Significance
Hypothalamic neural networks control hormone secretion to maintain body function. For gonadotropin-releasing hormone (GnRH) neurons, critical to fertility, release must be pulsatile. While it is clear that kisspeptin neurons trigger GnRH activity and pulses, how GnRH neurons return to baseline activity before the next stimulation is unknown. Here, we show 1) that the long-lasting response of GnRH neurons to kisspeptin depends on the degradation of phosphatidylinositol 4,5-bisphosphate (PIP2) and its slow resynthesis keeping canonical transient receptor potential channels opened and 2) a mechanism whereby nitric oxide facilitates PIP2 resynthesis and GnRH neuron recovery from kisspeptin activation. Defining the overlap in kisspeptin and nitric oxide signaling pathways may provide an avenue for fertility treatment.
Keywords: fertility, GnRH, kisspeptin, pulse, nitric oxide
Abstract
Fertility relies upon pulsatile release of gonadotropin-releasing hormone (GnRH) that drives pulsatile luteinizing hormone secretion. Kisspeptin (KP) neurons in the arcuate nucleus are at the center of the GnRH pulse generation and the steroid feedback control of GnRH secretion. However, KP evokes a long-lasting response in GnRH neurons that is hard to reconcile with periodic GnRH activity required to drive GnRH pulses. Using calcium imaging, we show that 1) the tetrodotoxin-insensitive calcium response evoked by KP relies upon the ongoing activity of canonical transient receptor potential channels maintaining voltage-gated calcium channels in an activated state, 2) the duration of the calcium response is determined by the rate of resynthesis of phosphatidylinositol 4,5-bisphosphate (PIP2), and 3) nitric oxide terminates the calcium response by facilitating the resynthesis of PIP2 via the canonical pathway guanylyl cyclase/3′,5′-cyclic guanosine monophosphate/protein kinase G. In addition, our data indicate that exposure to nitric oxide after KP facilitates the calcium response to a subsequent KP application. This effect was replicated using electrophysiology on GnRH neurons in acute brain slices. The interplay between KP and nitric oxide signaling provides a mechanism for modulation of the refractory period of GnRH neurons after KP exposure and places nitric oxide as an important component for tonic GnRH neuronal pulses.
Gonadotropin-releasing hormone (GnRH)-secreting neurons integrate multiple physiological and environmental cues and translate them into GnRH secretory patterns, to drive gonadotrophs, which in turn control the gonads. In both sexes, tonic GnRH pulses regulate gametogenesis and steroidogenesis via luteinizing hormone (LH) and follicle-stimulating pulses. Females require a GnRH surge to trigger LH surge and ovulation [reviewed in (1, 2)]. However, disruption of the normal pulsatile GnRH secretion impairs fertility in both sexes (3). Although insufficient for optimal reproductive health (4), the direct action of kisspeptins (KP) on GnRH neurons, via the KP receptor Kiss1r, is required for fertility (4–6). KP neurons play a critical regulatory role in the hypothalamic–pituitary–gonadal axis, including puberty onset (7–9), the preovulatory GnRH surge (5, 9, 10), and tonic GnRH pulses (6, 11). In rodents, two KP neuronal subpopulations exist with distinct functions: the anteroventral periventricular nucleus (AVPV) subpopulation, linked to puberty onset [reviewed in (12)] and preovulatory surge [reviewed in (13)], and the arcuate nucleus (ARC) subpopulation, also known as Kisspeptin-Neurokinin B-Dynorphin (KNDy) neurons, linked to tonic pulses (reviewed in ref. 14).
Exogenous KP at the GnRH cell body evokes a long-lasting increase in intracellular calcium levels ([Ca2+]i) (15), often leading to the summation of individual oscillations into [Ca2+]i plateaus (15, 16). This observation is in agreement with an increase in electrical activity where GnRH neurons in acute slices go from burst firing to tonic firing after KP application (17–19). One could argue that this prolonged response is an artifact of the exogenously applied KP. However, endogenously released KP by AVPV stimulation also evokes a long-lasting increase in firing rate (20). Under normal conditions, [Ca2+]i oscillations are driven by bursts of action potentials (AP) (21, 22). Yet, AP are not necessary for the KP-evoked [Ca2+]i response to occur, as it is driven by multiple effectors including transient receptor potential-canonical channels (TRPC), voltage-gated calcium channels (VGCC), and inositol 1,4,5-trisphosphate receptors (InsP3R) (15, 16, 19, 23). Thus, the versatility of Kiss1r signaling pathway underlies the functionality of KP projections along GnRH neuron processes (24), with KP locally applied on nerve terminals also evoking a long-lasting increase in [Ca2+]i (16).
While the long-lasting KP response is suitable to trigger the preovulatory surge, it seems incompatible with the KNDy model for tonic pulses. GnRH and LH pulses occur every ∼20 min in ovariectomized mice (25). Indeed, the KNDy model provides on-/off- signals for KNDy neurons, neurokinin B and dynorphin respectively, and an on-signal for GnRH neurons, KP. However, this model lacks an off-signal for GnRH neurons. KNDy neurons trigger GnRH/LH pulses via Kiss1r (26), but the “extinction” of KNDy neurons by dynorphin is not obligatory for the termination of GnRH/LH pulses in rodents (27, 28), and dynorphin is lacking in human KP-neurokinin neurons (29).
In fact, how the response in firing and [Ca2+]i to KP relate to GnRH secretion is unknown. In acute coronal brain slices, the KP-evoked increase in firing at the cell body cannot be repeated (19, 30). In contrast, in acute sagittal brain slices, using fast scan cyclic voltammetry, the KP-evoked GnRH secretion from cells in the preoptic area (POA) and fibers in the median eminence (ME) can be triggered repeatedly (31). The difference in repeatability at the cell body is puzzling. One explanation could be a technical consequence of brain slicing or conventional patch clamp. Another explanation could be a dissociation between firing and [Ca2+]i during the second KP application. In fact, the common feature of the KP-evoked increase in [Ca2+]i and GnRH secretion, at the GnRH neuron cell body and nerve terminal, is that it is AP-independent (15, 16, 31). The current study uses calcium imaging and electrophysiology to address the mechanisms that allow 1) [Ca2+]i in GnRH neurons to return to baseline after KP stimulation, and 2) GnRH neurons to respond to a second KP stimulation (i.e., repeatability) and shows nitric oxide as an important component for tonic GnRH neuronal pulses.
Results
GnRH/LH pulses are driven by kisspeptinergic inputs arising from KNDy neurons (26). The GnRH neuron distal process integrates these KP inputs (32) and the calcium response is action potential–independent (16). At 7 to 14 d in vitro, ∼90% of primary GnRH neurons from explants increase intracellular calcium in response to KP and express Kiss1r messenger RNA (15). The signaling pathway downstream Kiss1r identified in 7-div GnRH neurons is identical to the one identified in GnRH neurons from adult brain slices (15).
Because, like the GnRH neuron distal process (16), primary GnRH neurons from explants exhibit action potential–insensitive calcium signaling under KP stimulation (15), they were used for calcium imaging experiment as a tool to specifically study the recovery from this specific action potential–insensitive calcium signaling in a large number of GnRH neurons.
The Time Course of the KP-Evoked Calcium Response Is Constant.
The ultimate goal is to understand the mechanisms that lead to GnRH pulses. Since both KP-induced GnRH secretion (31) and calcium response to KP (15, 16, 19) are tetrodotoxin (TTX) insensitive, the paradigm chosen was as follows: 5 min in serum-free media (SFM) (cell culture medium) was followed by 5 min in SFM+TTX (0.5 µM) to establish the baseline (Fig. 1 B and C). SFM+TTX+KP (10 or 100 nM) was applied for 2 min between the 10th and 12th minutes and then washout in SFM+TTX for 30 min. The calcium responses evoked by the two KP doses had the same time-course. The time to 50% recovery was not different (Fig. 1 D and E, P = 0.3667). The dose of 100 nM was chosen for the rest of the experiments. Unless stated, pharmacological perturbations were applied after the 17th minute with SFM+TTX to avoid interfering with the onset and development of the full KP response.
Fig. 1.
