showed a significant effect on self-reported skin reaction to sun exposure, whereas no association was found for rs885479(G>A) and rs2228479(G>A). The inverted effect of latitude on the number of Gd-enhancing lesions in carriers and noncarriers of the rs1805008 variant is in line with the observation of increased photosensitivity and a higher grade of inflammation induced through sun exposure, combined with the reduced responsiveness to antiinflammatory stimuli of α-melanocyte–stimulating hormone in carriers of MC1R variant alleles (60, 61). The result should not be confounded by the major MS risk allele HLA-DRB1*15:01, since rs1805008 has been shown not to be in linkage disequilibrium with rs3135005, a nearly perfect proxy for HLA-DRB1*15:01 (62). The fact that this inverted association was not present across read-outs could be due to the fact that there was no evidence for correlation between the MSSS and the number of Gd-enhancing lesions. Weak or missing correlation between MRI activity and disability has been described previously, especially for early disease forms (63). Furthermore, as the effects of MC1R in this study were only nominally significant, the results must be interpreted with care and require confirmation in larger cohorts.
Limitations of the study were that no information regarding recent holidays abroad (e.g., in areas with very high or very low sun exposure), no information regarding individual clothing behavior, and no information regarding the use of sunscreen was available. However, as there was a strong correlation between sampling de-seasonalized serum vitD and latitude in both cohorts, no major bias seems to have been introduced by these factors.
In summary, this study provides evidence for an effect of UVR on severity and disease worsening in established MS. Although excessive sun exposure is not recommended due the risk of skin cancer, this argues for beneficial effects of moderate sun exposure in MS. Mechanistically, vitD is supported as one of the main mediators of UVR-effects, but a role for the type I IFN and the MC1R pathway is brought up, as well. Differential effects of UVR in carriers and noncarriers of MC1R variants were observed, suggesting detrimental effects of UVR in sun-sensitive patients. Previous therapy with IFN-β reduced the effect of latitude on severity and also seemed to nullify the association between latitude and vitD levels. This suggests that the effect of latitude is at least in part mediated through vitD. This is also complemented by the up-regulation of the vitD and the type I IFN pathway in immune cells of UVB-treated MS patients, which suggests involvement of both pathways in the mediation of UVR-effects.
Methods
Patients.
Data from the NationMS cohort, a prospective multicenter observational study, were used. In total, clinical data of 946 CIS/RRMS patients recruited between 2010 and 2017 in 21 centers in Germany, were obtained (64). Patients were followed-up and longitudinal data are available for 798 patients (Fig. 1). Included were patients with age ≥ 18 y, who were diagnosed with either CIS with fulfillment of three Barkhof criteria or confirmed RRMS according to revised 2005 McDonald criteria. All patients were treatment-naïve at baseline with onset of symptoms no longer than 2 y before inclusion. The study was approved by the lead ethics committee (Ethik-Kommission der Ruhr-Universität Bochum, registration no. 3714-10) and all local committees. Informed written consent was obtained from all participants and the study was performed according to the Declaration of Helsinki. For replication, data of 990 patients from the BIONAT cohort (Study identifier: NCT00942214), a French, multicenter cohort, at their baseline assessment, were acquired (65). BIONAT patients were not treatment-naïve before inclusion. Study investigators for NationMS and BIONAT can be found in SI Appendix. For RNA-seq, PBMC of five patients from our 2014 pilot study were used (18).
Genotyping and Estimation of Population Stratification.
Genotyping and QC wer performed as previously published (66, 67). Briefly, genotyping was performed using an Illumina OmniExpress chip and QC was performed in PLINK v1.90. Genotypes for the most common MC1R variants were extracted (rs1805008:C>T, rs885479:G>A, rs2228479:G>A), and population stratification was calculated by multidimensional scaling as previously published (66).
Serum vitD Measurements.
For NationMS, vitD was measured as the serum level of 25(OH)D3. Measurement was performed in NationMS for n = 761 patients with a commercially available kit for liquid chromatography-mass spectrometry (LC-MS/MS) (Recipe) on a Shimadzu 8040 LC–MS/MS instrument coupled to a UHPLC system (Nexera, Shimadzu). For BIONAT, total 25(OH)D2+3 measurements were available for 579 patients. Total vitD has been measured using a commercially available electro-chemiluminescence competitive 25-hydroxyvitamin D binding assay (Cobas, Roche), and quantified on a Roche Cobas 8000 e602 analyzer (Roche Diagnostics). As food-derived vitD2 only contributes little to total serum vitD levels, there are only minimal differences between total vitD and vitD3 serum levels.
Questionnaire on Photosensitivity.
Of the NationMS cohort, 229 patients were asked to report their skins potential reaction to sunlight at noon for 1 h without the use of sunscreen in summer. Possible answers were “tan,” “sunburn with a delayed tan,” “sunburn without tan and without blisters,” and “sunburn without tan and with blisters.” Patients were asked to report skin reddening, a feeling of heat and itching after 12 to 24 h as a sunburn, and liquid-filled skin alterations with a diameter ≥5 mm as blisters. The resulting variable was treated as ordinal.
