Abstract
Background
Endometrial cancer is one of the most common malignancies of the reproductive system. Effective and cost-effective screening method for populations at high risk is not available. This study aimed to investigate specimen adequacy and the influencing factors in microscale endometrial sampling biopsy and to evaluate the diagnostic accuracy and medical cost of biopsy in endometrial cancer and atypical hyperplasia screenings in comparison with hysteroscopic endometrial biopsy.
Methods
A total of 1551 patients at high risk for endometrial lesions who required hysteroscopic endometrial biopsy from November 2017 to August 2018 were included. Microscale endometrial sampling biopsy was performed, followed by hysteroscopic endometrial biopsy. We evaluated the specimen adequacy and influencing factors of microscale endometrial sampling. Diagnostic consistency between microscale endometrial sampling biopsy and hysteroscopic endometrial biopsy was evaluated. The sensitivity, specificity, positive predictive value, and negative predictive value of microscale endometrial sampling biopsy in screening for endometrial cancer and atypical hyperplasia were analyzed, and the medical costs of the two procedures were compared.
Results
The specimen adequacy was 81.2%. Patient age, menopausal status, endometrial thickness, and endometrial lesion type were correlated with specimen adequacy. There was good consistency in distinguishing benign and malignant endometrial diseases between microscale endometrial sampling biopsy and hysteroscopic biopsy (kappa 0.950, 95% CI 0.925–0.975). The sensitivity, specificity, positive predictive value, and negative predictive value of microscale endometrial sampling biopsy were 91.7%, 100.0%, 100.0%, and 99.3% for endometrial cancer screening, respectively, and 82.0%, 100.0%, 100.0%, and 99.4% for atypical hyperplasia screening. The medical cost of endometrial sampling biopsy was only 22.1% of the cost of hysteroscopic biopsy.
Conclusions
Microscale endometrial sampling biopsy is a minimally invasive alternative technique for obtaining adequate endometrial specimens for histopathological examination. It has the potential to be used in detecting endometrial cancer and atypical hyperplasia with high efficiency and low cost.
Keywords: Endometrial atypical hyperplasia, Endometrial cancer, Hysteroscopic endometrial biopsy, Microscale endometrial sampling biopsy
Introduction
Endometrial cancer is one of the most common malignancies of the reproductive system. The incidence of endometrial cancer has increased in recent years. The American Cancer Society (ACS) reported that 61,380 new cases of endometrial cancer were identified in the United States in 2017, and 10,920 patients died.[1] In 2018, it was estimated that 382,096 new cases of endometrial cancer were identified, and 89,929 patients died of endometrial cancer worldwide.[2] In China, the estimated incidence of endometrial cancer was 63.4/100,000 in 2015, and the mortality rate was 21.8/100,000.[3] Therefore, screening is critical for the early diagnosis of endometrial cancer.
Cervical cancer screening has gradually become a mature strategy and has been widely used in clinical practice. However, a cost-efficient screening procedure is not available for endometrial cancer screening. In traditional endometrial histology, the specimen is obtained mainly via dilatation and curettage (D&C) or hysteroscopy, both of which are invasive and costly. In recent years, various endometrial sampling devices have been used to harvest endometrial cells or tissue for screening. This procedure is less invasive and less expensive; however, the diagnostic accuracy reported in the literature was not consistent, and results from large sample-sized control studies are lacking.[4–6]
In this study, we obtained microscale endometrial specimens with a non-invasive endometrial sampling device in women at high risk for endometrial lesions who required hysteroscopic endometrial biopsy. We first evaluated the specimen adequacy and the factors influencing endometrial microtissue collection by the endometrial sampler, to determine the characteristics of patients who were more suitable for collecting endometrial tissue with this minimally invasive method. We further compared the diagnostic accuracy and medical cost between microscale endometrial sampling biopsy and hysteroscopic endometrial biopsy (the gold standard) to evaluate the significance of microscale endometrial biopsy in detecting endometrial malignant lesions (including endometrial cancer and endometrial atypical hyperplasia).
Methods
Ethical approval
The study was approved by the Ethics Committee of Peking University People's Hospital (IRB No. 2018 PHB 034-01). After signing an informed consent form, the patients underwent endometrial device sampling with microscale endometrial sampling biopsy, followed by hysteroscopic endometrial biopsy.
