Abstract
Background:
The Shexiang Baoxin Pill (MUSKARDIA) has been used for treating coronary artery disease (CAD) and angina for more than 30 years in China. Nevertheless, methodologically sound trials on the use of MUSKARDIA in CAD patients are scarce. The aim of the study is to determine the effects of MUSKARDIA as an add-on to optimal medical therapy (OMT) in patients with stable CAD.
Methods:
A total of 2674 participants with stable CAD from 97 hospitals in China were randomized 1:1 to a MUSKARDIA or placebo group for 24 months. Both groups received OMT according to local tertiary hospital protocols. The primary outcome was the occurrence of a major adverse cardiovascular event (MACE), defined as a composite of cardiovascular death, non-fatal myocardial infarction (MI), or non-fatal stroke. Secondary outcomes included all-cause mortality, non-fatal MI, non-fatal stroke, hospitalization for unstable angina or heart failure, peripheral revascularization, angina stability and angina frequency.
Results:
In all, 99.7% of the patients were treated with aspirin and 93.0% with statin. After 2 years of treatment, the occurrence of MACEs was reduced by 26.9% in the MUSKARDIA group (MUSKARDIA: 1.9% vs. placebo: 2.6%; odds ratio = 0.80; 95% confidence interval: 0.45–1.07; P = 0.2869). Angina frequency was significantly reduced in the MUSKARDIA group at 18 months (P = 0.0362). Other secondary endpoints were similar between the two groups. The rates of adverse events were also similar between the two groups (MUSKARDIA: 17.7% vs. placebo: 17.4%, P = 0.8785).
Conclusions:
As an add-on to OMT, MUSKARDIA is safe and significantly reduces angina frequency in patients with stable CAD. Moreover, the use of MUSKARDIA is associated with a trend toward reduced MACEs in patients with stable CAD. The results suggest that MUSKARDIA can be used to manage patients with CAD.
Trial registration
chictr.org.cn, No. ChiCTR-TRC-12003513
Keywords: MUSKARDIA, Stable coronary artery disease, Angina, Major adverse cardiovascular event
Introduction
Cardiovascular diseases (CVDs) are the leading causes of death in many countries, accounting for 31.5% of deaths worldwide and 45% of all deaths due to non-communicable diseases. The combined use of aspirin and statin is a standard (and effective) secondary prevention approach to reduce the risk of cardiovascular events in patients with stable coronary artery disease (CAD).[1–4] Nevertheless, some residual cardiovascular risks persist.[5–7] Moreover, many patients with CAD in China, particularly females, are intolerant to aspirin because of gastrointestinal reaction, exacerbated respiratory disease, gout, or hyperuricemia.[8–10] Therefore, novel approaches are urgently needed to reduce the residual CAD risk and as eventual alternatives for patients with intolerance to standard drugs.
Traditional Chinese medicine (TCM) may be a potential add-on treatment and has long been used for treating CAD, which is considered as heart Yang deficiency resulting from Qi inadequacy.[11,12] Shexiang Baoxin Pill (MUSKARDIA) has been used to treat CAD and angina for more than 35 years in China. MUSKARDIA is composed of bioactive components, including muscone, ginsenosides, storax, bufadienolides, cinnamic acid, arenobufagin, and borneol.[13–16] Preliminary studies have indicated that MUSKARDIA dilates coronary arteries[17] and increases coronary blood flow, relieving the symptoms of angina.[13,18] Nevertheless, there are few methodologically sound trials that have been conducted on the use of MUSKARDIA in patients with CAD.
Therefore, the aim of this multicenter, double-blind, placebo-controlled, phase IV, randomized clinical trial (RCT) was to examine the long-term efficacy, safety, and compliance of MUSKARDIA as an add-on treatment to optimal medical therapy (OMT) in patients with stable CAD.
Methods
Design and oversight
The MUSKARDIA trial is a randomized, double-blinded, placebo-controlled, phase IV trial conducted at 97 sites in China (chictr.org.cn, ChiCTR-TRC-12003513). The trial was designed and led by an executive steering committee. The protocol and amendments were approved by the ethics committee at each participating center. Written informed consent was obtained from all patients before enrollment. The funder (Shanghai Hutchison Pharmaceuticals) had no role in the study design or in the collection, analysis, and reporting of data. Data were reviewed regularly throughout the trial by an independent data and safety monitoring committee.
