Summary
Germinal centers (GCs) are confined anatomic regions where rapidly proliferating B cells undergo somatic mutation and selection and eventual differentiation into memory B cells or long-lived plasma cells. GCs are also the origin of malignancy, namely follicular lymphoma (FL), GC B cell-diffuse large B cell lymphoma (GCB-DBLCL), and Burkitt lymphoma (BL). GC B cell lymphomas maintain their GC transcriptional signatures and sustain many features of the GC microenvironment, including CD4+ T follicular helper (Tfh) cells. Tfh cells are essential for the formation and maintenance of GCs, providing critical helper signals such as CD40L. Large scale sequencing efforts have led to new insights about the tightly regulated selection mechanisms that are commonly targeted during GC B cell lymphomagenesis. For instance, HVEM, a frequently mutated surface molecule in GC-derived lymphomas, engages the inhibitory receptor BTLA on Tfh cells and loss of HVEM leads to exaggerated T cell help. Here we review current understanding of how Tfh cells contribute to the selection of GC B cells, with a particular emphasis on how Tfh cell signals may contribute to lymphomagenesis. The possibility of targeting Tfh cells for the treatment of GC-derived lymphomas is discussed.
Keywords: Germinal Center B cell, T follicular helper cell, Lymphoma, HVEM, BTLA, PD-1, PD-L1, Immunotherapy
Introduction
Germinal centers (GCs) are anatomic regions in lymphoid organs where activated B cells undergo somatic mutation and rapid proliferation, and give rise to memory B cells and long-lived antibody secreting plasma cells. GCs are organized into two main anatomical compartments, named based on their histological appearance in early staining techniques, a dark zone (DZ) and light zone (LZ). In the DZ, B cells express activation induced cytidine deaminase (AID) which mutates immunoglobulin (Ig) genes through deamination of cytosines, leading to Ig diversification. In the LZ, GC B cells test their newly mutated Ig proteins for improved affinity to antigen presented on the surface of specialized stromal cells called follicular dendritic cells (FDCs). If the B cell recognizes antigen, it internalizes the antigen for presentation to the limited number of CD4+ T follicular helper (Tfh) cells that reside in the LZ. Tfh cell positive selection drives expression of Myc, which determines GC B cell size and subsequent numbers of clonal cell divisions that occur in the DZ 1–4. Just as GC B cells are some of the most proliferative mammalian cells, dividing every 4–6 hours, they are also particularly sensitive to cell death with ~50% of GC B cells dying in 6 hours. DZ GC cells die due to deleterious AID-induced mutations and LZ GC B cells die by default if there is a lack of antigen engagement and subsequent T cell help.
Tfh cells are a subset of CD4+ T cells that require the transcription factor Bcl6, localize to B cell follicles and GCs, and depend on GC B cells for their maintenance 5,6. Tfh cells are not necessarily defined by their production of particular cytokines, but rather by their ability to support GC formation, select GC B cells, and determine GC B cell differentiation into memory B cells and plasma cells through cognate interactions. Tfh cell positive selection of GC B cells occurs predominantly through CD40L engagement of CD40 on the GC B cell. However, several cytokine and cell surface proteins contribute to the Tfh cell’s ability to promote the clonal expansion and selection of GC B cells.
Despite the fragility of GC B cells in the absence of sufficient Tfh cell positive selection signals, GC B cell selection can go awry. While overexpression of anti-apoptotic molecules such as Bcl2 and cell cycle promoting molecules such as c-Myc have long been recognized to drive lymphomagenesis, whether mutations occur that lead to exaggerated T cell help has been less clear 7. There are three main types of non-Hodgkin B cell lymphomas that originate in the GC and maintain a GC B cell transcriptional identity: follicular lymphoma (FL), GC-type diffuse large B cell lymphoma (GC-DLBCL), and Burkitt lymphoma (BL). Follicular lymphoma, in particular, maintains the normal GC architecture, such as the presence of Tfh cells and stromal cells, despite dyssynchronous LZ and DZ cycling 8–10. GC-DLBCL also maintains a CD4+ T cell and Tfh cell signature; in contrast the related Activated B cell (ABC)-DLBCL, that is thought to be a post-GC stage lymphoma, does not maintain these signatures 11,12. Burkitt lymphoma, which is largely defined by MYC translocations 13, is more similar to the DZ GC B cell stage and is not associated with CD4+ T cells. FL and GC-DLBCL association with Tfh cells and phenotypically normal stroma has led to the hypothesis that non-malignant immune cells support lymphomagenesis. Large scale sequencing of patient samples has provided novel insights about the regulatory pathways that are commonly targeted during GC B cell lymphomagenesis 11,14,15. In this review, we explore evidence for participation of Tfh cells in the formation and progression of GC B cell lymphomagenesis with a focus on the key molecular mediators of Tfh cell help. We will also discuss the role of GC B cells in supporting Tfh cell lymphomagenesis. We will discuss how the feedforward loop of Tfh cell support of GC B cells suggests that targeting molecular regulators of Tfh cells may be a method to inhibit early lymphomagenesis or thwart progressive disease.
Mutations in B cell lymphomagenesis
In follicular lymphoma, the first genetic hit in 90% of cases is the t(14;18)(q32;q21) chromosomal translocation of the anti-apoptotic Bcl2 gene with the IgH locus during Ig gene recombination in the bone marrow (BM). Although Bcl2 overexpression provides B cells with a survival advantage, it is not sufficient for lymphomagenesis as 10–100 per million circulating peripheral blood B cells in a healthy adult have this translocation 16. Bcl2-overexpressing GC B cells have increased survival and this is likely associated with an increased risk of abnormal selection and AID-induced transformation to a malignant state 8. Indeed, Bcl2 overexpression can occur in FL and GC-DLBCL, driving survival of GC B cells that normally do not express Bcl2. This correlates with the increased frequency of Bcl2 translocations in older people, and the evidence that Bcl2-overexpressing B cells give rise to memory B cells that preferentially undergo iterative rounds of GC entry, allowing for multiple rounds of mutagenesis and potential errors in selection 17,18.
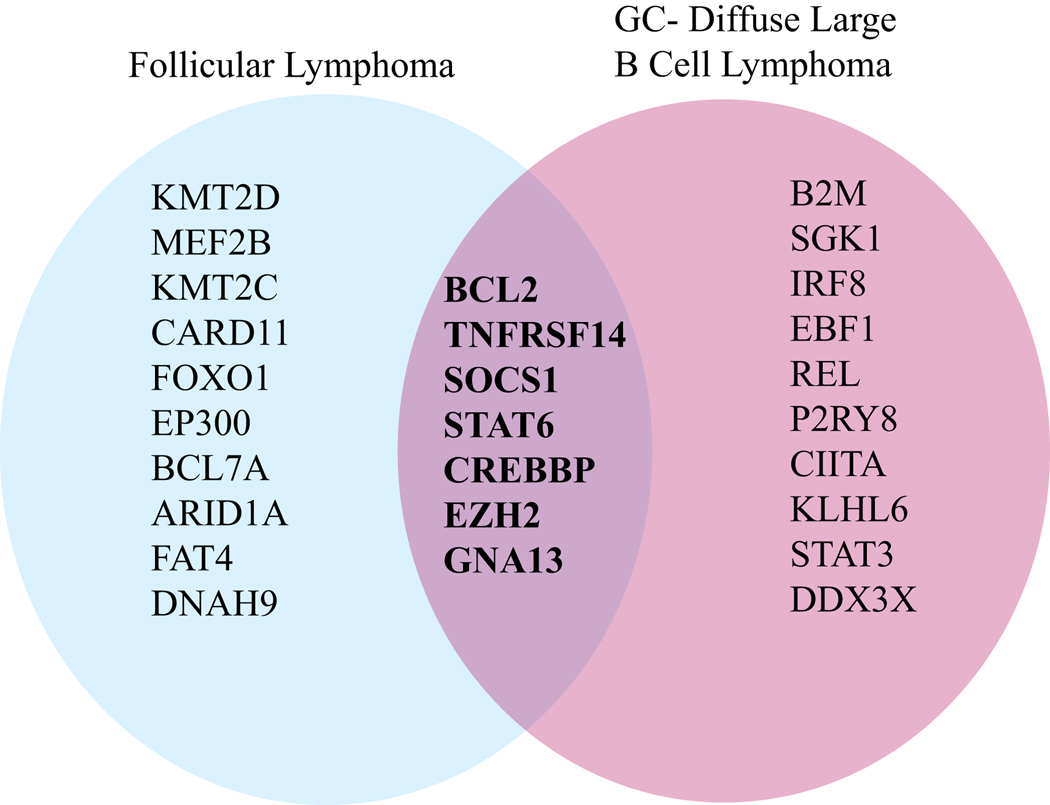
AID-mediated cytidine deamination can be recognized by base excision repair or mismatch repair mechanisms leading to either point mutations or double strand breaks followed by recombination. While AID is hypothesized to drive the majority of mutations that transform a mature B cell into a malignant cell, some lymphoma-associated mutations may arise independently of AID, perhaps as a consequence of the increased error prone DNA repair in GC B cells 19,20. Extensive sequencing has been done in GC-derived lymphomas to understand what mutations are driving lymphomagenesis. Surprisingly, only around half a dozen mutations are shared by FL and GC-DLBCL11, 14, 15, 21 (Figure 1). These include mutations in BCL2, TNFRSF14, SOCS1, STAT6, CREBBP, EZH2, and GNA13.
Figure 1. Common mutations in germinal center (GC)-derived lymphomas.
Venn diagram of mutations found in follicular lymphoma (FL) and germinal center diffuse large B cell lymphoma (GC-DLBCL) 11,14,15. Common mutations shared by FL and GC-DLBCL include BCL2, CREBBP, EZH2, GNA13, TNFRSF14, SOCS1, and STAT6.
Loss of function mutations in TNFRSF14 and SOCS1 and activating mutations in STAT6 enhance or override Tfh cell helper signals to GC B cells 21–23 and these molecules will be discussed in detail in later sections. CREBBP and EZH2 are epigenetic modifiers that have complex effects on GC B cell signaling and gene expression. Loss of function mutations in CREBBP promote unrestricted GC growth, in part through increasing CD40 signaling, and also reducing MHC-II expression, which alters mutant FL cell ability to present antigen to CD4+ T cells 24. Gain of function mutations in polycomb repressor complex-2 (PRC2) component EZH2 inhibits cell cycle checkpoints, promoting uncontrolled GC B cell proliferation 25,26. GNA13 is a signaling protein downstream of transmembrane G-protein coupled receptors S1PR2 and P2RY8 that confines B cells to the GC and inhibits Akt and possibly other signaling molecules, limiting survival signals 27–29. While mutations in some of the key positive regulators of GC B cells, such as CD40, ICOSL, and SLAMF and negative regulators of GC B cells, such as PD-L1/2 and Fas, are less selected in GC lymphomas, we suggest that these molecular mediators may often play an important, and yet underappreciated role in disease pathogenesis.
