Abstract
Background.
Donor-derived cell-free DNA (dd-cfDNA) is a useful biomarker of rejection that originates from allograft cells undergoing injury. Plasma levels <1% in kidney transplant recipients have a high negative predictive value for active allograft rejection. The utility of this biomarker in kidney transplant recipients receiving immune checkpoint inhibitor therapy is unknown.
Methods.
We describe a case in which serial dd-cfDNA monitoring facilitated the use of immune checkpoint inhibitor therapy, which is known to be associated with high rates of rejection, in a kidney transplant recipient with metastatic cancer.
Results.
A 72-y-old man with end-stage kidney disease secondary to autosomal dominant polycystic kidney disease underwent living unrelated kidney transplant in December 2010. His immunosuppression regimen included tacrolimus, mycophenolate, and prednisone. In July 2017, he presented with metastatic cutaneous squamous cell carcinoma. After his disease progressed through radiation therapy and cetuximab, he received pembrolizumab (antiprogrammed cell death protein 1). His dd-cfDNA level was undetectable at baseline, then increased during treatment but remained <1%. This trend, despite fluctuations in serum creatinine levels during therapy, allowed for continuation of pembrolizumab and successful treatment of his metastatic cancer without clinically evident allograft rejection. After discontinuation of pembrolizumab, dd-cfDNA levels fell below the level of detection. Genetic analysis of the cutaneous squamous cell carcinoma demonstrated a genetic profile distinct from the dd-cfDNA, indicating that tumor lysis did not impact increases in dd-cfDNA.
Conclusions.
Serial dd-cfDNA measurements may provide a useful, noninvasive biomarker for detecting allograft injury that may facilitate the use of immunomodulatory therapies in organ transplant recipients with cancer.
In kidney transplant recipients, early and accurate diagnosis of rejection is critical in ensuring long-term allograft survival. Donor-derived cell-free DNA (dd-cfDNA) originates from donor allograft cells undergoing injury or cell death. It can be detected in the plasma and urine of the recipient and has been proposed as a noninvasive biomarker of allograft rejection in solid organ transplant patients including kidney transplant recipients.1-3 The Circulating Donor-Derived Cell-Free DNA in Blood for Diagnosing Acute Rejection in Kidney Transplant Recipients (DART) study3 validated that plasma levels of dd-cfDNA >1% could discriminate active rejection from no rejection with a negative predictive value of 84% and a positive predictive value of 61%. Thus, this noninvasive biomarker holds the potential to reduce the need for invasive percutaneous needle allograft biopsies, which remain the standard method for diagnosing allograft rejection. This becomes particularly important when a change in immunosuppression may be needed, for example, when monitoring an allograft that is at high risk of rejection after administration of immune checkpoint inhibitor therapy to treat malignancy in transplant recipients.
Immune checkpoint inhibitors (eg, programmed death-ligand 1 antibodies) are a novel therapeutic class of drugs that trigger immune (including T cell) activation and can be used to treat patients with cancer. However, these therapies are associated with immune-mediated toxicities and, in transplant recipients, rejection of the allograft.4 In a recent comprehensive analysis, among 20 cases of solid organ transplant recipients with malignancies in whom checkpoint inhibitors were used, kidney was the most common allograft (65%) and rejection occurred in 61.5% cases (n = 8 of 13).4-6
This case report describes a kidney transplant recipient with metastatic cancer treated with immune checkpoint inhibitor therapy whose serial plasma dd-cfDNA measurements provided reassurance about the health of his allograft during his course of treatment. Our findings suggest that, in this patient population, dd-cfDNA levels may provide a useful, noninvasive biomarker for detecting allograft injury, thereby facilitating the administration of immunomodulatory cancer therapies.
MATERIALS AND METHODS
We report a case of a kidney transplant recipient with metastatic cutaneous squamous cell carcinoma (CSCC). The patient received treatment with pembrolizumab (antiprogrammed cell death protein 1 [anti-PD-1]) while continuing on low-dose tacrolimus and prednisone. The allograft was monitored with serial serum creatinine and plasma dd-cfDNA (AlloSure; CareDx, Brisbane, CA) levels. The dd-cfDNA assay measures the percent of dd-cfDNA in the total cell-free DNA circulating in plasma.7 Greater than 1.0% dd-cfDNA is associated with a probability of active allograft rejection.3 In a reference population of stable patients, the median dd-cfDNA value is 0.21%, and a >61% increase in dd-cfDNA from a prior sample exceeds the biological variability observed. To verify that the cfDNA was donor-derived and not recipient-derived (ie, from tumor lysis), the patient’s tumor DNA was extracted from a formalin-fixed paraffin-embedded CSCC biopsy specimen using AllPrep DNA/RNA FFPE Kit (Qiagen, Redwood City, CA). DNA was quantified using Qubit dsDNA HS Assay Kit (Thermo Fisher, Waltham, MA) and analyzed by AlloSure. Single-nucleotide polymorphism genotype profiles were determined and compared with the single-nucleotide polymorphism genotype profiles of cell-free DNA from serial patient plasma samples. This study was performed after approval from the Johns Hopkins Medicine Institutional Review Board.