The tetrodotoxin-insensitive calcium response to KP-10 in GnRH neurons. (A) High-magnification images of GnRH neurons in explants identified by their morphology (Left), recorded with calcium imaging (Middle), and confirmed by immunocytochemistry (Right). Arrows indicate the same neurons throughout the experiment. (Scale bar, 20 μm in all panels.) (B) A heat map showing changes in [Ca2+] levels in 49 GnRH neurons recorded simultaneously. Neurons were perfused for 5 min in a control medium, and then TTX was added for the rest of the recording. After 5 min in TTX, the cells received a 2-min application of KP-10. Optical density (arbitrary units) was measured, with cells showing an increase in calcium levels changing toward yellow. (C) Examples of traces showing changes in [Ca2+] levels (ΔF/F0) in five individual GnRH neurons (gray) and the average in black, evoked by 10 nM (Top) or 100 nM (Bottom) KP. The time of the arrow heads in C correspond to the time of arrow heads in D. (D) Average trace for changes in [Ca2+] levels (percent) evoked by 10 nM (open circles, n = 3 explants) and 100 nM (filled circles, n = 7 explants; reference trace for subsequent experiments). Each trace was normalized to its maximum to assess the recovery time at 50% (red horizontal line) for each dose (red vertical lines). The arrow heads represent the recovery period during which drugs were applied in subsequent experiments (i.e., once the response to KP had fully developed). (E) A bar graph depicting the average time (+SEM) at 50% recovery for explants used in D. The dotted line represents the value used as the reference (30.0 ± 1.4 min) without perturbations (KP 100 nM) for subsequent experiments. The average time at 50% recovery between 10 nM and 100 nM KP was not significantly different (nonparametric Mann–Whitney U test).
Maintenance of Calcium Response to KP Is Dependent on TRPC Channels and VGCC.
The balance between protein kinase and phosphatase activities is at the center of the well-characterized long-lasting phenomena, long-term potentiation, and long-term depression (33, 34). In neurons, calcium influx activates the calcium/calmodulin‐dependent protein kinase II (CaMKII). An autophosphorylation keeps the enzyme active. In vitro, both protein phosphatase 1 (PP1) and protein phosphatase 2A (PP2A) can dephosphorylate CaMKII (35) (i.e., reverse its calcium-independent activity). Since a physical association between Kiss1r and PP2A was identified in a heterologous expression system (36), the role of PP2A on the KP-evoked calcium response was tested with okadaic acid (OKA, 30 nM; inhibitor of PP1 and PP2A). The application of OKA did not affect the time course of the recovery (Fig. 2D, P = 0.3671). An activator of PP1 and PP2, C2 ceramide (also known as N-acetoyl-D-erythro-sphingosine; 20 µM), was also tested and had no effect upon the time course of the response to KP (Fig. 2D, P = 0.6061).
Fig. 2.
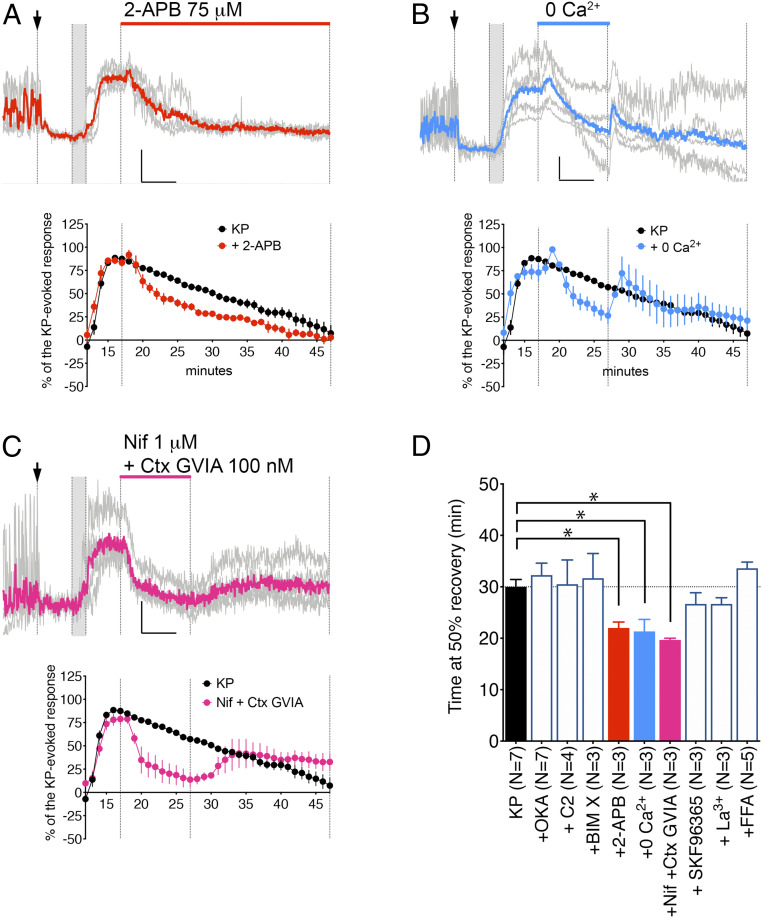
Canonical transient receptor potential channels and subsequently voltage-gated calcium channels feed the TTX-insensitive calcium response to KP. (A–C) Examples of traces showing changes in [Ca2+] levels in five individual GnRH neurons (gray) and the average (color) under the indicated perturbation. The first vertical line marks treatment with TTX (arrow indicates presence for the remaining recording period), the second and third vertical lines indicate treatment with KP (gray, 2 min), and the fourth vertical line marks beginning of experimental perturbation (length of perturbation is shown on top). (Inset) Horizontal bar = 5 min, vertical bar = 0.2 ΔF/F0. Below each graph is the average response for changes in [Ca2+] levels (percent) evoked by KP 100 nM without perturbation (control from Fig. 1D, black circles) or with perturbation (colored circles). (A) 2-APB curtailed the calcium response to KP, indicating the role of canonical TRPC in the maintenance of the calcium response. (B, C) Transient removal of extracellular calcium or blockade of L-type (Nifedipine [Nif]) and N-type (ω-Conotoxin GVIA [Ctx GVIA]) VGCC dampened the calcium response to KP but only during the perturbation, indicating the TRPC were still in an activated state and that TRPC worked synergistically with VGCC to maintain the calcium response. (D) A bar graph showing the average time (+SEM) at 50% recovery for GnRH cells recorded in A–C. Note that blocking or activating protein phosphatases 1/2 with OKA or C2, respectively, or protein kinase C with BIM X was ineffective, and other TRPC blockers (SKF96365, La3+, or flufenamic acid [FFA]) were not as effective as 2-APB. All average times at 50% recovery with 100 nM KP + drug were compared to the average time with 100 nM KP alone (black bar). Statistical significances (P < 0.05) were indicated with asterisks (nonparametric Mann–Whitney U test), and P values were listed in Table 1.
Multiple studies showed the ability of 2-aminoethoxydiphenyl borate (2-APB) to interfere with the onset of the KP response in GnRH neurons (15, 16, 19). The fact that extracellular, but not intracellular, 2-APB blocks KP-induced nonselective cation inward currents, identifies receptor-operated currents in the KP response and TRPC channels as mediators rather than store-operated currents (37). To determine whether the maintenance of the long-lasting calcium response to KP was dependent on persistent activation of TRPC channels, 2-APB (75 µM) was applied during the KP recovery period. Treatment with 2-APB decreased the time to reach 50% recovery (Fig. 2 A and D, P = 0.0083), consistent with activated TRPC channels feeding the KP-induced calcium response.
The Kiss1r downstream signaling pathway involves protein kinase C (PKC) (15, 38). Some TRPC channels are modulated by a diacylglycerol and PKC-dependent mechanism (39). Thus, the PKC inhibitor bisindolylmaleimide X (BIM X, 100 nM) was applied during the KP recovery period. BIM X did not change the time to reach 50% recovery (Fig. 2D, P = 0.9333), indicating that PKC activity does not determine the duration of the KP response.
2-APB is a reliable blocker for Ca2+ entry, less so for Ca2+ release (40). To confirm the calcium response to KP required an influx, regular SFM was switched to nominal calcium-free SFM (0 Ca2+) for 10 min. As predicted, the calcium response dropped during perfusion with calcium-free SFM (Fig. 2 B and D, P = 0.0083). Notably, the calcium levels went back to control levels upon calcium reintroduction, indicating the persistence of the activated state of TRPC channels, with or without calcium flow.
Cadmium, a broad-spectrum blocker of VGCC, and ω-conotoxin GVIA (Ctx GVIA) attenuate and antagonize the onset of KP response in GnRH neuron cell bodies (15) and nerve terminals (16), respectively. To test whether voltage-gated calcium channels (VGCC) contribute to the calcium influx during the long-lasting response, a mixture of nifedipine (Nif; 1 µM) and Ctx GVIA (100 nM), blockers of L-type and N-type VGCC, respectively, was used. The mixture was applied for 10 min during the KP recovery period. Although it effectively depressed the calcium plateau (Fig. 2 C and D, P = 0.0083), the calcium response resumed after its removal. While this indicated the contribution of VGCC to the calcium response, it also showed the ongoing activated state of TRPC channels.
Modulation of TRPC Channels by Nitric Oxide Decreases Recovery Time from KP Activation.