Statistical Analysis.
Disease severity outcomes were the MSSS and the number of Gd-enhancing lesions (baseline). For longitudinal analyses, the increase/decrease in EDSS 2 y after baseline and the time to relapse were assessed. Explanatory variables were the two measures of UVR-exposure vitD and latitude. For further confirmation, satellite data from NASA’s ozone monitoring instrument-dataset were used to generate coordinate- and time-specific estimates of UVR exposure for each patient (methodology and results are reported in the SI Appendix, Supplementary Methods and Table S3). The MSSS was modeled as a continuous variable and the influence of the UVR-exposure measures was assessed using linear models. In the NationMS cohort these analyses were adjusted for age, sex, body mass index (BMI = weight/height2), smoking (yes/no), alcohol (yes/no), month of assessment (treated as a categorical variable with 12 factor-levels), clinical subtype (CIS or RRMS), and the site/symptom of first disease manifestation (coded as categorical) (SI Appendix, Table S4). A random intercept was used to adjust for center variability, which was omitted when assessing the influence of latitude (latitude is intrinsic to the variability between centers and inclusion would lead to unresolvable multicollinearity). For the BIONAT cohort, these analyses were adjusted for age, sex, month of assessment, site/symptom of first manifestation, and prior medication (other covariates not available for BIONAT). Center variability was treated as in the NationMS cohort. For BIONAT, the interaction of vitD or latitude and prior treatment was tested as well, followed by subgroup analyses to obtain subgroup-specific estimates, as some medications have been shown to modulate vitD synthesis (29).
The number of Gd-enhancing lesions in NationMS was modeled using a zero-inflated negative binomial-model to account for overdispersion. Confounder-adjustment was done as in the linear model. Plots for Gd-enhancing lesions include a left y axis, showing the observed number of lesions, and a right y axis, showing the mean number of lesions over x. The influence of the MC1R genotype on skin reaction to sun exposure was modeled using cumulative link models (ordinal regression) and adjusted for age, sex, BMI, alcohol consumption, smoking, and population stratification (first three multidimensional scaling components). To assess whether MC1R genotype modifies the effect of UVR exposure on severity, an interaction term between MC1R genotype and measures of sun exposure (latitude or vitD) was added to the models for disease severity and adjusted as the prior models for disease severity plus population stratification. To assess the influence of vitD and latitude on the risk for relapses in the NationMS cohort, (mixed effects) Cox-regression was performed and adjusted as the baseline models for disease severity plus initial medication after baseline (therapy was initiated after baseline visit) and for whether medication was changed during the course of the study. Medications were treated as substance groups (e.g., IFN-β containing formulations from different suppliers were grouped as one IFN-β group). The difference between the EDSS 2 y after baseline and the baseline EDSS was calculated and declared as ΔEDSS. The ΔEDSS was modeled using linear and linear mixed models and adjusted as the baseline model plus the type of medication initiated after baseline visit and whether medication was changed during the study. Additionally, the models were adjusted for the number of lesions and the MSSS at baseline to account for differences in baseline severity and disease duration. Model fit was assessed by inspection of scaled quantile residuals (68). Model coefficients, Cis, and P values were reported from fully adjusted models with significance threshold of α = 0.05 using two-sided tests. Genetic analyses of the MC1R genotype that were not of confirmatory nature (i.e., assessing disease severity and not skin reaction) were assessed for genome-wide significance (α = 5 × 10−8).
UVB Phototherapy.
Patient samples from a previous study were used where patients had been irradiated with narrow-band UVB (311 nm). Irradiation had been performed in a chamber for phototherapy equipped with Philips TL-01 lamps (PDT 1200; Waldmann) for 6 wk (five sessions per week). Individual minimal erythema doses were determined and 60% of minimal erythema dose was used as the starting point. Per session, the dose was then increased by 100 mJ/cm2 until either the maximum dose (900 mJ/cm2) was reached or erythema occurred. A detailed description can be found in the original study (18).
RNA-Seq Analysis.
RNA-seq was performed using samples of five patients from our 2014 pilot study (18). PBMC samples were sorted for CD4 T cells, CD8 T cells, B cells, and monocytes. Samples were then prepared for sequencing using reagents from New England Biolabs and sequenced on an Illumina NextSeq. 500 machine. Bioinformatic analysis was done in R using Kallisto and edgeR. Results from Kallisto quantification can be found in Datasets S7–10. Pathway analysis was performed using reference gene sets from wikipathways for permutation tests. A detailed description of the RNA-seq workflow can be found in SI Appendix, SI Supplementary Methods.
Software.
Statistical analyses were conducted in R v3.6.0 using the packages stats, lme4, glmmTMB, ordinal, and DHARMa. To extract and process data from NASA’s ozone monitoring instrument dataset, the package RNetCDF was used. The remaining data were manipulated and plotted using the R packages tidyR, ggplot2, and RColorBrewer. For population stratification estimation, PLINK v1.90b5.2 was used. For analysis of RNA-seq data, Kallisto and edgeR were used. For pathway extraction from wikipathways, the package rWikiPathways was used.
