Significance
Infection/inflammation is a major contributor to male infertility, and macrophages are likely to be key players in both pathological progression and resolution of the inflammation. We report that macrophage populations in the epididymis and testis are derived from fetal and neonatal monocytes, which are self-maintaining during adulthood. Furthermore, during inflammation, circulating monocytes recruited to the epididymis and testis give rise to inflammatory macrophages that promote tissue damage. These data are significant for our understanding of the origins and maintenance of macrophage subpopulations in both organs; our results point to a fundamental mechanism underpinning male infertility by infection and inflammation, and pave the way for the development of innovative therapeutics to treat this important and common condition.
Keywords: testis, epididymis, macrophgaes, ontogeny, monocytes
Abstract
Macrophages are the principal immune cells of the epididymis and testis, but their origins, heterogeneity, development, and maintenance are not well understood. Here, we describe distinct populations of epididymal and testicular macrophages that display an organ-specific cellular identity. Combining in vivo fate-mapping, chimeric and parabiotic mouse models with in-depth cellular analyses, we found that CD64hiMHCIIlo and CD64loMHCIIhi macrophage populations of epididymis and testis arise sequentially from yolk sac erythro-myeloid progenitors, embryonic hematopoiesis, and nascent neonatal monocytes. While monocytes were the major developmental source of both epididymal and testicular macrophages, both populations self-maintain in the steady-state independent of bone marrow hematopoietic precursors. However, after radiation-induced macrophage ablation or during infection, bone marrow-derived circulating monocytes are recruited to the epididymis and testis, giving rise to inflammatory macrophages that promote tissue damage. These results define the layered ontogeny, maintenance and inflammatory response of macrophage populations in the male reproductive organs.
Macrophages are important effector cells of the innate immune system and play a critical role in host defense. They also contribute to tissue homeostasis, aid tissue repair, and promote the resolution of inflammation. In order to perform these diverse roles, macrophages are composed of different populations with distinct origins, which can change their phenotype and functions in response to the tissue microenvironment or upon encountering inflammatory cues (1, 2).
Tissue-resident macrophages arise from three distinct waves of embryonic hematopoiesis and maintain themselves by local self-renewal (3, 4). In mice, the first wave of embryonic hematopoiesis, termed “primitive hematopoiesis,” gives rise to primitive yolk sac (YS) macrophages (5). A second wave of YS hematopoiesis follows, which generates late erythro-myeloid progenitors (EMPs) that then migrate and colonize the fetal liver (FL). There they continue to generate myeloid progenitors, including FL monocytes (MOs), which contribute to most tissue macrophage populations, except microglia (6, 7). The third wave of hematopoiesis starts in the para-aortic splanchnopleural/aorta-gonad-mesonephros region, giving rise to immature hematopoietic stem cells (HSCs), which then migrate, colonize, and differentiate in the FL to establish definitive hematopoiesis (8, 9). Immediately before birth, hematopoiesis switches from the FL to the bone marrow (BM), and after 1 wk of age BM monocytes (BM-MOs) are generated and circulate in the blood, ready for recruitment into tissues in response to inflammatory cues (9). In some organs, such as the intestine, pancreas, and dermis, BM-MOs can contribute to the pool of tissue macrophages in the steady state/under inflammation and replace those derived during embryonic development (10–13), however it is not known whether this occurs in all organs or is restricted to specific situations.
Among the male reproductive organs, the epididymis and testis are unique immunological sites where macrophages represent the most prevalent immune cell population (14–16). Recent studies indicate that testicular macrophages have a phenotype and functional profile consistent with a role in maintaining immune tolerance/privilege in the testis (17, 18), but very little is known of the phenotype or function of epididymal macrophages. What we do know is that infection, inflammation, and autoimmunity in the male reproductive tract are common health issues, and also underlie around 15% of all cases of male infertility (19). So far, the possible role of macrophages in these conditions has received little attention. A recent report identified two macrophage populations in adult mouse testes: Interstitial macrophages (CD64hiMHCIIlo) and peritubular macrophages (CD64loMHCIIhi) (16). The CD64hiMHCIIlo population was found to originate from embryonic progenitors, whereas CD64loMHCIIhi cells appeared only postnatally in the testis and were thought to arise from BM-derived cells (16). However, the relative contributions of the embryonic progenitors to the adult testes-resident macrophages, and how testicular macrophages maintain themselves in adulthood are not yet defined. Furthermore, the origin and maintenance of epididymal macrophages in adulthood is completely lacking.
Here, we define the cellular identity, development, and maintenance of macrophage populations that reside in adult mouse testis and epididymis. Using immunophenotyping, fate-mapping analyses, long-term parabiosis, and BM transplantation, we observed that steady-state epididymis and testis contain two self-maintaining adult MO-independent macrophage populations: CD64hiMHCIIlo and CD64loMHCIIhi, both arising either embryonically from fetal MOs or immediately after birth from neonatal nascent blood MOs. However, when the macrophage niche was emptied by irradiation, engrafted BM-MOs differentiated into tissue-resident macrophages in both organs. During bacterial infection, the recruitment of BM-MOs was also associated with tissue damage. We provide an alternative view to the current paradigm that testicular CD64loMHCIIhi macrophages arise from blood monocytes in the steady state, showing instead that fetal/neonatal monocytes give rise to these populations which then self maintain during adulthood.
Results
Adult Epididymal and Testicular Macrophages Express a Distinct Phenotype and Transcriptional Signature.
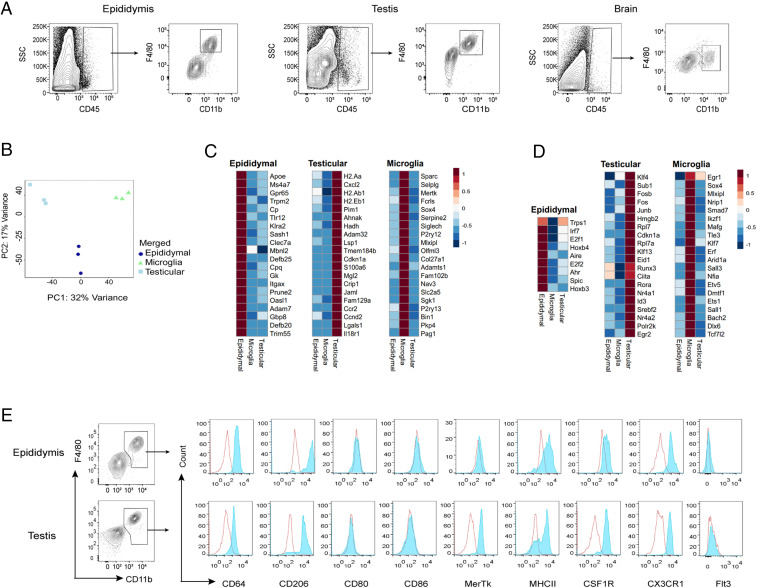
We first characterized the macrophage populations in the epididymides and testes of 9-wk-old male mice. Macrophages were readily identified by flow cytometry of live CD45+ cells after gating-out Ly6C+ MOs and CD11c+ dendritic cells (SI Appendix, Fig. S1). A distinct single population of macrophages (F4/80+CD11b+) was present in both the epididymides and testes, as is seen in the murine brain (Fig. 1A). Among the CD45+ immune cells, macrophages represented the largest population in both organs (SI Appendix, Fig. S2 A and B), and were also significantly more abundant in the epididymis than the testis (SI Appendix, Fig. S2C). Besides macrophages, we also detected dendritic cells (F4/80−CD11c+MHCII+) and MOs (F4/80−Ly6C+ and MHCII+) (SI Appendix, Fig. S2 A and B). Next, to confirm that these macrophages were bone fide tissue-resident cells and not intravascular leukocytes from within the tissues, we intravenously injected FITC-conjugated anti-CD45 antibodies just before killing the mice to label all of the blood leukocytes (20) (SI Appendix, Fig. S3A). Epididymal and testicular macrophages did not label with CD45-FITC, and thus were not intravascular leukocytes; in contrast, around 10% of Ly6c high MOs from within the epididymides and testes were labeled, pointing to a vascular origin (SI Appendix, Fig. S3).
Fig. 1.
Adult epididymal and testicular macrophages display a distinct phenotype and transcriptional signature. (A) Single-cell suspensions from epididymis, testis, and brain were analyzed by flow cytometry in 9- to 10-wk-old adult WT male mice. The full gating strategy is described in SI Appendix, Fig. S1A. Representative plots are shown. Data were pooled from at least three different experiments, each utilizing two mice. (B) Principal component (PC) analysis of RNA-seq data from macrophages isolated by FACS from epididymis, testis, and brain. (C) Heat map and hierarchical clustering of the top 20 uniquely expressed genes of epididymal, testicular, and brain macrophages. (D) Heat map and hierarchical clustering of differentially expressed transcription factor genes in macrophages isolated from epididymis, testis, and brain. For each individual experiment, testicular and epididymal macrophages were pooled from seven mice, respectively, whereas brain microglia were obtained from one mouse. Data were obtained from three independent experiments. (E) Expression of cell surface markers was measured by flow cytometry of cells from epididymis, and testis. Representative histograms show the expression of CD64, CD206, CD80, CD86, MerTK, MHCII, CSF1R, CX3CR1, and FLT3 within the F4/80+ CD11b+ population. The shaded blue line denotes the specific antibody and the open red line denotes the isotype control (n = 4).