RESULTS
A 72-y-old Caucasian man with autosomal dominant polycystic kidney disease underwent living unrelated kidney transplantation in December 2010. He received induction immunosuppression with low-dose thymoglobulin because of a history of cutaneous melanoma. His baseline serum creatinine was 1.1 mg/dL, and he was maintained on a stable immunosuppression regimen with tacrolimus 4 mg every 12 h, mycophenolate mofetil (MMF) 500 mg every 12 h, and prednisone 5 mg daily.
In June 2017, he developed CSCC with metastases to bones (vertebral bodies, left scapula), left parotid gland, and regional lymph nodes. His immunosuppression regimen was decreased to MMF 250 mg every 12 h and tacrolimus was switched to everolimus. His disease progressed through radiotherapy and cetuximab.
After a thorough discussion of the risks and benefits—including allograft rejection and loss and the need to initiate dialysis—the patient agreed to begin immune checkpoint inhibitor therapy. His MMF was stopped and everolimus was switched back to tacrolimus 2 mg every 12 h, aiming for trough levels of 3–5 ng/mL. A baseline dd-cfDNA (AlloSure; CareDx, Brisbane, CA) was obtained (Figure 1). A test of anti-HLA antibodies was negative for donor-specific antibodies. Pembrolizumab (anti-PD-1) was initiated in November 2017.
FIGURE 1.
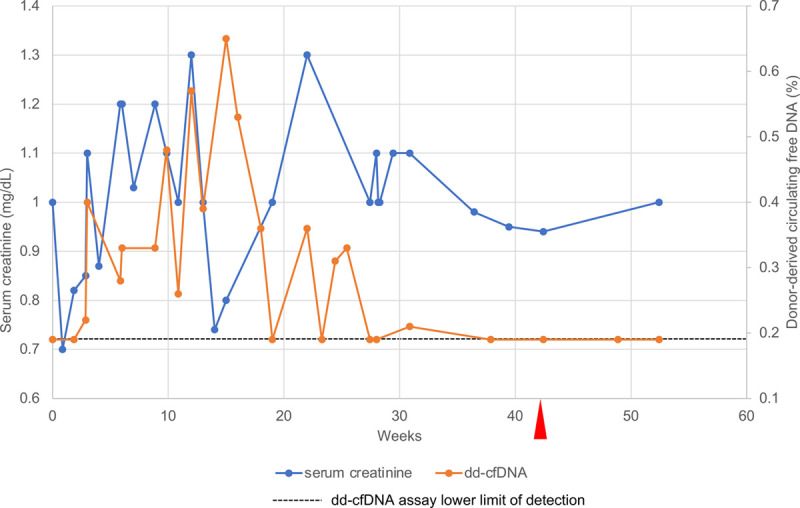
Serum creatinine (blue line, left vertical axis) and plasma donor-derived cell-free DNA (dd-cfDNA) (orange line, right vertical axis) levels during and after pembrolizumab (anti-PD-1) therapy. The dd-cfDNA assay measures the amount of dd-cfDNA as a percentage of the total cell-free DNA circulating in plasma. The dashed horizontal line marks the lower limit of detection of the dd-cfDNA assay (0.19%); points along this line indicate undetectable levels of dd-cfDNA. Red arrowhead indicates the patient’s final dose of pembrolizumab. anti-PD-1, anti-programmed cell death protein 1.
Serum creatinine and plasma dd-cfDNA levels were measured approximately weekly for 8 wk and then at least monthly (Figure 1). At the time of initiation of pembrolizumab, the serum creatinine level was 1.0 mg/dL and plasma dd-cfDNA was below the lower limit of detection (ie, <0.19%). Throughout the course of pembrolizumab therapy, the creatinine increased steadily to as high as 1.3 mg/dL, raising questions about whether allograft rejection was taking place and, if so, whether pembrolizumab could safely be continued. During that same period, plasma dd-cfDNA levels remained <1% (absolute value) with <61% increase from the prior test (relative change value). These metrics were consistent with a low likelihood of active rejection, allowing for continuation of pembrolizumab therapy, avoidance of an allograft biopsy, and continuation of his immunosuppressive medication regimen.