Tfh cell participation in follicular lymphoma
Tfh cells, defined by surface expression as CXCR5+PD-1+ICOS+CD4+ T cells, support GC B cells, which in turn maintain Tfh cells, providing a feedforward positive selection loop. In a mouse model of follicular lymphoma, in which the Vav promoter drives Bcl2 overexpression in hemopoietic cells, depletion of CD4+ T cells reduced the size of the GCs, suggesting that disease initiation and likely progression depends on T cells 30. Tfh cells in the FL tumor microenvironment have been demonstrated by several studies 9. Tfh cell gene signatures are also maintained in Bcl2-positive GC-DLBCL cases 12. An association of Tfh cells with EBV+ BL cases has not been reported 13, supporting the idea that BL is more DZ-like and less dependent on Tfh cell help signals, perhaps because BL cells constitutively express MYC. We will focus our review on what is known about the key molecular regulators of Tfh cell help to GC B cells and how they may play a role in GC B cell outgrowth and lymphomagenesis (Figure 2).
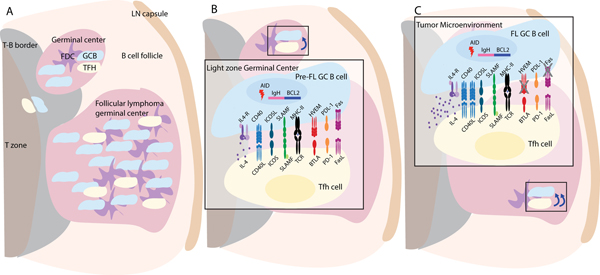
Figure 2. Follicular lymphoma (FL) germinal center B cells receive exaggerated helper cues from T follicular helper (Tfh) cells.
(A) Germinal center microenvironment in a LN with a follicular lymphoma tumor. The follicular lymphoma GC contains stromal follicular dendritic cells (FDC, purple), GC B cells (blue), and Tfh cells (yellow). (B) Magnification of GC light zone where a Bcl2 translocated GC B cell is presenting antigen to a Tfh cell and is transmitting and receiving normal positive selection (left of TCR-MHCII) and negative selection (right of TCR-MHCII) signals. (C) Magnification of a FL GC B cell’s interaction with a Tfh cell in which several of the key molecular regulators are altered by AID-induced mutations, as detailed in the text. In this example, there is increased IL-4 production, increased CD40L due to the absence of BTLA-HVEM, and mutations in Fas death domain that prevents FasL-mediated cell death. Small box in B and C shows relative strength of helper signal from Tfh cell to B cell.
Before discussing CD4+ T helper molecules, the role of cytotoxic lymphocytes in lymphomas should be mentioned. GC lymphoma cells have a high mutation burden and are susceptible to killing by cytolytic CD8 T cells and NK cells 31. It is therefore not surprising that GC lymphomas commonly show mutations in beta-2-microglobulin (b2m) and CD58 (LFA3). B2m is a component of all MHC class I molecules, and loss of this protein leads to loss of MHC class I expression and protection from CD8 mediated killing. Loss of MHC class I can, however, lead to recognition by NK cells. GC lymphomas can become resistant to NK cells through loss of CD58 expression, a surface counter receptor for the CD2 adhesion and activating molecule expressed on NK cells 32. Cytotoxic cells usually have very limited access to B cell follicles and GCs and it is possible that another mode of GC lymphoma evasion of cytotoxic cells is through continued CXCR5-CXCL13 mediated compartmentalization within the follicular microenvironment. CXCL13 is made by several types of stromal cells, including FDCs and marginal reticular cells (MRCs) and GC lymphomas, especially FLs, maintain their FDC networks and have strong expression of CXCL13 33. A signature feature of Tfh cells is their expression of CXCR5 and efficient ability to access lymphoid follicles. Human Tfh cells are also a source of CXCL13 though the role of Tfh CXCL13 in GCs remains unclear. While most cytotoxic cells are CXCR5 negative, in some situations they can be induced to express CXCR5 and this facilitates their access to GC microenvironments and elimination of GC lymphoma cells 34–36.
IL-4 – STAT6
IL-4 is a CD4 T cell derived cytokine known to support B cell proliferation, class-switching, and survival 37. Sequencing of human FL identified activating mutations in STAT6, the main IL-4R signaling mediator, and loss of function mutations in a suppressor of cytokine signaling, including IL-4R signaling, SOCS1 15. This is consistent with increased STAT6 signaling measured by p-STAT6 in formalin fixed FL sections compared to tonsil 38. Furthermore, IL-4 protein was identified to be 5-fold more abundant in FL samples compared to tonsils 39. Interestingly, normal Tfh cells express high amounts of IL-4 mRNA, yet produce small amounts of IL-4 protein 6. When malignant B cells and bystander healthy immune cells were sorted and their transcriptional signatures analyzed, Tfh cells were found to express high levels of IL-4 transcripts in FL 40. Furthermore, FL B cells in close proximity to Tfh cells were actively signaling through STAT6 as measured by p-STAT6, suggesting Tfh cells in FL are the major producers of IL-4. A follow up study, confirmed the high expression of IL-4 in CD25-low Tfh cells 41.
It remains unknown what is driving the elevated expression of IL-4 transcripts by FL Tfh cells compared to tonsillar Tfh cells. Unlike the increase seen in IL-21 expression, IL-4 expression was unchanged when the amount of antigen GC B cells presented to Tfh cell was increased, suggesting that TCR signaling in Tfh cells is not sufficient to increase IL-4 mRNA expression 42. IL-4 production in Tfh cells is uniquely regulated compared to Th2 cell IL-4, as Tfh cell IL-4 is transcriptionally regulated by CNS2, a 3’ enhancer in the IL-4 locus, but not by GATA-3 43. Understanding the production of IL-4 by Tfh cells and its consequential IL4-R – STAT6 signaling in FL B cells is further complicated by the results that the blockade or deletion of these signaling pathways results in minor alterations in the normal GC response 44. In the mouse, production of both IL-4 and IL-21 are required for a robust GC B cell response 45,46. Loss of IL-4 signaling into the B cell modestly reduces the size of the GC response in some studies. Two recent studies have found that IL-4 is preferentially expressed by Tfh cells later in the immune response, compared to IL-21 which is preferentially expressed early in the GC 47,48. IL-4 has also recently been shown to be uniquely able to induce high levels of Bcl6 protein, which is the transcription factor that is required for GC B cell formation and maintenance 49.
These data support a model in which Tfh cells in FL are most similar to late GC-Tfh cells. The independent data supporting high IL-4 and selection for activating STAT6 and loss of function SOCS1 mutations adds further support to the notion that IL-4 production by Tfh cells is supportive of FL B cells. Additionally, IL-4’s ability to stabilize Bcl6 protein may play an important role in maintaining GC B cell identity and preventing transformation into a post-GC B cell lymphoma. While the effect of IL-4 alone in driving GC B cell outgrowth is less clear based on the normal GC B cell evidence, it remains to be studied whether IL-21 is being co-produced by FL Tfh cells, such that FL B cells are receiving two complementary cytokine signals known to support GC B cell expansion.
CD40L – CD40
CD40, a TNFRSF member, predominantly promotes B cell survival, proliferation and class switching through its ability to signal via NFκB. In humans with mutations in CD40L-CD40 signaling, GCs cannot form and hyper-IgM immunodeficiency develops. FL B cells express CD40 and respond to CD40L. CD40 signaling in FL B cells increased the anti-apoptotic protein Bcl-XL and prolonged FL B cell survival in vitro, suggesting CD40 promotes the survival of FL B cells complementary to their overexpression of Bcl2 50,51. CD40 signaling can promote differentiation into plasma cells and CD40 amplifications occur in human plasma cell malignancies, yet none have been described in GC-derived lymphomas 52.
CD40L is expressed by naïve and activated CD4+ T cells, but not by CD8+ T cells 53,54. CD40L expression increases after TCR signaling, and Tfh cells contain pre-formed CD40L 55–57. The amount of preformed CD40L mobilized to the surface of Tfh cells increases in proportion to the amount of antigen encountered 21. One study found FL Tfh cells expressed 2.5-fold higher amounts of CD40L mRNA compared to tonsillar Tfh cells, suggesting FL Tfh cells may receive stronger TCR signals, though it is also possible that the FL samples were more enriched for the type of Tfh cells associated with GCs (GC-Tfh) that correspond to only a fraction of Tfh cells in healthy lymphoid tissue 41. CD40L protein is also expressed on T cells in the FL environment 58. Strong evidence for a positive role of CD40L in promoting lymphomagenesis comes from a mouse study in which the LTR of HTLV1 drives constitutive expression of CD40L in mature T cells; one third of these mice developed a GC lymphoma by 6 – 12 months of age 59. These data are in contrast to B cell constitutive CD40L transgenic mice which do not maintain the GC B cell response 60, suggesting that the cellular source and regulation of CD40L delivery is crucial to the signaling outcome.
As CD40 signaling is a master regulator of B cell responses, therapeutics have been developed for the purposes of immune modulation in transplantation, autoimmunity, and lymphoma. While several CD40 agonists have been developed to stimulate the immune response via costimulation of antigen presenting cells, CD40 blocking agents have also been developed. In a Phase I/II trial with a CD40 antagonist HCD122, CD40 blockade induced a response in a third of FL patients with refractory disease to rituximab treatment 61. Since HCD122 has been shown to induce antibody dependent cellular cytotoxicity (ADCC), it remains to be determined if the clinical efficacy is due to ADCC, blockade of CD40L-CD40 signaling, or both.
PD-1 – PD-L1/PD-L2
The inhibitory IgSF member PD-1 is a defining marker of normal and FL Tfh cells 62. PD-1 is also highly upregulated on exhausted CD8+ T cells that cannot appropriately perform cytotoxic functions, which has led to impressive immunotherapy benefit by blocking PD-1 to restore TCR sensitivity and T cell function in some cancers 63,64. Several studies have attempted to correlate the outcome of FL with PD-1+ T cells, but the results have not been consistent, likely due to heterogeneity of PD-1+ T cells within the FL environment 65,66. While the number of PD-1+ T cells did not correlate with outcome, the location of PD-1+ T cells in a follicular pattern was associated with a shorter time to transformation compared to a diffuse pattern 65. Although these results are difficult to interpret as the authors did not stain for CD4 or CD8, the finding that the diffuse PD-1+ cells co-stained for TIM-3, a marker of CD8+ T cell exhaustion, whereas the follicular PD-1+ cells did not, suggests that the diffuse T cells are CD8+ cells whereas the follicular cells may be Tfh cells. In another study, higher levels of PD-1 expression were found in CD4+ T cells compared to CD8+ T cells in FL 66. Amongst the CD4+CD25- PD-1+ cells in FL, there were 4 subsets: (1) CD27+CXCR5+ Tfh cells, (2) CD27+CXCR5- cells, (3), CD28-CCR4+ cells, (4) CD28-CCR4- cells. Interestingly, CD27+CXCR5+ Tfh cells and CD27+CXCR5-cells were correlated with better overall survival while CD28-CCR4+ cells and CD28-CCR4- cells were correlated with poor overall survival. These data suggest that non-Tfh CD4+ T cells with low expression of costimulatory molecules likely prevent a productive immune response to the FL or, alternatively, directly support FL B cell proliferation. While a higher frequency of PD-1+ Tfh cells in the FL microenvironment did not correlate with poor survival, these data do not rule out a possibility that PD-1 may be restraining the Tfh cell’s ability to support FL B cell survival. Interestingly, 40% of ABC-DLBCL have mutations or copy number gains of PD-L1, whereas only 10% of GC-DLBCL have increased PD-L1 expression 64,67. Increased expression of PD-L1 is associated with increased numbers of CD8+ T cells in the tumor microenvironment. However, these data suggest that increased PD-L1 is not as prevalent of a selection mechanism in GC-DLBCL compared to post-GC ABC-DLBCL, perhaps due to the inhibition of PD-1+ Tfh cells in GC-DLBCL tumors. It remains to be determined if PD-L1 expression alters Tfh cells in the GC-DLBCL tumor microenvironment and if PD-L1 is mutated in a manner that leads to loss of expression in some GC-DLBCL cases 67.