Supplementary Material
Acknowledgments
We thank Nicole Hessler and Prof. Inke R. König (University of Lübeck, Germany) for their statistical contributions; Prof. Markus Herrmann (University of Graz, Austria), Dr. Irene Pusceddu, and Manuela Lucchiari (Bolzano Hospital, Italy) for 25(OH)D3 measurements; Lise Scandella for technical assistance in Toulouse; Petra Kotte, Barbara Meyring, and Jila Abkari for technical assistance in Münster; and the Genomics Core Facility of the medical faculty of Münster University for RNA sequencing.
Footnotes
Competing interest statement: H.W. received honoraria and consultation fees from Bayer Healthcare, Biogen, Fresenius Medical Care, GlaxoSmithKline, GW Pharmaceuticals, Merck Serono, Novartis, Sanofi Genzyme, and Teva Pharma. K.L. is coinventor on patent no. US 2016/0158322 A1 entitled “NDP-MSH for treatment of inflammatory and/or neurodegenerative disorders of the CNS.” A.S. received speaker honoraria and/or travel compensation for activities with Almirall Hermal GmbH, Biogen, Merck, Novartis, Roche and Sanofi Genzyme, none related to this work. R.G. serves on scientific advisory boards for Teva Pharmaceutical Industries Ltd. (Teva), Biogen Idec, Bayer Schering Pharma, and Novartis; has received speaker honoraria from Biogen Idec, Teva, Bayer Schering Pharma, and Novartis; serves as editor for Therapeutic Advances in Neurological Diseases, and is on the editorial boards of Experimental Neurology and the Journal of Neuroimmunology; and receives research support from Teva, Biogen Idec, Bayer Schering Pharma, Genzyme, Merck Serono, and Novartis, none related to this work. L.K. receives honoraria for lecturing and travel expenses for attending meetings from Biogen, Merck, Sanofi Genzyme, Novartis and Roche; her research is funded by the German Ministry for Education and Research (BMBF), Deutsche Forschungsgemeinschaft (DFG), Interdisciplinary Center for Clinical Studies (IZKF) Muenster, Biogen, Merck, and Novartis. M.A.F. received honoraria for consultation and travel expenses from Biogen, Merck KGaA, Novartis, and Roche; his research is funded by the BMBF, DFG, Landesforschungsförderung Hamburg, Gemeinnützige Hertie-Stiftung, and Else Kröner-Fresenius-Stiftung. S.G.M. receives honoraria for lecturing, and travel expenses for attending meetings from Almirall, Amicus Therapeutics Germany, Bayer Health Care, Biogen, Celgene, Diamed, Genzyme, MedDay Pharmaceuticals, Merck Serono, Novartis, Novo Nordisk, ONO Pharma, Roche, Sanofi-Aventis, Chugai Pharma, QuintilesIMS, and Teva; his research is funded by the BMBF, DFG, Else Kröner Fresenius Foundation, German Academic Exchange Service, Hertie Foundation, IZKF Muenster, German Foundation Neurology and Almirall, Amicus Therapeutics Germany, Biogen, Diamed, Fresenius Medical Care, Genzyme, Merck Serono, Novartis, ONO Pharma, Roche, and Teva. R.H. has received grant support from Biogen, Genzyme-Sanofi, Merck Serono, Novartis, Teva, and personal fees from Actelion, Biogen, Genzyme-Sanofi, Medday, Merck Serono, Novartis, Roche, and Teva. T.K. has received travel expenses and personal compensation from Bayer Healthcare, Teva Pharma, Merck, Novartis Pharma, Sanofi-Aventis/Genzyme, Roche, and Biogen, as well as grant support from Bayer Schering AG, Novartis, and Chugai Pharma. U.Z. has received grants from DFG, BMBF, the European Research Council, Bristol Myers Squibb, Janssen Pharmaceuticals NV, Servier, and Biogen Idec, and personal consultation fees from Pfizer GmbH, Bayer Vital GmbH, and CorTec GmbH. F.Z. received funds for scientific consultation or research by DFG, BMBF, the Pharmaceutical Management Science Association, Novartis, Octapharma, Merck Serono, ONO Pharma, Biogen, Genzyme, Celgene, Roche, Sanofi Aventis. A.S.-M. received travel expenses and research support from Novartis. C.C.G. received speaker honoraria and travel expenses for attending meetings from Biogen, Euroimmun, Genzyme, MyLan, Novartis Pharma GmbH, and Bayer Health Care, none related to this study; her work is funded by Biogen, Novartis, the BMBF (01Gl1603A), the DFG (GR3946/3-1, SFB Transregio 128 A09), the European Union (Horizon2020, ReSToRE), the IZKF, and the Innovative Medizinische Forschung. D.B. receives lectures, congress invitation, board participation with Bayer, Biogen, Medday, Merck, Novartis, Roche, Sanofi Genzyme, and Teva. B.T. received personal speaker honoraria and consultancy fees as a speaker and advisor from Alexion, Bayer Healthcare, Biogen, CSL Behring, GILEAD, GRIFOLS, Merck Serono, Novartis, Octapharma, Roche, Sanofi Genzyme, Teva, and UCB Pharma; his university received unrestricted research grants from Biogen-Idec, Novartis, Teva, Bayer Healthcare, CSL-Behring, GRIFOLS, Octapharma, Sanofi Genzyme, and UCB Pharma. F.T.B. has received, through his institution, grant support and/or travel reimbursement to attend scientific meetings from Actelion, Bayer, Biogen, Merck, Novartis, Sanofi Genzyme, and Teva; he has received personal honoraria for speaking or advisory board activities from Actelion, Bayer, Biogen, Merck, Novartis, Roche, and Sanofi Genzyme; his research is funded, in part, by the DFG and the BMBF. C.W. has received institutional support from Novartis, Sanofi Genzyme, Biogen, and Roche. H.