Abstract
Background:
Recent studies suggest that a healthy diet helps to prevent the development of Alzheimer disease (AD). This study aimed to investigate whether spicy food consumption is associated with cognition and cerebrospinal fluid (CSF) biomarkers of AD in the Chinese population.
Methods:
We enrolled 55 AD patients and 55 age- and gender-matched cognitively normal (CN) subjects in a case-control study, as well as a cohort of 131 participants without subjective cognitive decline (non-AD) in a cross-sectional study. Spicy food consumption was assessed using the Food Frequency Questionnaire (FFQ). Associations of FFQ scores with cognition and CSF biomarkers of AD were analyzed.
Results:
In the case-control study, spicy food consumption was lower in AD patients than that in CNs (4.0 [4.0–8.0] vs. 8.0 [4.5–10.0], P < 0.001); FFQ scores were positively associated with Mini-Mental Status Examination scores in the total sample (r = 0.218, P = 0.014). In the cross-sectional study, the association between spicy food consumption and cognition levels was verified in non-AD subjects (r = 0.264, P = 0.0023). Moreover, higher FFQ scores were significantly associated with higher β-Amyloid (1–42) (Aβ42) levels and lower phospho-tau/Aβ42 and total tau/Aβ42 ratios in the CSF of non-AD subjects (P < 0.05).
Conclusion:
Spicy food consumption is closely related to higher cognition levels and reversed AD biomarkers in the CSF, suggesting that a capsaicin-rich diet might have the potential to modify the cognitive status and cerebral pathologies associated with AD.
Keywords: Alzheimer disease, Biomarker, Capsaicin, Cerebrospinal fluid, Cognition, Spicy food
Introduction
Alzheimer disease (AD) is the most common cause of age-related dementia among the elderly population.[1] To date, no effective therapy has been available to halt the progression of AD. Due to the failures of clinical trials in patients with advanced AD, the intervention for AD has shifted from treatment to prevention in at-risk populations.[2,3] Recent studies suggest that a healthy diet helps to prevent the development of AD.[4–6]
Spicy food is popular in many regions of China. Capsaicin is the major component in chili peppers, accounting for the spicy/pungent flavor. It is interesting to note that there is a geographic overlap between AD incidence and spicy food consumption in China. The incidence of AD in western China (3.99/1000 person-years) is lower than that in eastern China (5.58/1000 person-years),[7] while the proportion of dishes containing chilis is higher and the pungency degree is greater in western China than those in eastern China.[8,9] Previously, we reported that capsaicin-rich diet consumption was associated with better cognition and lower blood β-Amyloid (Aβ) levels in subjects aged 40 years and older.[10] These findings imply that a capsaicin-rich diet may be protective against AD. In the present study, we further investigated the association of spicy food consumption with cognition and AD biomarkers in cerebrospinal fluid (CSF) in the Chinese population.
Methods
Ethical approval
This study was approved by the Institutional Review Board at the Daping Hospital and Qingdao Municipal Hospital and conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from participants or their guardians.
Study subjects and clinical assessment
In the case-control study, 55 AD patients and 55 age- and gender-matched cognitively normal (CN) Chinese were recruited from the Daping Hospital from March to December 2018. The diagnosis of AD was defined by at least two neurologists in accordance with the “probable AD” criteria of the National Institute of Neurological and Communication Disorders and Stroke/Alzheimer Disease and Related Disorders Association.[11] Subjects were excluded for the following reasons: (1) a family history of dementia; (2) neurologic diseases such as hydrocephalus, Parkinson disease, epilepsy, inflammatory demyelinating diseases, stroke, brain trauma, and tumor; (3) other disorders that have the potential to affect CSF biomarker levels, including intracranial infections, intracranial hypertension, and hydrocephalus; (4) severe cardiac, pulmonary, hepatic, renal or hematological diseases, or malignant tumor; (5) enduring mental illness (eg, schizophrenia); and (6) inability to comply with the study assessment or refusal to participate.
The clinical evaluation of AD patients was performed according to our previous protocol.[12] Demographic data (including age, sex, and educational level) and medical history were collected. Fasting blood was sampled for the examination of routine blood tests, electrolytes, liver and kidney function, coagulation function, thyroid function, vitamin B12, pre-transfusion examination (human immunodeficiency virus, syphilis, and hepatitis C), and tumor markers. The cognitive and functional status were first assessed with the Mini-Mental Status Examination (MMSE) and Activities of Daily Living Scale. If the participant had abnormal cognitive status, other cognitive tests were evaluated, including the Auditory Verbal Learning Test, clock drawing test, Trail Making Test, Boston naming test, digit span test, Clinical Dementia Rating, Pfeiffer Outpatient Disability Questionnaire, and Hachinski Ischemic Score.