Study design
A total of 1551 consecutive female patients at high risk for endometrial lesions who required hysteroscopy with endometrial biopsy from November 2017 to August 2018 were included in this study. At least one of the following clinical conditions was present in the included patients: (1) abnormal uterine bleeding; (2) vaginal color Doppler ultrasound indicating an intrauterine occupational disease or abnormal thickening of the endometrium (endometrial thickness ≥5 mm in postmenopausal patients without hormone replacement therapy); (3) patients attending follow-up examinations after oral progesterone treatment for endometrial cancer or endometrial hyperplasia.
Patients were excluded if one of the following conditions was present: (1) a confirmed diagnosis of cervical cancer; (2) inability to tolerate hysteroscopic surgery due to severe systemic complications; (3) acute vaginitis or pelvic inflammatory disease; (4) postpartum women in the puerperal period or those who recently underwent abortion; (5) patients with endometrial hyperplasia treated with levonorgestrel intrauterine system (LNG-IUS).
Specimen collection and micropathological specimen preparation
Before performing hysteroscopy, misoprostol was placed in the vagina to prepare the uterine cervix, except in patients with suspected endometrial cancer. The patient was placed in the lithotomy position, and intravenous anesthesia was administered. After routine disinfection of the operative field, a speculum was used to expose the vagina and the cervix, and the endometrial sampling device (SAP-1; Saipujiuzhou, Beijing, China) was used to harvest microscale endometrial tissue. The device has been patented in China and approved for clinical use. The diameter of the device is 2.8 mm, and the length is 250 mm. It consists of an outer sheath, a harvesting loop, and a handle. The outer sheath is a flexible polypropylene plastic tube marked with scales to measure the depth of the uterine cavity. A slidable piston with a harvesting loop on its tip is contained within the outer sheath. Six serrated scrapers are evenly distributed along the harvesting loop, which can reach the basal layer of the endometrium but cannot pass through the basal layer. The shape of the harvesting loop is similar to the shape of the uterine cavity. The entire endometrium can be reached by 360° rotation. The harvesting loop can be retracted into the outer sheath before and after sampling to avoid contamination of the endometrial specimens by cells outside the uterine cavity [Figure 1]. The sampling procedure is shown in Figure 2. The four-step process of endometrial sampling by the SAP-1 device was performed only once in each patient. The specimen was fixed in a specific fixative agent, labeled, and sent for microscale tissue pathology. The specimen was processed in a microsample embedding machine (Saipujiuzhou, Beijing, China) to generate a microscale tissue specimen block, which was then dehydrated, waxed, sectioned, and stained with hematoxylin-eosin. After endometrial device sampling with microscale tissue biopsy, hysteroscopy (Olympus, Tokyo, Japan) was performed for endometrial tissue biopsy under direct hysteroscopic vision. The endometrial tissue specimens were fixed in 10% formaldehyde, embedded in paraffin wax, sectioned, and stained with hematoxylin-eosin.
Figure 1.
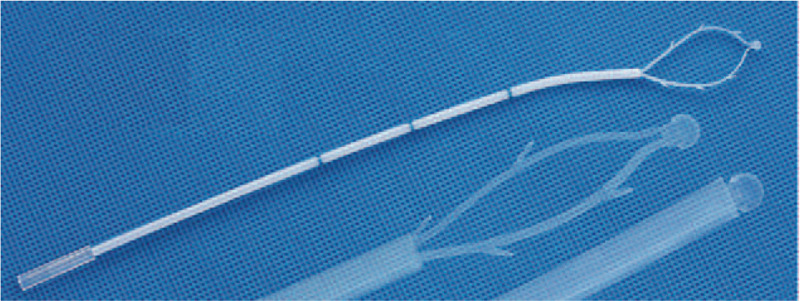
The SAP-1 sampling device.
Figure 2.