-P.H. has received fees for consulting, speaking, and serving on steering committees from Bayer Healthcare, Biogen, GeNeuro, MedImmune, Merck, Novartis, Opexa, Receptos Celgene, Roche, Sanofi Genzyme, CSL Behring, Octapharma, and Teva, with approval by the Rector of Heinrich-Heine University. H.T. received speaker honoraria from Bayer, Biogen, Fresenius, Genzyme, Merck, Novartis, Roche, Siemens, and Teva; he serves as section editor for the Journal of Neurology, Psychiatry, and Brain Research; H.T. and his institution receives research support from the BMBF, DMSG, Fresenius, Genzyme, Merck, and Novartis, none related to this work. A.B. has received personal compensation from Merck, Biogen, Bayer Vital, Novartis, Teva, Roche, Celgene, Alexion, and Sanofi Genzyme, and grants for congress trips and participation from Biogen, Teva, Novartis, Sanofi Genzyme, Celgene, and Merck, none related to this work. C.L. received an endowed professorship supported by the Novartis foundation, has received consulting and speaker’s honoraria from Biogen-Idec, Bayer Schering, Novartis, Sanofi Genzyme, and Teva; and has received research scientific grant support from Merck-Serono and Novartis. B.W. received research grants and/or honoria from Merck Serono, Biogen, Teva, Novartis, Sanofi Genzyme, Bayer Healthcare, Roche, and research grants from the Dietmar Hopp Foundation, the Klaus Tschira Foundation, the BMBF, and the DFG. U.K.Z. received honoraria for lecturing and/or travel expenses for attending meetings from Almirall, Alexion, Bayer, Biogen, Celgene, Merck Serono, Novartis, Octapharma, Roche, Sanofi Genzyme, and Teva, as well as grant support from the BMBF and European Union, none related to this study; his research is funded by the BMBF, German Ministry of Economy, DFG, and European Union. S.B. has received honoria and compensation for travel from Biogen Idec, Merck Serono, Novartis, Sanofi Genzyme, and Roche. M.S. has received honoraria for scientific lectures or consultancy from Alexion, Bayer Healthcare, Biogen, CSL Behring, Grifols, MedDay, Merck-Serono, Novartis, Roche, Sanofi Genzyme, Takeda, and Teva; his institution received research support from Biogen, Sanofi-Genzyme, and Merck-Serono. R.A.L. received personal compensation from Celgene, Bayer Healthcare, Teva Pharma, Merck, Novartis Pharma, Sanofi-Aventis/Genzyme, Roche, and Biogen, as well as grant support from Novartis and Merck. F.W. served on the scientific advisory board of Genzyme, Merck Serono, and Novartis; he has received travel funding and/or speaker honoraria from Bayer, Biogen, Teva, Merck Serono, Novartis, and Pfizer; he is employed by Sana and received research support from the BMBF, Merck Serono, and Novartis. F.P. reported other support from Bayer, Novartis, Biogen-Idec, Teva, Sanofi-Aventis/Genzyme, Merck Serono, Alexion, Chugai, MedImmune, Roche, and Shire outside the submitted work; he has a post as academic editor for PLoS One and is associate editor for Neurology: Neuroimmunology & Neuroinflammation; he has scientific advisory board membership for Novartis and consulting fees from Sanofi Genzyme, Biogen-Idec, MedImmune, Shire, and Alexion; and research support from Bayer, Novartis, Biogen Idec, Teva, Sanofi-Aventis/Genzyme, Alexion, Merck Serono, the German Research Council, Werth Stiftung, German Ministry of Education and Research, Arthur Arnstein Stiftung Berlin, EU FP7 Framework Program, Arthur Arnstein Foundation Berlin, Guthy Jackson Charitable Foundation, and National Multiple Sclerosis of the United States. B.H. has served on scientific advisory boards for Novartis; he has served as data monitoring and safety committee member for AllergyCare and TG therapeutics; he or his institution have received speaker honoraria from Desitin; he holds part of two patents, one for the detection of antibodies against KIR4.1 in a subpopulation of MS patients and one for genetic determinants of neutralizing antibodies to interferon. All conflicts are not relevant to the topic of the study. F.L. has served on the advisory board of Roche and received travel funding from Teva. M.M. received research support from Merck Serono and Novartis as well as travel expenses for attending meetings from Merck Serono. K.L.-H. has received research support (to Technische Universität München) from Novartis, honoraria from Novartis and F. Hoffmann-La Roche, and compensation for travel expenses from Merck Serono.