In the cross-sectional study, a total of 131 participants without memory complaints (non-AD) were enrolled from the Daping Hospital (n = 62) and the Qingdao Municipal Hospital (n = 69) from March to December 2018. These non-AD participants suffered from urinary diseases and planned to undergo surgery with lumbar anesthesia. Neuropsychological assessments and the Food Frequency Questionnaire (FFQ) were conducted the day before the surgery. CSF was collected after lumbar anesthesia at the beginning of surgery.
Assessment of spicy food consumption
Consumption of spicy food during the last 12 months was assessed with FFQ,[10] which includes four questions: (Q1) the degree of spicy food intake (no, mild, middle, or heavy); (Q2) the degree of food spiciness (no, mild, middle, or heavy); (Q3) the frequency of spicy food intake (less than once a week, approximately once a week, 2–6 times per week, or daily); and (Q4) the amount of spicy food intake (no, mild, middle, or heavy). The score for each question ranged from 1 (no) to 4 (heavy) points, and scores for all these questions were summed to obtain the total FFQ score, which could range from 4 to 16 points.
CSF collection
Lumbar punctures were conducted under local anesthesia. CSF was sampled and centrifuged within 2 h after collection at 2000 × g for 10 min at room temperature to eliminate cells and insoluble components, then aliquoted and stored at −80°C until use.
Measurement of β-amyloid (1–40) (Aβ40), β-amyloid (1–42) (Aβ42), total-tau (t-tau), and phospho-tau181 (p-tau181)
Levels of Aβ40, Aβ42, t-tau, and p-tau181 in CSF were measured using the following enzyme-linked immunosorbent assay kits: INNOTEST Aβ42, INNOTEST Aβ40, INNOTEST hTAU Ag t-tau, and INNOTEST p-tau181 (Innogenetics, Belgium).
Statistics
Continuous variables normally distributed are shown as mean ± standard deviation; those not normally distributed are presented as the median and interquartile range (25th–75th percentiles). Categorical variables are expressed as number (%). Statistical comparisons between the AD and CN groups were made using Student's t test or Mann-Whitney U test as applicable for continuous variables and χ2 test for categorical variables. Normality and equal-variance tests were performed for all assays. Since FFQ scores and several parameters were not normally distributed even after transformed or standardized, Spearman rank coefficients and partial correlation coefficients were used to analyze the correlations of FFQ scores with MMSE scores and CSF biomarkers, with adjustment for age, gender, and education which are important variates for cognition. Two-tailed P values < 0.05 were considered significant. All analyses were performed with SPSS 20.0 (SPSS Inc., Chicago, IL, USA).
Results
Characteristics of the study subjects
The basic information of the AD patients and CNs in the case-control study is shown in Table 1. AD patients had lower MMSE scores (14.0 [5.5–18.5] vs. 28.0 [27.0–29.0], P < 0.001), years of education (6.0 [6.0–9.0] vs. 12.0 [9.0–12.0], P < 0.001), and FFQ scores (4.0 [4.0–8.0] vs. 8.0 [4.5–10.0], P < 0.001) than CNs.
Table 1.
Characteristics of AD patients and CNs.
| Variables | AD (n = 55) | CN (n = 55) | P |
| Age (years), mean ± SD | 67.8 ± 8.1 | 65.5 ± 7.0 | 0.106 |
| Female, n (%) | 30 (54.5) | 28 (50.9) | 0.849 |
| Education (years), median (IQR) | 6.0 (6.0–9.0) | 12.0 (9.0–12.0) | <0.001 |
| MMSE, median (IQR) | 14.0 (5.5–18.5) | 28.0 (27.0–29.0) | <0.001 |
| FFQ, median (IQR) | 4.0 (4.0–8.0) | 8.0 (4.5–10.0) | <0.001 |
AD: Alzheimer disease; CN: Cognitively normal control; SD: Standard deviation; IQR: Interquartile range; MMSE: Mini-mental status examination; FFQ: Food frequency questionnaire.
Association between spicy food consumption and cognition
In the cohort containing AD patients and CNs, FFQ scores were positively associated with MMSE scores (r = 0.218, P = 0.014) after adjusting for education [Figure 1A]. In the cohort of non-AD participants, FFQ scores were also associated with MMSE scores (r = 0.264, P = 0.0023) after adjusting for education and region [Figure 1B]. These findings suggest that consumption of spicy food may protect against cognitive decline.