We next asked whether macrophages from the epididymis and testis display a unique gene-expression profile, as do microglia (21). We performed RNA-sequencing (RNA-seq) whole-genome expression profiling coupled with principal-component analysis of the differentially expressed genes (DEGs), which revealed distinct clustering of macrophages according to their organ of origin (Fig. 1B). At the gene-expression level, remarkably, epididymal macrophages were as different from testicular macrophages as from the microglia of the brain, exhibiting 290 and 400 DEGs, respectively, whereas 834 genes were differentially expressed between testicular macrophages and microglia (SI Appendix, Fig. S4 and Dataset S1). The epididymis expresses high levels of defensins as antimicrobial peptides (22), and accordingly we saw high expression of Def20 and Defb25 in these cells (Fig. 1C). Moreover, the epididymis contains an acidic luminal environment that is required to maintain spermatozoa in a quiescent and immotile state before ejaculation (23). Gpr65, which is highly expressed in epididymal macrophages (Fig. 1C), can sense pH by protonation of histidines on its extracellular domain; downstream signaling then suppresses proinflammatory cytokine production in macrophages and T cells and so reduces inflammation (24). Testicular macrophages expressed significantly higher levels of immunosuppressive genes, namely Mrc1, Dab2, Igf1, Lgals1, and Lgals3, as well as genes encoding transcription factors, such as Klf4, Rora, Nr4a2, Nr4a1, and Egr2 (Fig. 1 C and D). This corroborates previous findings that testicular macrophages display an immunosuppressive phenotype that maintains the immune privilege of the testes (17, 18). Thus, the pattern of DEGs we observed highlights links between the known biological features of the epididymis and testis and the macrophages that reside there.
Next, we investigated whether epididymal and testicular macrophages express common macrophage markers or have unique expression signatures. By flow cytometry, we found that both macrophage populations abundantly express CD64 (FcγR1), CX3CR1, and the mannose receptor CD206, while neither population expresses the costimulatory molecules CD80 or CD86 (Fig. 1E). In contrast to testicular macrophages, the expression of the tyrosine kinase receptor MerTK and colony stimulating factor-1 receptor (CSF1R) was low/heterogeneous in epididymal macrophages. We also detected heterogeneous expression of MHC class II within the macrophage populations of the epididymis and testis, suggesting the presence of MHCII+ and MHCII− subpopulations, as previously published (16), and in common with other resident macrophage populations (2). Of note, surface expression of FLT3, a classic marker of dendritic cells, was barely detected in either epididymal or testicular macrophages, confirming that these macrophage populations were not contaminated with dendritic cells (Fig. 1E). Taken together, these data suggest that epididymal and testicular macrophages share core markers with tissue macrophages of other organs, while concomitantly expressing a unique set of genes consistent with their organ-specific functions.
Epididymal and Testicular Macrophages Originate from Definitive Hematopoiesis.
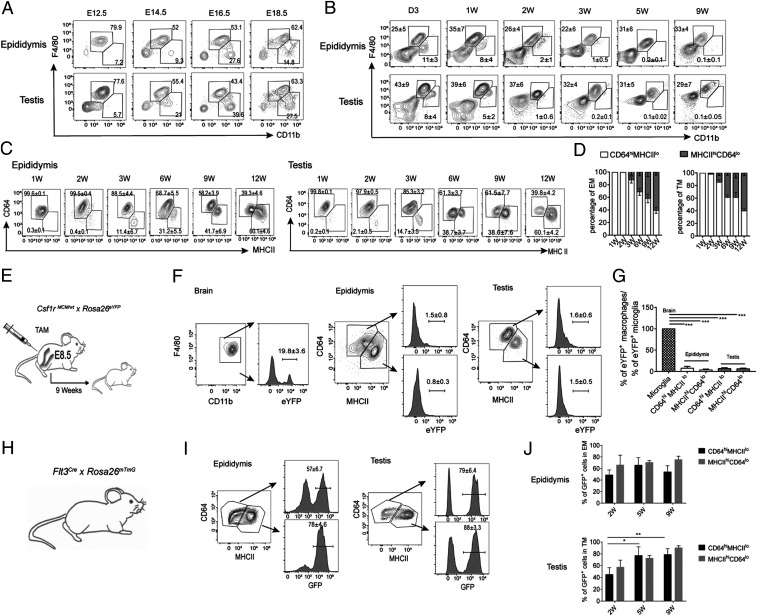
Most tissue-resident macrophages, such as microglia, Langerhans cells (LC), liver Kupffer cells (KCs), cardiac, and alveolar macrophages (AMs), derive from embryonic precursors that seed the respective organ before birth and maintain themselves locally throughout life (25). To understand the likely contribution of embryonic precursors to the testicular and epididymal macrophage populations, we monitored the emergence and numbers of F4/80+CD11b+ macrophages from embryonic day (E)12.5 until 2 mo of age in mice. At E12.5, we found that the majority of the macrophage populations are F4/80hiCD11blo, reminiscent of Myb-independent embryonic macrophages described previously (26), and suggesting their descent from YS hematopoiesis (Fig. 2A and SI Appendix, Fig. S5). From E14.5, an influx of F4/80loCD11bhi cells occurred in both mouse epididymis and testis, appearing at a similar time as MOs in other organs (26) (Fig. 2A). On postnatal day 3, epididymal and testicular macrophage populations comprised F4/80hiCD11blo macrophages and a F4/80loCD11bhi myeloid population; however, the number of F4/80loCD11bhi cells diminished shortly after birth, and by 3 wk of age epididymal and testicular macrophages were entirely F4/80hiCD11blo cells (Fig. 2B).
Fig. 2.
Epididymal and testicular macrophages originate from definitive hematopoiesis and are maintained in adulthood. Epididymides and testes were harvested before birth (A) (E12.5 to E18.5) and after birth (B) (D3 to 9 wk of age) from C57BL/6J WT mice for flow cytometric analyses. Contour plots show labeling for F4/80 and CD11b in embryonic and postnatal epididymis and testis. Embryonic data were obtained from three pregnant females for each time point with three to five embryos per animal that were pooled postbirth data were obtained from three to six mice. (C) F4/80+CD11b+ cells from epididymis and testis of C57BL/6J WT mice were analyzed for CD64 and MHCII expression from 1 to 12 wk of age. Representative flow cytometry plots indicate the percentage of CD64hiMHCIIlo and CD64loMHCIIhi populations in F4/80+ CD11b+ macrophages from each tissue. (D) Relative proportions of CD64hi and MHCIIhi macrophages within total F4/80+CD11b+ epididymal macrophage (EM) and testicular macrophage (TM) populations at different postnatal ages as indicated. Data were pooled from two different experiments with three to five mice at each time point (mean ± SD). (E) Csf1rMCM/WT × Rosa26eYFP pregnant dams were injected with TAM at E8.5 to label the CSF1R+ YS macrophages in embryos. (F) Progeny of dams in E were killed at 9 wk of age to determine the frequency of labeled CSF1R+ cells within the macrophage populations of the brain, epididymis, and testis. (G) Relative proportions of eYFP-expressing CSF1R+ macrophage populations in epididymis and testes normalized to the frequency of CSF1R expression in brain microglia (19.8 ± 3.6, n = 4; one-way ANOVA, ***P < 0.001). (H and I) The frequency of FLT3+ (tdTom−GFP+) cells in epididymal and testicular macrophages analyzed by flow cytometry in 9 wk old Flt3cre × Rosa-mTmG mice. Representative data from one of seven mice are presented. (J) Percentage of FLT3+ (tdTom−GFP+) cells within CD64hiMHCIIlo and CD64loMHCIIhi testicular and epididymal macrophage populations at 2 to 9 wk of age (mean ± SD, number of mice at 2 wk = 5, at 5 wk = 7, and at 9 wk = 7; two way ANOVA, *P < 0.05, **P < 0.01).
Adult testis contains two subpopulations of macrophages: CD64hiMHCIIlo and CD64loMHCIIhi, with different ontogeny (16). Given our finding of heterogeneous MHCII expression also in epididymal macrophages, we next sought to characterize the subpopulations in this tissue. We first monitored the relative frequencies of CD64hiMHCIIlo and CD64loMHCIIhi-positive cells within the F4/80+CD11b+ population at an interval between 1 and 12 wk of age. At 1 wk of age, both epididymis and testis contain a single distinct population of CD64hiMHCIIlo macrophages, suggesting an embryonic origin (Fig. 2 C and D). From the second week onward, a population of CD64loMHCIIhi cells appears, which progressively increases until it represents the majority of the cells at 12 wk of age, whereupon the proportion of the CD64loMHCIIhi population exceeds that of the CD64hiMHCIIlo population in both organs (Fig. 2 C and D). The appearance of distinct subpopulations of macrophages at different stages of development could suggest a layered ontogeny, with CD64hiMHCIIlo cells arising embryonically and CD64loMHCIIhi either being generated from prenatal MOs or adult hematopoiesis, or by conversion of CD64hiMHCIIlo macrophages.
