Significance
Genetic factors can concurrently influence perceptions of threatening and stressful events, like discriminatory experiences, and increase liability for anxiety and negative affect. The question has remained unsettled as to whether genetic susceptibility to anxiety and negative affect confounds the relationship between discrimination exposure and these phenotypes. In a national probability sample of noninstitutionalized, English-speaking respondents (n = 1,146), we found that discrimination was positively associated with anxiety and negative affect, operationalized by a common latent factor, even after accounting for genetic confounds. These findings suggest that discrimination is a risk factor for anxiety and related disorders rather than a result of common genetic liability alone. Reducing exposure to discrimination has the potential to improve mental health at the population level.
Keywords: discrimination, anxiety, negative affect, internalizing, polygenic scores
Abstract
An established body of research indicates that discrimination is associated with increased symptoms of anxiety and negative affect. However, the association cannot be interpreted unambiguously as an exposure effect because a common set of genetic factors can simultaneously contribute to increased liability for symptoms of anxiety, negative affect, and the perception of discrimination. The present study elucidates the association between discrimination and anxiety/negative affect by implementing strict genetic controls in a large sample of adults. We used data from the biomarker project of the Study of Midlife Development in the United States (MIDUS), a national probability sample of noninstitutionalized, English-speaking respondents aged 25 to 74 y. Participants who consented to provide genetic data were biologically unrelated and of European ancestry as determined by genotype principal components analysis (n = 1,146). A single structural regression model was fit to the data with three measures of discrimination specified to load onto a latent factor and six measures of anxiety and negative affect specified to load onto a second latent factor. After accounting for potential genetic confounds—polygenic scores for anxiety, depression, and neuroticism and the first five genetic principal components—greater discrimination was associated with greater anxiety/negative affect (β = 0.53, SE = 0.04, P < 0.001). Findings suggest that measures of perceived discrimination should be considered environmental risk factors for anxiety/negative affect rather than indices of genetic liability for anxiety, depression, or neuroticism. Clinical interventions and prevention measures should focus on ways to mitigate the impact of discrimination to improve mental health at the population level.
Anxiety disorders (ADs) are the most common mental illness in the United States, affecting over 40 million adults in the United States every year (1). ADs represent a variety of different disorders, including generalized anxiety disorder (GAD), panic disorder (PD), or phobias, that are generally characterized by excessive, persistent, and impairing worry or fear (2). Symptoms of ADs are also a common associated feature of depressive disorders, and those who meet diagnostic criteria for one or more AD are likely to meet criteria for a depressive disorder, a common pattern of comorbidity thought to be undergirded by genetic risk factors for neuroticism or emotional liability (2). Overall, ADs account for a substantial burden of morbidity and mortality as well as long-term work disability and absenteeism (3–5). For instance, ADs are associated with several chronic health conditions, including heart disease, hypertension, and diabetes (6, 7). Although the etiology of anxiety and related disorders remains unclear, familial and genetic factors have been established as risk factors.
Family and twin studies reveal that 20 to 40% of the variance contributing to ADs is heritable (8–10). For instance, Hettema et al. meta-analyzed two large twin studies and found the ∼32% of the variance for liability to GAD was attributable to additive genetic factors and that the same genes predispose men and women to GAD (8). With recent advances in molecular genetic studies, such as the genome-wide association studies (GWAS), researchers have identified chromosomal risk loci and susceptibility genes for ADs. A meta-analysis of seven GWAS with a total of nine samples of European ancestry (n = 17,310) found that certain single-nucleotide polymorphisms are associated with a lifetime diagnosis of GAD, PD, agoraphobia, social anxiety disorder, or specific phobias (11). Together, however, common familial environments and genetic predisposition do not fully explain liability to ADs, suggesting that the remaining variance is due to individual-specific environmental exposures.
Over the last two decades, research has shown that exposure to unfair treatment, often referred to as perceived discrimination, has deleterious effects on mental health (12, 13). In the first national study to assess the distribution, prevalence, and mental health correlates of major and everyday discrimination, Kessler et al. (14) found that discrimination was relatively common in the total population. Of the 3,032 US adults in the study (ages ranging from 25 to 74 y), ∼34% of participants reported experiencing at least one type of major discrimination, which is characterized as acute and observable discriminatory experiences (e.g., being denied a bank loan or having a promotion withheld). Approximately 61% of participants reported experiencing at least one type of everyday discrimination, which is a more minor form of interpersonal transgressions (e.g., being treated with less courtesy than other people). Using the same measures of discrimination, a national 2015 survey by the American Psychological Association documented that the prevalence of self-reported discrimination remains high, with 61% of American adults reporting everyday discrimination (15). Although racial/ethnic minorities report the highest levels of discrimination, Whites also report discrimination. For example, 11% of Whites compared to 34% of American Indians, 23% of Blacks, 19% of Latinos, and 11% of Asians reported experiences of everyday discrimination almost every day or at least once a week. People also experience discrimination based on factors that traverse racial demarcations, including gender, age, sexual orientation, physical appearance, and religion (16, 17).