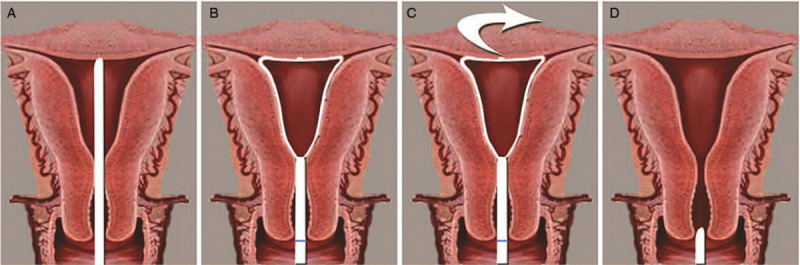
Steps for obtaining samples using the SAP-1 device. (A) The tip of the sampling device is reaching the fundus. (B) The sampling loop is opened. (C) The loop is rotated for sampling. (D) The sampling loop is retracted into the sheath, and the device is withdrawn from the uterine cavity.
Criteria for specimen adequacy
The specimen adequacy was evaluated by cooperation of the pathologists and the gynecologists who performed hysteroscopic operations. Samples that satisfied the following conditions were considered to be adequate samples: (1) Obvious scratch marks can be visualized in the intrauterine walls, fundus, and fallopian tube openings under hysteroscopy; (2) sufficient amounts of glandular epithelial cells are present, and the number of glands is ≥5;[7] (3) a clear pathological diagnosis can be determined. Criteria for specimen inadequacy includes: (1) scratch marks can be visualized in the intrauterine walls, but no scratch marks can be visualized in either the fundus or the fallopian tube openings under hysteroscopy; (2) tissue specimens are too small and insufficient for pathological diagnosis.
Result interpretation
Two groups of experts from the Department of Pathology who were blinded to the study determined the histological diagnoses. The histological diagnosis results are divided into the following four categories: (1) endometrial cancer; (2) endometrial atypical hyperplasia; (3) normal or benign endometrial changes (including proliferative endometrium, secretory endometrium, endometrial hyperplasia without atypical hyperplasia, and endometritis); (4) intrauterine occupational disease (including endometrial polyps and submucosal uterine fibroids).
Statistical analysis
The Chi-square test was used to compare specimen adequacy between pre- and post-menopausal women and those with benign and malignant endometrial lesions. Logistic regression analysis was used to assess the factors influencing specimen adequacy. Diagnostic agreement was compared between endometrial sampling biopsy and hysteroscopic endometrial biopsy with kappa statistics. The sensitivity, specificity, positive predictive value and negative predictive value of endometrial microscale tissue biopsy in detecting endometrial cancer and atypical hyperplasia were analyzed. Hysteroscopic endometrial biopsy was considered as the gold standard. SPSS software (version 22.0; IBM Corp, Armonk, NY, USA) was used for the statistical analysis. The medical costs of the two procedures were calculated and compared. A P < 0.05 was considered to be statistical significance.
Results
Specimen adequacy and influencing factors
The patients at high risk for endometrial lesions who required hysteroscopy with endometrial biopsy from November 2017 to August 2018 were consecutively recruited. Microscale endometrial sampling biopsy was performed before hysteroscopic endometrial biopsy in all of the recruited patients (n = 1616). Three of them who recently underwent abortions were excluded according to the exclusion criteria. Then, 62 patients who lacked a definite diagnosis due to insufficient endometrial samples on hysteroscopic biopsy were excluded. Thus, the number of enrolled patients in this study was 1551. The inclusion and exclusion criteria were not relevant with the severity of the disease. The clinical data of the 1551 patients are shown in Table 1. Specimen adequacy was confirmed in 1260 patients. The rate of specimen adequacy was 81.2% (1260/1551). The rate of specimen adequacy was significantly higher for premenopausal patients (89.6%, 1000/1116) than for postmenopausal patients (59.8%, 260/435; Pearson χ2 = 182.798, P < 0.001). The rate of specimen adequacy was significantly higher for endometrial cancer and atypical hyperplasia (97.1%, 166/171) than for benign diseases (79.3%, 1094/1380), including normal or benign endometrial changes and intrauterine occupational disease (Pearson χ2 = 69.090, P < 0.001).
Table 1.