This article is a PNAS Direct Submission.
3Complete lists of the German Competence Network Multiple Sclerosis (KKNMS) and BIONAT study investigators can be found in SI Appendix.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2018457118/-/DCSupplemental.
Contributor Information
Collaborators: Antonios Bayas, Susanne Rothacher, Stephanie Starke, Friedemann Paul, Judith Bellmann-Strobl, Janina Behrens, Jan-Markus Dörr, Rene Gieß, Joseph Kuchling, Ludwig Rasche, Ralf Gold, Andrew Chan, Gisa Ellrichmann, Anna Lena Fisse, Anna Gahlen, Thomas Grüter, Aiden Haghikia, Robert Hoepner, Ümmügülsün Koc, Carsten Lukas, Jeremias Motte, Kalliopi Pitarokoili, Anke Salmen, Ruth Schneider, Joanna Schöllhammer, Christoph Schroeder, Björn Ambrosius, Seray Demir, Clemens Warnke, Thomas Dehmel, Kathleen Ingenhoven, Ralf Linker, De-Hyung Lee, Alexandra Lämmer, Eva Sauer, Christoph Heesen, Jan-Patrick Stellmann, Martin Stangel, Lena Boenig, Stefan Gingele, Martin Hümmert, Philipp Schwenkenbecher, Thomas Skripuletz, Wolfram Suehs, Brigitte Wildemann, Mirjam Korporal-Kuhnke, Hanna Oßwald, Alexander Schwarz, Andrea Viehöver, Volker Limmroth, Kathrin Gerbershagen, Florian Then Bergh, Barbara Ettrich, Steffi Gray, Sarah Haars, Johannes Orthgieß, Nicole Schwanitz, Muriel Stoppe, Astrid Unterlauft, Sandra Paryjas, Stefan Bittner, Vinzenz Fleischer, Sergiu Groppa, Felix Lüssi, Johannes Piepgras, Timo Uphaus, Björn Tackenberg, Michael Pütz, Christian Eienbröker, Maria Seipelt, Reinhard Hohlfeld, Tania Kümpfel, Joachim Havla, Ingrid Meinl, Hannah Pellkofer, Elisabeth Schuh, Bernhard Hemmer, Lilian Aly, Achim Berthele, Viola Pongratz, Kirsten Brinkhoff, Dorothea Buck, Christiane Gasperi, Mirjam Hermisson, Muna-Miriam Hoshi, Miriam Kaminski, Ana Klein, Benjamin Knier, Markus Kowarik, Helena Kronsbein, Klaus Lehmann Horn, Meike Mitsdörffer, Verena Pernpeintner, Veit Rothhammer, Andrea Schweikert, Rebecca Selter, Mark Mühlau, Claus Zimmer, Jan Kirschke, Frank Weber, Heike Staufer, Matthias Knop, Sandra Nischwitz, Philipp Sämann, Heinz Wiendl, Sven Meuth, Luisa Klotz, Gerd Meyer zu Hörste, Julia Krämer, Lena Schünemann, Catharina Gross, Steffen Pfeuffer, Tobias Ruck, Selma Belgriri, Alexander Buchheister, Nora Bünger, Kerstin Göbel, Lucienne Kirstein, Nico Melzer, Ole Simon, Antje Echterhoff, Uwe Zettl, Alexander Winkelmann, Ulf Ziemann, Ahmed Abdelhak, Markus Kowarik, Markus Krumbholz, Margarete Paech, Christoph Ruschil, Maria-Ioanna Stefanou, Johannes Tünnerhoff, Lena Zeltner, Hajera Sheikh, Hayrettin Tumani, Tanja Fangerau, Florian Lauda, Daniela Rau, Daniela Taranu, André Huss, David Brassat, Béatrice Pignolet, Florence Bucciarelli, Lise Scandella, Christine Lebrun-Frenay, Marc Debouverie, Sophie Pittion-Vouyovitch, Bruno Brochet, Aurelie Ruet, Gilles Defer, Nathalie Derache, Jérôme de Sèze, David Laplaud, Sandrine Wiertlewski, Oliver Casez, Pierre Clavelou, Pierre Labauge, Jean Pelletier, Audrey Rico, Sandra Vukusic, Olivier Outteryck, Jean-Claude, Jean-Christophe Ouallet, Patrick Hautecoeur, Ayman Tourbah, Giovanni Castelnovo, Eric Berger, Hélène Zéphir, Philippe Cabre, William Camu, Eric Thouvenot, Thibault Moreau, Agnès Fromont, Caroline Papeix, Catherine Lubetzki, Patrick Vermersch, Mikael Cohen, and Lucien Rumbach
Data Availability.
