Abstract
BACKGROUND:
While sex differences in incidence of Alzheimer’s disease (AD) and potential explanations have received considerable attention, less attention has been paid to possible sex differences in genetic risk for AD.
OBJECTIVE:
We examined sex differences in genetic and environmental influences on disease risk and age at onset for All Dementia, AD Only, and Non-AD Dementia.
METHODS:
Twin pairs were drawn from the Swedish Twin Registry. All Dementia analysis included 9,467 pairs; AD only, 8,696 pairs; and non-AD dementia, 8,195 pairs. APOE analyses included 1,923 individual twins with measured ε4 alleles. Dementia diagnoses were based on clinical workup and national health registry linkage.
RESULTS:
Although within-pair correlations for All Dementia and AD Only were higher for women than for men, sex differences did not statistically differ for genetic or environmental etiology of All Dementia, AD Only, and Non-AD dementia. Similar results were observed when looking at specific genetic effects (APOE ε4). Co-twin control analyses indicated that among twin pairs discordant for dementia, female twins without dementia had approximately 40% greater risk of developing dementia, compared with their male counterparts, in the 2–5 years following the first twin’s diagnosis.
CONCLUSION:
For All Dementia, AD Only and Non-AD Dementia, genetic influences could be equated across sex. Co-twin analyses, however, suggest greater risk to female than to male co-twins of dementia cases even though sex differences in either genetic or shared environmental influences on the risk of dementia could not be differentiated.
Keywords: Alzheimer’s disease, Twin studies, Sex differences, ApoE epsilon 4
Genes have a substantial role in risk of Alzheimer’s disease and related dementias (ADRD) [1–8]. Earlier reports from the Swedish Twin Registry (STR) demonstrated that genetic sources of variability estimated from a twin design accounted for substantial liability to ADRD [2–4], but were inconclusive with respect to sex differences. Recent reports suggest a genetic basis for sex differences in ADRD, which are thought to underlie differences in degradation of brain structure and function [9]. Investigations of the molecular genetics of ADRD [10] have suggested that women might be more susceptible to effects of apolipoprotein (APOE) ε4 allele(s) that increase risk of ADRD compared to men [11,12] vis-à-vis neuro-anatomical and functional differences, including higher levels of AD-related biomarkers signaling neurodegeneration (e.g., tau) [10,11]. While there is more than one approach to the investigation of genetic basis of ADRD, recent multiplexing approaches demonstrating that polygenic scores account for about half of the observed heritability underlying ADRD phenotypes is but one example [13], quantitative genetic studies remain relevant for identifying the relative importance of genetic and environmental factors contributing to ADRD, specifically for guiding research on sex differences. As there are few published twin and sibling studies on sex differences in heritability, the purpose of this study is to test whether heritability estimates are either the same or different across sexes.
Previous twin studies of ADRD in the STR have reported on ADRD phenotypes based on clinical assessment only [2–4]. Sweden possesses national registries that can be used to identify ADRD cases from hospital discharge diagnoses and from causes of death. The current study builds on previous research by supplementing clinical assessment with use of national registries to follow individuals through years after their final clinical contact, creating a much larger sample with information on their dementia outcomes.
The first aim in this report, thus, is to use quantitative genetic methods in a larger sample of same-sex male and female twin pairs and opposite-sex twin pairs to test for sex differences in genetic influences contributing to dementia risk. Additive genetic influences were hypothesized to be stronger in female than in male twins, particularly when AD is considered alone compared to non-AD dementia types.
The second aim of the current study is to test sex-by-APOE ε4 interaction effects on ADRD risk and age at onset in a subsample of individuals from the STR. The APOE ε4 allele gene is the most potent genetic marker associated with late-onset AD risk. Most evidence indicates that two APOE ε4 allele copies are associated with higher rates of AD and younger age at onset in both men and women [11,14,15], with one copy conferring greater risk in women but not men [16,17]. However, some have reported a stronger association of AD risk with APOE ε4 in men showing greater risk than women [18]. Given the relative weight of available evidence we hypothesized that sex moderates effects of APOE ε4 on both risk and age at onset, with greater risk and younger age at onset observed in women than in men [19,20].
METHOD
Sample
The sample was drawn from four different studies of Swedish older adults, all from the Swedish Twin Registry [19]: one cross-sectional census of all Swedish twins age 65 and older and three longitudinal studies that followed representative subgroups within the STR. The Study of Dementia in Swedish Twins [21] (HARMONY), consists of 18,564 Swedish twins, all of whom were age 65 or older in 1998 at the time of a cross-sectional comprehensive census study of all living twins in Sweden born 1958 and earlier [22]. Of the total HARMONY sample initially screened, 11,211 twins were included in the current study. All twins who participated in HARMONY received cognitive screening over telephone, with those who performed poorly referred for an in-person clinical evaluation for dementia. The three remaining studies are longitudinal studies of aging in the STR – Aging in Men and Women [23] (GENDER) study, Origins of Variance in the Oldest Old: Octogenarian Twins [24] (OCTO-Twin), and the Swedish Adoption/Twin Study of Aging [25,26] (SATSA). These studies entailed repeated cognitive assessments. GENDER began in 1994, OCTO-Twin began in 1991, and SATSA began in 1984. GENDER was a longitudinal study of opposite-sexed twins born 1906–1925 and consists of 496 individual twins, both of whom participated in a cognitive assessment between age 69 and 79; 388 met inclusion criteria for the current study sample. OCTO-Twin consisted of 702 same-sexed individual twins, both of whom had to have lived to be at least aged 80 years or older at baseline in 1991; 572 met inclusion criteria for the current study sample. There were 2,840 twins who were eligible for participation in the first SATSA assessment in 1984, of whom 1,388 twins were included in the current study. In the three longitudinal studies, at each wave of cognitive testing, those who performed poorly were referred for further dementia evaluation. Some individuals participated in more than one study and so were included in the analysis sample once, with data from both studies combined. This resulted in a sample of 22,602, of whom 13,559 met inclusion criteria. Derivation of the study sample and numbers of twins of each zygosity are outlined in Figure 1. After all exclusions, the base sample consisted of 13,559 individual twins, including 5,758 women and 7,801 men from 9,467 unique families.
Figure 1.
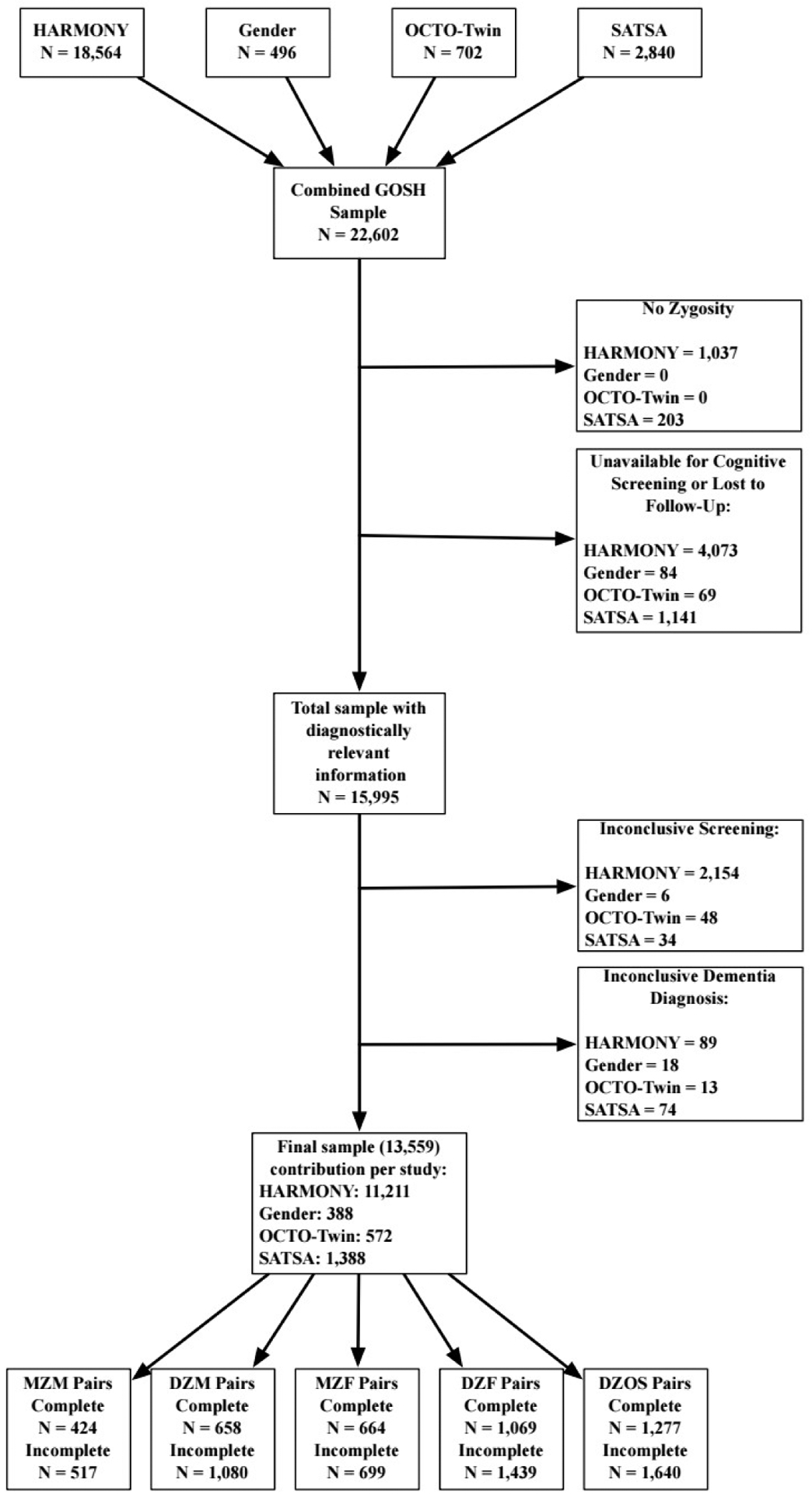
GOSH inclusion criteria for analytic sample.