During the normal GC response, the majority of evidence suggests that PD-1’s interaction with PD-L1 restrains the response. However, PD-1’s function in the Tfh cell and how it restrains the amount of help provided to GC B cells remains unclear despite several studies. PD-L1 is expressed in naïve B cells, and upregulated in GC B cells, whereas PD-L2 is not expressed in naïve B cells, but slightly increased in GC B cells 68. In one study, where PD-1 or its ligands were deficient, plasma cell output was decreased at 2 weeks and this decrease was sustained for greater than 1 year after NP-CGG immunization. In discordance with PC output, GC B cells were not increased, likely due to their increased cell death in the absence of PD-1 during this immunization. Interestingly, while the authors found that PD-1 deficiency led to increased Tfh cell numbers, these Tfh cells had reduced expression of helper cytokines such as IL-21 68. In another study, after helminth infection, PD-L1-deficiency led to increased GC B cells and Tfh cells 69. After immunizing with KLH/CFA and blocking PD-1, PD-L1, or PD-L2, it was found that PD-L1 blockade increased Tfh cells 5-fold, whereas PD-L2 blockade had no effect, and PD-1 blockade provided an intermediate 2-fold increase in Tfh cells, suggesting that PD-L2 is not upregulated during KLH/CFA or the blocking agent is not sufficient to reveal an effect 69. These data are further complicated as PD-1 is also expressed by T follicular regulatory (Tfr) cells, CD4+Foxp3+ cells that express CXCR5. PD-1 or PD-L1 deficiency, but not PD-L2 deficiency, led to increased Tfr cells rather than Tfh cells after immunization with MOG 70. Interestingly, PD-1 or PD-L1-deficiency or blockade increased GC B cell responses in a mouse model of type I diabetes, whereas PD-L2 blockade had no effect 71. While these studies suggest that PD-1 restrains Tfh cells, together they do not point to a mechanism of how PD-1 on the T cell restrains help to GC B cells. In a more recent study, mixed chimeras were generated with PD-L1 deficient and wild-type BM. While PD-L1 deficient B cells were increased in representation in the GC, memory B cell, and plasma cell compartments, they had reduced affinity to the immunogen, the hapten NP 5,72. These data most clearly suggest that PD-L1 expression on the GC B cell restricts the amount of T cell help and the stringency of GC B cell selection by Tfh cells. While the authors did not directly show PD-L1 in the GC B cell was regulating PD-1 in Tfh cells, they observed that PD-1 mutant antigen-specific Tfh cells supported larger GC B cell responses.
PD-1 is known to dampen T cell signaling by inhibiting TCR and CD28, and has been shown to signal predominantly through the phosphatase SHP-2 21,73,74. Although T cell deficiency in SHP-2 did not phenocopy many of PD-1’s known roles in CD8+ T cells, in the absence of SHP-2, the inhibitory phosphatase SHP-1 was capable of replacing SHP-2 in mediating inhibition 75,76. The relative importance of SHP-1 and SHP-2 for PD1 function in Tfh cells remains to be determined. Another set of recent observations has shown the existence of cis-interactions between PD-1/PD-L1 and PD-L1/CD80 on dendritic cells 77,78. Since B cells can express PD-1 and CD80 68, it may be the case that not all PD-L1 molecules expressed on GC B cells are capable of engaging with PD-1 on Tfh cells to restrain their TCR signaling.
The heterogeneity of PD-1+ T cells in FL and the role of PD-1 in restraining Tfh cell help to GC B cells may provide an explanation for the modest effects of PD-1 blockade in FL. If the majority of PD-1+ T cells in the FL microenvironment are exhausted CD8+ and CD4+ T cells that are failing to perform cytotoxic or immune priming functions, we would hypothesize that FL patients would likely have good clinical responses to anti-PD-1 therapy. However, if PD-1+ Tfh cells are present in nearly all FL microenvironments, we would hypothesize that blocking PD-1 in Tfh cells would increase helper signals to FL B cells and lead to poor clinical responses. In agreement with the diversity of PD-1+ T cells, FL clinical responses to PD-1 have only been effective in a third of patients, while in classical Hodgkin’s lymphoma nearly 90% of patients exhibit a response 63,64. This suggests that additional immunotherapy approaches that do not unleash Tfh cell helper signals need to be tested in FL, such as CD47 blockade, where combination therapy with rituximab has shown a response in 70% of patients in a small clinical trial 63,79. CD47 is present on GC B cells and is often upregulated on DLBCL and FL cells 80. CD47 binds SIRPα, an ITIM containing inhibitory receptor that is expressed on myeloid cells, transmitting an anti-phagocytic ‘don’t eat me signal’. Blockade of CD47 maybe an approach to enhance rituximab mediated ADCC. SIRPα is not expressed on T cells and therefore blocking antibodies to CD47 are unlikely to lead to augmented Tfh cell responses.
HVEM – BTLA
HVEM, TNFRSF member 14, is one of the most highly mutated surface receptors in GC-derived lymphomas, with the gene harboring mutations in approximately 50% of FL and GC-DLBCL cases 11,15,81. No mutations in TNFRSF14 have been documented in BL cases 13, supporting the idea that BL does not benefit from CD4+ T cell help. Although reports of TNFRSF14 mutations have been found in double BCL2 and MYC mutated lymphomas 82, it remains to be determined if TNFRSF14 mutations are occurring prior to the MYC translocations and if loss of TNFRSF14 is redundant with MYC overexpression. Mutations in TNFRSF14 are concentrated in the extracellular region of the encoded protein and a third are predicted to lead to truncations 15,81 and at least some mutations have been confirmed to cause a loss of HVEM surface expression 83.
As a TNFRSF molecule, HVEM can recruit TRAF2/TRAF5 and signal via NFκB, as well as through NIK and STAT3 84–86. Although it is unexpected that a potential NFκB signaling mediator is selectively lost during GC B cell lymphomagenesis, HVEM has been shown to be a tumor suppressor in mouse models of lymphomagenesis 21,81. HVEM has a complex signaling network that can promote reverse signaling through HVEM ligands. HVEM’s signaling network is conserved in mouse and human, with the exception of one partner (LTα) which only binds human HVEM. HVEM received its name Herpes virus entry mediator as it is the receptor for the gD subunit of HSV-1 and HSV-2. HVEM can bind to two TNFSF members, LIGHT and LTα through its cysteine rich domain 2 (CRD2). HVEM transmits reverse signals through its two IgSF ligands BTLA and CD160, which bind HVEM’s CRD1, as well as via a more recently identified synaptic adhesion molecule SALM5 86,87.
BTLA, also known as B and T lymphocyte attenuator, is the most highly expressed HVEM ligand in the GC 88,89. BTLA, an IgSF member, has 50% amino acid sequence homology between mouse and human. BTLA was shown biochemically to recruit the inhibitory phosphatases SHP-1 and SHP-2 to its cytoplasmic tail 90. While human BTLA has 4 cytoplasmic tyrosines and mouse only has 3, they are conserved in their ability to recruit SHP-1, SHP-2, and Grb-2, but do not recruit SHIP-1 90–92. In antigen-receptor-BTLA co-crosslinking studies, BTLA directly inhibited CD3ζ phosphorylation in T cells and inhibited BCR signaling by regulating Syk and Fyn in B cells 93,94. BTLA is upregulated in Tfh cells compared to naïve CD4+ T cells, and one study has suggested BTLA negatively regulates Tfh cell support of GC B cells by restricting IL-21 production 95,96. Although the BTLA-deficient TCR transgenic T cells in this model did support a modestly larger GC B cell response, IL-21 is a notoriously difficult to stain for cytokine and the study did not determine if IL-21 mRNA was elevated, leaving it unclear whether BTLA-mediated inhibition of IL-21 was the key mechanism of action of this HVEM ligand in Tfh cells.
While Tnfrsf14 transcripts are expressed at similar levels in naïve and GC B cells in mouse, HVEM surface protein is reduced on GC B cells 21. A similar pattern of HVEM mRNA and protein expression is evident in human follicular and GC B cells 97 and unpublished observations. A study of FL has confirmed the specificity of this low-level staining by identifying some cases that are surface positive and HVEM mutant cases that are surface negative 83. The apparent post-transcriptional downregulation of HVEM in GC B cells suggests these cells need to have tight control of HVEM protein abundance. However, what regulates HVEM protein expression in GC B cells remains unknown and is an area of active investigation.
Using a mixed chimeric mouse system, HVEM-deficiency was found to provide B cells with a competitive advantage in the GC compared to the follicular compartment 21. HVEM haploinsufficiency provided GC B cells with an intermediate advantage, suggesting that the amount of HVEM expressed on the B cell regulates competitiveness. HVEM-deficient B cells showed a competitive advantage at both the pre-GC and GC stages 21. HVEM-deficiency led to a small increase in the frequency of pre-GC and GC B cells entering into the cell cycle, providing at least a partial explanation for their increased representation. Following immunization with an NP-haptenated protein, HVEM-deficient GC B cells showed preferential expansion of the non-NP binding relative to the NP-binding B cells. Since the NP-binding cells tend to rapidly achieve high affinity, these data suggested HVEM-deficient B cells with lower affinity were being preferentially selected. In additional support for this model, HVEM-deficient NP-specific GC B cells had reduced high affinity W33L mutations compared to HVEM-expressing competitors in the same mice. Comparison with W33L mutation frequencies in control chimeras suggested that the most prominent effect was an increased stringency of selection for W33L mutations in wild-type cells that were in competition with HVEM-deficient cells. One model to explain these observations is that the wild-type GC B cells needed to acquire more antigen through a higher affinity BCR in order to receive competitive Tfh cell positive selection signals. These data are similar to what was observed in chimeric PD-L1-deficient GCs, where PD-L1-deficient GC B cells were preferentially expanded amongst the non-NP binding cells and had reduced high affinity mutations 72. While the data together suggest that negative regulators maintain the stringency of Tfh cell selection of GC B cells, the PD-L1 study did not observe an increase in high affinity mutations in the PD-L1-expressing GC B cells competing with the PD-L1 deficient GC B cells, suggesting that these regulatory molecules do not act in an identical manner.