Although the patient initially experienced tumor control (noncomplete response/nonprogressive disease per Response Evaluation Criteria in Solid Tumors [RECIST] v1.1),8 his disease progressed at 10 mo when positron emission tomography/computed tomography imaging revealed an fluorodeoxyglucose-avid lesion in the right acetabulum consistent with tumor growth. Pembrolizumab was discontinued and the patient received a course of radiotherapy. The dd-cfDNA level at this time was below the limit of detection.
Genetic analysis of the CSCC demonstrated that the tumor shared the same genotype as the recipient cfDNA. Thus, the rising dd-cfDNA seen during treatment with pembrolizumab was, in fact, donor-derived, not an artifact of immune-mediated tumor destruction.
Almost 3 y after the initiation of pembrolizumab, the patient’s kidney function is stable (serum creatinine 1.0–1.3 mg/dL), and he has no evidence of metastatic cancer. His dd-cfDNA level remains below the limit of detection.
DISCUSSION
This case highlights how serial dd-cfDNA monitoring facilitated the administration of an immune checkpoint inhibitor to a kidney transplant recipient with advanced cancer. In the setting of a fluctuating serum creatinine level, dd-cfDNA values reassured the clinical team about the health of the allograft, allowing avoidance of an allograft biopsy and guiding management of immunosuppressive medications.
Interestingly, there was a persistent rise in the level of dd-cfDNA while the patient was being treated with pembrolizumab. However, the relative increase from 1 sample to the next (ie, “biological variation” value3) was never >61%, and the absolute value was always <1%, the threshold beyond which rejection is suspected. These findings emphasize the importance of monitoring dd-cfDNA levels frequently, first to detect an emerging rejection, but also to avoid the false appearance of rejection when there has been >61% change between values because of infrequent monitoring.
The fluctuations in dd-cfDNA levels during the treatment period with pembrolizumab invite speculation. Pembrolizumab is associated with immune-mediated acute tubular necrosis and interstitial nephritis.9 In our patient’s case, the fluctuations in dd-cfDNA may have been a marker of renal injury that was subclinical for rejection. Indeed, his dd-cfDNA level returned to below the limit of detection after discontinuation of pembrolizumab. Similarly, immune checkpoint blocking agents are known to cause injury to various organs, which could increase the total amount of circulating cell-free DNA (the denominator of reported dd-cfDNA), thereby decreasing the percentage of dd-cfDNA. Oellerich et al10 have suggested that the absolute dd-cfDNA value instead of the percentage may be a more predictive biomarker of allograft injury that can be followed over time and used for clinical decision-making. Of note, the utility of dd-cfDNA monitoring in cases where in the tumor is of donor origin is unknown.
The use of dd-cfDNA as a marker of allograft rejection in the setting of immune checkpoint inhibitor therapy is further supported by a case from Hurkmans et al,11 who described a kidney transplant recipient treated with nivolumab (anti-PD-1) for metastatic melanoma. Acute rejection and allograft loss occurred within 12 d of initiation of therapy. The level of dd-cfDNA predicted the rejection by rising from a baseline of 0.9% to 2.9% within 1 wk and peaking at 23.1% on day 12. Allograft biopsy revealed extensive acute ischemic changes with capillary endothelial necrosis, edema, and hemorrhage. The allograft was eventually explanted and immunologic analysis demonstrated infiltration of alloreactive, nivolumab-saturated PD-1+ cytotoxic T cells. The rapidity with which the rejection occurred suggests that although the dd-cfDNA was predictive of eventual rejection, it might need to be measured very frequently after initiation of checkpoint inhibitor therapy in transplant recipients to allow for prompt administration of antirejection therapy.
Our study is limited in that an allograft biopsy was not performed. Thus, the presence, type, and degree of subclinical rejection cannot be confirmed.
CONCLUSIONS
dd-cfDNA detected in the plasma of transplant recipients may be a useful noninvasive marker of allograft rejection in patients treated with immune checkpoint inhibitors. Formal clinical trials testing immune checkpoint inhibitors in solid organ transplant recipients (eg, ClinicalTrials.gov identifier NCT03816332) are needed to better define appropriate monitoring intervals, thresholds for allograft biopsy, and necessary changes to immunosuppressive and antineoplastic treatment regimens.