Notes. “Unavailable for cognitive screening” refers to participants who did not participate in the cognitive screening; “Lost to follow-up” refers to participants who could not be located for additional follow-up assessments (e.g., participant emigrated); “Inconclusive screening” refers to participants who were unable to complete the cognitive screening or who performed borderline between intact and impaired with no informant available; and “inconclusive dementia diagnosis” refers to participants who were cognitively impaired but not demented.
The range of birth years was between 1893 and 1935. For twins’ last known age in the STR, mean age of male twins was 74.71 years (SD = 7.98) and mean age of women female twins was 76.23 years (SD = 8.64). The median years of education for all twins was 7 years (M = 8.48, SD = 2.95, Range: 0 – 25), typical for these birth cohorts in Sweden for whom 6 years was the required basic education. The international socio-economic index of occupational status mean was 38.11 (SD = 19.83, Range: 11.74 – 88.96), where 18 corresponds to agricultural workers, 38 corresponds to working in crafts or trades, and 68 represents professionals. Overall, twins reported relatively low scores on the cumulative illness rating scale, indicating number of physical disease categories endorsed out of 13 (M = 1.79, SD = 1.50, Range: 0 – 12).
The sample of pairs in the quantitative genetic analyses included both complete pairs and individual twins from incomplete pairs. Incomplete pairs arise from one twin’s refusing to participate or from an individual twin having been deceased prior to the baseline assessment. For purposes of analysis, we considered All Dementia, Alzheimer’s disease alone, and a residual category with other dementia diagnoses. The sample sizes of complete and incomplete pairs by disease concordance for All Dementia, AD Only, and Non-AD Dementia analyses are reported in Table 1. The analytic sample for sex-by-APOE ε4 was 1,740 individuals (12.83% of the total analytic sample) and is the subset of participants with available APOE ε4 data.
Table 1.
Sample sizes of concordant pairs, discordant pairs, and singletons by zygosity for each dementia phenotype
| All Dementia | MZ Male | DZ Male | MZ Female | DZ Female | DZ Opposite-Sex |
| 42 | 50 | 119 | 179 | 100 | |
| 80 | 147 | 115 | 270 | 306 | |
| 163 | 305 | 262 | 591 | 446 | |
| 354 | 775 | 437 | 848 | 1194 | |
| AD Only | MZ Male | DZ Male | MZ Female | DZ Female | DZ Opposite-Sex |
| 17 | 22 | 59 | 74 | 32 | |
| 35 | 76 | 62 | 155 | 160 | |
| 120 | 187 | 213 | 458 | 304 | |
| 399 | 846 | 490 | 963 | 1340 | |
| Non-AD Dementia | MZ Male | DZ Male | MZ Female | DZ Female | DZ Opposite-Sex |
| 7 | 11 | 10 | 26 | 18 | |
| 40 | 71 | 53 | 115 | 146 | |
| 79 | 152 | 149 | 291 | 242 | |
| 389 | 851 | 499 | 1003 | 1354 | |
| Pairs w/No Dementia Diagnosis | 302 | 461 | 430 | 620 | 871 |
| Totals | MZ Male | DZ Male | MZ Female | DZ Female | DZ Opposite-Sex |
| Any Dementia | 941 | 1738 | 1363 | 2508 | 2917 |
| AD Only | 873 | 1592 | 1254 | 2270 | 2707 |
| Non-AD Dementia | 822 | 1546 | 1141 | 2055 | 2631 |
Note. Concordant pairs are pairs where both twins are cases (i.e., diagnosed with dementia; discordant pairs are pairs where one twin is a case and the co-twin is a control; Singletons (case and control) comprise information on one twin where the co-twin is missing.
Dementia Assessment
Participants received cognitive screening at each contact, including telephone cognitive screening with all of HARMONY and anyone who missed a longitudinal wave in the other three studies. Those who performed poorly at a screening or declined since the prior wave, their co-twins, and a control sample of pairs who made few screening errors were referred for an in-person dementia diagnostic workup. The in-person battery included physical and neuropsychological assessment, blood panels, and depression screening [21,23–25]. The cognitive measures included episodic memory, perceptual speed, verbal ability, and visuospatial ability. A diagnostic consensus board assigned a clinical diagnosis using information from the in-person workup and medical records using DSM-III R or DSM-IV criteria for dementia (depending on year of evaluation), and NINCDS-ADRDA criteria for AD. The same consensus protocols were used across all four studies.
Those lost to follow-up by study design or participant refusal at a later wave (n = 1,294) were followed by registry linkage, using individual-level data from the Swedish National Patient Register (NPR) and Cause of Death Register (CDR). Registries contained International Classification of Disease (ICD) codes for dementia diagnoses (see Supplementary Appendix). Included here are diagnoses updated until December 31, 2013. Thus, every individual’s dementia status is up to and including their death or until the end of 2013.
Of the 3,665 individual twins in the analytic sample with a dementia diagnosis (clinical diagnosis = 2,070 and registry diagnosis = 1,595), 2,178 were diagnosed with AD and 1,487 with another form of dementia (mainly comprising vascular dementia, mixed type, and dementia not otherwise specified). There were 9,894 twin individuals determined to be cognitively normal.
Apolipoprotein-ε genotype
An APOE ε4 indicator was created (0 = no ε4 allele; 1 = one ε4 allele; 2 = two ε4 alleles) for those with an APOE genotype. Of the 1740 individuals with an APOE genotype, 0.46% were ε2/ε2 (n = 8), 12.24% were ε2/ε3 (n = 213), 3.05% were ε2/ε4 (n = 53), 52.76% were ε3/ε3 (n = 918), 27.47% were ε3/ε4 (n = 478), and 4.02% were ε4/ε4 (n = 70). Individuals with APOE 24 were included as having one ε4 allele, as studies on the sex differences in effects of APOE ε4 generally include APOE 24 groups [11,17,27–29].
Age at onset
Individuals who were clinically diagnosed with dementia received an age at onset derived from information collected during their in-person clinical assessment by comparing cognitive performance at successive longitudinal testing, that is, through informant description of symptoms and onset, medical records from the participant’s physician, or both where available [30]. For individuals whose diagnosis came from the NPR or CDR, we do not have an age at onset, only a date at which the individual was discharged from a hospital or date of death. Analyses with other Swedish samples have determined that for twins with a clinically-established age at onset, the first hospital discharge date is 3–5 years later, within a fairly narrow window [31,32]. Consequently, we inferred age at onset by subtracting, conservatively, three years from NPR date of discharge or CDR age at death. For diagnoses determined from the CDR, a small amount of normally distributed random error (σ2 = 0.66) was added to individuals’ age at onset in the CDR to model naturally occurring variability in age at onset so that the interval from onset to death would not be a constant across individuals who died at the same age.