Using gain and loss of function approaches, we demonstrated that the growth restraining actions of HVEM in GC B cells depended on BTLA expression by T cells 21. Although BTLA is highly expressed in B cells and HVEM–BTLA have been shown to engage in cis interactions in HEK-293 cells 90,98, BTLA-deficiency in B cells did not give them a growth advantage in GCs, suggesting BTLA is not acting in cis to regulate B cell responses 21. In contrast to these results, HVEM-BTLA cis-interactions were presented to explain findings that sh-RNA targeting of Btla in Vav-Bcl2 BM chimeras phenocopied the increased GC lymphomagenesis seen in HVEM-knockdown Vav-Bcl2 BM chimeras 81,99,100. We reconcile the findings by noting that the global hematopoietic shRNA knockdown approach would be expected to reduce BTLA in Tfh cells as well as in B cells. In accord with the model that BTLA acts in trans to restrain GC B cell responses, no mutations have been reported in the coding region of BTLA in B cell lymphomas. It has been suggested that BTLA expression is lost in a significant fraction of FLs through epigenetic regulation of KMT2D methylation 81, and an additional report observed a diversity of BTLA expression in FL samples 101. Further work will be needed to understand if heterogeneity of human BTLA expression in the GC and follicular B cell compartments is relevant to lymphomagenesis.
Early evidence that HVEM mutations may be enhancing the ability of FL cells to present antigen to T cells came from the observation that FL patients with BM transplantation had increased rates of graft versus host disease 83. Using FL cells as antigen presenting cells in allogeneic mixed lymphocyte reactions, they found HVEM-mutated FL cells induced more division in both CD4+ and CD8+ T cells than HVEM-expressing FL cells. When HVEM-expressing FL cells were incubated with a BTLA antagonist both allogeneic CD4+ and CD8+ T cell proliferation increased, while the antagonist had no effect on the response elicited by HVEM-mutated FL cells. These findings suggest that HVEM on FL cells inhibits signaling in allogeneic T cells through engagement of BTLA 83.
Planar lipid bilayer imaging of human T cells showed co-localization of SHP-1 with BTLA that was largely reversed when BTLA’s ITIM/ITSM motif was mutated; BTLA did not colocalize with SHP-2 21. These results have been supported by two independent studies that found BTLA preferentially recruits SHP-1 in mouse CD4+ T cells 76. In addition to its ability to directly dephosphorylate CD3ζ 93, BTLA inhibited phosphorylation of ZAP-70 and PKC-θ in human Tfh cells 21. Analysis of mice homozygous for a cytoplasmic domain mutant of BTLA that lacks all three tyrosines including its SHP-1 recruiting ITIM/ITSM motif, confirmed that BTLA reverse signaling was required for HVEM function in B cells 21. These data suggest that HVEM-expressing B cells are restrained in the help they receive through trans signaling into BTLA expressing Tfh cells during cell-cell contacts. Experiments in mice lacking SHP-1 or SHP-2 in T cells further supported the conclusion that BTLA signaling via SHP-1 into the Tfh cell modulates the amount of help provided to HVEM-expressing B cells 21.
While early T cell-B cell interactions can last for an hour, in the GC the majority of interactions take place on the range of minutes. Based on finding that HVEM-deficiency led to an intrinsic growth advantage in B cells, it was inferred that HVEM-deficient B cells must receive greater help than their wild-type counterparts during these brief interactions. CD40L is the main helper factor known to be pre-formed in Tfh cells. When Tfh cells were exposed in vitro to B cells presenting increasing amounts of MHC-peptide complexes, an analog upregulation of CD40L was observed 21, indicating that the extent of mobilization of preformed CD40L to the Tfh cell surface is tuned to the TCR signal strength. In accord with this notion, HVEM-deficient B cells induced greater CD40L surface mobilization in Tfh cells when compared to HVEM-expressing B cells displaying the same amount of MHC-peptide. Furthermore, CD40 recruitment to the immune synapse was decreased when HVEM was included in lipid bilayers, suggesting that CD40L mobilization to the synapse is reduced by HVEM-BTLA interactions. While these data do not rule out additional factors that HVEM-BTLA may be regulating, such as cytokines, they suggest that BTLA inhibits Tfh cell help by restraining the amount of CD40 signals delivered to GC B cells (Figure 3). In further support of this model, HVEM-deficient GC B cells competing with wild-type GC B cells exhibited an increased Myc gene signature 21.
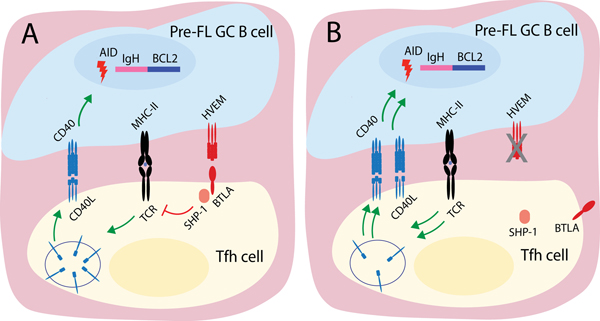
Figure 3. Model of BTLA action as a cell-extrinsic suppressor of GC lymphomagenesis.
(A) HVEM-expressing GC B cell recruits BTLA to immunological synapse. BTLA signals into Tfh cell through SHP-1, which reduces the strength of TCR signal and amount of preformed CD40L mobilized to the cell surface. (B) In the absence of HVEM, BTLA is not recruited to the immune synapse and the amount of preformed CD40L mobilized to the surface is increased. The HVEM-deficient B cell receives increased CD40 signaling.
In planar lipid bilayer studies, the presence of HVEM in the bilayer transformed the stable, centered immune synapse into a motile, polarized kinapse structure 21. These results suggested that BTLA inhibits strong contacts with HVEM-expressing cells. Surprisingly, 2-photon microscopy-based imaging of Tfh cells interacting with HVEM-deficient and wild-type GC B cells in intact lymph nodes did not reveal differences in the time of contact or surface area of interactions (unpublished observations). Further work is needed to determine if there are differences in interaction stability early versus late in the GC response.
In models of Bcl2-overexpression, HVEM-deficiency continued to provide GC B cells with an advantage and it led to accelerated lymphomagenesis, suggesting that HVEM-deficiency was acting in a separate pathway from Bcl2 81. Overexpression of Bcl2 in mice lacking BTLA in T cells led to similar pre-malignant outgrowths as occurred in Bcl2 over-expressing HVEM-deficient mice. These data provide some of the first evidence of a cell-extrinsic tumor suppressor distinct from the mechanism of CD8+ and NK cell immune surveillance. The ways in which increased signaling via CD40 and other T cell-derived helper factors cooperates with Bcl2-overexpression in lymphoma development remains to be fully elucidated.
Given HVEM’s complex signaling biology and the expression of several HVEM ligands on cells in immune microenvironments, it remains to be determined if loss of HVEM in GC-derived lymphomas alters parameters in addition to the BTLA-HVEM axis. While a third of TNFRSF14 mutations are truncating, the remaining two-thirds may still preserve HVEM protein expression but alter its ability to engage ligands. For instance, HVEM-Y61A, a mutation that disrupts binding to BTLA and CD160 98, is found in FL patients. In particular, it will be interesting to learn if loss of HVEM or particular mutations alter FL cell interactions with CD160 expressing cytotoxic CD8+ T cells or NK cells and immune surveillance in the tumor microenvironment. It will also be interesting to learn if LIGHT engages with HVEM on B cells to determine GC B cell outcomes, and if HVEM can signal into the B cell. Lastly, given BTLA’s high expression on B cells it will be important to better understand if the ability of this molecule to negatively regulate BCR signaling is relevant to lymphomagenesis or other B cell-mediated diseases.
ICOS – ICOSL
ICOS, or inducible T cell co-stimulator, is required for Tfh cell differentiation and maintenance 5. ICOS, which signals through PI3K, is the dominant co-stimulatory molecule for Tfh cells and it functions analogously to CD28 once T cells are in the GC 5,102. ICOSL is not known to signal and is downregulated on GC B cells compared to follicular B cells. However, ICOSL is upregulated after CD40 signaling on GC B cells, suggesting that GC B cells that have received the most T cell help enter into a positive feedback loop that amplifies this help. Indeed, when ICOSL-high cells versus -low cells were analyzed for high affinity NP-binding mutations, the highest ICOSL expressing GC B cells had increased high affinity mutations. When Tfh cells were stimulated in vitro with activating antibodies to CD3 and ICOS, Tfh cells increased their mobilization of CD40L to the T cell surface compared to cells activated by anti-CD3 alone, suggesting ICOS signaling enhances T cell helper signals to GC B cells 5,103. While in a competitive setting with WT cells, ICOSL-deficient GC B cells formed brief immune synapses with decreased surface area of engagement with Tfh cells 5,103. Together these data suggest that ICOSL expression on GC B cells is crucial for positive selection by ICOS-expressing Tfh cells.
Surprisingly, increases in ICOSL expression have not been reported in GC B cell lymphomas. However, ICOSL is more highly expressed in GC-DLBCL compared to ABC-DLBCL, suggesting ICOSL may be maintained and beneficial in a more GC-like lymphoma microenvironment 104. Although ICOSL transcripts were expressed highly in FL cells compared to a B cell line, ICOSL protein was not detected on FL cells in sections. Despite this, ICOS has been shown to be highly expressed on FL T cells and specifically on a subset of CD25+CXCR5+PD-1+ CD4+ T cells 105. While one group suggested that ICOS is defining a Treg population that is directly suppressing FL B cells through in vitro experiments, it has also been shown that ICOS expressing CD4+ T cells in the FL microenvironment are IL4-expressing non-regulatory Tfh cells 41. Further study on the role of ICOS-ICOSL signaling in GC lymphomas is needed.
FasL – Fas
GC B cells are known to be intrinsically prone to cell death given their low expression of Bcl2. Another feature that poises GC B cells for apoptosis is their high expression of the cell death receptor Fas (CD95). Fas is a TNFRSF molecule that, upon engagement by FasL, initiates the death inducing signaling complex, leading to Caspase-8 activation and apoptosis 106. Fas is selectively lost in a number of GC-derived lymphomas, as well as antibody driven autoimmune diseases. Fas mutations are found in GC-DLBCL and in some cases of FL and transformed FL 11,107. Fas mutations likely arise during the GC response and can lead to dominant negative mutations in the death domain or loss of expression 108. In autoimmune lymphoproliferative syndrome (ALPS), patients with inherited mutations in the Fas death domain have a 14-fold increased likelihood in developing non-Hodgkin B cell lymphomas later in life 109. Interestingly, healthy family members of ALPS patients that have Fas mutations are at increased risk of lymphomagenesis likely due to their accumulation of somatic hypermutations during GC responses 110.
In mice, Fas has varying impacts on GC size and B cell affinity maturation. Fas is highly expressed on activated and GC B cells. A Fas mutation in the mouse called lpr leads to lymphoproliferation and autoimmunity, yet normal sized peak GC responses after acute immunization. However, while WT GCs begin to contract three weeks post immunization, Fas-deficient GCs are maintained at this time point 111. In another study, conditional deletion of Fas in class-switched B cells was sufficient to lead to increased GC B cell responses 112. Although FasL transcript was increased in activated T cells and presumably present on Tfh cells 113, FasL is difficult to stain for and it has yet to be shown that Tfh cells can negatively select GC B cells through FasL. In addition to the elusive source of FasL in the GC, contradictory results have also been reported for the role of Fas in regulating GC size. Loss of Fas in GC B cells led to increased somatic hypermutation, loss of antigen reactivity, and preferential differentiation into plasma cells late in the response in a more recent study 114. Together, these data are inconclusive on whether Fas is a crucial negative regulator of ongoing GC responses, but may suggest Fas is necessary for the proper contraction and differentiation of the late GC response and suppression of lymphomagenesis. In a setting in which the intrinsic mitochondrial sensitivity to cell death is askew, such as in t(14;18) GC B cells, Fas may play a heightened role in maintaining stringent GC B cell selection. Such a role has been demonstrated in the setting of c-Myc overexpression 115.