Clinical characteristics of the 1551 patients at high risk for endometrial lesions who required hysteroscopic endometrial biopsy
| Clinical Characteristics | Premenopausal (n = 1116) | Postmenopausal (n = 435) | Total (n = 1551) |
| Age (years), median (range) | 40 (20–56) | 58 (37–83) | 44 (20–83) |
| Indication, n | |||
| Intrauterine anomalies∗ | 411 | 226 | 637 |
| Abnormal Uterine bleeding | 437 | 156 | 593 |
| Intrauterine anomalies∗ Plus abnormal uterine bleeding | 168 | 53 | 221 |
| Follow-up examination of endometrial lesions after progesterone treatment | 100 | 0 | 100 |
| Pathological diagnosis, n | |||
| Endometrial cancer | 50 | 71 | 121 |
| Endometrial atypical hyperplasia | 45 | 5 | 50 |
| Normal or benign endometrial changes | 528 | 94 | 622 |
| Intrauterine Occupational disease† | 493 | 265 | 758 |
| Specimen adequacy, n | |||
| Adequacy | 1000 | 260 | 1260 |
| Inadequacy | 116 | 175 | 291 |
B-mode ultrasound suggested an intrauterine occupational disease or abnormal thickening of the endometrium. † Uterine submucosal fibroids and endometrial polyps.
The results showed that age, menopausal status, endometrial thickness, and endometrial lesion types were correlated with specimen adequacy. The rate of specimen inadequacy was 3.162-fold higher for postmenopausal women than for premenopausal women. The rate of specimen inadequacy was 21.343-fold higher for patients with endometrial benign diseases than for patients with endometrial cancer. The factors affecting specimen adequacy are shown in Table 2.
Table 2.
Analysis of the factors affecting specimen adequacy for circular endometrial device sampling with microscale tissue biopsy.
| Univariate analysis | Multivariate analysis | |||||
| Items | Specimen inadequacy (n = 291) | Specimen adequacy (n = 1260) | OR (95% CI) | P | OR (95% CI) | P |
| Age (years), mean ± standard deviation | 52.15 ± 11.92 | 43.29 ± 10.93 | 1.068 (1.056–1.081) | <0.001 | 1.029 (1.009–1.050) | 0.004 |
| Menopausal status, n | ||||||
| No | 116 | 1000 | 1.0 | <0.001 | 1.0 | <0.001 |
| Yes | 175 | 260 | 5.802 (4.423–7.612) | 3.162 (1.988–5.028) | ||
| Endometrial lesion type, n | ||||||
| Endometrial cancer | 2 | 119 | 1.0 | <0.001 | 1.0 | <0.001 |
| Endometrial atypical hyperplasia | 3 | 47 | 3.798 (0.615–23.458) | 0.151 | 13.544 (2.093–87.647) | 0.006 |
| Normal or benign endometrial changes | 85 | 537 | 9.418 (2.285–38.812) | 0.002 | 21.343 (5.017–90.801) | <0.001 |
| Intrauterine Occupational disease | 201 | 557 | 21.471 (5.259–87.668) | <0.001 | 30.947 (7.367–130.008) | <0.001 |
| Indication, n | ||||||
| Intrauterine anomalies | 165 | 472 | 1.0 | <0.001 | 1.0 | 0.037 |
| Bleeding | 88 | 505 | 2.147 (1.188–3.882) | 0.011 | 0.815 (0.585–1.135) | 0.226 |
| Intrauterine anomalies plus abnormal uterine bleeding | 24 | 197 | 1.070 (0.582–1.967) | 0.826 | 0.571 (0.341–0.957) | 0.034 |
| Follow-up examination of endometrial lesions after progesterone treatment | 14 | 86 | 0.748 (0.369–1.516) | 0.421 | 1.641 (0.823–3.272) | 0.160 |
| Endometrial thickness (mm), mean ± standard deviation | 5.84 ± 4.63 | 9.37 ± 4.99 | 0.834 (0.806–0.863) | <0.001 | 0.885 (0.853–0.917) | <0.001 |
We also estimated specimen adequacy according to endometrial thickness. The endometrial thicknesses from 1551 patients were used to plot a receiver operating characteristic (ROC) curve for the factors affecting specimen adequacy. The area under the curve (AUC) was 0.722 (95% CI 0.687–0.756), and assuming a cut-off value of endometrial thickness of 6.5 mm, the estimated sensitivity and specificity for specimen adequacy were 71.4% and 66.7%, respectively.