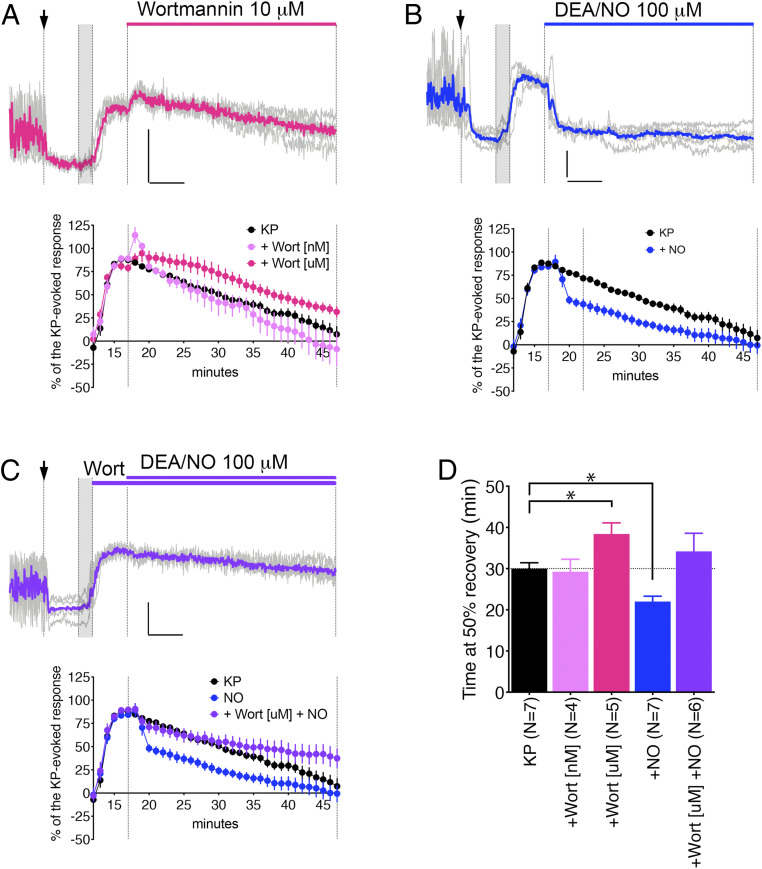
The degradation of phosphatidylinositol 4,5-bisphosphate (PIP2) is required for the activation of TRPC (37). Preventing the resynthesis of PIP2 increases the duration of the KP-evoked TRPC current (37). To test whether the resynthesis of PIP2 also sets the time course of the KP response, phosphatidylinositol 4-kinase (PI4K) was blocked with wortmannin (10 µM). The time at 50% recovery was increased (Fig. 3 A and D, P = 0.0278). When wortmannin was applied at a dose specific to phosphoinositide 3-kinase (PI3K, 10 nM), it had no effect (Fig. 3D, P = 0.7545). A dense fiber network containing the neuronal nitric oxide synthase (nNOS) exists in the median eminence (41–43) and endothelial nitric oxide synthase (eNOS) produces nitric oxide in the median eminence (44). Since nitric oxide modulates PIP2 and calcium responses to growth factors (45–47), and the time at 50% recovery was increased when PIP2 resynthesis was blocked, we tested whether promoting PIP2 resynthesis with nitric oxide would facilitate the recovery from KP stimulation. A 30-min application of a nitric oxide–releasing compound, DEA/NO (100 µM, 12 min into solution at the time the perfusion starts), abruptly stopped the KP-evoked calcium response (Fig. 3 B and D, P = 0.0041). To ensure the effect of nitric oxide was mediated by the stimulation of PIP2 resynthesis via PI4K, rather than inhibition of TRPC channels (48), DEA/NO was applied with wortmannin (10 µM) and failed to affect the time course of the KP-evoked calcium response (Fig. 3 C and D, P = 0.4254). Finally, to test whether the KP-evoked calcium response was transiently paused or terminated by nitric oxide, experiments were performed in which the application of DEA/NO lasted for only 5 min. This 5-min application of nitric oxide was sufficient to stop the KP-evoked calcium response (Fig. 4 A and F, P = 0.0239). Notably, no rebound appeared after removal of DEA/NO, indicating that the TRPC channels were in fact deactivated.
Fig. 3.
Resynthesis of PIP2 defines the duration of the TTX-insensitive calcium response to KP. (A–C) Examples of traces showing changes in [Ca2+] levels in five individual GnRH neurons (gray) and the average (color) under the indicated perturbation. The first vertical line marks treatment with TTX (arrow, indicates presence for the remaining recording period), the second and third vertical lines indicate treatment with KP (gray, 2 min), and the fourth vertical line marks beginning of experimental perturbation (length of perturbation is shown on top). (Inset) Horizontal bar = 5 min, vertical bar = 0.2 ΔF/F0. Below each graph is the average response for changes in [Ca2+] levels (percent) evoked by KP 100 nM without perturbation (control from Fig. 1D, black circles) or with perturbation (colored circles). (A) Preventing the resynthesis of PIP2 by blocking PI4K/PI3K with Wortmannin [μM, micromolar range] prolonged the calcium response to KP. This effect did not occur when PI3K alone was inhibited (Wortmannin [nM, nanomolar range]). (B) Facilitating synthesis of PIP2 with nitric oxide (DEA/NO) shortened the calcium response to KP. (C) Blocking PI4K/PI3K with Wortmannin [μM] abolished the effect of DEA/NO. (D) The bar graph represents the average time (+SEM) at 50% recovery for explants used in A, B, and C. All average times at 50% recovery with 100 nM KP + drug were compared to the average time with 100 nM KP alone (black bar). Statistical significances (P < 0.05) were indicated with asterisks (nonparametric Mann–Whitney U test), and P values were listed in Table 1.
Fig. 4.
Nitric oxide facilitates PIP2 resynthesis via its canonical pathway. (A–E) Examples of traces showing changes in [Ca2+] levels in five individual GnRH neurons (gray) and the average (color) under the indicated perturbation. The first vertical line marks treatment with TTX (arrow, indicates presence for the remaining recording period), the second and third vertical lines indicate treatment with KP (gray, 2 min), and the fourth vertical line marks beginning of experimental perturbation (length of perturbation is shown on top). (Inset) Horizontal bar = 5 min, vertical bar = 0.2 ΔF/F0. Below each graph is the average response for changes in [Ca2+] levels (percent) evoked by KP 100 nM without perturbation (control from Fig. 1D, black circles) or with perturbation (colored circles). (A) Similar to 30-min DEA/NO application, a 5-min application of DEA/NO shortened the calcium response and it did not resume after the removal of DEA/NO, indicating the deactivation of TRPC channels. (B, C) The effect of DEA/NO was partially mimicked by a cell permeable analog of cGMP, 8-pGTP-cGMP. (D) The effect of DEA/NO was antagonized by pretreatment with ODQ, a blocker of guanylyl cyclase (GC), indicating the canonical nitric oxide signaling pathway via GC/cGMP/protein kinase G. (E) Blocking Rho kinase with Y27632 did not prevent the effect of DEA/NO. (F) The bar graph represents the average time (+SEM) at 50% recovery for explants used in A–C. All average times at 50% recovery with 100 nM KP + drug were compared to the average time with 100 nM KP alone (black bar). Statistical significances (P < 0.05) were indicated with asterisks (nonparametric Mann–Whitney U test), and P values were listed in Table 1.
Canonically, nitric oxide activates soluble guanylyl cyclase, which in turn produces 3′,5′-cyclic guanosine monophosphate (cGMP) (49). cGMP activates its downstream effector, PKG (50). The less classical alternative is nitric oxide–induced posttranslational modifications such as S-nitrosylation and nitration (51, 52). To define the signaling pathway activated with DEA/NO, first we tested whether a membrane-permeable analog of cGMP, 8-pCPT-cGMP (cGMP, 200 µM), could mimic the effect of DEA/NO. cGMP was applied for 5 or 10 min and in both cases, it dampened the KP-evoked calcium response (Fig. 4 B, C, and F, P = 0.0061 and P = 0.0083, respectively). Second, we tested whether the synthesis of cGMP mediated the effect of DEA/NO by blocking the guanylyl cyclase prior to its application. DEA/NO failed to inhibit the KP-evoked calcium response in GnRH neurons pretreated for 17 min with a blocker of guanylyl cyclase, ODQ (30 µM) (Fig. 4 D and F, P = 0.7636). Both experiments point toward the classical nitric oxide pathway ending the KP-evoked calcium response. The common downstream effector of PKG, RhoA/Rho kinase (ROCK), links PKG activity to PIP2 synthesis (47, 53). A 17-min preincubation of GnRH neurons with Y-27632 (ROCK inhibitor, 10 µM) did not prevent DEA/NO to terminate the KP-evoked calcium response (Fig. 4 E and F, P = 0.0204), suggesting a different effector than ROCK downstream PKG.
Nitric Oxide Exposure Facilitates a Second KP-Evoked Response in GnRH Cells.