Due to the downregulation of Fas and its apoptosis promoting signaling in many GC-derived lymphomas, it is not straightforward to consider how FasL-Fas signaling might be restored. Current approaches take advantage of what is known about molecules used to dampen or heighten Fas signaling. One group has suggested that CD74 (the invariant chain) might be a regulator of Fas signaling and that an anti-CD74 antibody, milatuzumab, might act to enhance Fas mediated cell death 116, though other mechanisms of anti-CD74 action are likely. Another approach is to inhibit NFκB signaling, which provides resistance to extrinsic cell death signaling through a Nedd8 inhibitor called Pevonedistat 117. It remains to be determined how Fas-mediated cell death can best be enhanced as a treatment for lymphomagenesis.
SAP – SLAMF
Signaling lymphocytic activation molecule (SLAM)-associated protein or SAP (encoded by SH2D1A) is a T cell signaling molecule that is mutated or lost in patients with X-linked lymphoproliferative (XLP) syndrome 118. SAP is composed of a single SH2 domain and its functions include blocking the recruitment of SHP1 and SHP2 to SLAM proteins. SLAM family members are homotypic adhesion receptors that are involved in the formation of immune synapses between T and B cells, with SLAMF6 (Ly108) and SLAMF5 (CD84) being most important. XLP is thought to arise because NK and CD8+ T cells in SAP-deficient patients are ineffective in killing EBV-infected B cells due to exaggerated negative signaling via SLAM-family proteins. As well as an extreme susceptibility to EBV infection, patients harboring SH2D1A mutations have a 200-fold increased risk in developing lymphoma compared to the normal population, likely due to uncontrolled EBV, and 30% have an extranodal BL type presentation in the ileum. XLP patients have not been reported to have increases in other types of GC-derived lymphomas 119.
In the mouse, loss of SAP prevents T cells from forming stable conjugates with B cells, but not other antigen presenting cells 120. Despite the relatively normal expression of helper molecules, SAP-deficient Tfh cells are altered in their ability to engage GC B cells because SLAMF6 and SLAMF5 send inhibitory signals into SAP-deficient T cells, and as a result the GC response fails. The importance of SAP in Tfh cell function in supporting GC cells might explain why XLP patients do not have an increased incidence of non-EBV associated GC lymphomas. In this regard, it is interesting to consider that a lack of B cell killing ability might be expected to have a similar effect to loss of b2m or LFA3/CD58, which are common mutations in GC-derived lymphomas 31; thus the restricted impact of SAP-deficiency may reflect the need for Tfh cells to be able to provide positive signals during lymphomagenesis. While the negative role of SLAM family molecules is clearly evident when SAP is lacking, the physiological role of SLAM molecules in SAP replete T cells is less clear. SLAM signaling can contribute to Tfh cell IL-4 production 121 though recent analysis of mice lacking all SLAM family members did not reveal an essential role for these molecules in the GC response 122. More studies of these SLAM-family deficient mice may help reveal how these molecules influence events necessary for antibody diversification and affinity maturation in GCs 123.
Tfh cell-derived lymphomas
In addition to GC B cell derived lymphomas, two types of Tfh cell-derived lymphomas, angioimmunoblastic T cell lymphoma (AITL) and Tfh-type peripheral T cell lymphoma (Tfh-PTCL), originate from the GC reaction. Tfh cell lymphomas frequently maintain a GC microenvironment and around half maintain EBV+ B cells and clonal outgrowths of blasting B cells in the tumor microenvironment 124. A case study reported depletion of B cells using rituximab combined with chemotherapy was a successful treatment for a relapsed AITL patient with EBV reactivation 125 making it important to determine how broadly these T lymphomas depend on B cells. A small clinical trial of AITL patients observed an association of EBV copies in PBMCs with shorter progression free survival, but did not find a correlation with B cell density in tumors or a clinical benefit of adding rituximab to CHOP 126. Since the study compared their results to historical CHOP responses in AITL patients, they may have overestimated the clinical response to CHOP in their elderly cohort, masking a positive benefit of rituximab 126.
Tfh cell-derived lymphomas are characterized by epigenetic modifications and often have inactivating mutations in TET2 and DNMT2 and activating mutations in IDH2. Additionally, several TCR-related signaling genes are mutated and activate the pathway. In 60–70% of AITL and Tfh-PTCL cases, RHOA harbors a G17V function altering mutation 114. This point mutation was sufficient to increase Tfh cells and drive an AITL-like disease in Tet2-deficient mice 127. Additional activating mutations in the TCR signaling pathway, namely PLCG1, PIK3CA, VAV, FYN, LCK mutations, are found in both AITL and Tfh-PTCL 116. In addition to CD28 mutations, a CTLA4-CD28 fusion gene has been discovered, transforming an inhibitory signal into an activating signal 124. These data provide further evidence that AITL and Tfh-PTCL cells depend on engaging with antigen presenting GC B cells in the tumor microenvironment.
Given ICOS’s ability to drive Tfh cell differentiation and helper signals to B cells, it is not surprising that multiple mechanisms of regulation of ICOS mRNA has been described, such as by Roquin and miR-146a 117. It seems reasonable to speculate that the ICOS-ICOSL positive feedback loop contributes to Tfh cell maintenance in the GC-derived lymphoma microenvironment. In a mouse model in which Roquin, a protein that negatively regulates Icos mRNA stability, was heterozygously mutated, ICOS expression was increased and Tfh cells and GC B cells spontaneously outgrew and when the mice were aged AITL developed 128. Additionally, in a mouse model of AITL driven by RHOA G17V, ICOS was strongly upregulated 118. Although Roquin mutations were not reported in a small cohort of human AITL patients 129, analysis of more patients is needed.
An ICOS blocking antibody MEDI 570 is being tested for the treatment of AITL and other Tfh cell-derived lymphomas in humans 119. Given that Tfh cell-derived lymphomas often maintain normal GC architecture, similar to their malignant B cell counterparts, it seems likely that blocking this potent positive regulator of GC responses could reduce outgrowth in both B and T cell lymphomas.
The dependence of SAP—SLAMF signaling for Tfh cell interactions with B cells raises the possibility that overexpression of SAP, SLAM, SLAMF5, and SLAMF6 could contribute to GC lymphomagenesis. While alterations in their expression have not been described, SAP expression is maintained in AITL, suggesting productive AITL Tfh cell immune contacts with B cells are beneficial 120. Interestingly, AITL cells continue to express high levels of PD-1 130. More analysis will be needed to determine whether PD-1 can engage growth regulatory pathways in AITL cells.
The HVEM—BTLA axis may be a regulatory pathway suppressing Tfh cell lymphomas. Interestingly, high expression of BTLA mRNA in tumor samples correlated with positive clinical outcomes in AITL 131. We hypothesize that the high levels of BTLA mRNA could be maintained on Tfh cells to inhibit TCR signaling strength when interacting with HVEM-expressing B cells, thereby limiting tumor growth. In accord with this logic, a TNFRSF14 mutation has been found in one AITL sample in which the entire tumor was sequenced 130. These data raise the possibility that loss of HVEM in B cells in the tumor environment could be driving Tfh cell outgrowth.
Therapeutic approaches
By reviewing the key molecular regulators of Tfh cell help to GC B cells and their possible roles in GC B cell outgrowth and lymphomagenesis, we have discussed several novel therapies to stop lymphoma progression. Blunt approaches can be considered if a patient is at high risk of developing GC-derived lymphomas or failed prior therapies. These include depleting Tfh cells through anti-CD4 antibodies and blocking CD40-CD40L, ICOS-ICOSL and IL4-IL4R signaling axes. Restoring negative regulation of GC B cell responses by enhancing PD-1-PD-L1 and BTLA-HVEM axes could inhibit GC B cell outgrowth. This has been demonstrated in a mouse model of FL where anti-CD19 CAR-T cells delivered soluble HVEM to the tumor microenvironment and likely inhibited Tfh cell help to FL B cells by engaging BTLA. Augmenting FasL-Fas interaction in lymphomas that retain this pathway could have therapeutic benefit. Better definition of the dependency of some GC lymphomas on T cells, and of tumor suppressors that are acting in trans, may identify more opportunities for restoring tumor suppressors using CAR T cell-type approaches or other surface receptor-targeted therapeutic strategies. It is also possible that targeting the cues that organize GCs and foster interactions between GC B cells and Tfh cells, such as CXCL13 and CXCR5, may be an approach to interrupt T cell support of lymphoma cells.
Conclusion
The study of mutations arising in B and T cell lymphomas is providing important insights about the normal mechanisms acting in GC responses and this work, in turn, is proving informative about the drivers of lymphomagenesis. While the tumor microenvironment is unquestionably crucial for lymphomagenesis 31, the potential of supportive T cells to be targeted during lymphomagenesis is only just beginning to be appreciated. The evidence discussed in this review suggests that targeting T cell help during GC-derived lymphomagenesis can provide a complementary approach to B cell targeted therapies for treating these lymphomas.
Acknowledgements
We thank Jagan Muppidi and Marissa Chou for helpful comments on the manuscript. M.A.M. was supported by 5F30AI131496 from the National Institute of Allergy and Infectious Diseases, J.G.C. is a Howard Hughes Medical Institute Investigator. Work discussed in this review was supported in part by NIH grant AI045073.
Footnotes
Conflict of Interest: Dr. Cyster is on the scientific advisory board of MiroBio Ltd.