To determine the relative contribution of YS macrophages to the adult testicular and epididymal pools, we took advantage of a fate-mapping system in which expression of a tamoxifen (TAM)-inducible Cre recombinase (MerCreMer) occurs under the control of the Csf1r promoter (Csf1rMCM) in YS EMPs (26). We crossed Csf1rMCM/wt mice with a Rosa26eYFP reporter mouse that expresses enhanced yellow fluorescent protein (eYFP). Cre-mediated recombination in the F1 progeny was induced by a single i.p. injection of 4-hydroxytamoxifen (OH-TAM) into pregnant female mice at E8.5 (Fig. 2E). TAM injection induced irreversible expression of eYFP in YS-derived macrophage progenitors and their derivatives, but not in HSCs: In Csf1rMCM/wtxRosa26eYFP mice ∼20% of brain microglia, acting as positive controls, were eYFP+ at 9 wk of age (Fig. 2F). In contrast, less than 5% of CD64hiMHCIIlo and CD64loMHCIIhi macrophage populations in both epididymis and testis expressed eYFP at the same age (Fig. 2F), with no significant difference in labeling between the two subsets in either tissue (Fig. 2G). After normalization to the labeling efficiency in microglia, we saw that YS EMPs contribute a relatively small number of cells to the macrophage populations in testis and epididymis (Fig. 2G).
We therefore asked whether embryonic or adult HSCs might rather be the main source of epididymal and testicular macrophages. We used Flt3Cre mice, which efficiently track fetal and adult HSC progenitors and their progeny (27), crossed with Rosa26mTmG reporter mice (Fig. 2H), and measured the frequency of Flt3Cre+ cells in the epididymis and testis between 2 and 9 wk of age. We saw robust labeling of both subsets of testicular and epididymal macrophages from 2 wk, and by 9 wk around 50 to 60% of CD64hiMHCIIlo and around 80% of CD64loMHCIIhi epididymal macrophages expressed GFP (Fig. 2 I and J); similarly, in the testis, around 75 to 85% of CD64hiMHCIIlo and 85 to 90% of CD64loMHCIIhi macrophages expressed GFP at this time (Fig. 2 I and J). Thus, definitive hematopoiesis plays a significant role in the development of the epididymal and testicular macrophage pool, and this contribution remains stable into early adulthood (5 vs. 9 wk), indicating an embryonic or neonatal MO contribution.
Epididymal and Testicular Macrophages Are Maintained Independently of Blood MOs and Adult Hematopoiesis.
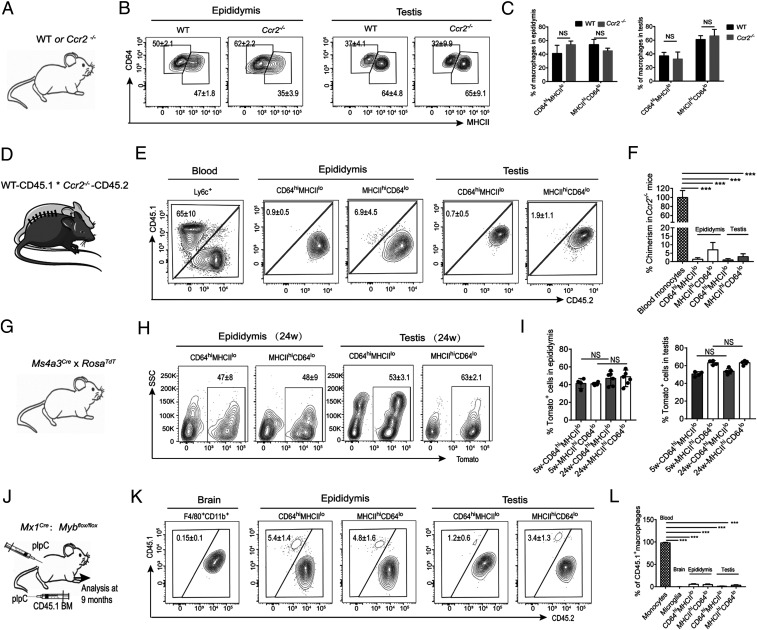
Although the above data show that both subpopulations of testicular and epididymal macrophages by majority arise from early (embryonic or neonatal) HSCs, it was possible that this contribution was replaced over time by blood MOs. Therefore, we compared the macrophage populations in these tissues under steady-state conditions in WT and chemokine receptor 2 (Ccr2−/−) -deficient mice, which lack peripheral blood MOs due to defective egress of Ly6Chi MOs from the BM (28) (Fig. 3A). The numbers of CD64hiMHCIIlo and CD64loMHCIIhi macrophage populations in both organs were similar in WT and Ccr2−/− mice (Fig. 3 B and C). Conversely, intestinal macrophages, which largely originate from blood MOs, were significantly reduced in the Ccr2−/− mice (12, 28) (SI Appendix, Fig. S6). To further confirm this finding, we established parabiosis between CD45.1+ WT mice and CD45.2+ Ccr2−/− mice for 6 mo and then asked whether CD45.1+ monocyte-derived cells were present within the Ccr2−/− mouse testicular and epididymal macrophage populations (Fig. 3D). In blood, ∼65% of Ly6Chi MOs were of donor origin, indicating efficient establishment of the chimeras, and in accordance with previous findings (29) (Fig. 3E). In both epididymis and testis, only around 1% of CD64hiMHCIIlo macrophages were of CD45.1 origin within the CD45.2+ Ccr2−/− mice. In contrast 5 to 7% of CD64loMHCIIhi in epididymis and 2% in testis were of CD45.1 origin in the CD45.2+ Ccr2−/− mice (Fig. 3 E and F). This indicates that replacement by blood MOs plays only a minor role in the maintenance/turnover of the resident epididymal and testicular macrophage populations.
Fig. 3.
Epididymal and testicular macrophages are maintained independently of blood MOs and adult hematopoiesis. (A and B) Percentage of CD64hiMHCIIlo and CD64loMHCIIhi macrophages in F4/80+CD11b+ cells of epididymis and testis in 9-wk-old WT and Ccr2−/− mice. (C) Percentage of CD64hiMHCIIlo and CD64loMHCIIhi macrophage populations in epididymis and testis of 9-wk-old WT and Ccr2−/− mice. Data from three to five individual mice (Welch’s t test, mean ± SD). (D) Parabiotic mice were generated by surgically connecting the blood circulatory systems of C57BL/6J CD45.1 and Ccr2−/− CD45.2 mice for 6 mo. (E) Chimerism in monocytes from blood and CD64hiMHCIIlo and CD64loMHCIIhi macrophage populations from epididymis and testis were analyzed by flow cytometry. Plots are representative of six parabionts. (F) Mean percent chimerism in Ccr2−/− mouse MOs and CD64hiMHCIIlo and CD64loMHCIIhi macrophage populations from epididymis and testis. Histogram represents the summary of all of the parabionts. The one-way ANOVA was employed for statistical analysis. ***P < 0.001. (G–I) Five- and 24-wk-old Ms4a3Cre-RosaTdT mice were analyzed for the percentage of Ms4a3Cre/TdT+ cells in epididymal and testicular CD64hiMHCIIlo and CD64loMHCIIhi macrophages. Representative data from one of four mice are presented (one-way ANOVA; mean ± SD). (I) Mean percentage of dtTomato+ cells in in CD64hiMHCIIlo and CD64loMHCIIhi epididymal and testicular macrophages of Ms4a3Cre-RosaTdT mice (n = 4). (J–L) Six- to 8-wk-old male Cd45.2;Mx1-Cre;Mybf/f mice were treated with poly (I:C) (pIpC) to induce the deletion of Myb-floxed alleles. Mice were infused with 107 BM cells from CD45.1 mice and the host mice were examined after 9 mo for donor (CD45.1) and host (CD45.2) origins of macrophage populations in the brain, testis, and epididymis. Expression of CD45.1 and CD45.2 was examined in live CD64hiMHCIIlo and CD64loMHCIIhi-positive cells. (L) Mean percentage of CD45.1 macrophages in blood MOs and macrophages of brain, epididymis, and testis (one-way ANOVA; mean ± SD; n = 4, *P < 0.05, **P < 0.01, ***P < 0.001, NS: not significant).
To confirm and extend this finding, we employed Ms4a3Cre-RosaTdT reporter mice that effectively label MO-derived macrophages with tdTomato, but not lymphocytes or dendritic cells (13) (Fig. 3G). At 24 wk of age ∼40 to 55% of both CD64hiMHCIIlo and CD64loMHCIIhi epididymal macrophages, respectively, were labeled with tdTomato (Fig. 3H). Similarly, we observed a high frequency of tdTomato labeling in the testicular CD64hiMHCIIlo (around 50 to 55%) and CD64loMHCIIhi (around 60 to 65%) macrophage populations (Fig. 3H). In agreement with the above data, labeling of tdTomato+ cells did not increase with progressing age, indicating that, similar to the FLT3-Cre data, ∼50 to 80% of epididymal and testicular macrophages are derived from MOs (HSCs) early in development, but there is minimal contribution of blood MOs to these macrophage populations in adult life (Fig. 3 H and I).