The increase in firing evoked by KP was rarely repeatable in GnRH neurons as measured in acute brain slices (54). Since the present studies demonstrate that nitric oxide facilitates the time to 50% recovery to KP application in GnRH neurons, we applied KP twice, with and without DEA/NO between, to determine whether the repeatability of the KP-evoked calcium response would be altered. KP was applied between the 10 and 12th min and a second time between the 37 and 39th min (i.e., 25 min later). Notably, during the second application, KP evoked a small decrease in the first KP-evoked calcium response (Fig. 5A). However, after its removal, the calcium response returned to levels similar to that recorded after the first KP application (i.e., ∼5% increase of the first response added from the second exposure to KP) (Fig. 5B). During the next paradigm, DEA/NO was applied between the 17th and 32nd min. As previously described, DEA/NO dampens the first KP-evoked calcium response. In contrast to cells that receive KP twice, without DEA/NO in between, cells that received KP twice with DEA/NO in between had a larger response to the second KP application, ∼25% increase (Fig. 5 A and B, P = 0.0039). This showed that the mechanism we identified above (i.e., DEA/NO-induced PIP2 resynthesis and TRPC channel deactivation) facilitated the repeatability of the KP calcium response.
Fig. 5.
Exposure to nitric oxide facilitates a second response to KP. (A) Average traces for changes in [Ca2+] levels (percent) evoked by two exposures to KP 100 nM with (blue circles) or without (black circles) DEA/NO application in between KP application. TTX was added at the arrow and remained present for the whole recording period. (B) A bar graph showing the ratio between the two responses to KP (KP2/KP1) when not exposed to DEA/NO (black) or exposed to DEA/NO (blue) after KP1. The ratio of KP2/KP1 calcium responses was greater when DEA/NO was applied between KP application. Statistical significance (P < 0.05) is indicated with an asterisk (unpaired t test with Welch’s correction). (C) Two examples of electrophysiological recordings showing the first response and the second response to KP, when artificial cerebrospinal fluid (aCSF) only (Top trace) or DEA/NO (Bottom trace) was used in between KP applications. (bars: 2 min, 50 pA). (D) A bar graph showing the firing rate before and after KP1 and KP2, when aCSF only (black) or DEA/NO (blue) was used in between KP applications. The firing rates (aCSF, KP1, and washout [aCSF and NO/aCSF]) were similar between both paradigms (KP–KP [black] and KP–NO–KP [blue]), but the firing rate after KP2 was statistically greater when DEA/NO was applied between KP applications. Statistical significance (P < 0.05) was indicated with an asterisk (nonparametric Mann–Whitney U test).
The KP firing response also relies upon the activation of TRPC channels and lack of repeatability in primary GnRH neurons in acute slices (19, 55). Thus, using this different model, we examined whether DEA/NO application would allow the KP firing response to repeat. We repeated this paradigm (KP twice, with or without DEA/NO in between) on GnRH cells in acute brain slices, monitoring electrical activity with loose patch recordings. To our surprise, the KP-evoked increase in firing was partially repeatable in cells which had returned to baseline activity in the KP twice group without DEA/NO (Fig. 5C). However, the application of DEA/NO allowed a larger response to occur with the second application of KP (Fig. 5 C and D, P = 0.0451).
Discussion
To date, the majority of studies examining the effect of KP on GnRH cells focus on the initiation of the KP response [reviewed in (56)]. The present study investigates how KP evokes a long-lasting response in GnRH neurons and how GnRH neurons can return to baseline to allow repetitive responses to KP, which is the keystone for the KNDy model to work. We show that 1) the ongoing activity of TRPC channels, subsequently activating L-/N-type VGCC, maintains the calcium response, 2) the ongoing activity of the TRPC channels is determined by the kinetics of PIP2 resynthesis, 3) nitric oxide accelerates PIP2 resynthesis and terminates the KP response, via a protein kinase G-dependent mechanism, and 4) nitric oxide exposure between two subsequent exposures to KP enhances a second calcium and firing response.
Previous study using heterologous expression systems showed Kiss1r internalization over prolonged exposure to KP (57). However, a 2- to 5-min stimulation, sufficient to elicit a GnRH/LH pulse in vivo (26), did not lead to Kiss1r internalization. In our paradigm, the calcium response fully developed in GnRH neurons within the 3 min following the removal of KP. Thus, this study focused on the signaling pathway downstream of the Kiss1r controlling return to baseline of GnRH neurons after KP stimulation.
Electrophysiological studies identified the phospholipase C pathway downstream of the Kiss1r and implicated Ba2+-sensitive potassium channels, flufenamic acid (FFA)-sensitive TRP channels, 2-APB-sensitive TRP channels, and/or InsP3R in the KP response (19, 55). Studies using calcium imaging have shown that at the cell body, the sarco/endoplasmic reticulum calcium transport ATPase blocker, thapsigargin, is ineffective on the KP-evoked increase in [Ca2+]i, suggesting that InsP3-dependent calcium stores are not solely involved in the calcium response to KP (15). Both 2-APB or FFA reduced the percentage of cells responding to KP but neither totally abolished the response, suggesting that TRP channels are not the only components of the calcium response to KP. Only 2-APB combine with TTX were effective, indicating the facilitation of a plasma membrane component by TRPC channels (15). At the GnRH neuron nerve terminal, similar conclusions were reached (16). The only difference in the calcium response to KP between the GnRH neuron cell body and nerve terminal is the relative contribution of InsP3-dependent calcium stores (16). While the role of TRPC channels in the onset of the KP response, firing and calcium, is well documented (15, 16, 19, 37, 55), their ongoing activity throughout the long-lasting response is an important new point to acknowledge while thinking about the reversal of the KP response.
Consistent with previous data (16), our data show that VGCC were activated and remained active throughout the KP response. However, our experiments reveal that VGCC did not drive the response, being downstream TRPC channels. Indeed, usually TRPC do not mediate a calcium response but work in synergy with VGCC (58). Hence, for the GnRH neuron, a return to baseline activity required deactivation of TRPC channels. On one hand, PKC is known to facilitate the KP response (15, 37) and to regulate the activity of TRPC channels (39). On the other hand, PP2A associates with Kiss1r in heterologous system (36), can be activated by calcium influx through TRPC channels (59), and is a well-known player in neuronal long-lasting phenomena (33, 34). However, neither manipulation impacted the duration of the calcium response.
The KP-induced TRPC activation requires PIP2 depletion, with TRPC4alpha being the sensor (37, 60). Hence, the logical reciprocal to deactivate TRPC would be the replenishment of PIP2. Indeed, blocking PI4K (i.e., preventing the resynthesis of PIP2) prolonged the calcium response, which is in agreement with an enhanced cationic current (37). In hippocampal cultures, nitric oxide increases membrane PIP2 (46, 61) and in GnRH neurons, nitric oxide terminated the KP-evoked calcium response. When PI4K was blocked, DEA/NO had no effect, supporting the idea that it facilitates PIP2 resynthesis but does not directly inhibit TRPC (62) or VGCC (63) via S-nitrosylation. Similarly, S-nitrosylation of β-arrestin could not account for accelerated internalization and a subsequent arrest of calcium response (64). In fact, the DEA/NO effect was partially mimicked by a membrane-permeant cGMP and blocked by a guanylyl cyclase inhibitor, supporting the canonical NO–sGC–cGMP signaling pathway (65). Although Rho-kinase is the link between PKG and PIP2 synthesis (47), our data with 17-min Rho-kinase blocker pretreatment did not show an effect and one would expect longer exposure to decrease PIP2 membrane content. Thus, further experiments are needed to identify the link between PKG and PIP2 synthesis.
Notably, our data indicate that DEA/NO application between two KP applications enhances the second response to KP, both calcium and firing. Surprisingly, in our hands, the KP effect on GnRH neuron firing was repeatable in cells that had recovered, at least partially, from the first KP application. This observation is in contrast with other studies (19, 30). One explanation might be the method used. One study used gramicidin-perforated patch clamp which is known to induce membrane lipid redistribution (66). The other study used whole-cell patch clamp which is known to alter PIP2, hence ionic channel function (67, 68), with current run-down being the apparent consequence of cytosol dialysis (69). However, the effect of RF9 on GnRH neurons is also mediated by Kiss1R and shown to be repeatable (70). Thus, the variable response of GnRH neurons to a second application of KP remains unclear. However, the experiments in the present study clearly indicate that nitric oxide allowed GnRH neurons to generate a greater response to the second KP application. While many parameters for the DEA/NO application might need optimization to achieve a full reset, it is obvious that nitric oxide facilitated the second response to KP. One could argue [Ca2+]i remained higher in KP–KP than KP–NO–KP, even after KP2. Yet, the increase in [Ca2+]i preKP2-postKp2 (Δ[Ca2+]i) was greater in KP–NO–KP than KP-KP (i.e., greater ratio KP2/KP1 in KP–NO–KP than KP–KP). Return to baseline and changes in [Ca2+]i were judged more relevant because 1) neuropeptide secretion responds to calcium dynamics (71) and 2) tonic secretion is not sustainable as it leads to fatigue (72).