In the study by Kessler et al., both major and everyday discrimination were positively associated with psychological distress and major depression but were not associated with GAD (14). Subsequent laboratory and community-based studies found extensive evidence that discrimination is adversely associated with a broad range of psychiatric disorders, including anxiety disorders, depression, and posttraumatic stress disorders (12, 13, 18–23). The evidence for the link between discrimination and ADs, however, cannot be interpreted unequivocally as shared genetic vulnerabilities could be confounding factors that explain the associations between perceived discrimination and ADs.
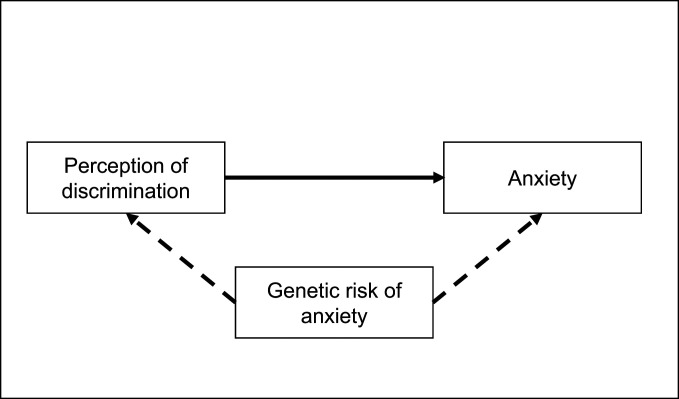
Expression of certain genes may influence emotional arousal and vigilance even to nonemotional and neutral stimuli (24, 25). This, therefore, opens the possibility that the perception of threatening and stressful environmental events, like discriminatory experiences and social exclusion, and their emotional sequelae may be by-products of genetic liability for social or generalized anxiety. When a genetic variant directly affects more than one phenotype this is called pleiotropy (26). This possibility is illustrated in Fig. 1, by which the same set of genes may influence both anxiety and perception of discrimination. The purpose of the present study was to examine the association between perceived discrimination and anxiety while accounting for relevant genetic factors. Identifying whether and to what extent the discrimination–anxiety association remains after accounting for potential genetic confounds is valuable for clarifying etiology, which may have high potential impact for prevention efforts and clinical interventions.
Fig. 1.
Conceptual framework of the potential confounding effect of genetic risk of anxiety on the relationship between discrimination and anxiety.
Methods
Sample.
Participants in the present study enrolled in the Study of Midlife in the United States (MIDUS) Biomarker Project (27). Additional information on MIDUS and its recruitment methods and data collection is found elsewhere (28). Biologically unrelated adults of European ancestry—as determined by genotype principal components analysis (29)—were considered for this study (n = 1,189). Participants with missing data for educational attainment were excluded from the analyses, resulting in a final analytic sample of 1,146 participants.
Measures.
Discrimination.
Three self-report scales were used to measure discrimination and other forms of social exclusion. Everyday discrimination (30) was measured using a nine-item scale asking participants how often on a daily basis they experience different forms of interpersonal transgressions, including “being treated with less courtesy than other people,” “treated with less respect than other people,” and “receiving poorer service than other people at restaurants or stores.” Each item was rated on a 4-point scale (1 = Often, 2 = Sometimes, 3 = Rarely, 4 = Never). Scale scores were constructed by taking the sum of reverse-coded values, such that higher scores reflect higher levels of everyday discrimination.
Major discrimination (14) was measured by asking participants how many times in their lives they have been discriminated against based on their race, ethnicity, gender, age, religion, physical appearance, and other social identities. Items included being “discouraged by a teacher or advisor from seeking higher education,” being “denied a scholarship,” and being “prevented from renting or buying a home in the neighborhood you wanted.” Scale scores for major discrimination were constructed by taking the sum of endorsed questions irrespective of frequency (i.e., 1 = event occurred one or more times, 0 = event never occurred).
Chronic job discrimination was measured using a 12-item adapted scale (31, 32), assessing discrimination exposure in the workplace. Items included how often participants think they were unfairly given the jobs that no one else wanted to do, how often are participants watched more closely than other workers, and how often does your supervisor or boss use ethnic, racial, or sexual slurs or jokes. Questions were rated on a 5-point scale (1 = Once a week or more, 2 = A few times a month, 3 = A few times a year, 4 = Less than once a year, 5 = Never). Scale scores were calculated by taking the sum of the values of reverse-coded items, such that higher scores reflected higher levels of chronic job discrimination. All data are publicly available on the MIDUS Colectica portal, along with extensive documentation regarding how each measure was calculated (https://midus.colectica.org/).
Anxiety and negative affect.
Six scales were used to measure anxiety and negative affect: the Spielberger trait anxiety scale (33), the Liebowitz social anxiety scale (34), GAD (35), neuroticism (36), negative affect (37), and the negative affect subscale of the Positive and Negative Affect Schedule [PANAS (38)].