Data Analysis
Quantitative genetic twin analyses include tetrachoric within-pair (twin) correlations to describe intrapair similarity and provide a suggestion of the genetic and environmental influences underlying liability to dementia. Monozygotic (MZ) twin correlations are expected to be higher than the dizygotic (DZ) twin correlations because MZ twins share their entire genotype while DZ twins share 50% of their segregating genes, on average. When MZ twin correlations (rMZ) are not at least twice as great as DZ twin correlations (rDZ), shared environmental influences contribute to liability to dementia, regardless of zygosity. Finally, nonshared environmental influences are inferred from imperfect MZ correlations (1-rMZ), as only unique environmental experiences contribute to MZ twin differences.
Next, sex-limitation models (Figure 2) for binary outcomes (i.e., threshold model for liabilities) [33,34] were used to estimate the magnitudes of genetic and environmental influences underlying liability to dementia and to test whether the variance components are the same between men and women. The logic of sex-limitation models is the same as traditional univariate twin models (commonly referred to as “ACE models”), with slight modifications to account for opposite sex DZ pairs [33], which we describe below. All ACE models consist of three variance components. First, genetic variance components (A) refer to the additive influence of genotype that makes twins more similar to one another. Second, shared environmental variance components (C) refer to any environmental factor that makes twins (or siblings) similar to one another (e.g., books in the house during childhood). Third, nonshared environmental variance components (E) refer to any environmental factor that makes siblings dissimilar to one another (e.g., if one twin sustained a head injury but not the other). The E component also includes measurement error.
Figure 2.
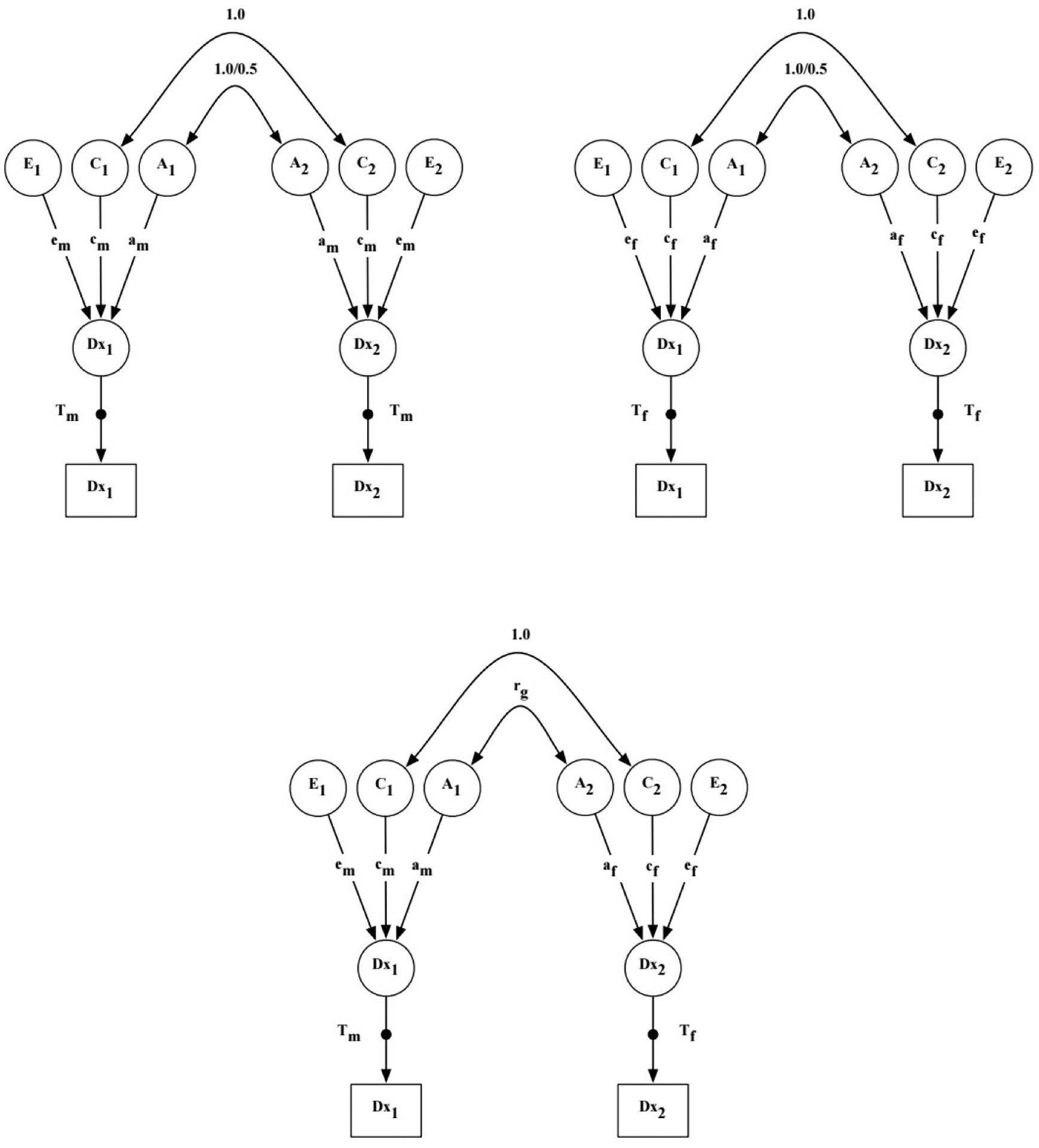
Sex-limitation ACE model for binary outcomes.
Notes. A = additive genetic effects; C = shared environmental effects; E = nonshared environmental effects; Dx = dementia diagnosis; T = threshold for diagnosis of dementia; m = male twin; f = female twins; rg = genetic correlation between opposite-sex DZ twins; 1 = twin 1; 2 = twin 2. The genetic correlation between twins’ A effects is 1.0 for MZ twins and 0.5 for DZ twins. The shared environmental correlation is 1.0 for all zygosity groups. The black dot project from the latent Dx variables (circles) to the observed Dx variables (squares) represent the threshold estimates for the categorical outcome (i.e., threshold model for liabilities).
In the sex limitation models, genetic and environmental variance components are estimated separately for each sex and compared to determine whether they are qualitatively or quantitatively the same or different in men and women. Qualitative sex differences in genetic influences are inferred when the genetic correlation between opposite-sex DZ twins (rDZOS) is not equivalent to the assumption that DZ twins share one-half of their segregating genes. In sex limitation models, the genetic correlation in the DZOS group, thus, is an estimated parameter that is compared to the fixed genetic correlation in the same-sex DZ groups (0.5). Quantitative sex differences refer to whether the same ACE model fits for men as for women. Model estimation proceeded as follows: Model 1 is a baseline model that allows the ACE parameter estimates to vary across sex, including the genetic correlation within opposite-sex DZ twin pairs. Model 2 tests for qualitative sex differences by testing whether the genetic correlation between opposite sex twins can be set to 0.5, as in same-sex DZ pairs. Model 3 tests for quantitative sex differences by setting all ACE parameter estimates equal across sex. If Model 3 resulted in significant loss of model fit, we tested 3 additional models that constrained each ACE parameter to be equal across sex to identify which, if any, parameters were equal across sex. Analyses were performed for All Dementia, AD Only, and Non-AD Dementia. All models were adjusted for age effects, both age and age-squared, as they may be confounded with shared environmental effects. Models were estimated in Mplus 8.2 [34] using a weighted least squares estimator to take into the account the binary nature of diagnostic outcomes. Chi-square difference testing of nested models was performed using the DIFFTEST function in Mplus, which is a two-step procedure implemented to obtain chi-square distributed estimates for model testing. In order to include incomplete pairs in the quantitative genetic analyses where age is a covariate, we assigned the last known age of the participating twin to the missing co-twin.
Using a co-twin control approach, we next estimated sex differences in risk of All Dementia for a subset of twins (N = 848 women; 473 men) who survived beyond their co-twins’ diagnosis of dementia. Kaplan-Meier estimates show survival until dementia for twins from the point in time when their co-twin became demented. Cox regression addresses whether there is a sex difference in the potentially elevated risk for dementia to a co-twin due to the first twin’s having become demented. We retained the proportionality assumption in our modeling and used the Efron approximation method for handling ties. All analyses were performed using the survival package (version 2.4.4–1.1) in R 3.5.1 [35].