The ability of nitric oxide to terminate the KP response is of particular importance. Nitric oxide, possibly one of the earliest signaling molecules in evolution (73), is at the center of reproductive neuroendocrinology (review in (50)). In females, nitric oxide regulates ovulation, likely through a nitrergic connection between KP and GnRH neurons. Endothelial cells express Kiss1r (74, 75) and median eminence fragments exhibit eNOS-synthetized nitric oxide pulses whose frequency is compatible with GnRH pulses (44), yet mice lacking eNOS are fertile (76). KP neurons contact nitric oxide-synthesizing neurons expressing Kiss1r (77), and nNOS-containing fibers contact GnRH neurons in the POA (78). Accordingly, female nNOS knockout are infertile. However, males are also infertile (79), implying an additional interaction. A dense nNOS-containing fiber network exists in the median eminence (41–43). Some evidently belong to the hypothalamo-neurohypophysial system (80). However, while the nNOS-containing fibers contacting GnRH cell bodies are glutamatergic, the nNOS-containing fibers contacting GnRH nerve terminals are GABAergic (81). These GABAergic nNOS fibers likely come from the ARC since only this nucleus contains GABAergic nitrergic neurons, while other nuclei mostly contain glutamatergic nitrergic neurons (81). This population also stands out by its resistance to NADPH-diaphorase staining, which is usually seen in nitric oxide positive cells (43, 82). The different sites of action for nitric oxide are supported by functional changes evoked by nitric oxide and its downstream cGMP/PKG pathway: at the GnRH neuron cell body, nitric oxide inhibits GnRH firing (78); at the GnRH neuron distal process, it facilitates GnRH release (44, 83).
KP signaling pathways allow subcellular regions of GnRH neurons to have specific roles (2). The integration of KP inputs at the GnRH neuron cell body is critical for the GnRH/LH surge, while the integration of KP inputs at the GnRH neuron distal process is critical for GnRH/LH pulses (32). Hence, nitrergic modulation of GnRH neuron cell body relates to the preovulatory surge while nitrergic modulation of GnRH neuron distal process relates to GnRH pulsatility. In the preoptic area, the release of nitric oxide restrains GnRH neurons during the estrogen-negative feedback, but increased nitric oxide production is a landmark for GnRH/LH surge during the estrogen-positive feedback (84). Kisspeptinergic stimulation of nNOS-expressing neurons in the preoptic area mimics their activation during the estrogen-positive feedback (77). At the median eminence, nitric oxide stimulates GnRH release in males and females (44, 83) and estrogen enhances the amplitudes of nitric oxide pulses, but not its frequency (44). To date, the stimulation of GnRH release by nitric oxide is explained by the retraction of tanycyte end-feet (84). However, these nitric oxide–mediated morphological changes occur only during proestrus to facilitate the GnRH/LH surge (85). Yet, the role of nitric oxide pulses at the median eminence for GnRH/LH pulses was hypothesized (44). The present study focused upon the action potential–insensitive signaling that normally occurs at the distal GnRH neuron process under kisspeptinergic inputs and its nitrergic modulation.
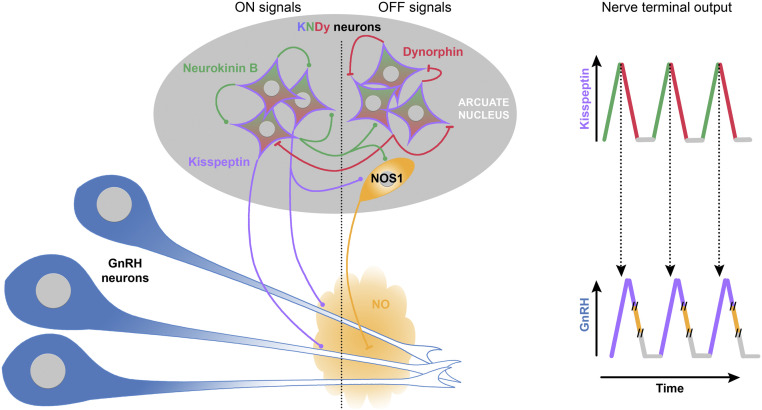
To date, research has focused on how KP can evoke GnRH secretion looking at the onset of the response in GnRH neurons and how nitric oxide modulates GnRH secretion looking at its action upon firing rate at the GnRH cell body (50). However, an anatomical relationship between KP fibers and NOS1 neurons in the ARC is found in mouse, sheep, and nonhuman primate (77, 86, 87). To our knowledge, no equivalent study exists in human but NOS1 is present in the infundibular nucleus (88) where human GnRH neurons receive the heaviest innervation (89). In the present report, we highlight a common mechanism between KP and nitric oxide, (i.e., degradation and resynthesis of PIP2 respectively, independent of firing activity). While the calcium response of GnRH neurons to KP requires depletion of PIP2 (37), a subsequent response requires its resynthesis. The rate of the PIP2 replenishment sets the refractory period for the efficiency of the next response to KP. Nitric oxide facilitates the next response to KP, by facilitating PIP2 resynthesis, subsequently deactivating TRPC channels and restoring their availability. For appropriate timing of KP/nitric oxide interplay, nitric oxide might be synthetized by NOS1 neurons in response to a Kiss1 neuron signal, the location of these NOS1 neurons remains to determined. However, given its unique rapid and unrestrained diffusibility, nitric oxide might provide the break for the calcium response in a pool of GnRH neurons simultaneously and restore their ability to respond to the next kisspeptinergic stimulation (Fig. 6). Thus, the PIP2 signaling overlap between KP and nitric oxide may be an essential component for GnRH neuron pulses and provide the response to the long-sought-after question (90–93) (i.e., synchronization within the GnRH neuronal population).
Fig. 6.
Proposed model for pulsatile GnRH/LH release controlled by KNDy neurons. KNDy neurons are an interconnected population of neurons in the arcuate nucleus that coexpress KP (purple), neurokinin B (green), and dynorphin (red). The division of KNDy neurons in the diagram is artificial and serves to depict ON- (Left) and OFF- (Right) signals. According to the KNDy model, GnRH neurons are activated by KP, ON-signal (round inputs), and Neurokinin B, and dynorphin participates in an autoregenerative mechanism driving bouts of activity within the KNDy population. Neurokinin B evokes neuronal excitation (ON-signal, round inputs). Subsequently, released dynorphin evokes neuronal inhibition (OFF-signal, flat inputs). This alternation of excitation and inhibition leads to episodic KP release onto GnRH neurons (blue). The contribution of each input into the episodic release of KP is depicted on the top right. The original KNDy model suggests that episodic KP release is sufficient to trigger pulsatile GnRH release. However, this model does not take into consideration that KP evokes a long-lasting calcium response in GnRH neurons that hampers the ability of GnRH neurons to respond to the next kisspeptinergic event. Here, we propose the addition of a third partner, neuronal nitric oxide synthase (NOS1)-expressing neurons (orange). In this model, NOS1-expressing neurons, activated by KNDy neurons, via KP or neurokinin B or other unknown coexpressed neuropeptides, release nitric oxide (OFF-signal, flat inputs) in the vicinity of GnRH neurons. Highly diffusible, nitric oxide provides a global reset of the signaling pathway components within GnRH neurons necessary for the next kisspeptinergic event. The contribution of each input into the episodic release of GnRH is depicted on the bottom right, where NO release occurs sometime after kisspeptinergic stimulation (time break, //).
Materials and Methods
Animals.
Embryos collected from timed-pregnant NIH Swiss mice were used to generate primary explant cultures (94) (SI Appendix). Adult GnRH-green fluorescent protein (GnRH-GFP, MGI:6158458) male mice (2 to 4 mo old, gonad intact) were used for electrophysiological recordings (95) (SI Appendix). All procedures were approved by the National Institute of Neurological Disorders and Stroke, Animal Care and Use Committee and performed in accordance with NIH guidelines.
Calcium Imaging.
Explants (14 to 18 d in culture) were used as previously described (15), and the phenotype of the recorded cells was confirmed with immunocytochemistry for GnRH (Fig. 1A) (SI Appendix).
Electrophysiology.
Male mice were chosen to avoid the possible influence of fluctuating circulating steroids. Electrophysiology was performed as previously described (96) (SI Appendix).
Statistical Analysis.
Calcium imaging analysis.
Regions of interest were drawn around GnRH neurons. Changes in optical densities over time were detected in iVision and analyzed in Matlab (Mathworks, Natick, MA). All files are processed with the minimum user inputs to avoid bias (SI Appendix). The graphs represent the mean ± SEM of independent recordings from at least three explants (N represents the number of explants). For each trace, the time at which the average trace is midway from maximal response and baseline (time at 50% recovery in minutes) was determined (Fig. 1 D and E). Experimental values were compared to KP control (100 nM) values using a nonparametric Mann–Whitney U test. All results are summarized in Table 1.
Table 1.