Figure 1.
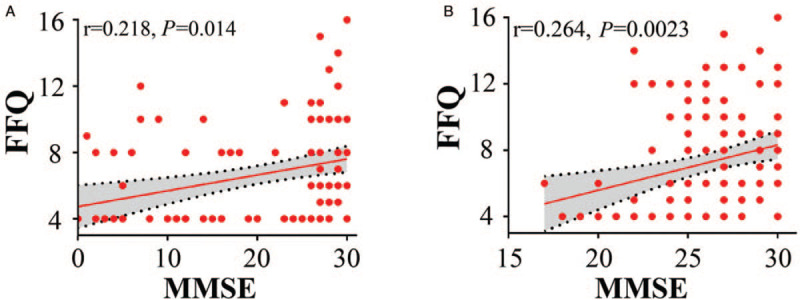
Association between FFQ and MMSE scores. (A) FFQ scores were positively associated with MMSE scores in AD patients and CNs; N = 110. (B) FFQ scores were positively associated with MMSE scores in non-AD participants; N = 131. Partial correlation analysis: ∗P < 0.05, two-tailed. AD: Alzheimer disease; CNs: Cognitively normal; FFQ: Food frequency questionnaire; MMSE: Mini-mental status examination.
Associations between spicy food consumption and AD biomarkers in CSF
Next, we investigated the association between spicy food consumption and AD biomarkers in the CSF of non-AD participants [Table 2]. FFQ scores were positively associated with the Aβ42 levels but negatively associated with p-tau181/Aβ42 and t-tau/Aβ42 ratios in CSF. After adjustment for age, sex, and education, FFQ scores were also associated with Aβ42 levels, p-tau181/Aβ42, and t-tau/Aβ42 ratios in CSF. Since ratios of t-tau/Aβ42 and p-tau/Aβ42 in CSF best discriminated Pittsburgh compound-B (PiB)-positive from PiB-negative individuals,[13] these findings imply that spicy food consumption may affect amyloid pathologies and neurodegeneration.
Table 2.
Correlation of FFQ with MMSE scores and AD biomarkers in CSF of non-AD participants.
| Concentration (pg/mL) | Unadjusted∗ | Adjusted† | |||
| Biomarker | (n = 131) | R | P | R | P |
| Aβ42 | 844.200 ± 312.900 | 0.311 | <0.001 | 0.325 | <0.001 |
| Aβ40 | 9619.800 ± 5168.000 | 0.062 | 0.483 | 0.170 | 0.054 |
| Aβ42/Aβ40 | 0.105 ± 0.056 | 0.189 | 0.030 | 0.092 | 0.300 |
| t-Tau | 194.900 ± 86.900 | 0.045 | 0.609 | 0.053 | 0.551 |
| p-Tau181 | 42.700 ± 15.000 | 0.023 | 0.797 | 0.074 | 0.406 |
| p-Tau181/t-Tau | 0.233 ± 0.045 | –0.045 | 0.612 | –0.055 | 0.535 |
| t-Tau/Aβ42 | 0.247 ± 0.115 | –0.311 | <0.001 | –0.181 | 0.041 |
| p-Tau181/Aβ42 | 0.056 ± 0.026 | –0.358 | <0.001 | –0.223 | 0.011 |
∗Spearman correlation analysis acted as a univariate analysis. †Partial correlation analysis adjusted for age, gender, and education years. Data are shown as the mean ± Standard deviation. FFQ: Food frequency questionnaire; MMSE: Mini-mental status examination; AD: Alzheimer disease; CSF: Cerebrospinal fluid; Aβ42: β-Amyloid (1–42); Aβ40: β-Amyloid (1–40).
Discussion
In the present study, we found that spicy food consumption was lower in AD patients than that in CNs. In non-AD participants, spicy food consumption was correlated with MMSE scores and the AD core biomarkers Aβ42, p-tau181/Aβ42 ratio, and t-tau/Aβ42 ratio in CSF. These findings imply that a capsaicin-rich diet might offer protective effects against cognitive decline and AD-type pathologies.
Our previous study found that a capsaicin-rich diet was related to cognition and blood biomarkers in subjects aged 40 years and older.[10] In the present study, we further showed that a capsaicin-rich diet was associated with cerebral AD core biomarkers. Moreover, recent animal studies from our and other groups suggested that consumption of capsaicin was able to reduce brain Aβ pathology, attenuate neurodegeneration, and improve cognition in AD transgenic animals.[14,15] Taken together, our findings suggest that a capsaicin-rich diet not only offers protection against cognitive decline but also may modify AD-type pathologies in the brain. However, a recent study suggested that higher spicy food intake was associated with worse memory decline in an open cohort study in China.[16] However, cognitive status was assessed in that study with subjectively self-reported memory loss, and participants with higher spicy food consumption in this study were less educated. Whether a capsaicin-rich diet is protective against AD in humans needs further investigation in the future.