References
- 1.Finkin S, Hartweger H, Oliveira TY, Kara EE, Nussenzweig MC. Protein Amounts of the MYC Transcription Factor Determine Germinal Center B Cell Division Capacity. Immunity. 2019;51(2):324–336.e325. doi: 10.1016/j.immuni.2019.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ersching J, Efeyan A, Mesin L, et al. Germinal Center Selection and Affinity Maturation Require Dynamic Regulation of mTORC1 Kinase. Immunity. 2017;46(6):1045–1058.e1046. doi: 10.1016/j.immuni.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luo W, Weisel F, Shlomchik MJ. B Cell Receptor and CD40 Signaling Are Rewired for Synergistic Induction of the c-Myc Transcription Factor in Germinal Center B Cells. Immunity. 2018;48(2):313–326.e315. doi: 10.1016/j.immuni.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gitlin AD, Mayer CT, Oliveira TY, et al. HUMORAL IMMUNITY. T cell help controls the speed of the cell cycle in germinal center B cells. Science. 2015;349(6248):643–646. doi: 10.1126/science.aac4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wan Z, Lin Y, Zhao Y, Qi H. T FH cells in bystander and cognate interactions with B cells. Immunological Reviews. 2019;288(1):28–36. doi: 10.1111/imr.12747. [DOI] [PubMed] [Google Scholar]
- 6.Crotty S. T Follicular Helper Cell Biology: A Decade of Discovery and Diseases. Immunity. 2019;50(5):1132–1148. doi: 10.1016/j.immuni.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mlynarczyk C, Fontán L, Melnick A. Germinal center‐derived lymphomas: The darkest side of humoral immunity. Immunological Reviews. 2019;288(1):214–239. doi: 10.1111/imr.12755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bende RJ, Smit LA, van Noesel CJM. Molecular pathways in follicular lymphoma. Leukemia. 2007;21(1):18–29. doi: 10.1038/sj.leu.2404426. [DOI] [PubMed] [Google Scholar]
- 9.Ochando J, Braza MS. T follicular helper cells: a potential therapeutic target in follicular lymphoma. Oncotarget. 2017;8(67):112116–112131. doi: 10.18632/oncotarget.22788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milpied P, Cervera-Marzal I, Mollichella M-L, et al. Human germinal center transcriptional programs are de-synchronized in B cell lymphoma. Nat Immunol. August 2018:1–19. doi: 10.1038/s41590-018-0181-4. [DOI] [PubMed] [Google Scholar]
- 11.Schmitz R, Wright GW, Huang DW, et al. Genetics and Pathogenesis of Diffuse Large B-Cell Lymphoma. N Engl J Med. 2018;378(15):1396–1407. doi: 10.1056/NEJMoa1801445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iqbal J, Meyer PN, Smith LM, et al. BCL2 Predicts Survival in Germinal Center B-cell-like Diffuse Large B-cell Lymphoma Treated with CHOP-like Therapy and Rituximab. Clinical Cancer Research. 2011;17(24):7785–7795. doi: 10.1158/1078-0432.CCR-11-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmitz R, Young RM, Ceribelli M, et al. Burkitt lymphoma pathogenesis and therapeutic targets from structural and functional genomics. Nature. 2012;490(7418):116–120. doi: 10.1038/nature11378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kridel R, Chan FC, Mottok A, et al. Histological Transformation and Progression in Follicular Lymphoma: A Clonal Evolution Study. Mardis ER, ed. PLoS Med. 2016;13(12):e1002197–25. doi: 10.1371/journal.pmed.1002197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lackraj T, Goswami R, Kridel R. Pathogenesis of follicular lymphoma. Best Practice & Research Clinical Haematology. 2018;31(1):2–14. doi: 10.1016/j.beha.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Rabkin CS, Hirt C, Janz S, Dolken G. t(14;18) Translocations and Risk of Follicular Lymphoma. JNCI Monographs. 2008;2008(39):48–51. doi: 10.1093/jncimonographs/lgn002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sungalee S, Mamessier E, Morgado E, et al. Germinal center reentries of BCL2-overexpressing B cells drive follicular lymphoma progression. J Clin Invest. 2014;124(12):5337–5351. doi: 10.1172/JCI72415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y, Hernandez AM, Shibata D, Cortopassi GA. BCL2 translocation frequency rises with age in humans. Proceedings of the National Academy of Sciences. 1994;91(19):8910–8914. doi: 10.1073/pnas.91.19.8910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Álvarez-Prado ÁF, Pérez-Durán P, Pérez-García A, et al. A broad atlas of somatic hypermutation allows prediction of activation-induced deaminase targets. Journal of Experimental Medicine. 2018;215(3):761–771. doi: 10.1084/jem.20171738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsukamoto T, Nakano M, Sato R, et al. High-risk follicular lymphomas harbour more somatic mutations including those in the AID-motif. Scientific Reports. October 2017:1–10. doi: 10.1038/s41598-017-14150-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mintz MA, Felce JH, Chou MY, et al. The HVEM-BTLA Axis Restrains T Cell Help to Germinal Center B Cells and Functions as a Cell- Extrinsic Suppressor in Lymphomagenesis. Immunity. 2019;51(2):310–323.e317. doi: 10.1016/j.immuni.2019.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Losman JA, Chen XP, Hilton D, Rothman P. Cutting edge: SOCS-1 is a potent inhibitor of IL-4 signal transduction. The Journal of Immunology. 1999;162(7):3770–3774. [PMC free article] [PubMed] [Google Scholar]
- 23.Turqueti-Neves A, Otte M, da Costa OP, et al. B-cell-intrinsic STAT6 signaling controls germinal center formation. Eur J Immunol. 2014;44(7):2130–2138. doi: 10.1002/eji.201344203. [DOI] [PubMed] [Google Scholar]
- 24.Hashwah H, Schmid CA, Kasser S, et al. Inactivation of CREBBP expands the germinal center B cell compartment, down-regulates MHCII expression and promotes DLBCL growth. Proc Natl Acad Sci USA. 2017;114(36):9701–9706. doi: 10.1073/pnas.1619555114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Béguelin W, Popovic R, Teater M, et al. EZH2 Is Required for Germinal Center Formation and Somatic EZH2 Mutations Promote Lymphoid Transformation. Cancer Cell. 2013;23(5):677–692. doi: 10.1016/j.ccr.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Béguelin W, Rivas MA, Fernández MTC, et al. EZH2 enables germinal centre formation through epigenetic silencing of CDKN1A and an Rb-E2F1 feedback loop. Nat Commun. October 2017:1–16. doi: 10.1038/s41467-017-01029-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muppidi JR, Schmitz R, Green JA, et al. Loss of signalling via Gα13 in germinal centre B-cell-derived lymphoma. Nature. 2014;516(7530):254–258. doi: 10.1038/nature13765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Green JA, Suzuki K, Cho B, et al. The sphingosine 1-phosphate receptor S1P2 maintains the homeostasis of germinal center B cells and promotes niche confinement. Nat Immunol. 2011;12(7):672–680. doi: 10.1038/ni.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu E, Wolfreys FD, Muppidi JR, Xu Y, Cyster JG. S-Geranylgeranyl-L-glutathione is a ligand for human B cell-confinement receptor P2RY8. Nature. 2019;567(7747):244–248. doi: 10.1038/s41586-019-1003-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Egle A. VavP-Bcl2 transgenic mice develop follicular lymphoma preceded by germinal center hyperplasia. Blood. 2004;103(6):2276–2283. doi: 10.1182/blood-2003-07-2469. [DOI] [PubMed] [Google Scholar]
- 31.Basso K, Dalla-Favera R. Germinal centres and B cell lymphomagenesis. Nat Rev Immunol. 2015;15(3):172–184. doi: 10.1038/nri3814. [DOI] [PubMed] [Google Scholar]
- 32.Challa-Malladi M, Lieu YK, Califano O, et al. Combined genetic inactivation of β2-Microglobulin and CD58 reveals frequent escape from immune recognition in diffuse large B cell lymphoma. Cancer Cell. 2011;20(6):728–740. doi: 10.1016/j.ccr.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang K-C, Huang X, Medeiros LJ, Jones D. Germinal centre-like versus undifferentiated stromal immunophenotypes in follicular lymphoma. J Pathol. 2003;201(3):404–412. doi: 10.1002/path.1478. [DOI] [PubMed] [Google Scholar]
- 34.Tang J, Zha J, Guo X, Shi P, Xu B. CXCR5+CD8+ T cells present elevated capacity in mediating cytotoxicity toward autologous tumor cells through interleukin 10 in diffuse large B-cell lymphoma. International Immunopharmacology. 2017;50:146–151. doi: 10.1016/j.intimp.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 35.Valentine KM, Hoyer KK. CXCR5+ CD8 T Cells: Protective or Pathogenic? Front Immunol. 2019;10:68–10. doi: 10.3389/fimmu.2019.01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ayala VI, Deleage C, Trivett MT, et al. CXCR5-Dependent Entry of CD8 T Cells into Rhesus Macaque B-Cell Follicles Achieved through T-Cell Engineering. Silvestri G, ed. Journal of Virology. 2017;91(11):132–17. doi: 10.1128/JVI.02507-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paul WE. History of interleukin-4. Cytokine. 2015;75(1):3–7. doi: 10.1016/j.cyto.2015.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rawal S, Chu F, Zhang M, et al. Cross Talk between Follicular Th Cells and Tumor Cells in Human Follicular Lymphoma Promotes Immune Evasion in the Tumor Microenvironment. The Journal of Immunology. 2013;190(12):6681–6693. doi: 10.4049/jimmunol.1201363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Calvo KR, Dabir B, Kovach A, et al. IL-4 protein expression and basal activation of Erk in vivo in follicular lymphoma. Blood. 2008;112(9):3818–3826. doi: 10.1182/blood-2008-02-138933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pangault C, Ame-Thomas P, Ruminy P, et al. Follicular lymphoma cell niche: identification of a preeminent IL-4-dependent T(FH)-B cell axis. Leukemia. 2010;24(12):2080–2089. doi: 10.1038/leu.2010.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ame-Thomas P, Le Priol J, Yssel H, et al. Characterization of intratumoral follicular helper T cells in follicular lymphoma: role in the survival of malignant B cells. Leukemia. 2012;26(5):1053–1063. doi: 10.1038/leu.2011.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shulman Z, Gitlin AD, Weinstein JS, et al. Dynamic signaling by T follicular helper cells during germinal center B cell selection. Science. 2014;345(6200):1058–1062. doi: 10.1126/science.1257861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vijayanand P, Seumois G, Simpson LJ, et al. Interleukin-4 Production by Follicular Helper T Cells Requires the Conserved Il4 Enhancer Hypersensitivity Site V. Immunity. 2012;36(2):175–187. doi: 10.1016/j.immuni.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kopf M, Le Gros G, Coyle AJ, Kosco-Vilbois M, Brombacher F. Immune responses of IL-4, IL-5, IL-6 deficient mice. Immunological Reviews. 1995;148(1):45–69. doi: 10.1111/j.1600-065x.1995.tb00093.x. [DOI] [PubMed] [Google Scholar]