Finally, for the APOE by sex moderation analyses, mixed-effects ordinal regression analysis [36] and linear mixed-effects regression analysis [37] were used to predict liability to dementia and age at onset, respectively, as a function of sex, age, age-squared, and number of APOE ε4 alleles. Age was centered on the sample mean year of birth. Mixed ordinal regression is appropriate for handling nonindependence of twins from the same family. In mixed-effects regression analyses, parameters were estimated using restricted maximum likelihood estimation, which provides unbiased parameter estimates compared to maximum likelihood estimation [37]. In both analyses, we tested three models: sex only (Model 1), sex and APOE ε4 (Model 2), and a sex-by-APOE ε4 interaction model (Model 3). Alpha cut-offs were set at .05 for all analyses.
Results
Among the men, 1,303 (22.63%) were diagnosed with dementia, including 709 AD and 594 non-AD dementia cases. Among the women, 2,362 (30.28%) were diagnosed with dementia, including 1,469 AD and 893 non-AD dementia cases.
Quantitative Genetic Results
Tetrachoric twin correlations (Table 2) followed the predicted pattern that MZ twins would be more highly correlated than DZ twins. Twin correlations for All Dementia and AD Only are stronger for women than for men, although with overlapping confidence intervals. For women but not men, the confidence intervals for MZ and DZ pairs were nonoverlapping, and therefore were statistically different from one another, suggesting genetic influences. As with All Dementia, the confidence intervals for MZ and DZ female correlations of AD Only were nonoverlapping, indicating that they too were statistically different from one another, indicating genetic influences. For Non-AD Dementia, for women and men, the confidence intervals for MZ and DZ pairs overlapped, suggesting no statistically significant genetic influences.
Table 2.
Tetrachoric twin correlations and 95% confidence intervals for All Dementia, Alzheimer’s Disease Only, and Non-Alzheimer’s Disease Dementia
| MZ Male | DZ Male | MZ Female | DZ Female | DZ Opposite-Sex | |
|---|---|---|---|---|---|
| All Dementia | .66 (.53 – .78) | .49 (.37 – .62) | .80 (.73 – .86) | .61 (.54 – .69) | .45 (.35 – .54) |
| AD Only | .78 (.66 – .91) | .60 (.45 – .75) | .86 (.80 – .93) | .66 (.57 – .75) | .48 (.35 – .61) |
| Non-AD Dementia | .45 (.20 – .71) | .44 (.23 – .64) | .62 (.44 – .79) | .54 (.44 – .79) | .34 (.18 – .50) |
Model fitting results (Table 3) suggest no qualitative (Model 2) or quantitative (Model 3) sex differences in the genetic and environmental variance components underlying All Dementia, AD Only, and Non-AD Dementia. All parameters could be set equal without significant loss of model fit.
Table 3.
Model fit statistics of sex-limitation ACE models for All dementia, AD only, and non-AD Dementia
| All Dementia Models | χ2 | df | Model Comparison | Δχ2 | Δdf | p | RMSEA |
|---|---|---|---|---|---|---|---|
| 1. ACE unconstrained, rG estimated | 256.02 | 44 | - | - | - | 0.000 | 0.05 |
| 2. ACE unconstrained, rG = 0.5 | 257.95 | 45 | 1 | 0.32 | 1 | 0.569 | 0.05 |
| 3. ACE constrained, rG = 0.5 | 265.26 | 48 | 2 | 5.27 | 3 | 0.153 | 0.05 |
| AD Only Models | χ2 | df | Model Comparison | Δχ2 | Δdf | p | RMSEA |
| 1. ACE unconstrained, rG estimated | 131.89 | 44 | - | - | - | 0.000 | 0.03 |
| 2. ACE unconstrained, rG = 0.5 | 132.88 | 45 | 1 | 0.06 | 1 | 0.803 | 0.03 |
| 3. ACE constrained, rG = 0.5 | 135.57 | 48 | 2 | 0.62 | 3 | 0.892 | 0.03 |
| Non-AD Dementia Models | χ2 | df | Model Comparison | Δχ2 | Δdf | p | RMSEA |
| 1. ACE unconstrained, rG estimated | 195.89 | 44 | - | - | - | 0.000 | 0.05 |
| 2. ACE unconstrained, rG = 0.5 | 197.37 | 45 | 1 | 0.17 | 1 | 0.681 | 0.05 |
| 3. ACE constrained, rG = 0.5 | 203.05 | 48 | 2 | 3.34 | 3 | 0.342 | 0.04 |
Notes. RMSEA = root mean square error of approximation. A = additive genetic variance component; C = shared environmental variance component; E = nonshared environmental variance component; χ2 = chi-square estimate; df = degrees of freedom; Δχ2 = change in chi-square between nested models; Δdf = change in degrees of freedom between nested models; p indicates probability of difference in chi-square estimates of nested models; RMSEA = root mean square error of approximation.
Table 4 presents the proportion of variance attributed to genetic and environmental influences by sex when the genetic correlation for opposite-sex DZ is equal to 0.5 (corresponding to Model 2, Table 3) for disease liability of each dementia phenotype. For All Dementia, genetic influences accounted for 41% of the variability in disease liability in men and 59% in women. Although these point estimates are dissimilar, model testing did not support quantitative sex differences and the 95% confidence intervals for the parameter estimates are overlapping. When men and women were constrained to be equal (corresponding to Model 3, Table 3), genetic influences accounted for 56% of the variance in disease liability (.95 CI: .36 – .73), shared environmental influences accounted for 11% of the variance, but was not statistically significant (.95 CI: −.03 – .24), and nonshared environmental influences accounted for 33% of the variance (.95 CI: .25 – .39).
Table 4.
Parameter estimates (and 95% confidence intervals) of sex limitation ACE models for All Dementia, AD only, and Non-AD Dementia
| All Dementia | |||||||
|---|---|---|---|---|---|---|---|
| Group | A | C | E | rg | Thresholds | bAge | bAge2 |
| Male | .41 (.03 – .79) | .15 (−.15 – .44) | .44 (.30 – .59) | 1.69 | 1.09 (0.96 – 1.22) | −0.14 (−0.19 – 0.09) | |
| Female | .59 (.34 – .83) | .13 (−.06 – .32) | .28 (.19 – .37) | 1.73 | 1.59 (1.48 – 1.70) | −0.18 (−0.21 – −0.14) | |
| DZOS | 0.5 | ||||||
| Alzheimer’s Disease Alone | |||||||
| Group | A | C | E | ||||
| Male | .65 (.36 – .94) | .10 (−.12 – .33) | .25 (.13 – .36) | 1.89 | 0.87 (0.72 – 1.03) | −0.06 (−0.12 – 0.01) | |
| Female | .54 (.24 – .85) | .24 (−.02 – .49) | .22 (.13 – .32) | 1.89 | 1.03 (0.93 – 1.14) | −0.05 (−0.09 – −0.01) | |
| DZOS | 0.5 | ||||||
| Non-Alzheimer’s Disease Alone | |||||||
| Group | A | C | E | ||||
| Male | .10 (−.44 – .64) | .28 (−.14 – .69) | .62 (.38 – .86) | 2.13 | 1.43 (1.26 – 1.61) | −0.28 (−0.35 – −0.21) | |
| Female | .53 (.09 – .96) | .11 (−.20 – .41) | .37 (.17 – .58) | 2.41 | 2.43 (2.18 – 2.69) | −0.48 (−0.56 – −0.39) | |
| DZOS | 0.5 | ||||||
Note. Parameter estimates come from the full model (Model 2), with rg set to 0.50. For All Dementia, AD Only, and Non-AD Dementia, A, C, and E can be set equal in men and women without significant loss of model fit. A = additive genetic variance component; C = shared environmental variance component; E = nonshared environmental variance component
For AD Only, genetic influences accounted for 65% of the variability in disease liability in men and 54% of the variability in women when estimates were modeled separately for men and women (Model 2, Table 3). Again, there was no support for sex differences in these estimates. In the final constrained model (Model 3, Table 3), genetic influences accounted for 59% of the variability in disease liability (95% CI: .37 – .77); shared environmental influences accounted for 18% of the variability, but was not statistically significant (95% CI: .00 – .33); and nonshared environmental influences accounted for 23% of the variability (95% CI: .16 – .29).