AD is a complicated neurodegenerative disease, and healthy lifestyles, including diet patterns, have been suggested to be able to lower the incidence of AD.[17] As part of multidomain lifestyle interventions, a healthy diet has shown beneficial effects in preventing cognitive impairment in several trials.[18,19] Additionally, capsaicin has been proven to be potentially therapeutic to many diseases, including obesity, hypertension, and atherosclerosis,[20–22] which also act as contributing risk factors for AD.[23–25] Taken together, our current and previous findings suggest that a capsaicin-rich diet may protect against AD.
This study has some strengths and limitations. This is a rare study examining the correlation between the consumption of spicy food and CSF biomarkers in humans. However, for technical reasons, we did not test capsaicin levels in the blood and CSF or analyze the correlation of capsaicin levels with biomarkers and cognitive function. The investigation of capsaicin-rich diet habits was based on a retrospective subjective scale, which could generate recall bias in the consumption of spicy food. Although FFQ scores were significantly associated with CSF biomarkers, the correlation coefficient was small, suggesting a weak association. This may be caused by other confounders, such as comprehensive diet patterns and concomitant diseases. Moreover, the sample size was limited in the present study, and more participants should be enrolled in the future, especially participants from different regions of China.
In conclusion, our findings suggest that spicy food consumption is correlated with cognition levels and AD biomarkers in CSF, indicating that capsaicin might have the potential to modify cognitive dysfunction and cerebral pathologies associated with AD.
Funding
This study was supported by grants from the National Natural Science Foundation of China (NSFC) (Nos. 81625007, 91749206, and 81600949).
Conflicts of interest
None.
Footnotes
How to cite this article: Tian DY, Wang J, Sun BL, Wang Z, Xu W, Chen Y, Shen YY, Li HY, Chen DW, Zhou FY, Yi X, Zeng GH, Xu ZQ, Chen LY, Yu JT, Wang YJ. Spicy food consumption is associated with cognition and cerebrospinal fluid biomarkers of Alzheimer disease. Chin Med J 2021;134:173–177. doi: 10.1097/CM9.0000000000001318
References
- 1.Wang J, Gu BJ, Masters CL, Wang YJ. A systemic view of Alzheimer disease—insights from amyloid-beta metabolism beyond the brain. Nat Rev Neurol 2017; 13:703.doi: 10.1038/nrneurol.2017.147. [DOI] [PubMed] [Google Scholar]
- 2.Kivipelto M, Mangialasche F, Ngandu T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat Rev Neurol 2018; 14:653–666. doi: 10.1038/s41582-018-0070-3. [DOI] [PubMed] [Google Scholar]
- 3.Sperling R, Mormino E, Johnson K. The evolution of preclinical Alzheimer's disease: implications for prevention trials. Neuron 2014; 84:608–622. doi: 10.1016/j.neuron.2014.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer's disease: an analysis of population-based data. Lancet Neurol 2014; 13:788–794. doi: 10.1016/S1474-4422(14)70136-X. [DOI] [PubMed] [Google Scholar]
- 5.Solomon A, Mangialasche F, Richard E, Andrieu S, Bennett DA, Breteler M, et al. Advances in the prevention of Alzheimer's disease and dementia. J Intern Med 2014; 275:229–250. doi: 10.1111/joim.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu W, Tan L, Wang HF, Jiang T, Tan MS, Tan L, et al. Meta-analysis of modifiable risk factors for Alzheimer's disease. J Neurol Neurosurg Psychiatry 2015; 86:1299–1306. doi: 10.1136/jnnp-2015-310548. [DOI] [PubMed] [Google Scholar]
- 7.Yuan J, Zhang Z, Wen H, Hong X, Hong Z, Qu Q, et al. Incidence of dementia and subtypes: a cohort study in four regions in China. Alzheimers Dement 2016; 12:262–271. doi: 10.1016/j.jalz.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 8.Wang S, Cheng L, He S, Xie D. Regionalpungency degree in China and its correlation with typical climate factors. J Food Sci 2019; 84:31–37. doi: 10.1111/1750-3841.14408. [DOI] [PubMed] [Google Scholar]