Trait anxiety was measured using a 20-item scale asking participants to rate the frequency of occurrence of state anxiety and trait anxiety. Items included feeling tired, lacking self-confidence, and being in a state of tension or turmoil as they think over their recent concerns and interests. Positively valanced items were reverse-coded before computing summed scale scores, such that higher values on the scale indicate higher levels of trait anxiety.
Social anxiety was measured by asking participants to rate how much fear or anxiety they generally feel in certain situations including talking to people in authority, talking with people they don’t know very well, and returning goods to a store. Scales scores were computed as the mean of all items.
GAD was measured by asking participants how often—over the past 12 mo—they experienced symptoms of anxiety, including feeling restless because of worry, feeling “keyed up, on edge, or had a lot of nervous energy,” and having sore or aching muscles because of tension. All questions were rated on a 4-point scale (1 = most days, 2 = about half the days, 3 = less than half the days, 4 = never). A nominal variable was created, such that participants who answered “most days” to three or more items were coded as having GAD (∼2% = Yes; ∼98% = No).
Neuroticism was measured using a self-report scale, whereby participants were asked how much a series of temperaments accurately describe them, specifically “moody,” “worrying,” “nervous,” and “calm.” All adjectives were rated on a 4-point scale (1 = A lot, 2 = Some, 3 = A little, 4 = Not at all). The necessary items were reverse-coded before calculating mean scores, such that higher values indicate higher levels of neuroticism.
Negative affect was measured using two self-reported scales. The first scale asked how often in the past 30 d they experienced negative emotions, including feeling “restless or fidgety” and feeling “hopeless.” The second scale asked participants how often they felt afraid, jittery, irritable, ashamed, and upset. All items measuring negative affect were rated on a 5-point scale (1 = All of the time, 2 = Most of the time, 3 = Some of the time, 4 = A little of the time, 5 = None of the time). Scale scores were constructed by calculating the mean across the sets of items. For both scales, items were reverse-coded before computing mean scores so higher values reflected higher levels of negative affect.
Genotyping, imputation, and polygenic risk scoring.
Information about MIDUS’s genotype calling and DNA collection methods is reported in detail elsewhere (29). Briefly, PLINK (39) was used to analyze all genomic data and conduct quality control checks. Genotypes were imputed using Eagle (40) and minimac3 (41) software via the Michigan Imputation Server pipeline and the 1000 Genomes phase 3 reference panel. Single-nucleotide polymorphisms that deviated from Hardy–Weinberg equilibrium (P < 0.001), with ambiguous strand orientation, or had greater than 5% missing calls were removed (29). Using the Polygenic Risk Score software, PRSice 2.0, polygenic risk scores were calculated for physical and behavioral health outcomes using an a priori P value threshold of 1.0 (42) and summary statistic weights from current GWAS for each phenotype (11, 43, 44). For this analysis, we selected three polygenic risk scores, one for anxiety and the other two for closely related phenotypes (proxy phenotypes), depression and neuroticism.
Data Analysis.
To increase content validity and decrease unsystematic measurement error, focal study variables (i.e., discrimination and anxiety) were operationalized using a confirmatory factor analysis (CFA) model, estimated using robust weighted least squares (45, 46). Latent factors were scaled using unit loading identification by fixing the factor loading of the first indicator to equal 1. Factor variances were freely estimated, and simple structure was assumed, such that every indicator loaded onto only one factor and covariances between residual variances were fixed to zero. The precision of estimated factor loadings, multiple regression coefficients, and residual errors were evaluated using 95% nonparametric bootstrapped (1,000 draws) confidence intervals. Data were prepared for analysis using R version 3.2.1 and exported for inferential analyses using the MplusAutomation package (47). Inferential analyses were conducted using Mplus 8.1 (48).
A single structural regression model was fit to the data, whereby three measures of discrimination were specified to load onto a latent factor and six measures of anxiety and negative affect were specified to load onto a second latent factor. The second factor, which captures variance that is common to the different measures of anxiety and negative affect, is also regressed on the first latent factor, which captures variance that is common to the different measures of discrimination. Both discrimination and anxiety factors are also regressed on a set of exogenous covariates, including polygenic scores for anxiety, depression, and neuroticism, the first five genetic principal components, the highest level of education completed by participants, dummy-coded biological sex as determined by genotype, and chronological age (reported in years). Consequently, the structural regression coefficient predicting anxiety from discrimination is adjusted for the effects of the other variables in the model. Put differently, the structural regression coefficient tests whether discrimination is related to anxiety after implementing strict genetic controls, including polygenic risk for anxiety, depression, neuroticism, and the first five genetic principal components, in addition to demographic covariates (i.e., age, sex, and education).
Results
The average age of participants was ∼54 y (SD = 12.61 y; Table 1). Approximately 49% of the sample was female. Approximately 3% of the sample did not graduate from high school, 15% graduated from high school, 15% attended 1 to 2 y of college but did not receive a degree, ∼5% attended 3 or 4 y of college but did not receive a degree, ∼9% had a vocational or associates degree, 25% had a bachelor’s degree, 4% attended some graduate school but did not receive a degree, 18% had a master’s degree, and 5% had a doctoral degree (i.e., PhD, EdD, MD, DDS, LLB, LLD, or JD).