For Non-AD Dementia, genetic influences accounted for 10% of the variability in disease liability in men and 53% of the variability in women when estimates were modeled separately for men and women (Model 2, Table 3). In the final constrained model (Model 3, Table 3), genetic influences accounted for 31% of the variability in disease liability, but was not statistically significant (95% CI: −.08 – .65); shared environmental influences accounted for 19% of the variability, but was not statistically significant (95% CI: −.08 – .41); and nonshared environmental influences accounted for 50% of the variability (95% CI: .33 – .65).
Cox Regression
There were 848 female and 473 male twin pairs for the analysis. Proportions of MZ and DZ pairs similar for the sexes. Of the total 1,321 co-twins, 680 were right censored due to death or were still living without dementia. Figure 3 shows Kaplan-Meier estimates of the survivor function in the left panel and shows that female twins are more likely to be diagnosed with dementia following their co-twin’s diagnosis than are male twins. Corresponding cumulative hazard functions are presented in the right panel of Figure 3.
Figure 3.
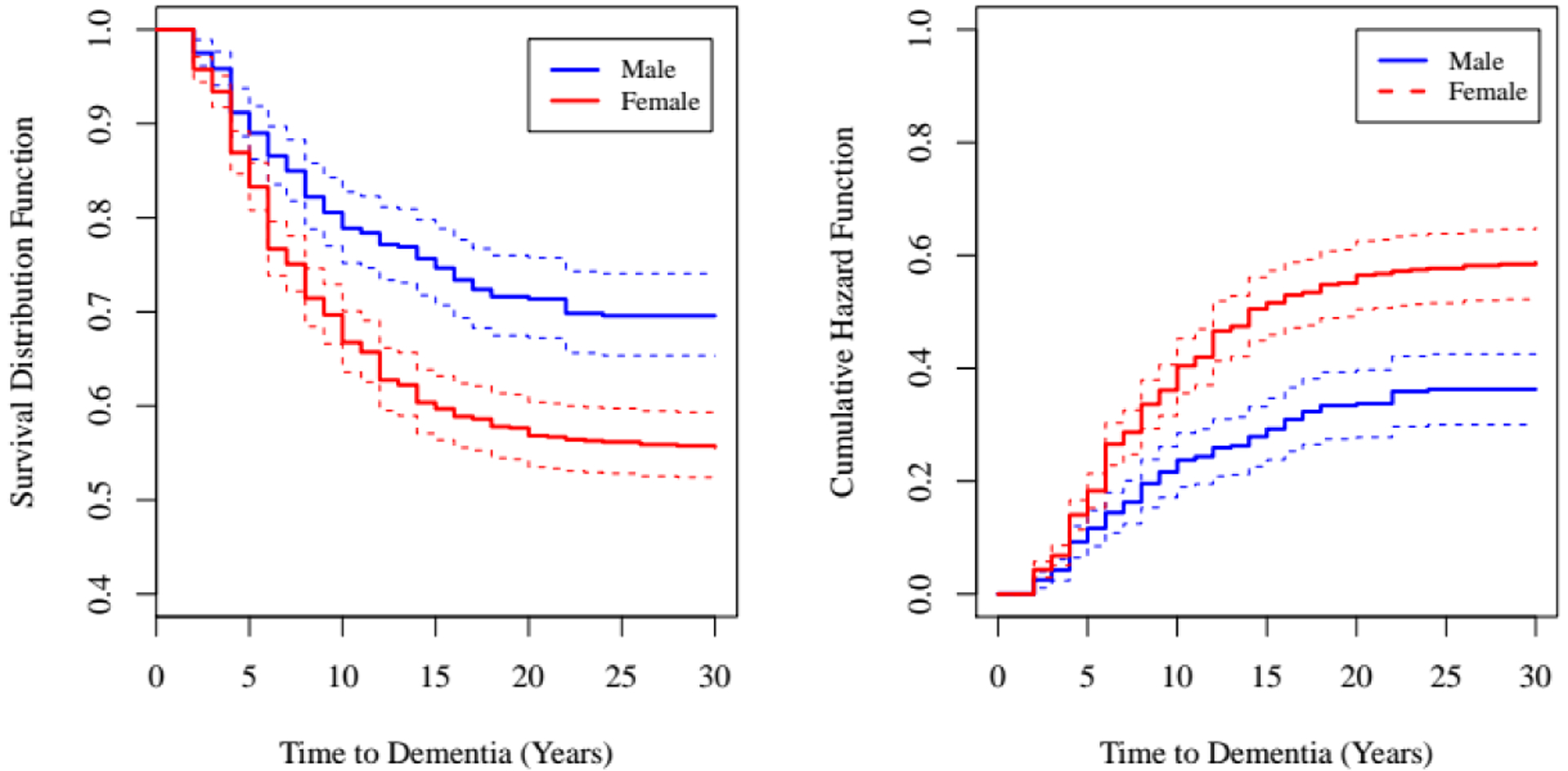
Kaplan-Meier estimates of the survivor function (left) and cumulative hazard function (right) by sex.
Notes. Estimates are presented with thicker lines and 95% confidence intervals are given with thinner lines.
Results from Cox regression models suggest that women with co-twins who were diagnosed with dementia had a greater risk of dementia than men with co-twins who were diagnosed with dementia (log hazard: 0.53, SE = 0.10, p = .001; hazard ratio: 1.39 [.95 CI: 1.39 – 2.08]), particularly in the 2–5 years following the co-twins’ diagnosis. Results did not change when co-twin sex was entered as a predictor in the model to account for twins from opposite sex pairs (−2LL = 1.21, df = 1, p = .271). Thus, results from this co-twin analysis were consistent with greater twin correlations for All Dementia in females compared to males.
Sex-by-APOE Interaction
Table 5 presents the number of individual cases with a diagnosis of All Dementia, AD Only, and Non-AD Dementia and corresponding age at onset by APOE ε4 allele status (none, one, or two). Visual inspection of the observed data (Figure 4) conform expected pattern of differences: women’s expected age at onset of both All Dementia and AD is younger with each additional allele whereas men’s expected age at onset is younger only in men who have two APOE ε4 alleles.
Table 5.
Number of individual male and female twins diagnosed with All Dementia, AD only, and Non-AD Dementia out of the total number of individual twins with available APOE ε4 data.
| All Dementia | AD Only | Non-AD Dementia | ||||
|---|---|---|---|---|---|---|
| Case/Total | Age at onset (SD) | Case/Total | Age at onset (SD) | Case/Total | Age at onset (SD) | |
| Male | 333/697 | 78.85 (7.60) | 165/529 | 79.43 (7.41) | 168/532 | 78.29 (7.77) |
| 187/697 | 79.08 (7.98) | 87/529 | 80.01 (8.16) | 100/532 | 78.28 (7.77) | |
| 124/697 | 79.08 (7.03) | 65/529 | 79.87 (5.84) | 59/532 | 78.20 (8.11) | |
| 22/697 | 75.66 (7.00) | 13/529 | 73.35 (6.84) | 9/532 | 79.00 (6.10) | |
| Female | 584/1043 | 80.35 (8.17) | 377/836 | 79.81 (8.11) | 207/666 | 81.33 (8.19) |
| 347/1043 | 81.78 (7.86) | 211/836 | 81.41 (7.82) | 136/666 | 82.35 (7.90) | |
| 200/1043 | 78.79 (8.22) | 144/836 | 77.99 (8.20) | 56/666 | 80.86 (7.98) | |
| 37/1043 | 75.34 (7.34) | 22/836 | 76.36 (6.91) | 15/666 | 73.83 (7.93) | |
| Total Cases | 917/1740 | 79.81 (8.00) | 542/1365 | 79.69 (7.90) | 375/1198 | 79.97 (8.14) |
Figure 4.