Diagnostic consistency and validation in detecting endometrial cancer and atypical hyperplasia
For the 1260 cases with adequate specimens, the diagnostic agreement of microscale endometrial sampling biopsy and hysteroscopic biopsy was compared. First, we analyzed the diagnostic consistency according to the four categories of pathological results mentioned in the “Result interpretation” section. The kappa value was 0.248 (95% CI 0.217–0.279), as shown in detail in Table 3.
Table 3.
Consistency of the biopsy results from endometrial sampling and hysteroscopy, n (%).
| Hysteroscopic biopsy (gold standard) | |||||
| Microscale endometrial sampling biopsy | Endometrial cancer | Endometrial atypical hyperplasia | Normal or benign endometrial changes | Intrauterine occupational disease | Total |
| Endometrial cancer | 60 (4.8) | 0 | 0 | 0 | 60 (4.8) |
| Endometrial atypical hyperplasia | 51 (4.1) | 41 (3.3) | 0 | 0 | 92 (7.3) |
| Normal or benign endometrial changes | 8 (0.6) | 6 (0.5) | 537 (42.6) | 520 (41.3) | 1071 (85.0) |
| Intrauterine occupational disease | 0 | 0 | 0 | 37 (2.9) | 37 (2.9) |
| Total | 119 (9.4) | 47 (3.7) | 537 (42.6) | 557 (44.2) | 1260 (100.0) |
To further evaluate the diagnostic consistency of the two procedures in distinguishing between endometrial malignant and benign lesions, we defined two new diagnostic categories as follows: (1) malignant endometrial lesions, including endometrial atypical hyperplasia and cancer; (2) benign endometrial changes, including normal endometrium, benign endometrial lesions and uterine occupational lesions. The results showed that pathological diagnosis by the two procedures had good consistency in detecting benign and malignant endometrial diseases, with a kappa value of 0.950 (95% CI 0.925–0.975), as shown in Table 4.
Table 4.
Consistency of endometrial sampling and hysteroscopy in diagnosing malignant and benign endometrial changes, n (%).
| Hysteroscopic Biopsy (gold standard) | |||
| Microscale Endometrial Sampling Biopsy | Malignant Endometrial Lesions | Benign Endometrial Changes | Total |
| Malignant endometrial lesions∗ | 152 (12.1) | 0 | 152 (12.1) |
| Benign endometrial changes† | 14 (1.1) | 1094 (86.8) | 1108 (87.9) |
| Total | 166 (13.2) | 1094 (86.8) | 1260 (100.0) |
Including endometrial atypical hyperplasia and cancer.
Including normal endometrium, benign endometrial lesions, and uterine occupational lesions.
In this study, 121 cases of endometrial cancer and 50 cases of endometrial atypical hyperplasia were identified. The sensitivity, specificity, and positive and negative predictive values of microscale endometrial sampling biopsy were 91.7% (111/121), 100.0%, 100.0%, and 99.3% (1430/1440) for detecting endometrial cancer, respectively, and 82.0% (41/50), 100.0%, 100.0%, and 99.4% (1501/1510) for detecting endometrial atypical hyperplasia.
Medical cost comparison
Microscale endometrial sampling biopsy with endometrial sampling device can be performed in outpatient gynecological clinics. The overall medical cost was $44.49, including $24.22 for the endometrial sampling device, $5.47 for endometrial sampling, and $14.79 for histopathological examination. Hospital admission is required for hysteroscopic endometrial biopsy. Excluding hospitalization expenses, the surgery-related costs were $201.34, including $170.13 for hysteroscopy, $16.42 for D&C, and $14.79 for pathological examination. Clearly, the medical cost was considerably lower for microscale endometrial sampling biopsy than for hysteroscopic biopsy; the cost of the former procedure was only 22.1% (44.49/201.34) of the cost of the latter.
Discussion
Histology is the gold standard for evaluating endometrial conditions. Traditionally, endometrial samples for histologic analysis can be obtained by hysteroscopy or D&C, and these methods are considered to be reliable. However, these procedures are not suitable for screening because they are invasive, complex, expensive, and painful and are associated with certain surgical risks and complications.