Study population
Patients aged ≥18 years were eligible for the study if they presented with stable ischemic myocardial symptoms for at least 1 month and had at least one of the following events according to their hospital records or follow-up/health examination report: (1) history of acute myocardial infarction (MI) over 6 months; (2) history of percutaneous coronary intervention (PCI) or coronary artery bypass graft surgery (CABG) over 6 months; and (3) epicardial coronary stenosis of ≥ 50% in at least one major branch as indicated by coronary computed tomography (CT) angiography or coronary angiography. The key exclusion criteria were: (1) patients preparing to receive PCI or CABG; (2) serious CVDs (sustained severe angina [Canadian Cardiovascular Society IV], refractory heart failure, cardiogenic shock, severe aortic stenosis, or aortic insufficiency; (3) severe respiratory diseases; (4) diabetes with inadequate glycemic control (fasting blood glucose > 200 mg/dL or 11.1 mmol/L for more than twice within 1 month before enrollment); (5) hypertension with inadequate blood pressure control (systolic pressure ≥180 mmHg or diastolic pressure ≥110 mmHg before enrollment); (6) severe liver or kidney disease; or (7) any other severe diseases such as malignant tumor, severe anemia, or severe renal artery stenosis. Detailed inclusion and exclusion criteria are listed in Supplementary Table S1.
Randomization and blinding
Patients were randomly assigned 1:1 to receive oral MUSKARDIA (Hehuang Pharmaceutical Co., Shanghai, China) or placebo for 24 months. Central randomization with a block size of four was used to generate grouping codes. No stratification was applied. The codes were prepared in sealed envelopes and opened after a patient met the eligibility criteria and signed the consent form. The placebo had the exact same appearance and taste as the MUSKARDIA (bitter, black-brown, lustrous pill) and was kindly donated by the Shanghai Hutchison Pharmaceuticals Company. The patients, investigators, and core study staff were blinded to treatment allocation.
Treatment
Before the initiation of trial drug administration, all study patients entered a 28-day run-in period during which they received standard therapy for stable CAD according to the guidelines. Patients were then allocated to either oral MUSKARDIA (two pills, three times daily, 135 mg in total) or placebo (two pills, three times daily, 135 mg in total). The patients were instructed to take medications about 30 min after each meal. Patients continued with the study medication for 24 consecutive months or until the development of a major adverse event (AE). Interruption of the study drug for more than 14 consecutive days was considered as a protocol violation. Patients were allowed to receive other prescription medication, except TCM, for CVDs. The participants were followed up at 1, 3, 6, 9, 12, 18, and 24 months.
Endpoints and assessments
The primary composite efficacy endpoint was the occurrence of a major adverse cardiovascular event (MACE), defined as cardiovascular death, non-fatal MI, or non-fatal stroke. The secondary endpoints included all-cause mortality, non-fatal MI, non-fatal stroke, hospitalization for unstable angina or heart failure and coronary angioplasty (PCI or CABG), patient compliance, angina stability and angina frequency. All primary and secondary endpoint events were adjudicated by a blinded, independent clinical endpoint committee. Compliance was defined as the proportion of prescribed medication taken by the patients. Angina stability and angina frequency were assessed with the Seattle Angina Questionnaire. Other prespecified exploratory endpoint measures consisted of liver and renal functions and concomitant medication.
Safety endpoints comprised the number of total AEs and severe AEs (SAEs) by 24 months. Vital signs and electrocardiogram were assessed at each visit, whereas the physical examination and laboratory parameters were assessed every 6 months.
Statistical analysis
According to the ACTION[19] and EUROPA[20] studies, an event rate of 5.0% per year was estimated for the placebo (control) group. With a planned sample size of 2700, the overall study would have 80% power at a two-sided α of 0.05 to detect a 30% relative risk reduction in the MUSKARDIA group. All reported P values were two-sided. P < 0.05 was considered statistically significant.
All statistical analyses were performed using SAS version 9.2 (SAS Institute, NY, USA). For variables not normally distributed, medians and interquartile ranges were reported; otherwise, means and standard deviations were reported.
All efficacy and safety analyses were performed in the full analysis set (FAS) / safety set (SS), defined as randomized patients who received at least one dose of study medication. The analysis of the primary endpoint was based on Kaplan-Meier estimates of cumulative incidence. The hazard ratio and 95% confidence interval (CI) were estimated based on Cox proportional hazards models. All-cause mortality, non-fatal MI, non-fatal stroke, hospitalization for unstable angina or heart failure and peripheral revascularization, compliance, angina stability and angina frequency were compared between the two groups using the independent sample t test or Pearson chi-square test or Fisher exact test, as appropriate. The odds ratio (OR) was calculated by logistic regression. All statisticians were blinded to group allocation.
Results
Study population
A total of 2674 patients from 97 centers were enrolled and randomized between July 2011 and August 2015. At one site with only one patient enrolled, the patient dropped out before the run-in period for logistic reasons. The study flowchart is shown in Supplementary Figure 1. No unblinding in response to AE was undertaken before the data lock. Further, 2673 patients received a study drug: 1342 in the MUSKARDIA group and 1331 in the placebo group. The mean age of the total population was 63.8 years; 70.8% were male. The overall baseline aspirin was 99.7%. The overall statin use was 93.0%. In addition, 31.6% of the patients were under isosorbide mononitrate medication at enrollment. The Seattle Angina Questionnaire showed that the frequency of angina at baseline was similar in the two groups (MUSKARDIA: 20.1%; controls: 19.4%). As shown in Table 1, all baseline characteristics were comparable between groups.