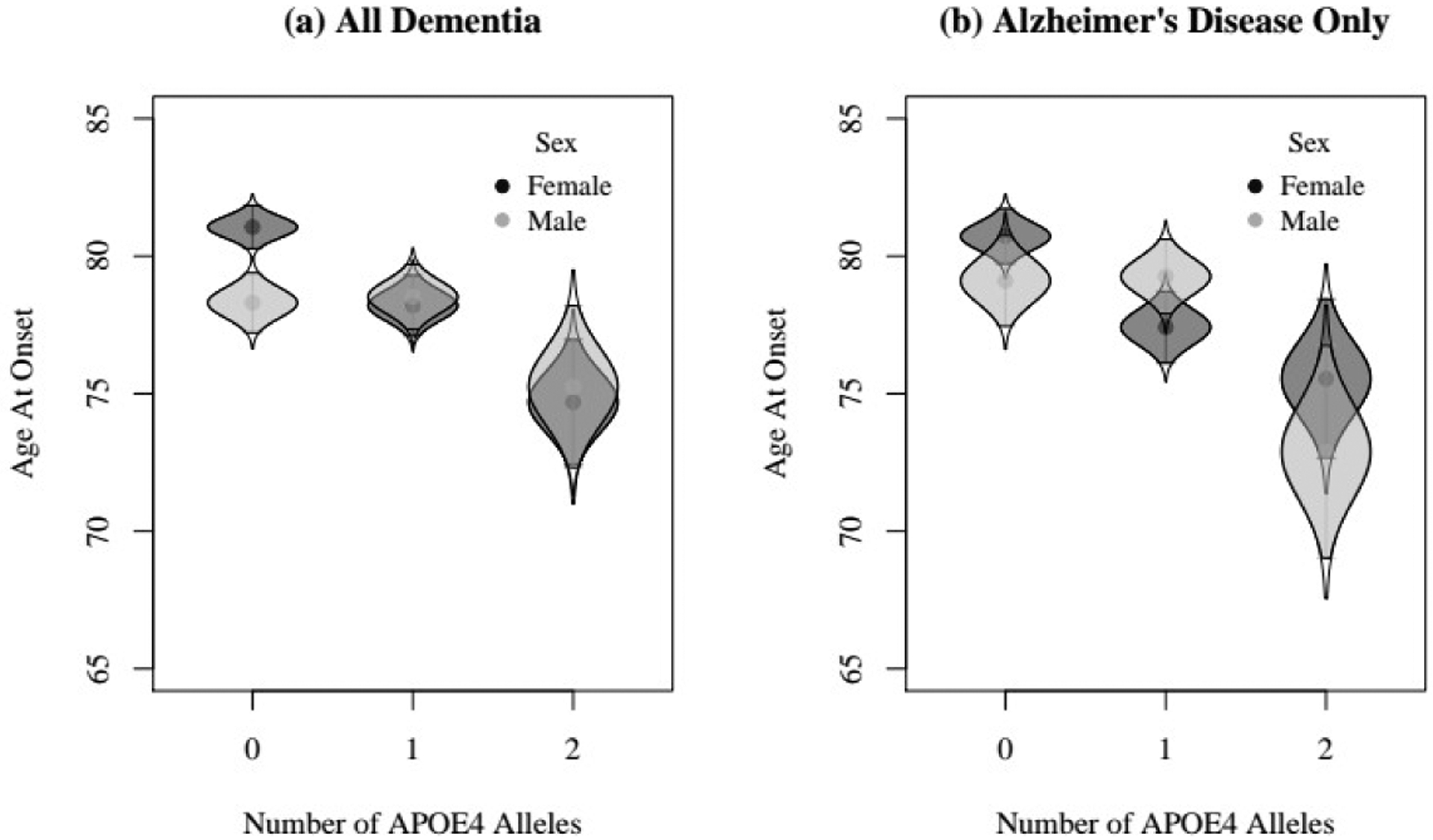
Plot of raw means of age at onset for male and female twins who were diagnosed with dementia (a) and only twins who were diagnosed with Alzheimer’s disease (b) as a function of APOE ε4 status.
Notes. Density distributions of each group are provided for each group to illustrate within-group dispersion. The 95% confidence intervals encompass the shaded region of each density distribution for each allele group.
Table 6 shows results from testing for sex differences in the effects of APOE ε4 on disease occurrence (Table 6a) and age at onset (Table 6b) of All Dementia and AD Only. Log odds are presented in Table 6a. Odds ratios (OR) can be ascertained by taking the exponent of the parameter estimates. Odds ratios greater than 1.00 indicate excess risk of dementia and AD while odds ratios less than 1.00 indicate decreased risk. There were significant main effects of sex on both All Dementia (OR: 1.32, 95% CI: 1.03 – 1.71) and AD Only (OR: 2.03, 95% CI: 1.42 – 2.92), reflecting the greater rate of dementia in women compared to men (Model 1). As well, there were significant main effects of APOE ε4 on All Dementia (OR: 3.35, 95% CI: 2.55 – 4.39) and AD Only (OR: 5.16, 95% CI: 3.40 – 7.83), reflecting the greater risk conferred by number of APOE ε4 alleles carried (Model 2). There was not a significant interaction effect between APOE ε4 and sex on either All Dementia or AD Only, as indicated by the similar deviance and AIC values and worse BIC value in Model 3 as Model 2.
Table 6.
Parameter estimates of mixed-effects ordinal regression analyses for All dementia and AD only occurrence and linear mixed-effects regression analyses for age at onset of All dementia and AD only
| a) Mixed Ordinal Regression Results for Disease Occurrence | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All Dementia | Alzheimer’s Disease Only | |||||||||||
| Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | |||||||
| Model Parameters | Estimate | SE | Estimate | SE | Estimate | SE | Estimate | SE | Estimate | SE | Estimate | SE |
| Fixed Effects | ||||||||||||
| Intercept | 0.24 | 0.12 | −0.21 | 0.12 | −0.18 | 0.13 | −0.84 | 0.18 | −1.38 | 0.19 | −1.33 | 0.21 |
| Sex | 0.27 | 0.13 | 0.28 | 0.13 | 0.24 | 0.15 | 0.72 | 0.19 | 0.71 | 0.18 | 0.63 | 0.22 |
| APOE4 | - | - | 1.21 | 0.14 | 1.14 | 0.20 | - | - | 1.64 | 0.21 | 1.51 | 0.29 |
| Sex*APOE4 | - | - | - | - | 0.13 | 0.25 | - | - | - | - | 0.23 | 0.34 |
| Random Effects | ||||||||||||
| σ2Intercept | 1.74 | 0.48 | 1.22 | 0.41 | 1.24 | 0.41 | 3.63 | 1.13 | 2.71 | 0.94 | 3.32 | 1.06 |
| Fit Statistics | ||||||||||||
| Deviance | 2281.40 (5) | 2182.16 (6) | 2181.91 (7) | 1690.03 (5) | 1589.83 (6) | 1589.35 (7) | ||||||
| AIC | −1145.70 | −1097.08 | −1097.96 | −850.01 | −800.91 | −801.68 | ||||||
| BIC | −1158.45 | −1112.38 | −1115.81 | −862.32 | −815.68 | −818.90 | ||||||
| b) Mixed Effects Regression Results for Age at Onset | ||||||||||||
| All Dementia | Alzheimer’s Disease Only | |||||||||||
| Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | |||||||
| Model Parameters | Estimate | SE | Estimate | SE | Estimate | SE | Estimate | SE | Estimate | SE | Estimate | SE |
| Fixed Effects | ||||||||||||
| Intercept | 79.30 | 0.38 | 80.11 | 0.42 | 79.83 | 0.48 | 79.23 | 0.54 | 80.09 | 0.59 | 80.08 | 0.68 |
| Sex | −0.12 | 0.46 | −0.14 | 0.45 | 0.29 | 0.57 | −0.81 | 0.61 | −0.85 | 0.61 | −0.84 | 0.79 |
| APOE4 | - | - | −1.65 | 0.35 | −1.10 | 0.57 | - | - | −1.58 | 0.45 | −1.57 | 0.78 |
| Sex*APOE4 | - | - | - | - | −0.87 | 0.72 | - | - | - | - | −0.02 | 0.95 |
| Random Effects | ||||||||||||
| σ2Intercept | 8.43 | - | 8.03 | - | 7.91 | - | 12.54 | - | 12.22 | - | 12.22 | - |
| σ2Residual | 32.99 | - | 32.41 | - | 32.46 | - | 28.35 | - | 27.77 | - | 27.77 | - |
| Fit Statistics | ||||||||||||
| Deviance (df) | 6010.90 (6) | 5989.20 (7) | 5987.70 (8) | 3542.90 (6) | 3530.90 (7) | 3530.90 (8) | ||||||
| Δχ2/Δdf | - | 21.70 (1) | 1.50 (1) | - | 12.00 (1) | 0.001 (1) | ||||||
| p-value | - | < .001 | .221 | - | < .001 | .981 | ||||||
| AIC | 6022.90 | 6003.20 | 6003.70 | 3554.90 | 3544.90 | 3546.90 | ||||||
| BIC | 6051.90 | 6036.90 | 6042.30 | 3580.70 | 3575.00 | 3581.30 | ||||||
Notes. In the models in Section a, odds ratios can be ascertained by exponentiating parameter estimates. AIC = Akaike Information Criterion; BIC = Bayesian Information Criterion.