The emergence of endometrial sampling devices has provided new possibilities for endometrial cancer screening.[8] In this study, the endometrial sampling device was used to harvest microscale endometrial tissue by circular scraping, which can be called “intrauterine brushing.” In contrast with other endometrial collection devices (such as suction devices), the blades on the collection ring of the device brush the endometrial tissue that has not fallen off. After the collection ring is opened in the uterine cavity, it is compatible with the uterine cavity, thus ensuring comprehensiveness of specimen collection as much as possible. The four walls and bilateral corners of the uterine cavity can be sampled. Considering the thin structure and flexible material of the device, it can be freely inserted into and withdrawn from the uterine cavity without requiring dilation of the cervix, making this procedure minimally invasive, painless, safe, and convenient.
In previous studies, various endometrial sampling devices, such as aspiration devices or the pipelle, Tao brush, and SAP-1 samplers, were used to harvest exfoliated endometrial cells to screen for endometrial cancer, i.e., the endometrial cytological test (ECT). The endometrial cell sampling adequacy rate of these devices is 73.9% to 100.0% for the pipelle sampler, 89.9% to 100.0% for the Tao brush sampler and 96.3% for the SAP-1 sampler.[9,10] However, the ECT cannot provide information about the morphology of the endometrial glands, the proportions of the glands, and the stroma; therefore, this test cannot replace histopathological diagnosis.[11] Moreover, the ECT lacks the cytological diagnostic criteria unanimously accepted by cytologists.[12] Thus, the ECT has not been widely accepted and used in practice for endometrial cancer screening. More and more studies have evaluated the significance of endometrial cytology in endometrial cancer screening.[13,14]
In this study, microscale endometrial tissue was collected by the endometrial sampling device for pathological examination to determine the pathological type and degree of cell differentiation. The diagnostic criteria used with this method were the same as those used for pathological diagnosis with D&C or hysteroscopy. Therefore, endometrial device sampling for microscale endometrial tissue has great potential for the screening and diagnosis of endometrial lesions. Previously, our research group performed microscale tissue sampling with the SAP-1 device before hysteroscopy (169/182) or D&C (13/182). The rate of specimen adequacy was 81.3%. Menopausal status, endometrial thickness, and endometrial lesion type are important factors affecting the rate of specimen adequacy.[15] Other researchers have reported that endometrial sampling yields insufficient tissue for definite pathological diagnosis in 6% to 33% of cases.[16–20] A recent review indicated that the incidence of insufficient tissue following endometrial sampling was 28.8% (23.0%–35.0%). Significant independent factors associated with an increased risk of insufficient tissue were menopausal status and endometrial thickness less than 8 mm.[21] In this study, the rate of endometrial tissue specimen adequacy was 81.2%, consistent with previous studies. Patient age, menopausal status, and endometrial disease types were risk factors for specimen adequacy, while endometrial thickness was a protective factor for specimen adequacy.
The purpose of this study was to evaluate the possibility of using microscale endometrial sampling biopsy with a sampler to detect endometrial cancer in patients undergoing hysteroscopy in current clinical practice. Therefore, the heterogeneity of the patients was relatively high. Due to the inclusion of 758 cases (65.9%) of intrauterine occupational disease, for which the sample adequacy rate was only 73.5%, the overall sample adequacy rate was reduced. For those with endometrial thickness less than 6.5 mm, especially postmenopausal patients, microscale endometrial sampling biopsy should be used with caution to avoid missed diagnoses due to sample inadequacy. Therefore, the circular endometrial sampling device for microscale tissue biopsy is recommended for use in patients with an endometrial thickness greater than 6.5 mm and in patients with suspected endometrial cancer and atypical hyperplasia.
The results of the diagnostic consistency analysis suggested that microscale endometrial sampling biopsy has poor value in diagnosing intrauterine occupational diseases, possibly due to the tough tissue beyond the endometrium in these lesions and the difficulty of obtaining sufficient specimens. Thus, the sampling device is suitable for endometrial biopsy. Uterine occupational diseases beyond the endometrium should be diagnosed via hysteroscopy with direct biopsy. However, microscale endometrial sampling biopsy was efficient in differentiating malignant endometrial lesions from benign endometrial diseases. Moreover, the sensitivity, specificity, and positive and negative predictive values of microscale endometrial sampling biopsy were satisfactory for detecting endometrial cancer and atypical hyperplasia.