The transcription factor Myb is essential for definitive hematopoiesis (30, 31). To determine whether BM HSCs contribute to adult testicular and epididymal macrophages, we treated Cd45.2;Mx1-Cre;Mybf/f mice with poly (I:C) to induce the deletion of Myb-floxed alleles, then reconstituted them with BM cells from CD45.1 mice (Fig. 3J). By conditionally deleting the Myb gene, we could ablate HSCs and their progeny, enabling transplantation of donor HSCs without irradiation or other preconditioning (32). After 9 mo, we measured the proportions of donor (CD45.1) and host (CD45.2) cells within the macrophage populations of the brain, testis, and epididymis (Fig. 3K). All blood MO were of donor CD45.1 origin after 9 mo, indicative of complete BM chimerism (Fig. 3L). In contrast, and consistent with our results in Ccr2−/− and parabiotic mice, the relative contribution of donor-derived CD45.1+ cells to the host epididymis, testis, and brain (as control) was minimal (Fig. 3 K and L). Taking these data together, we find our multipronged analysis clearly shows that epididymal and testicular macrophages are long-lived and that neither circulatory MOs nor adult definitive HSCs contribute significantly to their pool in adult life.
Next, we investigated whether the lack of replenishment of the macrophage population by MO-derived macrophages in the epididymis and testis was balanced by in situ proliferation or alternatively a long life span of the resident macrophages. We measured bromodeoxyuridine (BrdU) incorporation to determine the proliferation rate of epididymal and testicular macrophages under steady-state conditions. This showed significant turnover of both the testicular and epididymal macrophage populations at 1 and 2 wk of age, which reduced to almost zero from 4 wk onward (SI Appendix, Fig. S7). Taken together with the above data, this suggests that the embryonically or neonatally seeded monocytic cells are proliferating rapidly to fill the niche, then stop when it’s full and do not proliferate again in the steady state except for a minimal level of self-maintenance, and that replacement by circulatory MOs is dispensable for the maintenance of both populations.
Following Irradiation BM Cells Repopulate the Empty Niche of Epididymal and Testicular Macrophages.
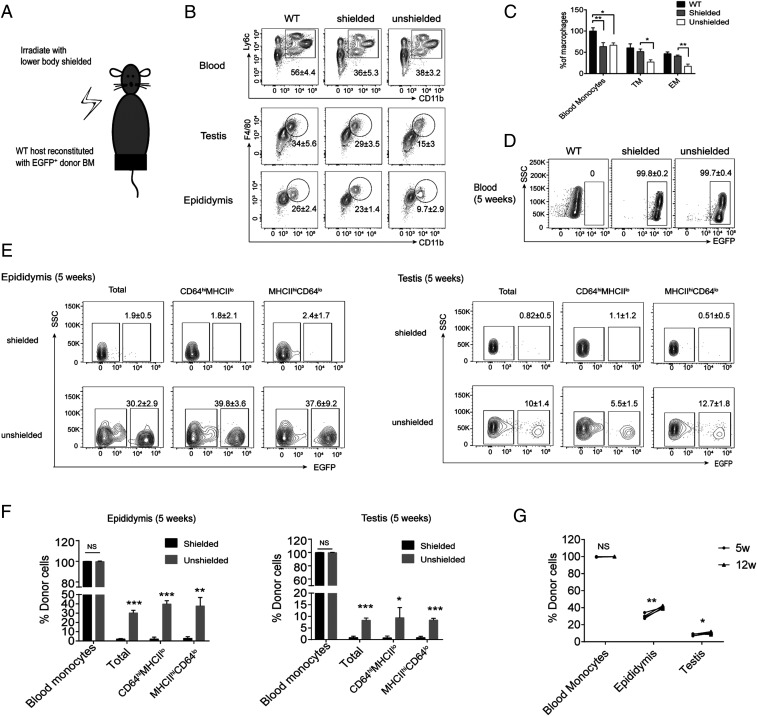
To determine whether BM-derived cells could repopulate an ablated macrophage niche in the epididymis and testis, we lethally irradiated WT mice with or without protecting the inguinal scrotal region, then adoptively transferred EGFP+ donor BM cells and analyzed their subsequent frequency in the epididymis and testis (Fig. 4A). One day after irradiation, we confirmed the partial ablation of epididymal and testicular macrophages and the efficacy of shielding (Fig. 4 B and C). Five and 12 wk after BM transplantation, all BM-derived cells in blood expressed EGFP, confirming complete chimerism (Fig. 4D). In the epididymides and testes of shielded mice there was a negligible frequency of EFGP+ cells in either the CD64hiMHCIIlo or CD64loMHCIIhi populations; however, in unshielded irradiated mice, EFGP+ cell numbers increased significantly in CD64hiMHCIIlo and CD64loMHCIIhi macrophages over the time period investigated (Fig. 4 E–G). Of note, ablation efficiency of both epididymal and testicular macrophages was the same (SI Appendix, Fig. S8 A–C), but epididymal macrophage replenishment by BM cells occurred at a substantially higher rate than that of testicular macrophages (SI Appendix, Fig. S8D), highlighting a potential difference in the ability of the two tissues to be repopulated by MO-derived cells. Together, these data show that, as in other organs (33, 34), BM-derived cells can repopulate both the macrophage populations of the epididymis and testis when the niche is empty, albeit perhaps to differing extents, but not in the steady state when the niche is full.
Fig. 4.
Following irradiation, BM cells repopulate the empty niche of epididymal and testicular macrophages. (A) Six-week-old C57BL/6J mice were lethally irradiated with or without the inguinal/scrotal region shielded, before EGFP+ BM cells were injected into the tail vein. (B and C) After 1 d, efficiency of blood MO and epididymal and testicular macrophage ablation was determined by flow cytometry (two-way ANOVA; mean ± SD; n = 5). (D and E) After 5 wk the frequency of EGFP+ cells in blood MOs and in macrophage populations of epididymis and testis was measured by flow cytometry. (F) Mean percentage of EGFP+ cells in blood MOs, and in CD64hiMHCIIlo and CD64loMHCIIhi testicular and epididymal macrophage populations at 5 wk after irradiation. (Welch’s t test; mean ± SD; n = 5). (G) At 5 and 12 wk after irradiation, the frequency of EGFP+ cells of donor were determined by flow cytometry in blood MOs, and in macrophage populations of epididymis and testis (Welch’s t test; mean ± SD; n = 5, *P < 0.05, **P < 0.01, ***P < 0.001, NS: not significant).
CSF-1 Is Required for Epididymal and Testicular Macrophage Survival.
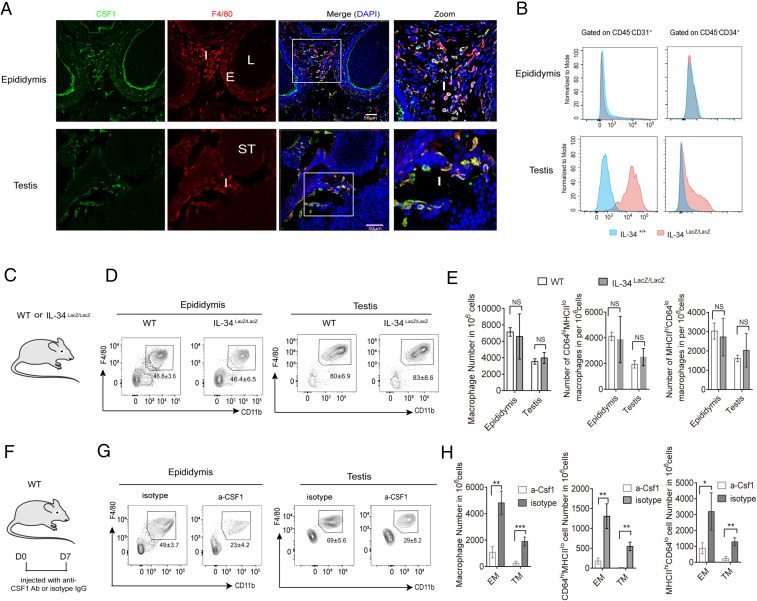
The resident macrophage populations of all organs in adult mice are controlled by CSF1R, and its cognate ligands colony stimulating factor-1 (CSF-1) and interleukin-34 (IL-34) (35, 36). However, the role of CSF-1 and IL-34 in the maintenance of both CD64hiMHCIIlo and CD64loMHCIIhi macrophages in epididymis and testis is not known. We found that CSF-1 was expressed exclusively in the interstitial space where it colocalized with F4/80+ cells in the testis; while in the cauda epididymidis, CSF-1 was expressed in the interstitial space as well as on the apical part of the epithelial cells bordering the lumen (Fig. 5A). Of note, the colocalization pattern of CSF-1 and F4/80 was similar in all of the regions (i.e., caput, corpus, and cauda epididymidis). As there is no commercially available anti–IL-34 antibody suitable for immunofluorescence microscopy, we performed FACS-Gal flow cytometry to measure the expression of IL-34 in the epididymis and the testis of adult Il34LacZ/+ reporter mice and WT control mice (Fig. 5B). We did not observe any IL-34 expression in the epididymis, while in the testis IL-34 was expressed in CD45– cells, mostly CD31+ and CD34+ cells (Fig. 5B). As we detected IL-34 synthesis in testis but not in epididymis, it was possible that testicular macrophages require both IL-34 and CSF-1 for survival, whereas epididymal macrophages depend solely on CSF-1 for survival. Therefore, we asked whether either population was affected in mice deficient in IL-34 (IL34LacZ/LacZ) (37) (Fig. 5C) and found that they were not (Fig. 5 D and E). We next blocked CSF1R signaling in mice using an anti-CSF1 antibody, as this exclusively bind CSF-1 and block interaction to CSF1R, without influencing the interaction of IL-34 with CSF1R. In contrast to IL-34–deficient mice, blocking of CSF1R signaling with an anti–CSF-1 antibody, significantly reduced the number of F4/80+CD11b+ macrophages in both tissues (Fig. 5F) and in both the CD64hiMHCIIlo and the CD64loMHCIIhi populations in each tissue (Fig. 5 G and H). Thus CSF-1 but not IL-34 is required for the survival of epididymal and testicular macrophages.