Data and R code is available from the authors upon reasonable request. Full results from RNA-seq can be found in Datasets S1–S10.
References
- 1.Reich D. S., Lucchinetti C. F., Calabresi P. A., Multiple sclerosis. N. Engl. J. Med. 378, 169–180 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sospedra M., Martin R., Immunology of multiple sclerosis. Annu. Rev. Immunol. 23, 683–747 (2005). [DOI] [PubMed] [Google Scholar]
- 3.Olsson T., Barcellos L. F., Alfredsson L., Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat. Rev. Neurol. 13, 25–36 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Smolders J., Torkildsen Ø., Camu W., Holmøy T., An update on vitamin D and disease activity in multiple sclerosis. CNS Drugs 33, 1187–1199 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bikle D., Christakos S., New aspects of vitamin D metabolism and action—Addressing the skin as source and target. Nat. Rev. Endocrinol. 16, 234–252 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Lehmann U., et al. , Bioavailability of vitamin D(2) and D(3) in healthy volunteers, a randomized placebo-controlled trial. J. Clin. Endocrinol. Metab. 98, 4339–4345 (2013). [DOI] [PubMed] [Google Scholar]
- 7.DeLuca H. F., Plum L., UVB radiation, vitamin D and multiple sclerosis. Photochem. Photobiol. Sci. 16, 411–415 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Mirzaei F., et al. , Gestational vitamin D and the risk of multiple sclerosis in offspring. Ann. Neurol. 70, 30–40 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mokry L. E., et al. , Vitamin D and risk of multiple sclerosis: A Mendelian randomization study. PLoS Med. 12, e1001866 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rhead B., et al. , Mendelian randomization shows a causal effect of low vitamin D on multiple sclerosis risk. Neurol. Genet. 2, e97 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernard J. J., Gallo R. L., Krutmann J., Photoimmunology: How ultraviolet radiation affects the immune system. Nat. Rev. Immunol. 19, 688–701 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Langer-Gould A., et al. , MS sunshine study: Sun exposure but not vitamin D is associated with multiple sclerosis risk in Blacks and Hispanics. Nutrients 10, 268 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hedström A. K., Olsson T., Kockum I., Hillert J., Alfredsson L., Low sun exposure increases multiple sclerosis risk both directly and indirectly. J. Neurol. 267, 1045–1052 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trend S., et al. , Short-term changes in frequencies of circulating leukocytes associated with narrowband UVB phototherapy in people with clinically isolated syndrome. Sci. Rep. 9, 7980 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hart P. H., et al. , A randomised, controlled clinical trial of narrowband UVB phototherapy for clinically isolated syndrome: The PhoCIS study. Mult. Scler. J. Exp. Transl. Clin. 4, 2055217318773112 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hauser S. L., et al. , Prevention of experimental allergic encephalomyelitis (EAE) in the SJL/J mouse by whole body ultraviolet irradiation. J. Immunol. 132, 1276–1281 (1984). [PubMed] [Google Scholar]
- 17.Becklund B. R., Severson K. S., Vang S. V., DeLuca H. F., UV radiation suppresses experimental autoimmune encephalomyelitis independent of vitamin D production. Proc. Natl. Acad. Sci. U.S.A. 107, 6418–6423 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Breuer J., et al. , Ultraviolet B light attenuates the systemic immune response in central nervous system autoimmunity. Ann. Neurol. 75, 739–758 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Irving A. A., Marling S. J., Seeman J., Plum L. A., DeLuca H. F., UV light suppression of EAE (a mouse model of multiple sclerosis) is independent of vitamin D and its receptor. Proc. Natl. Acad. Sci. U.S.A. 116, 22552–22555 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ascherio A., et al. , Vitamin D as an early predictor of multiple sclerosis activity and progression. JAMA Neurol. 71, 306–314 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cortese M.et al.; BENEFIT Study Group , Vitamin D, smoking, EBV, and long-term cognitive performance in MS: 11-year follow-up of BENEFIT. Neurology 94, e1950–e1960 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Camu W., et al. , Cholecalciferol in relapsing-remitting MS: A randomized clinical trial (CHOLINE). Neurol. Neuroimmunol. Neuroinflamm. 6, e597 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smolders J., et al. , Vitamin D3 supplementation and neurofilament light chain in multiple sclerosis. Acta Neurol. Scand. 141, 77–80 (2020). [DOI] [PubMed] [Google Scholar]