Calcium imaging summary
| Drugs (n explants) | Time at 50% recovery (min) | P value |
| KP 10 nM (n = 3) | 27.0 ± 1.0 | 0.3667 |
| KP 100 nM (n = 7) | 30.0 ± 1.4 | — |
| + OKA (n = 7) | 32.3 ± 2.3 | 0.3671 |
| + C2 (n = 4) | 30.5 ± 4.72 | 0.6061 |
| + BIM X (n = 3) | 31.7 ± 4.8 | 0.9333 |
| + 2-APB (n = 3) | 22.0 ± 1.2 | 0.0083* |
| + 0 Ca2+ (n = 3) | 21.3 ± 2.3 | 0.0083* |
| + Nif + Ctx GVIA (n = 3) | 19.7 ± 0.3 | 0.0083* |
| + SKF96365 (n = 3) | 26.7 ± 2.2 | 0.2333 |
| + La3+ (n = 3) | 26.7 ± 1.2 | 0.1500 |
| + FFA (n = 5) | 33.6 ± 1.2 | 0.1174 |
| + Wort [nM] (n = 4) | 29.3 ± 3.0 | 0.7545 |
| + Wort [uM] (n = 5) | 38.4 ± 2.7 | 0.0278* |
| + NO 30 min (n = 7) | 22.0 ± 1.3 | 0.0041* |
| + Wort [uM] + NO 30 min (n = 6) | 34.2 ± 4.4 | 0.4254 |
| + NO 5 min (n = 5) | 23.0 ± 3.0 | 0.0239* |
| + cGMP 5 min (n = 4) | 19.3 ± 0.9 | 0.0061* |
| + cGMP 10 min (n = 3) | 20.7 ± 1.2 | 0.0083* |
| + ODQ + NO 30 min (n = 4) | 33.0 ± 4.3 | 0.7636 |
| + Y27632 + NO 30 min (n = 6) | 21.7 ± 2.1 | 0.0204* |
*P < 0.05.
To test the repeatability of the KP response, KP was applied on explants twice (i.e., after a 25-min washout period) with and without DEA/NO (100 µM) in between (SI Appendix). The amplitude of the responses was determined by subtracting the baseline value to the response value, and the amplitude of the second application was normalized to the amplitude of the first application. Unpaired nonparametric Mann–Whitney U test was used to compare the second KP response.
Electrophysiology analysis.
Analysis was performed with Clampfit 10 over 5-min windows before and after KP. KP was applied twice, with or without DEA/NO (100 µM) in between (SI Appendix). The responses to the second KP application between cells exposed or not to DEA/NO were compared using an unpaired t test with Welch’s correction.
Drugs.
The drugs (name, abbreviation, doses, target, and providers) are listed in Table 2.
Table 2.
Drug list
| Chemical names | Abbreviations | Doses | Targets | Providers, catalog no. |
| Tetrodotoxin | TTX | 0.5 µM | Na+ channel blocker | Tocris, 1069 and Cayman, 14964 |
| Kisspeptin-10 | KP | 10 or 100 nM | Kiss1r agonist | Tocris, 4243 and Phoenix Pharmaceuticals Inc., 048–56 |
| Okadaic acid | OKA | 30 nM | Protein phosphatase 1 and 2A inhibitor | Cayman, 10011490 |
| N-acetoyl-D-erythro-sphingosine | C2 ceramide | 20 µM | Protein phosphatase A2 activator | Cayman, 62510 |
| Bisindolylmaleimide X | BIM X | 100 nM | Protein kinase C inhibitor | Cayman, 17511 |
| 2-Aminoethoxydi-phenylborane | 2-APB | 75 µM | TRPC1, TRPC3, TRPC5, and TRPC6 blocker & IP3 receptor blocker | Cayman, 64970 |
| Nifedipine | Nif | 1 µM | L-type voltage-gated calcium channel blocker | Sigma, N7634 |
| ω-conotoxin GVIA | Ctx GVIA | 100 nM | N-type voltage-gated calcium channel blocker | Sigma, C9915 |
| SKF96365 | SKF96365 | 30 µM | TRPC6, TRPC7 blocker | Tocris, 1147 |
| Lanthanum | La3+ | 100 µM | TRPC3, TRPC4/5, TRPC6, TRPC7 blocker | Sigma, 449830 |
| Flufenamic acid | FFA | 100 µM | TRPC3, TRPC5, and TRPC7 inhibitor | Sigma, F9005 |
| Wortmannin | Wort [nM] | 100 nM | PI3K inhibitor | Cayman 10010591 |
| Wortmannin | Wort [µM] | 10 µM | PI4K inhibitor | |
| Diethylamine NONOate | DEA/NO | 100 µM | NO donor | Cayman 82100 |
| 8-pCPT-cGMP | cGMP | 200 µM | GMP-dependent protein kinase activator | Sigma, C5438 |
| 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one | ODQ | 30 µM | NO-sensitive guanylyl cyclase inhibitor | Cayman, 81410 |
| Y27632 | Y27632 | 10 µM | ROCK-I and ROCK-II inhibitor | MedChemExpress, HY-10583 |
Supplementary Material
Acknowledgments
This work was supported by the Intramural Research Program of the NIH, National Institute of Neurological Disorders and Stroke (Grant ZIA-NS-002824).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2012339118/-/DCSupplemental.
Data Availability.
All study data are included in the article and SI Appendix.
References
- 1.Herbison A. E., “Physiology of the adult gonadotropin-releasing hormone neuronal network” in Knobil and Neill’s Physiology of Reproduction, Knobil E., Neill J., Eds. (Elsevier, ed. 4, 2015), pp. 399–467. [Google Scholar]
- 2.Constantin S., Progress and challenges in the search for the mechanisms of pulsatile GnRH secretion. Front. Endocrinol. (Lausanne) 8, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsutsumi R., Webster N. J., GnRH pulsatility, the pituitary response and reproductive dysfunction. Endocr. J. 56, 729–737 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.León S., et al. , Direct actions of kisspeptins on GnRH neurons permit attainment of fertility but are insufficient to fully preserve gonadotropic Axis Activity. Sci. Rep. 6, 19206 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clarkson J., d’Anglemont de Tassigny X., Moreno A. S., Colledge W. H., Herbison A. E., Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J. Neurosci. 28, 8691–8697 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeo S. H., Clarkson J., Herbison A. E., Kisspeptin-gpr54 signaling at the GnRH neuron is necessary for negative feedback regulation of luteinizing hormone secretion in female mice. Neuroendocrinology 100, 191–197 (2014). [DOI] [PubMed] [Google Scholar]
- 7.de Roux N., et al. , Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc. Natl. Acad. Sci. U.S.A. 100, 10972–10976 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Funes S., et al. , The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem. Biophys. Res. Commun. 312, 1357–1363 (2003). [DOI] [PubMed] [Google Scholar]
- 9.Pineda R., et al. , Critical roles of kisspeptins in female puberty and preovulatory gonadotropin surges as revealed by a novel antagonist. Endocrinology 151, 722–730 (2010). [DOI] [PubMed] [Google Scholar]
- 10.Adachi S., et al. , Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J. Reprod. Dev. 53, 367–378 (2007). [DOI] [PubMed] [Google Scholar]
- 11.Dungan H. M., et al. , The role of kisspeptin-GPR54 signaling in the tonic regulation and surge release of gonadotropin-releasing hormone/luteinizing hormone. J. Neurosci. 27, 12088–12095 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clarkson J., Han S. K., Liu X., Lee K., Herbison A. E., Neurobiological mechanisms underlying kisspeptin activation of gonadotropin-releasing hormone (GnRH) neurons at puberty. Mol. Cell. Endocrinol. 324, 45–50 (2010). [DOI] [PubMed] [Google Scholar]
- 13.Clarkson J., Herbison A. E., Oestrogen, kisspeptin, GPR54 and the pre-ovulatory luteinising hormone surge. J. Neuroendocrinol. 21, 305–311 (2009). [DOI] [PubMed] [Google Scholar]
- 14.Moore A. M., Coolen L. M., Porter D. T., Goodman R. L., Lehman M. N., KNDy cells revisited. Endocrinology 159, 3219–3234 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Constantin S., Caligioni C. S., Stojilkovic S., Wray S., Kisspeptin-10 facilitates a plasma membrane-driven calcium oscillator in GnRH-1 neurons. Endocrinology 150, 1400–1412 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iremonger K. J., Porteous R., Herbison A. E., Spike and neuropeptide-dependent mechanisms control GnRH neuron nerve terminal Ca2+ over diverse time scales. J. Neurosci. 37, 3342–3351 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han S. K., et al. , Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J. Neurosci. 25, 11349–11356 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Constantin S., Iremonger K. J., Herbison A. E., In vivo recordings of GnRH neuron firing reveal heterogeneity and dependence upon GABAA receptor signaling. J. Neurosci. 33, 9394–9401 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu X., Lee K., Herbison A. E., Kisspeptin excites gonadotropin-releasing hormone neurons through a phospholipase C/calcium-dependent pathway regulating multiple ion channels. Endocrinology 149, 4605–4614 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu X., et al. , Frequency-dependent recruitment of fast amino acid and slow neuropeptide neurotransmitter release controls gonadotropin-releasing hormone neuron excitability. J. Neurosci. 31, 2421–2430 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Constantin S., Wray S., Gonadotropin-releasing hormone-1 neuronal activity is independent of hyperpolarization-activated cyclic nucleotide-modulated channels but is sensitive to protein kinase a-dependent phosphorylation. Endocrinology 149, 3500–3511 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee K., Duan W., Sneyd J., Herbison A. E., Two slow calcium-activated afterhyperpolarization currents control burst firing dynamics in gonadotropin-releasing hormone neurons. J. Neurosci. 30, 6214–6224 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X. B., Spergel D. J., Kisspeptin inhibits high-voltage activated Ca2+ channels in GnRH neurons via multiple Ca2+ influx and release pathways. Neuroendocrinology 96, 68–80 (2012). [DOI] [PubMed] [Google Scholar]