Table 1.
Baseline characteristics of patients with coronary artery disease in the full analysis set.
| Variables | Placebo (n = 1327) | MUSKARDIA (n = 1335) |
| Age | ||
| Mean (SD) | 63.7 (9.9) | 63.9 (9.8) |
| < 65 years, n (%) | 682 (51.4) | 696 (52.1) |
| ≥65 years, n (%) | 645 (48.6) | 639 (47.9) |
| Males, n (%) | 935 (70.5) | 951 (71.2) |
| History of coronary disease, mean (SD), years | 3.0 (3.7) | 3.0 (3.8) |
| BMI, mean (SD), kg/m2 | 24.5 ± 3.0 | 24.5 ± 3.0 |
| Systolic blood pressure, mean (SD), mm Hg | 127.6 (12.8) | 127.9 (13.0) |
| Diastolic blood pressure, mean (SD), mm Hg | 76.0 (8.4) | 75.8 (8.4) |
| Medical history, n (%) | ||
| Diabetes | 376 (28.3) | 340 (25.5) |
| Hypertension | 764 (57.6) | 742 (55.6) |
| Chronic kidney disease | 417 (33.5) | 445 (35.7) |
| Atrial fibrillation | 9 (0.7) | 10 (0.7) |
| Heart failure | 3 (0.2) | 5 (0.4) |
| Baseline medication, n (%) | ||
| Aspirin | 1322 (99.6) | 1331 (99.7) |
| Statins | 1238 (93.3) | 1237 (92.7) |
| Isosorbide mononitrate | 416 (31.3) | 426 (31.9) |
| Clopidogrel | 674 (50.8) | 702 (52.6) |
| β-blockers | 981 (73.9) | 997 (74.7) |
| CCB | 463 (34.9) | 480 (36.0) |
| ARB | 395 (29.8) | 377 (28.2) |
| ACEI | 381 (28.7) | 379 (28.4) |
| Anti-angina drugs | 78 (5.9) | 68 (5.1) |
| SAQ quality of life, mean (SD) | ||
| Physical limitation | 81.1 (17.1) | 80.1 (17.4) |
| Angina stability | 61.0 (22.8) | 62.5 (22.8) |
| Angina frequency | 82.3 (20.1) | 82.3 (19.4) |
| Satisfaction with treatment | 74.0 (15.0) | 73.5 (14.9) |
| Cognition of disease | 59.4 (20.8) | 59.8 (20.0) |
ARB: Angiotensin receptor blocker; ACEI: Angiotensin-converting enzyme inhibitor; BMI: Body mass index; CCB: Calcium channel blocker; MUSKARDIA: Shexiang Baoxin Pill; SAQ: Seattle angina questionnaire; SD: Standard deviation.
Efficacy endpoints
The incidence of the primary endpoint (MACE) was 1.9% (26/1335) in the MUSKARDIA group compared with 2.6% (34/1327) in the placebo group at 24 months (OR = 0.80; 95% CI: 0.45–1.07; P = 0.2869). From 18 months, the Kaplan-Meier curves of the two groups diverged, with a 26.9% reduction in the occurrence of MACE in the MUSKARDIA group after 2 years of treatment compared with the placebo group [Figure 1]. For every 1000 CAD patients, there were, on average, 3.5 fewer MACEs were reported in the MUSKARDIA group per year. The comparisons of the occurrence rates of MACE at different time points are listed in Supplementary Table S2.
Figure 1.
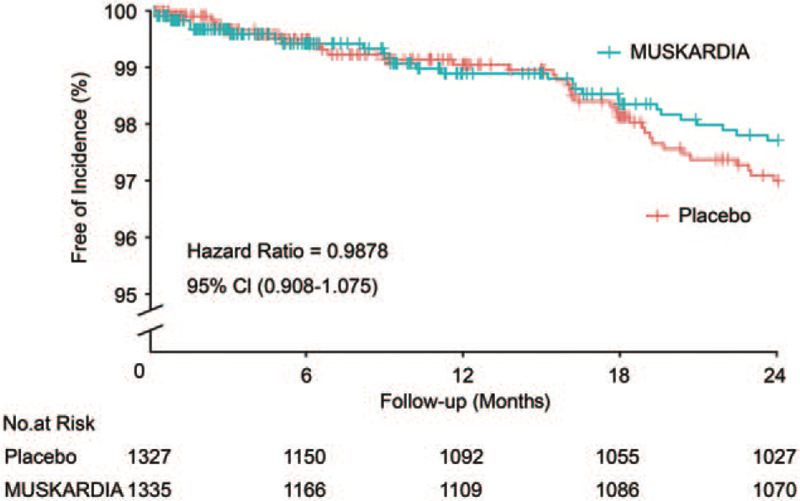
Cumulative Kaplan-Meier estimates of the time to the first major adverse cardiovascular event. No significant difference was observed between the Shexiang Baoxin Pill and placebo groups during the 24-month trial period (P = 0.2215). A trend of gradual curve diversion emerged after 18 months of treatment.