- 24.Hupperts R.et al.; SOLAR Study Group , Randomized trial of daily high-dose vitamin D3 in patients with RRMS receiving subcutaneous interferon β-1a. Neurology 93, e1906–e1916 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alla S., et al. , An investigation of the relationship between latitude and multiple sclerosis severity in New Zealand. Mult. Scler. 22, 705–707 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Nakamura Y.et al.; Japan Multiple Sclerosis Genetics Consortium , Latitude and HLA-DRB1*04:05 independently influence disease severity in Japanese multiple sclerosis: A cross-sectional study. J. Neuroinflammation 13, 239 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morra V. B., et al. , Interferon-beta treatment decreases cholesterol plasma levels in multiple sclerosis patients. Neurology 62, 829–830 (2004). [DOI] [PubMed] [Google Scholar]
- 28.Feng X., et al. , Vitamin D enhances responses to interferon-beta in MS. Neurol. Neuroimmunol. Neuroinflamm. 6, e622 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stewart N., et al. , Interferon-β and serum 25-hydroxyvitamin D interact to modulate relapse risk in MS. Neurology 79, 254–260 (2012). [DOI] [PubMed] [Google Scholar]
- 30.Løken-Amsrud K. I., et al. , Vitamin D and disease activity in multiple sclerosis before and during interferon-β treatment. Neurology 79, 267–273 (2012). [DOI] [PubMed] [Google Scholar]
- 31.Fitzgerald K. C., et al. , Association of vitamin D levels with multiple sclerosis activity and progression in patients receiving interferon beta-1b. JAMA Neurol. 72, 1458–1465 (2015). [DOI] [PubMed] [Google Scholar]
- 32.Morgan M. D., et al. , Genome-wide study of hair colour in UK Biobank explains most of the SNP heritability. Nat. Commun. 9, 5271 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.García-Borrón J. C., Abdel-Malek Z., Jiménez-Cervantes C., MC1R, the cAMP pathway, and the response to solar UV: Extending the horizon beyond pigmentation. Pigment Cell Melanoma Res. 27, 699–720 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mykicki N., et al. , Melanocortin-1 receptor activation is neuroprotective in mouse models of neuroinflammatory disease. Sci. Transl. Med. 8, 362ra146 (2016). [DOI] [PubMed] [Google Scholar]
- 35.Arnason B. G., Berkovich R., Catania A., Lisak R. P., Zaidi M., Mechanisms of action of adrenocorticotropic hormone and other melanocortins relevant to the clinical management of patients with multiple sclerosis. Mult. Scler. 19, 130–136 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Priemel M., et al. , Bone mineralization defects and vitamin D deficiency: Histomorphometric analysis of iliac crest bone biopsies and circulating 25-hydroxyvitamin D in 675 patients. J. Bone Miner. Res. 25, 305–312 (2010). [DOI] [PubMed] [Google Scholar]
- 37.Gómez-Alonso C., et al. , Vitamin D status and secondary hyperparathyroidism: The importance of 25-hydroxyvitamin D cut-off levels. Kidney Int. Suppl., S44–S48 (2003). [DOI] [PubMed] [Google Scholar]
- 38.Urru S. A., Antonelli A., Sechi G. M., , MS Working Group , Prevalence of multiple sclerosis in Sardinia: A systematic cross-sectional multi-source survey. Mult. Scler. 26, 372–380 (2020). [DOI] [PubMed] [Google Scholar]
- 39.Friedman A. P., Do hyporesponsive genetic variants of the melanocortin 1 receptor contribute to the etiology of multiple sclerosis? Med. Hypotheses 62, 49–52 (2004). [DOI] [PubMed] [Google Scholar]
- 40.Hart P. H., Gorman S., Finlay-Jones J. J., Modulation of the immune system by UV radiation: More than just the effects of vitamin D? Nat. Rev. Immunol. 11, 584–596 (2011). [DOI] [PubMed] [Google Scholar]
- 41.Correale J., Farez M. F., Modulation of multiple sclerosis by sunlight exposure: Role of cis-urocanic acid. J. Neuroimmunol. 261, 134–140 (2013). [DOI] [PubMed] [Google Scholar]
- 42.Bäärnhielm M., et al. , Sunlight is associated with decreased multiple sclerosis risk: No interaction with human leukocyte antigen-DRB1*15. Eur. J. Neurol. 19, 955–962 (2012). [DOI] [PubMed] [Google Scholar]
- 43.Hossein-nezhad A., Spira A., Holick M. F., Influence of vitamin D status and vitamin D3 supplementation on genome wide expression of white blood cells: A randomized double-blind clinical trial. PLoS One 8, e58725 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vukić M., et al. , Relevance of vitamin D receptor target genes for monitoring the vitamin D responsiveness of primary human cells. PLoS One 10, e0124339 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mendes M. M., Hart K. H., Lanham-New S. A., Botelho P. B., Suppression of parathyroid hormone as a proxy for optimal vitamin D status: Further analysis of two parallel studies in opposite latitudes. Nutrients 12, 942 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tetradis S., Bezouglaia O., Tsingotjidou A., Parathyroid hormone induces expression of the nuclear orphan receptor Nurr1 in bone cells. Endocrinology 142, 663–670 (2001). [DOI] [PubMed] [Google Scholar]