For age at onset of all dementia, sex was not a statistically significant predictor of age at onset for either All Dementia or AD Only, with similar age at onset for men and women. Effects of APOE ε4 were additively related to younger age at onset, regardless of sex (All dementia: b = −1.65, SE = 0.35, p < .05; AD only: b = −1.57, SE = 0.78, p < .05). However, there was not a significant sex-by-APOE ε4 interaction, as indicated by the nonsignificant likelihood ratio test between Model 2 and Model 3 (Table 6b).
Discussion
Genetic factors account for a large proportion of the variability in Alzheimer’s disease and related dementias [3,5,38], but there is no resolution as to whether genetic factors are sex-specific. In the few studies that have investigated sex differences in heritability of ADRD, genetic factors account for about 60% of the variance in ADRD outcomes with no significant sex difference [2,3]. With a larger sample of twins in the present study compared to previous reports, the current results suggest with greater confidence that the genetic etiology of ADRD may be similar for women and men. Secondarily, these findings show that heritability of All Dementia is largely attributed to AD Dementia types and that familial risk is indeed different for women than men conditional upon one twin already carrying a diagnosis of dementia.
Current findings confirm results in previous reports from various samples drawn from the Swedish Twin Registry, but with a larger sample. Heritability estimates of AD Only across sex in the current study (h2 =.59) is comparable to a previous report using only the HARMONY sample (h2 = .58) [3]. Heritability of non-AD dementia was small, and not statistically significant. We note, however, that although sex differences in both genetic and environmental influences on ADRD were null, the extent to which similar etiological processes interact with sex-specific biological factors (e.g., hormones) and psychosocial risk factors (e.g., the tendency for women to be caregivers rather than to be recipients of caregiving) remains unknown. The current findings only clarify that main effects of genetic and environmental etiological factors appear to be more similar than different.
Co-twin analyses indicated a significant sex difference with non-demented female co-twins of dementia cases having approximately 40% greater risk of dementia than non-demented male co-twins of dementia cases. While greater survival of women compared to men may play a role [31], women’s risk of dementia, as compared to men’s, was primarily elevated in the 2–5 years following the co-twins’ diagnosis. In other words, the effect is unlikely to be due solely to longevity. This difference implies either greater genetic influences on dementia risk among females compared to males, greater shared environmental influences, or both.
In the APOE ε4 subset, sex was found to be a significant predictor of disease liability but not age at onset for All Dementia and AD Only. There was no interaction between sex and number of APOE ε4 alleles in dementia risk or age at onset. This result contrasts with some [11,17], but not all previous reports [20]. Moreover, the sample size was small and the pattern seemed consistent with sex moderation.
One implication of the current findings is that All Dementia results were driven primarily by AD Only rather than Non-AD Dementia. This observation derives from the similarity of heritability estimates between All Dementia and AD Only analyses. One implication of this conclusion is that the measurement of any form of dementia in population-based studies likely reflects pathology nearer to AD-type dementias than non-AD type dementias.
In the current study, heritability estimates of AD were comparable to results from the Alzheimer’s Disease Genetics Consortium where all single nucleotide polymorphisms (SNPs) accounted for 53.24% of the variability in AD [39]. Yet at the same time, nonshared environmental factors accounted for greater than a quarter of the liability of AD, suggesting that lifestyle decisions people independently make play an important role in AD risk. Like the cognitive ability literature [40], we expect that environmental context moderates genetic variability underlying liability to AD.
The current findings should be interpreted in view of several identified limitations. First, twins were followed using dementia diagnoses from both clinical assessments and registry-based sources. Validity studies have shown registries to provide reliable identification of ADRD cases with no evidence of sex differences in sensitivity of the registries [32], but registry-based diagnosis does not identify all individuals who actually have ADRD. Second, the sample consisted of twins who had participated in four different studies, three with longitudinal in-person cognitive follow-up and one reliant on registry follow-up after the initial census to identify dementia cases. Third, the Swedish sample, with its absence of ethnic diversity, potentially limits the generalizability of the results to other populations of interest. Fourth, quantitative genetic studies only identify global, unmeasured genetic and environmental influences. While we followed up with testing effects of APOE genotype on risk and age at onset of ADRD, inferences about other specific genes that might contribute to sex differences in additive genetic variance must be made using genomic data sets [39]. Fifth, power is low in covariance structure analyses that include categorical indicators as primary outcomes [41], with individual group sizes of 5000 or greater needed to reject a truly false null hypothesis for genetic effects like those observed in the current study. We, thus, cannot conclude definitively null sex differences in heritability of ADRD, as the results in part may reflect statistical power. As is true in all studies estimating genetic influences from twin designs for a binary outcome, and similar to the challenges with genomic data, large sample sizes are required.
Overall, the current study reaffirms previous findings that heritability estimates of Any Dementia and AD Only account for 55–60% of the variability in diagnoses. Further, given the increase in sample size, the current results reflect greater confidence in the genetic and environmental estimates of Any Dementia, AD Only and Non-AD Dementia, which decreases ambiguity about heritability of AD and related dementias. Notably, heritability of All Dementia is largely attributed to AD. Finally, with respect to sex differences, while quantitative differences in genetic and environmental influences were null, post-hoc analyses suggest that familial risk is higher for women than men conditional upon one twin already carrying a diagnosis of dementia.
Supplementary Material
Acknowledgements:
This work was supported by the National Institutes of Health, National Institute on Aging (T32 AG000037-37, R01 AG060470, RF1 AG058068, R01 AG08724, R01 AG17561, and R01 AG028555) and the Alzheimer’s Association (AARF-17-505302).
Footnotes
Conflicts of interest: The authors have no conflict of interest to report.