- 45.Reinhardt RL, Liang H-E, Locksley RM. Cytokine-secreting follicular T cells shape the antibody repertoire. Nat Immunol. 2009;10(4):385–393. doi: 10.1038/ni.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zotos D, Coquet JM, Zhang Y, et al. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J Exp Med. 2010;207(2):365–378. doi: 10.1084/jem.20091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gonzalez DG, Cote CM, Patel JR, et al. Nonredundant Roles of IL-21 and IL-4 in the Phased Initiation of Germinal Center B Cells and Subsequent Self-Renewal Transitions. J Immunol. 2018;201(12):3569–3579. doi: 10.4049/jimmunol.1500497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weinstein JS, Herman EI, Lainez B, et al. TFH cells progressively differentiate to regulate the germinal center response. Nat Immunol. 2016;17(10):1197–1205. doi: 10.1038/ni.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chevrier S, Kratina T, Emslie D, Tarlinton DM, Corcoran LM. IL4 and IL21 cooperate to induce the high Bcl6 protein level required for germinal center formation. Immunol Cell Biol. 2017;95(10):925–932. doi: 10.1038/icb.2017.71. [DOI] [PubMed] [Google Scholar]
- 50.Ghia P, Boussiotis VA, Schultze JL, et al. Unbalanced expression of bcl-2 family proteins in follicular lymphoma: contribution of CD40 signaling in promoting survival. Blood. 1998;91(1):244–251. [PubMed] [Google Scholar]
- 51.Travert M, Ame-Thomas P, Pangault C, et al. CD40 ligand protects from TRAIL-induced apoptosis in follicular lymphomas through NF-kappaB activation and up-regulation of c-FLIP and Bcl-xL. J Immunol. 2008;181(2):1001–1011. doi: 10.4049/jimmunol.181.2.1001. [DOI] [PubMed] [Google Scholar]
- 52.Hömig-Hölzel C, Hojer C, Rastelli J, et al. Constitutive CD40 signaling in B cells selectively activates the noncanonical NF-κB pathway and promotes lymphomagenesis. Journal of Experimental Medicine. 2008;205(6):1317–1329. doi: 10.1084/jem.20080238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lesley R, Kelly LM, Xu Y, Cyster JG. Naive CD4 T cells constitutively express CD40L and augment autoreactive B cell survival. Proceedings of the National Academy of Sciences. 2006;103(28):10717–10722. doi: 10.1073/pnas.0601539103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.MacLennan IC. Germinal centers. Annu Rev Immunol. 1994;12(1):117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 55.Casamayor-Palleja M, Khan M, MacLennan IC. A subset of CD4+ memory T cells contains preformed CD40 ligand that is rapidly but transiently expressed on their surface after activation through the T cell receptor complex. Journal of Experimental Medicine. 1995;181(4):1293–1301. doi: 10.1084/jem.181.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iezzi G, Sonderegger I, Ampenberger F, Schmitz N, Marsland BJ, Kopf M. CD40–CD40L cross-talk integrates strong antigenic signals and microbial stimuli to induce development of IL-17-producing CD4+ T cells. Proceedings of the National Academy of Sciences. 2009;106(3):876–881. doi: 10.1073/pnas.0810769106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koguchi Y, Buenafe AC, Thauland TJ, et al. Preformed CD40L Is Stored in Th1, Th2, Th17, and T Follicular Helper Cells as Well as CD4+8− Thymocytes and Invariant NKT Cells but Not in Treg Cells. Mosley RL, ed. PLoS ONE. 2012;7(2):e31296–14. doi: 10.1371/journal.pone.0031296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carbone A, Gloghini A, Gruss HJ, Pinto A. CD40 ligand is constitutively expressed in a subset of T cell lymphomas and on the microenvironmental reactive T cells of follicular lymphomas and Hodgkin’s disease. Am J Pathol. 1995;147(4):912–922. [PMC free article] [PubMed] [Google Scholar]
- 59.Sacco MG, Ungari M, Catò EM, et al. Lymphoid abnormalities in CD40 ligand transgenic mice suggest the need for tight regulation in gene therapy approaches to hyper immunoglobulin M (IgM) syndrome. Cancer Gene Ther. 2000;7(10):1299–1306. doi: 10.1038/sj.cgt.7700232. [DOI] [PubMed] [Google Scholar]
- 60.Kishi Y, Aiba Y, Higuchi T, et al. Augmented Antibody Response with Premature Germinal Center Regression in CD40L Transgenic Mice. The Journal of Immunology. 2010;185(1):211–219. doi: 10.4049/jimmunol.0901694. [DOI] [PubMed] [Google Scholar]
- 61.Fanale M, Assouline S, Kuruvilla J, et al. Phase IA/II, multicentre, open-label study of the CD40 antagonistic monoclonal antibody lucatumumab in adult patients with advanced non-Hodgkin or Hodgkin lymphoma. British Journal of Haematology. 2013;164(2):258–265. doi: 10.1111/bjh.12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Myklebust JH, Irish JM, Brody J, et al. High PD-1 expression and suppressed cytokine signaling distinguish T cells infiltrating follicular lymphoma tumors from peripheral T cells. Blood. 2013;121(8):1367–1376. doi: 10.1182/blood-2012-04-421826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mulder TA, Wahlin BE, Österborg A, Palma M. Targeting the Immune Microenvironment in Lymphomas of B-Cell Origin: From Biology to Clinical Application. Cancers (Basel). 2019;11(7). doi: 10.3390/cancers11070915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu-Monette ZY, Xiao M, Au Q, et al. Immune Profiling and Quantitative Analysis Decipher the Clinical Role of Immune-Checkpoint Expression in the Tumor Immune Microenvironment of DLBCL. Cancer Immunology Research. 2019;7(4):644–657. doi: 10.1158/2326-6066.CIR-18-0439. [DOI] [PubMed] [Google Scholar]
- 65.Smeltzer JP, Jones JM, Ziesmer SC, et al. Pattern of CD14+ follicular dendritic cells and PD1+ T cells independently predicts time to transformation in follicular lymphoma. Clinical Cancer Research. 2014;20(11):2862–2872. doi: 10.1158/1078-0432.CCR-13-2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang ZZ, Kim HJ, Villasboas JC, et al. Mass Cytometry Analysis Reveals that Specific Intratumoral CD4+ T Cell Subsets Correlate with Patient Survival in Follicular Lymphoma. Cell Rep. 2019;26(8):2178–2193.e3. doi: 10.1016/j.celrep.2019.01.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Godfrey J, Tumuluru S, Bao R, et al. PD-L1 gene alterations identify a subset of diffuse large B-cell lymphoma harboring a T-cell–inflamed phenotype. Blood. 2019;133(21):2279–2290. doi: 10.1182/blood-2018-10-879015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Good-Jacobson KL, Szumilas CG, Chen L, Sharpe AH, Tomayko MM, Shlomchik MJ. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nat Immunol. 2010;11(6):535–542. doi: 10.1038/ni.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hams E, McCarron MJ, Amu S, et al. Blockade of B7-H1 (programmed death ligand 1) enhances humoral immunity by positively regulating the generation of T follicular helper cells. J Immunol. 2011;186(10):5648–5655. doi: 10.4049/jimmunol.1003161. [DOI] [PubMed] [Google Scholar]
- 70.Sage PT, Francisco LM, Carman CV, Sharpe AH. The receptor PD-1 controls follicular regulatory T cells in the lymph nodes and blood. Nat Immunol. 2012;14(2):152–161. doi: 10.1038/ni.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Martinov T, Swanson LA, Breed ER, et al. Programmed Death-1 Restrains the Germinal Center in Type 1 Diabetes. J Immunol. 2019;203(4):844–852. doi: 10.4049/jimmunol.1801535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shi J, Hou S, Fang Q, Liu X, Liu X, Qi H. PD-1 Controls Follicular T Helper Cell Positioning and Function. Immunity. 2018;49(2):264–274.e264. doi: 10.1016/j.immuni.2018.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yokosuka T, Takamatsu M, Kobayashi-Imanishi W, Hashimoto-Tane A, Azuma M, Saito T. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. Journal of Experimental Medicine. 2012;209(6):1201–1217. doi: 10.1084/jem.20112741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hui E, Cheung J, Zhu J, et al. T cell costimulatory receptor CD28 is a primary target for PD-1–mediated inhibition. Science. 2017;355(6332):1428–1433. doi: 10.1126/science.aaf1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rota G, Niogret C, Dang AT, et al. Shp-2 Is Dispensable for Establishing T Cell Exhaustion and for PD-1 Signaling In Vivo. Cell Rep. 2018;23(1):39–49. doi: 10.1016/j.celrep.2018.03.026. [DOI] [PubMed] [Google Scholar]
- 76.Celis-Gutierrez J, Blattmann P, Zhai Y, et al. Quantitative Interactomics in Primary T Cells Provides a Rationale for Concomitant PD-1 and BTLA Coinhibitor Blockade in Cancer Immunotherapy. Cell Rep. 2019;27(11):3315–3330.e3317. doi: 10.1016/j.celrep.2019.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sugiura D, Maruhashi T, Okazaki I-M, et al. Restriction of PD-1 function by cis-PD-L1/CD80 interactions is required for optimal T cell responses. Science. 2019;364(6440):558–566. doi: 10.1126/science.aav7062. [DOI] [PubMed] [Google Scholar]
- 78.Zhao Y, Harrison DL, Song Y, Ji J, Huang J, Hui E. Antigen-Presenting Cell-Intrinsic PD-1 Neutralizes PD-L1 in cis to Attenuate PD-1 Signaling in T Cells. Cell Rep. 2018;24(2):379–390.e6. doi: 10.1016/j.celrep.2018.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Advani R, Flinn I, Popplewell L, et al. CD47 Blockade by Hu5F9-G4 and Rituximab in Non-Hodgkin’s Lymphoma. N Engl J Med. 2018;379(18):1711–1721. doi: 10.1056/NEJMoa1807315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chao MP, Alizadeh AA, Tang C, et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell. 2010;142(5):699–713. doi: 10.1016/j.cell.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Boice M, Salloum D, Mourcin F, et al. Loss of the HVEM Tumor Suppressor in Lymphoma and Restoration by Modified CAR-T Cells. Cell. 2016;167(2):405–418.e413. doi: 10.1016/j.cell.2016.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bouska A, Bi C, Lone W, et al. Adult high-grade B-cell lymphoma with Burkitt lymphoma signature: genomic features and potential therapeutic targets. Blood. 2017;130(16):1819–1831. doi: 10.1182/blood-2017-02-767335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kotsiou E, Okosun J, Besley C, et al. TNFRSF14 aberrations in follicular lymphoma increase clinically significant allogeneic T-cell responses. Blood. April 2016:1–40. doi: 10.1182/blood-2015-10-679191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hsu H, Solovyev I, Colombero A, Elliott R, Kelley M, Boyle WJ. ATAR, a novel tumor necrosis factor receptor family member, signals through TRAF2 and TRAF5. J Biol Chem. 1997;272(21):13471–13474. doi: 10.1074/jbc.272.21.13471. [DOI] [PubMed] [Google Scholar]
- 85.Shui J-W, Larange A, Kim G, et al. HVEM signalling at mucosal barriers provides host defence against pathogenic bacteria. Nature. 2012;488(7410):222–225. doi: 10.1038/nature11242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ward-Kavanagh LK, Lin WW, Sedy JR, Ware CF. The TNF Receptor Superfamily in Co-stimulating and Co-inhibitory Responses. Immunity. 2016;44(5):1005–1019. doi: 10.1016/j.immuni.2016.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhu Y, Yao S, Augustine MM, et al. Neuron-specific SALM5 limits inflammation in the CNS via its interaction with HVEM. Science Advances. 2016;2(4):e1500637-e1500637. doi: 10.1126/sciadv.1500637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sedy JR, Gavrieli M, Potter KG, et al. B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nat Immunol. 2004;6(1):90–98. doi: 10.1038/ni1144. [DOI] [PubMed] [Google Scholar]