ACKNOWLEDGMENTS
The authors thank Marica Grskovic, PhD for assistance with genetic analyses, and Sham Dholakia, MBBS and Bethany Dale, PhD for helpful discussions.
Footnotes
Published online 15 January, 2021.
A.B. was supported by a Transplant Nephrology Training Grant from CareDx. D.C.B. receives institutional research grant funding from CareDx, speaking honoraria from CareDx and Veloxis and is a consultant for Allovir, Amplyx, Argenyx, CareDx, Medeor, Natera, Sanofi, and Veloxis. E.J.L. receives institutional research grant funding from Bristol-Myers Squibb, Merck, and Regeneron and is a consultant for Array BioPharma, Bristol-Myers Squibb, EMD Serono, MacroGenics, Novartis, Merck, Regeneron, and Sanofi Genzyme. The other authors declare no conflicts of interest.
D.C.B. was supported by the National Institute of Diabetes and Digestive and Kidney Diseases U01 DK116042-01 and R01DK102981. This study was supported by the Bloomberg-Kimmel Institute for Cancer Immunotherapy (to M.D.S. and E.J.L.), the Barney Family Foundation (to M.D.S. and E.J.L.), Moving for Melanoma of Delaware (to M.D.S. and E.J.L.), the Laverna Hahn Charitable Trust (to M.D.S. and E.J.L.)., and gifts from Raymond and Melody Ranelli (D.C.B. and E.J.L.)
This study was presented in part as an abstract at the American Society of Nephrology Meeting; November 9, 2019; Washington, DC and and Onco-Nephrology Symposium at Memorial Sloan Kettering Cancer Center in NYC; December 7, 2019.
L.L., S.A., A.B., A.A., D.O., M.D.S., D.C.B., and E.J.L. have participated in the clinical evaluation, laboratory workup, and management of the patient, along with drafting and reviewing of the article. All authors provided final approval of the article version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
REFERENCES
- 1.Gielis EM, Ledeganck KJ, De Winter BY, et al. Cell-free DNA: an upcoming biomarker in transplantation. Am J Transplant. 2015; 15:2541–2551 [DOI] [PubMed] [Google Scholar]
- 2.Snyder TM, Khush KK, Valantine HA, et al. Universal noninvasive detection of solid organ transplant rejection. Proc Natl Acad Sci U S A. 2011; 108:6229–6234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bloom RD, Bromberg JS, Poggio ED, et al. ; Circulating Donor-Derived Cell-Free DNA in Blood for Diagnosing Active Rejection in Kidney Transplant Recipients (DART) Study Investigators. Cell-free DNA and active rejection in kidney allografts. J Am Soc Nephrol. 2017; 28:2221–2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aguirre LE, Guzman ME, Lopes G, et al. Immune checkpoint inhibitors and the risk of allograft rejection: a comprehensive analysis on an emerging issue. Oncologist. 2019; 24:394–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwatra V, Karanth NV, Priyadarshana K, et al. Pembrolizumab for metastatic melanoma in a renal allograft recipient with subsequent graft rejection and treatment response failure: a case report. J Med Case Rep. 2017; 11:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lipson EJ, Bagnasco SM, Moore J, Jr, et al. Tumor regression and allograft rejection after administration of anti-PD-1. N Engl J Med. 2016; 374:896–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grskovic M, Hiller DJ, Eubank LA, et al. Validation of a clinical-grade assay to measure donor-derived cell-free DNA in solid organ transplant recipients. J Mol Diagn. 2016; 18:890–902 [DOI] [PubMed] [Google Scholar]
- 8.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–247 [DOI] [PubMed] [Google Scholar]
- 9.Izzedine H, Mathian A, Champiat S, et al. Renal toxicities associated with pembrolizumab. Clin Kidney J. 2019; 12:81–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oellerich M, Shipkova M, Asendorf T, et al. Absolute quantification of donor-derived cell-free DNA as a marker of rejection and graft injury in kidney transplantation: results from a prospective observational study. Am J Transplant. 2019; 19:3087–3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hurkmans DP, Verhoeven JGHP, de Leur K, et al. Donor-derived cell-free DNA detects kidney transplant rejection during nivolumab treatment. J Immunother Cancer. 2019; 7:182. [DOI] [PMC free article] [PubMed] [Google Scholar]