In this study, the costs of the two procedures were compared. The results showed that the overall medical cost was considerably lower for microscale endometrial sampling biopsy than for hysteroscopic biopsy.
In summary, specimen adequacy for pathological examination can be achieved using endometrial device sampling with microscale tissue biopsy. However, this procedure has limited significance in diagnosing endometrial benign lesions, especially intrauterine occupational lesions. Due to the higher sensitivity, specificity, and agreement rate and the significantly lower cost, microscale endometrial sampling biopsy can be used as a screening method for endometrial cancer and atypical hyperplasia. Further, hysteroscopic biopsy should be performed when specimen adequacy is not achieved via device sampling. Therefore, this method is not intended to replace hysteroscopy or D&C for endometrial cancer diagnosis but should be used as a screening method for endometrial cancer in high-risk women so that fewer must undergo invasive hysteroscopy.
Acknowledgements
The authors thank Xin Yang, Chao Zhao, Hong-Lan Zhu, Yang Zhao and Xiao-Wei Li from the Department of Gynecology and Obstetrics, Peking University People's Hospital, and Ming-Xia Li from the Department of Gynecology and Obstetrics, People's Liberation Army (PLA) Medical School, Chinese PLA General Hospital for their contributions in case collection and sampling of endometrium (both endometrial device sampling with microscale tissue biopsy and hysteroscopic endometrial biopsy).
Funding
This study was supported by grants from the Special Projects for Strengthening Basic Research of Peking University (No. BMU2018JC005) and the Application Research and Achievement Popularization of Clinical Characteristics in Capital from Beijing Municipal Science and Technology Commission (No. z161100000516227).
Conflicts of interest
This study was supported by Application Research and Achievement Popularization of Clinical Characteristics in Capital from the Beijing Municipal Science & Technology Commission (No. z161100000516227), which was undertaken by the enterprise Saipujiuzhou, Beijing, China. Peking University People's Hospital is one of the participants in this research. The authors declare no direct financial transactions with the enterprise. The research design, data collection, data analysis and article writing were all independently completed by researchers of Peking University People's Hospital, and the research data were independently kept by Peking University People's Hospital. The researchers have full access to all data in this study and are fully responsible for the completeness of the data and the accuracy of the data analysis.
Footnotes
How to cite this article: Zhang G, Wang Y, Liang XD, Zhou R, Sun XL, Wang JL, Wei LH. Microscale endometrial sampling biopsy in detecting endometrial cancer and atypical hyperplasia in a population of 1551 women: a comparative study with hysteroscopic endometrial biopsy. Chin Med J 2021;134:193–199. doi: 10.1097/CM9.0000000000001109
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics. CA Cancer J Clin 2017 2017; 67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016; 66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 4.Narice BF, Delaney B, Dickson JM. Endometrial sampling in low-risk patients with abnormal uterine bleeding: a systematic review and meta-synthesis. BMC Fam Pract 2018; 19:135.doi: 10.1186/s12875-018-0817-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tumrongkunagon S, Suknikhom W. Histological sampling of endometrial tissue: comparison between the MedGyn® endosampler and formal fractional curettage in patients with abnormal uterine bleeding. Asian Pac J Cancer Prev 2019; 20:3527–3531. doi: 10.31557/apjcp.2019.20.11.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Utida GM, Kulak J., Jr Hysteroscopic and aspiration biopsies in the histologic evaluation of the endometrium, a comparative study. Medicine (Baltimore) 2014; 98:e17183.doi: 10.1097/md.0000000000017183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang T, Zhou R, Liu C, Shen D. Influence of sampling satisfaction using endometrial sampling device and related factors for pathology diagnostic accordance rate (in Chinese). Chin J Obstet Gynecol 2014; 49:655–658. doi: 10.3760/cma.j.issn.0529-567x.2014.09.004. [PubMed] [Google Scholar]