- 89.Murphy KM, Nelson CA, Sedy JR. Balancing co-stimulation and inhibition with BTLA and HVEM. Nat Rev Immunol. 2006;6(9):671–681. doi: 10.1038/nri1917. [DOI] [PubMed] [Google Scholar]
- 90.Watanabe N, Gavrieli M, Sedy JR, et al. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat Immunol. 2003;4(7):670–679. doi: 10.1038/ni944. [DOI] [PubMed] [Google Scholar]
- 91.Gavrieli M, Murphy KM. Association of Grb-2 and PI3K p85 with phosphotyrosile peptides derived from BTLA. Biochem Biophys Res Commun. 2006;345(4):1440–1445. doi: 10.1016/j.bbrc.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 92.Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 Associate with Immunoreceptor Tyrosine-Based Switch Motif of Programmed Death 1 upon Primary Human T Cell Stimulation, but Only Receptor Ligation Prevents T Cell Activation. The Journal of Immunology. 2004;173(2):945–954. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- 93.Wu T-H, Zhen Y, Zeng C, Yi H- F, Zhao Y. B and T lymphocyte attenuator interacts with CD3ζ and inhibits tyrosine phosphorylation of TCRζ complex during T-cell activation. Immunol Cell Biol. 2007;85(8):590–595. doi: 10.1038/sj.icb.7100087. [DOI] [PubMed] [Google Scholar]
- 94.Vendel AC, Calemine-Fenaux J, Izrael-Tomasevic A, Chauhan V, Arnott D, Eaton DL. B and T lymphocyte attenuator regulates B cell receptor signaling by targeting Syk and BLNK. J Immunol. 2009;182(3):1509–1517. [DOI] [PubMed] [Google Scholar]
- 95.Chtanova T, Tangye SG, Newton R, et al. T Follicular Helper Cells Express a Distinctive Transcriptional Profile, Reflecting Their Role as Non-Th1/Th2 Effector Cells That Provide Help for B Cells. The Journal of Immunology. 2004;173(1):68–78. doi: 10.4049/jimmunol.173.1.68. [DOI] [PubMed] [Google Scholar]
- 96.Kashiwakuma D, Suto A, Hiramatsu Y, et al. B and T lymphocyte attenuator suppresses IL-21 production from follicular Th cells and subsequent humoral immune responses. J Immunol. 2010;185(5):2730–2736. doi: 10.4049/jimmunol.0903839. [DOI] [PubMed] [Google Scholar]
- 97.Duhen T, Pasero C, Mallet FO, Barbarat B, Olive D, Costello RGT. LIGHT costimulates CD40 triggering and induces immunoglobulin secretion; a novel key partner in T cell-dependent B cell terminal differentiation. Eur J Immunol. 2004;34(12):3534–3541. doi: 10.1002/eji.200425598. [DOI] [PubMed] [Google Scholar]
- 98.Cheung TC, Oborne LM, Steinberg MW, et al. T cell intrinsic heterodimeric complexes between HVEM and BTLA determine receptivity to the surrounding microenvironment. J Immunol. 2009;183(11):7286–7296. doi: 10.4049/jimmunol.0902490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huet S, Sujobert P, Salles G. From genetics to the clinic: a translational perspective on follicular lymphoma. Nature Publishing Group. 2018;18(4):224–239. doi: 10.1038/nrc.2017.127. [DOI] [PubMed] [Google Scholar]
- 100.Verdière L, Mourcin F, Tarte K. Microenvironment signaling driving lymphomagenesis. Current Opinion in Hematology. 2018;25(4):335–345. doi: 10.1097/MOH.0000000000000440. [DOI] [PubMed] [Google Scholar]
- 101.Carreras J, Lopez-Guillermo A, Kikuti YY, et al. High TNFRSF14 and low BTLA are associated with poor prognosis in Follicular Lymphoma and in Diffuse Large B-cell Lymphoma transformation. JCEH. 2019;59(1):1–16. doi: 10.3960/jslrt.19003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Weber JP, Fuhrmann F, Feist RK, et al. ICOS maintains the T follicular helper cell phenotype by down-regulating Krüppel-like factor 2. Journal of Experimental Medicine. 2015;212(2):217–233. doi: 10.1084/jem.20141432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu D, Xu H, Shih C, et al. T-B-cell entanglement and ICOSL-driven feed-forward regulation of germinal centre reaction. Nature. October 2014. doi: 10.1038/nature13803. [DOI] [PubMed] [Google Scholar]
- 104.Xu-Monette ZY, Li L, Byrd JC, et al. Assessment of CD37 B-cell antigen and cell of origin significantly improves risk prediction in diffuse large B-cell lymphoma. Blood. 2016;128(26):3083–3100. doi: 10.1182/blood-2016-05-715094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Le KS, Thibult ML, Just-Landi S, et al. Follicular B Lymphomas Generate Regulatory T Cells via the ICOS/ICOSL Pathway and Are Susceptible to Treatment by Anti-ICOS/ICOSL Therapy. Cancer Res. 2016;76(16):4648–4660. doi: 10.1158/0008-5472.CAN-15-0589. [DOI] [PubMed] [Google Scholar]
- 106.Koncz G, Hueber A-O. The Fas/CD95 Receptor Regulates the Death of Autoreactive B Cells and the Selection of Antigen-Specific B Cells. Front Immunol. 2012;3:207. doi: 10.3389/fimmu.2012.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lossos IS, Gascoyne RD. Transformation of follicular lymphoma. Best Practice & Research Clinical Haematology. 2011;24(2):147–163. doi: 10.1016/j.beha.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Müschen M, Rajewsky K, Krönke M, Küppers R. The origin of CD95-gene mutations in B-cell lymphoma. Trends Immunol. 2002;23(2):75–80. doi: 10.1016/s1471-4906(01)02115-9. [DOI] [PubMed] [Google Scholar]
- 109.Straus SE, Jaffe ES, Puck JM, et al. The development of lymphomas in families with autoimmune lymphoproliferative syndrome with germline Fas mutations and defective lymphocyte apoptosis. Blood. 2001;98(1):194–200. doi: 10.1182/blood.v98.1.194. [DOI] [PubMed] [Google Scholar]
- 110.Bride K, Teachey D. Autoimmune lymphoproliferative syndrome: more than a FAScinating disease. F1000Res. 2017;6:1928–16. doi: 10.12688/f1000research.11545.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Takahashi Y, Ohta H, Takemori T. Fas is required for clonal selection in germinal centers and the subsequent establishment of the memory B cell repertoire. Immunity. 2001;14(2):181–192. doi: 10.1016/s1074-7613(01)00100-5. [DOI] [PubMed] [Google Scholar]
- 112.Hao Z, Duncan GS, Seagal J, et al. Fas Receptor Expression in Germinal-Center B Cells Is Essential for T and B Lymphocyte Homeostasis. Immunity. 2008;29(4):615–627. doi: 10.1016/j.immuni.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kondo E, Yoshino T, Nishiuchi R, et al. Expression of Fas ligand mRNA in germinal centres of the human tonsil. J Pathol. 1997;183(1):75–79. doi:. [DOI] [PubMed] [Google Scholar]
- 114.Butt D, Chan TD, Bourne K, et al. FAS Inactivation Releases Unconventional Germinal Center B Cells that Escape Antigen Control and Drive IgE and Autoantibody Production. Immunity. 2015;42(5):890–902. doi: 10.1016/j.immuni.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 115.Totten S, Gaucher D, Morin RD, et al. FAS Mutations Accelerate Lymphoma Growth and Induce Therapeutic Resistance. Blood. 2014;124(21):3018–3018. doi: 10.1182/blood.V124.21.3018.3018. [DOI] [Google Scholar]
- 116.Berkova Z, Tao R-H, Samaniego F. Milatuzumab – a promising new immunotherapeutic agent. Expert Opinion on Investigational Drugs. 2009;19(1):141–149. doi: 10.1517/13543780903463854. [DOI] [PubMed] [Google Scholar]
- 117.Paiva C, Godbersen JC, Rowland T, et al. Pevonedistat, a Nedd8-activating enzyme inhibitor, sensitizes neoplastic B-cells to death receptor-mediated apoptosis. Oncotarget. 2017;8(13):21128–21139. doi: 10.18632/oncotarget.15050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Engel P, Eck MJ, Terhorst C. The SAP and SLAM families in immune responses and X-linked lymphoproliferative disease. Nat Rev Immunol. 2003;3(10):813–821. doi: 10.1038/nri1202. [DOI] [PubMed] [Google Scholar]
- 119.Fouquet G, Marcq I, Debuysscher V, et al. Signaling lymphocytic activation molecules Slam and cancers: friends or foes? Oncotarget. 2018;9(22):16248–16262. doi: 10.18632/oncotarget.24575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Qi H, Cannons JL, Klauschen F, Schwartzberg PL, Germain RN. SAP-controlled T–B cell interactions underlie germinal centre formation. Nature. 2008;455(7214):764–769. doi: 10.1038/nature07345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yusuf I, Kageyama R, Monticelli L, et al. Germinal Center T Follicular Helper Cell IL-4 Production Is Dependent on Signaling Lymphocytic Activation Molecule Receptor (CD150). The Journal of Immunology. 2010;185(1):190–202. doi: 10.4049/jimmunol.0903505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Huang B, Gomez-Rodriguez J, Preite S, Garrett LJ, Harper UL, Schwartzberg PL. CRISPR-Mediated Triple Knockout of SLAMF1, SLAMF5 and SLAMF6 Supports Positive Signaling Roles in NKT Cell Development. Unutmaz D, ed. PLoS ONE. 2016;11(6):e0156072–19. doi: 10.1371/journal.pone.0156072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cannons JL, Qi H, Lu KT, Dutta M, Gomez-Rodriguez J. Optimal germinal center responses require a multistage T cell: B cell adhesion process involving integrins, SLAM-associated protein, and CD84. Immunity. 2010;32(2):253–265. doi: 10.1016/j.immuni.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schmitz N, de Leval L. How I manage peripheral T-cell lymphoma, not otherwise specified and angioimmunoblastic T-cell lymphoma: current practice and a glimpse into the future. British Journal of Haematology. 2016;176(6):851–866. doi: 10.1111/bjh.14473. [DOI] [PubMed] [Google Scholar]
- 125.Kasahara H, Kakimoto T, Saito H, et al. Successful treatment with rituximab for angioimmunoblastic T-cell lymphoma. Leukemia Research Reports. 1AD;2(1):36–38. doi: 10.1016/j.lrr.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Delfau-Larue M-H, de Leval L, Joly B, et al. Targeting intratumoral B cells with rituximab in addition to CHOP in angioimmunoblastic T-cell lymphoma. A clinicobiological study of the GELA. Haematologica. 2012;97(10):1594–1602. doi: 10.3324/haematol.2011.061507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cortes JR, Ambesi-Impiombato A, Couronné L, et al. RHOA G17V Induces T Follicular Helper Cell Specification and Promotes Lymphomagenesis. Cancer Cell. 2018;33(2):259–273.e7. doi: 10.1016/j.ccell.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ellyard JI, Chia T, Rodriguez-Pinilla S-M, et al. Heterozygosity for Roquinsan leads to angioimmunoblastic T-cell lymphoma-like tumors in mice. Blood. 2012;120(4):812–821. doi: 10.1182/blood-2011-07-365130. [DOI] [PubMed] [Google Scholar]
- 129.Auguste T, Travert M, Tarte K, et al. ROQUIN/RC3H1 Alterations Are Not Found in Angioimmunoblastic T-Cell Lymphoma. Galardy PJ, ed. PLoS ONE. 2013;8(6):e64536–4. doi: 10.1371/journal.pone.0064536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Odejide O, Weigert O, Lane AA, et al. A targeted mutational landscape of angioimmunoblastic T-cell lymphoma. Blood. 2014;123(9):1293–1296. doi: 10.1182/blood-2013-10-531509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Iqbal J, Weisenburger DD, Greiner TC, et al. Molecular signatures to improve diagnosis in peripheral T-cell lymphoma and prognostication in angioimmunoblastic T-cell lymphoma. Blood. 2010;115(5):1026–1036. doi: 10.1182/blood-2009-06-227579. [DOI] [PMC free article] [PubMed] [Google Scholar]