Table 1.
Descriptive statistics for demographic variables, polygenic scores, and indicators of discrimination and anxiety
| Variables | n | Missing, % | Mean | Median | SD | Skew | Kurtosis | ||||
| Continuous variables | |||||||||||
| Age | 1,146 | 3.61 | 54.49 | 54.00 | 12.56 | −0.06 | −0.69 | ||||
| Level of education | 1,146 | 3.61 | 8.15 | 9.00 | 2.41 | −0.21 | −0.98 | ||||
| Daily discrimination | 1,137 | 4.37 | 12.61 | 11.00 | 4.33 | 1.25 | 1.26 | ||||
| Lifetime discrimination | 1,117 | 6.05 | 0.89 | 0.00 | 1.45 | 2.16 | 5.32 | ||||
| Job discrimination | 776 | 34.73 | 10.79 | 10.00 | 4.56 | 1.07 | 0.98 | ||||
| Trait anxiety | 1,146 | 3.61 | 34.05 | 33.00 | 8.92 | 0.84 | 0.46 | ||||
| Social anxiety | 1,146 | 3.61 | 1.86 | 1.80 | 0.53 | 0.56 | −0.01 | ||||
| Neuroticism | 1,145 | 3.70 | 2.04 | 2.00 | 0.62 | 0.45 | −0.06 | ||||
| Negative affect | 1,139 | 4.20 | 1.50 | 1.33 | 0.58 | 1.82 | 3.65 | ||||
| PANAS | 1,137 | 4.37 | 1.53 | 1.40 | 0.51 | 1.49 | 2.85 | ||||
| PRS: anxiety | 1,146 | 3.61 | 0.00 | 0.01 | 1.00 | −0.02 | −0.09 | ||||
| PRS: depression | 1,146 | 3.61 | 0.00 | −0.02 | 1.00 | −0.10 | −0.11 | ||||
| PRS: neuroticism | 1,146 | 3.61 | 0.00 | −0.01 | 1.00 | −0.09 | 0.03 | ||||
| Nominal response | |||||||||||
| Nominal variables | (0) | (1) | Missing | ||||||||
| Sex | Frequency | 568 | 578 | 34 | |||||||
| Percent | ∼48% | ∼49% | ∼3% | ||||||||
| GAD | Frequency | 1,125 | 21 | 34 | |||||||
| Percent | ∼95% | ∼2% | ∼3% | ||||||||
(0) = does not meet diagnostic criteria, (1) = meets diagnostic criteria. For sex (0) = female, (1) = male. For level of education (1) = No school/some grade school (grades 1 to 6), (2) = Eighth grade/junior high school (grades 7 and 8), (3) = Some high school (grades 9 to 12, No Diploma or GED), (4) = GED (general education diploma), (5) = Graduated from high school, (6) = 1 to 2 y of college, no degree yet, (7) = 3 or 4 y of college, no degree yet, (8) = Graduated from 2 y of college, vocational school, or obtained associate degree, (9) = Graduated from a 4- or 5-y college or obtained a bachelor’s degree, (10) = Attended some graduate school, no graduate degree yet, (11) = Master’s degree, (12) = PhD, EdD, MD, DDS, LLB, LLD, JD, etc.
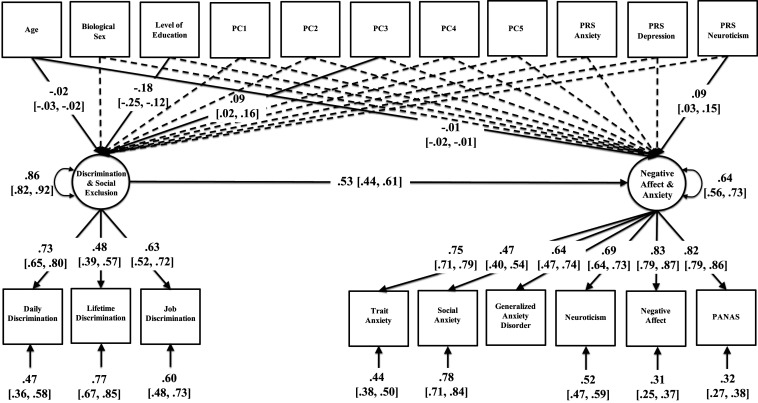
The CFA model showed good fit to the data (χ2 = 334.62, degrees of freedom = 103, P < 0.001; root mean square error of approximation (RMSEA) = 0.044, 90% confidence interval for RMSEA = 0.039, 0.050; comparative fit index (CFI) = 0.923; Tucker–Lewis index (TLI) = 0.900). Standardized estimates are reported in Fig. 2, including factor loadings, residual variances, and the structural regression coefficient that quantifies the magnitude of interdependence between discrimination and anxiety/negative affect, after accounting for variation associated with polygenic risk for anxiety, polygenic risk for depression, polygenic risk for neuroticism, the first five genetic principal components, and demographic covariates (age, biological sex, and educational attainment). Multiple regression coefficients for polygenic risk scores, genetic principal components, and demographic covariates are reported in Table 2.