- 9.Sun D, Lv J, Chen W, Li S, Guo Y, Bian Z, et al. Spicy food consumption is associated with adiposity measures among half a million Chinese people: the China Kadoorie Biobank study. BMC Public Health 2014; 14:1293.doi: 10.1186/1471-2458-14-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu CH, Bu XL, Wang J, Zhang T, Xiang Y, Shen LL, et al. The associations between a capsaicin-rich diet and blood amyloid-beta levels and cognitive function. J Alzheimers Dis 2016; 52:1081–1088. doi: 10.3233/JAD-151079. [DOI] [PubMed] [Google Scholar]
- 11.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984; 34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 12.Li J, Wang YJ, Zhang M, Xu ZQ, Gao CY, Fang CQ, et al. Vascular risk factors promote conversion from mild cognitive impairment to Alzheimer disease. Neurology 2011; 76:1485–1491. doi: 10.1212/WNL.0b013e318217e7a4. [DOI] [PubMed] [Google Scholar]
- 13.Schindler SE, Gray JD, Gordon BA, Xiong C, Batrla-Utermann R, Quan M, et al. Cerebrospinal fluid biomarkers measured by Elecsys assays compared to amyloid imaging Alzheimer's Dement’ 2018; 14:1460–1469. doi: 10.1016/j.jalz.2018.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du Y, Fu M, Huang Z, Tian X, Li J, Pang Y, et al. TRPV1 activation alleviates cognitive and synaptic plasticity impairments through inhibiting AMPAR endocytosis in APP23/PS45 mouse model of Alzheimer's disease. Aging Cell 2020; 19:e13113.doi: 10.1111/acel.13113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J, Sun BL, Xiang Y, Tian DY, Zhu C, Li WW, et al. Capsaicin consumption reduces brain amyloid-beta generation and attenuates Alzheimer's disease-type pathology and cognitive deficits in APP/PS1 mice. Transl Psychiatry 2020; 10:230.doi: 10.1038/s41398-020-00918-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi Z, El-Obeid T, Riley M, Li M, Page A, Liu J. High chili intake and cognitive function among 4582 adults: an open cohort study over 15 years. Nutrients 2019; 11:1183.doi: 10.3390/nu11051183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scheltens P, Blennow K, Breteler MM, de Strooper B, Frisoni GB, Salloway S, et al. Alzheimer's disease. Lancet 2016; 388:505–517. doi: 10.1016/S0140-6736(15)01124-1. [DOI] [PubMed] [Google Scholar]
- 18.Scarmeas N, Luchsinger JA, Schupf N, Brickman AM, Cosentino S, Tang MX, et al. Physical activity, diet, and risk of Alzheimer disease. JAMA 2009; 302:627–637. doi: 10.1001/jama.2009.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morris MC, Tangney CC. Diet and prevention of Alzheimer disease. JAMA 2010; 303:2519–2520. doi: 10.1001/jama.2010.844. [DOI] [PubMed] [Google Scholar]
- 20.Zhang LL, Yan Liu D, Ma LQ, Luo ZD, Cao TB, Zhong J, et al. Activation of transient receptor potential vanilloid type-1 channel prevents adipogenesis and obesity. Circ Res 2007; 100:1063–1070. doi: 10.1161/01.RES.0000262653.84850.8b. [DOI] [PubMed] [Google Scholar]
- 21.Yang D, Luo Z, Ma S, Wong WT, Ma L, Zhong J, et al. Activation of TRPV1 by dietary capsaicin improves endothelium-dependent vasorelaxation and prevents hypertension. Cell Metab 2010; 12:130–141. doi: 10.1016/j.cmet.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edwards JG. TRPV1 in the central nervous system: synaptic plasticity, function, and pharmacological implications. Prog Drug Res 2014; 68:77–104. doi: 10.1007/978-3-0348-0828-6_3. [DOI] [PubMed] [Google Scholar]
- 23.Luchsinger JA, Cheng D, Tang MX, Schupf N, Mayeux R. Central obesity in the elderly is related to late-onset Alzheimer disease. Alzheimer Dis Assoc Disord 2012; 26:101–105. doi: 10.1097/WAD.0b013e318222f0d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rafael H, Fernandez E, Ayulo V. Hypertension directly relates to Alzheimer disease. J Alzheimers Dis 2002; 4:329–330. doi: 10.3233/JAD-2002-4409. [PubMed] [Google Scholar]
- 25.Roher AE, Esh C, Rahman A, Kokjohn TA, Beach TG. Atherosclerosis of cerebral arteries in Alzheimer disease. Stroke 2004; 35:2623–2627. doi: 10.1161/01.STR.0000143317.70478.b3. [DOI] [PubMed] [Google Scholar]