- 24.Yip S. H., Boehm U., Herbison A. E., Campbell R. E., Conditional viral tract-tracing delineates the projections of the distinct kisspeptin neuron populations to gonadotropin-releasing hormone (GnRH) neurons in the mouse. Endocrinology 156, 2582–2594 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Czieselsky K., et al. , Pulse and surge profiles of luteinizing hormone secretion in the mouse. Endocrinology 157, 4794–4802 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Han S. Y., McLennan T., Czieselsky K., Herbison A. E., Selective optogenetic activation of arcuate kisspeptin neurons generates pulsatile luteinizing hormone secretion. Proc. Natl. Acad. Sci. U.S.A. 112, 13109–13114 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grachev P., et al. , Suppression of the GnRH pulse generator by neurokinin B involves a κ-opioid receptor-dependent mechanism. Endocrinology 153, 4894–4904 (2012). [DOI] [PubMed] [Google Scholar]
- 28.Mostari P., et al. , dynorphin-kappa opioid receptor signaling partly mediates estrogen negative feedback effect on LH pulses in female rats. J. Reprod. Dev. 59, 266–272 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hrabovszky E., et al. , Low degree of overlap between kisspeptin, neurokinin B, and dynorphin immunoreactivities in the infundibular nucleus of young male human subjects challenges the KNDy neuron concept. Endocrinology 153, 4978–4989 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dumalska I., et al. , Excitatory effects of the puberty-initiating peptide kisspeptin and group I metabotropic glutamate receptor agonists differentiate two distinct subpopulations of gonadotropin-releasing hormone neurons. J. Neurosci. 28, 8003–8013 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glanowska K. M., Moenter S. M., Differential regulation of GnRH secretion in the preoptic area (POA) and the median eminence (ME) in male mice. Endocrinology 156, 231–241 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang L., et al. , Different dendritic domains of the GnRH neuron underlie the pulse and surge modes of GnRH secretion in female mice. eLife 9, e53945 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colbran R. J., Protein phosphatases and calcium/calmodulin-dependent protein kinase II-dependent synaptic plasticity. J. Neurosci. 24, 8404–8409 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mullasseril P., Dosemeci A., Lisman J. E., Griffith L. C., A structural mechanism for maintaining the ‘on-state’ of the CaMKII memory switch in the post-synaptic density. J. Neurochem. 103, 357–364 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shonesy B. C., Jalan-Sakrikar N., Cavener V. S., Colbran R. J., CaMKII: A molecular substrate for synaptic plasticity and memory. Prog. Mol. Biol. Transl. Sci. 122, 61–87 (2014). [DOI] [PubMed] [Google Scholar]
- 36.Evans B. J., et al. , Physical association of GPR54 C-terminal with protein phosphatase 2A. Biochem. Biophys. Res. Commun. 377, 1067–1071 (2008). [DOI] [PubMed] [Google Scholar]
- 37.Zhang C., Bosch M. A., Rønnekleiv O. K., Kelly M. J., Kisspeptin activation of TRPC4 channels in female GnRH neurons requires PIP2 depletion and cSrc kinase activation. Endocrinology 154, 2772–2783 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang C., Rønnekleiv O. K., Kelly M. J., Kisspeptin inhibits a slow afterhyperpolarization current via protein kinase C and reduces spike frequency adaptation in GnRH neurons. Am. J. Physiol. Endocrinol. Metab. 304, E1237–E1244 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Venkatachalam K., Zheng F., Gill D. L., Regulation of canonical transient receptor potential (TRPC) channel function by diacylglycerol and protein kinase C. J. Biol. Chem. 278, 29031–29040 (2003). [DOI] [PubMed] [Google Scholar]
- 40.Bootman M. D., et al. , 2-aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J. 16, 1145–1150 (2002). [DOI] [PubMed] [Google Scholar]
- 41.Bhat G. K., et al. , Histochemical localization of nitric oxide neurons in the hypothalamus: Association with gonadotropin-releasing hormone neurons and co-localization with N-methyl-D-aspartate receptors. Neuroendocrinology 62, 187–197 (1995). [DOI] [PubMed] [Google Scholar]
- 42.Herbison A. E., Simonian S. X., Norris P. J., Emson P. C., Relationship of neuronal nitric oxide synthase immunoreactivity to GnRH neurons in the ovariectomized and intact female rat. J. Neuroendocrinol. 8, 73–82 (1996). [DOI] [PubMed] [Google Scholar]
- 43.Ng Y. K., Xue Y. D., Wong P. T., Different distributions of nitric oxide synthase-containing neurons in the mouse and rat hypothalamus. Nitric Oxide 3, 383–392 (1999). [DOI] [PubMed] [Google Scholar]
- 44.Knauf C., et al. , Evidence for a spontaneous nitric oxide release from the rat median eminence: Influence on gonadotropin-releasing hormone release. Endocrinology 142, 2343–2350 (2001). [DOI] [PubMed] [Google Scholar]
- 45.Clementi E., et al. , Nitric oxide action on growth factor-elicited signals. Phosphoinositide hydrolysis and [Ca2+]i responses are negatively modulated via a cGMP-dependent protein kinase I pathway. J. Biol. Chem. 270, 22277–22282 (1995). [DOI] [PubMed] [Google Scholar]
- 46.Micheva K. D., Holz R. W., Smith S. J., Regulation of presynaptic phosphatidylinositol 4,5-biphosphate by neuronal activity. J. Cell Biol. 154, 355–368 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taoufiq Z., Eguchi K., Takahashi T., Rho-kinase accelerates synaptic vesicle endocytosis by linking cyclic GMP-dependent protein kinase activity to phosphatidylinositol-4,5-bisphosphate synthesis. J. Neurosci. 33, 12099–12104 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen J., Crossland R. F., Noorani M. M. Z., Marrelli S. P., Inhibition of TRPC1/TRPC3 by PKG contributes to NO-mediated vasorelaxation. Am. J. Physiol. Heart Circ. Physiol. 297, H417–H424 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Snyder S. H., Nitric oxide: First in a new class of neurotransmitters. Science 257, 494–496 (1992). [DOI] [PubMed] [Google Scholar]
- 50.Chachlaki K., Garthwaite J., Prevot V., The gentle art of saying NO: How nitric oxide gets things done in the hypothalamus. Nat. Rev. Endocrinol. 13, 521–535 (2017). [DOI] [PubMed] [Google Scholar]
- 51.Sen N., Snyder S. H., Protein modifications involved in neurotransmitter and gasotransmitter signaling. Trends Neurosci. 33, 493–502 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bian K., Ke Y., Kamisaki Y., Murad F., Proteomic modification by nitric oxide. J. Pharmacol. Sci. 101, 271–279 (2006). [DOI] [PubMed] [Google Scholar]
- 53.Sunico C. R., González-Forero D., Domínguez G., García-Verdugo J. M., Moreno-López B., Nitric oxide induces pathological synapse loss by a protein kinase G-, Rho kinase-dependent mechanism preceded by myosin light chain phosphorylation. J. Neurosci. 30, 973–984 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu X., Herbison A. E., Kisspeptin regulation of neuronal activity throughout the central nervous system. Endocrinol. Metab. (Seoul) 31, 193–205 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang C., Roepke T. A., Kelly M. J., Rønnekleiv O. K., Kisspeptin depolarizes gonadotropin-releasing hormone neurons through activation of TRPC-like cationic channels. J. Neurosci. 28, 4423–4434 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Piet R., de Croft S., Liu X., Herbison A. E., Electrical properties of kisspeptin neurons and their regulation of GnRH neurons. Front. Neuroendocrinol. 36, 15–27 (2015). [DOI] [PubMed] [Google Scholar]