- 47.Barbalat R., Lau L., Locksley R. M., Barton G. M., Toll-like receptor 2 on inflammatory monocytes induces type I interferon in response to viral but not bacterial ligands. Nat. Immunol. 10, 1200–1207 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mori M., Kuwabara S., Paul F., Worldwide prevalence of neuromyelitis optica spectrum disorders. J. Neurol. Neurosurg. Psychiatry 89, 555–556 (2018). [DOI] [PubMed] [Google Scholar]
- 49.Gurevich M., et al. , Transcriptional response to interferon beta-1a treatment in patients with secondary progressive multiple sclerosis. BMC Neurol. 15, 240 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Comabella M., et al. , A type I interferon signature in monocytes is associated with poor response to interferon-beta in multiple sclerosis. Brain 132, 3353–3365 (2009). [DOI] [PubMed] [Google Scholar]
- 51.Yamada T., et al. , Constitutive aryl hydrocarbon receptor signaling constrains type I interferon-mediated antiviral innate defense. Nat. Immunol. 17, 687–694 (2016). [DOI] [PubMed] [Google Scholar]
- 52.Lange C. M., et al. , Vitamin D receptor and Jak-STAT signaling crosstalk results in calcitriol-mediated increase of hepatocellular response to IFN-α. J. Immunol. 192, 6037–6044 (2014). [DOI] [PubMed] [Google Scholar]
- 53.Munger K. L., et al. , Molecular mechanism underlying the impact of vitamin D on disease activity of MS. Ann. Clin. Transl. Neurol. 1, 605–617 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gehrke N., et al. , Oxidative damage of DNA confers resistance to cytosolic nuclease TREX1 degradation and potentiates STING-dependent immune sensing. Immunity 39, 482–495 (2013). [DOI] [PubMed] [Google Scholar]
- 55.Kemp M. G., Lindsey-Boltz L. A., Sancar A., UV light potentiates STING (stimulator of interferon genes)-dependent innate immune signaling through deregulation of ULK1 (Unc51-like kinase 1). J. Biol. Chem. 290, 12184–12194 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Skopelja-Gardner S., et al. , The early local and systemic type I interferon responses to ultraviolet B light exposure are cGAS dependent. Sci. Rep. 10, 7908 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reefman E., Kuiper H., Limburg P. C., Kallenberg C. G., Bijl M., Type I interferons are involved in the development of ultraviolet B-induced inflammatory skin lesions in systemic lupus erythaematosus patients. Ann. Rheum. Dis. 67, 11–18 (2008). [DOI] [PubMed] [Google Scholar]
- 58.Barrat F. J., Crow M. K., Ivashkiv L. B., Interferon target-gene expression and epigenomic signatures in health and disease. Nat. Immunol. 20, 1574–1583 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morand E. F.et al.; TULIP-2 Trial Investigators , Trial of anifrolumab in active systemic lupus erythematosus. N. Engl. J. Med. 382, 211–221 (2020). [DOI] [PubMed] [Google Scholar]
- 60.Healy E., et al. , Melanocortin-1-receptor gene and sun sensitivity in individuals without red hair. Lancet 355, 1072–1073 (2000). [DOI] [PubMed] [Google Scholar]
- 61.Barnetson R. S., et al. , [Nle(4)-D-Phe(7)]-alpha-melanocyte stimulating hormone, significantly increased pigmentation and decreased UV damage in fair-skinned Caucasian volunteers. J. Invest. Dermatol. 127, 2680 (2007). [DOI] [PubMed] [Google Scholar]
- 62.Dwyer T., et al. , Melanocortin 1 receptor genotype, past environmental sun exposure, and risk of multiple sclerosis. Neurology 71, 583–589 (2008). [DOI] [PubMed] [Google Scholar]
- 63.Kappos L.et al.; Gadolinium MRI Meta-analysis Group , Predictive value of gadolinium-enhanced magnetic resonance imaging for relapse rate and changes in disability or impairment in multiple sclerosis: A meta-analysis. Lancet 353, 964–969 (1999). [DOI] [PubMed] [Google Scholar]
- 64.von Bismarck O., et al. , Treatment choices and neuropsychological symptoms of a large cohort of early MS. Neurol. Neuroimmunol. Neuroinflamm. 5, e446 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Outteryck O.et al.; BIONAT Network; CFSEP , A prospective observational post-marketing study of natalizumab-treated multiple sclerosis patients: Clinical, radiological and biological features and adverse events. The BIONAT cohort. Eur. J. Neurol. 21, 40–48 (2014). [DOI] [PubMed] [Google Scholar]
- 66.Andlauer T. F., et al. , Novel multiple sclerosis susceptibility loci implicated in epigenetic regulation. Sci. Adv. 2, e1501678 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dankowski T.et al.; German Competence Network for Multiple Sclerosis (KKNMS) , Successful replication of GWAS hits for multiple sclerosis in 10,000 Germans using the exome array. Genet. Epidemiol. 39, 601–608 (2015). [DOI] [PubMed] [Google Scholar]
- 68.Dunn P. K., Smyth G. K., Randomized quantile residuals. J. Comput. Graph. Stat. 5, 1–10 (1996). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and R code is available from the authors upon reasonable request. Full results from RNA-seq can be found in Datasets S1–S10.