Fig. 2.
Path diagram of a confirmatory factor analysis model of the relation between discrimination/social exclusion and anxiety/negative affect controlling for genetic and demographic covariates. Standardized estimates are reported with 95% nonparametric bootstrapped confidence intervals in brackets. Estimates for dashed pathways (P > 0.05) were omitted from the diagram to ease visualization (Table 2). Factor loadings, residual variances, and the structural regression coefficient were significantly different from zero (P < 0.001).
Table 2.
Effects of polygenic risk scores, genetic principal components, and demographic covariates on discrimination and anxiety factors
| Outcome: anxiety factor | Outcome: discrimination factor | |||||
| Independent variable | β | SE | P | β | SE | P |
| PC1 | 0.04 | 0.05 | 0.377 | −0.02 | 0.05 | 0.710 |
| PC2 | 0.01 | 0.03 | 0.672 | −0.04 | 0.04 | 0.293 |
| PC3 | −0.01 | 0.03 | 0.734 | 0.09 | 0.04 | 0.019 |
| PC4 | −0.04 | 0.04 | 0.192 | 0.01 | 0.04 | 0.805 |
| PC5 | 0.05 | 0.04 | 0.248 | −0.05 | 0.05 | 0.252 |
| PRS: anxiety | 0.02 | 0.03 | 0.478 | −0.01 | 0.04 | 0.862 |
| PRS: depression | −0.01 | 0.03 | 0.915 | 0.06 | 0.04 | 0.106 |
| PRS: neuroticism | 0.09 | 0.03 | 0.004 | 0.03 | 0.04 | 0.473 |
| Sex | −0.11 | 0.06 | 0.065 | 0.00 | 0.07 | 0.990 |
| Age | −0.01 | 0.00 | <0.001 | −0.02 | 0.000 | <0.001 |
| Education | −0.06 | 0.03 | 0.054 | −0.18 | 0.03 | <0.001 |
β = multiple regression coefficient, P = pD|Ho.
Factor loadings onto latent discrimination and anxiety factors were moderate to large (range of λ = 0.47 and 0.83) and statistically significant (P < 0.001). Polygenic risk for neuroticism had a significant and positive effect on anxiety/negative affect (β = 0.09, SE = 0.03, P = 0.004). In addition, age was negatively associated with anxiety/negative affect (β = −0.01, SE = 0.003, P < 0.001) and educational attainment was marginally associated with anxiety/negative affect (β = −0.06, SE = 0.03, P = 0.054). Similarly, age (β = −0.02, SE = 0.003, P < 0.001) and educational attainment (β = −0.18, SE = 0.03, P < 0.001) were negatively associated with discrimination/social exclusion. The third genetic principal component was also significantly associated with discrimination/social exclusion (β = 0.09, SE = 0.04, P = 0.019). Importantly, after accounting for the effects of genetic confounds and demographic covariates, the association between discrimination/social exclusion and anxiety/negative affect was appreciable and statistically significant (β = 0.53, SE = 0.05, P < 0.001). In a sensitivity analysis that estimated the same model using maximum likelihood with robust standard errors, the size and precision of effects remained largely unchanged.
Discussion
The present study used a large sample of genotyped adults to test whether discrimination (and different forms of social exclusions) is associated with the experience of anxiety and negative affect after implementing state-of-the-art genetic controls. Results indicate a high degree of interdependence between discrimination and anxiety, even after accounting for increased genetic liability for anxiety, depression, neuroticism, and other potential genetic and sociodemographic confounds (e.g., genetic principal components, chronological age, biological sex, and education). These findings corroborate systematic reviews that found a strong association between discrimination and psychiatric disorders, including anxiety disorder, depression, and lifetime DSM‐IV disorders (12, 13, 18, 49). However, these previous studies did not account for potential genetic confounds, which left open the possibility that the cooccurrence of discrimination and psychiatric disorders was the result of pleiotropy (i.e., the expression of two or more phenotypes from the same set of genetic factors). Individuals with high genetic liability to anxiety may hold beliefs that others view them negatively and have enhanced emotional arousal to threatening and stressful events. This, in turn, may increase the likelihood to perceive and label stressful events as discriminatory. However, results of the present study are more consistent with discrimination acting as an environmental stressor for symptoms of anxiety and negative affect. Consequently, the present study contributes further to the growing body of evidence that discrimination operates like other stressors.