In terms of person-year analysis for the primary endpoint the occurrence rates of MACE were 1.2% (27.0/2232.5) in the MUSKARDIA group and 1.6% (35.0/2209.6) in the placebo group (OR = 0.70; 95% CI: 0.44–1.18; P = 0.1986). The subgroup analysis showed that MUSKARDIA was superior to placebo in females and in the body mass index (BMI) < 24 kg/m2 subgroups [Figure 2].
Figure 2.

Subgroup analysis indicated benefits of Shexiang Baoxin Pill in females and patients with BMI < 24 kg/m2.
For individual MACE endpoints, there were no significant differences in all-cause mortality (0.37% in the MUSKARDIA group vs. 0.23% in the placebo group), non-fatal MI (0.97% in the MUSKARDIA group vs. 1.51% in the placebo group), and non-fatal stroke (0.67% in the MUSKARDIA group vs. 0.90% in the placebo group). The detailed comparisons on efficacy endpoints are shown in Supplementary Table S3. At 18 months, the MUSKARDIA group had significantly higher scores than the placebo group for angina stability (P = 0.0458) and angina frequency (P = 0.0362). No significant differences were observed in angina stability (P = 0.9104) and angina frequency (P = 0.0742) at 24 months [Figure 3]. Treatment compliance was similar between groups, with 84.5% the patients in the MUSKARDIA group and 82.0% in the placebo group achieving ≥70% compliance in FAS [Supplementary Table S4].
Figure 3.

Angina assessed by the Seattle angina questionnaire. Shexiang Baoxin Pill improved angina stability (A) and frequency (B) in patients with stable coronary artery disease at 18 months.
Safety endpoints and key laboratory results
In the SS, 236 patients (17.7%) in the MUSKARDIA group had at least 1 AE, which could be compared with 231 patients (17.1%) in the placebo group (P = 0.8785). The total numbers of AEs were 443 in the MUSKARDIA group and 477 in the placebo group. SAEs occurred in 47 patients (3.5%) in the MUSKARDIA group and 41 patients (3.1%) in the placebo group. Table 2 summarizes the centrally adjudicated clinical events, clinical AEs, and laboratory abnormalities.
Table 2.
Adverse events of patients with coronary artery disease undergoing different treatment.
| Variables | Placebo (n = 1327) | MUSKARDIA (n = 1335) |
| Had ≥1 AE | 231 (17.4) | 236 (17.7) |
| Had ≥1 SAE | 41 (3.1) | 47 (3.5) |
| Cardiovascular events | ||
| Unstable angina | 11 (0.8) | 7 (0.5) |
| Atrial fibrillation | 1 (0.1) | 3 (0.2) |
| Acute MI | 4 (0.3) | 3 (0.2) |
| Stable angina | 21 (1.6) | 16 (1.2) |
| Old MI | 2 (0.2) | 0 |
| Hepatobiliary disease | ||
| Liver dysfunction | 3 (0.2) | 2 (0.1) |
| Liver discomfort | 0 | 1 (0.1) |
| Gamma glutamyl transferase | 5 (0.4) | 3 (0.2) |
| Elevated alanine aminotransferase | 3 (0.2) | 4 (0.3) |
| Elevated aspartate aminotransferase | 3 (0.2) | 2 (0.1) |
| Elevated blood cholesterol | 7 (0.5) | 4 (0.3) |
| Elevated blood triglycerides | 18 (1.4) | 15 (1.1) |
| Elevated transaminase | 2 (0.2) | 1 (0.1) |
| Renal and urinary system diseases | ||
| Chronic kidney failure | 1 (0.1) | 0 |
| Urinary incontinence | 1 (0.1) | 0 |
| Renal failure | 0 | 1 (0.1) |
| Renal pain | 1 (0.1) | 0 |
| Acute kidney injury | 0 | 1 (0.1) |
| Hematuria | 3 (0.2) | 1 (0.1) |
| Elevated serum creatinine | 5 (0.4) | 5 (0.4) |
| Proteinuria | 9 (0.7) | 9 (0.7) |
| Elevated uric acid | 14 (1.1) | 14 (1.0) |
| Metabolic and nutritional diseases | ||
| New onset type 2 diabetes | 1 (0.1) | 3 (0.2) |
| Hypoglycemia | 0 | 1 (0.1) |
| Hypercholesterolemia | 1 (0.1) | 0 |
| Hypertriglyceridemia | 2 (0.2) | 2 (0.1) |
| Hyperuricemia | 6 (0.5) | 3 (0.2) |
| Hyperglycemia | 0 | 1 (0.1) |
| Hyperlipidemia | 12 (0.9) | 10 (0.7) |
| Loss of appetite | 1 (0.1) | 0 |
| Diabetes | 1 (0.1) | 2 (0.1) |
| Peripheral edema | 1 (0.1) | 0 |
| Dyslipidemia | 1 (0.1) | 0 |
Data are shown as n (%). AE: Adverse event; MI: Myocardial infarction; MUSKARDIA: Shexiang Baoxin Pill; SAE: Serious adverse event.