- 57.M. Leet al., Dynamic kisspeptin receptor trafficking modulates kisspeptin-mediated calcium signaling. J. Mol. Endocrinol. 28, 16–27 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gees M., Colsoul B., Nilius B., The role of transient receptor potential cation channels in Ca2+ signaling. Cold Spring Harb. Perspect. Biol. 2, a003962 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marasa B. S., et al. , Induced TRPC1 expression increases protein phosphatase 2A sensitizing intestinal epithelial cells to apoptosis through inhibition of NF-kappaB activation. Am. J. Physiol. Cell Physiol. 294, C1277–C1287 (2008). [DOI] [PubMed] [Google Scholar]
- 60.Otsuguro K., et al. , Isoform-specific inhibition of TRPC4 channel by phosphatidylinositol 4,5-bisphosphate. J. Biol. Chem. 283, 10026–10036 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Micheva K. D., Buchanan J., Holz R. W., Smith S. J., Retrograde regulation of synaptic vesicle endocytosis and recycling. Nat. Neurosci. 6, 925–932 (2003). [DOI] [PubMed] [Google Scholar]
- 62.Yoshida T., et al. , Nitric oxide activates TRP channels by cysteine S-nitrosylation. Nat. Chem. Biol. 2, 596–607 (2006). [DOI] [PubMed] [Google Scholar]
- 63.Zhou M.-H., Bavencoffe A., Pan H.-L., Molecular basis of regulating high voltage-activated calcium channels by S-nitrosylation. J. Biol. Chem. 290, 30616–30623 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ozawa K., et al. , S-nitrosylation of beta-arrestin regulates beta-adrenergic receptor trafficking. Mol. Cell 31, 395–405 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Honda A., et al. , Spatiotemporal dynamics of guanosine 3′,5′-cyclic monophosphate revealed by a genetically encoded, fluorescent indicator. Proc. Natl. Acad. Sci. U.S.A. 98, 2437–2442 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Beaven A. H., et al. , Gramicidin A channel formation induces local lipid redistribution I: Experiment and simulation. Biophys. J. 112, 1185–1197 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Suh B.-C., Hille B., PIP2 is a necessary cofactor for ion channel function: How and why? Annu. Rev. Biophys. 37, 175–195 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu L., et al. , Gαq sensitizes TRPM8 to inhibition by PI(4,5)P2 depletion upon receptor activation. J. Neurosci. 39, 6067–6080 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hume J. R., Leblanc R. N., A whole-cell patch clamp technique which minimizes cell dialysis. Mol. Cell. Biochem. 80, 49–57 (1988). [DOI] [PubMed] [Google Scholar]
- 70.Liu X., Herbison A. E., RF9 excitation of GnRH neurons is dependent upon Kiss1r in the adult male and female mouse. Endocrinology 155, 4915–4924 (2014). [DOI] [PubMed] [Google Scholar]
- 71.Muschol M., Salzberg B. M., Dependence of transient and residual calcium dynamics on action-potential patterning during neuropeptide secretion. J. Neurosci. 20, 6773–6780 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.MacGregor D. J., Leng G., Phasic firing in vasopressin cells: Understanding its functional significance through computational models. PLoS Comput. Biol. 8, e1002740 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Murad F., Barber R., A hypothesis about cellular signaling with nitric oxide in the earliest life forms in evolution. Free Radic. Biol. Med. 47, 1325–1327 (2009). [DOI] [PubMed] [Google Scholar]
- 74.Mead E. J., Maguire J. J., Kuc R. E., Davenport A. P., Kisspeptins are novel potent vasoconstrictors in humans, with a discrete localization of their receptor, G protein-coupled receptor 54, to atherosclerosis-prone vessels. Endocrinology 148, 140–147 (2007). [DOI] [PubMed] [Google Scholar]
- 75.Ramaesh T., et al. , Kisspeptin-10 inhibits angiogenesis in human placental vessels ex vivo and endothelial cells in vitro. Endocrinology 151, 5927–5934 (2010). [DOI] [PubMed] [Google Scholar]
- 76.Huang P. L., et al. , Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature 377, 239–242 (1995). [DOI] [PubMed] [Google Scholar]
- 77.Hanchate N. K., et al. , Kisspeptin-GPR54 signaling in mouse NO-synthesizing neurons participates in the hypothalamic control of ovulation. J. Neurosci. 32, 932–945 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Clasadonte J., Poulain P., Beauvillain J. C., Prevot V., Activation of neuronal nitric oxide release inhibits spontaneous firing in adult gonadotropin-releasing hormone neurons: A possible local synchronizing signal. Endocrinology 149, 587–596 (2008). [DOI] [PubMed] [Google Scholar]
- 79.Gyurko R., Leupen S., Huang P. L., Deletion of exon 6 of the neuronal nitric oxide synthase gene in mice results in hypogonadism and infertility. Endocrinology 143, 2767–2774 (2002). [DOI] [PubMed] [Google Scholar]
- 80.Vanhatalo S., Soinila S., Nitric oxide synthase in the hypothalamo-pituitary pathways. J. Chem. Neuroanat. 8, 165–173 (1995). [DOI] [PubMed] [Google Scholar]
- 81.Chachlaki K., et al. , Phenotyping of nNOS neurons in the postnatal and adult female mouse hypothalamus. J. Comp. Neurol. 525, 3177–3189 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bredt D. S., et al. , Nitric oxide synthase protein and mRNA are discretely localized in neuronal populations of the mammalian CNS together with NADPH diaphorase. Neuron 7, 615–624 (1991). [DOI] [PubMed] [Google Scholar]
- 83.Rettori V., et al. , Role of nitric oxide in the control of luteinizing hormone-releasing hormone release in vivo and in vitro. Proc. Natl. Acad. Sci. U.S.A. 90, 10130–10134 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bellefontaine N., et al. , Nitric oxide as key mediator of neuron-to-neuron and endothelia-to-glia communication involved in the neuroendocrine control of reproduction. Neuroendocrinology 93, 74–89 (2011). [DOI] [PubMed] [Google Scholar]
- 85.Prevot V., Dutoit S., Croix D., Tramu G., Beauvillain J. C., Semi-quantitative ultrastructural analysis of the localization and neuropeptide content of gonadotropin releasing hormone nerve terminals in the median eminence throughout the estrous cycle of the rat. Neuroscience 84, 177–191 (1998). [DOI] [PubMed] [Google Scholar]
- 86.Bedenbaugh M., et al. , Neuroanatomical relationship of nNOS to GnRH and kisspeptin neurons in adult female sheep and primates. Neuroendocrinology 107, 218–227 (2018). [DOI] [PubMed] [Google Scholar]
- 87.Bedenbaugh M. N., et al. , Kisspeptin, gonadotrophin-releasing hormone and oestrogen receptor α colocalise with neuronal nitric oxide synthase neurones in prepubertal female sheep. J. Neuroendocrinol. 30, 10.1111/jne.12560 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Egberongbe Y. I., et al. , The distribution of nitric oxide synthase immunoreactivity in the human brain. Neuroscience 59, 561–578 (1994). [DOI] [PubMed] [Google Scholar]
- 89.Hrabovszky E., Liposits Z., Afferent neuronal control of type-I gonadotropin releasing hormone neurons in the human. Front. Endocrinol. (Lausanne) 4, 130 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Voliotis M., et al. , The origin of GnRH pulse generation: An integrative mathematical-experimental approach. J. Neurosci. 39, 9738–9747 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gordan J. D., Attardi B. J., Pfaff D. W., Mathematical exploration of pulsatility in cultured gonadotropin-releasing hormone neurons. Neuroendocrinology 67, 2–17 (1998). [DOI] [PubMed] [Google Scholar]
- 92.LeBeau A. P., Van Goor F., Stojilkovic S. S., Sherman A., Modeling of membrane excitability in gonadotropin-releasing hormone-secreting hypothalamic neurons regulated by Ca2+-mobilizing and adenylyl cyclase-coupled receptors. J. Neurosci. 20, 9290–9297 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vidal A., Clement F., A dynamical model for the control of the GnRH neurosecretory system. J. Neuroendocrinol. 22, 1251–1266 (2010). [DOI] [PubMed] [Google Scholar]
- 94.Fueshko S., Wray S., LHRH cells migrate on peripherin fibers in embryonic olfactory explant cultures: An in vitro model for neurophilic neuronal migration. Dev. Biol. 166, 331–348 (1994). [DOI] [PubMed] [Google Scholar]
- 95.Spergel D. J., Krüth U., Hanley D. F., Sprengel R., Seeburg P. H., GABA- and glutamate-activated channels in green fluorescent protein-tagged gonadotropin-releasing hormone neurons in transgenic mice. J. Neurosci. 19, 2037–2050 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Constantin S., Wray S., Nociceptin/Orphanin-FQ inhibits gonadotropin-releasing hormone neurons via G-protein-gated inwardly rectifying potassium channels. eNeuro, 10.1523/ENEURO.0161-18.2018 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and SI Appendix.