Discriminatory experiences can lead to stress responses characterized by enhanced spontaneous amygdala activity and heightened physiological arousal and negative affect (12, 50, 51). Although the mechanism remains unclear, studies have indicated that greater exposure to discrimination is associated with alterations in cortisol output (52), multisystem physiological dysregulation (53, 54), increase in inflammation (55), shorter telomere length (56), and impairment of the prefrontal cortex’s function (57). Even the anticipation of discrimination can lead to physiological and emotional dysregulation (12). These pathways can potentially lead to poor mental and physical health (58). While further research is needed to understand the mechanisms underpinning the association between discrimination exposure and psychiatric disorders, greater attention is also needed to understand the pathways that bridge the gap between polygenic liability for psychiatric disorders and the phenotypic expression of psychiatric symptoms. A key direction for future studies is the simultaneous use of sequence-based and omics-based technology to measure epigenetic markers that impact the regulation or expression of genes but do not change the DNA sequence itself.
Our findings are subject to important limitations. We used a cohort of White-identifying respondents who were predominately of European ancestry as determined by genetic principal components. Thus, inferences based on study findings are limited to White adults living in the United States. While the effects of discrimination seem to be similar across racial/ethnic groups (58), racial/ethnic minorities experience more discrimination than their White counterparts, placing them at an increased risk for poor mental health (17). In addition, questions remain about the extent to which self-reported experiences of discrimination by Whites are truly equivalent to those of more socially stigmatized groups. Our findings need to be replicated using a diverse sample of adults to increase generalizability. In particular, the finding of a strong association between perceived discrimination and anxiety after accounting for genetic confounds should be replicated in an independent sample of genotyped adults. For this to be achieved in a racially diverse sample, future GWAS discovery efforts need to focus on non-European cohorts to increase the predictive validity of resulting polygenic risk scores in diverse samples (59).
In addition, this study focused on general discrimination that encompassed different forms of social exclusion. Race-based discrimination or other forms of discrimination (e.g., sexual orientation) may have different effects on anxiety. Although there is some evidence to suggest that discriminatory experiences affect health, irrespective of certain physical attributions (12), some stigmatized identities may be more common than others or have unique adverse effects on mental health when exposed to discrimination. The present study also conducted a cross-sectional analysis of the association between discrimination and anxiety, while controlling for genetic confounds. Although the present study helps rule out common genetic liability as a potential confound of the association between discrimination and anxiety, the cross-sectional design precludes determining the temporal order of discrimination and anxiety. Future longitudinal, genetically informative designs stand to benefit from establishing the temporal direction of effects, in addition to controlling for potential genetic confounds. Finally, polygenic risk scores are continuing to be refined as GWAS grow in size and discover more variants that are associated with the expression of complex phenotypes. Nevertheless, our study lays the foundation for future research to improve model performance and increase the generalizability of findings.
Conclusions
Exposure to discrimination was associated with anxiety, even after adjusting for genetic controls. This suggests that the association between discrimination and anxiety is not explained by known genetic variants of anxiety, depression, or neuroticism. These findings highlight the importance of increased awareness among health researchers and clinicians of discrimination as a potential pathogenic factor for mental illness. Further research is needed to illuminate the psychological and physiological pathways by which discrimination can affect health and to identify the optimal societal interventions to reduce the prevalence of discrimination and the needed psychosocial and clinical interventions to minimize its negative effects.
Acknowledgments
The MIDUS study is supported by the John D. and Catherine T. MacArthur Foundation Research Network, National Institute on Aging (P01-AG020166), and the National Institute on Aging (U19-AG051426). Biomarker data collection was further supported by the National Institute of Health National Center for Advancing Translational Sciences (NCATS) Clinical and Translational Science Award (CTSA) program as follows: UL1TR001409 (Georgetown), UL1TR001881 (UCLA), and 1UL1RR025011 (UW). The development of the manuscript was partially supported by Cancer Disparities Research Network/Geographic Management Program (GMaP) Region 4 funded by 3 P30 CA006927-52S2 and by the Clinical & Translational Science Institute Mentored Career Development Award (KL2 TR002545).
Footnotes
The authors declare no competing interest.
Data Availability.
Data analyzed in this manuscript are archival data and may be accessed via the MIDUS Collectica Portal (https://midus.colectica.org/). Data, analysis scripts, and figure files are also available through the Open Science Framework (https://osf.io/ukg8q).
References
- 1.Anxiety and Depression Association of America , Facts & Statistics. https://adaa.org/about-adaa/press-room/facts-statistics. Accessed 17 November 2019.