Fig. 5.
CSF-1 is essential for the survival of testicular and epididymal macrophages. (A) Confocal microscopy image of sections of cauda epididymidis and testis of WT C57BL6J mice stained with DAPI and labeled with anti-F4/80 and anti–CSF-1 (E, epithelium; I, interstitium; L, lumen; ST, seminiferous tubules) (magnification, 20× objective). (B) Histograms show expression of IL-34 in CD45−CD31+ and CD45−34+ cells from epididymis and testis of WT and IL-34LacZ/+mice (n = 3 to 5). (C and D) Flow cytometry density plots of the F4/80+CD11b+ macrophage populations in epididymis and testis of Il34LacZ/LacZ and WT mice (n = 5). (E) Average number of CD64hiMHCIIlo and CD64loMHCIIhi macrophage populations of epididymis and testis in Il34LacZ/LacZ and Il34+/+ mice (Welch’s t test; mean ± SD; n = 5). (F) WT C57BL/6J mice were injected with anti–CSF-mAb or isotype control. (G) Seven days after the first injection, the frequency of F4/80+CD11b+ macrophage populations in the epididymis and testis was measured by flow cytometry. (H) The number of CD64hiMHCIIlo and CD64loMHCIIhi macrophage populations of epididymis and testis in anti–CSF-mAb or isotype control injected mice. Welch’s t test. was employed for statistical analysis. (mean ± SD; n = 5) *P < 0.05, **P < 0.01, ***P < 0.001, NS: not significant.
Bacterial Infection Drives Proliferation of Resident Testicular and Epididymal Macrophage Populations and Recruitment of Blood MOs.
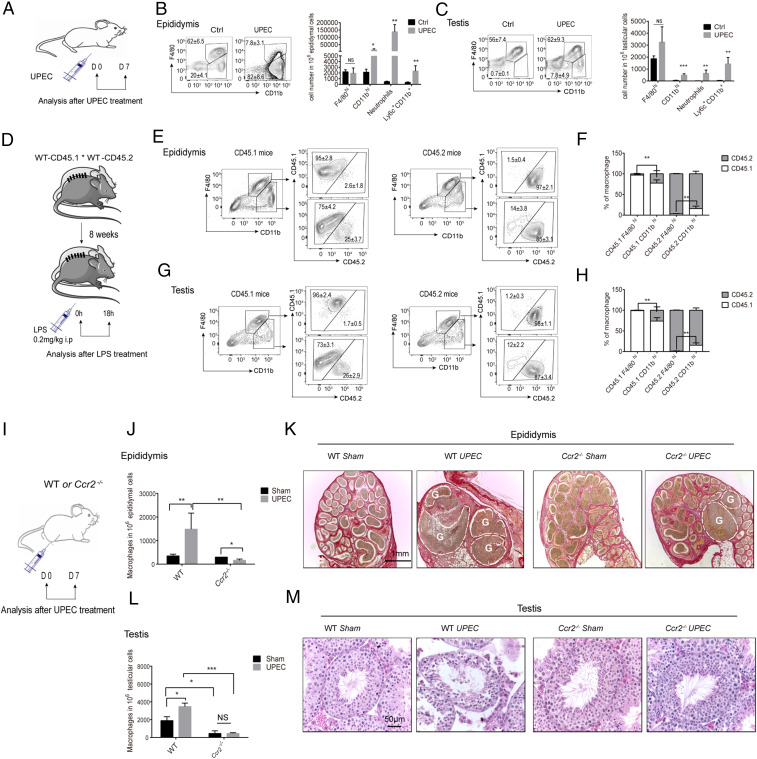
In males, bacteria may travel through the urethra to the epididymides and testes, causing epididymitis or combined epididymo-orchitis (38, 39). Uropathogenic Escherichia coli (UPEC) is a gram-negative bacterium that commonly infects the male genito-urinary tract and is often the main pathogen found in urine samples of patients diagnosed with epididymitis or combined epididymo-orchitis (40). To examine the inflammatory effects of UPEC on the epididymal and testicular immune compartments, we injected bacteria into both vasa deferentia of mice, close to the epididymides, and monitored their effects on the cell populations and tissues by flow cytometry 1 wk later (Fig. 6A). UPEC infection increased the number of inflammatory MOs (Ly6c+CD11b+F4/80− up 6.6-fold in testis, 33.7-fold in epididymis), neutrophils (Ly6G+, 286.6 fold in testis, 34.7-fold in epididymis), and inflammatory macrophages (Ly6c+MHCII+F4/80lo CD11bhi, 6.5-fold in testis, 10.3-fold in epididymis) (Fig. 6 B and C). Numbers increased significantly more in the epididymis, which is reminiscent of the MO influx data in the above-mentioned irradiation model (SI Appendix, Fig. S9). Similarly, the frequencies of inflammatory macrophages, inflammatory MOs (testis: 9.1-fold; epididymis: 11.3-fold), and neutrophils (testis: 8.7-fold; epididymis: 10.2-fold) were increased as observed 18 h after intraperitoneal injection of lipopolysaccharide (LPS, a gram-negative bacterial cell wall component) (SI Appendix, Fig. S10).
Fig. 6.
Bacterial infection drives proliferation of resident testicular and epididymal macrophage populations and recruitment of blood MOs. (A) WT C57BL/6J mice were injected with saline or UPEC. Seven days postinfection, macrophage and immune cell populations were measured in (B) epididymis and (C) testis by flow cytometry. Data are representative of at least two independent experiments. Mean numbers of F4/80hiCD11blo resident macrophages, F4/80loCD11bhi MO-derived macrophages, Ly6hiCD11bhi MOs, and Ly6G+ neutrophils are presented (Welch’s t test; mean ± SD; saline control n = 6; LPS n = 6). (D) WT CD45.1 and CD45.2 parabionts were established for 8 wk before injection of LPS. After 18 h the chimerism of F4/80hiCD11blo and F4/80loCD11bhi macrophages was measured in (E) epididymis and (G) testis, with mean data from all parabionts shown in F and H. (Welch’s t test, mean ± SD, saline control = 5, LPS = 5). Flow cytometry plots are representative of one of five parabionts. (I) WT and Ccr2−/− mice were injected with UPEC or saline (sham) and after 7 d the number of macrophages in (J) epididymis and (L) testis were counted. Data were pooled from two different experiments (two-way ANOVA; saline control = 4, UPEC Ccr2−/− = 4. ***P < 0.001, **P < 0.01, *P < 0.01. Day 7 tissue sections from UPEC-infected or saline-injected WT and Ccr2−/− mice were stained with (K) Sirius red (cauda epididymidis [left 2 images], cauda and distal part of corpus epididymidis [right 2 images]) and (M) hematoxylin and eosin (testis) (magnification, 20× objective). In K, dashed line and G = granuloma.
During inflammation the macrophage population at the affected site swells, both by rapid recruitment of blood MOs and by proliferation of resident macrophages (41, 42). To understand whether blood MOs contribute to the pool of testicular and epididymal macrophages under inflammatory conditions, we established parabiotic CD45.1/CD45.2 mice, then exposed them to LPS and measured the proportion of donor MO-derived cells in the host reproductive tissues (Fig. 6D). While again the chimerism was low in resident macrophages (F4/80hiCD11blo) of both testis and epididymis, it was high in the infiltrating macrophage populations (F4/80loCD11bhi) (Fig. 6 E–H). This indicates that blood MOs give rise to the inflammatory macrophage populations. To further confirm these data, we also compared macrophage numbers in WT and Ccr2-deficient mice after UPEC infection (Fig. 6I). Under infectious conditions, we saw that numbers of the F4/80loCD11bhi macrophage populations were reduced in the epididymis and testis of Ccr2−/− mice (Fig. 6 J and L).
We also asked whether UPEC infection affected resident macrophage proliferation by administrating mice BrdU in their drinking water for 7 d from the initiation of the infection. In the testis, only resident macrophages were positive for BrdU, while in the epididymis both resident and inflammatory macrophages were labeled (SI Appendix, Fig. S11). Taken together, our results indicate that both testicular and epididymal macrophage numbers increase during inflammation through blood MO ingress and local proliferation.