Discussion
TCM has long been used to treat CAD in China. However, the evidence regarding the efficacy and safety from large scale RCTs is lacking. Therefore, this study aimed to determine the effects of MUSKARDIA on stable CAD as an add-on to OMT by enrolling 2673 patients with stable CAD from 97 sites across China. Our results showed that add-on MUSKARDIA to standard aspirin and statin in patients with stable CAD was safe and significantly reduced angina frequency at 18 and 24 months. In addition, a trend towards reduced MACE was observed in the MUSKARDIA group.
The bioactive components of MUSKARDIA include muscone, ginsenosides, storax, bufadienolides, cinnamic acid, arenobufagin, and borneol.[13,14,16] Muscone has been shown to have beneficial effects on cardiac remodeling in animal models of CAD.[21] Bufadienolides are compounds that are toxic at high doses, but are beneficial at low doses to control heart failure.[22] Ginsenosides exert cardioprotective functions through anti-oxidative activity, inhibiting platelet adhesion, promoting vasoconstriction, improving lipid profile, and regulating ion channels.[23] Cinnamic acid is known for the management of diabetes and its complications[24] while borneol is known for its anti-ischemia effects.[25] Therefore, taken together, the combination of different compounds in MUSKARDIA might be beneficial for patients with CAD, as supported by data from preliminary studies.[13,17,18]
Despite OMT, CAD patients might still face residual cardiovascular risk.[5–7] Indeed, Bhatt et al[5] showed that among 45,227 patients with stable CAD, the risk of MACE was about 12%. The ACTION trial reported the occurrences of 1.53 to 1.64 per 100 person-years for death and 4.60 to 4.75 per 100 person-years for the primary endpoint, which was the combination of death, acute MI, refractory angina, new overt heart failure, debilitating stroke, and peripheral revascularization.[19] In the recently published Cardiovascular Outcomes for People Using Anticoagulation Strategies (COMPASS) trial the occurrence of MACE was 4% to 6% after 1.95 years.[26]
The combined use of aspirin and statin is a standard approach to reduce the risk of cardiovascular events in patients with stable CAD in a secondary prevention context.[1–4] Several studies showed that the long-term use of statin decreased the residual risk of MACE in CAD patients.[27–32] In a study on 7657 CAD patients, the use of both aspirin and statin reduced the risk of MACE over 10 years.[32] The confirm registry showed that statin but not aspirin reduced the risk of MACE in CAD patients.[33] A previous study reported that statins and aspirin improved the long-term clinical outcomes after PCI.[34] In a recent Chinese study the incidence of MACE was 1.8% in CAD patients treated with statin.[35] In the present study the 24-month MACE rate for the overall study population was 2.3% for the MUSKARDIA group and 3.1% for the placebo group. These rates of occurrence were lower than those observed in other populations,[5,19,26] but similar to that found in a recent Chinese study.[35] The relatively low MACE rate might have several explanations. Notably, the proportion of patients on optimal therapy at baseline was high in the present study, with 99.7% on aspirin and 93.0% on statin, these values were much higher than those reported in previous studies.[27–32] Moreover, the compliance with medication was high during the 24-month study period (good compliance for more than 80% of the study patients), which was also considerably higher than previous findings.[27–32] Second, the patients enrolled in this trial had a relatively low risk of recurrent CVD. The inclusion criterion for coronary stenosis was ≥50% in one or more coronary arteries compared with ≥50% in two or more coronary arteries in the COMPASS trial.[26] Other trials, such as the Intravascular Cooling in the Treatment of Stroke trial, reported MACE rates of > 20%, but they enrolled patients who were at higher risks.[36] Nevertheless, the unexpectedly low MACE rate might underpower the results of the present study given the sample size. The Kaplan-Meier curves showed a separation after 18 months of treatment, indicating that MUSKARDIA had a trend towards a superior effect compared with the placebo. This delay in curve separation was in accordance with the characteristic slow action of TCM.[37] In other words, an underpowered sample size and a relatively short treatment period might be the main causes of the borderline neutral results on the occurrence of MACE, despite a numerical reduction of 26.9%. On the contrary, the subgroup analyses revealed statistically significant results in females and patients with BMI < 24 kg/m2. This result might provide a hint of treatment option for Asian females who generally have a lower BMI and higher gastric intolerance to aspirin.