- 2.American Psychiatric Association , Diagnostic and statistical manual of mental disorders. BMC Med. 17, 133–137 (2013). [Google Scholar]
- 3.Miloyan B., Bulley A., Bandeen-Roche K., Eaton W. W., Gonçalves-Bradley D. C., Anxiety disorders and all-cause mortality: Systematic review and meta-analysis. Soc. Psychiatry Psychiatr. Epidemiol. 51, 1467–1475 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Player M. S., Peterson L. E., Anxiety disorders, hypertension, and cardiovascular risk: A review. Int. J. Psychiatry Med. 41, 365–377 (2011). [DOI] [PubMed] [Google Scholar]
- 5.Hendriks S. M., et al. , Long-term work disability and absenteeism in anxiety and depressive disorders. J. Affect. Disord. 178, 121–130 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Bhattacharya R., Shen C., Sambamoorthi U., Excess risk of chronic physical conditions associated with depression and anxiety. BMC Psychiatry 14, 10 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tully P. J., Cosh S. M., Baune B. T., A review of the affects of worry and generalized anxiety disorder upon cardiovascular health and coronary heart disease. Psychol. Health Med. 18, 627–644 (2013). [DOI] [PubMed] [Google Scholar]
- 8.Hettema J. M., Neale M. C., Kendler K. S., A review and meta-analysis of the genetic epidemiology of anxiety disorders. Am. J. Psychiatry 158, 1568–1578 (2001). [DOI] [PubMed] [Google Scholar]
- 9.Scherrer J. F., et al. , Evidence for genetic influences common and specific to symptoms of generalized anxiety and panic. J. Affect. Disord. 57, 25–35 (2000). [DOI] [PubMed] [Google Scholar]
- 10.Smoller J. W., Block S. R., Young M. M., Genetics of anxiety disorders: The complex road from DSM to DNA. Depress. Anxiety 26, 965–975 (2009). [DOI] [PubMed] [Google Scholar]
- 11.Otowa T., et al. , Meta-analysis of genome-wide association studies of anxiety disorders. Mol. Psychiatry 21, 1391–1399 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis T. T., Cogburn C. D., Williams D. R., Self-reported experiences of discrimination and health: Scientific advances, ongoing controversies, and emerging issues. Annu. Rev. Clin. Psychol. 11, 407–440 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paradies Y., et al. , Racism as a determinant of health: A systematic review and meta-analysis. PLoS One 10, e0138511 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kessler R. C., Mickelson K. D., Williams D. R., The prevalence, distribution, and mental health correlates of perceived discrimination in the United States. J. Health Soc. Behav. 40, 208–230 (1999). [PubMed] [Google Scholar]
- 15.American Psychological Association , Stress in America: The impact of discrimination. Stress Am. Surv., 2016 (2016). [Google Scholar]
- 16.Williams D. R., Lawrence J. A., Davis B. A., Vu C., Understanding how discrimination can affect health. Health Serv. Res. 54 (suppl. 2), 1374–1388 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cuevas A. G., Williams D. R., “Perceived discrimination and health: Integrative findings” in The Oxford Handbook of Integrative Health Science, Ryff C. D., Krueger R. F., Eds. (Oxford University Press, 2018). [Google Scholar]
- 18.Williams D. R., Mohammed S. A., Discrimination and racial disparities in health: Evidence and needed research. J. Behav. Med. 32, 20–47 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hopkins P. D., Shook N. J., A review of sociocultural factors that may underlie differences in African American and European American anxiety. J. Anxiety Disord. 49, 104–113 (2017). [DOI] [PubMed] [Google Scholar]
- 20.de Freitas D. F., et al. , Psychological correlates of perceived ethnic discrimination in Europe: A meta-analysis. Psychol. Violence 8, 712 (2018). [Google Scholar]
- 21.Kirkinis K., Pieterse A. L., Martin C., Agiliga A., Brownell A., Racism, racial discrimination, and trauma: A systematic review of the social science literature. Ethn. Health, 1–21 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Carter R. T., et al. , A meta-analytic review of racial discrimination: Relationships to health and culture. Race Soc. Probl. 11, 15–32 (2019). [Google Scholar]
- 23.Carter R. T., Lau M. Y., Johnson V., Kirkinis K., Racial discrimination and health outcomes among racial/ethnic minorities: A meta‐analytic review. J. Multicult. Couns. Devel. 45, 232–259 (2017). [Google Scholar]
- 24.Kobiella A., et al. , How the serotonin transporter 5-HTTLPR polymorphism influences amygdala function: The roles of in vivo serotonin transporter expression and amygdala structure. Transl. Psychiatry 1, e37 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nordquist N., Oreland L., Serotonin, genetic variability, behaviour, and psychiatric disorders—a review. Ups. J. Med. Sci. 115, 2–10 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Avinun R., The E is in the G: Gene–Environment–Trait correlations and findings from genome-wide association studies. Perspect. Psychol. Sci., 1745691619867107 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dienberg Love G., Seeman T. E., Weinstein M., Ryff C. D., Bioindicators in the MIDUS national study: Protocol, measures, sample, and comparative context. J. Aging Health 22, 1059–1080 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radler B., The Midlife in the United States (MIDUS) series: A national longitudinal study of health and well-being. Open Health Data 2, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin J. D., Mann F. D., Krueger R. F., Low cardiac vagal control is associated with genetic liability for elevated triglycerides and risky health behaviors. Biol. Psychol. 153, 107892 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams D. R., Yan Yu, Jackson J. S., Anderson N. B., Racial differences in physical and mental health: Socio-economic status, stress and discrimination .. J. Health Psychol. 2, 335–351 (1997). [DOI] [PubMed] [Google Scholar]