Leukocytic infiltration is a hallmark of epididymo-orchitis (38, 43), but the possible role of macrophage populations in the pathology of this condition is unknown. To determine the effect of inflammatory MOs and macrophages on tissue damage and disease progression, we examined testicular and epididymal integrity in UPEC-infected WT and Ccr2−/− mice. In WT mice we observed in the sham animals that the morphology of the cauda epididymidis was normal, with visible cross-sections of multiple epididymal ducts, intact epithelium, and the lumen filled with spermatozoa. However, in UPEC-infected epididymis, the major histological alterations were restricted mainly to the cauda epididymidis with epithelial exfoliation, infiltration of immune cells and interstitial fibrosis, the presence of abscesses and granuloma all leading to epididymal duct narrowing/obstruction which is particularly evident in UPEC infected WT mice (Fig. 6K). In contrast, epididymal damage was much less pronounced in UPEC-infected Ccr2−/−-deficient mice, which was associated with a reduced influx of inflammatory Ly6chi MOs and macrophages. Similarly, focal impairment of spermatogenesis induced by UPEC infection in WT mice was ameliorated in Ccr2−/−-deficient mice (Fig. 6M). In accordance with previous studies, we did not observe gross morphological alterations in the corpus or caput epididymidis of UPEC-infected mice (38).
Discussion
Here we show that epididymal and testicular macrophages express unique sets of signature genes and transcription factors, respectively, which are distinct to those of other tissue macrophages investigated, such as microglia. The survival and maintenance of macrophages from both reproductive organs is controlled by CSF-1. Our multipronged lineage-tracing and fate-mapping analysis showed that the epididymal and testicular macrophage subsets, CD64hiMHCIIlo and CD64loMHCIIhi, arise in distinct waves from the YS, embryonic and early hematopoiesis, and are self-maintained in adult life with minimal contribution from circulatory MOs. However, when their respective niche is ablated, BM-MOs populate the epididymis and testis and differentiate into resident macrophages. These findings close an important gap in the understanding of tissue macrophage biology in the male reproductive system and have significant implications for our understanding of male infertility.
Most resident tissue macrophages arise from two distinct waves of embryonic hematopoiesis. They are derived from early and late EMPs in the YS and then self-maintain without contribution from BM-MOs (7, 27). In accordance with previous findings (26), we identified a distinct population of F4/80hiCD11blo macrophages at E12.5, but from E14.5 onward F4/80loCD11bhi fetal MOs appeared in both the testis and epididymis. This indicates that epididymal and testicular macrophage populations, akin to most other investigated organs, are contributed to by both YS EMPs and embryonic hematopoiesis before birth (7, 27). Fate-mapping analyses with Csf1rMCM and Flt3Cre mice suggest that the main precursors of the populations of CD64hiMHCIIlo and CD64loMHCIIhi macrophages of adult epididymis and testis are derived from embryonic or adult HSC with a minor proportion of early YS EMPs.
During adulthood, embryonically established tissue-resident macrophages are gradually replaced by BM-MOs after the establishment of adult hematopoiesis (10, 11, 13, 28). However, the time and rate of replacement of tissue resident macrophages subsets by BM-MOs varies from organ to organ (13). During steady-state development brain microglia, epidermal LCs, liver KCs, and lung AMs are minimally replaced with circulatory monocytes (7). In contrast, arterial macrophages are replenished immediately after birth by BM-MOs and thereafter maintain locally by self-renewal (44). Different again are the intestinal and dermal macrophages, which are continuously replaced by BM-MOs (10, 28), with rates of replacement varying between the different subsets (10, 12, 13). Using Ccr2−/−, parabiotic, Ms4a3Cre-RosaTdT reporter, and BM-chimeric mouse models, we systematically examined the contribution of BM-MOs (adult HSC) to the pool of CD64hiMHCIIlo and CD64loMHCIIhi macrophage populations of adult epididymis and testis. Here, the number of epididymal and testicular macrophages was similar in both Ccr2−/− and WT mice, while as control—in accordance with a previously published study (28)—Ccr2−/− mice displayed a dramatic decrease in the number of intestinal macrophages; similarly, an exchange of epididymal and testicular macrophages did not occur between parabiotic mice or in BM-chimeric mice in which HSC transplantation was enabled genetically as a means to avoid irradiation-induced tissue inflammation. Our results show that CD64hiMHCIIlo and CD64loMHCIIhi macrophage populations in epididymis and testis have no monocyte contribution in adult life. Fate-mapping with Ms4a3 mice traces origin and replacement rates of tissue-resident macrophages from the pool of BM-MO (13). Our results with Ms4a3Cre-RosaTdT reporter mice showed efficient tdTomato labeling of CD64hiMHCIIlo and the CD64hiMHCIIlo macrophage populations; however, in agreement with the parabiosis and BM chimera data, both macrophage populations showed no contribution from monocytes with increasing age. This result indicates that around 50 to 60% of CD64hiMHCIIlo and CD64loMHCIIhi macrophage populations of epididymis and testis arise from nascent BM-MO/HSC very early in life, like arterial macrophages (44), as replacement of macrophages was not observed by blood MOs in adult life. In addition, our results highlight that CD64hiMHCIIlo and CD64loMHCIIhi macrophages in both organs derived from same the ontogenic precursors and that their heterogeneity could be attributed to different stages of maturation or different localization within the organs, as for other tissue-resident macrophage populations (2). The appearance of CD64loMHCIIhi macrophages in both the testis and epididymis coincides with the initiation of spermatogenesis at 2 wk of age (commencement of meiosis), and spermatogenesis at 3 wk (first haploid spermatids). Hence, it is plausible that neoantigens present on newly arrived germ cells could induce the maturation of macrophages by acquiring MHCII expression in both reproductive organs, as happens to LCs and arterial macrophages (44, 45). However, future studies are necessary to elucidate this further.
Our results and a recent publication from Lokka et al. (46) are in contrast to a previous study on testicular macrophage origin (16), which claimed that the CD64hiMHCIIlo macrophages arise from embryonic progenitors, while the CD64loMHCIIhi macrophage population exclusively arose from BM-derived progenitors. Mossadegh-Keller et al. (16) transferred BM cells into the liver of neonatal mice and showed their differentiation into CD64loMHCIIhi testicular macrophages. Using a similar strategy, Scott et al. (33) have also demonstrated that BM cells injected in the neonatal period can differentiate into liver KCs, which are normally exclusively derived from embryonic precursors. Considering these data alongside our own findings, we propose that the engraftment of injected BM-derived cells into the testis during the neonatal period occurs due to the partial availability of the macrophage niche that results from ongoing reproductive organ growth, rather than the physiological process that would otherwise occur. Altogether, our fate-mapping data with Csf1rMCM, Flt3Cre, and Ms4a3Cre mice showed that the resident macrophage populations of epididymis and testis (e.g., CD64hiMHCIIlo and CD64loMHCIIhi derived from different waves of hematopoiesis) initially arise from primitive YS macrophages, followed by fetal MOs and, finally, from nascent BM-derived cells. Once established in the niche, epididymal, and testicular macrophages self-maintain for long periods of time without replenishment from blood MOs, like other tissue resident macrophages (33, 47–49).
Infection, inflammation, and autoimmunity of the reproductive tract can contribute to male infertility, and growing evidence implicates infiltrating MOs and macrophages in the process (38, 50). In two inflammation models, we show that organ damage is correlated with a major influx of MOs/macrophages, with numbers further increasing by local proliferation. Reduced influx of blood MOs/inflammatory macrophages in Ccr2−/− UPEC-infected mice was associated with less-severe tissue damage in the epididymis and testis. Of note, the influx of immune cells with concomitant damage was more pronounced in the epididymis as compared to the testis, suggestive of a mechanism to preserve the immune privilege status of the testis. Moreover, we observed distinct morphological changes along the different regions of the epididymis following UPEC infection. As reported previously, the cauda is affected most severely by gross morphological changes following UPEC infection in WT mice, while the corpus and caput epididymidis remain mostly intact (38). Similarly, systemic administration of LPS resulted in a stronger proinflammatory response in the cauda epididymidis as compared to the other regions (51). This region-specific inflammatory response in epididymitis could be attributed to a varied composition of immune cell populations present in the different regions of the epididymis (15). In this light, for some organs two subsets of macrophages (Lyve1hiMHCIIlo and Lyve1loMHCIIhi) were described displaying distinct functions (2). While Lyve1loMHCIIhi macrophages exhibit immunogenic properties due to a high expression of MHCII, the Lyve1hiMHCIIlo macrophages play an important role in inflammation resolution, repair, fibrosis, and wound healing. Similarly, the epididymis and testis also contain two distinct sets of macrophages defined by CD64hiMHCIIlo and CD64loMHCIIhi.