Nevertheless, the present trial confirmed the relative long-term safety of MUSKARDIA. For this 2-year TCM add-on treatment, the levels of creatinine clearance, serum alanine aminotransferase, and serum aspartate aminotransferase were similar between the two groups throughout the trial under strict monitoring. Safety, particularly long-term safety, is a major concern for the use of TCM. The present study addressed this concern, at least regarding MUSKARDIA, with the help of a large-scale RCT in CAD for a 2-year period. Nevertheless, additional studies are required to examine the adverse effects of TCM over a longer period.
Angina stability and angina frequency scores were significantly reduced at 18 months in the MUSKARDIA group compared with the placebo group. This time point was in accordance with the separation of the Kaplan-Meier curves for MACE. These results might suggest that the chronic administration of MUSKARDIA ≥18 months might be essential to induce better coronary circulation and thus result in lower angina frequency.
The present trial has several limitations. First, the trial was registered after the enrollment of the first study patient. For historical reasons, China had not adopted the tradition of trial registration at the time of trial preparation in 2011. Still, there was no exposure of preliminary results or bias induced from the delay of registration. Between the randomization of the first patient (07/2011) and trial registration (04/2012), no change in the trial protocol or statistical analysis plan was made and no unblinding was undertaken. Second, the 2-year treatment might just allow the starting action of MUSKARDIA, but not reveal its maximal beneficial effects. The follow-up after 2 years was not conducted, leading to loss of precious information beyond the trial. The underestimation of the occurrence of the primary endpoint resulted in a relatively small sample size and the underpowered sample size probably contributed to the negative results for the primary endpoint. Finally, several important variables were not examined (eg, inflammatory markers and oxidative stress status).
In conclusion, as an add-on therapy to aspirin and statin, the 24-month use of MUSKARDIA is safe and reduced angina frequency in patients with CAD. Moreover, a 26.9% reduction in MACE was found in the MUSKARDIA group compared with the placebo group; however, this reduction did not reach statistical significance.
Acknowledgements
This study was funded by the Shanghai Science and Technology Committee and Shanghai Hutchison Pharmaceuticals Company.
Conflicts of interest
None.
Supplementary Material
Footnotes
How to cite this article: Ge JB, Fan WH, Zhou JM, Shi HM, Ji FS, Wu Y, Zhao YL, Qian J, Jin YZ, Liu YW, Wang SH, He SH, Yang P, Wu J, Lu F, Hou ZS. Efficacy and safety of Shexiang Baoxin pill in patients with stable coronary artery disease: a multicenter, double-blind, placebo-controlled phase IV randomized clinical trial. Chin Med J 2021;134:185–192. doi: 10.1097/CM9.0000000000001257
References
- 1.Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010; 376:1670–1681. doi: 10.1016/s0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith SC, Jr, Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the american heart association and american college of cardiology foundation. Circulation 2011; 124:2458–2473. doi: 10.1161/CIR.0b013e318235eb4d. [DOI] [PubMed] [Google Scholar]
- 3.Anderson TJ, Gregoire J, Pearson GJ, Barry AR, Counture P, Dawes M, et al. 2016 Canadian cardiovascular society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol 2016; 32:1263–1282. doi: 10.1016/j.cjca.2016.07.510. [DOI] [PubMed] [Google Scholar]
- 4.Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, et al. 2016 European guidelines on cardiovascular disease prevention in clinical practice: the sixth joint task force of the European society of cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European association for cardiovascular prevention & rehabilitation (EACPR). Eur Heart J 2016; 37:2315–2381. doi: 10.1093/eurheartj/ehw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatt DL, Eagle KA, Ohman EM, Hirsch AT, Goto S, Mahoney EM, et al. Comparative determinants of 4-year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA 2010; 304:1350–1357. doi: 10.1001/jama.2010.1322. [DOI] [PubMed] [Google Scholar]