References
- [1].Gatz M, Pedersen NL, Berg S, Johansson B, Johansson K, Mortimer JA, Posner SF, Viitanen M, Winblad B, Ahlbom A (1997) Heritability for Alzheimer’s disease: The Study of Dementia in Swedish Twins. Journals Gerontol. Ser. A Biol. Sci. Med. Sci 52A, M117–M125. [DOI] [PubMed] [Google Scholar]
- [2].Gatz M, Fiske A, Reynolds CA, Wetherell JL, Johansson B, Pedersen NL (2003) Sex differences in genetic risk for dementia. Behav. Genet 33, 95–105. [DOI] [PubMed] [Google Scholar]
- [3].Gatz M, Reynolds CA, Fratiglioni L, Johansson B, Mortimer JA, Berg S, Fiske A, Pedersen NL (2006) Role of genes and environments for explaining Alzheimer’s disease. Arch. Gen. Psychiatry 63, 168–74. [DOI] [PubMed] [Google Scholar]
- [4].Pedersen NL, Gatz M, Berg S, Johansson B (2004) How heritable Is Alzheimer’s disease late in life? Findings from Swedish twins. Ann. Neurol 55, 180–185. [DOI] [PubMed] [Google Scholar]
- [5].Bergem ALM, Engedal K, Kringlen E (1997) The role of heredity in late-onset Alzheimer disease and vascular dementia. Arch. Gen. Psychiatry 54, 264–270. [DOI] [PubMed] [Google Scholar]
- [6].Räihä I, Kaprio J, Koskenvuo M, Rajala T, Sourander L (1996) Alzheimer’s disease in Finnish twins. Lancet 347, 573–578. [DOI] [PubMed] [Google Scholar]
- [7].Ertekin-Taner N, Graff-Radford N, Younkin LH, Eckman C, Adamson J, Schaid DJ, Blangero J, Hutton M, Younkin SG (2001) Heritability of plasma amyloid β in typical late-onset Alzheimer’s disease pedigrees. Genet. Epidemiol 21, 19–30. [DOI] [PubMed] [Google Scholar]
- [8].Brandt J, Welsh KA, Breitner JC, Folstein MF, Helms M, Christian JC (1993) Hereditary influences on cognitive functioning in older men. A study of 4000 twin pairs. Arch. Neurol 50, 599–603. [DOI] [PubMed] [Google Scholar]
- [9].Mazure CM, Swendsen J (2016) Sex differences in Alzheimer’s disease and other dementias. Lancet Neurol. 15, 451–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Brouwers N, Sleegers K, Van Broeckhoven C (2008) Molecular genetics of Alzheimer’s disease: An update. Ann. Med 40, 562–583. [DOI] [PubMed] [Google Scholar]
- [11].Altmann A, Tian L, Henderson VW, Greicius MD (2014) Sex modifies the APOE-related risk of developing Alzheimer disease. Ann. Neurol 75, 563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Snyder HM, Asthana S, Bain L, Brinton R, Craft S, Dubal DB, Espeland MA, Gatz M, Mielke MM, Raber J, Rapp PR, Yaffe K, Carrillo MC (2016) Sex biology contributions to vulnerability to Alzheimer’s disease: A think tank convened by the Women’s Alzheimer’s Research Initiative. Alzheimer’s Dement. 12, 1186–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sims R, Hill M, Williams J (2020) The multiplex model of the genetics of Alzheimer’s disease. Nat. Neurosci 38, 30–34. [DOI] [PubMed] [Google Scholar]
- [14].Ungar L, Altmann A, Greicius MD (2014) Apolipoprotein E, gender, and Alzheimer’s disease: An overlooked, but potent and promising interaction. Brain Imaging Behav. 8, 262–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Miech RA, Breitner JCS, Zandi PP, Khachaturian AS, Anthony JC, Mayer L (2002) Incidence of AD may decline in the early 90s for men, later for women: The Cache County study. Neurology 58, 209–218. [DOI] [PubMed] [Google Scholar]
- [16].Riedel BC, Thompson PM, Brinton RD (2016) Age, APOE and Sex: Triad of Risk of Alzheimer’s Disease. J. Steroid Biochem. Mol. Biol [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Farrer LiA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-vance MA, Risch N, van Duijn CM (1997) Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: A meta-analysis. J. Am. Med. Assoc 278, 1349–1356. [PubMed] [Google Scholar]
- [18].Qiu C, Kivipelto M, Agüero-Torres H, Winblad B, Fratiglioni L (2004) Risk and protective effects of the APOE gene towards Alzheimer’s disease in the Kungsholmen project: variation by age and sex. J. Neurol. Neurosurg. Psychiatry 75, 828–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lichtenstein P, Sullivan PF, Cnattingius S, Gatz M, Johansson S, Carlström E, Björk C, Svartengren M, Wolk A, Klareskog L, de Faire U, Schalling M, Palmgren J, Pedersen NL (2006) The Swedish Twin Registry in the third millennium: An update. Twin Res. Hum. Genet 9, 875–882. [DOI] [PubMed] [Google Scholar]
- [20].Neu SC, Pa J, Kukull W, Beekly D, Kuzma A, Gangadharan P, Wang LS, Romero K, Arneric SP, Redolfi A, Orlandi D, Frisoni GB, Au R, Devine S, Auerbach S, Espinosa A, Boada M, Ruiz A, Johnson SC, Koscik R, Wang JJ, Hsu WC, Chen YL, Toga AW (2017) Apolipoprotein E genotype and sex risk factors for Alzheimer disease: A meta-analysis. JAMA Neurol. 74, 1178–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gatz M, Fratiglioni L, Johansson B, Berg S, Mortimer J a., Reynolds C a., Fiske A, Pedersen NL (2005) Complete ascertainment of dementia in the Swedish Twin Registry: The HARMONY study. Neurobiol. Aging 26, 439–447. [DOI] [PubMed] [Google Scholar]
- [22].Lichtenstein P, De Faire U, Floderus B, Svartengren M, Svedberg P, Pedersen NL (2002) The Swedish Twin Registry: A unique resource for clinical, epidemiological and genetic studies. J. Intern. Med 252, 184–205. [DOI] [PubMed] [Google Scholar]
- [23].Gold CH, Malmberg B, McClearn GE, Pedersen NL, Berg S (2002) Gender and health: A study of older unlike-sex twins. J. Gerontol. Soc. Sci 57B, S168–S176. [DOI] [PubMed] [Google Scholar]
- [24].McClearn GE, Johansson B, Berg S, Pedersen NL, Ahern F, Petrill SA, Plomin R (1997) Substantial genetic influence on cognitive abilities in twins 80 or more years old. Science (80-.) 276, 1560–1563. [DOI] [PubMed] [Google Scholar]
- [25].Finkel D, Pedersen NL (2004) Processing speed and longitudinal trajectories of change for cognitive abilities: The Swedish Adoption/Twin Study of Aging. Aging, Neuropsychol. Cogn 11, 325–345. [Google Scholar]
- [26].Gatz M, Pedersen NL (2012) Study of Dementia in Swedish Twins. Twin Res. Hum. Genet 16, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].De Luca V, Orfei MD, Gaudenzi S, Caltagirone C, Spalletta G (2016) Inverse effect of the APOE epsilon4 allele in late- and early-onset Alzheimer’s disease. Eur. Arch. Psychiatry Clin. Neurosci 266, 599–606. [DOI] [PubMed] [Google Scholar]
- [28].Sundermann EE, Tran M, Maki PM, Bondi MW (2018) Sex differences in the association between apolipoprotein E ε4 allele and Alzheimer’s disease markers. Alzheimer’s Dement. Diagnosis, Assess. Dis. Monit 10, 438–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Protas HD, Chen K, Langbaum JBS, Fleisher AS, Alexander GE, Lee W, Bandy D, De Leon MJ, Mosconi L, Buckley S, Truran-Sacrey D, Schuff N, Weiner MW, Caselli RJ, Reiman EM (2013) Posterior cingulate glucose metabolism, hippocampal glucose metabolism, and hippocampal volume in cognitively normal, late-middle-aged persons at 3 levels of genetic risk for alzheimer disease. JAMA Neurol. 70, 320–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Fiske A, Gatz M, Aadnøy B, Pedersen NL (2005) Assessing age of dementia onset: validity of informant reports. Alzheimer Dis. Assoc. Disord 19, 128–134. [DOI] [PubMed] [Google Scholar]
- [31].Beam CR, Kaneshiro C, Jang JY, Reynolds CA, Pedersen NL, Gatz M (2018) Differences between women and men in incidence rates of dementia and Alzheimer’s disease. J. Alzheimer’s Dis 64, 1077–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Rizzuto D, Feldman AL, Karlsson IK, Dahl Aslan AK, Gatz M, Pedersen NL (2018) Detection of dementia cases in two Swedish health registers: A validation study. J. Alzheimer’s Dis 61, 1301–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Neale MC, Maes HHM (2004) Methodology for genetic studies of twins and families, Springer; Netherlands, Dordrecht. [Google Scholar]
- [34].Muthén LK, Muthén BO Mplus user’s guide.
- [35].Therneau TM (2015) A package for survival analysis in S.
- [36].Hedeker D, Gibbons RD (1996) MIXOR: A computer program for mixed-effects ordinal regression analysis. Comput. Methods Programs Biomed 49, 157–176. [DOI] [PubMed] [Google Scholar]
- [37].Bates D, Maechler M, Bolker BM, Walker S (2015) Fitting linear mixed-effects models using lme4. J. Stat. Softw 67, 1–48. [Google Scholar]
- [38].Gatz M, Reynolds CA, Finkel D, Pedersen NL, Walters E (2010) Dementia in Swedish twins: Predicting incident cases. Behav. Genet 40, 768–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ridge PG, Hoyt KB, Boehme K, Mukherjee S, Crane PK, Haines JL, Mayeux R, Farrer LA, Pericak-vance MA, Schellenberg GD, Kauwe JSK, Genetics D, Adgc C (2016) Neurobiology of aging assessment of the genetic variance of late-onset Alzheimer’s disease. Neurobiol. Aging 41, 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Beam CR, Turkheimer E (2017) Gene-environment correlation as a source of stability and diversity in development In Gene-environment transactions in developmental psychopathology: The role in intervention research, Tolan PH, Leventhal BL, eds. Springer, Cham, Switzerland, pp. 111–130. [Google Scholar]
- [41].Neale MC, Eaves LJ, Kendler KS (1994) The power of the classical twin study to resolve variation in threshold traits. Behav. Genet 24, 239–258. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