In relation to the functional properties of these macrophage subsets in other organs, we believe that CD64loMHCIIhi and CD64hiMHCIIlo macrophages will play a similar role in maintaining homeostasis in both reproductive organs and possibly a tolerogenic environment. In contrast to testicular macrophages, epididymal macrophages express Itgax (CD11c) (52). This could imply that epididymal macrophages have greater plasticity than testicular macrophages and could more easily shift their phenotype from inflammatory properties to wound-healing/inflammation resolution. In addition, high expression of CD11c could enable epididymal macrophages to efficiently capture and process antigens for activation of adaptive immunity (52). Our results call for further research to understand the spatiotemporal relationship between infiltrating MOs and resident macrophages, which will significantly advance knowledge into the mechanisms of male infertility and possible treatments for this condition.
In summary, this study demonstrates that the epididymis and testis harbor distinct macrophage populations. Our data provide functional insights to better understand the biology of epididymal and testicular macrophages in steady-state and disease conditions.
Limitations.
Our multifaceted approach clearly suggests that both macrophage populations of epididymis and testis are derived from fetal/neonatal MOs; however, we could not clearly ascertain the proportions of their origin from fetal or neonatal MOs. A fate-mapping strategy is not yet available to distinguish fetal or neonatal MO-derived macrophages, hence a new fate-mapping model is warranted to distinguish these two cellular sources.
Materials and Methods
Animals.
C57BL/6J CD45.2 (Jackson Laboratory), Csf1rMerCreMer (53), Flt3Cre (54), Ccr2−/− (55), Ms4a3Cre (13), Mx1Cre (56), Myb floxed (Mybf/f) (57), Il34LacZ/LacZ (37), Rosa26eYFP (58), Rosa26mT/mG (59), C57BL/6-Tg(CAG-EGFP)131Osb/LeySopJ (60), and C57BL/6J CD45.1 (61) mice have been previously described. All mice used in this study were housed under special pathogen-free conditions at the animal facility of: the Justus Liebig University, Giessen, Germany; Ludwig-Maximilians-University, Munich, Germany; Toronto General Research Institute, facility, Toronto, Canada; Shanghai Jiao Tong University School of Medicine, Shanghai, China; The first Affiliated Hospital of Zhengzhou University, Zhengzhou, China; and the University of Zurich, Zurich, Switzerland. The animal experiments performed in this study were approved by the respective local animal authority.
Tissue Preparation and Flow Cytometry.
Mice were killed by cervical dislocation in deep isoflurane anesthesia and organs were retrieved, minced, and enzymatically digested to single-cell suspensions, as described previously (26) with minor modifications (SI Appendix, SI Materials and Methods). Obtained single-cell suspensions were subjected to flow cytometry using a MACSQuant Analyzer 10 flow cytometer (Miltenyi Biotec) or a BD Biosciences LSR Fortessa (BD Bioscience) and data were analyzed with the FlowJo software version X (Tree Star) (SI Appendix, SI Materials and Methods).
BrdU Labeling and Analysis.
Male C57BL/6J mice were injected intraperitoneally with BrdU (0.1 mg/g body weight; n = 5; Sigma) or saline (control n = 5) and killed after 4 h. Epididymides and testes were collected and digested to obtain single-cell suspensions. Subsequently, BrdU+ cells were labeled by using an anti-BrdU antibody (Biolegend) followed by flow cytometric analysis.
Genetic Tracing of YS Macrophages.
For fate-mapping analysis of YS macrophages, TAM-inducible Csf1rMerCreMer mice were crossed with Rosa26eYFP reporter mice, as described previously (26). Recombination was induced in Csf1rMerCreMer × Rosa26eYFP embryos by single injection of 75 μg per gram (body weight) of OH-TAM (Sigma) into pregnant females at E8.5. OH-TAM was supplemented with 37.5 μg per gram Progesterone (Sigma) to counteract the mixed estrogen agonist effects of TAM, which can result in fetal abortion (26, 32). Observation of vaginal plugs at 0.5 d postcoitum was used as criteria to estimate the embryonic development. The frequency of YFP+ cells in brain, epididymides, and testes were analyzed at 9 wk of age by flow cytometry, as described in SI Appendix, SI Materials and Methods.
Fate Mapping with FLT3Cre Mice.
To examine the contribution of fetal HSC to the pool of adult epididymal and testicular macrophages, Flt3Cre/WT mice were crossed with Rosa26mT/mG reporter mice as described previously (62). Epididymides and testes were analyzed at 2, 5, and 9 wk of age by flow cytometry, as described in SI Appendix, SI Materials and Methods.
Fate-Mapping with Ms4a3Cre Mice.
The contribution of FL monocytes to the pool of adult epididymal and testicular macrophages was examined by crossing Ms4a3Cre/WT mice with RosaTtdTom/tdTom reporter mice (13). Epididymides and testes were analyzed at 5 and 24 wk of age by flow cytometry, as described in SI Appendix, SI Materials and Methods.
In Vivo Labeling of Vascular Immune Cells.
Nine- to 10-wk-old C57BL/6J mice were injected intravenously with FITC-conjugated anti-mouse CD45 antibody (0.5 µg in 200 µL PBS) and killed 5 min later. Epididymis and testis were harvested and analyzed by flow cytometry.
Irradiation and Generation of BM-Chimeric Mice.
Six-week-old C57BL/6J mice were anesthetized by intraperitoneal injection of 5% chloral hydrate then exposed to a single dose of 8.5-Gy γ-irradiation with or without shielding of the inguinal scrotal region with a lead sheet to protect the testis and epididymis from irradiation. After irradiation and recovery from anesthesia, 5 × 106 EGFP+ donor BM cells were adoptively transferred into the tail vein of the mice. Mice were kept on 0.03% enrofloxacin in drinking water for up to 1 wk before and after irradiation. Reconstituted mice were analyzed 5 wk and 12 wk later.
FACS-Gal Assay.
Cells were resuspended with staining buffer (1× HBSS, 2% FCS, 10 mM Hepes buffer [pH 7.2], 1% penicillin/streptomycin) before 2 mM FDG solution (Fluorescein di[β-d-galactopyranoside], Sigma, F2756) was added to the cell suspension, which was incubated at 37 °C for 1 min, then transferred to ice-cold staining buffer and kept on ice for 1.5 h. After incubation, cells were washed with PBS by centrifugation at 400 × g for 5 min at 4 °C. Pelleted cells were resuspended in FACS buffer (2 mM EDTA, 2% FCS in PBS) before analysis by flow cytometry.
Depletion of Macrophages in the Epididymis and Testis.
Anti–CSF-1 mAb (200 μg; clone 5A1, Bio X Cell) or isotype control (rat IgG1, clone HRPN, Bio X Cell) were injected intraperitoneally into adult C57BL/6 male mice every other day for 6 d. Seven days after the first injection, epididymides and testes were retrieved to analyze the macrophage population in these organs by flow cytometry.
RNA-Seq Analyses.
Flow-sorted brain microglia, epididymides, and testes macrophages were used for RNA-seq analyses (SI Appendix, SI Materials and Methods).
Supplementary Material
Acknowledgments
We thank Suada Froehlich for her help with flow cytometry, immunofluorescence, and immunohistochemistry analyses; Dr. Lucy Robinson of Insight Editing London for critical review and editing of the manuscript/assistance preparing the manuscript; and Mr. Helge Hudel for his advice on statistical analyses. This study was supported by Deutsche Forschungsgemeinschaft Grants BH 93/1-4 (to S.B.) and SFB 914, project A10 (to C. Schulz), and National Natural Science Foundation of China 81901466 (to M.W.). The fellowship support of the China Scholarship Council (M.W.) and Justus-Liebig University of Giessen (M.W.) are gratefully acknowledged.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. S.J. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2013686117/-/DCSupplemental.
Data Availability.
The RNA-seq results from macrophages of epididymis, testis, and brain were deposited in the Gene Expression Omnibus database, https://www.ncbi.nlm.nih.gov/geo (accession no. GSE132471). All study data are included in the article and SI Appendix.