- 8.Singh P, Zhou R, Liu C, Shen D. Abnormal Uterine Bleeding-evaluation by Endometrial Aspiration. J Midlife Health 2018; 9:32–35. doi: 10.4103/jmh.JMH_109_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du J, Li Y, Lv S, Wang Q, Sun C, Dong X, et al. Endometrial sampling devices for early diagnosis of endometrial lesions. J Cancer Res Clin Oncol 2016; 142:2515–2522. doi: 10.1007/s00432-016-2215-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wen J, Chen R, Zhao J, Dong Y, Yang X, Liao QP. Combining endometrium sampling device and surepath preparation to screen for endometrial carcinoma: a validation study. Chin Med J 2015; 128:648–653. doi: 10.4103/0366-6999.151664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Q, Wang Q, Zhao L, Han L, Sun C, Ma S, et al. Endometrial cytology as a method to improve the accuracy of diagnosis of endometrial cancer: case report and meta-analysis. Front Oncol 2019; 9:256.doi: 10.3389/fonc.2019.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Norimatsu Y, Yamaguchi T, Taira T, Abe H, Sakamoto H, Takenaka M, et al. Inter-observer reproducibility of endometrial cytology by the Osaki Study Group method: utilising the Becton Dickinson SurePath(™) liquid-based cytology. Cytopathology 2016; 27:472–478. doi: 10.1111/cyt.12342. [DOI] [PubMed] [Google Scholar]
- 13.Yanaki F, Hirai Y, Hanada A, Ishitani K, Matsui H. Liquid-based endometrial cytology using surepath™ is not inferior to suction endometrial tissue biopsy in clinical performance for detecting endometrial cancer including atypical endometrial hyperplasia. Acta Cytol 2017; 61:133–139. doi: 10.1159/000455890. [DOI] [PubMed] [Google Scholar]
- 14.Zhang H, Wen J, Xu PL, Chen R, Yang X, Zhou LE, et al. Role of liquid-based cytology and cell block in the diagnosis of endometrial lesions. Chin Med J 2016; 126:1459–1463. doi: 10.4103/0366-6999.183431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li MX, Zhou R, Liu C, Shen DH, Zhao LJ, Wang JL, et al. Direct uterine sampling using the SAP-l sampler device to detect endometrial lesions during histopathological examination. Eur J Gynaecol Oncol 2017; 38:221–226. [PubMed] [Google Scholar]
- 16.Bakour SH, Khan KS, Gupta JK. Controlled analysis of factors associated with insufficient sample on outpatient endometrial biopsy. BJOG 2000; 107:1312–1314. doi: 10.1111/j.1471-0528.2000. tb11627.x. [DOI] [PubMed] [Google Scholar]
- 17.Gordon SJ, Westgate J. The incidence and management of failed pipelle sampling in a general outpatient clinic. Aust N Z J Obstet Gynaecol 1999; 39:115–118. doi: 10.1111/j.1479-828x.1999. tb03460.x. [DOI] [PubMed] [Google Scholar]
- 18.Williams AR, Brechin S, Porter AJ, Warner P, Critchley HO. Factors affecting adequacy of pipelle and Tao brush endometrial sampling. BJOG 2008; 115:1028–1036. doi: 10.1111/j.1471-0528.2008.01773.x. [DOI] [PubMed] [Google Scholar]
- 19.Xie B, Qian C, Yang B, Ning C, Yao X, Du Y, et al. Risk factors for unsuccessful office-based endometrial biopsy: a comparative study of office-based endometrial biopsy (pipelle) and diagnostic dilation and curettage. J Minim Invasive Gynecol 2018; 25:724–729. doi: 10.1016/j.jmig.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 20.van Doorn HC, Opmeer BC, Burger CW, Duk MJ, Kooi GS, Mol BW. Inadequate office endometrial sample requires further evaluation in women with postmenopausal bleeding and abnormal ultrasound results. Int J Gynaecol Obstet 2007; 99:100–104. doi: 10.1016/j.ijgo.2007.05.040. [DOI] [PubMed] [Google Scholar]
- 21.Aue-Aungkul A, Kleebkaow P, Kietpeerakool C. Incidence and risk factors for insufficient endometrial tissue from endometrial sampling. Int J Womens Health 2018; 10:453–457. doi: 10.2147/IJWH.S172696. [DOI] [PMC free article] [PubMed] [Google Scholar]