- 6.Lin FJ, Tseng WK, Yin WH, Yeh HI, Chen JW, Wu CC. Residual risk factors to predict major adverse cardiovascular events in atherosclerotic cardiovascular disease patients with and without diabetes mellitus. Sci Rep 2017; 7:9179.doi: 10.1038/s41598-017-08741-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Bacquer D, Dallongeville J, Kotseva K, Cooney MT, Pajak A, Deckers JW, et al. Residual risk of cardiovascular mortality in patients with coronary heart disease: the EUROASPIRE risk categories. Int J Cardiol 2013; 168:910–914. doi: 10.1016/j.ijcard.2012.10.051. [DOI] [PubMed] [Google Scholar]
- 8.Latib A, Ielasi A, Ferri L, Chieffo A, Godino C, Carlino M, et al. Aspirin intolerance and the need for dual antiplatelet therapy after stent implantation: a proposed alternative regimen. Int J Cardiol 2013; 165:444–447. doi: 10.1016/j.ijcard.2011.08.080. [DOI] [PubMed] [Google Scholar]
- 9.Fan Y, Feng S, Xia W, Qu L, Li X, Chen S, et al. Aspirin-exacerbated respiratory disease in China: a cohort investigation and literature review. Am J Rhinol Allergy 2012; 26:e20–22. doi: 10.2500/ajra.2012.26.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xue Y, Feng ZW, Li XY, Hu ZH, Xu Q, Wang Z, et al. The efficacy and safety of cilostazol as an alternative to aspirin in Chinese patients with aspirin intolerance after coronary stent implantation: a combined clinical study and computational system pharmacology analysis. Acta Pharmacol Sin 2017; doi: 10.1038/aps.2017.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hao P, Jiang F, Cheng J, Ma L, Zhang Y, Zhao Y. Traditional Chinese medicine for cardiovascular disease: evidence and potential mechanisms. J Am Coll Cardiol 2017; 69:2952–2966. doi: 10.1016/j.jacc.2017.04.041. [DOI] [PubMed] [Google Scholar]
- 12.Wang J, Lin F, Guo LL, Xiong XJ, Fan X. Cardiovascular Disease, Mitochondria, and Traditional Chinese Medicine. Evid Based Complement Alternat Med 2015; 2015:143145.doi: 10.1155/2015/143145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou Z, Shen W, Yu L, Xu C, Wu Q. A Chinese patent medicine, Shexiang Baoxin Pill, for Non-ST-elevation acute coronary syndromes: A systematic review. J Ethnopharmacol 2016; 194:1130–1139. doi: 10.1016/j.jep.2016.11.024. [DOI] [PubMed] [Google Scholar]
- 14.Fang HY, Zeng HW, Lin LM, Chen X, Shen XN, Fu P, et al. A network-based method for mechanistic investigation of Shexiang Baoxin Pill's treatment of cardiovascular diseases. Sci Rep 2017; 7:43632.doi: 10.1038/srep43632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang S, Peng C, Jiang P, Fu P, Tao J, Han L, et al. Simultaneous determination of seven bufadienolides in rat plasma after oral administration of Shexiang Baoxin Pill by liquid chromatography-electrospray ionization-tandem mass spectrometry: application to a pharmacokinetic study. J Chromatogr B Analyt Technol Biomed Life Sci 2014; 967:255–263. doi: 10.1016/j.jchromb.2014.07.038. [DOI] [PubMed] [Google Scholar]
- 16.Chang W, Han L, Huang H, Wen B, Peng C, Lv C, et al. Simultaneous determination of four volatile compounds in rat plasma after oral administration of Shexiang Baoxin Pill (SBP) by HS-SPDE-GC-MS/MS and its application to pharmacokinetic studies. J Chromatogr B Analyt Technol Biomed Life Sci 2014; 963:47–53. doi: 10.1016/j.jchromb.2014.05.047. [DOI] [PubMed] [Google Scholar]
- 17.Zhang KJ, Zhu JZ, Bao XY, Zheng Q, Zheng GQ, Wang Y. Shexiang Baoxin pills for coronary heart disease in animal models: preclinical evidence and promoting angiogenesis mechanism. Front Pharmacol 2017; 8:404.doi: 10.3389/fphar.2017.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang LJ, Luo XP, Wang Y. Evaluation on tolerability and safety of long-term administration with shexiang baoxin pill in patients with coronary heart disease of stable angina pectoris. Zhongguo Zhong Xi Yi Jie He Za Zhi 2008; 28:399–401. [PubMed] [Google Scholar]
- 19.Poole-Wilson PA, Lubsen J, Kirwan BA, Van Dalen FJ, Wagener G, Danchin N, et al. Effect of long-acting nifedipine on mortality and cardiovascular morbidity in patients with stable angina requiring treatment (ACTION trial): randomised controlled trial. Lancet 2004; 364:849–857. doi: 10.1016/s0140-6736(04)16980-8. [DOI] [PubMed] [Google Scholar]
- 20.Fox KM, Henderson JR, Bertrand ME, Ferrari R, Remme WJ, Simoons ML. The European trial on reduction of cardiac events with perindopril in stable coronary artery disease (EUROPA). Eur Heart J 1998; 19: Suppl J: J52–J55. [PubMed] [Google Scholar]