- 31.Bobo L., Suh S. A., Surveying Racial Discrimination: Analyses from a Multiethnic Labor Market (Russell Sage Foundation, 1995). [Google Scholar]
- 32.McNeilly M. D., et al. , The perceived racism scale: A multidimensional assessment of the experience of white racism among African Americans. Ethn. Dis. 6, 154–166 (1996). [PubMed] [Google Scholar]
- 33.Spielberger C. D., State-Trait Anxiety Inventory: A Comprehensive Bibliography (Consulting Psychologists Press, 1989). [Google Scholar]
- 34.Fresco D. M., et al. , The Liebowitz social anxiety scale: A comparison of the psychometric properties of self-report and clinician-administered formats. Psychol. Med. 31, 1025–1035 (2001). [DOI] [PubMed] [Google Scholar]
- 35.Wang P. S., Berglund P., Kessler R. C., Recent care of common mental disorders in the United States : Prevalence and conformance with evidence-based recommendations. J. Gen. Intern. Med. 15, 284–292 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keyes C. L., Shmotkin D., Ryff C. D., Optimizing well-being: The empirical encounter of two traditions. J. Pers. Soc. Psychol. 82, 1007–1022 (2002). [PubMed] [Google Scholar]
- 37.Mroczek D. K., Kolarz C. M., The effect of age on positive and negative affect: A developmental perspective on happiness. J. Pers. Soc. Psychol. 75, 1333–1349 (1998). [DOI] [PubMed] [Google Scholar]
- 38.Watson D., Clark L. A., Tellegen A., Development and validation of brief measures of positive and negative affect: The PANAS scales. J. Pers. Soc. Psychol. 54, 1063–1070 (1988). [DOI] [PubMed] [Google Scholar]
- 39.Chang C. C., et al. , Second-generation PLINK: Rising to the challenge of larger and richer datasets. Gigascience 4, 7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loh P.-R., et al. , Reference-based phasing using the haplotype reference consortium panel. Nat. Genet. 48, 1443–1448 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Das S., et al. , Next-generation genotype imputation service and methods. Nat. Genet. 48, 1284–1287 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Euesden J., Lewis C. M., O’Reilly P. F., PRSice: Polygenic risk score software. Bioinformatics 31, 1466–1468 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okbay A.et al.; LifeLines Cohort Study , Genetic variants associated with subjective well-being, depressive symptoms, and neuroticism identified through genome-wide analyses. Nat. Genet. 48, 624–633 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wray N. R.et al.; eQTLGen; 23andMe; Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium , Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat. Genet. 50, 668–681 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Enders C. K., Bandalos D. L., The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Struct. Equ. Modeling 8, 430–457 (2001). [Google Scholar]
- 46.Schafer J. L., Graham J. W., Missing data: Our view of the state of the art. Psychol. Methods 7, 147–177 (2002). [PubMed] [Google Scholar]
- 47.Hallquist M. N., Wiley J. F., MplusAutomation: An R package for facilitating large-scale latent variable analyses in Mplus. Struct. Equ. Modeling 25, 621–638 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muthén L., Muthén B., Mplus User’s Guide (Muthén & Muthén, Los Angeles, ed. 8, 1998–2017). [Google Scholar]
- 49.Pascoe E. A., Smart Richman L., Perceived discrimination and health: A meta-analytic review. Psychol. Bull. 135, 531–554 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harrell J. P., Hall S., Taliaferro J., Physiological responses to racism and discrimination: An assessment of the evidence. Am. J. Public Health 93, 243–248 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clark U. S., Miller E. R., Hegde R. R., Experiences of discrimination are associated with greater resting amygdala activity and functional connectivity. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 3, 367–378 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Busse D., Yim I. S., Campos B., Marshburn C. K., Discrimination and the HPA axis: Current evidence and future directions. J. Behav. Med. 40, 539–552 (2017). [DOI] [PubMed] [Google Scholar]
- 53.Brody G. H., et al. , Perceived discrimination among African American adolescents and allostatic load: A longitudinal analysis with buffering effects. Child Dev. 85, 989–1002 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cuevas A. G., et al. , The association between perceived discrimination and allostatic load in the Boston Puerto Rican Health Study. Psychosom. Med. 81, 659–667 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kershaw K. N., et al. , Self-reported experiences of discrimination and inflammation among men and women: The multi-ethnic study of atherosclerosis. Health Psychol. 35, 343–350 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu S. Y., Kawachi I., Discrimination and telomere length among older adults in the United States. Public Health Rep. 132, 220–230 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berger M., Sarnyai Z., “More than skin deep”: Stress neurobiology and mental health consequences of racial discrimination. Stress 18, 1–10 (2015). [DOI] [PubMed] [Google Scholar]
- 58.Williams D. R., Lawrence J. A., Davis B. A., Racism and health: Evidence and needed research. Annu. Rev. Public Health 40, 105–125 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stryjecki C., Alyass A., Meyre D., Ethnic and population differences in the genetic predisposition to human obesity. Obes. Rev. 19, 62–80 (2018). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data analyzed in this manuscript are archival data and may be accessed via the MIDUS Collectica Portal (https://midus.colectica.org/). Data, analysis scripts, and figure files are also available through the Open Science Framework (https://osf.io/ukg8q).