References
- 1.Lavin Y., Mortha A., Rahman A., Merad M., Regulation of macrophage development and function in peripheral tissues. Nat. Rev. Immunol. 15, 731–744 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chakarov S., et al. , Two distinct interstitial macrophage populations coexist across tissues in specific subtissular niches. Science 363, eaau0964 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Hoeffel G., Ginhoux F., Fetal monocytes and the origins of tissue-resident macrophages. Cell. Immunol. 330, 5–15 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Ginhoux F., Guilliams M., Tissue-resident macrophage ontogeny and homeostasis. Immunity 44, 439–449 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Palis J., Robertson S., Kennedy M., Wall C., Keller G., Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development 126, 5073–5084 (1999). [DOI] [PubMed] [Google Scholar]
- 6.Frame J. M., McGrath K. E., Palis J., Erythro-myeloid progenitors: “Definitive” hematopoiesis in the conceptus prior to the emergence of hematopoietic stem cells. Blood Cells Mol. Dis. 51, 220–225 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoeffel G., et al. , C-Myb(+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity 42, 665–678 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin Y., Yoder M. C., Yoshimoto M., Lymphoid progenitor emergence in the murine embryo and yolk sac precedes stem cell detection. Stem Cells Dev. 23, 1168–1177 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orkin S. H., Zon L. I., Hematopoiesis: An evolving paradigm for stem cell biology. Cell 132, 631–644 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tamoutounour S., et al. , Origins and functional specialization of macrophages and of conventional and monocyte-derived dendritic cells in mouse skin. Immunity 39, 925–938 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Calderon B., et al. , The pancreas anatomy conditions the origin and properties of resident macrophages. J. Exp. Med. 212, 1497–1512 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaw T. N., et al. , Tissue-resident macrophages in the intestine are long lived and defined by Tim-4 and CD4 expression. J. Exp. Med. 215, 1507–1518 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Z., et al. , Fate mapping via Ms4a3-expression history traces monocyte-derived cells. Cell 178, 1509–1525.e19 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Voisin A., et al. , Comprehensive overview of murine epididymal mononuclear phagocytes and lymphocytes: Unexpected populations arise. J. Reprod. Immunol. 126, 11–17 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Battistone M. A., et al. , Region-specific transcriptomic and functional signatures of mononuclear phagocytes in the epididymis. Mol. Hum. Reprod. 26, 14–29 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mossadegh-Keller N., et al. , Developmental origin and maintenance of distinct testicular macrophage populations. J. Exp. Med. 214, 2829–2841 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang M., et al. , Characterization of the micro-environment of the testis that shapes the phenotype and function of testicular macrophages. J. Immunol. 198, 4327–4340 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Bhushan S., et al. , Differential activation of inflammatory pathways in testicular macrophages provides a rationale for their subdued inflammatory capacity. J. Immunol. 194, 5455–5464 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Schuppe H. C., Meinhardt A., Immune privilege and inflammation of the testis. Chem. Immunol. Allergy 88, 1–14 (2005). [DOI] [PubMed] [Google Scholar]
- 20.Tagliani E., et al. , Coordinate regulation of tissue macrophage and dendritic cell population dynamics by CSF-1. J. Exp. Med. 208, 1901–1916 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gautier E. L.et al.; Immunological Genome Consortium , Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat. Immunol. 13, 1118–1128 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Com E., et al. , Expression of antimicrobial defensins in the male reproductive tract of rats, mice, and humans. Biol. Reprod. 68, 95–104 (2003). [DOI] [PubMed] [Google Scholar]
- 23.Carr D. W., Usselman M. C., Acott T. S., Effects of pH, lactate, and viscoelastic drag on sperm motility: A species comparison. Biol. Reprod. 33, 588–595 (1985). [DOI] [PubMed] [Google Scholar]
- 24.Tcymbarevich I., et al. , Lack of the pH-sensing receptor TDAG8 [GPR65] in macrophages plays a detrimental role in murine models of inflammatory bowel disease. J. Crohn’s Colitis 13, 245–258 (2019). [DOI] [PubMed] [Google Scholar]
- 25.Perdiguero E. G., Geissmann F., The development and maintenance of resident macrophages. Nat. Immunol. 17, 2–8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schulz C., et al. , A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336, 86–90 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Gomez Perdiguero E., et al. , Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature 518, 547–551 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bain C. C., et al. , Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat. Immunol. 15, 929–937 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dick S. A., et al. , Self-renewing resident cardiac macrophages limit adverse remodeling following myocardial infarction. Nat. Immunol. 20, 29–39 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mucenski M. L., et al. , A functional c-myb gene is required for normal murine fetal hepatic hematopoiesis. Cell 65, 677–689 (1991). [DOI] [PubMed] [Google Scholar]
- 31.Sumner R., Crawford A., Mucenski M., Frampton J., Initiation of adult myelopoiesis can occur in the absence of c-Myb whereas subsequent development is strictly dependent on the transcription factor. Oncogene 19, 3335–3342 (2000). [DOI] [PubMed] [Google Scholar]
- 32.Stremmel C., et al. , Inducible disruption of the c-myb gene allows allogeneic bone marrow transplantation without irradiation. J. Immunol. Methods 457, 66–72 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scott C. L., et al. , Bone marrow-derived monocytes give rise to self-renewing and fully differentiated Kupffer cells. Nat. Commun. 7, 10321 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van de Laar L., et al. , Yolk sac macrophages, fetal liver, and adult monocytes can colonize an empty niche and develop into functional tissue-resident macrophages. Immunity 44, 755–768 (2016). [DOI] [PubMed] [Google Scholar]
- 35.Chitu V., Stanley E. R., Colony-stimulating factor-1 in immunity and inflammation. Curr. Opin. Immunol. 18, 39–48 (2006). [DOI] [PubMed] [Google Scholar]
- 36.Wang Y., et al. , IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat. Immunol. 13, 753–760 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greter M., et al. , Stroma-derived interleukin-34 controls the development and maintenance of langerhans cells and the maintenance of microglia. Immunity 37, 1050–1060 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klein B., et al. , Differential tissue-specific damage caused by bacterial epididymo-orchitis in the mouse. Mol. Hum. Reprod. 26, 215–227 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhushan S., et al. , Uropathogenic E. coli induce different immune response in testicular and peritoneal macrophages: Implications for testicular immune privilege. PLoS One 6, e28452 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pilatz A., et al. , Acute epididymitis revisited: Impact of molecular diagnostics on etiology and contemporary guideline recommendations. Eur. Urol. 68, 428–435 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Davies L. C., et al. , Distinct bone marrow-derived and tissue-resident macrophage lineages proliferate at key stages during inflammation. Nat. Commun. 4, 1886 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jenkins S. J., et al. , Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science 332, 1284–1288 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lang T., et al. , Uropathogenic Escherichia coli modulates innate immunity to suppress Th1-mediated inflammatory responses during infectious epididymitis. Infect. Immun. 82, 1104–1111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ensan S., et al. , Self-renewing resident arterial macrophages arise from embryonic CX3CR1(+) precursors and circulating monocytes immediately after birth. Nat. Immunol. 17, 159–168 (2016). [DOI] [PubMed] [Google Scholar]
- 45.Tripp C. H., et al. , Ontogeny of Langerin/CD207 expression in the epidermis of mice. J. Invest. Dermatol. 122, 670–672 (2004). [DOI] [PubMed] [Google Scholar]
- 46.Lokka E., et al. , Generation, localization and functions of macrophages during the development of testis. Nat. Commun. 11, 4375 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hashimoto D., et al. , Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 38, 792–804 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chorro L., et al. , Langerhans cell (LC) proliferation mediates neonatal development, homeostasis, and inflammation-associated expansion of the epidermal LC network. J. Exp. Med. 206, 3089–3100 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bain C. C., et al. , Long-lived self-renewing bone marrow-derived macrophages displace embryo-derived cells to inhabit adult serous cavities. Nat. Commun. 7, ncomms11852 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nicolas N., et al. , Testicular activin and follistatin levels are elevated during the course of experimental autoimmune epididymo-orchitis in mice. Sci. Rep. 7, 42391 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang F., et al. , Lipopolysaccharide-induced testicular dysfunction and epididymitis in mice: A critical role of tumor necrosis factor alpha. Biol. Reprod. 100, 849–861 (2019). [DOI] [PubMed] [Google Scholar]
- 52.Mendelsohn A. C., et al. , From initial segment to cauda: A regional characterization of mouse epididymal CD11c(+) mononuclear phagocytes based on immune phenotype and function. Am. J. Physiol. Cell Physiol., 10.1152/ajpcell.00392.2020 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qian B. Z., et al. , CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 475, 222–225 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Benz C., Martins V. C., Radtke F., Bleul C. C., The stream of precursors that colonizes the thymus proceeds selectively through the early T lineage precursor stage of T cell development. J. Exp. Med. 205, 1187–1199 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boring L., et al. , Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J. Clin. Invest. 100, 2552–2561 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kühn R., Schwenk F., Aguet M., Rajewsky K., Inducible gene targeting in mice. Science 269, 1427–1429 (1995). [DOI] [PubMed] [Google Scholar]
- 57.Lieu Y. K., Kumar A., Pajerowski A. G., Rogers T. J., Reddy E. P., Requirement of c-myb in T cell development and in mature T cell function. Proc. Natl. Acad. Sci. U.S.A. 101, 14853–14858 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Srinivas S., et al. , Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 1, 4 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Muzumdar M. D., Tasic B., Miyamichi K., Li L., Luo L., A global double-fluorescent Cre reporter mouse. Genesis 45, 593–605 (2007). [DOI] [PubMed] [Google Scholar]
- 60.Okabe M., Ikawa M., Kominami K., Nakanishi T., Nishimune Y., ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett. 407, 313–319 (1997). [DOI] [PubMed] [Google Scholar]
- 61.Schluns K. S., Williams K., Ma A., Zheng X. X., Lefrançois L., Cutting edge: Requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J. Immunol. 168, 4827–4831 (2002). [DOI] [PubMed] [Google Scholar]
- 62.Boyer S. W., Schroeder A. V., Smith-Berdan S., Forsberg E. C., All hematopoietic cells develop from hematopoietic stem cells through Flk2/Flt3-positive progenitor cells. Cell Stem Cell 9, 64–73 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The RNA-seq results from macrophages of epididymis, testis, and brain were deposited in the Gene Expression Omnibus database, https://www.ncbi.nlm.nih.gov/geo (accession no. GSE132471). All study data are included in the article and SI Appendix.