- 21.Wang X, Meng H, Chen P, Yang N, Lu X, Wang ZM, et al. Beneficial effects of muscone on cardiac remodeling in a mouse model of myocardial infarction. Int J Mol Med 2014; 34:103–111. doi: 10.3892/ijmm.2014.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffmann D. Medical Herbalism: The Science and Practice of Herbal Medicine. Rochester: Healing Arts Press; 2003. [Google Scholar]
- 23.Lee CH, Kim JH. A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. J Ginseng Res 2014; 38:161–166. doi: 10.1016/j.jgr.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adisakwattana S. Cinnamic acid and its derivatives: mechanisms for prevention and management of diabetes and its complications. Nutrients 2017; 9:163.doi: 10.3390/nu9020163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kong QX, Wu ZY, Chu X, Liang RQ, Xia M, Li L. Study on the anti-cerebral ischemia effect of borneol and its mechanism. Afr J Tradit Complement Altern Med 2014; 11:161–164. doi: 10.4314/ajtcam.v11i1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anand SS, Bosch J, Eikelboom JW, Connolly SJ, Diaz R, Widimsky P, et al. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet 2017; 391:219–229. doi: 10.1016/s0140-6736(17)32409-1. [DOI] [PubMed] [Google Scholar]
- 27.Larsen AI, Tomey MI, Mehran R, Nilsen DW, Kirtane AJ, Witzenbichler B, et al. Comparison of outcomes in patients with ST-segment elevation myocardial infarction discharged on versus not on statin therapy (from the Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction Trial). Am J Cardiol 2014; 113:1273–1279. doi: 10.1016/j.amjcard.2014.01.401. [DOI] [PubMed] [Google Scholar]
- 28.Lenderink T, Boersma E, Gitt AK, Zeymer U, Wallentin L, Van de Werf F, et al. Patients using statin treatment within 24 h after admission for ST-elevation acute coronary syndromes had lower mortality than non-users: a report from the first Euro Heart Survey on acute coronary syndromes. Eur Heart J 2006; 27:1799–1804. doi: 10.1093/eurheartj/ehl125. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz GG, Olsson AG, Ezekowitz MD, Ganz P, Oliver MF, Waters D, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA 2001; 285:1711–1718. doi: 10.1001/jama.285.13.1711. [DOI] [PubMed] [Google Scholar]
- 30.Stenestrand U, Wallentin L. Swedish Register of Cardiac Intensive C. Early statin treatment following acute myocardial infarction and 1-year survival. JAMA 2001; 285:430–436. doi: 10.1001/jama.285.4.430. [DOI] [PubMed] [Google Scholar]
- 31.Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. JAMA 2007; 297:177–186. doi: 10.1001/jama.297.2.177. [DOI] [PubMed] [Google Scholar]
- 32.Wei L, Fahey T, MacDonald TM. Adherence to statin or aspirin or both in patients with established cardiovascular disease: exploring healthy behaviour vs. drug effects and 10-year follow-up of outcome. Br J Clin Pharmacol 2008; 66:110–116. doi: 10.1111/j.1365-2125.2008.03212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schulman-Marcus J, Hartaigh BO, Giambrone AE, Gransar H, Valenti V, Berman DS, et al. Effects of cardiac medications for patients with obstructive coronary artery disease by coronary computed tomographic angiography: results from the multicenter CONFIRM registry. Atherosclerosis 2015; 238:119–125. doi: 10.1016/j.atherosclerosis.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kubota N, Kasai T, Miyauchi K, Njaman W, Kajimoto K, Akimoto Y, et al. Therapy with statins and aspirin enhances long-term outcome of percutaneous coronary intervention. Heart Vessels 2008; 23:35–39. doi: 10.1007/s00380-007-1007-8. [DOI] [PubMed] [Google Scholar]
- 35.Xie G, Sun Y, Myint PK, Patel A, Yang X, Li M, et al. Six-month adherence to Statin use and subsequent risk of major adverse cardiovascular events (MACE) in patients discharged with acute coronary syndromes. Lipids Health Dis 2017; 16:155.doi: 10.1186/s12944-017-0544-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirsch A, Windhausen F, Tijssen JG, Verheugt FW, Cornel JH, de Winter RJ, et al. Long-term outcome after an early invasive versus selective invasive treatment strategy in patients with non-ST-elevation acute coronary syndrome and elevated cardiac troponin T (the ICTUS trial): a follow-up study. Lancet 2007; 369:827–835. doi: 10.1016/S0140-6736(07)60410-3. [DOI] [PubMed] [Google Scholar]
- 37.Lam TP. Strengths and weaknesses of traditional Chinese medicine and Western medicine in the eyes of some Hong Kong Chinese. J Epidemiol Community Health 2001; 55:762–765. doi: 10.1136/jech.55.10.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


